Abstract
Objective
This study aims to investigate the factors affecting development of acute kidney injury (AKI) in patients with severe hypothyroidism.
Methods
This retrospective observational study involved patients with primary hypothyroidism and thyroid stimulating hormone (TSH) levels of more than 50 mIU/L at their review in the endocrinology outpatient clinic, between January 2015 and April 2021. Factors affecting the development of AKI were examined by logistic regression analysis.
Results
A total of 100 patients, 20 (11 male (M), 9 female (F)) in the AKI (case) group and 80 (23 M, 57 F) patients in control group, were included in our study. The median age of the case group (56 years, interquartile range (IQR) 44.3–68.5) was significantly higher than the control group (49 years, IQR 32.3–60; p = 0.027), and the ratio of males to females was significantly higher in the case group (p = 0.001). Multivariate logistic regression analyses showed that hypothyroidism diagnosed after the age of 60 years (odds ratio (OR) 59.674, 95% confidence intervals (CI) 5.955–598.031; p = 0.001), free triiodothyronine (FT3) < 1.3 pg/mL (OR 17.151, 95% CI 2.491–118.089; p = 0.004) and creatine kinase (CK) > 1000 U/L (OR 1.522, 95% CI 1.602–82.848; p = 0.015) were predictors for the development of AKI in patients with severe hypothyroidism.
Conclusion
We recommend close follow-up and monitoring of patients with AKI caused by severe hypothyroidism if patients who are diagnosed at age > 60 years, CK > 1000 U/L or FT3 < 1.3 pg/mL.
Keywords: Hypothyroidism, Acute kidney injury, Creatine kinase, Free T3
INTRODUCTION
Thyroid and renal function have well-documented associations. Thyroid hormones (TH) are required for kidney growth and for development and maintenance of water and electrolyte balance. Thyroid dysfunction affects kidney function, and renal failure affects thyroid hormone metabolism both peripherally and centrally via the hypothalamic-pituitary-thyroid axis (1, 2). TH are necessary for the kidney to complete its functional growth and for the development, preservation and maintenance of glomerular function (3). Overt hypothyroidism in particular has been linked to a reduction in glomerular filtration rate (GFR) and renal blood flow (RBF) (4). Cases of acute kidney injury (AKI) due to severe hypothyroidism have been reported in the literature. Since AKI due to severe hypothyroidism is rare, the number of cases is insufficient for publication beyond case series (5-8). The glomerular function is more impaired than tubular function during severe hypothyroidism. In severe hypothyroidism, a reduction in RBF has been documented, which is most likely due to the overall hypodynamic circulation, reduced cardiac output due to myocardial impairment, sinus bradycardia, and the reduced inotropy and chronotropy due to lower levels of TH (9). However, the risk factors of AKI due to severe hypothyroidism has not been fully elucidated. This study aimed to investigate the factors that affect the development of AKI in patients with severe hypothyroidism.
MATERIALS AND METHODS
Study design
This study was designed as a retrospective, cross-sectional study. The local ethical committee approved the study protocol (07.07.2020/1601), and the study was conducted following the ethical principles stated in the Declaration of Helsinki.
The study included patients who were reviewed in our hospital between January 2015 and April 2021 and had primary hypothyroidism with thyroid stimulating hormone (TSH) levels of more than 50 mIU/L. At the time of presentation, each patient's demographic information (gender and age), medication history and atherosclerotic risk factors (history of smoking, alcohol use, dyslipidaemia, hypertension (HT) and diabetes mellitus (DM)) were documented. Patients were excluded if they were currently pregnant or under 18 years of age, with myxedema coma, had an GFR<15 mL/min/1.73 m2, were receiving maintenance renal replacement therapy, had chronic liver disease, concomitant malignancies, stroke, pulmonary embolism, immunological disease or chronic heart failure, or had recently received treatments known to affect renal function, such as nonsteroidal anti-inflammatory drugs, diuretics, contrast agents or certain antibiotics. The height and weight of the participants were recorded, and the body mass index (BMI) was calculated by dividing the weight (kg) by the square of their height (m).
All blood samples were taken after eight or more hours of fasting. All laboratory analyses were performed in the same laboratory. Free thyroxine (FT4), free triiodothyronine (FT3) and TSH were analysed using the electrochemiluminescence immunoassay method (Roche Diagnostics GmbH, D-68298 Mannheim, Germany). The GFR was estimated using the National Kidney Foundation's simplified Modification of Diet in Renal Disease (MDRD) calculation, as follows (6,7): GFR = 175 × (serum creatinine)-1.154 × (age)-0.203 × (0.742 if female) × (1.212 if African American), where units for GFR are mL/min/1.73 m2 and creatinine mg/dL.
Patients with severe hypothyroidism who had normal renal functions in the examinations performed in the last 3 months in their medical records before admission and who were diagnosed with AKI at the time of admission were included in the case group. Acute kidney injury was defined as an increase in creatinine levels of 0.3 mg/dL (26.4 µmol/L) or 50% from baseline in less than 48 hours, or a reduction in urine output to less than 0.5 mL/kg/h for more than six hours (10). Patients with AKI were included in the case group and patients without AKI in the control group.
Statistical analysis
Kolmogorov-Smirnov and Shapiro-Wilk tests were used to test for normality. Correlation of risk factors with outcome were analysed independently (univariate analysis) by either Student’s t-test or Mann-Whitney U-test where applicable. The differences in proportions between groups were compared by using the chi-squared or Fisher’s exact test, where appropriate. Descriptive statistics were used to summarise the data and are presented as median (interquartile range (IQR) or odds ratio (OR)) for skewed continuous variables, and count with the percentage of total for categorical variables. In order to define risk factors for AKI secondary to severe hypothyroidism, univariate and multivariate binary logistic regression analyses were performed; ORs and confidence intervals (CI) were calculated. All statistical analyses were carried out on Windows using the SPSS v23 programme (SPSS Inc., Chicago, IL, USA). A p value of less than 0.05 was taken as statistically significant.
RESULTS
Between January 2015 and April 2021, 2226 patients with TSH > 50 mIU/L were identified. A total of 100 patients, with complete retrospective demographic and laboratory data were included in our study. Twenty patients (11 males (M), 9 females (F)) were included in the AKI group (cases) and 80 (23 M, 57 F) were included in the control group (Fig. 1). The median age of the AKI group was 56 years (IQR 44.3–68.5), and the median age of the control group 49 years (IQR 32.3–60). The median age, the median age at diagnosis of hypothyroidism and the proportion of males were significantly higher in the cases than in the control group (p = 0.027, p = 0.001 and p = 0.001 respectively). There was no difference between the groups in weight, BMI, duration of hypothyroidism, rate of newly diagnosed or daily levothyroxine dose per kg. The rate of treatment (TH replacement therapy) discontinuation and duration of treatment discontinuation were significantly higher in the case group (p = 0.040 and p = 0.049 respectively). While there was no difference in the rates of cardiovascular disease (CVD), heart failure, obstructive sleep apnoea or hyperlipidemia, the rates of HT and DM were significantly higher in the case group (p < 0.001 and p = 0.002 respectively). Alcohol consumption, cigarette use, fibrate use as risk factors for rhabdomyolysis and AKI did not differ between the groups, but the rate of statin use was found to be higher in the case group (p = 0.002) (Table 1).
Figure 1.
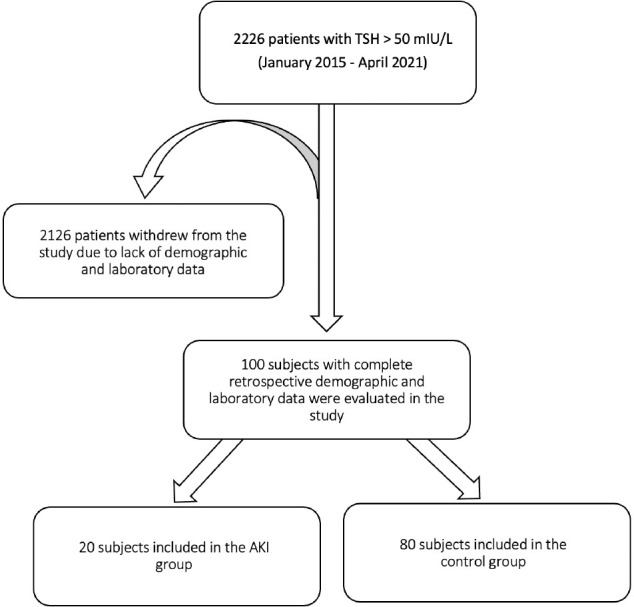
Study flow diagram.
Table 1.
Comparison of demographic data and risk factors between groups
| Variable | Cases (n = 20) | Controls (n = 80) | |
|---|---|---|---|
| Median (IQR) or % | Median (IQR) or % | p | |
| Gender (M / F) | 11/9 (55% / 45%) | 23/57 (28.7% / 71.3%) | 0.027* |
| Age (years) | 62.5 (44.3–68.5) | 49 (32.3–60) | 0.001* |
| Weight (kg) | 75 (70–83) | 74 (66–80) | 0.52 |
| BMI (kg/m2) | 26.9 (25.7–33) | 27.2 (23.9–30.6) | 0.773 |
| Age at diagnosis of hypothyroidism (years) | 55.5 (42–64.3) | 35.5 (25–52.3) | 0.001* |
| Duration of hypothyroidism (months) | 36 (1.3–69) | 60 (3.5–110.5) | 0.357 |
| Hypothyroid aetiology | |||
| Chronic autoimmune thyroiditis | 45/80 (56.3%) | 13/20 (65%) | 0.799 |
| Post-operative | 31/80 (39.7%) | 6/20 (30%) | |
| Post-RAI | 4/80 (5%) | 1/20 (5%) | |
| New diagnosis (%) | 7 (35%) | 24 (30.8%) | 0.717 |
| HT (%) | 12 (80%) | 8 (10%) | <0.001* |
| DM (%) | 6 (30%) | 5 (6.2%) | 0.002* |
| Heart failure (%) | 1 (5%) | 2 (2.5%) | 0.558 |
| CVD (%) | 3 (15%) | 3 (3.8%) | 0.058 |
| OSA (%) | 3 (15%) | 5 (6.2%) | 0.197 |
| HL (%) | 2 (10%) | 3 (3.8%) | 0.251 |
| Treatment discontinuation rate (%) | 11/13 (84.6%) | 30/56 (53.6%) | 0.040 |
| Duration of treatment discontinuation (months) | 3 (0.8–6.8) | 1 (0.5–3) | 0.049 |
| Levothyroxine dose (mcg/kg/d) | 1.2 (1.1–1.6) | 1.3 (1.1–1.7) | 0.355 |
| Alcohol consumption (%) | 1 (5%) | 4 (5%) | 1.000 |
| Cigarette smoking (%) | 8 (40%) | 27 (33.8%) | 0.600 |
| Statin use (%) | 5 (25%) | 3 (3.8%) | 0.002* |
| Fibrate use (%) | 1 (5%) | 1 (1.2%) | 0.987 |
BMI: body mass index; RAI: radioactive iodine; HT: hypertension; DM: diabetes mellitus; CVD: cardiovascular disease; OSA: obstructive sleep apnoea; HL: hyperlipidaemia. *Statistically significant (p < 0.05).
There was a significant difference between the groups in levels of urea, creatinine, GFR, creatine kinase (CK), uric acid, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, FT4, FT3 and potassium (p = 0.001, p < 0.001, p < 0.001, p = 0.010, p < 0.001, p = 0.036, p = 0.047, p = 0.001, p < 0.001 and p = 0.027 respectively) (Table 2).
Table 2.
Comparison of laboratory data between groups
| Cases (n = 20) | Controls (n = 80) | ||
|---|---|---|---|
| Median (IQR) | Median (IQR) | p | |
| FBG (70–110 mg/dL) | 89 (81–131) | 91 (82–100) | 0.847 |
| Urea (12–43 mg/dL) | 38.5 (30–62.5) | 28 (19.8–50) | 0.001* |
| Creatinine (0.5–1.1 mg/dL) | 1.35 (1.3–1.6) | 0.86 (0.7–1.2) | <0.001* |
| GFR (>60 mL/min/1.73 m2) | 57 (51–60) | 97.3 (58–80.8) | <0.001* |
| AST (0–50 U/L) | 34 (23–47) | 21 (15–30) | 0.048 |
| ALT (0–50 U/L) | 26 (19.9–49) | 23 (16–28) | 0.389 |
| CK (0–170 U/L) | 706.5 (167.3–1110.3) | 180 (107.3–319.8) | 0.010* |
| LDH (0–248 U/L) | 296 (227–442) | 244.5 (210.5–300.3) | 0.174 |
| Uric acid (1.5–6 mg/dl) | 6.1 (5.1–7.2) | 4.6 (4–5.7) | <0.001* |
| Albumin (3.5–5.2 g/dL) | 4.5 (4–4.6) | 4.3 (4–4.5) | 0.113 |
| Total cholesterol (0–200 mg/dL) | 307 (215–379) | 269 (212–304.3) | 0.074 |
| Triglycerides (0–150 mg/dL) | 142 (111–201) | 156 (104.5–212.8) | 0.697 |
| LDL-cholesterol (0–150 mg/dL) | 179.5 (120.5–250.5) | 120 (61–190.5) | 0.036* |
| HDL-cholesterol (0–60 mg/dL) | 52 (41.5–75.5) | 67 (52–131.3) | 0.047* |
| TSH (0.4–5.3 mIU/L) | 148.6 (105.1–237.3) | 106.5 (73.7–178.4) | 0.088 |
| FT4 (0.6–1.2 ng/dL) | 0.2 (0.01–0.5) | 0.56 (0.4–0.7) | 0.001* |
| FT3 (2.6–4.4 pg/mL) | 1.3 (0.7–1.9) | 2.3 (1.8–2.7) | <0.001* |
| Na (135–146 mmol/L) | 141 (139–142) | 140 (139–142) | 0.282 |
| K (3.5–5.3 mmol/L) | 4.4 (4.2–4.7) | 4.2 (4–4.5) | 0.027* |
| CRP (0–5 mg/L) | 3.3 (3–5.6) | 3 (0.8–3.7) | 0.226 |
| ESR (1–23 mm/h) | 14 (9.3–28.8) | 13.5 (8.8–23.3) | 0.856 |
IQR: interquartile range; FBG: fasting blood glucose; GFR: glomerular filtration rate; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CK: creatine kinase; LDH: lactate dehydrogenase; LDL: low-density lipoprotein; HDL: high-density lipoprotein; TSH: thyroid stimulating hormone; FT4: free thyroxine; FT3: free triiodothyronine; Na: sodium; K: potassium; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate. *Statistically significant (p < 0.05).
The binary logistic regression analyses are shown in Table 3. In the univariate logistic regression analyses, male gender (OR 0.330, 95% CI 0.121–0.902; p = 0.031), age > 60 years (OR 0.222, 95% CI 0.080–0.621; p = 0.004), hypothyroidism diagnosed after age 60 years (OR 0.127, 95% CI 0.035–0.511; p = 0.462), presence of DM (OR 0.156, 95% CI 0.042–0.580; p = 0.006), presence of HT (OR 0.074, 95% CI 0.023–0.235; p < 0.001), hyperuricaemia (OR 0.110, 95% CI 0.024–0.503; p = 0.004), CK > 1000 U/L (OR 0.246, 95% CI 0.082–0.739; p = 0.012), FT4 < 0.2 ng/dL (OR 0.241, 95% CI 0.083–0.700; p = 0.009), FT3 < 1.3 pg/mL (OR 0.080, 95% CI 0.019–0.345; p = 0.001) and statin use (OR 0.120, 95% CI 0.026–0.557; p = 0.007) were statistically significant predictors for the occurrence of AKI due to severe hypothyroidism. Multivariate logistic regression analyses showed that age greater than 60 when diagnosed with hypothyroidism (OR 59.674, 95% CI 5.955–598.031; p = 0.001), FT3 < 1.3 pg/mL (OR 17.151, 95% CI 2.491–118.089; p = 0.004), CK > 1000 U/L (OR 11.522, 95% CI 1.602–82.848; p = 0.015) were predictors for the development of AKI associated with severe hypothyroidism.
Table 3.
Univariate and multivariate binary logistic regression analysis
| Univariate binary logistic regression | Multivariate binary logistic regression | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| Variable | OR | Lower | Upper | p | OR | Lower | Upper | p |
| Gender (male) | 0.330 | 0.121 | 0.902 | 0.031* | ||||
| Age > 60 years | 0.222 | 0.080 | 0.621 | 0.004* | ||||
| Age > 60 years at diagnosis of hypothyroidism | 0.127 | 0.035 | 0.462 | 0.002* | 59.674 | 5.955 | 598.031 | 0.001* |
| DM | 0.156 | 0.042 | 0.580 | 0.006* | ||||
| HT | 0.074 | 0.023 | 0.235 | <0.001* | ||||
| Hyperuricaemia | 0.110 | 0.024 | 0.503 | 0.004* | ||||
| CK > 1000 U/L | 0.246 | 0.082 | 0.739 | 0.012* | 11.522 | 1.602 | 82.848 | 0.015* |
| FT3 < 1.3 pg/mL | 0.080 | 0.019 | 0.345 | 0.001* | 17.151 | 2.491 | 118.089 | 0.004* |
| FT4 < 0.2 ng/dL | 0.241 | 0.083 | 0.700 | 0.009* | ||||
| Statin use | 0.120 | 0.026 | 0.557 | 0.007* | ||||
OR: odds ratio; CI: confidence interval; DM: diabetes mellitus; HT: hypertension; CK: creatine kinase; FT4: free thyroxine; FT3: free triiodothyronine. *Statistically significant (p < 0.05).
DISCUSSION
This is the first study in the literature to examine the risk factors for AKI in patients with severe hypothyroidism. Male gender, age greater than 60 years, age at diagnosis of hypothyroidism greater than 60, statin use, presence of DM, presence of HT, hyperuricaemia, CK > 1000 U/L, FT4 < 0.2 ng/dL, FT3 < 1.3 pg/mL were identified as possible contributing factors to the development of AKI in the univariate analyses. In the multivariate analyses, age at diagnosis of hypothyroidism > 60 years, CK > 1000 U/L and FT3 < 1.3 pg/mL were observed as predictors for development of AKI.
Severe hypothyroidism can have various effects on the biological structure and functioning of the kidneys. It can affect kidney functions by disrupting hemodynamics, causing a decrease in GFR, a decrease in cardiac functions and deterioration in renal tubular functions (1, 3, 4). In severe hypothyroidism, histological abnormalities such as thickening of both tubular and glomerular basement membranes have been observed (11). In some studies, proteinuria has been observed in hypothyroid patients and is associated with the severity of hypothyroidism (12). In addition, hypothyroidism has been linked to a variety of glomerulopathies, including membranous glomerulopathy, minimum-change nephropathy and membranoproliferative glomerulonephritis, with thyroxine therapy alleviating urine protein loss (13, 14).
Rhabdomyolysis is an important and life-threatening complication of severe hypothyroidism. Cases of AKI due to rhabdomyolysis caused by hypothyroidism have been reported in the literature (15, 16). The risk of rhabdomyolysis increases with a CK value of >1000 U/L (17, 18), and that CK > 1000 U/L increases the risk of AKI three-fold (16). Supporting this, in our study, CK values were significantly higher in the AKI group, and CK > 1000 U/L was observed as a risk factor for the development of AKI.
Male gender was shown to be another factor that increased the risk of AKI due to severe hypothyroidism in our study. Epidemiological studies have observed that chronic kidney disease is more common in females (19). This is because females have a longer life expectancy than males and that GFR decreases with age. However, the risk of AKI and the proportion of patients with chronic kidney disease receiving renal replacement therapy were found to be higher in males in epidemiological studies. Possible reasons for this include the protective effects of oestrogen in women, the harmful effects of testosterone, and an unhealthy lifestyle and busy work-life in males (19-21).
Studies have reported that TSH levels increase with age, and follow-up is recommended until high TSH levels are >8 mIU/L, especially in older patients (22). As age increases, renal function decreases; patients become more susceptible to prerenal AKI, and GFR may decrease (23). Hypothyroidism has been linked to decreased renal plasma flow (RPF), and inadequate effective RBF. The hypodynamic condition of the vascular system caused by hypothyroidism is hypothesised to be the cause of the reduction in GFR and RBF (24). High TSH levels have been found to be negatively correlated with RPF, RBF and GFR due to high vascular resistance in afferent renal arterioles. It has also been shown that the TH relaxes arteries and reduces arterial resistance (4). The elderly are at risk of renal dysfunction due to the hypodynamic circulation caused by severe hypothyroidism. In support of this, our study showed that being over 60 years old or age greater than 60 at the time of diagnosis of hypothyroidism were risk factors for development of AKI in patients with severe hypothyroidism.
Although the TSH levels in the AKI and control groups were not different in our study, other studies has shown that an increase in TSH is related to a decrease in RBF and a decline in GFR (4). The fact that all of the patients in this study had severe hypothyroidism may explain for the lack of differences in TSH values. Besides, it has been observed that FT4 levels were strongly associated with changes in renal function before and also , after levothyroxine treatment (25). Peripheral TH may cause activation of the renin-angiotensin and sympathetic systems, and affect renal vascular compliance through actions on the nuclear receptor (26). TH, especially FT3, are particularly associated with renal tubular function, which induces increased expression of mRNA encoding alpha and beta subunits of the Na+/K+-ATPase enzyme (27). It was observed that low FT3 levels, especially in chronic kidney disease, have been related to a higher risk of cardiovascular disease and mortality (2). In our study, it was observed that FT3 below the median value in both the univariate and multivariate analyses was a statistically significant risk factor for the development of AKI in patients with severe hypothyroidism.
The length of time that a patient has been hypothyroid, independent of the TSH level, may also increase the risk of renal dysfunction. In our study, duration of treatment discontinuation in the AKI group was found to be statistically higher than in the control group. Although the duration of treatment discontinuation did not appear to affect the development of AKI, the authors believe that a longer duration of treatment discontinuation has a greater effect on the decrease in RBF and GFR (1). The hypodynamic circulation due to TH deficiency may also be implicated in AKI formation in the chronic period.
There are many studies in the literature regarding the relationship between serum uric acid levels and kidney function. A negative association has been found in most studies, but not confirmed in others (28). Furthermore, as an inflammatory marker, high blood uric acid levels have various physiological effects on renal function, hypertension, and CVD development, including endothelial dysfunction (28, 29). In the current study, hyperuricaemia, in addition to the vascular and renal implications of severe hypothyroidism, was discovered to be a risk factor for AKI in the univariate analysis.
Due to the effects of hypothyroidism on the renal glomerular and vascular structure, the presence of a disease that affecting kidney baseline function may increase the risk of AKI. Hypertension and diabetes are the most common causes of chronic kidney disease and are associated with poor renal outcomes, including in patients with hypothyroidism (30). In diabetic patients, a reduction in GFR is seen in parallel with an increase in TSH. In addition, the incidence of diabetic nephropathy increases with increasing TSH values (31). It is already predictable that the presence of traditional AKI risk factors such as DM and HT will increase the risk of AKI in patients with severe hypothyroidis, as in our study.
Myopathy due to severe hypothyroidism, elevated CK, hypovolaemia and electrolyte abnormalities increases the risk of rhabdomyolysis (15). In cross-sectional studies, statin therapy increases the risk of rhabdomyolysis due to the possibility of inducing immune-mediated necrotising myopathy and CK elevation, and via other mechanisms (32, 33). For these possible reasons, in our study, statin use was one of the factors affecting the development of AKI in patients with severe hypothyroidism.
The present study, being retrospective, and investigating a rare disease, has several limitations. First, all the patients were recruited from a single centre, and the sample size was relatively small. Second, several potentially relevant factors, such as the duration of chronic illness, genetic predisposition and race, were not investigated. However, there is little research on severe hypothyroidism; our study focused on patients with AKI related to severe hypothyroidism, which is rarer. Randomized prospective studies with more patients participating in terms of risk factors for AKI in patients with severe hypothyroidism independent of classical AKI risk factors (such as DM and HT) will contribute to the literature.
In conclusion, AKI due to severe hypothyroidism is an important and life-threatening complication. We recommend close follow-up and monitoring in hypothyroid patients who are diagnosed at age > 60 years, or whose CK is greater than 1000 U/L, or FT3 less than 1.3 pg/mL.
Acknowledgement
Preparation for publication of this article is supported by the Society of Endocrinology and Metabolism of Turkey.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Iglesias P, Bajo MA, Selgas R, Díez JJ. Thyroid dysfunction and kidney disease: An update. Rev Endocr Metab Disord. 2017;18(1):131–144. doi: 10.1007/s11154-016-9395-7. [DOI] [PubMed] [Google Scholar]
- 2.El Ters M, Patel S, Norby S. Hypothyroidism and reversible kidney dysfunction: An essential relationship to recognize. Endocr Pract. 2014;20(5):490–499. doi: 10.4158/EP12084.RA. [DOI] [PubMed] [Google Scholar]
- 3.Wang K, Xie K, Gu L, Xu B, Chen J, Lou Q. Association of thyroid function with the estimated glomerular filtration rate in a large Chinese euthyroid population. Kidney Blood Press Res. 2018;43(4):1075–1083. doi: 10.1159/000491069. [DOI] [PubMed] [Google Scholar]
- 4.Tsuda A, Inaba M, Ichii M, Ochi A, Ohno Y, Nakatani S. Relationship between serum TSH levels and intrarenal hemodynamic parameters in euthyroid subjects. Eur J Endocrinol. 2013;169(1):45–50. doi: 10.1530/EJE-13-0026. [DOI] [PubMed] [Google Scholar]
- 5.Han B, Cheng T, Ye L, Sui C, Yang L, Lin D, Qiao J, Lu Y. Comparison between acute kidney injury (AKI) and non-AKI patients secondary to severe hypothyroidism. Clin Case Reports. 2018;6(6):1066–1069. doi: 10.1002/ccr3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghayur A, Elahi Q, Patel C, Raj R. Rhabdomyolysis-induced acute kidney injury in a patient with non-compliance to levothyroxine therapy. Endocrinol Diabetes Metab. Case Reports. 2021;2021:21–0034. doi: 10.1530/EDM-21-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baghi MA, Sirajudeen J, Naushad VA, Alarbi KS, Benshaban N. Severe hypothyroidism-induced rhabdomyolysis: A case report. Clin Case Reports. 2021;9(12):1–4. doi: 10.1002/ccr3.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigo C, Gamakaranage CSSSK, Epa DS, Gnanathasan A, Rajapakse S. Hypothyroidism causing paralytic ileus and acute kidney injury-Case report. Thyroid Res. 2011;4(1):7. doi: 10.1186/1756-6614-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mooraki A, Broumand B, Neekdoost F, Amirmokri P, Bastani B. Reversible acute renal failure associated with hypothyroidism: Report of four cases with a brief review of literature. Nephrology. 2003;8(2):57–60. doi: 10.1046/j.1440-1797.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RL, Kellum JA, Shah S V, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):1–8. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulur O, Dal K, Ertugrul DT, Eser M, Kaplan Efe F, Karakaya S, Şahin K, Baser S, Ata N, Aybal Kutlugun A, Beyan E. Renal function improves with the treatment of hypothyroidism. Endocr Res. 2017;42(3):246–251. doi: 10.1080/07435800.2017.1293686. [DOI] [PubMed] [Google Scholar]
- 12.Chang YC, Chang CH, Yeh YC, Chuang LM, Tu YK. Subclinical and overt hypothyroidism is associated with reduced glomerular filtration rate and proteinuria: A large cross-sectional population study. Sci. Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-19693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajji R, Derbali F, Mnafgui K, Zaribi S, Elleuch M, Kammoun N, Jallali Z. A Transient Proteinuria: An Unusual Complication of Hypothyroidism. Am. J. Med. Case Reports. 2014;2(11):237–239. [Google Scholar]
- 14.Paydas S, Gokel Y. Different renal pathologies associated with hypothyroidism. Ren Fail. 2002;24(5):595–600. doi: 10.1081/jdi-120013962. [DOI] [PubMed] [Google Scholar]
- 15.Cai Y, Tang L. Rare acute kidney injury secondary to hypothyroidism-induced rhabdomyolysis. Yonsei Med J. 2013;54(1):172–176. doi: 10.3349/ymj.2013.54.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Abdellati E, Eyselbergs M, Sirimsi H, Hoof VV, Wouters K, Verbrugghe W, Jorens PG. An observational study on rhabdomyolysis in the intensive care unit. Exploring its risk factors and main complication: Acute kidney injury. Ann. Intensive. 2013 Care;3(1):1–8. doi: 10.1186/2110-5820-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weibrecht K, Dayno M, Darling C, Bird SB. Liver Aminotransferases Are Elevated with Rhabdomyolysis in the Absence of Significant Liver Injury. J. Med. Toxicol. 2010;6(3):294–300. doi: 10.1007/s13181-010-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott KR, Simmons Z, Boyer PJ. Hypothyroid myopathy with a strikingly elevated serum creatine kinase level. Muscle and Nerve. 2002;26(1):141–144. doi: 10.1002/mus.10128. [DOI] [PubMed] [Google Scholar]
- 19.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–164. doi: 10.1038/nrneph.2017.181. [DOI] [PubMed] [Google Scholar]
- 20.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James MT, Grams ME, Woodward M, Elley CR, Green JA, Wheeler DC, de Jong P, Gansevoort RT, Levey AS, Warnock DG, Sarnak MJ, CKD Prognosis Consortium A Meta-analysis of the Association of Estimated GFR, Albuminuria, Diabetes Mellitus, and Hypertension With Acute Kidney Injury. Am J Kidney Dis. 2015;66(4):602–612. doi: 10.1053/j.ajkd.2015.02.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duntas LH, Yen PM. Diagnosis and treatment of hypothyroidism in the elderly. Endocrine. 2019;66(1):63–69. doi: 10.1007/s12020-019-02067-9. [DOI] [PubMed] [Google Scholar]
- 23.Denic A, Glassock RJ, Rule AD. Structural and Functional Changes With the Aging Kidney. Adv. Chronic Kidney Dis. 2016;23(1):19–28. doi: 10.1053/j.ackd.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moses MA SS. ed. 7. Philadelphia: Lippincott-Raven; 1996. The kidneys and electrolyte metabolism in hypothyroidism. [Google Scholar]
- 25.Den Hollander JG, Wulkan RW, Mantel MJ, Berghout A. Correlation between severity of thyroid dysfunction and renal function. Clin. Endocrinol. (Oxf) 2005;62(4):423–427. doi: 10.1111/j.1365-2265.2005.02236.x. [DOI] [PubMed] [Google Scholar]
- 26.Ichihara A, Kobori H, Miyashita Y, Hayashi M, Saruta T. Differential effects of thyroid hormone on renin secretion, content, and mRNA in juxtaglomerular cells. Am J Physiol. 1998;274(1):224–231. doi: 10.1152/ajpendo.1998.274.2.e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gicks GG, Ismail-beigiq F, Edelman IS. Thyroidal Regulation of Rat Renal and Hepatic Na , K-ATPase Gene Expression. J Biol Chem. 1988;263(32):16610–16618. [PubMed] [Google Scholar]
- 28.Li L, Yang C, Zhao Y, Zeng X, Liu F, Fu P. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol. 2014;15:122. doi: 10.1186/1471-2369-15-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005;16(12):3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 30.Jiang G, Luk AOY, Tam CHT, Xie F, Carstensen B, Lau ESH, Lim CKP, Lee HM, Ng ACW, Ng MCY, Ozaki R, Kong APS, Chow CC, Yang X, Lan HY, Tsui SKW, Fan X, Szeto CC, So WY, Chan JCN, Ma RCW, Hong Kong Diabetes Register TRS Study Group Progression of diabetic kidney disease and trajectory of kidney function decline in Chinese patients with Type 2 diabetes. Kidney Int. 2019;95(1):178–187. doi: 10.1016/j.kint.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Keskin H, Cadirci K, Gungor K, Karaaslan T, Usta T, Ozkeskin A, Musayeva A, Yesildal F, Isman F, Zengin HY. Association between TSH Values and GFR Levels in Euthyroid Cases with Metabolic Syndrome. Int J Endocrinol. 2021;2021:8891972. doi: 10.1155/2021/8891972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta S, Blaivas M, Ike RW, Crofford LJ. Polymyositis evolving after rhabdomyolysis associated with HMG-CoA reductase inhibitors: A report of two cases. J Clin Rheumatol. 2001;7(5):332–335. doi: 10.1097/00124743-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Mammen AL, Chung T, Christopher-Stine L, Rosen P, Rosen A, Doering KR, Casciola-Rosen LA. Autoantibodies against 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase (HMGCR) in Patients with Statin-Associated Autoimmune Myopathy. Arthritis Rheum. 2011;63(3):713–721. doi: 10.1002/art.30156. [DOI] [PMC free article] [PubMed] [Google Scholar]


