Abstract
Theranostic agents that can be sensitively and specifically activated by the tumor microenvironment (TME) have recently attracted considerable attention. In this study, TME-activatable 3,3′,5,5′-tetramethylbenzidine (TMB)-copper peroxide (CuO2)@poly(lactic-co-glycolic acid) (PLGA)@red blood cell membrane (RBCM) (TCPR) nanoparticles (NPs) for second near-infrared photoacoustic imaging-guided tumor-specific photothermal therapy were developed by co-loading CuO2 NPs and TMB into PLGA camouflaged by RBCMs. As an efficient H2O2 supplier, once exposed to a proton-rich TME, CuO2 NPs can generate H2O2 and Cu2+, which are further reduced to Cu+ by endogenous glutathione. Subsequently, the Cu+-mediated Fenton-like reaction produces cytotoxic ·OH to kill the cancer cells and induce TMB-mediated photoacoustic and photothermal effects. Combined with the RBCM modification-prolonged blood circulation, TCPR NPs display excellent specificity and efficiency in suppressing tumor growth, paving the way for more accurate, safe, and efficient cancer theranostics.
Keywords: Nanoparticles, Photoacoustic imaging, Second near-infrared region, Tumor microenvironment, Self-sufficient H2O2
Graphical Abstract
1. Introduction
Theranostic nanoplatforms, which integrate diagnostic and therapeutic functions, have paved the way for cancer management to achieve the goal of personalized medicine, owing to their unique advantages, including engineerable functionalities, early detection of tumors, visualization of the spatiotemporal biodistribution of nanoagents, and real-time assessment of therapeutic effects [1], [2], [3], [4], [5], [6]. Among various theranostic nanoplatforms, optical theranostic nanoagents have drawn increasing attention due to their minimal invasiveness, high specificity, and spatial-temporal selectivity.
Photothermal therapy (PTT), which causes cancer cell death by using incident light energy to induce hyperthermia using photothermal agents (PTAs), has substantial potential in oncotherapy. Compared to other diagnostic methods, photoacoustic imaging (PAI) has the advantages of excellent tissue penetrability and low scattering and dissipation in biological tissues; hence, PAI-based theranostic nanoplatforms have drawn increasing attention among all developed nanoplatforms. PAI/PTT theranostic nanoplatforms show great promise for tackling cancers.
Most of the reported theranostic agents lack tumor specificity, and the diagnostic signals and therapeutic effects are always in “on” mode. Intravenous (i.v.) injected nanoplatforms, which are prone to phagocytosis and retention by the reticuloendothelial system, not only lead to non-specific interference during cancer diagnosis, but also cause side effects during treatment. Therefore, it is preferable to design stimuli-responsive theranostic agents that can be exclusively activated in a specific focal area to overcome the aforementioned barriers.
Unlike normal tissues, the tumor microenvironment (TME) is generally characterized by hypoxia, acidosis, overexpressed H2O2, and high interstitial fluid pressure [7]. Taking advantage of these unique characteristics, TME-activatable theranostic agents (ATAs) have gained broad interest as the diagnostic and therapeutic features they induce can be “switched on” in tumors and “switched off” in normal tissues, which facilitates precise tumor detection and ablation with minimal non-specific damage. Such theranostic systems can be built by employing chemical reactions that are initiated exclusively at the tumor sites [8], [9], [10], [11], [12], [13], [14], [15]. For instance, H2O2-activated agents provide a feasible choice for constructing TME-activated ATAs [16], [17], [18], [19], [20], [21]. However, the H2O2 levels in the TME are often insufficient for ATAs to realize efficient cancer theranostics, and integrating H2O2-activated ATAs with self-sufficient H2O2 could be an effective strategy to tackle this challenge.
PTT, which causes cancer cell death by using light energy to induce hyperthermia via PTAs, has substantial potential in oncotherapy owing to its minimal invasiveness and short treatment period [22]. Exploring PTAs with high photothermal conversion efficiency (PCE) and strong near-infrared (NIR) absorption, especially in the second near-infrared (NIR-II) band (1000–1350 nm), is critical for improving the antitumor efficacy of PTT [23], [24], [25]. Noble metal nanoparticles (NPs), metal chalcogen NPs, polyoxometalates, semiconducting polymers, and MXene nanomaterials are the most common NIR-II-absorbing materials [26], [27], [28]. Among these, organic PTAs based on NIR-II-absorbing p-conjugated semiconducting polymers have been used for PTT and PAI of deep-seated tumors owing to their high extinction coefficient, good biocompatibility, flexible structure, and engineerable physicochemical properties. Although the PCE of PTAs has dramatically improved in recent years, the side effects induced by strong hyperthermia on adjacent normal tissues cannot be ignored. Thus, there is an urgent need to develop novel PTAs with enhanced specificity and efficiency to improve the patient therapeutic outcomes, while reducing the potential side effects.
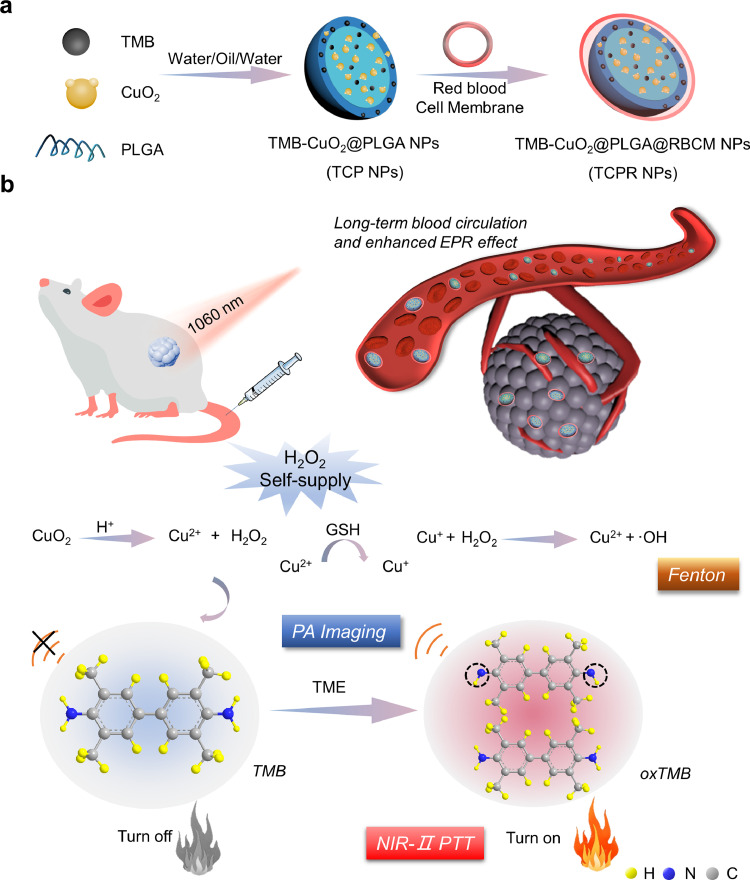
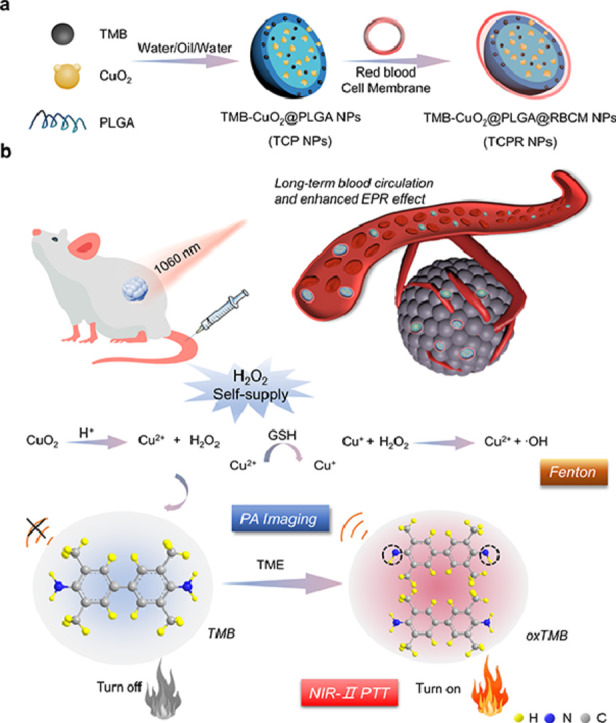
Copper peroxide (CuO2) NPs, with a high H2O2 self-production capacity, and 3,3′,5,5′-tetramethylbenzidine (TMB), with excellent hydroxyl radical (·OH)-activated photothermal efficiency in the NIR-II region, were co-loaded into biodegradable poly(lactic-co-glycolic acid) (PLGA), which was subsequently coated with the red blood cell membrane (RBCM) of mice to fabricate TMB-CuO2@PLGA@RBCM (TCPR) NPs for in vivo TME-activated theranostics (Scheme 1).
Scheme 1.
(a, b) Schematic illustration of the synthesis of 3,3′,5,5′-tetramethylbenzidine (TMB)-copper peroxide (CuO2)@poly(lactic-co-glycolic acid) (PLGA)@red blood cell membrane (RBCM) (TCPR) nanoparticles (NPs) and the mechanism of H2O2-activated specific photothermal therapy (PTT) guided by second near-infrared (NIR-II) photoacoustic imaging (PAI).
Specifically, TCPR NPs present various desirable properties: (1) With the aid of RBCMs, TCPR NPs can be efficiently accumulated in tumor locations due to the enhanced permeability and retention (EPR) effect. (2) The loaded CuO2 in TCPR can generate H2O2 via the reaction between CuO2 and protons in the mildly acidic TME to increase the concentration of H2O2 in situ. (3) The Fenton-like reaction mediated by Cu ions and H2O2 (both endogenous and supplied) can efficiently generate sufficient •OH to oxidize TMB for NIR-II PAI and PTT. The theranostic performance of the rationally designed nanosystem was investigated in vitro and in vivo.
2. Section of Experimentation
2.1. Materials
The Shanghai Xinbao Fine Chemical Factory supplied CuCl2·2H2O. H2O2, CH2Cl2, NaOH, and PLGA were purchased from Nanjing Chemical Reagent Co. Ltd. Poly(vinylpyrrolidone) (PVP), polyvinyl alcohol (PVA), and TMB were obtained from Adamas-beta Co. Ltd.
2.2. Characterization
A scanning electron microscope (SEM, S4800; Hitachi, Japan) was used to characterize the morphology of the samples, and a Zetasizer Nano series (Nano ZS90; Malvern Instrument Ltd.) was used to characterize the hydrodynamic size distribution and zeta potential. Similarly, an ultraviolet (UV)–visible (Vis)–NIR spectrometer (UV-3600; Shimadzu, Japan) was used to measure the absorption spectrum, whereas inductively coupled plasma mass spectrometry (NeXion 300X) was used to measure the concentration of Cu. An infrared camera (FLIR) was used to capture the thermal pictures, and photoacoustic (PA) imaging was done using LOIS-3D (TomoWave Laboratories, USA). Flow cytometry (BD FACSCalibur) was used to detect the apoptotic rate of the cells, and biochemical analysis was performed using an automatic biochemical analyzer (Chemray 800; Leidu Life Technology, China). Lastly, an X-ray photoelectron spectroscopy (XPS) analysis was performed to further confirm the valence state of Cu.
2.3. Synthesis of CuO2, TCP, and TCPR NPs
PVP (500 mg) was dissolved in a CuCl2·2H2O (1 mL, 0.05 M) solution, and NaOH (10 mL, 0.01 M) and H2O2 (100 L) were then added to the mixture, from which the CuO2 NPs were obtained via ultrafiltration after 30 min of stirring.
PLGA (10 mg) was dissolved in 2 mL of CH2Cl2, and a mixture of 1% PVA (500 µL) and CuO2 NP solution (200 mg mL−1, 250 µL) was added. The microemulsion bubbles were formed twice using an ultrasonic cell grinder for 15 min, and TMB (5 mg mL−1, 7 mL) in dimethyl sulfoxide (DMSO) was then mixed with the microemulsion. Finally, the prepared solution was progressively added to 6 mL of 2% PVA solution under ultrasonic pulverization for 10 min. The TMB-CuO2@PLGA (TCP) NPs were collected via centrifugation after stirring overnight at 25 °C.
While RBCM was obtained from fresh blood of mice, hypotonic phosphoric acid buffer (1:50) was added to red blood cells (RBCs) and placed in the refrigerator overnight. The RBC solution was then purified via centrifugation (9000 rpm/min) to obtain pure RBCM, while the TCP NPs were stirred with RBCM for two hours to obtain TCPR NPs using the nanoprecipitation method.
2.4. Detection of H2O2
KMnO4 (10 µg mL−1) solution was treated with H2O2, phosphate-buffered saline (PBS), and TCPR NPs to detect the production of H2O2. Subsequently, UV–Vis–NIR spectra ranging from 400–1100 nm was measured. The presence of H2O2 was detected by the appearance and increase in the absorbance of oxTMB at 650 nm, which could also react with KI to generate I3−, resulting in an absorbance peak at 350 nm. Finally, the H2O2 concentration was determined by plotting a standard curve.
2.5. Photothermal Effect
The FLIR thermal camera was used to measure the temperature curves of TCPR NPs, which were incubated for 8 h for TMB oxidization before measuring UV–Vis absorption spectra, with varying concentrations at various pH values under 1060 nm laser irradiation. Meanwhile, the temperature change associated with TCPR NP dispersion was also observed in a quartz tube under laser irradiation with various power intensities (1.0, 0.8, 0.6, 0.4, and 0.2 W cm−2), and their optical stability (100 mg L−1) under acidic conditions (pH 5.5) was also tested. The following equation was used to calculate the PCE (η):
| (1) |
The symbol η denotes the PCE, while the cell surface area is denoted by “s” and the heat transfer coefficient is denoted by “h”. Similarly, the equilibrium temperature is TMax, ambient temperature is TSur, and I is the incident laser power. When a quartz cell and water are irradiated with laser light, QDis is the baseline energy generated, and A1060 is the absorbance of the sample at 1060 nm. “hs” is calculated from substituting equations:
| (2) |
The mass and capacity of pure water are “m” and “c,” respectively, and the following formula can be used to compute the time constant (τ):
| (3) |
| (4) |
in which θ is the driving force temperature of the solution.
2.6. In vitro cytotoxicity assay
Mouse breast cancer cells (4T1) and human-immortalized keratinocytes (HaCaTs) were cultured in a medium (Roswell Park Memorial Institute-1640 for 4T1 and Dulbecco's minimal essential medium for HaCaTs purcharsed form Wuhan Servicebio technology Co. Ltd. containing 10% fetal bovine serum and 1% penicillin-streptomycin in the presence of 5% CO2 at 37 °C). In regards to the cytotoxicity assay, a 96-well plate was inoculated with HaCaTs (2.0 × 104 cells per well), and the cells were incubated with different concentrations of TCPR NPs in a 100 µL complete culture medium. After a 12-h incubation period, each well received 20 µL of 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (5 mg mL−1), and the plate was kept at 37 °C for additional four hours. DMSO (200 µL) was used to dissolve the formazan crystals in living cells, and the absorbance of the purple-colored formazan/DMSO solution at 490 nm was measured using a microplate reader (MK3, Thermo Scientific, America) for the calculation of cell viability.
Finally, a 2,7-dichlorodi-hydrofluorescein diacetate probe was used to detect intracellular •OH. Dead cells emitted red fluorescence when stained with propidium iodide (PI), whereas live cells were stained with calcein acetoxymethyl ester (Calcein-AM), which emitted green fluorescence.
In a 6-well plate, 4T1 cells (5 × 106 cells per well) were seeded and incubated overnight. Different substances (PBS, TPR, TPR + NIR, TCPR, and TCPR + NIR) were added to each well at a concentration of 100 µg mL−1. The laser irradiation group received 10 min of 1060 nm laser irradiation (1 W cm−2) after a 4-h incubation, and the cells were cultured for another 8 h before conducting western blotting analysis.
2.7. NIR-II PA imaging
TCPR NP aqueous suspensions (25, 50, 75, and 100 µg mL−1) were simultaneously injected into PA glass tubes, and the PA intensity of the samples under 1060 nm laser irradiation was subsequently determined.
To detect the in vivo PA signals at the tumor sites, 4T1 tumor-bearing mice were i.v. injected with 200 µL of TCPR NPs (500 µg mL−1), and placed into the LOIS-3D machine to gather PA signals.
2.8. Animal models
The Yangzhou University Comparative Medicine Centre provided female mice (BALB/c, 5–6 weeks old), and 4T1 cells were inoculated subcutaneously in the right rear to build a local tumor model. All animal experiments were monitored and authorized by the School of Pharmaceutical Science, Nanjing Tech University. Female Balb/c mice were ordered from the Comparative Medicine Centre of Yangzhou University's animal ethics (permit number:SCXK (SU) 2017-0007).
2.9. In vivo assay
The 4T1 tumor-bearing mice were randomly distributed into five groups (n = 4) and i.v. injected with 100 µL of the following formulations when the tumor volume reached 100–120 mm3: (1) control (PBS), (2) CuO2 NPs, (3) TPR NPs + NIR, (4) TCPR NPs, and (5) TCPR NPs + NIR. The concentration of the NPs was 4 mg/kg. After administration for 8 h, 10 min of laser irradiation (0.5 W cm−2, 1060 nm) was applied to the 4T1 tumor-bearing mice in groups (3) and (5), and tumor volumes and body weights were measured every two days.
2.10. Intratumoral dihydroethidium (DHE) staining and copper ion detection
While frozen sections of fresh tumors were stored at –20 °C, DHE could enter the cells and be oxidized by intracellular reactive oxygen species (ROS) to emit red fluorescence. The brighter the red fluorescence, the higher were the cellular ROS levels.
Tumor tissue sections were treated with ethanolic ruby acid and sodium acetate solutions to detect Cu ions, while excess rubric acid combined to form dark green-black rubric Cu salt precipitates.
2.11. In vivo histochemical analysis
The mice were sacrificed at the end of the treatment, and their spleen, liver, heart, kidney, lung, and tumor tissues were fixed with 4% paraformaldehyde (Wuhan Servicebio technology Co. Ltd.). Ki-67 and hematoxylin and eosin (H&E) were used to stain the tissues before examination under an optical microscope.
3. Results and discussion
The smart TME-triggered theranostic system is expected to remarkably improve the efficiency of tumor management and resultant patient outcomes. Hence, TME-responsive PTT is extremely beneficial to patients. As illustrated in Scheme 1, CuO2 NPs (∼12 nm) were first synthesized according to a previous study [29], and the crystal structure was confirmed by the XRD spectrum (Fig. S1). TCP NPs were fabricated by co-loading TMB and CuO2 NPs into a water/oil/water (w/o/w) double emulsion formed by PLGA to realize NIR-II PAI-guided tumor-specific PTT. RBCM was employed to modify TCP NPs (TCPR NPs) via self-assembly to achieve long-term blood circulation.
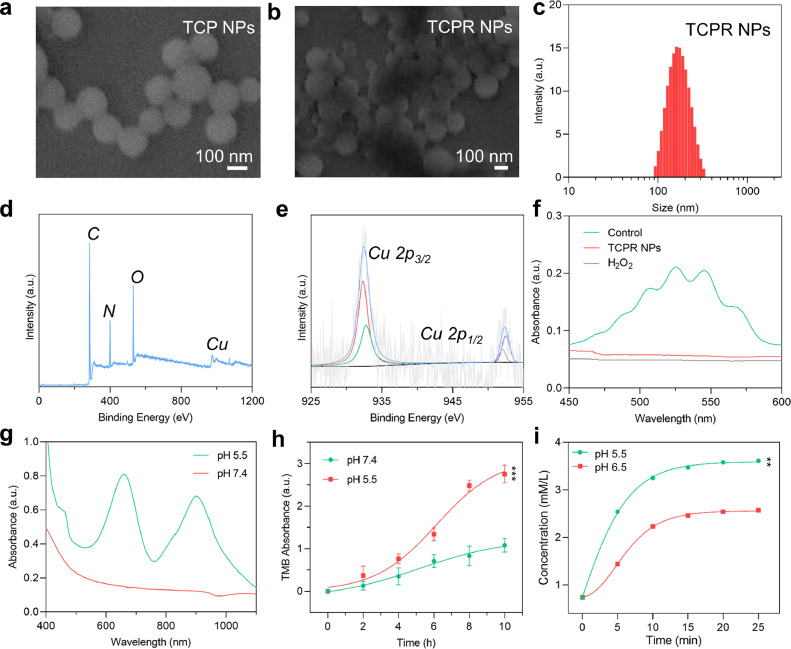
As shown in Fig. 1a, the TCP NPs exhibited a regular homogeneous spherical shape, with an average diameter of 124 nm. The roughness of the surface of the TCPR NPs increased after surface modification with RBCM (Fig. 1b). In turn, the low zeta potential of TCPR NPs compared to that of TCP NPs further confirmed effective surface modification (Fig. S2). The hydrodynamic size distribution of TCPR NPs in water was ∼125 nm (Fig. 1c), which is consistent with the SEM results. The atomic composition was confirmed by XPS (Fig. 1d and S3). The Cu 2p1/2 and Cu 2p3/2 peaks of the XPS Cu spectrum in Fig. 1e deconvoluted into their peak at 932.3 eV from Cu+ and at 952.5 eV from Cu2+ [30]. KMnO4 was chosen as the detection probe to investigate the H2O2 generation ability of the developed TCPR NPs, whose characteristic UV–Vis–NIR absorption peaks would dissolve due to H2O2 oxidation. As shown in Fig. 1f, five distinct KMnO4 peaks were identified in the control group, but when KMnO4 was treated with H2O2 or TCPR NPs, the characteristic absorption disappeared, indicating the H2O2 generating capability of TCPR NPs. The existence of Cu+/Cu2+ redox couples together with the H2O2 generating capability of TCPR NPs verified the H2O2 self-supply property of TCPR NPs in an acidic environment (CuO2 + 2H+ → Cu2+ + H2O2). Under acidic conditions, the Fenton-like reaction mediated by hydrolyzed Cu ions and endogenous/generated H2O2 produces •OH, further oxidizing TMB into oxTMB (Fig. S4). The absorption spectra of TCPR NPs were analyzed at pH 7.4 (to mimic normal tissue) and pH 5.5 (to mimic tumor tissue) to assess the selectivity of TCPR NPs for acidity. The characteristic oxTMB absorption spectrum showed that the release rate was faster at pH 5.5, within 10 h of the test, with the release amount corresponding to pH 5.5, which was nearly three times that at pH 7.4, confirming the specificity of TCPR in acidic environments (Fig. 1g). Furthermore, the release of TMB at pH 5.5 reached a maximum at 10 h, which was nearly 3-fold the release at pH condition of 7.4 (Fig. 1h). In addition, KI was used as the probe to detect self-supplied H2O2 at pH 5.5, and pH 6.5. At pH 5.5, the generation rate of H2O2 was significantly higher than that at pH 7.4, as shown in Fig. 1i. These results revealed that the recently obtained TCPR NPs possess the capability of H2O2 self-supplementary and specific release of TMB in an acidic environment, enabling self-enhanced and TME-activated PTT.
Fig. 1.
(a,b) Scanning electron microscope (SEM) images of 3,3′,5,5′-tetramethylbenzidine (TMB)-copper peroxide (CuO2)@poly(lactic-co-glycolic acid) (PLGA) (TCP) and TCP@red blood cell membrane (RBCM) (TCPR) nanoparticles (NPs). (c) Hydrodynamic size distribution of TCPR NP solution (polydispersity index: 0.258). (d) X-ray photoelectron spectroscopy (XPS) survey spectrum. (e) Cu 2p XPS spectra at high resolution. Ultraviolet (UV)–visible (Vis)–near-infrared (NIR) absorption spectra of (f) KMnO4 incubated with PBS (Control), TCPR NPs, and H2O2 for detecting the generation of H2O2, (g) TCPR NPs at pH 5.5 and 7.4. (h) Absorbance of the released TMB at different time points under pH 5.5 and 7.4. (i) Concentration changes of H2O2 with time changes generated by TCPR NPs at pH 6.5 and 5.5 (***p < 0.001, **p < 0.01).
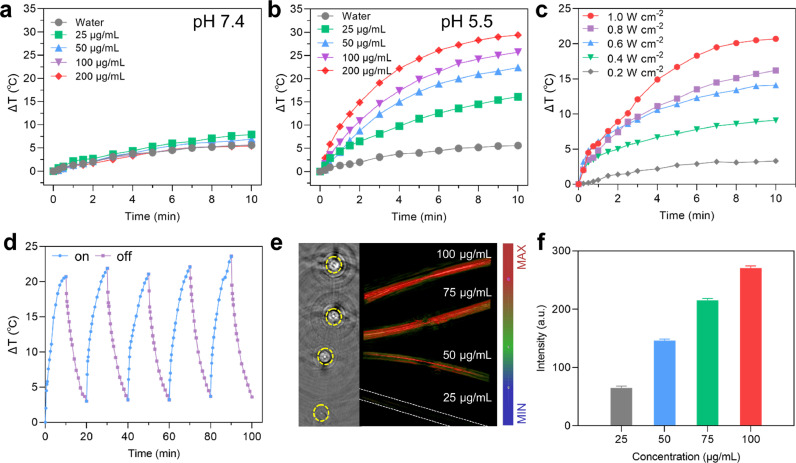
Compared with the NIR-I biowindow (750–1000 nm), the NIR-II biowindow (1000–1350 nm) has very recently attracted more attention due to its higher maximum allowable exposure (MPE) to laser (e.g., 0.33 W cm−2 for 808 nm light, 1 W cm−2 for 1060 nm light), as well as its deeper tissue penetration depth. We further evaluated the photothermal performance of TCPR NPs under different conditions. As shown in Fig. 2a–c, TCPR NPs showed concentration-, pH-, and laser power density-dependent photothermal efficiency. Impressively, benefiting from the effective generation of H2O2 under acidic conditions (pH 5.5), the temperature of the TCPR NPs solution (100 µg mL−1) increased by over 25 °C, which was significantly higher than that in the neutral pH condition. This again confirmed the selective oxidation of TMB by introducing CuO2 NP and ensured tumor-specific PTT with minimal side effects to adjacent normal tissues (Fig. 2a and b). Under 1060 nm laser irradiation, the TCPR NPs presented an excellent PCE efficiency (ƞ = 54%, Fig. S5) and outstanding photothermal stability (Fig. 2d) as a photothermal agent.
Fig. 2.
(a,b) Quantitative temperature curve of TCPR NPs with different concentrations at pH 7.4 and 5.5 (1060 nm, 1 W cm−2, 10 min). (c) Temperature variations of TCPR NPs (100 µg mL−1) irradiated by 1060 nm laser at different power densities. (d) Photothermal stability of TCPR NPs exposed to laser irradiation (1060 nm, 1 W cm−2) for 5 on/off cycles. (e) Photoacoustic (PA) images of TCPR NP suspensions at various concentrations (25–100 µg mL−1). (f) Quantitative PA intensities corresponding to the PA signals in (e).
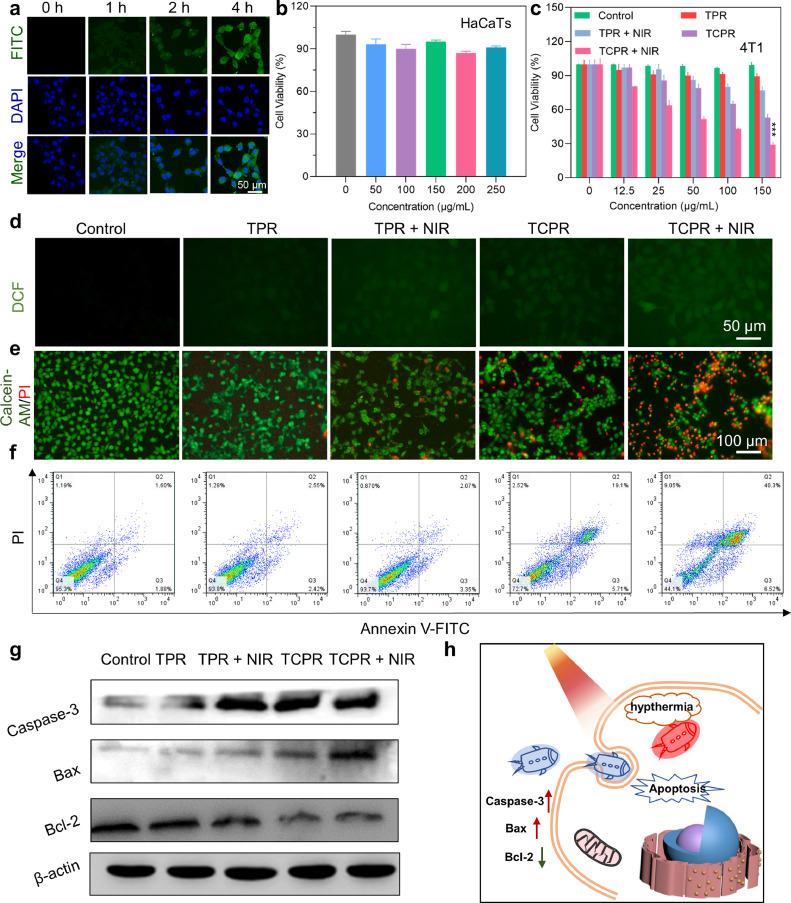
As expected, TCPR NPs at pH 5.5 showed desirable PAI performance and a concentration-dependent increase in PA signal (Fig. 2e and f), which revealed that TCPR NPs exhibit a TME-responsive photothermal effect and satisfactory PAI performance for directing and assessing the treatment process. Inspired by their superior physicochemical properties, the anticancer effects of the TCPR NPs were further tested at the cellular level. By incubating 4T1 cells with fluorescein isothiocyanate (FITC)-labeled TCPR NPs for different time intervals, we found that TCPR NPs were effectively endocytosed within 4 h and were distributed in both the nucleus and cytoplasm (Fig. 3a). TCPR NPs at concentration of 0-250 µg mL−1 exhibited no apparent toxicity to HaCaTs (Fig. 3b), whereas they were dose-dependently cytotoxic to 4T1 tumor cells, presumably because cancer cells have a relatively higher H2O2 level than normal cells. 4T1 cells were treated with nanoparticles of different formulations, with or without laser irradiation, to further investigate the anticancer mechanism of TCPR NPs. The relative survival rate of cancer cells treated with TPR nanoparticles (without CuO2) did not decrease significantly during laser irradiation, as seen in Fig. 3c. Interestingly, when 4T1 cells were cultured with the TCPR NPs for 6 h, a large number (∼40%) of cancer cells were killed, which could be due to the Fenton-like reaction of Cu ions with the self-supplied H2O2 and endogenous H2O2., which is demonstrated by the significantly increased ROS levels in the TCPR and TCPR + NIR groups (Fig. 3d).
Fig. 3.
(a) Cellular uptake and localization of fluorescein isothiocyanate (FITC)-labeled TCPR NPs (green fluorescence) at a concentration of 100 µg mL−1 at different time points. The cell nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI). (b) Cell viability of human-immortalized keratinocytes (HaCaTs) incubated with TCPR NPs at various concentrations. (c) Viability of 4T1 cells cultured with NPs of TPR or TCPR at different concentrations with and without laser irradiation (10 min, 1 W cm−2, 1060 nm). (d) Reactive oxygen species (ROS) staining for intracellular radical detection (incubated with NPs [50 µg mL−1] for 8 h). (e) Live/dead staining of 4T1 cells after treatment (50 µg mL−1) under laser irradiation (10 min, 1 W cm−2, 1060 nm). (f) Annexin-V-FITC/propidium iodide (PI) flow cytometry assay. (g) Western blotting assay of caspase-3, B-cell lymphoma (Bcl)-2, and Bcl-2-associated X (Bax) protein expression. (h) Schematic illustration of cell apoptosis caused by TCPR NP-induced hypothermia (***p < 0.001).
To a certain extent, the laser promoted ROS production, and the relative cell viability in the TCPR NPs + NIR group decreased remarkably, indicating the highly efficient tumor-killing effect of this TCPR NP-based treatment, which could be attributed to the oxidation of TMB. Calcein-AM and PI were used to stain live and dead cells, respectively, to further confirm the treatment performance of TCPR NPs (Fig. 3e). Although the TPR, TPR + NIR, and TCPR groups exhibited limited lethality to 4T1 cells and most of them showed green fluorescence, significant red fluorescence was observed in the TCPR group, which indicated an effective anticancer effect triggered by the prominent synergistic effect of enhanced PTT. Flow cytometric analysis was used to quantify cell apoptosis and necrosis. As shown in Fig. 3f, the annexin V-FITC/PI double staining assay was used to examine apoptotic and necrotic cells. While almost no apoptotic or necrotic cells were observed when 4T1 cells were treated with TPR and TPR+NIR groups, 27.33% of apoptotic/necrotic cells were induced when treated with TCPR NPs alone, and 55.87% of apoptotic/necrotic cells in the TCPR group were observed. With the assistance of TME responsiveness and laser irradiation, TCPR NPs demonstrated excellent cytotoxicity in tumor cells and a favorable synergistic therapeutic effect. Additionally, as the key regulators of cell apoptosis and necrosis, the expression levels of the B-cell lymphoma (Bcl)-2 family (Bcl-2-associated X protein (Bax) and Bcl-2) and caspase family proteins (Caspase-3) in 4T1 cells after different treatments (PBS, TPR, TPR + NIR, TCPR, and TCPR + NIR) were analyzed by western blotting (Fig. 3g). Bcl-2 was downregulated, Bax was upregulated, and its value gradually decreased after the promotion of cell apoptosis, consistent with the upregulation of Caspase-3. As discussed above, the TCPR NPs self-supplied with H2O2 in cancer cells could activate TMB oxidization with Cu ions, which would induce a potent photothermal effect and kill cancer cells by activating the apoptotic/necrotic cascade (Fig. 3h).
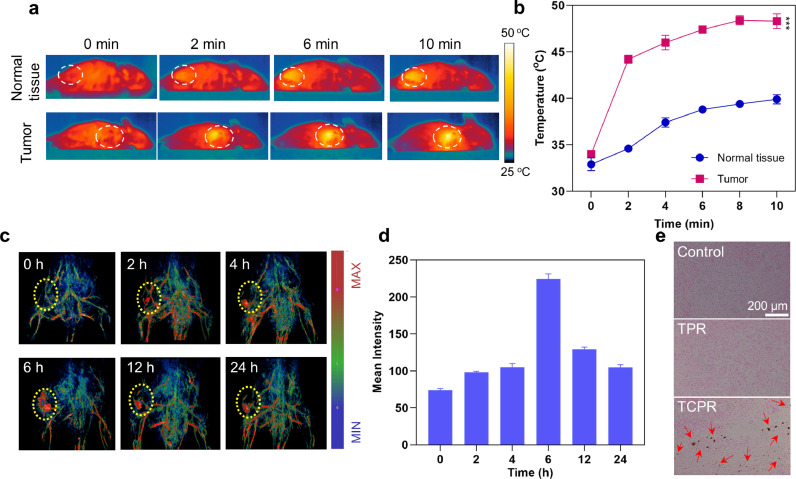
Bioimaging provides information on lesions and monitors the spatiotemporal distribution of nanomedicines, thus enabling more precise phototherapy and reducing the potential damage to normal tissues. Here, we investigated the in vivo photothermal performance of TCPR NPs using the absorption of TCPR in the NIR-II range, in which T was injected subcutaneously into normal tissue and intratumorally (Fig. 4a and b). Notably, while the temperature in normal tissue only increased to 39.9 °C, the tumor temperature increased to 48.4 °C after 10 min of laser irradiation (1060 nm, 1 W cm−2), indicating that TCPR NPs could be triggered explicitly by the TME while maintaining stealth in normal tissues. As the TME-triggered imaging ability of the TCPR NPs could be used to guide in vivo therapy, PAI was further used to assess the in vivo performance of TCPR NPs after i.v. injection. As shown in Fig. 4d and e, the PA signal of the tumor site appeared 2 h post-injection and gradually increased over time. The maximum tumor accumulation of the NPs occurred at 6 h, after which the PA signal intensity was gradually attenuated. Copper red acid staining was conducted to confirm the presence of TCPR NPs in the tumor. In turn, the copper salt deposition was dark green and black, and the nuclei were red. In the comparison between the control and TPR groups, copper salt deposition was observed in the TCPR group (Fig. 4c), which confirmed the apparent tumor uptake of the TCPR nanoparticles.
Fig. 4.
(a) Photothermal pictures of the treatment with TCPR NPs (50 µg mL−1, 200 µL), which were irradiated with 1060 nm laser (1 W cm−2) at the tumor sites for 10 min. (b) Corresponding temperature change in (a). (c) Copper red acid staining of tumor tissues. (d) PA images of mice at various time points after the intravenous injection of TCPR NPs. (e) Corresponding PA signal intensities in (d) (***p < 0.001).
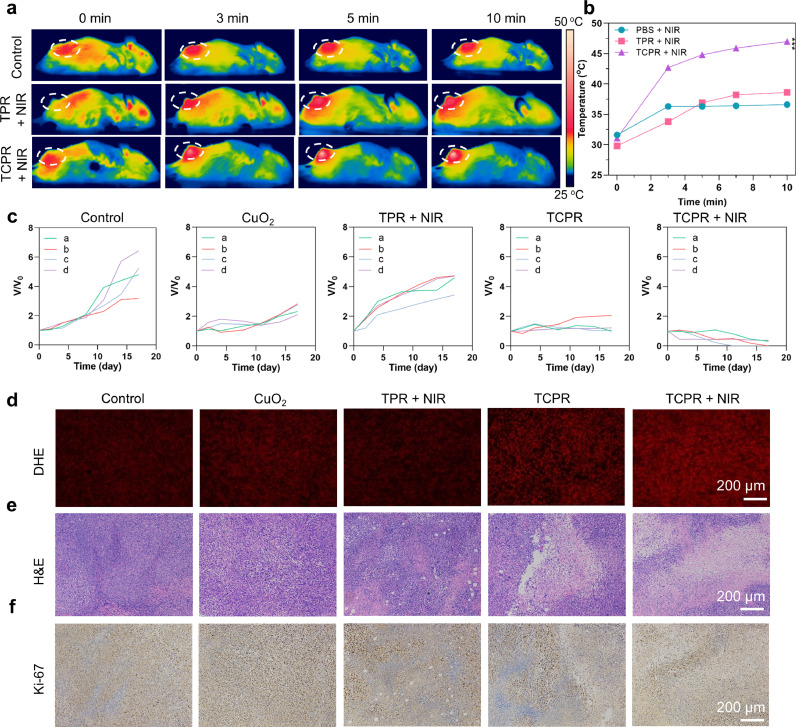
Mice were randomly distributed into five groups and injected with different formulations through the tail vein (CuO2, TPR + NIR, TCPR, TCPR + NIR, and control, n = 4) to assess the in vivo antitumor performance of TCPR NPs-based PTT. The temperature of the tumor injected with the TCPR NPs increased to 47.2 °C 6 h later, with 10 min of laser irradiation, while the control group and TPR + NIR group only increased to approximately 36 °C, which confirmed the photothermal effect (Fig. 5a and b).
Fig. 5.
(a) Photothermal pictures of mice treated under 1060 nm laser irradiation (1 W cm−2) for different time points after being injected with TPR or TCPR NPs (200 µg mL−1, 50 µL) and phosphate-buffered saline (PBS.) (b) Temperature changes in mice tumor sites corresponding to (a). (c) Variation in relative tumor volume after different treatments. (d–f) Dihydroethidium (DHE; red fluorescent signal indicates ROS concentration), hematoxylin and eosin (H&E), and Ki-67 staining of tumors with various treatments (***p < 0.001).
Under laser irradiation, the reaction between endogenous H2O2 and TPR NPs suppressed tumor growth, even in the absence of CuO2 NPs. The growth of the tumors treated with CuO2 and TCPR nanoparticles exhibited some inhibition, which might be due to the chemodynamic effect induced by the hydrolyzed Cu ions with endogenous and/or self-generated H2O2. On the other hand, the TCPR-NIR group showed significantly suppressed tumor growth (∼100% reduction in tumor volume compared to the control) after 10 min of laser irradiation, demonstrating the excellent therapeutic efficacy of TCPR NPs due to the self-supplied H2O2 and activated PTT (Fig. 5c and S6). Intratumoral ROS levels were determined by DHE staining (Fig. 5d). Compared to the other groups, TCPR NP treatment resulted in higher ROS levels in tumors, which were further elevated by laser irradiation-induced hyperthermia. H&E staining demonstrated apoptosis and necrosis of the tumor cells caused by PTT (Fig. 5e). The most severe damage was observed in the TCPR and TCPR + NIR groups. Furthermore, the Ki-67 (brown staining indicates cell proliferation) immunohistochemistry assay inhibited tumor cell proliferation (Fig. 5f), which showed the high antitumor efficiency of TME-responsive therapy based on TCPR NPs.
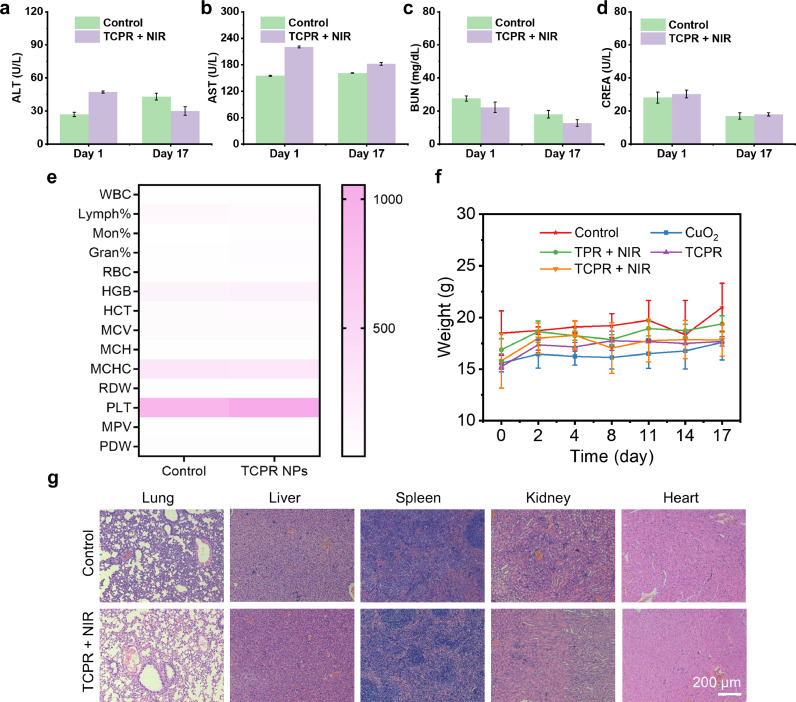
The long-term biosafety of TCPR NPs was further assessed via complete blood panel and blood biochemistry analyses, with all experimental groups maintaining normal levels of critical functional markers, such as alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, and creatinine, in the blood. The levels of aminotransferase in the tested groups did not change, which demonstrated that TCPR NPs were kidney- and liver-compatible (Fig. 6a–d). The evaluated fourteen common indexes for routine blood analysis were within the normal ranges, indicating the negligible blood toxicity of TCPR NPs for 17 days (Fig. 6e). Furthermore, the weights of the mice also showed no abnormal changes (Fig. 6f), indicating negligible systematic toxicity of the treatment. Moreover, histological examination of the major organs of mice injected with PBS (as control) and TCPR NPs was performed to assess the potential toxicity (Fig. 6g), with no pathogenic abnormalities or inflammation observed in both groups. Collectively, these findings demonstrate the in vivo biocompatibility of TCPR NPs, paving the way for their future use as theranostic agents.
Fig. 6.
(a–d) Alanine aminotransferase (ALT), creatinine (CREA), blood urea nitrogen (BUN), and aspartate aminotransferase (AST) levels in mice after receiving TCPR NPs via an intravenous (i.v.) injection. (e) Analysis of the complete blood panel of mice after i.v. injection of TCPR NPs, including white blood cells (WBCs), hematocrit (HCT), red blood cell distribution width (RDW), platelet distribution width (PDW), hemoglobin (HGB), mean corpuscular hemoglobin (MCH), platelets (PLTs), red blood cells (RBCs), mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean platelet volume (MPV), lymphocyte (Lymph%), monocyte (Mon%), and granulocyte (Gran%). (f) The body weights of mice fluctuate during different treatments. (g) H&E staining of major organs in the control and TCPR + NIR groups.
4. Conclusion
In conclusion, an activatable theranostic nanoreactor was developed for TME-triggered NIR-II PTT under PAI guidance. The obtained TCPR NPs, with their rational design, showed impressive on–off switch-ability for cancer-specific therapy and outstanding pH-responsive activities to generate H2O2 and Cu ions, which further resulted in the generation of •OH via a Fenton-like reaction and oxidized TMB, turning on the photothermal effect via the generation of oxTMB. TCPR NPs, which also benefited from the camouflage of RBCM, achieved long-term blood circulation and successfully accumulated in tumor tissues via the EPR effect. Moreover, theranostic TCPR NRs could be specifically activated at the tumor site to achieve high-efficiency PTT under 1060 nm laser irradiation without harming the normal tissues. Therefore, use of activatable TCPR NRs has proven to be a feasible approach for tumor-specific imaging and treatment with minimal side effects.
Declaration of competing interest
The authors declare that they have no conflicts of interest in this work.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (62120106002, 51803091, 61935004, 22175089), Jiangsu Province Policy Guidance Plan (BZ2019014), Jiangsu Provincial key research and development plan (BE2021711), Natural Science Foundation of Shandong Province (ZR2020KB018), Taishan scholars construction special fund of Shandong Province, Natural Science Foundation of Ningbo (202003N40448), and the Jiangsu postgraduate research innovation program (KYCX21_1103).
Biographies

Nan Yang is assiduously studying for a doctoral degree in the Institute of Advanced Materials, Nanjing Tech University (Nanjing Tech), Nanjing 211800, China, supervised by Prof. Xiaochen Dong. Her current research focuses on the development of functional nanomaterials with good biocompatibility and innovative strategies for designing tumor microenvironment-responsive.

Xiaochen Dong(BRID: 09638.00.66037) obtained his Ph.D. degree from Zhejiang University. Then he joined the School of Materials Science and Engineering at Nanyang Technological University as a postdoctoral. In 2012, he joined the Institute of Advanced Materials, Nanjing Tech University as a Full Professor. He has published more than 200 papers, including Adv. Mater., ACS Nano, etc. The current research involves biophotonics and bioelectronics, flexible electronics.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.fmre.2022.04.021.
Contributor Information
Xuejiao Song, Email: xjsong@njtech.edu.cn.
Yewei Zhang, Email: zhangyewei@njmu.edu.cn.
Xiaochen Dong, Email: iamxcdong@njtech.edu.cn.
Appendix. Supplementary materials
References
- 1.Kumar R., Shin W.S., Sunwoo K., et al. Small conjugate-based theranostic agents: an encouraging approach for cancer therapy. Chem. Soc. Rev. 2015;44:6670–6683. doi: 10.1039/c5cs00224a. [DOI] [PubMed] [Google Scholar]
- 2.Xing J., Gong Q., Akakuru O.U., et al. Research advances in integrated theranostic probes for tumor fluorescence visualization and treatment. Nanoscale. 2020;12:24311–24330. doi: 10.1039/d0nr06867e. [DOI] [PubMed] [Google Scholar]
- 3.Yan N., Wang X., Lin L., et al. Gold nanorods electrostatically binding nucleic acid probe for in vivo microRNA amplified detection and photoacoustic imaging-guided photothermal therapy. Adv. Funct. Mater. 2018;28 [Google Scholar]
- 4.Zhao X., Yang C.X., Chen L.G., et al. Dual-stimuli responsive and reversibly activatable theranostic nanoprobe for precision tumor-targeting and fluorescence-guided photothermal therapy. Nat. Commun. 2017;8:14998. doi: 10.1038/ncomms14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu M., Zhang H., Tie C., et al. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat. Commun. 2018;9:4777. doi: 10.1038/s41467-018-07250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu G., Yu S., Saha M.L., et al. A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat. Commun. 2018;9:4335. doi: 10.1038/s41467-018-06574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong F., Yang N., Wang X., et al. Tumor microenvironment-responsive intelligent nanoplatforms for cancer theranostics. Nano Today. 2020;32 [Google Scholar]
- 8.Zhang L., Wang Z., Zhang Y., et al. Erythrocyte membrane cloaked metal–organic framework nanoparticle as biomimetic nanoreactor for starvation-activated colon cancer therapy. ACS Nano. 2018;12:10201–10211. doi: 10.1021/acsnano.8b05200. [DOI] [PubMed] [Google Scholar]
- 9.Xie C., Zhen X., Lyu Y., et al. Nanoparticle regrowth enhances photoacoustic signals of semiconducting macromolecular probe for in vivo imaging. Adv. Mater. 2017;29 doi: 10.1002/adma.201703693. [DOI] [PubMed] [Google Scholar]
- 10.Chen D., Tang Y., Zhu J., et al. Photothermal-pH-hypoxia responsive multifunctional nanoplatform for cancer photo-chemo therapy with negligible skin phototoxicity. Biomaterials. 2019;221 doi: 10.1016/j.biomaterials.2019.119422. [DOI] [PubMed] [Google Scholar]
- 11.Bakthavatsalam S., Sleeper M.L., Dharani A., et al. Leveraging γ-glutamyl transferase to direct cytotoxicity of copper dithiocarbamates against prostate cancer cells. Angew. Chem. Int. Ed. 2018;57:12780–12784. doi: 10.1002/anie.201807582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi B., Yan Q., Tang J., et al. Hydrogen sulfide-activatable second near-infrared fluorescent nanoassemblies for targeted photothermal cancer therapy. Nano Lett. 2018;18:6411–6416. doi: 10.1021/acs.nanolett.8b02767. [DOI] [PubMed] [Google Scholar]
- 13.Chen D., Tang Q., Zou J., et al. pH-responsive PEG–doxorubicin-encapsulated Aza-BODIPY nanotheranostic agent for imaging-guided synergistic cancer therapy. Adv. Healthc. Mater. 2018;7 doi: 10.1002/adhm.201701272. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Huang X., Tang Y., et al. A light-induced nitric oxide controllable release nano-platform based on diketopyrrolopyrrole derivatives for pH-responsive photodynamic/photothermal synergistic cancer therapy. Chem. Sci. 2018;9:8103–8109. doi: 10.1039/c8sc03386b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou J., Wang P., Wang Y., et al. Penetration depth tunable BODIPY derivatives for pH triggered enhanced photothermal/photodynamic synergistic therapy. Chem. Sci. 2018;10:268–276. doi: 10.1039/c8sc02443j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan K., Xi J., Fan L., et al. In vivo guiding nitrogen-doped carbon nanozyme for tumor catalytic therapy. Nat. Commun. 2018;9:1440. doi: 10.1038/s41467-018-03903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao L., Fan K., Yan X. Iron oxide nanozyme: a multifunctional enzyme mimetic for biomedical applications. Theranostics. 2017;7:3207–3227. doi: 10.7150/thno.19738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golchin J., Golchin K., Alidadian N., et al. Nanozyme applications in biology and medicine: an overview. Artif. Cells Nanomed. Biotechnol. 2017;45:1069–1076. doi: 10.1080/21691401.2017.1313268. [DOI] [PubMed] [Google Scholar]
- 19.Cao C., Yang N., Dai H., et al. Recent advances in phase change material based nanoplatforms for cancer therapy. Nanoscale Adv. 2021;3:106–122. doi: 10.1039/d0na00622j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang N., Xiao W., Song X., et al. Recent advances in tumor microenvironment hydrogen peroxide-responsive materials for cancer photodynamic therapy. Nano-Micro Lett. 2020;12:15. doi: 10.1007/s40820-019-0347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong W.K., Yao X., Jana D., et al. Efficient production of reactive oxygen species from Fe3O4/ZnPC coloaded nanoreactor for cancer therapeutics in vivo. Small Struct. 2020;1 [Google Scholar]
- 22.Jung H.S., Verwilst P., Sharma A., et al. Organic molecule-based photothermal agents: an expanding photothermal therapy universe. Chem. Soc. Rev. 2018;47:2280–2297. doi: 10.1039/c7cs00522a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai Y., Wei Z., Song C., et al. Optical nano-agents in the second near-infrared window for biomedical applications. Chem. Soc. Rev. 2019;48:22–37. doi: 10.1039/c8cs00494c. [DOI] [PubMed] [Google Scholar]
- 24.Ge X., Fu Q., Bai L., et al. Photoacoustic imaging and photothermal therapy in the second near-infrared window. New J. Chem. 2019;43:8835–8851. [Google Scholar]
- 25.Lyu Y., Li J., Pu K. Second near-infrared absorbing agents for photoacoustic imaging and photothermal therapy. Small Methods. 2019;3 [Google Scholar]
- 26.Zhang X.Q., Chen X., Cheng X.B., et al. Highly stable lithium metal batteries enabled by regulating the solvation of lithium ions in nonaqueous electrolytes. Angew. Chem. Int. Ed. 2018;57:5301–5305. doi: 10.1002/anie.201801513. [DOI] [PubMed] [Google Scholar]
- 27.Ding X., Liow C.H., Zhang M., et al. Surface plasmon resonance enhanced light absorption and photothermal therapy in the second near-infrared window. J. Am. Chem. Soc. 2014;136:15684–15693. doi: 10.1021/ja508641z. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y., Zhen W., Wang Y., et al. One-dimensional Fe2P acts as a Fenton agent in response to NIR-II light and ultrasound for deep tumor synergetic theranostics. Angew. Chem. Int. Ed. 2019;58:2407–2412. doi: 10.1002/anie.201813702. [DOI] [PubMed] [Google Scholar]
- 29.Lin L.S., Huang T., Song J., et al. Synthesis of copper peroxide nanodots for H2O2 self-supplying chemodynamic therapy. J. Am. Chem. Soc. 2019;141:9937–9945. doi: 10.1021/jacs.9b03457. [DOI] [PubMed] [Google Scholar]
- 30.Karikalan N., Karthik R., Chen S.M., et al. Sonochemical synthesis of sulfur doped reduced graphene oxide supported CuS nanoparticles for the non-enzymatic glucose sensor applications. Sci. Rep. 2017;7:2494. doi: 10.1038/s41598-017-02479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.