Abstract
Background
Thromboprophylaxis for medically ill patients during hospitalization and postdischarge remains underutilized. Clinical decision support (CDS) may address this need if embedded within workflow, interchangeable among electronic health records (EHRs), and anchored on a validated model.
Objectives
The purpose of this study was to assess the clinical impact of a universal EHR-integrated CDS tool based on the International Medical Prevention Registry on Venous Thromboembolism plus D-Dimer venous thromboembolism model.
Methods
This was a cluster randomized trial of 4 tertiary academic hospitals from December 21, 2020 to January 21, 2022. Inpatients over age 60 with key medical illnesses were eligible. We embedded CDS at admission and discharge. Hospitals were randomized to intervention (CDS; n = 2) vs usual care (n = 2) groups. The primary outcome was rate of appropriate thromboprophylaxis. Secondary outcomes included venous, arterial, and total thromboembolism, major bleeding, and all-cause mortality through 30 days postdischarge.
Results
After exclusions, 10,699 of 19,823 patients were analyzed. Intervention group tool adoption was 77.8%. Appropriate thromboprophylaxis was increased at intervention hospitals, both inpatient (80.1% vs 72.5%, OR: 1.52, 95% CI: 1.39-1.67) and at discharge (13.6% vs 7.5%, OR: 1.93, 95% CI: 1.60-2.33). There were fewer venous (2.7% vs 3.3%, OR: 0.80, 95% CI: 0.64-1.00), arterial (0.25% vs 0.70%, OR: 0.35, 95% CI: 0.19-0.67), and total thromboembolisms (2.9% vs 4.0%, OR: 0.71, 95% CI: 0.58-0.88) at intervention hospitals. Major bleeding was rare and did not differ between groups. Mortality was higher at intervention hospitals (9.1% vs 7.0%, OR: 1.32, 95% CI: 1.15-1.53).
Conclusions
EHR-embedded CDS increased appropriate thromboprophylaxis and reduced thromboembolism without increasing major bleeding in medically ill inpatients. Mortality was higher at intervention hospitals.
Key words: arterial thromboembolism, clinical decision support, clinical prediction rule, IMPROVE-DD VTE score, medical inpatient, thromboprophylaxis, venous thromboembolism
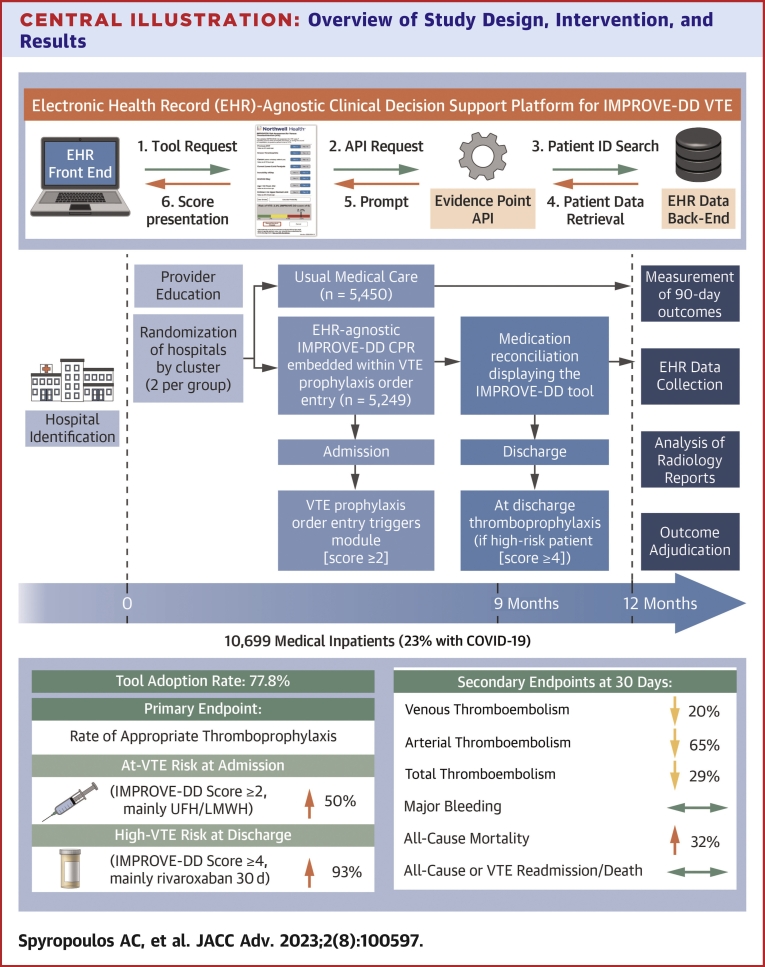
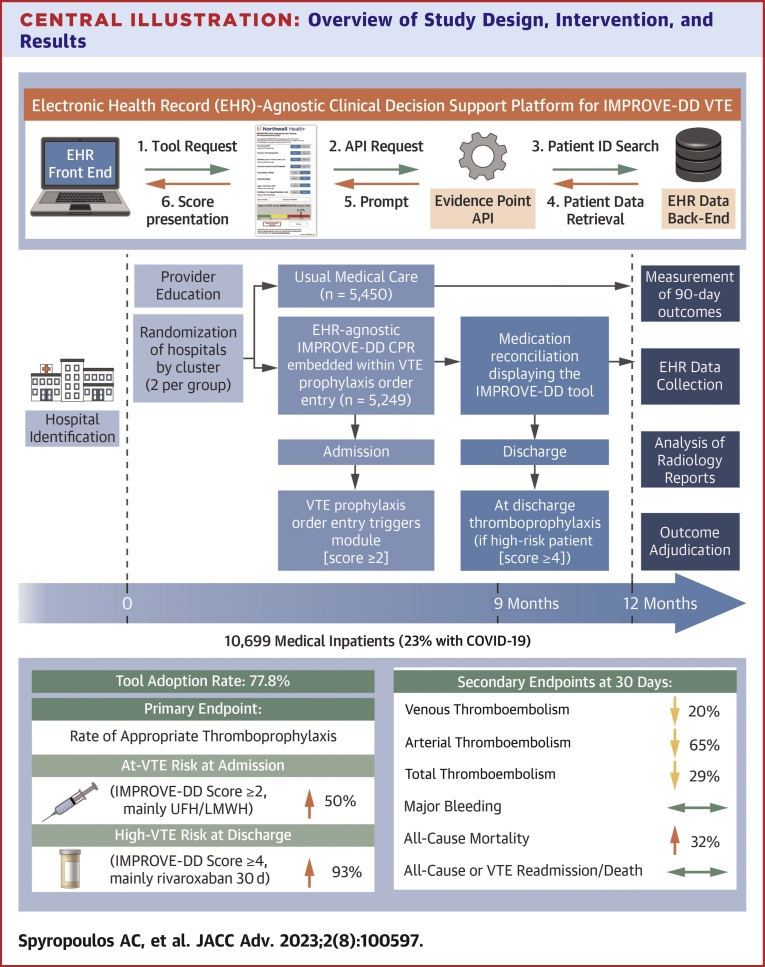
Central Illustration
Hospital-acquired venous thromboembolism (VTE) in medical inpatients during hospital stay and within 3 months after hospitalization remains a major cause of morbidity and mortality and constitutes the majority of the populational burden of VTE.1, 2, 3 Appropriate in-hospital and extended postdischarge thromboprophylaxis in medical inpatients reduces the risk of symptomatic and fatal VTE.4,5 Recently, extended postdischarge thromboprophylaxis in this population has been shown to reduce arterial thromboembolism including nonhemorrhagic stroke.6, 7, 8 Antithrombotic guidelines recommend thromboprophylaxis in hospitalized medical patients with extended postdischarge thromboprophylaxis recommended in some guidelines using an individualized approach or in high-risk COVID-19 patients,9,10 while prevention of VTE is ranked the number one strategy to improve patient safety in hospitals.9,11,12
Despite these data, thromboprophylaxis in medical inpatients both in-hospital and immediately postdischarge continues to be underutilized.4 Of health system-wide approaches, electronic alerts incorporating venous thrombosis risk models and national health programs for venous thrombosis prevention have been shown to increase the proportion of hospitalized patients that receive appropriate thromboprophylaxis and decrease the incidence of symptomatic, and rarely fatal, VTE.13, 14, 15 However, both of these interventions are problematic or difficult to apply outside of national health systems.
Clinical decision support (CDS) tools can assist providers in adopting best practices to potentially improve care.16 Our health informatics group developed a novel universal platform for integrating CDS into any electronic health record (EHR) and demonstrated its effectiveness in increasing adoption of evidence-based best practice17,18 We designed a multicenter cluster randomized trial to evaluate whether use of a universal, platform-agnostic, EHR-embedded VTE risk assessment model with integrated CDS would: 1) increase rates of appropriate thromboprophylaxis; and thus 2) reduce thromboembolism compared to usual medical care in hospitalized medically ill patients.
Methods
Trial oversight
The IMPROVE-DD (International Medical Prevention Registry on Venous Thromboembolism plus D-Dimer) study was an investigator-initiated, multicenter, cluster randomized trial (NCT04768036) conducted at 4 New York tertiary academic hospitals that were selected based on historical admissions for medical illness diagnoses and supported by Janssen Scientific Affairs, LLC. The implemented clinical decision-support tool was developed as part of an Agency for Healthcare Research and Quality grant (R18HS026196). The funders had no role in trial design or conduct, data collection or analysis, or writing of the manuscript. The study was approved by the health system institutional review board overseeing all study hospitals with waiver of informed consent for this quality implementation study and was conducted in accordance with principles of the Declaration of Helsinki (Supplemental Appendix). It followed the Consolidated Standards of Reporting Trials guideline. The authors assume responsibility for the accuracy and completeness of the data, analyses, and protocol.
Patients
Patients were enrolled consecutively upon admission to study hospitals from December 21, 2020, to January 21, 2022. Eligible inpatients were medically ill (nonsurgical, nonobstetrical) individuals aged over 60 years with a primary diagnosis of at least one of 5 medical illness categories and additional risk factors per the IMPROVE-DD VTE score (Supplemental Tables 1 and 2). All patients were assumed to have had relative immobility for at least 1 day during hospitalization, utilizing a more pragmatic definition of immobility for the score.19 Patients requiring therapeutic anticoagulation prehospitalization or within the first 24 hours of hospitalization were excluded, as were those with a history of atrial fibrillation. IMPROVE-DD VTE risk factors, relevant laboratory parameters, biometrics, comorbidities, and medications were captured via electronic data capture. In addition, patients for whom the tool was not available due to technical or other reasons were further excluded in order to measure the actual impact of deploying the tool.20 Full criteria are listed in the protocol.
Trial procedures
Hospitals were randomized using a 1:1 allocation into 2 study groups, clustered at the hospital level: the intervention group where the IMPROVE-DD VTE clinical decision-support tool was deployed or the control group with usual medical care. Hospital selection was based on hospital size and historical admissions for acute medical illness diagnoses. Baseline education was conducted for all sites via web-based modules of thromboembolism risk assessment using the score. The IMPROVE-DD VTE score is a refinement of the original IMPROVE VTE score,21 which incorporates elevated D-dimer to improve model discrimination,22 and has been validated in acutely ill hospitalized medical patients, including those with COVID-19, using established cutoffs.23, 24, 25 The original IMPROVE VTE score is endorsed by antithrombotic guidelines in hospitalized medical patients.11 The tool leverages an EHR-agnostic platform, enabling integration of CDS with theoretically any EHR or health information exchange system (Supplemental Figure 1). The platform is web-based and offers bidirectional communication and workflow integration with the “host” EHR via open standards, such as substitutable medical applications, reusable technologies on fast healthcare interoperability resource and CDS hooks, or vendor-specific application programming interfaces. All sites operated within Sunrise Clinical Manager 18.4 (Allscripts Healthcare Solutions, Inc) and were integrated using Allscripts application programming interfaces. At intervention sites, providers encountered 3 launch points for the tool within patient records: the admission history and physical note, thromboprophylaxis order entry, and the discharge medication reconciliation. Though the tool was a required element of all 3 workflows, opt-out capabilities were incorporated that included identification of nonmedical (surgical) patients, nonavailability of the tool, and high bleed risk criteria as judged by the treating physician. Launching the tool presented a graphical modified IMPROVE-DD VTE calculator with buttons to select risk factors (Supplemental Figure 1). To enhance accuracy and usability, risk factors were prepopulated based on patient-specific EHR data when available. Scores captured through the tool were written to the EHR and auto-populated within thromboprophylaxis order entry. Thromboprophylaxis options (including type, dose, and duration of postdischarge anticoagulant) were based on IMPROVE-DD VTE risk score cutoffs as well as biometrics and creatinine clearance. On admission, scores of at least 2 prompted options for inpatient pharmacologic thromboprophylaxis, preferably with low molecular weight heparin or unfractionated heparin. At discharge, scores of 4 or more displayed a statement within the medication reconciliation recommending extended postdischarge thromboprophylaxis, preferably with rivaroxaban 10 mg once daily for 30 days. During the study, the COVID-19 pandemic necessitated health system guidance on thromboprophylaxis that mirrored established IMPROVE-DD VTE score thresholds for at-risk and high-risk COVID-19 patients over 60 years of age.
An algorithm was developed to simulate IMPROVE-DD VTE scores at control sites where the tool was not deployed (Supplemental Appendix). This algorithm was validated in the control population via manual chart review and showed a concordance of 100%. All patients in the trial had a minimum IMPROVE-DD VTE score of 2, given age and immobility criteria.
Outcomes
The primary outcome was the rate of appropriate thromboprophylaxis for at-risk inpatients (score 2-3) and high-risk inpatients (score ≥4). Appropriate thromboprophylaxis was defined as a range of subcutaneous doses for heparins and fondaparinux, as well as low-dose direct oral anticoagulants; escalated doses were considered appropriate for COVID inpatients. The full appropriate thromboprophylaxis algorithm is available on page 5 of the Supplemental Appendix. The algorithm was based on our institution’s policies derived from professional society guidelines and high-quality data for thromboprophylaxis of hospitalized medical patients, including those with COVID-19, in the inpatient and immediate postdischarge period.5,9,11,26 Secondary outcomes prespecified in the final version of the protocol included rates of venous, arterial, and total thromboembolism through 30 days postdischarge; major bleeding through 30 days postdischarge; and all with follow-up to 90 days postdischarge; and all-cause mortality through 30 days postdischarge. Additional composite outcomes included: 1) all-cause readmission or death through 30 days; and 2) VTE readmission or death through 30 days with follow-up to 90 days.
Thromboembolic events were diagnosed with imaging studies that included programmatic radiology report language coding for capturing positive studies and were validated by chart review. Major bleeding was defined according to the International Society on Thrombosis and Haemostasis criteria.27 Both thromboembolic and major bleeding events were adjudicated by manual chart review by 3 reviewers with kappa scores of 0.9 for any event type. Disagreements were resolved by an experienced reviewer (A.C.S. or M.G.). Adjudication criteria are included within Supplemental Appendix.
Statistical analysis
Sample size calculation was based on a secondary outcome—rate of VTE—which required a larger sample size than the primary outcome. Assuming a VTE rate of 1.5% in the control group and 0.9% in the intervention group (40% relative risk reduction, OR: 0.60),13 a sample size of 5,324 subjects per group would achieve 80% power (chi-square test α = 0.05).
For the primary outcome—rate of appropriate thromboprophylaxis—we extrapolated historical system data24 to estimate a rate of 55% in the control group. Assuming an 11% absolute increase in appropriate thromboprophylaxis13,28 in the intervention group (66%, OR: 0.63), the calculated sample size would achieve 99% power to detect a significant difference. These calculations account for the design effect (1.03)29 introduced by the intracluster correlation inherent in the cluster design.
Random effects logistic regression using the logit link function (to account for clustering within hospitals) was used to determine whether there was a difference in rates of appropriate prophylaxis during the inpatient or postdischarge periods; mortality from admission up to 30 days postdischarge; venous, arterial, and total thromboembolic events or major bleeding (each outcome analyzed separately) from admission up to 30 days postdischarge (and separately for 90-day follow-up); the composite of 30-day all-cause readmission or death; and the composite of 30-day VTE readmission or death with 90-day follow-up. All outcome analyses were performed using SAS version 9.4. Demographics were analyzed using the R programming language in Jamovi version 2.2.5.30,31
Results
Characteristics of the population
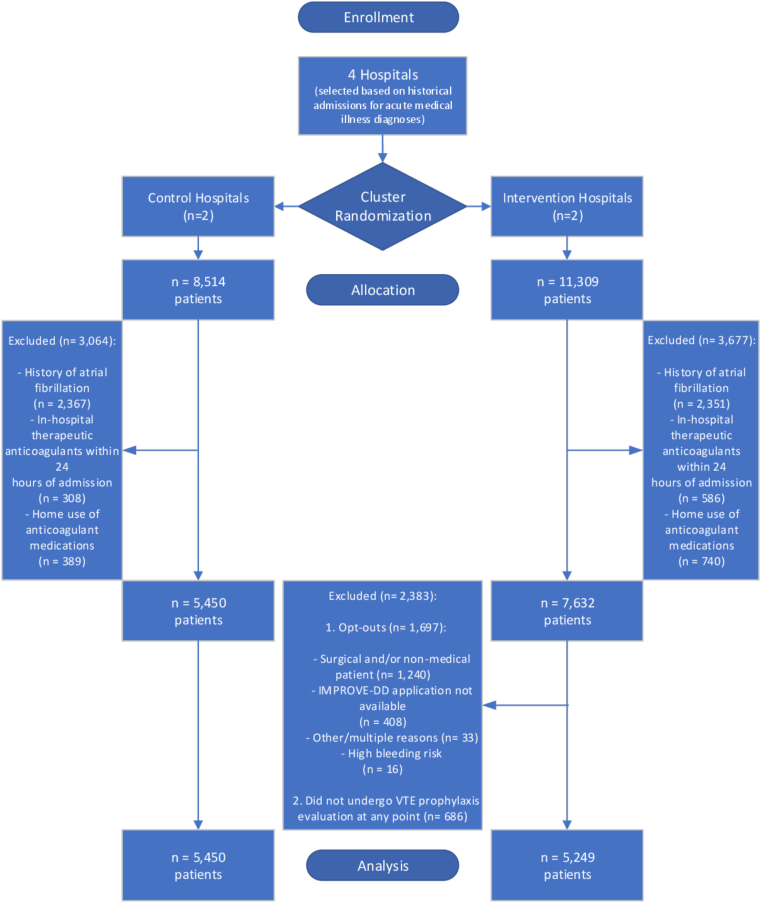
After cluster randomization of 4 academic tertiary hospitals, the 2 hospitals in the control group had 8,514 patients, and the 2 hospitals in the intervention group had 11,309 patients with a defined medical illness that met inclusion criteria. Of these, 3,064 patients in the control group and 3,677 patients in the intervention group met exclusionary criteria and were excluded from the analysis. Of the remaining patients at the intervention group, 2,383 patients were excluded due to provider opt-out from the tool (n = 1,697) or noncompletion of any thromboembolism risk assessment (n = 686). Thus, 10,699 patients were analyzed (5,249 at intervention sites and 5,450 at control sites) (Figure 1). Provider adoption rates of the tool at intervention sites were 77.8% (22.2% opted out of the tool). Baseline characteristics were broadly comparable between groups, though White race was more represented in the control group. The intervention group admitted numerically more patients with COVID-19 with correspondingly greater antiviral use, and numerically more patients in the control group had inflammatory/rheumatic disease (Table 1).
Figure 1.
CONSORT Study Flow Diagram
CONSORT = Consolidated Standards of Reporting Trials; IMPROVE-DD = International Medical Prevention Registry on Venous Thromboembolism Plus D-Dimer; VTE = venous thromboembolism.
Table 1.
Baseline and Other Characteristics of Patients in Intervention and Control Groups
| Intervention Group (n = 5,249) | Control Group (n = 5,450) | Standardized Differencesc | |
|---|---|---|---|
| Age, y | 75.1 ± 9.9 | 74.3 ± 9.6 | 0.08 |
| Sex | |||
| Male | 2,492 (47.5%) | 2,522 (46.3%) | 0.02 |
| Female | 2,757 (52.5%) | 2,928 (53.7%) | N/A |
| Race | |||
| Caucasian/White | 2,340 (44.6%) | 3,743 (68.7%) | 0.50 |
| African American/Black | 1,075 (20.5%) | 607 (11.1%) | 0.26 |
| Asian | 812 (15.5%) | 205 (3.8%) | 0.40 |
| Other/multiracial/unknown | 1,022 (19.5%) | 895 (16.4%) | N/A |
| Ethnicity | |||
| Not Hispanic or Latino | 4,629 (88.2%) | 4,725 (86.7%) | 0.05 |
| Hispanic or Latino | 475 (9.0%) | 575 (10.6%) | 0.05 |
| Declined/unknown | 145 (2.8%) | 150 (2.8%) | 0.00 |
| BMI, kg/m2 | 27.5 ± 7.8 | 28.2 ± 8.0 | 0.09 |
| Charlson comorbidity index age adjusted | 3.5 ± 1.2 | 3.5 ± 1.4 | 0.00 |
| Acute medical illness | |||
| Acute infectious disease/sepsis | 2,920 (53.6%) | 3,277 (62.4%) | 0.18 |
| COVID-19 | 1,355 (25.8%) | 1,097 (20.1%) | 0.14 |
| Heart failure | 441 (8.4%) | 338 (6.2%) | 0.08 |
| Severe lung disease | 1,014 (19.3%) | 1,086 (19.9%) | 0.02 |
| Ischemic stroke | 410 (7.8%) | 514 (9.4%) | 0.06 |
| Inflammatory disease including rheumatic diseases | 107 (2.0%) | 592 (10.9%) | 0.37 |
| IMPROVE-DD VTE risk factors | |||
| Previous VTE | 365 (7.0%) | 542 (9.9%) | 0.10 |
| Known thrombophilia | 44 (0.8%) | 43 (0.8%) | 0.00 |
| Cancer active or history within 5 y | 1,316 (25.3%) | 1,543 (28.3%) | 0.07 |
| Current lower limb paralysis | 102 (2.0%) | 220 (4.0%) | 0.12 |
| ICU/CCU stay during current hospitalization | 206 (4.0%) | 484 (8.9%) | 0.20b |
| D-dimer ≥2× upper normal limit if available | 588 (11.3%) | 751 (13.8%) | 0.08b |
| IMPROVE-DD VTE risk score | |||
| 2 or 3 | 3,183 (60.6%) | 2,992 (54.9%) | 0.12 |
| 4 or more | 2,066 (39.4%) | 2,458 (45.1%) | N/A |
| D-dimer mean, ng/mL | 998.7 ± 2861.6 | 1,196.3 ± 3994.4 | 0.06 |
| Hemoglobin mean, g/dL | 12.1 ± 2.2 | 12.2 ± 2.1 | 0.05 |
| Platelet count mean, K/μL | 242.3 ± 106.2 | 238.1 ± 96.6 | 0.04 |
| Creatinine serum mean, mg/dL | 1.4 ± 1.4 | 1.3 ± 1.2 | 0.08 |
| Mean duration of index hospitalization, d | 8.1 ± 8.8 | 6 ± 6.6 | 0.27b |
| Mean duration of thromboprophylaxis by length of stay, % | 93.4 ± 41.2 | 86 ± 47.2 | 0.17b |
| Use of medications | |||
| Corticosteroids | 2,024 (38.6%) | 1,961 (36.0%) | 0.05b |
| Antivirals (Remdesivir) | 952 (18.1%) | 530 (9.7%) | 0.24b |
| Antiplatelets | 2,484 (47.3%) | 2,752 (50.5%) | 0.06 |
| Aspirin | 2,345 (44.7%) | 2,606 (47.8%) | 0.06 |
| P2Y12 inhibitorsa | 788 (15%) | 886 (16.3%) | 0.03 |
| Cilostazol | 30 (0.6%) | 32 (0.6%) | 0.00 |
| Anticoagulants | |||
| Inpatient thromboprophylaxis | 4,582 (87.3%) | 4,354 (79.9%) | |
| Enoxaparin | 2,659 (50.7%) | 2,656 (48.7%) | |
| UFH | 1,787 (34.0%) | 1,609 (29.5%) | |
| Rivaroxaban | 48 (0.9%) | 14 (0.3%) | |
| Apixaban | 88 (1.7%) | 75 (1.4%) | |
| Postdischarge thromboprophylaxis | 481 (9.2%) | 372 (6.9%) | |
| Enoxaparin | 17 (0.3%) | 36 (0.7%) | |
| UFH | 1 (0.0%) | 3 (0.1%) | |
| Rivaroxaban | 355 (6.8%) | 140 (2.6%) | |
| Apixaban | 108 (2.1%) | 193 (3.5%) |
Values are mean ± SD or n (%).
Modified IMPROVE-DD risk scores range from 0 to 14, with higher scores indicating a higher risk of venous thromboembolism (minimal clinically important difference is 2).
The normal range for D-Dimer levels was defined according to the local laboratory criteria.
BMI = body mass index; CCU = coronary care unit; ICU = intensive care unit; IMPROVE-DD = International Medical Prevention Registry on Venous Thromboembolism plus D-Dimer; N/A = not applicable; UFH = unfractionated heparin; VTE = venous thromboembolism.
P2Y12 inhibitors include clopidogrel, prasugrel, ticagrelor.
Nonbaseline variables.
Standardized differences of >0.10 are considered significant.
30-day study outcomes
Among 10,699 inpatients analyzed, appropriate thromboprophylaxis rates for at-risk VTE patients were higher at intervention sites than control sites (80.1% vs 72.5%, OR: 1.52, 95% CI: 1.39 to 1.67, P < 0.001). Among 5,021 high-VTE-risk patients who had not had thromboembolic or bleeding events during index admission, 526 (10.5%) received appropriate extended thromboprophylaxis at discharge, including 13.6% at intervention sites vs 7.5% at control sites (OR: 1.93, 95% CI: 1.60-2.33, P < 0.001) (Table 2).
Table 2.
Primary and Secondary Outcomes at 30 Days Posthospital Discharge
| Intervention Group (n = 5,249) | Control Group (n = 5,450) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Primary outcome | ||||
| Appropriate thromboprophylaxis | 4,203/5,249 (80.1%) | 3,951/5,450 (72.5%) | 1.52 (1.39-1.67) | <0.001 |
| Appropriate postdischarge thromboprophylaxis | 331/2,433 (13.6%) | 195/2,588 (7.5%) | 1.93 (1.60-2.33) | <0.001 |
| Secondary outcomes | ||||
| VTE | 141/5,249 (2.7%) | 182/5,450 (3.3%) | 0.80 (0.64-1.00) | 0.048 |
| ATE | 13/5,249 (0.25%) | 38/5,450 (0.70%) | 0.35 (0.19-0.67) | <0.001 |
| Total TEa | 152/5,249 (2.9%) | 219/5,450 (4.0%) | 0.71 (0.58-0.88) | 0.002 |
| Major bleeding | 8/5,249 (0.15%) | 12/5,450 (0.22%) | 0.69 (0.28-1.69) | 0.42 |
| All-cause mortality | 478/5,249 (9.1%) | 383/5,450 (7.0%) | 1.32 (1.15-1.53) | <0.001 |
| Other secondary outcomes | ||||
| All-cause readmission/death | 845/4,882 (17.3%) | 922/5,142 (17.9%) | 0.96 (0.86-1.06) | 0.41 |
| VTE-related readmission/death | 136/4,882 (2.8%) | 114/5,142 (2.2%) | 1.26 (0.98-1.62) | 0.07 |
Values are n/N (%) unless otherwise indicated.
ATE = arterial thromboembolism; VTE = venous thromboembolism.
Includes VTE and ATE.
There were fewer venous (2.7% vs 3.3%, OR: 0.80, 95% CI: 0.64-1.00, P = 0.048), arterial (0.25% vs 0.70%, OR: 0.35, 95% CI: 0.19-0.67, P < 0.001), and total thromboembolic events (2.9% vs 4.0%, OR: 0.71, 95% CI: 0.58-0.88, P = 0.002) at intervention sites from admission through 30 days postdischarge (Table 2). Major bleeding was rare during hospitalization through 30 days postdischarge (0.15% vs 0.22% at intervention vs control sites) (Table 2). Mortality was higher at intervention sites from admission through 30 days postdischarge (9.1% vs 7.0%, OR: 1.32, 95% CI: 1.15-1.53, P < 0.001) (Table 2). Components of thromboembolic and major bleeding events at 30 days are reported in Supplemental Table 3.
Of all patients, 10,024 were discharged alive. Among these, 1,767 (17.6%) had an all-cause readmission or died within 30 days postdischarge, including 922/5,142 (17.9%) control patients and 845/4,882 (17.3%) intervention patients, a nonsignificant difference. Similarly, there was no difference in the composite of death or VTE readmission at 30 days postdischarge (Table 2).
Outcomes during study follow-up
Significant reductions in arterial (0.32% vs 0.86%, OR: 0.37, 95% CI: 0.21-0.65, P < 0.001) and total thromboembolic events (3.3% vs 4.5%, OR: 0.72, 95% CI: 0.59-0.88, P = 0.001) were maintained through the 90-day postdischarge follow-up period. The reduction in VTE events through the follow-up study period missed statistical significance (3.0% vs 3.7%, OR: 0.81 95% CI: 0.66-1.00, P = 0.052). Major bleeding was rare and did not differ among groups through the follow-up period (0.19% vs 0.25% at intervention vs control sites, respectively). Components of thromboembolic and major bleed events through the follow-up period are reported in Supplemental Table 4. There was increased death or VTE readmission at intervention vs control sites (4.9% vs 3.7%, OR: 1.34, 95% CI: 1.10-1.63, P < 0.004) during the 90 day postdischarge follow-up period.
Discussion
This multicenter cluster randomized trial of hospitalized medically ill patients (including those with COVID-19) revealed that universal, EHR-integrated CDS using a validated VTE risk assessment model (IMPROVE-DD VTE) had high adoption rates, increased rates of appropriate thromboprophylaxis (including appropriate extended thromboprophylaxis at discharge), and reduced venous, arterial, and total thromboembolism without an increase in major bleeding, compared to usual medical care at 30 days. Mortality was increased in the intervention group (Central Illustration).
Central Illustration.
Overview of Study Design, Intervention, and Results
(Top left) Schematic of informatics workflow of implemented CDS tool. (Bottom left) Study timeline. (Right) Outcomes including tool adoption rate and prespecified primary and secondary endpoints. API = application programming interface; CDS = clinical decision support; CPR = clinical prediction rule; EHR = electronic health record; IMPROVE-DD = International Medical Prevention Registry on Venous Thromboembolism Plus D-Dimer; LMWH = low molecular weight heparin; UFH = unfractionated heparin; VTE = venous thromboembolism.
Previous randomized trials have shown increased appropriate thromboprophylaxis and reduced VTE using electronic or physician alerts,13,28 while multifaceted programs combining educational activities, audit and feedback, and human intervention have been less effective than alerts.14 One national VTE prevention program that combined a paper-based VTE risk assessment tool, website resources, audit and data collection tools, and educational activities revealed over time increased appropriate thromboprophylaxis and reduced VTE-related death.15 However, all of these interventions have limitations: lack of interchangeability in EHRs with risk of operator fatigue; major time, resource, and labor inputs; cost; and difficulty of implementation outside of national health systems. Our group previously developed a novel universal platform for CDS integrated into clinician workflow that can be used in any EHR and demonstrated its effectiveness in increasing adoption of evidence-based best practice.17,18 Our study has implications in preventing venous (and arterial) thromboembolism at a health system level, as it has shown not only high adoption of evidence-based best practice (77.8%) but also reductions in hard outcomes, namely thromboembolism, utilizing this platform-agnostic tool.
All patients in our trial at both control and intervention sites had a minimum IMPROVE-DD VTE score of 2, indicating at least a moderate VTE risk group that would be candidates for in-hospital pharmacologic thromboprophylaxis. The 72.5% appropriate thromboprophylaxis rate at control sites was higher than our assumption of 55% and nearly identical to the 72.6% rate of pharmacologic thromboprophylaxis of at least moderately performing hospitals in a quality improvement cohort study of VTE prevention in a large network of U.S. health systems.32 Despite this metric, our study found a significantly approximate 8% absolute increase in appropriate thromboprophylaxis at intervention sites, suggesting more robust reductions in thromboembolism may be achievable in many low-performance hospitals outside of academic settings. Moreover, while intervention sites had a greater average hospital length of stay (8.1 days vs 6.0 days), these sites also showed a greater duration of thromboprophylaxis as a percentage of stay (93.4% vs 86.0%). This may be explained by a heightened attention to thromboprophylaxis as a result of using the CDS tool, a potential ancillary benefit of our intervention. Previous randomized trials or quality improvement initiatives that did not mandate extended postdischarge thromboprophylaxis in medical inpatients did not reduce rates of VTE despite increases in appropriate thromboprophylaxis.32,33 Our study found a significantly approximate 6% absolute increase in appropriate extended thromboprophylaxis at hospital discharge using established cutoff scores for high-risk patients per the IMPROVE-DD VTE score, which has previously been shown to identify high-risk medical inpatients that benefit from extended postdischarge thromboprophylaxis.34 Large registries have shown that a sizeable minority (approximately 20%-25%) of inpatients with acute medical illness, including those with COVID-19, are at elevated risk for postdischarge thromboembolism.21,35 Despite established benefits, historical rates of extended postdischarge prophylaxis have been very low.13,24,33 This relatively high rate of extended thromboprophylaxis at hospital discharge may have been a key factor in the tool’s ability to decrease VTE at 30 days in the intervention sites despite relatively high appropriate thromboprophylaxis in the control sites. Lastly, our study found significantly fewer arterial thromboembolic events, including numerically fewer ischemic stroke, myocardial infarction, and systemic embolism events in the intervention vs control sites, confirming recent evidence from randomized trials of the ability of extended postdischarge thromboprophylaxis to reduce arterial thromboembolism in hospitalized medical patients, in which 49% of subjects had background antiplatelet therapy.6, 7, 8,36 In addition to common risk factors and common pathophysiological mechanisms of inflammation, hypercoagulability, and endothelial injury that are shared between VTE and arterial thromboembolism, there has been a greater appreciation of the coagulation cascade and thrombin playing a larger role in what was thought to be mostly platelet-derived mechanisms of arterial thrombosis, through neutrophil extracellular traps providing the scaffolding to activate platelets and initiate clotting via the intrinsic pathway as well as activation of protease-activated receptors on platelet surfaces.37,38
Despite reductions in major thromboembolism in our trial, all-cause mortality was higher at intervention sites, which suggests that nonthrombotic mechanisms may have played a role in mortality. We conducted this trial during the initial phases of the COVID-19 pandemic in the United States, during which large numbers of COVID-19 patients were admitted to our health system, where internal published data revealed mortality rates of 26.6% in patients older than 65 years of age and 88.1% in patients receiving mechanical ventilation.39 Intervention sites admitted more patients with COVID-19 (25.8%) compared with control sites (20.1%) (standard difference 0.14). Although this small imbalance of COVID-19 inpatients may have contributed to the differences in mortality seen between groups where thromboprophylactic strategies may have had limited roles in reducing risk,9,40 other factors such as differences in baseline characteristics and medical illness categories, as well as hospital-specific differences in mortality rates during the pandemic, may have played a role as well.
Our study has several strengths and limitations. Strengths include the large sample size that was well powered for clinical outcomes and event adjudication, which add robustness and generalizability to our conclusions. A major strength is our CDS tool’s ability to be integrated theoretically into any EHR, thus operationalizing our study results broadly. The 77.8% adoption rate of the tool due to key features of the tool’s usability such as provider workflow integration, minimal clinician data entry, automatic triggering, and specific and actionable tool recommendations, compares very favorably with much lower provider adoption rates seen previously for CDS tools.41,42 The relatively high rate of appropriate thromboprophylaxis at control sites, likely due to system-wide educational and audit activities heightening awareness of best practices, would be expected to dampen the impact of our intervention, which nevertheless significantly increased appropriate thromboprophylaxis and reduced major thromboembolism. Our trial included a relatively small number of clusters (4) in which unseen population differences could magnify selection bias, although there were a large number of patients per cluster (∼2,500), which is a study strength.43 Preimplementation data pulls demonstrated comparable annual admissions for defined acute medical illness across sites. In addition, the comparability of the 2 groups, including similar numbers of at-VTE-risk and high-VTE-risk patients, argues against selection bias and is similar to group comparability of other intervention trials of heterogenous hospitalized medically ill populations.28 The notable exception was COVID-19 hospitalization at intervention sites, a condition for which optimal thromboprophylaxis had not been established when the initial protocol was approved, and as such, may be a group that requires further study with the use of the tool. Our decision to exclude the opt-out users of the CDS tool in the intervention sites in order to measure the tool’s performance during actual application is consistent with our study results conducted as part of a formal impact analysis of the tool.20 However, our study was not able to assess the tool’s effectiveness when it was unavailable for use due to technical or administrative reasons. Furthermore, the nature of our intervention as a hospital-level quality implementation avoided pitfalls of clustered trials, such as attrition bias. Lastly, the results of our trial would not apply to low VTE risk populations.
Conclusions
An integrated universal EHR-embedded CDS tool utilizing a validated VTE risk model increased appropriate thromboprophylaxis and reduced thromboembolism without increasing major bleeding in medical inpatients. Mortality was higher at intervention sites, which included more patients with COVID-19.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: This cluster randomized trial is the largest multicenter study to date investigating the impact of universal EHR-embedded CDS on rates of appropriate thromboprophylaxis (both during hospitalization and at discharge) and major thromboembolism in medically ill patients.
COMPETENCY IN PATIENT CARE: In 10,699 medically ill patients at 4 tertiary academic hospitals, an EHR-embedded IMPROVE-DD VTE CDS tool had high adoption (77.8%), increased appropriate thromboprophylaxis during hospitalization (80.1% vs 72.5%) and at discharge (13.6% vs 7.5%) vs usual care, and reduced major thromboembolism without increasing major bleeding. Mortality was higher at intervention sites.
TRANSLATIONAL OUTLOOK 1: A universal thromboprophylaxis CDS tool can increase appropriate thromboprophylaxis and reduce major thromboembolism in hospitalized medical inpatients if embedded within provider workflow during hospitalization and at discharge and anchored on a validated thromboembolism risk tool.
TRANSLATIONAL OUTLOOK 2: These benefits may be more robust in lower-performing hospitals for thromboprophylaxis outside of academic settings. A broad impact can be achieved as the CDS tool is interchangeable among different EHRs.
Funding support and author disclosures
This work was supported by Janssen Scientific Affairs, LLC, and by funding from the Broxmeyer Fellowship in Clinical Thrombosis. The implemented clinical decision-support tool was developed as part of an Agency for Healthcare Research and Quality grant (R18HS026196). The funders had no role in trial design or conduct; data collection or analysis; preparation, review, or writing of the manuscript; or decision to submit the manuscript for publication. Dr Spyropoulos has received honoraria from Janssen, Bayer, Bristol Myers Squibb, Pfizer, and Sanofi; and research grants from Janssen and Boehringer Ingelheim; and is a member of the ATLAS Group, an academic research organization. Dr Goldin has received honoraria from Janssen outside of the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
As per Northwell Health policy, our data dictionary, claims codes, CDS structure, and group-level data are available upon request. The trial was presented at the American Heart Association Scientific Sessions (November 7, 2022).
Footnotes
Trial Registration:clinicaltrials.gov identifier NCT04768036: https://clinicaltrials.gov/ct2/show/NCT04768036.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental information, tables, and figures, please see the online version of this paper.
Supplementary data
References
- 1.Raskob G. As part of ISTH Steering Committee for World Thrombosis Day. Venous thromboembolism: a call for risk assessment in all hospitalised patients. Thromb Haemost. 2016;116:777–779. doi: 10.1160/TH16-09-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer F.A., Lessard D., Emery C., Reed G., Goldberg R.J. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167:1471–1475. doi: 10.1001/archinte.167.14.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jha A.K., Larizgoitia I., Audera-Lopez C., Prasopa-Plaizier N., Waters H., Bates D.W. The global burden of unsafe medical care: analytic modelling of observational studies. BMJ Qual Saf. 2013;22:809–815. doi: 10.1136/bmjqs-2012-001748. [DOI] [PubMed] [Google Scholar]
- 4.Spyropoulos A.C., Ageno W., Cohen A.T., Gibson C.M., Goldhaber S.Z., Raskob G. Prevention of venous thromboembolism in hospitalized medically ill patients: a U.S. perspective. Thromb Haemost. 2020;120:924–936. doi: 10.1055/s-0040-1710326. [DOI] [PubMed] [Google Scholar]
- 5.Bajaj N.S., Vaduganathan M., Qamar A., et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: a trial sequential and cumulative meta-analysis. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raskob G.E., Spyropoulos A.C., Spiro T.E., et al. Benefit-risk of rivaroxaban for extended thromboprophylaxis after hospitalization for medical illness: pooled analysis from MAGELLAN and MARINER. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.121.021579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson C.M., Chi G., Halaby R., et al. Extended-duration Betrixaban reduces the risk of stroke versus standard-dose enoxaparin among hospitalized medically ill patients: an APEX trial substudy (acute medically ill venous thromboembolism prevention with extended duration Betrixaban) Circulation. 2017;135:648–655. doi: 10.1161/CIRCULATIONAHA.116.025427. [DOI] [PubMed] [Google Scholar]
- 8.Spyropoulos A.C., Ageno W., Albers G.W., et al. Post-discharge prophylaxis with rivaroxaban reduces fatal and major thromboembolic events in medically ill patients. J Am Coll Cardiol. 2020;75:3140–3147. doi: 10.1016/j.jacc.2020.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulman S., Sholzberg M., Spyropoulos A.C., et al. ISTH guidelines for antithrombotic treatment in COVID-19. J Thromb Haemost. 2022;20(10):2214–2225. doi: 10.1111/jth.15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolaides A.N., Fareed J., Kakkar A.K., et al. Prevention and treatment of venous thromboembolism--International Consensus Statement. Int Angiol. 2013;32:111–260. [PubMed] [Google Scholar]
- 11.Schunemann H.J., Cushman M., Burnett A.E., et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2:3198–3225. doi: 10.1182/bloodadvances.2018022954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shojania K.G., Duncan B.W., McDonald K.M., Wachter R.M., Markowitz A.J. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ) 2001:1–10. 1-668. [PMC free article] [PubMed] [Google Scholar]
- 13.Kucher N., Koo S., Quiroz R., et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352:969–977. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 14.Kahn S.R., Diendere G., Morrison D.R., et al. Effectiveness of interventions for the implementation of thromboprophylaxis in hospitalised patients at risk of venous thromboembolism: an updated abridged Cochrane systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-024444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts L.N., Durkin M., Arya R. Annotation: developing a national programme for VTE prevention. Br J Haematol. 2017;178:162–170. doi: 10.1111/bjh.14769. [DOI] [PubMed] [Google Scholar]
- 16.Shah N.R., Seger A.C., Seger D.L., et al. Improving acceptance of computerized prescribing alerts in ambulatory care. J Am Med Inform Assoc. 2006;13:5–11. doi: 10.1197/jamia.M1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGinn T.G., McCullagh L., Kannry J., et al. Efficacy of an evidence-based clinical decision support in primary care practices: a randomized clinical trial. JAMA Intern Med. 2013;173:1584–1591. doi: 10.1001/jamainternmed.2013.8980. [DOI] [PubMed] [Google Scholar]
- 18.McGinn T. Putting meaning into meaningful use: a roadmap to successful integration of evidence at the point of care. JMIR Med Inform. 2016;4:e16. doi: 10.2196/medinform.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spyropoulos A.C., Ageno W., Albers G.W., et al. Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med. 2018;379:1118–1127. doi: 10.1056/NEJMoa1805090. [DOI] [PubMed] [Google Scholar]
- 20.McGinn T.G., Guyatt G.H., Wyer P.C., et al. Users' guides to the medical literature - XXII: how to use articles about clinical decision rules. JAMA. 2000;284:79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 21.Spyropoulos A.C., Anderson F.A., Jr., FitzGerald G., et al. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140:706–714. doi: 10.1378/chest.10-1944. [DOI] [PubMed] [Google Scholar]
- 22.Gibson C.M., Spyropoulos A.C., Cohen A.T., et al. The IMPROVEDD VTE risk score: incorporation of D-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. TH Open. 2017;1:e56–e65. doi: 10.1055/s-0037-1603929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldin M., Lin S.K., Kohn N., et al. External validation of the IMPROVE-DD risk assessment model for venous thromboembolism among inpatients with COVID-19. J Thromb Thrombolysis. 2021;52:1032–1035. doi: 10.1007/s11239-021-02504-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg D., Eichorn A., Alarcon M., McCullagh L., McGinn T., Spyropoulos A.C. External validation of the risk assessment model of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) for medical patients in a tertiary health system. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahan C.E., Liu Y., Turpie A.G., et al. External validation of a risk assessment model for venous thromboembolism in the hospitalised acutely-ill medical patient (VTE-VALOURR) Thromb Haemost. 2014;112:692–699. doi: 10.1160/TH14-03-0239. [DOI] [PubMed] [Google Scholar]
- 26.Kahn S.R., Lim W., Dunn A.S., et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e195S–e226S. doi: 10.1378/chest.11-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulman S., Kearon C., Subcommittee on control of Anticoagulation of the Scientific and Standardization Committee of the International Society on T, Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 28.Piazza G., Rosenbaum E.J., Pendergast W., et al. Physician alerts to prevent symptomatic venous thromboembolism in hospitalized patients. Circulation. 2009;119:2196–2201. doi: 10.1161/CIRCULATIONAHA.108.841197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Killip S., Mahfoud Z., Pearce K. What is an intracluster correlation coefficient? crucial concepts for primary care researchers. Ann Fam Med. 2004;2:204–208. doi: 10.1370/afm.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heinzen E., Sinnwell J., Atkinson E., Gunderson T., Dougherty G. 2018. Arsenal: An Arsenal of 'R' Functions for Large-Scale Statistical Summaries. [R package] [Google Scholar]
- 31.The Jamovi Project . 2021. Jamovi (Version 1.6) [Google Scholar]
- 32.Flanders S.A., Greene M.T., Grant P., et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174:1577–1584. doi: 10.1001/jamainternmed.2014.3384. [DOI] [PubMed] [Google Scholar]
- 33.Piazza G., Anderson F.A., Ortel T.L., et al. Randomized trial of physician alerts for thromboprophylaxis after discharge. Am J Med. 2013;126:435–442. doi: 10.1016/j.amjmed.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Spyropoulos A.C., Lipardi C., Xu J., et al. Modified IMPROVE VTE risk score and elevated D-dimer identify a high venous thromboembolism risk in acutely ill medical population for extended thromboprophylaxis. TH Open. 2020;4:e59–e65. doi: 10.1055/s-0040-1705137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannis D., Allen S.L., Tsang J., et al. Postdischarge thromboembolic outcomes and mortality of hospitalized patients with COVID-19: the CORE-19 registry. Blood. 2021;137:2838–2847. doi: 10.1182/blood.2020010529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spyropoulos A.C., Goldin M., Ageno W., et al. Rivaroxaban plus Aspirin for extended thromboprophylaxis in acutely ill medical patients: insights from the MARINER trial. TH Open. 2022;06:e177–e183. doi: 10.1055/s-0042-1750379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prandoni P., Bilora F., Marchiori A., et al. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348:1435–1441. doi: 10.1056/NEJMoa022157. [DOI] [PubMed] [Google Scholar]
- 38.Goldin M., Koulas I., Weitz J.I., Spyropoulos A.C. State-of-the-Art mini review: dual-pathway inhibition to reduce arterial and venous thromboembolism. Thromb Haemost. 2022;122(8):1279–1287. doi: 10.1055/a-1778-1083. [DOI] [PubMed] [Google Scholar]
- 39.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim L., Garg S., O'Halloran A., et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US Coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2021;72:e206–e214. doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bright T.J., Wong A., Dhurjati R., et al. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012;157:29–43. doi: 10.7326/0003-4819-157-1-201207030-00450. [DOI] [PubMed] [Google Scholar]
- 42.Moxey A., Robertson J., Newby D., Hains I., Williamson M., Pearson S.A. Computerized clinical decision support for prescribing: provision does not guarantee uptake. J Am Med Inform Assoc. 2010;17:25–33. doi: 10.1197/jamia.M3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahan B.C., Forbes G., Ali Y., et al. Increased risk of type I errors in cluster randomised trials with small or medium numbers of clusters: a review, reanalysis, and simulation study. Trials. 2016;17:438. doi: 10.1186/s13063-016-1571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.