Abstract
Serological and molecular epidemiological studies indicate that Borna disease virus (BDV) can infect humans and is possibly associated with certain neuropsychiatric disorders. We examined brain tissue collected at autopsy from four schizophrenic patients and two healthy controls for the presence of BDV markers in 12 different brain regions. BDV RNA and antigen was detected in four brain regions of a BDV-seropositive schizophrenic patient (P2) with a very recent (2 years) onset of disease. BDV markers exhibited a regionally localized distribution. BDV RNA was found in newborn Mongolian gerbils intracranially inoculated with homogenates from BDV-positive brain regions of P2. Human oligodendroglia (OL) cells inoculated with brain homogenates from BDV-positive gerbils allowed propagation and isolation of BDVHuP2br, a human brain-derived BDV. Virus isolation was also possible by transfection of Vero cells with ribonucleoprotein complexes prepared from BDV-positive human and gerbil brain tissues. BDVHuP2br was genetically closely related to but distinct from previously reported human- and animal-derived BDV sequences.
Borna disease virus (BDV) causes central nervous system (CNS) disease in several vertebrate species that is manifested by behavioral abnormalities and diverse pathology (41). BDV has been molecularly characterized as a nonsegmented, negative-strand RNA virus. Based on its unique genetic and biological features, BDV is the prototypic member of a new family, Bornaviridae, within the order Mononegavirales (11, 44).
Horses and sheep have been regarded as the main natural hosts of BDV (41). In these species BDV can cause Borna disease (BD), an often fatal immune system-mediated neurologic disease. Evidence, however, indicates that the natural host range of BDV is wider than originally thought (8, 21, 30, 31, 40, 41, 49, 50). Moreover, asymptomatic naturally infected animals of different species have been documented worldwide, suggesting that the prevalence and geographic distribution of BDV may have been underestimated (2, 19–21, 34, 40, 41). Experimentally, BDV has a wide host range from birds to rodents and nonhuman primates (21, 40, 41). The age, immune status, and genetics of the host, as well as viral factors, significantly influence the course of BDV infection (21, 40, 41). Heightened viral gene expression in limbic system structures, together with astrocytosis and neuronal structural alterations within the hippocampus, are histopathological hallmarks of BDV infection (15, 16). Inflammatory cells are frequently, but not necessarily, seen in the brains of BDV-infected animals.
Seroepidemiological studies have consistently shown an increased BDV seroprevalence in neuropsychiatric patients (4, 15, 21, 29, 40). Moreover, higher BDV RNA prevalences have been documented in peripheral blood mononuclear cells of neuropsychiatric patients (10 to 50% of patients) than of healthy blood donors (0 to 4.6% of donors) (6, 27, 28, 37, 43). BDV antigen and RNA have also been detected in human brain samples collected at autopsy from individuals with a history of mental disorders (12, 17, 42), as well as in clinical samples of grade 4 glioblastomas (36) and from brain tissue of some apparently healthy controls (18). These findings together indicate that BDV can infect humans and persist in the CNS and that it is possibly associated with certain mental disorders. BDV has been isolated from peripheral blood mononuclear cells (three cases) (5) and from granulocytes (one case) (39), but not from brain tissue, of psychiatric patients. However, BDV has not been implicated as a human pathogen yet.
Here we document for the first time the isolation of BDV from human brain. BDV was isolated from brain tissue collected at autopsy from a BDV-seropositive schizophrenic patient referred to as P2. Histopathological examination revealed mild inflammatory changes in the hippocampus of this patient. BDV RNA and antigen were detected in brain tissue from patient P2 and exhibited a regionally localized distribution. BDV was isolated by intracranial inoculation of newborn gerbils with brain homogenates from patient P2 and subsequent inoculation of OL cells with homogenates from BDV-positive gerbil brain tissues. We also succeeded in isolating BDV by transfecting Vero cells with ribonucleoprotein (RNP) complexes prepared from brain tissue of P2 or from gerbil brain found to be BDV positive upon inoculation with brain tissue from P2. Sequence analysis showed a high degree of sequence conservation between this human brain isolate of BDV (BDVHuP2br) and previously reported human- and animal-derived BDV sequences (2, 7, 10, 40). Nevertheless, based on its unique nucleotide substitutions, BDVHuP2br was found to be genetically distinct from previously reported partial human- and animal-derived BDV sequences.
MATERIALS AND METHODS
Patients.
Brain tissue samples collected at autopsy from four Japanese schizophrenic patients (P1 to P4) and two Japanese healthy control individuals (H1 and H2) were used in these studies (Table 1). Informed consent was obtained from the subjects' relatives. Information obtained from clinical records and interviews with subjects' relatives indicated that control individuals H1 and H2 were free of any history of psychiatric disorders. Patients P1, P3, and P4 were diagnosed with schizophrenia without having depressive episodes but did have other symptoms including auditory hallucinations and delusion. P2 was originally diagnosed with schizophrenia including symptoms of hallucination and delusion. About 6 months after the initial diagnosis, P2 was admitted to the Kagawa Medical College Hospital and remained hospitalized for more than one year. P2 was not an intravenous drug abuser and did not have a history of using cardiotoxic drugs. During hospitalization, P2 developed mild symptoms of diabetes that were corrected by diet changes and did not require medication. P2 was treated with the neuroleptic haroperidol (0.2 g/kg/day [body weight, 70 kg]). P2 gradually developed depression, anxiety, and general fatigue. His mental condition alternated between periods of acute psychosis with hallucinations and delusions and periods of depression. Diagnostic classification was made according to DMS-III R criteria, American Psychiatry Association. Additional clinical information about these cases is summarized in Table 1. None of the patients or control cases owned cats or horses.
TABLE 1.
Patients examined for detection of BDV markers in brain
| Subject | Age (yr) | Age (yr) at disease onset | Sex | Clinical diagnosis (DSM-III-R) | Cause of death | Antibodies to BDVa in:
|
|
|---|---|---|---|---|---|---|---|
| Serum (p24/p40) | CSF (p24/p40) | ||||||
| P1 | 33 | 20 | Female | Schizophrenia (disorganized type) | Heart failure | −/− | −/− |
| P2 | 26 | 24 | Male | Schizophrenia (undifferentiated type) | Cardiac Infarction | +/− | −/− |
| P3 | 29 | 21 | Female | Schizophrenia (disorganized type) | Neglected cold | −/− | −/− |
| P4 | 40 | 20 | Male | Schizophrenia (paranoid type) | Heart failure | −/− | −/− |
| H1 | 22 | Female | Suffocation | −/− | −/− | ||
| H2 | 18 | Male | Sudden death | −/− | −/− | ||
The presence of serum or CSF antibodies to BDV was examined by Western blot analysis. After preabsorption with purified GST (10 μg/ml) for 1 h at 37°C, serum (dilution 1/100) and CSF (undiluted) samples were reacted with membranes blotted with purified GST-p24, GST-p40, or GST alone. Samples were considered positive when they reacted with the recombinant GST-p24 or GST-p40 but not with GST.
Tissue collection and preparation.
Autopsies were performed within 8 to 12 h of death. Brain tissue from each individual was dissected into olfactory bulb, cerebral cortex (frontal, temporal, and occipital lobes), hippocampus, fornix, hypophysis, cingulate gyrus, lateral ventricle (medial wall), pons, cerebellum, and medulla oblongata. Fixed tissue in 3% paraformaldehyde–phosphate-buffered saline and unfixed frozen tissue was prepared for each individual. For histopathological examination, paraffin-embedded sections of fixed tissue were sectioned at 4 μm and stained with hematoxylin-eosin. Blood and cerebrospinal fluid (CSF) samples were obtained from each individual via syringe from the heart and spinal column, respectively, 6 to 8 h postmortem. Cells and fluid phase were separated by centrifugation. Plasma, CSF, and the pellet containing total blood cells were stored at −20°C.
Detection of BDV-specific antibodies.
Plasma and CSF antibodies to BDV were detected by Western blot analysis as described previously (2). BDV recombinant antigens corresponding to the viral full-length nucleoprotein (NP) (p40) and phosphoprotein (P) (p24) from BDV He80 (10, 23) were expressed as fusion proteins with the glutathione S-transferase (GST) protein (2, 37). GST alone was used as a negative control antigen. GST-p40, GST-p24, and GST recombinant proteins were expressed and purified by using glutathione-Sepharose 4B column chromatography (Pharmacia Biotech AB, Uppsala, Sweden). Rabbit polyclonal sera to BDV p40 and p24, and normal rabbit serum were used as positive and negative control sera, respectively. Protein Mr values were estimated by comparing their mobilities to those of marker proteins from a calibration kit (Bio-Rad).
Detection of BDV RNA by RT-PCR.
BDV p40 and p24 RNA sequences were detected by reverse transcription (RT)-nested PCR as described previously (28, 43, 46). Briefly, total RNA was extracted from frozen brain tissue and total blood cells using an RNA isolation kit (ISOGEN; Nippon Gene Co., Tokyo, Japan). RNA (2 μg) was reverse transcribed using 200 U of SuperScript II RNaseH-minus reverse transcriptase (GIBCO BRL) and random hexamers. First-round PCR was performed using one-quarter of the cDNA product and two sets of primers to amplify BDV p40 and p24 sequences. The primers were nucleotides (nt) 242 to 261 and 511 to 489 for p40 and nt 1387 to 1405 and 1865 to 1847 for p24. Second-round PCR was done using one-fifth of the first-round PCR product and the nested primer pairs nt 259 to 278 and 483 to 464 for p40 and nt 1443 to 1461 and 1834 to 1816 for p24. The nucleotide positions correspond to those in the antigenome polarity of the BDVHe80 RNA (10). As a control of RNA quality, aliquots of each RNA sample were used to amplify by RT-PCR a 197-bp fragment of the housekeeping cellular mRNA glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primers used to amplify GAPDH sequences and the internal probe used for Southern blot hybridization of GAPDH were based on the human sequences.
Amplification of BDV genomic RNA by RT-PCR was done by priming the RT reaction mixture with a BDV-specific sense primer corresponding to nt 1 to 53 in the BDV genome. This method can amplify a full-length cDNA of BDV (8.9 kb) (45). Subsequent first and second rounds PCR were performed using the primer pairs for p40 and p24 described above.
PCR products were separated by agarose gel electrophoresis (1.5% agarose). The products were visualized by ethidium bromide staining, blotted onto a nylon membrane (Hybond-H+; Amersham), and analyzed by Southern blot hybridization with 32P-labeled probes corresponding to internal sequences of the amplified p40 and p24 PCR products.
To prevent possible contamination of the samples with BDV amplicons and plasmid DNA containing BDV sequences, RNA extractions and cDNA amplifications were done in separated rooms following strict rules of separation between pre- and post-PCR environments.
In situ hybridization.
Paraffin-embedded 4-μm-thick brain sections were deparaffinized and processed for in situ hybridization (ISH) as described previously (12). Full-length BDV-p24 digoxigenin-labeled sense and antisense probes were generated by in vitro transcription using T7 RNA polymerase. Hybridization to the RNA probes was detected by using and alkaline phosphatase-labeled anti-digoxigenin polyclonal antibody (Boehringer Mannheim) and nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate as substrates for the alkaline phosphatase reaction. Sections were counterstained with methyl green.
Immunohistochemical (IHC) studies.
To detect BDV antigens, formalin-fixed, paraffin-embedded tissue blocks corresponding to the hippocampal region and other brain regions of patient P2 were sectioned at 7 μm thick and collected onto slides which had been triple-coated with alum-gelatin. The coated sections were baked at 58°C for 1 h, deparaffinized in Histoclear (National Laboratories, Bridgeport, Conn.), and hydrated. They were then treated for 30 min in 0.3% H2O2 in methanol (100%), washed in Tris-buffered saline (TBS) (pH 7.4), and subjected to blockade of nonspecific sites with 10% normal goat serum. The sections were immunolabeled with a mouse polyclonal serum to BDV essentially as described previously (12). Briefly, sections were incubated overnight at 4°C with a 1/50 dilution of the mouse serum. Binding of the first antibody was detected with a biotinylated goat anti-mouse immunoglobulin G serum followed by incubation with Avidin D-HRP (ABC ELITE; Vector laboratories, Burlingame, Calif.). The reaction was developed using 40 mg of diaminobenzidine in 100 ml of Tris-HCl (pH 7.4) containing 45 μl of 30% H2O2. Sections from BDV persistently infected and mock-infected control rat brains processed in the same manner were used as positive and negative controls, respectively, for the immunolabeling reaction. In addition, sections were stained with normal mouse serum. In each case, three sections were processed to verify the reproducibility of the staining.
Inoculation of newborn gerbils with brain cell homogenates.
Pregnant Mongolian gerbils were purchased from SLC, Shizuoka, Japan. Newborn gerbils were intracerebrally (i.c.) inoculated, within 24 h of birth, with 30 μl of brain homogenates from patient P2 or from gerbils. Brain homogenates were prepared in phosphate-buffered saline by repeatedly passing minced brain tissue through 18-, 21-, and 27-gauge needles followed by freezing and thawing.
Rescue of BDV by inoculation of OL cells with brain homogenates.
Brain homogenates from patient P2 or from the brain and spinal cord of gerbils previously inoculated with brain material from patient P2 were used to inoculate OL cells which were maintained in tissue culture, being passaged every 5 days. OL is an established human oligodendroglial cell line generated by Y. Iwasaki at the Wistar Institute, Philadelphia, Pa., in the early 1980s. Consistent with their oligodendroglia origin, OL cells express high levels of CNPase (2′,3′-cyclic nucleotide 3′-phosphodiesterase) but do not express the astrocytic marker glial fibrillary acidic protein. Expression of viral antigen was monitored by immunofluorescence (IF) using a monoclonal antibody (HN182) that specifically recognizes BDV p40 (35).
Rescue of BDV by transfection of Vero cells with RNP complexes from BDV-positive brain samples. (i) RNP preparation.
Brain homogenates (10%, wt/vol) from hippocampus, cerebellum, cerebral cortex, and pons of patient P2 and from the cerebrum and spinal cord from gerbils were made in phosphate-buffered saline–2% fetal bovine serum by ultrasonication at 0°C followed by repeated passage of the homogenate through a 21-gauge needle. The homogenate was then clarified by centrifugation at 3,000 × g for 10 min at 4°C. Clarified supernatant was adjusted to 0.5% NP-40, incubated for 15 min at 20°C, and then centrifuged through a 20% sucrose cushion containing 0.5% NP-40. A pellet containing the nuclear fraction was resuspended in buffer I (140 mM NaCl, 1.5 mM MgCl2, 10 mM Tris-HCl [pH 8.5]), and sodium deoxycholate and Tween 40 were added to final concentrations of 0.4 and 0.8%, respectively. After incubation for 3 min on ice, samples were centrifuged for 5 min at 800 × g at 4°C. Pellets were resuspended in buffer II (100 mM KCl, 5 mM MgCl2, 0.5 mM CaCl2, 10 mM Tris-HCl [pH 8.5]) and digested with RNase-free DNase and micrococcal nuclease at 100 and 20 μg/ml, respectively, for 10 min at 37°C. After addition of EGTA to inactivate the micrococcal nuclease, samples were adjusted to 0.5% NP-40, layered on a discontinuous glycerol gradient (50 to 25% [vol/vol]) containing 150 mM NaCl, 2 mM dithiothreitol, and 10 mM Tris-HCl [pH 8.5], and centrifuged at 180,000 × g for 120 min at 4°C. The pellet containing RNP was resuspended in buffer HB (10 mM KCl, 1.5 mM MgCl, 5 mM dithiothreitol, 10 mM Tris-HCl [pH 7.5]) containing 40% glycerol and stored at −80°C. For RNP prepared from BDV-infected rat brain, the presence of genomic RNA (ca. 9 kb) and viral NP (p40) was verified by Northern blot hybridization and Western blot analysis, respectively. A similar biochemical characterization was not feasible for RNP prepared from human brain material obtained at autopsy due to their low levels.
(ii) RNP transfection.
The procedures for RNP transfection were similar to those previously described (9). Briefly, Vero cell monolayers were washed with OptiMem containing 100 μg of gelatin per ml (OptiMem-G) and treated for 30 min at room temperature with 300 μg of DEAE-dextran per ml (5 × 105 Da) and 0.25% dimethyl sulfoxide in OptiMem-G. After being washed once with OptiMem-G, RNP complexes were diluted in OptiMem-G and allowed to adsorb to cells for 60 min at room temperature. Cells were then washed twice with OptiMem-G and left in complete medium. Expression of BDV antigen was monitored by IF using a mouse polyclonal serum to BDV.
Northern blot analysis.
Total RNA was extracted from cells using TRI-Reagent (Molecular Research Center, Cincinnati, Ohio) as recommended by the manufacturer. RNA (5 μg) was size fractionated by 2.2 M formaldehyde–agarose gel electrophoresis, transferred by capillarity with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) to a MagnaGraph nylon membrane (MSI, Westboro, Mass.), and UV cross-linked. The membrane was hybridized to the indicated probes using Quikhyb (Stratagene, La Jolla, Calif.). DNA probes were labeled with [alpha-32P] dCTP by using random hexamers (Pharmacia LKB). Hybridization proceeded for 3 h at 68°C, and the blot was washed twice at low stringency (68°C in 2× SSC–0.2% sodium dodecyl sulfate) and twice at high stringency (68°C in 0.2× SSC–0.2% sodium dodecyl sulfate). The blot was exposed to a Biomax MR film (Kodak, Rochester, N.Y.).
Sequence analysis of BDV PCR products.
RNA extracted from BDV antigen-positive OL cells was reverse transcribed using a BDV-specific sense primer corresponding to nt 1 to 23. cDNAs were amplified by nested PCR using the p40 and p24 primer sets described above. PCR products directly obtained from brain tissue of patient P2 by using a BDV-specific sense primer corresponding to nt 1 to 53 were also included in the sequence analysis. The PCR products were cloned in pUC18 (Pharmacia Biotech AB), and four randomly selected clones of each cloned PCR product were sequenced by following the protocol supplied with the Dye Primer cycle-sequencing kit (Applied Biosystems) using the −21M13 Dye Primer and the M13 Reverse Dye Primer, in a 373 DNA sequencer. Nucleotide sequences were analyzed using GENETYX-MAC (Software Development Co., Ltd, Tokyo, Japan).
RESULTS
Detection of BDV RNA in brain tissue obtained from P2 at autopsy.
Plasma and CSF samples from four patients (P1 to P4) and two healthy control individuals (H1 and H2) (Table 1) were examined for the presence of BDV-specific antibodies by Western blot analysis using purified recombinant GST-p40 and GST-p24 proteins as target antigens. None of the plasma or CSF samples showed immunoreactivity with the control GST protein. Only P2 was found to be BDV seropositive by this assay. Plasma but not CSF from P2 had antibodies that specifically recognized GST-p24 in a Western blot (Table 1).
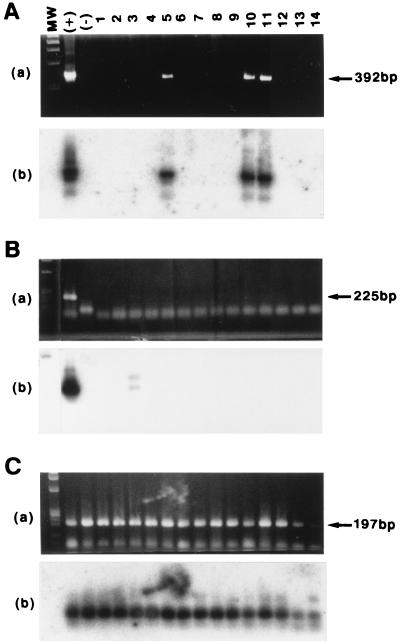
We next wanted to investigate whether there was a correlation between the detection of antibodies to BDV in plasma and the presence of viral RNA in brain tissue and blood. Brain tissue samples from patients and controls were dissected into 12 different regions (Table 2). Total RNA extracted from each brain region and total blood cells was reverse transcribed using random hexamers as primers, and the cDNAs were subjected to nested PCR using specific primers to amplify fragments of 225 and 392 bp from BDV p40 and p24 genes, respectively. BDV RNA sequences were detected only for P2 (Table 2). PCR products with the expected size of 392 bp for BDV p24 were obtained with RNA extracted from the hippocampus, pons, and cerebellum but not other brain regions of P2 (Fig. 1). The specificity of the PCR products was verified by Southern blot hybridization (Fig. 1A panel b). BDV p40 sequences were detected only in RNA from the temporal lobe of P2 and as a very weak signal after Southern blot hybridization (Fig. 1B).
TABLE 2.
Detection of BDV RNA in brain tissue and blood from four patients and healthy controls by RT-nested PCR and ISH
| Brain region | RT-PCR detection of BVD RNAa in:
|
ISH detection of BVD RNAb in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P1
|
P2
|
|||||||||
| P1 | P2 | P3 | P4 | H1 | H2 | Antisense | Sense | Antisense | Sense | |
| Olfactory bulb | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Cerebral cortex | ||||||||||
| Frontal lobe | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Temporal lobe | −/− | −/+ | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Occipital lobe | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Hippocampus | −/− | +/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | −/− |
| Fornix | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Hypophysis | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Cingulate gyrus | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Lateral ventricle (medial wall) | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Pons | −/− | +/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | −/− |
| Cerebellum | −/− | +/− | −/− | −/− | −/− | −/− | −/− | −/− | +/− | −/− |
| Medulla oblongata | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Blood | −/− | −/− | −/− | −/− | −/− | −/− | ||||
RNA isolated from the indicated brain regions was reverse transcribed using random hexamers, and the cDNAs were analyzed by nested PCR to amplify p24 and p40 BDV sequences as described in Materials and Methods. Results for p24 and p40 are indicated on the left and right, respectively, of the slash mark.
Sections from the indicated brain regions of patients P1 and P2 were analyzed by ISH using antisense and sense p24 and p40 riboprobes as described in Materials and Methods. Results are indicated as described in footnote a.
FIG. 1.
Detection of BDV RNA in tissue samples from patient P2. (A and B) A total of 14 RNA samples (1, olfactory bulb; 2, frontal lobe of cerebral cortex; 3, temporal lobe of cerebral cortex; 4, occipital lobe of cerebral cortex; 5, hippocampus; 6, fornix; 7, hypophysis; 8, cingulate gyrus; 9, medial wall of the lateral ventricle; 10, pons; 11, cerebellum; 12, medulla oblongata; 13, blood; 14, CSF) prepared from patient P2 were subjected to RT with random hexamers followed by nested PCR using primers to amplify DNA fragments with predicted sizes of 392 and 225 bp, corresponding to p24 (A) and p40 (B) sequences, respectively. RNA from BDV-infected (+) and uninfected (−) OL cells was used as positive and negative controls, respectively. (C) As a control for RNA quality, cDNAs were also amplified with specific primers to generate a 197-bp DNA fragment of the GAPDH housekeeping cellular gene. PCR products were resolved by agarose gel electrophoresis and visualized by staining with ethidium bromide (panels a). The specificity of the RT-PCR products was determined by Southern blot hybridization using 32P-labeled probes corresponding to internal sequences of the PCR products (panels b). MW, size markers (φX174 DNA HaeIII fragments).
A 192-bp fragment of the GAPDH cellular gene could be amplified in all the RNA samples analyzed (Fig. 1C and data not shown). PCR products of 225 and 392 bp, corresponding to BDV p24 and p40 sequences, respectively, were amplified from BDV-infected OL cells but not from uninfected control cells (Fig. 1). Omission of reverse transcriptase in the RT step resulted in the absence of BDV and GAPDH PCR products (data not shown).
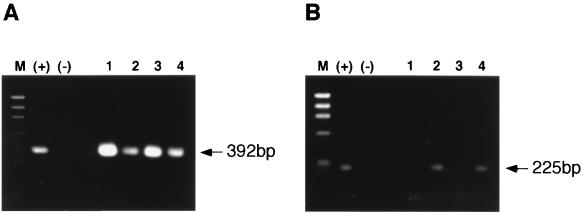
Priming of the RT reaction mixture with random hexamers did not allow us to distinguish between amplification of mRNA or genomic viral RNA sequences. To detect BDV genomic RNA in the BDV-positive samples, we performed the RT step using a BDV-specific primer of sense polarity (mRNA) corresponding to nt 1 to 53 in the BDV genome RNA. The corresponding cDNA was subsequently amplified by nested PCR using the same p40 and p24 primer pairs described above. PCR products of 392 bp (p24) were detected in the hippocampus, pons, cerebellum, and temporal lobe of P2 (Fig. 2A). PCR products of 225 bp (p40) were also readily detected by ethidium bromide staining in the pons and temporal lobe of P2 (Fig. 2B). For unknown reasons, clear detection of genome-derived p40 sequences in hippocampus and cerebellum of P2 required the use of Southern blot hybridization (results not shown). Together, these findings indicated that BDV genomic sequences were present in these brain regions.
FIG. 2.
Detection of BDV genomic RNA in brain tissue from patient P2. The same RNA samples extracted from the hippocampus (lane 1), pons (lane 2), cerebellum (lane 3), and temporal lobe of the cerebral cortex (lane 4), used in Fig. 1, were reverse transcribed using a BDV-specific primer of antigenomic polarity and complementary to nucleotides 1 to 53. The resulting cDNA was amplified by nested PCR, using the same primers as in Fig. 1, to amplify p24 (A) and p40 (B) sequences. PCR products were resolved by agarose gel electrophoresis and visualized by staining with ethidium bromide. RNA from BDV-infected (+) and uninfected (−) OL cells were used as positive and negative controls, respectively. M, size marker (φX174 DNA HaeIII fragments).
Histopathological analysis of brain tissue from patient P2.
Neuronal damage within the hippocampal region together with astrocytosis are characteristic histopathological findings in naturally and experimentally BDV-infected animals. Microscopy examination of hematoxylin-eosin-stained sections from different brain regions showed a mild cell infiltration, as well as chromatolysis of neuronal nuclei and satellitosis, especially around blood vessels in the hippocampus of P2 (data not shown). These histopathological findings were not observed in hippocampi from BDV RNA-negative patients and controls. Histopathological changes were not observed in any other P2 brain region examined, including the pons, cerebellum, and cerebral cortex, which were BDV RNA positive.
Cellular distribution of BDV markers in brain tissue from patient P2.
With the aim of examining the cellular distribution of BDV in the brain of P2 and determining whether detection of BDV RNA correlated with the presence of viral antigen, we performed ISH and IHC studies. ISH using a BDV-p24 antisense riboprobe revealed positive cells in the hippocampus, pons, and cerebellum of P2 but not in the same brain regions of P1, used as a control (Fig. 3). The cerebellum had the largest number of BDV-positive cells, followed by the hippocampus, whereas only very few positive cells were seen in the pons. The hybridization signal appeared to be located mainly in the nuclei of neurons. BDV RNA-positive neurons were found in hippocampal regions exhibiting immune cell infiltrates, but infiltrated cells did not show hybridization to BDV p24 antisense riboprobe. Eight other brain regions from P2 that were BDV negative by RT-PCR were also negative by ISH using a BDV p24 antisense riboprobe (Table 2). In addition, ISH results were negative for all brain regions analyzed from patient P1. ISH with a BDV p40 antisense riboprobe did not show positive cells in any of the brain regions examined from P1 and P2 (Table 2). In addition, no BDV RNA-positive cells were observed when the same brain regions were analyzed by ISH using BDV p40 or p24 sense riboprobes (Table 2). This finding suggested that only low levels of BDV genomic RNA species were present in these brain regions. BDV antigen was not detected in hippocampal sections from P1, the only other patient whose samples were analyzed by IHC.
FIG. 3.
Detection by ISH of BDV RNA in brain tissue from patient P2. Sections from the hippocampus (A), cerebellum (B), and pons (C) from P1 and P2, which were negative and positive for BDV p24 RNA (Fig. 1A), respectively, were subjected to ISH using a BDV p24 antisense riboprobe. Magnification, ×558.
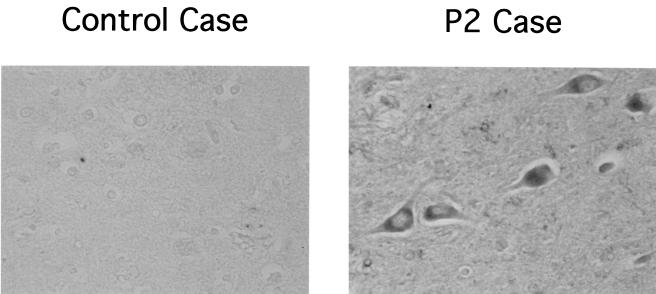
To examine whether detection of BDV RNA in the hippocampus of P2 correlated with the presence of viral antigen, we conducted IHC studies using serum from BDV-infected mice. This serum recognizes BDV p40 (NP), p24 (P), and p16 (M) antigens in BDV-infected cells. BDV-specific immunoreactivity was detected in neurons and to a lesser extent also in astrocytes in the hippocampal formation of P2 (Fig. 4).
FIG. 4.
Detection of BDV antigen in the brain of patient P2. Hippocampal sections from patient P2 and a healthy control were stained with a mouse polyclonal serum to BDV by procedures described in Materials and Methods. Photomicrographs were taken using a 10× ocular and a 40× objective.
Isolation of BDV from brain tissue from patient P2.
We next investigated whether it was possible to isolate BDV from BDV-positive brain tissue samples from patient P2. For this purpose we employed a variety of experimental approaches. Our first approach consisted of the use of cell homogenates from the cerebellum and hippocampus of patient P2 to directly inoculate OL cells. OL is a human oligodendroglial cell line highly susceptible to BDV, which has been previously used for the isolation of BDV from human PBMC (5). Inoculated OL cells were subcultured every 3 to 4 days. No BDV antigen expression could be detected after more than 30 passages in tissue culture.
Undaunted by this result, we then attempted the isolation of BDV from P2 brain tissue by passages in laboratory animals. We have recently documented that newborn Mongolian gerbils are highly permissive for BDV replication in brain following intracerebral (i.c.) virus inoculation (35). Newborn gerbils were inoculated i.c. with cell homogenates from the hippocampus and cerebellum of patient P2, and virus replication was examined at 10 and 20 days postinoculation. For this, RNA extracted from several tissues was analyzed by RT-nested PCR combined with Southern blot hybridization to detect BDV p40. BDV RNA was detected only in brain and spinal cord tissue samples at 20 but not at 10 days postinoculation (Fig. 5A). Cell homogenates prepared from BDV RNA-positive gerbil brains were used to attempt to perform subsequent i.c. passages in newborn gerbils of this human-derived BDV, designated BDVHuP2br. Gerbil brain and spinal cord tissues from the second and third passages were also positive for BDV RNA (Fig. 5B). We next tried to isolate BDVHuP2br by inoculating OL cells with cell homogenates from the cerebrum, cerebellum, and spinal cord of BDV RNA-positive gerbils and subjecting these cells to serial passages in tissue culture. The appearance of BDV antigen in the inoculated OL cells was monitored by IF using a mouse monoclonal antibody to BDV p40. BDV-positive cells were detected only in OL cells that were inoculated with a cell homogenate from the gerbil cerebellum. BDV-positive OL cells were first detected 45 days after inoculation, and the numbers increased during serial passages, reaching more than 70% of the population at 100 days p.i. (Fig. 6).
FIG. 5.
Replication of human brain-derived BDV in gerbils. (A) Detection of BDV RNA in gerbils inoculated with brain tissue from patient P2. Newborn gerbils were inoculated with a brain homogenate (hippocampus plus cerebellum) from patient P2. At 10 (upper panel) and 20 (lower panel) days after inoculation, total RNAs from different tissues and brain regions were prepared and analyzed by RT-PCR using primers to amplify a 225-bp DNA fragment of the BDV p40 open reading frame. The samples were as follows: 1, brain; 2, spinal cord; 3, olfactory nerve; 4, sciatic nerve; 5, heart; 6, lung; 7, liver; 8, spleen; 9, kidney; 10, thymus; 11, lymph node; 12, eye; 13, blood. (B) Detection of BDV RNA during the second and third passages of BDVHubrP2 in gerbil brains. Newborn gerbils were inoculated with brain homogenates from first (upper panel) and second (lower panel) passages of BDVHubrP2 in gerbil brains. RNA from the brain (lane 1) and spinal cord (lane 2) was prepared on day 20 postinoculation, and analyzed by RT-PCR as described for panel A. RNAs from BDV-infected (+) and uninfected (−) OL cells were used as positive and negative controls, respectively. PCR products were resolved by agarose gel electrophoresis and visualized by staining with ethidiume bromide (top). The specificity of the PCR products was determined by Southern blot hybridization using a 32P-labeled probe corresponding to internal sequences of the PCR product (bottom).
FIG. 6.
Isolation of BDV from the brain of a gerbil inoculated with brain material from patient P2. OL cells were inoculated with a cell homogenate prepared from the brain tissue of a gerbil 20 days after being inoculated with a brain homogenate (hippocampus plus cerebellum) from patient P2 (●). As a control, OL cells were inoculated with a cell homogenate from the brain of a control gerbil prepared 20 days after its inoculation with uninfected MDCK cells (○). BDV antigen expression was monitored by IF with monoclonal antibody to BDV p40 (HN182) for up to 120 days after inoculation. Cells were passaged (dilution, 1:10) every 5 days.
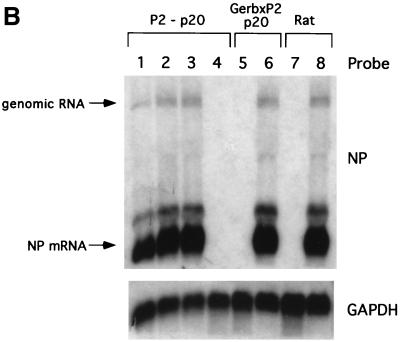
The rationale for the third approach used for the isolation of BDVHuP2br was based in our previous observation that the nuclei of BDV-infected cells contain relatively high levels of BDV RNP complexes that are infectious upon transfection into susceptible cells (9). We used RNP isolated from BDV-positive brain tissues of P2 and gerbils to transfect Vero cells using procedures described previously (9). Transfected cells were subcultured every 4 to 5 days, and expression of viral antigen was monitored by IF using a rabbit polyclonal serum to BDV NP and also serum from BDV-infected mice (Fig. 7). We performed several independent isolation attempts, including five from P2 hippocampus, six from P2 cerebellum, and four each from P2 pons and cerebral cortex. BDV infectivity was successfully rescued once from the hippocampus and pons, twice from the cerebellum, and not at all from the cerebral cortex. Control experiments included transfection of Vero cells with RNP prepared from total brain of 12-week-old rats infected at birth with BDV He80 and mock-infected control rats. BDV infectivity was rescued from all three BDV chronically infected rats, whereas no infectivity was rescued from any of the three mock-infected control rats. In addition, RNP were prepared from BDV-positive gerbil cerebrum, cerebellum, and spinal cord tissues. Preparations of RNP from spinal cord tissue systematically exhibited high toxicity on Vero cells and could not be further analyzed by this assay. BDV infectivity was found in gerbil cerebellum in three out of four attempts, whereas two attempts to isolate it from gerbil cerebrum were unsuccessful.
FIG. 7.
Replication of BDV in Vero cells upon transfection with RNP prepared from brain tissue from patient P2 or from gerbils inoculated with P2 brain tissue. (A) RNP were prepared from the indicated brain regions from patient P2 and from a gerbil inoculated with P2 brain tissue. RNP prepared from brain tissue of BDV-infected and mock-infected rats were used as positive and negative controls, respectively. Vero cells were transfected with the indicated RNP preparation as described in Materials and Methods. Expression of BDV antigen at passage 20 (p20) or 7 (p7) was detected by IF using a mouse polyclonal serum to BDV. (B) RNA was extracted from transfected cells and analyzed by Northern blot hybridization using a BDV NP-specific probe.
Sequence analysis of BDVHuP2br.
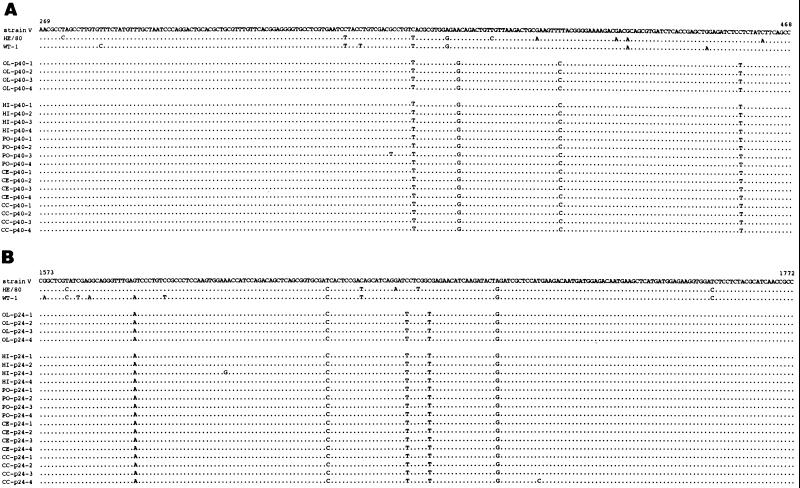
To molecularly characterize BDVHuP2br isolate and assess its relationship to BDV sequences previously reported from humans and naturally infected animals, we obtained partial sequences of p40 and p24 from BDVHuP2br. For this, we used RNA extracted from BDVHuP2br-infected OL cells to amplify BDV p40 and p24 sequences using RT-PCR procedures described above, followed by cloning and sequencing of the amplified PCR products. In addition, we cloned and sequenced BDV p40 and p24 PCR products directly derived from hippocampus, pons, and cerebral cortex tissues of P2. We sequenced four randomly selected clones of each cloned PCR product. Both BDV p40 and p24 consensus sequences obtained from BDVHuP2br-infected OL cells were identical to those directly derived from brain tissue of P2 (Fig. 8). BDVHuP2br p40 and p24 sequences were genetically very closely related to BDV strains V and He80, both of which were originally derived from horses with BD. Nonetheless, within the 200 nt of the p40 and p24 segments sequenced, BDVHuP2br showed unique substitutions compared to strain V and He80 (7, 10). Within p40, BDVHuP2br had four (2%) and three (1.5%) substitutions with respect to strains V and He80, respectively, whereas within p24, BDVHuP2br had five (2.5%) and three (1.5%) substitutions with respect to strains V and He80, respectively (Fig. 8).
FIG. 8.
Comparison of p40 and p24 nucleotide sequences between BDVHubrP2 isolate and horse-derived BDV sequences. Total RNA extracted from OL cells persistently infected with the BDVHubrP2 isolate was amplified by RT-PCR using the p24 and p40 primers described in Materials and Methods. The PCR products were cloned, and four randomly selected clones of each PCR product were sequenced, as described in Materials and Methods. These clones were named OL. PCR products directly amplified from BDV genomic RNA present in the hippocampus (HI), pons (PO), cerebellum (CE), and cerebral cortex (temporal lobe) (CC) of patient P2 were also cloned and sequenced. The nucleotide sequences of p40 and p24 regions spanning nt 269 to 468 and 1573 to 1772, respectively, of four randomly selected clones for each individual PCR product are shown. The sequences of the corresponding p40 and p24 regions for three BDV horse isolates, strain V, He/80, and WT-1, are also shown.
DISCUSSION
Serological data and molecular epidemiological studies indicate that BDV can infect humans and is possibly associated with certain neuropsychiatric illnesses (4, 6, 15, 21, 27–29, 37, 40, 43, 49, 50). Moreover, several studies have reported the detection of BDV RNA in brain tissue collected at autopsy from patients with clinical records of mental disorders (12, 17, 18, 36, 42). Consistent with these findings, we have documented here the detection of BDV RNA and antigen in brain tissue collected at autopsy from a schizophrenic patient. Our results showed evidence of a regionally localized distribution of BDV RNA, as well as possible differences in expression levels of BDV p40 and p24 mRNA in brain tissue from patient P2. We detected BDV RNA in only 4 of 12 different brain regions examined. This finding underscores the importance of examining several brain regions in studies aimed at investigating an association between persistence of BDV in the CNS and human mental disorders. As with other nonsegmented, negative-strand RNA viruses, the BDV NP is usually expressed at high levels in infected cells. Unexpectedly, however, BDV p24 but not p40 RNA sequences were detected in the hippocampus, cerebellum, and pons of patient P2 (Fig. 1 and 3). In contrast, p40 but not p24 RNA sequences were detected in the temporal lobe by RT-PCR. Levels of p40 in the temporal lobe appeared to be very low, which probably prevented its detection by ISH. These findings suggest that expression levels of p40 and p24 may be significantly different depending on the brain region. Nevertheless, we cannot rule out the possibility that differences in the sensitivity of RT-PCR and ISH procedures in detecting p40 and p24 also contributed to these results. As expected, BDV genome RNA was also detected in brain regions positive for p24 and p40 (Fig. 2). Nevertheless, specific hybridization was not observed when ISH was performed with a p24 sense probe, suggesting that genome RNA was present only at very low levels in these brain regions. BDV antigen-expressing cells could be detected in the hippocampus, cerebellum, pons, and temporal lobe of patient P2. Expression levels of BDV antigen seemed to be very low based on the intensity of the immunoreactivity observed, with the exception of the hippocampus, which appeared to harbor a higher viral antigen load.
Using tissue from patient P2, we succeeded in isolating for the first time BDV from the human brain. Several investigators have reported unsuccessful attempts to isolate BDV from brain tissue and CSF of individuals found to be BDV seropositive and having clinical symptoms compatible with a BDV infection (41). It is worth noting that studies aimed at detecting and isolating BDV from the human brain have mainly included tissue collected at autopsy from elderly patients. In contrast, our investigation included samples from relatively young schizophrenic patients. The natural course of BDV infection in humans is unknown. It is conceivable that an infection with BDV early in life could influence the onset and/or clinical course of certain neuropsychiatric disorders. However, many years later, histopathological signs of virus infection in brain may be absent and the BDV load in brain may be greatly decreased, making virus detection and isolation difficult. Autopsy data indicate that inflammatory changes in the brain are rarely associated with schizophrenia and bipolar disorders (51). We observed mild inflammatory changes in the hippocampus of patient P2. It is plausible that this case provided us with the rare opportunity of examining a very early phase of BDV infection in humans. Immunocompetent adult rats infected (i.c) with BDV usually develop an immune system-mediated CNS disease with a clinical and histopathological picture very similar to that described for animals with naturally acquired BD (38). The extensive inflammatory reaction associated with BD leads to significant neuronal destruction. In contrast, and interestingly, only subtle and fleeting inflammatory changes can be seen in the brain parenchyma of rats chronically infected with BDV since birth, although the animals exhibit neurobehavioral and neurochemical abnormalities (3, 14, 15, 25).
We first unsuccessfully attempted to isolate BDV by direct inoculation of OL cells with brain homogenates from P2. Isolation of several neurotropic viruses which grow poorly in cultured cells can be facilitated by the use of passages in laboratory animals. Gerbils have been used for the isolation of a variety of neurotropic viruses (1, 22, 47). Moreover, we have documented that newborn gerbils are highly susceptible to experimental infection with BDV (35). Newborn gerbils inoculated with hippocampus or cerebellum homogenates prepared from P2 brain tissue harbored BDV RNA in their brains and spinal cords. This human-derived BDV (BDVHuP2br) isolate could be serially passed in newborn gerbil brain and rescued by inoculating OL cells with cerebellum homogenates from BDV-positive gerbils. We could also rescue BDVHuP2br by transfecting Vero cells with BDV RNP prepared from the cerebellum of BDV-positive gerbils. BDV was found to be present in the cerebrum of newborn gerbils inoculated with BDV derived from persistently infected MDCK cells (MDCK/BDV) (35). However, we were unable to rescue BDVHuP2br from the cerebrum of BDV-positive gerbils. It is possible that MDCK/BDV and BDVHuP2br exhibit differences in their ability to replicate and propagate in gerbil brain. BDVHuP2br could be rescued, although at very low efficiency, by transfection of Vero cells with RNP prepared from the hippocampus, cerebellum, and pons of patient P2. The amount of purified RNP that we used to transfect Vero cells corresponded to approximately 1 ml of a 10% (wt/vol) brain cell homogenate. This represented about 50 times more than the maximum amount of brain cell homogenate used to inoculate OL cells without causing significant cytoxicity. These results suggest that levels of infectious BDV present in the brain of patient P2 were below those suitable for direct isolation by infection of OL cells but could be amplified by passages in gerbil brain. We cannot rule out the possibility that during its replication in gerbil brain, BDVHuP2br acquired mutations, conferring on it an increased ability to replicate in OL cells. Based on our findings, it seems reasonable to propose the use of passages in newborn gerbil brain to attempt virus isolation from human brain tissue suspected of harboring a BDV infection. It is worth noting that experimentally infected horses also exhibit a region-localized CNS expression of BDV and that virus isolation from infected horse brain tissue was difficult (26).
Both partial BDV p40 and p24 consensus sequences obtained from BDVHuP2br-infected OL cells were identical to those directly derived from brain tissue of P2. This, however, does not rule out the possibility that the BDVHuP2br isolate may differ in other positions with respect to the virus present in the brain of P2. Consistent with previous reports, the BDVHuP2br isolate exhibited a high degree of sequence conservation compared to BDV sequences derived from naturally infected animals of different species and also those derived from human PBMC. Nonetheless, BDVHuP2br had unique substitutions in both p40 and p24. It is worth noting that a single amino acid change can be responsible for drastically altered virus phenotypes, including changes in tropism and pathogenicity (13, 24). It remains to be determined whether BDVHuP2br exhibits biological properties different from those described for characterized animal isolates.
The source of BDV and routes for human infection are unknown. Infected blood cells, both lymphocytes and macrophages, are thought to facilitate access to the CNS by several neurotropic viruses. BDV RNA has been detected in human PBMC, raising concern about whether BDV can be transmitted by blood products. These findings, however, remain highly controversial due to conflicting results obtained by different investigators. In a recent report it has been proposed that the granulocyte fraction, rather than PBMC, harbors BDV in human blood (39). Various degrees of contaminating granulocytes present in PBMC preparations could explain discrepancies in BDV detection in blood. BDV-infected granulocytes in blood, although present at very low frequency, could reach the CNS and initiate the establishment of a long-term chronic infection in the CNS.
Persistent virus infections of the CNS can cause slowly progressive neurological disorders associated with diverse pathological manifestations (13, 32, 33, 48, 51). This observation, together with epidemiological data and clinical findings, has led to the proposal that viruses can contribute to a variety of neurological diseases. Therefore, there is much interest in identifying potential viral culprits. The findings reported here add strength to the hypothesis that BDV might represent one of the environmental cofactors known to contribute to neuropsychiatric disorders whose etiologies remain elusive.
ACKNOWLEDGMENTS
This work was partly supported by a Grant-in-Aid for BDV Research from the Ministry of Health and Welfare and Grants-in-Aid for Scientific Research (A and B) from the Ministry of Education, Science, Sports and Culture, Japan, and by NIH grants NS12428 and MH57063 (to J.C.T.).
REFERENCES
- 1.Anderson G W, Jr, Slone T W, Peter C J. The gerbil, Meriones unguiculatus, a model for Rift Valley fever viral encephalitis. Arch Virol. 1988;102:187–196. doi: 10.1007/BF01310824. [DOI] [PubMed] [Google Scholar]
- 2.Bahmani M K, Nowrouzian I, Nakaya T, Nakamuar Y, Hagiwara K, Takahashi H, Rad M A, Ikuta K. Varied prevalence of Borna disease virus infection in Arabic, thoroughbred and their cross-bred horses in Iran. Virus Res. 1996;45:1–13. doi: 10.1016/0168-1702(96)01355-x. [DOI] [PubMed] [Google Scholar]
- 3.Bautista J R, Schwartz G J, de la Torre J C, Moran T H, Carbone K M. Early and persistent abnormalities in rats with neonatally acquired Borna disease virus infection. Brain Res Bull. 1994;34:31–40. doi: 10.1016/0361-9230(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 4.Bode L. Human infections with Borna disease virus and potential pathogenic implications. Curr Top Microbiol Immunol. 1995;190:103–130. doi: 10.1007/978-3-642-78618-1_7. [DOI] [PubMed] [Google Scholar]
- 5.Bode L, Durrwald R, Rantam F A, Ferszt R, Ludwig H. First isolates of infectious human Borna disease virus from patients with mood disorders. Mol Psychiatry. 1996;1:200–212. [PubMed] [Google Scholar]
- 6.Bode L, Zimmermann W, Ferszt R, Steinbach F, Ludwig H. Borna disease virus genome transcribed and expressed in psychiatric patients. Nat Med. 1995;95:232–236. doi: 10.1038/nm0395-232. [DOI] [PubMed] [Google Scholar]
- 7.Briese T, Schneemann A, Lewis A J, Park Y S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caplazi P, Waldvogel A, Stitz L, Braun U, Ehrensperger F. Borna disease in naturally infected cattle. J Comp Pathol. 1994;94:65–72. doi: 10.1016/s0021-9975(05)80112-4. [DOI] [PubMed] [Google Scholar]
- 9.Cubitt B, de la Torre J C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J Virol. 1994;68:1371–1381. doi: 10.1128/jvi.68.3.1371-1381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cubitt B, Oldstone C, de la Torre J C. Sequence and genome organization of Borna disease virus. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Torre J C, Gonzalez-Dunia D, Cubitt B, Mallory M, Mueller-Lantzsch M, Grasser F A, Hansen L A, Masliah E. Detection of Borna disease virus antigen and RNA in human autopsy brain samples from neuropsychiatric patients. Virology. 1996;223:272–282. doi: 10.1006/viro.1996.0479. [DOI] [PubMed] [Google Scholar]
- 13.de la Torre J C, Oldstone M B A. The anatomy of viral persistence: Mechanisms of persistence and associated disease. Adv Virus Res. 1996;46:311–343. doi: 10.1016/s0065-3527(08)60075-5. [DOI] [PubMed] [Google Scholar]
- 14.Dittrich W, Bode L, Ludwig H, Kao M, Schneider K. Learning deficiencies in Borna disease virus-infected but clinically healthy rats. Biol Psychiatry. 1989;89:818–828. doi: 10.1016/0006-3223(89)90122-4. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Dunia D, Sauder C, de la Torre J C. Borna disease virus and the brain. Brain Res. 1997;44:647–664. doi: 10.1016/S0361-9230(97)00276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosztonyi G, Ludwig H. Borna disease—neuropathology and pathogenesis. In: Koprowski H, Lipkin W I, editors. Borna disease virus. Berlin, Germany: Springer-Verlag KG; 1995. pp. 39–74. [PubMed] [Google Scholar]
- 17.Haga S, Motoi Y, Ikeda K, Group J B S. Borna disease virus and neuropsychiatric disorders. Lancet. 1997;350:592–593. doi: 10.1016/s0140-6736(05)63183-2. [DOI] [PubMed] [Google Scholar]
- 18.Haga S, Yoshimura M, Motoi Y, Arima K, Aizawa T, Ikuta K, Tashiro M, Ikeda K. Detection of Borna disease virus genome in normal human brain tissue. Brain Res. 1997;770:307–309. doi: 10.1016/s0006-8993(97)00903-7. [DOI] [PubMed] [Google Scholar]
- 19.Hagiwara K, Kawamoto S, Takahashi H, Nakamuar Y, Nakaya T, Hiranuma T, Ishihara C, Ikuta K. High prevalence of Borna disease virus infection in healthy sheep in Japan. Clin Diagn Lab Immunol. 1997;4:339–344. doi: 10.1128/cdli.4.3.339-344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagiwara K, Nakaya T, Nakamura Y, Asahi S, Takahashi H, Ishihara C, Ikuta K. Borna disease virus RNA in peripheral blood mononuclear cells obtained from healthy dairy cattle. Med Microbiol Immunol. 1996;185:145–151. doi: 10.1007/s004300050024. [DOI] [PubMed] [Google Scholar]
- 21.Hatalski C G, Lewis A J, Lipkin W I. Borna disease. Emerging Infect Dis. 1997;3:129–135. doi: 10.3201/eid0302.970205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayles L B. Susceptibility of the Mongolian gerbil (Meriones unguiculatus) to Western equine encephalitis. Can J Microbiol. 1972;18:941–944. doi: 10.1139/m72-145. [DOI] [PubMed] [Google Scholar]
- 23.Herzog S, Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 24.Holland, J. J. 1992. Genetic diversity of RNA viruses. Curr. Top. Microbiol. Immunol. 176. [PubMed]
- 25.Hornig M, Weissenbock H, Horscroft N, Lipkin W I. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz J B, Alstad D, Jenny A L, Carbone K M, Rubin S A, Waltrip R W., II Clinical, serologic, and histopathologic characterization of experimental Borna disease in ponies. J Vet Diagn Investig. 1998;10:338–343. doi: 10.1177/104063879801000405. [DOI] [PubMed] [Google Scholar]
- 27.Kishi M, Nakaya T, Nakamura Y, Kakinuma M, Takahashi T A, Sekiguchi S, Uchikawa M, Tadokoro K, Ikeda K, Ikuta K. Prevalence of Borna disease virus RNA in peripheral blood mononuclear cells from blood donors. Med Microbiol Immunol. 1995;95:135–138. doi: 10.1007/BF00224350. [DOI] [PubMed] [Google Scholar]
- 28.Kishi M, Nakaya T, Nakamura Y, Zhong Q, Ikeda K, Senjo M, Kakinuma M, Kato S, Ikuta K. Demonstration of human Borna disease virus RNA in human peripheral blood mononuclear cells. FEBS Lett. 1995;95:293–297. doi: 10.1016/0014-5793(95)00406-y. [DOI] [PubMed] [Google Scholar]
- 29.Lipkin W I, Hatalski C G, Briese T. Neurobiology of Borna disease virus. J Neurovirol. 1997;3:S17–S20. [PubMed] [Google Scholar]
- 30.Lundgren A L, Ludwig H. Clinically diseased cats with non-suppurative meningoencephalomyelitis have Borna disease virus-specific antibodies. Acta Vet Scand. 1993;93(34):101–103. doi: 10.1186/BF03548230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malkinson M, Weisman Y, Perl S, Ashash E. A Borna-like disease in ostriches in Israel. In: Koprowski H, Lipkin W I, editors. Borna disease virus. Berlin, Germany: Springer-Verlag KG; 1995. pp. 31–38. [Google Scholar]
- 32.Mohammed A M, Norrby E, Kristensson K. Viruses and behavioral changes: a review of clinical and experimental findings. Rev Neurosci. 1993;4:267–280. doi: 10.1515/revneuro.1993.4.3.267. [DOI] [PubMed] [Google Scholar]
- 33.Morozov P V. Research on the viral hypothesis of mental disorders. In: Mendlewicz S, Praag H M, editors. Advances in biological psychiatry. S. New York, N.Y: Karger; 1983. [Google Scholar]
- 34.Nakamura Y, Asahi S, Nakaya T, Bahmani M K, Saitoh S, Yasui K, Mayama H, Hagiwara K, Ishihara C, Ikuta K. Demonstration of Borna disease virus RNA in peripheral blood mononuclear cells derived from domestic cats in Japan. J Clin Microbiol. 1996;96:188–191. doi: 10.1128/jcm.34.1.188-191.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura Y, Nakaya T, Hagiwara K, Momiyama N, Kagawa Y, Taniyama H, Ishihara C, Sata T, Kurata T, Ikuta K. High susceptibility of Mongolian gerbil (Meriones unguiculatus) to Borna disease virus. Vaccine. 1999;17:480–489. doi: 10.1016/s0264-410x(98)00222-9. [DOI] [PubMed] [Google Scholar]
- 36.Nakaya T, Tada M, Takahashi H, Fujiwara S, Sakuma S, Sawamura Y, Abe H, Ikuta K. Expression of Borna disease virus messages in clinical samples from patients with brain malignant tumors. Proc Jpn Acad. 1996;72:157–162. [Google Scholar]
- 37.Nakaya T, Takahashi H, Nakamura Y, Asahi S, Tobiume M, Kuratsune H, Kitani T, Yamanishi K, Ikuta K. Demonstration of Borna disease virus RNA in peripheral blood mononuclear cells derived from Japanese patients with chronic fatigue syndrome. FEBS Lett. 1996;96:145–149. doi: 10.1016/0014-5793(95)01439-x. [DOI] [PubMed] [Google Scholar]
- 38.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Behavioral disease in rats caused by immunopathological responses to persistent Borna disease virus. Science. 1983;220:1401–1402. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 39.Planz O, Rentzsch C, Batra A, Batra A, Winkler T, Buttner M, Rziha H-J, Stitz L. Pathogenesis of Borna disease virus: granulocyte fractions of psychiatric patients harbor infectious virus in the absence of antiviral antibodies. J Virol. 1999;73:6251–6256. doi: 10.1128/jvi.73.8.6251-6256.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richt J A, Pfeuffer I, Christ M, Frese K, Bechter K, Herzog S. Borna disease virus infection in animals and humans. Emerging Infect Dis. 1997;3:343–352. doi: 10.3201/eid0303.970311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rott R, Becht H. Natural and experimental Borna disease in animals. In: Koprowski H, Lipkin W I, editors. Borna disease virus. Berlin, Germany: Springer-Verlag KG; 1995. pp. 17–30. [DOI] [PubMed] [Google Scholar]
- 42.Salvatore M, Morzunov S, Schwemmle M, Lipkin W I, Group B S. Borna disease virus in brains of North American and European people with schizophrenia and bipolar disorder. Lancet. 1997;349:1813–1814. doi: 10.1016/s0140-6736(05)61693-5. [DOI] [PubMed] [Google Scholar]
- 43.Sauder C, Muller A, Cubitt B, Mayer J, Steinmetz J, Trabest W, Ziegler B, Wanke K, Mueller-Lantzsch N, de la Torre J C, Grasser F A. Detection of Borna disease virus (BDV) antibodies and BDV RNA in psychiatric patients: evidence for high sequence conservation of human blood-derived BDV RNA. J Virol. 1996;70:7713–7724. doi: 10.1128/jvi.70.11.7713-7724.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;95:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 45.Shoya Y, Kobayashi T, Koda T, Lai P K, Tanaka H, Koyama T, Ikuta K, Kakinuma M, Kishi M. Amplification of a full-length Borna disease virus (BDV) cDNA from total RNA of cells persistently infected with BDV. Immunology. 1997;41:481–486. doi: 10.1111/j.1348-0421.1997.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 46.Sierra-Honigmann A M, Rubin S A, Estafanous M G, Yolken R H, Carbone K M. Borna disease virus in peripheral blood mononuclear and bone marrow cells of neonatally and chronically infected rats. J Neuroimmunol. 1993;93:31–32. doi: 10.1016/0165-5728(93)90160-z. [DOI] [PubMed] [Google Scholar]
- 47.Suss J, Beziat P, Ramelow C, Kahl O. Tick-borne encephalitis virus (TBEV)-specific RT-PCR for characterization of natural foci of TBE and for other applications. Zentbl Bakteriol. 1997;286:125–138. doi: 10.1016/s0934-8840(97)80084-9. [DOI] [PubMed] [Google Scholar]
- 48.ter Meulen V. Virus-cell interactions in the nervous system. Semin Neurosci. 1991;3:81–173. [Google Scholar]
- 49.Waltrip R W, II, Buchanan R W, Summerfelt A, Breier A, Carpenter W T, Jr, Bryant N L, Rubin S A, Carbone K M. Borna disease virus antibodies and the deficit syndrome of schizophrenia. Schizophr Res. 1997;23:253–257. doi: 10.1016/s0920-9964(96)00114-4. [DOI] [PubMed] [Google Scholar]
- 50.Waltrip R W, II, Buchanan R W, Summerfelt A, Breier A, Carpenter W T, Jr, Bryant N L, Rubin S A, Carbone K M. Borna disease virus and schizophrenia. Psychiatry Res. 1995;56:33–44. doi: 10.1016/0165-1781(94)02600-n. [DOI] [PubMed] [Google Scholar]
- 51.Yolken R H, Fuller Torrey E. Viruses, schizophrenia, and bipolar disorder. Clin Microbiol Rev. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]