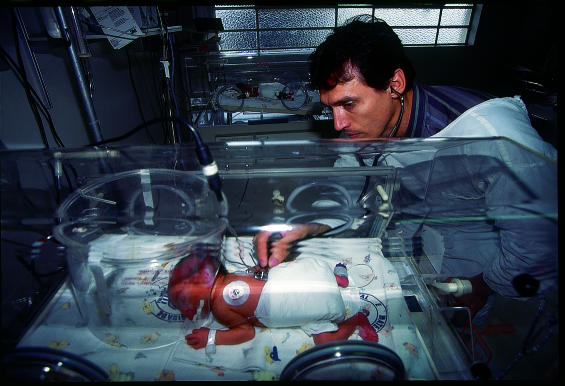
A new drug trial on infants in Latin America has been criticised as unethical because the control group of babies in the trial will be given a placebo rather than another, effective drug. Critics point out that it contravenes the newly revised Declaration of Helsinki, which states that new treatments should always be tested against the best current method, where that exists (BMJ 2000;321:913).
Discovery Laboratories of Doylestown, Pennsylvania, has planned a placebo controlled study in Latin America of sinapultide (Surfaxin), a new drug for treating the idiopathic respiratory distress syndrome in premature newborn infants.
Public Citizen, a Washington based, non-profit making, consumer watchdog, has written to Tommy Thompson, the new US health secretary, condemning the study design as “exploitative.” The control group of 325 premature infants will be treated with placebo, instead of a lifesaving surfactant drug approved by the Food and Drug Administration (FDA). Many infants given placebo are likely to die unnecessarily, an analysis by Public Citizen has shown.
In its letter to Mr Thompson, Public Citizen cited internal FDA documents showing that the administration was against placebo controlled trials of surfactants in the idiopathic respiratory distress syndrome. It also pointed out that the use of a placebo in the study violated the Declaration of Helsinki, which states: “The benefits, risks, burdens and effectiveness of a new method should be tested against those of the best current prophylactic, diagnostic and therapeutic methods.”
Dr Robert Capetola, head of Discovery Laboratories, said that his company had made a large commitment to what was not only a South American study but an international one. The proposed study would have three arms: the new synthetic drug (sinapultide), another previously approved surfactant drug (beractant), and a placebo.
He said that the company will provide training, support, equipment, and also sinapultide—if proved effective—at a very low cost throughout the countries in which the study is conducted (one or more of Bolivia, Ecuador, Peru, and Mexico) for 10 years.
Alternative designs of the trial were rejected as likely to increase the length of the study by as much as two years, resulting in thousands of additional deaths and debilitating lung conditions among infants.
The FDA has not given the go-ahead for the sponsor's proposed Latin American trial and said that the complex issues that the trial raised were the reason it was discussed at a recent “scientific rounds” meeting.
The administration objects to placebo controlled trials of surfactants for the idiopathic respiratory distress syndrome in the United States because “we have available approved surfactants that are the standard of care.”
Dr Sidney Wolfe, the director of Public Citizen's health research group, said: “The infants who would get placebos are being used by the company for reasons having to do with corporate bottom lines in order to get their drug approved.”
Public Citizen is also asking the new Office of Human Research Protections in the Department of Health and Human Services to use its influence to stop the study immediately.
Figure.
JON SPAULL/PANOS PICTURES
Some premature babies will receive only a placebo