Abstract
Background
In the SVR (Single Ventricle Reconstruction) Trial, 1-year survival in recipients of right ventricle to pulmonary artery shunts (RVPAS) was superior to that in those receiving modified Blalock-Taussig-Thomas shunts (MBTTS), but not in subsequent follow-up. Cost analysis is an expedient means of evaluating value and morbidity.
Objectives
The purpose of this study was to evaluate differences in cumulative hospital costs between RVPAS and MBTTS.
Methods
Clinical data from SVR and costs from Pediatric Health Information Systems database were combined. Cumulative hospital costs and cost-per-day-alive were compared serially at 1, 3, and 5 years between RVPAS and MBTTS. Potential associations between patient-level factors and cost were explored with multivariable models.
Results
In total, 303 participants (55% of the SVR cohort) from 9 of 15 sites were studied (48% MBTTS). Observed total costs at 1 year were lower for MBTTS ($701,260 ± 442,081) than those for RVPAS ($804,062 ± 615,068), a difference that was not statistically significant (P = 0.10). Total costs were also not significantly different at 3 and 5 years (P = 0.21 and 0.32). Similarly, cost-per-day-alive did not differ significantly for either group at 1, 3, and 5 years (all P > 0.05). In analyses of transplant-free survivors, total costs and cost-per-day-alive were higher for RVPAS at 1 year (P = 0.05 for both) but not at 3 and 5 years (P > 0.05 for all). In multivariable models, aortic atresia and prematurity were associated with increased cost-per-day-alive across follow-up (P < 0.05).
Conclusions
Total costs do not differ significantly between MBTTS and RVPAS. The magnitude of longitudinal costs underscores the importance of efforts to improve outcomes in this vulnerable population.
Key words: economic analysis, hypoplastic left heart syndrome, outcomes research, pediatrics
Central Illustration
Despite concerted efforts to improve outcomes, children with hypoplastic left heart syndrome (HLHS) and other single ventricle lesions are at risk for early mortality and morbidity.1, 2, 3 The SVR (Pediatric Heart Network Single Ventricle Reconstruction Trial) sought to determine whether the use of a right ventricle to pulmonary artery shunt (RVPAS) instead of a modified Blalock-Taussig-Thomas shunt (MBTTS) (Central Illustration) would improve clinical outcomes after Norwood reconstruction. In SVR, randomization to RVPAS conferred a 10% absolute reduction in mortality relative to MBTTS at 12 months.2 However, in longer follow-up, the differences in transplant-free survival are no longer statistically significant.5,6 RVPAS has been associated with increased morbidity, specifically, reduced pulmonary artery growth2 and more frequent reinterventions.1,7 Moreover, concern has been raised that RVPAS may increase the likelihood of ventricular dysfunction5,8 and/or ventricular arrhythmias.9
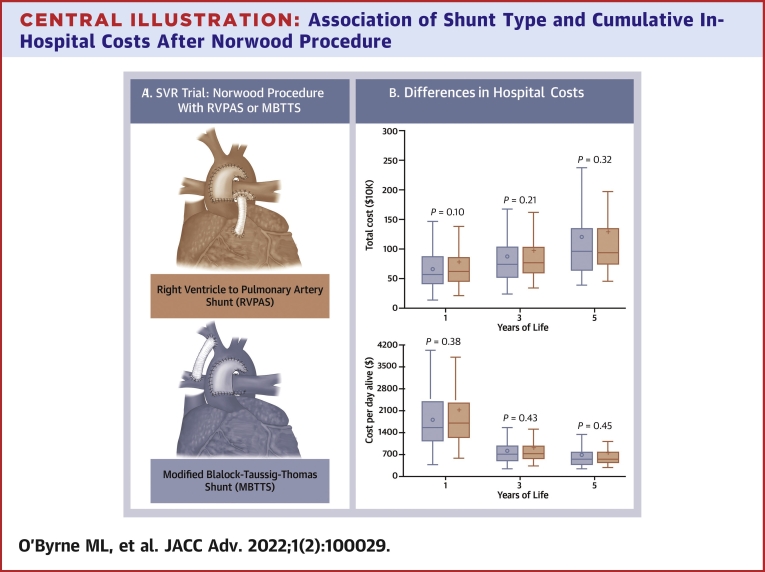
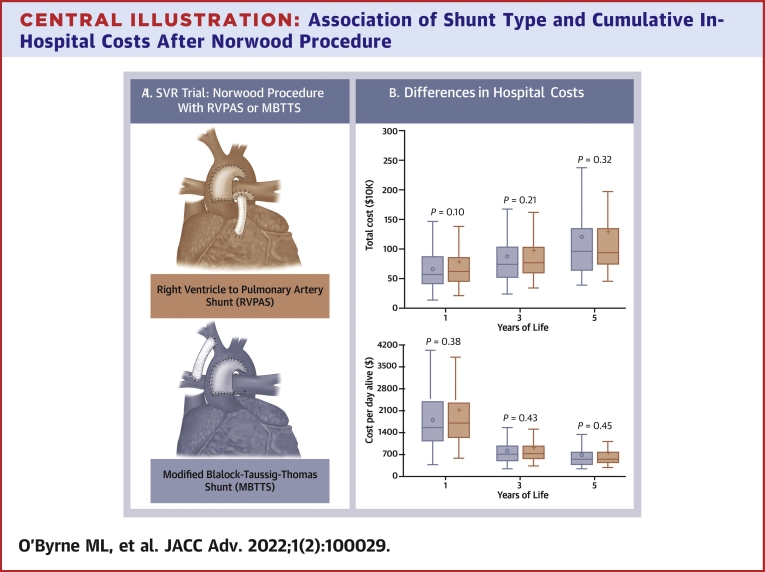
Central Illustration.
Association of Shunt Type and Cumulative In-Hospital Costs After Norwood Procedure
(A) Norwood procedure with right ventricle to pulmonary artery shunt (Top) and modified Blalock-Taussig-Thomas shunt (Bottom). Image from Menon et al.4(B) Total costs and cost-per-day alive. Box-and-whiskers plot depicting the accumulation of total cumulative costs (Top) and cost-per-day-alive (Bottom) for Blalock-Taussig-Thomas Shunt (MBTTS) (blue) and right ventricle to pulmonary artery shunt (RVPAS) (red). The solid line in the box depicts the median cost, the open circle depicts the mean cost, the box depicts the range between the 25th and 75th percentiles, and the whiskers depict 1.5 times the interquartile range. SVR = single ventricle reconstruction.
The optimal shunt type at Norwood remains unsettled. An obstacle to comparing longer term outcomes in this population is the range of adverse events (AEs) that can occur, which vary in both their frequency and severity. Counts of these events are both underpowered and complicated by the variety of AEs. Composite outcomes are expedient but conflate AEs of different severities (eg, uncomplicated reintervention and death), potentially implying a false equivalency. Attrition, which inevitably reduces statistical power, is another significant obstacle. Costs can serve a complementary outcome measure, not only of value but also as an integrated measure of morbidity (since sicker patients tend to accrue more costs).10, 11, 12, 13
To study whether RVPAS or MBTTS conferred a cost advantage, we combined clinical data from the SVR trial and hospital cost data from the Pediatric Health Information Systems (PHIS) database, leveraging the strengths of the clinical trial (randomization and prospective data collection) and the administrative database (expedient multicenter cost data). We hypothesized: 1) that the initial mortality benefit for RVPAS would result in short-term cost savings; and 2) that late morbidity would be associated with diminution of the initial cost benefit and ultimately higher costs for RVPAS than for MBTTS.
Methods
Data sources
The Pediatric Heart Network (PHN), funded by the National Heart, Lung, and Blood Institute, conducted the SVR I and SVR II trials, the designs of which have been described.14 In summary, 555 neonates undergoing Norwood operation at 15 centers in North America from 2005 to 2008 were randomized to either MBTTS or RVPAS to provide pulmonary blood flow, with data collected about preoperative condition and longitudinal follow-up through 6 years.5,6
PHIS is an administrative database that contains data from inpatient, emergency department, ambulatory surgery, and observation encounters from 52 not-for-profit, tertiary care pediatric hospitals in North America affiliated with the Children’s Hospital Association (Overland Park, Kansas). No Canadian SVR sites contributed data to PHIS during the study period. Data quality and reliability are assured through a joint effort between Children’s Hospital Association and participating hospitals. Participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures, as well as resource utilization data (eg, pharmacy products, radiologic studies, and laboratory studies). Data are deidentified at the time of data submission and are subjected to a number of reliability and validity checks.
The proposed study was reviewed as an ancillary study by the PHN Ancillary Studies and SVR Trial Committees. Data use agreements with both Children’s Hospital Association and HealthCore (the data-coordinating center for PHN) were obtained. The institutional review board of The Children’s Hospital of Philadelphia reviewed the project and determined that it was exempt from review, as it did not constitute research with human participants in accordance with the Common Rule (45 CFR 46.102(f)). Because of the terms of data-use agreements with Children’s Hospital Association, the combined data set will not be made available. Data from SVR are available from PHN after a review, or as a deidentified public-use data set. Statistical code and methods will be made available upon request.
Prospectively collected data from the SVR I trial and SVR II follow-up study were combined with data from PHIS by linking these 2 data sets using a previously validated method15 of probabilistic matching, which utilized indirect identifiers for the incident hospitalization, specifically hospital, sex, date of admission (±1 day), date of discharge (±1 day), and date of birth (±1 day) as described previously.16,17 Ninety-eight percent of trial subjects from PHIS centers were matched.17 A comparison of measurable participant level factors was performed, demonstrating that the proportion of participants of Hispanic ethnicity was higher at trial centers contributing data to PHIS than at centers not contributing data to PHIS (P = 0.03), whereas the prevalence of associated anatomic diagnosis was higher in the nonmatched cohort (P = 0.003). There were otherwise no significant differences (data not shown).
Study design/study measures
The study was a secondary analysis of a clinical trial with prospective longitudinal data collection2,14 in which additional hospital cost data were added to a subset of the original study population by merging trial data with administrative data. The primary exposure was the randomization-assigned shunt type (MBTTS vs RVPAS). The primary outcome was total measured hospital costs associated with inpatient and observation admissions at 1, 3, and 5 years of age (costs accrued from date of birth through first, third, and fifth birthdays). A complementary primary outcome was cost-per-day-alive (to mitigate the bias introduced by early mortality).13 Total medical costs from recorded inpatient and observation admissions were measured. Outpatient costs, nonmedical costs, and lost productivity were not evaluated. Two additional limitations of PHIS as a data source are: 1) hospital encounters were only captured if they occurred at the hospital at which the initial Norwood operation was performed; and 2) data about observation (ie, short-stay encounters) were not recorded uniformly at all PHIS hospitals during the study period. Although these limitations made overall estimates of longitudinal cost less accurate, randomization at center level mitigated the bias introduced, since the primary comparison is in costs between shunt types.
Costs in PHIS were calculated from proprietary hospital-specific cost-to-charge ratios and adjusted for regional wage-price indices. We acknowledge that there are several methods for deriving costs of medical care (goods and services).18 Cost-to-charge ratios are a standard factor for generating relative costs between subjects. Other methods such as standardized costing (estimation of the costs of individual goods and services) may also provide accurate estimates of total cost, but a recent analysis demonstrated that cost-to-charge ratios and standardized costing achieve consistent similar estimates of costs.19 All costs were adjusted for inflation to 2019 United States dollars using the consumer price index for medical care.
An important obstacle to accurate depiction of costs is the high rate of death and/or heart transplantation, especially in early follow-up. Differences in the rate of early deaths/transplant (which are well established in this cohort2) create the potential for bias. Generally, sicker patients accumulate higher costs, and costs preceding an in-hospital death are likely large. However, after death, costs no longer accrue. The effect on analysis would be small in the short term and increases as survivor follow-up time accrues. A similar pattern could occur with heart transplantation, with very high costs leading up to transplant. Since heart transplant recipients are qualitatively different from patients undergoing staged palliation, we also chose to censor costs after date of transplantation in our primary analysis. Finally, withdrawal from the study (or transfer of care to a different hospital where costs could not be counted) would have a similar effect. To mitigate bias from attrition, 2 steps were taken in terms of defining the outcomes. First, costs were measured at 3 time points (1, 3, and 5 years). These intervals were chosen pre hoc, under the expectation that the rate of cost accumulation would decrease as patients aged. For total costs, individuals who died, underwent transplant, or were lost to follow-up in 1 time period were excluded from analysis in subsequent time periods.
Study analysis
Participant demographics and clinical data are summarized using conventional descriptive statistics. Continuous variables are summarized as median (interquartile range) or mean ± SD, while categorical variables are summarized as percentages and counts. Comparison of baseline characteristics has been presented previously.2 However, the cohorts included are restricted to participants receiving care at hospitals contributing data to PHIS during the study period. To demonstrate that initial block randomization remained effective in the study population, their baseline characteristics stratified by shunt assignment at the outset of the study are compared. To ensure that attrition did not result in disproportionate distribution of patient-level factors between shunt type cohorts, the distribution of these covariates is re-evaluated for participants who survived without a transplant through 1 year of follow-up and through Fontan completion.
The primary analysis is a comparison of the 2 primary outcomes (total cost and cost-per-day-alive) at 1, 3, and 5 years between MBTTS and RVPAS groups. Because the study population was randomized, no adjustments for covariates are performed. Cost data are invariably right-skewed because of the potential for extreme high costs, and the optimal statistical techniques to compare costs remain unsettled. Techniques that mitigate skew (eg, nonparametric tests or gamma distribution in multivariable modeling) provide a measure of central tendency about the “most likely” costs an individual might incur, which are useful in describing center-level variation.10,16,20, 21, 22, 23 However, parametric statistics (expressing central tendency and variation using mean ± SD and adjusted analyses using linear regression) are recommended in clinical trials because they best represent the cost per individual of each treatment group24 and more useful for calculations of cost-effectiveness. For this reason, parametric tests (Student’s t-test) were used for primary analyses. To evaluate whether the use of parametric statistics introduced bias, nonparametric tests (Wilcoxon rank sum) were also performed.
Several preplanned sensitivity analyses were performed. First, to mitigate bias introduced by early censoring due to mortality or heart transplantation, total costs and cost-per-day-alive through 3 and 5 years were calculated in participants who survived without transplant through the end of the period, allowing the exploration of differences in cost that arose from differences in mortality and those that arose from differences in ongoing care of survivors. Second, because of variability in the timing of Fontan, total costs and cost-per-day-alive were compared based on completion of stages of palliation (Norwood operation, superior cavopulmonary connection operation, and Fontan completion). Finally, to determine if rates of heart transplantation introduced a cost difference between RVPAS and MBTTS recipients, total costs and cost-per-day-alive were compared including the costs accrued in participants after heart transplantation.
Two preplanned secondary analyses were performed. First, total costs and cost-per-day-alive were compared in participants with aortic atresia. The survival benefit of MBTTS vs RVPAS has been more robust in this population,2,5,6 and this subanalysis explores further the degree to which differences in mortality influence cost. Finally, to evaluate whether pre-Norwood patient-level factors influenced total costs and cost-per-day-alive at 1, 3, and 5 years, a multivariable mixed effects models was used. Fixed effects were shunt type, aortic atresia, associated conditions,2,14 non-HLHS anatomy, and gestational age <38 weeks along with a random intercept for hospital. Because the goal of these analyses was to see what the “most likely” effect of each covariate was on cost, a generalized linear model assuming a gamma distribution was used to mitigate the effect of skew in the data25 as has been done in previous studies of similar data.10,21,26 A second model was calculated assuming a Gaussian distribution on untransformed data. Although this model is more influenced by extreme values (ie, skew), this is an alternative strategy.24 Using both these modeling strategies provides bracketed (least effected by skew and most effected by skew) estimates of the effects. Models were also calculated including age to explore whether there was a measurable interaction between shunt type and the trajectory of accruing costs.
There were no missing data in terms of clinical covariates, so no formal compensation was performed. A threshold of statistical significance of P < 0.05 was used. Primary analyses were defined pre hoc, so no adjustments for multiple comparisons were performed. All analyses were performed using (SAS v9.4, SAS Institute).
Results
Study population
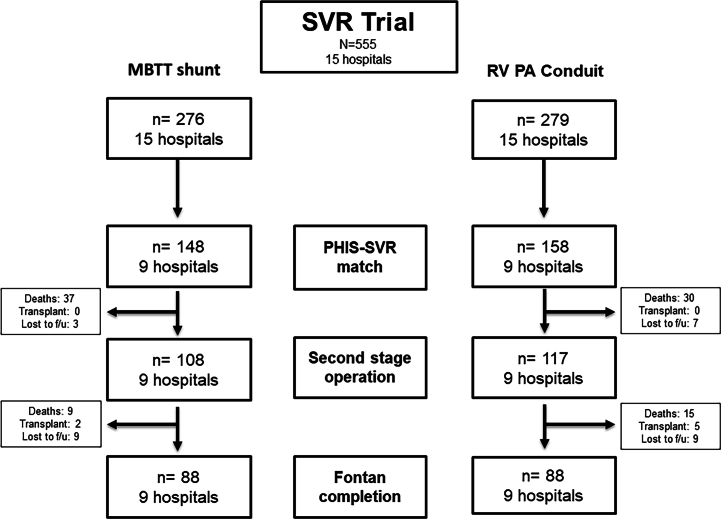
Matching SVR to PHIS records produced a study population of 303 participants (48% MBTTS) treated at 9 hospitals (Figure 1). This study population is 55% of the original study population and 66% (9/15) of the original SVR trial centers. Their characteristics are described in Table 1. There were no significant differences in the characteristics of the MBTTS and RVPAS cohorts. Although attrition was seen (Figure 1), the distribution of covariates did not change in a disproportionate fashion between MBTTS and RVPAS cohorts in subsequent follow-up (Supplemental Tables 1 and 2).
Figure 1.
Study Population
MBTT = modified Blalock-Taussig-Thomas shunt; PHIS = Pediatric Health Information Systems Database; RV PA = right ventricle to pulmonary artery; SVR = single ventricle reconstruction.
Table 1.
Study Population
| MBTTS (n = 148) | RVPAS (n = 158) | P Value | |
|---|---|---|---|
| Hospitals | 0.69 | ||
| 1 | 3% (5) | 7% (11) | |
| 2 | 9% (14) | 12% (19) | |
| 3 | 9% (13) | 8% (12) | |
| 4 | 12% (18) | 11% (17) | |
| 5 | 9% (14) | 12% (18) | |
| 6 | 30% (44) | 27% (42) | |
| 7 | 15% (22) | 13% (20) | |
| 8 | 1% (2) | 0% (0) | |
| 9 | 11% (16) | 10% (15) | |
| Female sex | 39% (57) | 37% (57) | 0.81 |
| Race | 0.47 | ||
| White | 80% (118) | 81% (124) | |
| Black | 17% (25) | 14% (21) | |
| Other/missing | 3% (5) | 6% (9) | |
| Hispanic ethnicity | 20% (29) | 28% (43) | 0.10 |
| Birth weight (kg) | 3.1 ± 0.6 | 3.0 ± 0.5 | 0.08 |
| Gestational age <38 wk (%) | 30% (45) | 31% (43) | 1.00 |
| Age at Norwood (d) | 5.1 ± 3.3 | 5.1 ± 3.3 | 0.94 |
| Payer | 0.90 | ||
| Private | 41% (60) | 40% (61) | |
| Public | 39% (58) | 42% (64) | |
| Other | 20% (30) | 19% (29) | |
| Primary diagnosis | 0.44 | ||
| HLHS | 87% (129) | 86% (132) | |
| Critical AS | 1% (1) | 0% (0) | |
| Single RV with outflow tract obstruction | 5% (8) | 3% (5) | |
| RV dominant AV canal | 5% (7) | 6% (10) | |
| Other | 2% (3) | 5% (7) | |
| Prenatal diagnosis | 74% (110) | 82% (126) | 0.13 |
| Aortic atresia | 66% (97) | 66% (102) | 0.90 |
| Pulmonary vein stenosis | 3% (5) | 4% (6) | 1.00 |
| Associated diagnosis | 30% (44) | 25% (38) | 0.36 |
Values are % (n).
AS = aortic stenosis; AV = atrioventricular; HLHS = hypoplastic left heart syndrome; MBTTS = modified Blalock-Taussig-Thomas shunts; RVPAS = right ventricle to pulmonary artery shunts.
Cost outcomes
The point estimate of the observed total cost in the first year of life for participants randomized to MBTTS ($701,260 ± 442,081) suggested an advantage over RVPAS (804,062 ± 615,068), but the difference was not statistically significant (P = 0.10, Table 2, Central Illustration B). Over time, the point estimate continued to favor MBTTS over RVPA, but the difference at 3 and 5 years was still not statistically significant (P = 0.21 and P = 0.32). Evaluation of these differences using nonparametric tests did not change the observed associations (data not shown). When expressing differences in terms of different stages of palliation (Norwood, interstage, superior cavopulmonary connection, and Fontan completion), no difference was seen in costs between the 2 populations (Supplemental Table 3A).
Table 2.
Accumulated Total Costs
| MBTTS (n = 148) | RVPAS (n = 154) | P Value | |
|---|---|---|---|
| Total costs | |||
| 1 y | $701,260 ± 442,081 | $804,062 ± 615,068 | 0.10 |
| 3 y | $864,071 ± 640,518 | $978,333 ± 920,990 | 0.21 |
| 5 y | $996,437 ± 828,270 | $1,105,594 ± 1,040,145 | 0.32 |
| Total daily costs | |||
| 1 y | $5,613 ± 8,265 | $6,596 ± 10,990 | 0.38 |
| 3 y | $4,967 ± 85,554 | $5,877 ± 11,268 | 0.43 |
| 5 y | $4,912 ± 8,579 | $5,784 ± 11,300 | 0.45 |
Values are mean ± SD in US$.
MBTTS = modified Blalock-Taussig-Thomas shunts; RVPAS = right ventricle to pulmonary artery shunts.
In terms of cost-per-day-alive, the point estimate in the first year of life suggested that costs were lower for MBTTS ($5,613 ± 8,265) than those for RVPAS ($6,596 ± 10,990), but the difference was not significant (P = 0.38) (Table 2, Central Illustration B). As with total costs, there were no significant differences in total costs between MBTTS and RVPA over the first 3 years and 5 years of life either (P = 0.43 and P = 0.45, respectively).
In sensitivity analyses excluding participants who died or underwent heart transplantation, at 1 year, total costs for MBTTS ($656,004 ± 331,984) were lower than those for RVPAS ($776,547 ± 556,160; P = 0.05) (Table 3). This can also be expressed as cost-per-day-alive ($1,796 ± 909 for MBTTS and $2,126 ± 1,523 for RVPAS; P = 0.05) (Table 3). At 3 and 5 years, no significant differences were demonstrated for either outcome (Table 3).
Table 3.
Accumulated Total Costs Excluding Subjects Who Died or Underwent Heart Transplantation
| MBTTS | RVPAS | P Value | |
|---|---|---|---|
| Total costs | |||
| 1 y, n = 107 (72%), 114 (74%) | $656,004 ± 331,984 | $776,547 ± 556,160 | 0.05 |
| 3 y, n = 95 (64%), 95 (62%) | $875,549 ± 623,776 | $972,656 ± 926,326 | 0.40 |
| 5 y, n = 56 (38%), 66 (43%) | $1,209,790 ± 1,092,009 | $1,290,784 ± 1,241,318 | 0.71 |
| Total daily costs | |||
| 1 y, n = 107 (72%), 114 (74%) | $1,796 ± 909 | $2,126 ± 1,523 | 0.05 |
| 3 y, n = 95 (64%), 95 (62%) | $799 ± 569 | 888 ± 845 | 0.40 |
| 5 y, n = 56 (38%), 66 (43%) | $662 ± 598 | 707 ± 680 | 0.71 |
Values are mean ± SD in US$.
For each time period, the number of participants and the percentage of the total cohort are listed.
MBTTS = modified Blalock-Taussig-Thomas shunts; RVPAS = right ventricle to pulmonary artery shunts.
In the subgroup of patients with aortic atresia, mortality at 1 year was 20% with no significant difference between shunt types (P = 0.73), which was not significantly different from the risk of mortality in the participants without aortic atresia (P = 0.11). Mortality, risk of transplant, and composite outcomes were not significantly different between MBTTS and RVPAS recipients over the rest of follow-up (data not shown). In the subgroup of patients with aortic atresia (n = 97 MBTTS and n = 102 RVPAS), total costs at 1 year again appeared to favor MBTTS ($684,423 ± 450,507) over RVPAS ($834,718 ± 649,222), but the difference was again not statistically significant (P = 0.06) (Supplemental Table 4). Over the rest of the follow-up, there was no difference in total costs at 3 and 5 years (P = 0.18 and 0.12, respectively). Cost-per-day-alive at 1 year appeared to favor MBTTS ($5,667 ± 8,127) over RVPAS ($7,437 ± 12,038), but this difference was not significant (P = 0.23), and no significant difference was demonstrated at 3 and 5 years (P = 0.30 and 0.32, respectively).
Models evaluating the association between patient-level factors and cost
In multivariable models, no significant association was demonstrated between shunt type and total costs at 1, 3, or 5 years (P = 0.11, P = 0.16, and P = 0.22, respectively) (Supplemental Table 5). Race, sex, associated diagnosis, non-HLHS cardiac anatomy, aortic atresia, and prematurity were also not significantly associated with total cost.
In models of cost-per-day-alive, several factors that were associated with increased cost were identified (Supplemental Table 6). Aortic atresia was associated with increased cost per day alive at 1 year (cost ratio: 1.39; 95% confidence interval [CI]: 1.04-1.86; P = 0.02). This association remained significant at 3 years (cost ratio: 1.49; 95% CI: 1.04-2.13; P = 0.03) and 5 years (cost ratio: 1.49; 95% CI: 1.03-2.15; P = 0.03). Prematurity was also associated with increased cost at 1 (cost ratio: 1.50; 95% CI: 1.14-1.98; P = 0.005), 3 (cost ratio: 1.64; 95% CI: 1.17-2.31; P = 0.004), and 5 years (cost ratio: 1.65; 95% CI: 1.16-2.35; P = 0.005).
Alternative models for longitudinal total costs were calculated to see if there was an interaction between cost and shunt type (Supplemental Table 7), but they did not demonstrate a significant association (P = 0.70). Models assuming a Gaussian distribution for costs were also calculated (Supplemental Tables 8 and 9).
Discussion
In this analysis combining clinical trial data from SVR and cost data from PHIS, no significant differences in total measured medical costs or cost-per-day-alive were seen between participants randomized to MBTTS vs those to RVPAS. However, there were differences in the accumulation of morbidity and costs between the 2 shunt types. RVPAS participants, although have lower mortality in the original trial, accrued higher costs, among survivors, that matched the higher costs associated with early death in the MBTTS cohort. The study also provides longitudinal measurement of the absolute magnitude of costs of surgical single ventricle palliation and identifies some patient-level factors associated with higher cost. Identifying a higher value strategy between the MBTTS and RVPAS would require incorporation of clinical outcomes to calculate the marginal cost-effectiveness of the 2 strategies27,28 and, therefore, is not possible at this time.
The public health impact of surgical palliation of HLHS and related anatomic defects is out of proportion to its incidence. Despite concerted efforts, 5-year transplant-free mortality remains <70%,5,29, 30, 31, 32, 33, 34 and survivors experience significant morbidity.32 Patients with single ventricle heart disease consume a disproportionate amount of health care resources.35, 36, 37, 38 To our knowledge, there are no contemporary series detailing cumulative costs of other resource-intensive pediatric conditions (eg, extreme prematurity or pediatric cancers). In a study of hospital admissions at US primary pediatric hospitals and their associated costs, HLHS admissions accounted for 0.15% of admissions (the 63rd most prevalent) but incurred the seventh highest total costs.35 Although several studies have reported the costs of initial stage I palliation,16,17,39, 40, 41 individual operations,42 or single-center cohorts,43 this is, to our knowledge, the first report of the longitudinal costs of staged palliation in a multi-institutional US cohort. Future research combining data about cost, morbidity, and patient-centered/patient-reported outcomes (eg, health-related quality of life) may help guide decision-making in the care of these vulnerable patients.
The current study quantifies the resources expended to complete single ventricle palliation, totaling approximately $1,000,000 (of in-hospital costs) regardless of shunt type. This estimate does not include costs of outpatient follow-up visits for this high-risk condition, home-monitoring programs, and parents’ lost wages and/or productivity. Consistent with previous series,42 the current study demonstrates that early costs (Norwood through the second-stage palliation) are disproportionately high, consuming ∼70% of the costs accrued over the first 5 years of life, and analysis restricted to survivors demonstrate that subjects who die early accrue disproportionately high costs. This, unsurprisingly, is also the period with the highest likelihood of mortality, morbidity, and unplanned reintervention.1,2,7,44,45 These findings underscore the importance of identifying potentially modifiable factors and care practices (underlying morbidity and cost) during this critical period to identify how best to care for this high-risk population.44,46, 47, 48, 49, 50, 51
Although smaller in magnitude than in the first year, costs continue to accrue even after the second-stage operation. This is consistent with data from the Australia and New Zealand Fontan Registry cohort, which demonstrated that after a peak in the first year of life, costs continued to accrue at a steady rate through childhood and into adolescence.52 Previously, concern has been raised about the long-term risk incurred by the ventriculotomy performed as part of the RVPAS. In the current analysis, it appears that in survivors, the use of RVPAS is significantly more costly, consistent with previous studies demonstrating that transcatheter reintervention is more common in the RVPAS at the stage of analysis of SVR data.5, 6, 7 Even though the rate of reintervention appears higher across 6 years of follow-up, the difference in the point estimates of cost do not appear to diverge. This may be due to attrition and type II error, but it does not support the hypothesis that the ventriculotomy or other factors are associated with higher risk of long-term morbidity. Longer follow-up and a larger number of participants are necessary to sufficiently address this question.
In the current analysis, we also attempted to explore whether patient factors influenced longitudinal costs. The fact that aortic atresia and prematurity were associated with increased cost-per-day-alive but not with total costs suggests that they are associated with increased risk of morbidity, which has been demonstrated previously.53 Since these are not modifiable factors, increased vigilance in longitudinal evaluation (including home-monitoring programs and longitudinal surveillance) in these higher risk patients may have the potential to improve care and/or reduce costs. In the current analysis, we cannot identify other surgical or medical practice variations that might underlie differences in costs that have been observed in other series.16,20,22,23,54 Further research is necessary to evaluate which practices could benefit these vulnerable patients.
Study Limitations
An important limitation to this study is its generalizability. As a clinical trial, study sites were large academic centers. The performance of these sites is not necessarily generalizable. For individual centers, implementation of these results should be made in light of local experience and comfort level with different strategies. The ultimate decision about shunt type remains one based on both patient characteristics and the experience of the center. Data from this study also cannot shed light on the expected costs of relatively novel approaches to treatment of children with HLHS and other forms of single ventricle heart disease.55, 56, 57, 58, 59, 60, 61
There are several other limitations to acknowledge. The study was a clinical trial with power calculated for its primary endpoint (1-year transplant-free survival).14 Although cost data usually are statistically expedient, the current study was limited to a subset of the initial trial and suffers from both the attrition noted previously and the tremendous variability in costs, which all increase the likelihood of type II error. Unmeasured confounding is always a potential limitation, but as noted, this should have been mitigated by the original trial’s randomized design (which uniformly distributed both measured and unmeasured confounders). As noted previously, PHIS does not include outpatient costs. These are unlikely to be differential between RVPAS and MBTTS shunt recipients and, on a unit cost basis, relatively small in magnitude compared to inpatient and observation costs but, over time, may represent significant total costs. To our knowledge, no other studies have evaluated these costs. Finally, the optimal study design to identify the optimal strategy is analysis of marginal cost-effectiveness (the quotient of the difference in costs and the difference in quality-adjust life years between 2 or more strategies), but to our knowledge, there are no standard methods for measuring utility in children.
Conclusions
Despite these limitations, we conclude that early term costs are influenced by shunt type with higher likelihood of transplant and mortality increasing the costs of recipients of MBTTS, but with the suggestion that reintervention and other sources of morbidity are associated with higher costs for RVPAS. There is no evidence that these costs diverge at 5 years of follow-up. The study also underscores that single ventricle palliation consumes a large magnitude of health care resources, with the majority spent in the first year of life.
Funding support and author disclosures
The SVR trial was funded by the Pediatric Heart Network/National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068288, HL068290, HL068292, and HL085057). Dr O'Byrne received support from NHLBI (K23 HL130420-01). The project also received assistance from The Pediatric Heart Network’s Integrated CARDiac Data and Outcomes Collaborative (iCARD). The Pediatric Heart Network Ancillary Studies Committee and Single Ventricle Reconstruction Trial Committee reviewed the proposed research and manuscript for appropriateness but did not participate in the drafting of the paper. The views expressed are solely of the authors and do not reflect those of the funders and other supporting groups. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Costs (which are reflective of morbidity) overall did not differ between participants randomized to a Blalock-Taussig-Thomas shunt and those to a RVPAS. However, analysis of survivors suggests that the cost of living with a RVPAS is higher, consistent with higher reintervention rates, while higher mortality rates increased costs of the Blalock-Taussig-Thomas shunt strategy. Differences in cost were derived from differences in the first year of life where the majority of total costs were accrued. Efforts to improve care should focus on improving outcomes in this period.
TRANSLATIONAL OUTLOOK: Although it did not reveal significant differences in costs between shunt types, this analysis of the SVR trial leads to a better understanding of the economic and medical impact of operative palliation for single ventricle heart disease.
Footnotes
The study cohort was collected as part of a clinical trial. The original PHN SVR trial is registered at ClinicalTrials.gov (NCT00115934).
An analysis of data from the Single Ventricle Reconstruction Trial and Pediatric Health Information System (PHIS) Database.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Ohye R.G., Schonbeck J.V., Eghtesady P., et al. Cause, timing, and location of death in the single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:907–914. doi: 10.1016/j.jtcvs.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohye R.G., Sleeper L.A., Mahony L., et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–1992. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Brien S.M., Clarke D.R., Jacobs J.P., et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 4.Menon S.C., Erickson L.K., McFadden M., Miller D.V. Effect of ventriculotomy on right-ventricular remodeling in hypoplastic left heart syndrome: a histopathological and echocardiography correlation study. Pediatr Cardiol. 2013;34:354–363. doi: 10.1007/s00246-012-0462-x. [DOI] [PubMed] [Google Scholar]
- 5.Newburger J.W., Sleeper L.A., Frommelt P.C., et al. Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation. 2014;129:2013–2020. doi: 10.1161/CIRCULATIONAHA.113.006191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newburger J.W., Sleeper L.A., Gaynor J.W., et al. Transplant-free survival and interventions at 6 years in the SVR trial. Circulation. 2018;137:2246–2253. doi: 10.1161/CIRCULATIONAHA.117.029375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill K.D., Rhodes J.F., Aiyagari R., et al. Intervention for re-coarctation in the single ventricle reconstruction trial: incidence, risk and outcomes. Circulation. 2013;128(9):954–961. doi: 10.1161/CIRCULATIONAHA.112.000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill G.D., Frommelt P.C., Stelter J., et al. Impact of initial norwood shunt type on right ventricular deformation: the single ventricle reconstruction trial. J Am Soc Echocardiogr. 2015;28:517–521. doi: 10.1016/j.echo.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall E.J., Smith A.H., Fish F.A., et al. Association of shunt type with arrhythmias after Norwood procedure. Ann Thorac Surg. 2018;105:629–636. doi: 10.1016/j.athoracsur.2017.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Byrne M.L., Glatz A.C., Faerber J.A., et al. Interhospital variation in the costs of pediatric/congenital cardiac catheterization laboratory procedures: analysis of data from the pediatric health information systems database. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ooi Y.K., Kelleman M., Ehrlich A., et al. Transcatheter versus surgical closure of atrial septal defects in children: a value comparison. J Am Coll Cardiol Intv. 2016;9:79–86. doi: 10.1016/j.jcin.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 12.O'Byrne M.L., DeCost G., Katcoff H., et al. Resource utilization in the first 2 years following operative correction for tetralogy of Fallot: study using data from the optum's de-identified clinformatics data mart insurance claims database. J Am Heart Assoc. 2020;169:e2–e13. doi: 10.1161/JAHA.120.016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Byrne M.L., Glatz A.C., Huang Y.-S.V., et al. Comparative costs of management strategies for neonates with symptomatic tetralogy of Fallot. J Am Coll Cardiol. 2022;79(12):1170–1180. doi: 10.1016/S0735-1097(22)02161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohye R.G., Gaynor J.W., Ghanayem N.S., et al. Design and rationale of a randomized trial comparing the Blalock-Taussig and right ventricle-pulmonary artery shunts in the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136:968–975. doi: 10.1016/j.jtcvs.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasquali S.K., Jacobs J.P., Shook G.J., et al. Linking clinical registry data with administrative data using indirect identifiers: implementation and validation in the congenital heart surgery population. Am Heart J. 2010;160:1099–1104. doi: 10.1016/j.ahj.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McHugh K.E., Pasquali S.K., Hall M.A., Scheurer M.A. Cost variation across centers for the Norwood operation. Ann Thorac Surg. 2018;105:851–856. doi: 10.1016/j.athoracsur.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McHugh K.E., Pasquali S.K., Hall M.A., Scheurer M.A. Impact of postoperative complications on hospital costs following the Norwood operation. Cardiol Young. 2016;26:1303–1309. doi: 10.1017/S1047951115002498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glick H.A., Doshi J.A., Sonnad S.S., Polsky D. Economic Evaluation in Clinical Trials. 1st ed. Oxford University Press; 2007. Valuing medical service use; pp. 31–57. [Google Scholar]
- 19.Pasquali S.K., Chiswell K., Hall M., et al. Estimating resource utilization in congenital heart surgery. Ann Thorac Surg. 2020;110:962–968. doi: 10.1016/j.athoracsur.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasquali S.K., Jacobs M.L., He X., et al. Variation in congenital heart surgery costs across hospitals. Pediatrics. 2014;133:e553–e560. doi: 10.1542/peds.2013-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Byrne M.L., Glatz A.C., Song L., et al. Association between variation in preoperative care before arterial switch operation and outcomes in patients with transposition of the great arteries. Circulation. 2018;138:2119–2129. doi: 10.1161/CIRCULATIONAHA.118.036145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasquali S.K., Sun J.L., d'Almada P., et al. Center variation in hospital costs for patients undergoing congenital heart surgery. Circ Cardiovasc Qual Outcomes. 2011;4:306–312. doi: 10.1161/CIRCOUTCOMES.110.958959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasquali S.K., Jacobs J.P., Bove E.L., et al. Quality-cost relationship in congenital heart surgery. Ann Thorac Surg. 2015;100:1416–1421. doi: 10.1016/j.athoracsur.2015.04.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glick H.A., Doshi J.A., Sonnad S.S., Polsky D. Economic Evaluation in Clinical Trials. 1st ed. Oxford University Press; 2007. Analyzing cost; pp. 89–114. [Google Scholar]
- 25.Manning W.G., Basu A., Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24:465–488. doi: 10.1016/j.jhealeco.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 26.O'Byrne M.L., Shinohara R.T., Grant E.K., et al. Increasing propensity to pursue operative closure of atrial septal defects following changes in the instructions for use of the Amplatzer Septal Occluder device: an observational study using data from the Pediatric Health Information Systems Database. Am Heart J. 2017;192:85–97. doi: 10.1016/j.ahj.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porter M.E. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 28.Glick H.A., Doshi J.A., Sonnad S.S., Polsky D. vol 1. Oxford University Press; 2007. Comparing cost and effect: point estimates for cost-effectiveness ratios and net monetary effects; pp. 133–142. (Economic Evaluation in Clinical Trials). [Google Scholar]
- 29.Mahle W.T., Spray T.L., Wernovsky G., Gaynor J.W., Clark B.J. Survival after reconstructive surgery for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation. 2000;102:III136–III141. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 30.Mascio C.E., Irons M.L., Ittenbach R.F., et al. Thirty years and 1663 consecutive Norwood procedures: has survival plateaued? J Thorac Cardiovasc Surg. 2019;158:220–229. doi: 10.1016/j.jtcvs.2018.12.117. [DOI] [PubMed] [Google Scholar]
- 31.Poh C.L., d'Udekem Y. Life after surviving Fontan surgery: a meta-analysis of the incidence and predictors of late death. Heart Lung Circ. 2018;27:552–559. doi: 10.1016/j.hlc.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Allen K.Y., Downing T.E., Glatz A.C., et al. Effect of Fontan-associated morbidities on survival with intact Fontan circulation. Am J Cardiol. 2017;119:1866–1871. doi: 10.1016/j.amjcard.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Cao J.Y., Phan K., Ayer J., Celermajer D.S., Winlaw D.S. Long term survival of hypoplastic left heart syndrome infants: meta-analysis comparing outcomes from the modified Blalock-Taussig shunt and the right ventricle to pulmonary artery shunt. Int J Cardiol. 2018;254:107–116. doi: 10.1016/j.ijcard.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 34.Menon S.C., Keenan H.T., Weng H.Y.C., et al. Outcome and resource utilization of infants born with hypoplastic left heart syndrome in the Intermountain West. Am J Cardiol. 2012;110:720–727. doi: 10.1016/j.amjcard.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 35.Keren R., Luan X., Localio R., et al. Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166:1155–1164. doi: 10.1001/archpediatrics.2012.1266. [DOI] [PubMed] [Google Scholar]
- 36.Pinto N.M., Waitzman N., Nelson R., Minich L.L., Krikov S., Botto L.D. Early childhood inpatient costs of critical congenital heart disease. J Pediatr. 2018;203:371–379.e7. doi: 10.1016/j.jpeds.2018.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burstein D.S., Shamszad P., Dai D., et al. Significant mortality, morbidity and resource utilization associated with advanced heart failure in congenital heart disease in children and young adults. Am Heart J. 2019;209:9–19. doi: 10.1016/j.ahj.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Nandi D., Rossano J.W., Wang Y., Jerrell J.M. Risk factors for heart failure and its costs among children with complex congenital heart disease in a medicaid cohort. Pediatr Cardiol. 2017;38:1672–1679. doi: 10.1007/s00246-017-1712-8. [DOI] [PubMed] [Google Scholar]
- 39.Kogon B.E., Kanter K., Alsoufi B., Maher K., Oster M.E. Outcomes and hospital costs associated with the Norwood operation: beyond morbidity and mortality. Cardiol Young. 2015;25:853–859. doi: 10.1017/S1047951114002224. [DOI] [PubMed] [Google Scholar]
- 40.Anderson B.R., Ciarleglio A.J., Salavitabar A., Torres A., Bacha E.A. Earlier stage 1 palliation is associated with better clinical outcomes and lower costs for neonates with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2015;149:205–210.e1. doi: 10.1016/j.jtcvs.2014.07.094. [DOI] [PubMed] [Google Scholar]
- 41.Gong C.L., Song A.Y., Horak R., et al. Impact of confounding on cost, survival, and length-of-stay outcomes for neonates with hypoplastic left heart syndrome undergoing stage 1 palliation surgery. Pediatr Cardiol. 2020;41:996–1011. doi: 10.1007/s00246-020-02348-5. [DOI] [PubMed] [Google Scholar]
- 42.Dean P.N., Hillman D.G., McHugh K.E., Gutgesell H.P. Inpatient costs and charges for surgical treatment of hypoplastic left heart syndrome. Pediatr. 2011;128:e1181–e1186. doi: 10.1542/peds.2010-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen J.E., Madsen N.L., Bishop L., Morales D.L.S., Anderson J.B. Longitudinal health care cost in hypoplastic left heart syndrome palliation. Pediatr Cardiol. 2018;39:1210–1215. doi: 10.1007/s00246-018-1885-9. [DOI] [PubMed] [Google Scholar]
- 44.Ghanayem N.S., Allen K.R., Tabbutt S., et al. Interstage mortality after the Norwood procedure: results of the multicenter single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabbutt S., Ghanayem N., Ravishankar C., et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: a report from the pediatric heart network single ventricle reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:882–895. doi: 10.1016/j.jtcvs.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudd N.A., Frommelt M.A., Tweddell J.S., et al. Improving interstage survival after Norwood operation: outcomes from 10 years of home monitoring. J Thorac Cardiovasc Surg. 2014;148:1540–1547. doi: 10.1016/j.jtcvs.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 47.Oster M.E., Ehrlich A., King E., et al. Association of interstage home monitoring with mortality, readmissions, and weight gain. Circulation. 2015;132:502–508. doi: 10.1161/CIRCULATIONAHA.114.014107. [DOI] [PubMed] [Google Scholar]
- 48.Petit C.J., Fraser C.D., Mattamal R., Slesnick T.C., Cephus C.E., Ocampo E.C. The impact of a dedicated single-ventricle home-monitoring program on interstage somatic growth, interstage attrition, and 1-year survival. J Thorac Cardiovasc Surg. 2011;142:1358–1366. doi: 10.1016/j.jtcvs.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 49.Gardner M.M., Mercer-Rosa L., Faerber J., et al. Association of a home monitoring program with interstage and stage 2 outcomes. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hansen J.H., Furck A.K., Petko C., et al. Use of surveillance criteria reduces interstage mortality after the Norwood operation for hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2012;41:1013–1018. doi: 10.1093/ejcts/ezr190. [DOI] [PubMed] [Google Scholar]
- 51.Goldstein B.H., O'Byrne M.L., Petit C.J., et al. Differences in cost of care by palliation strategy for infants with ductal-dependent pulmonary blood flow. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.118.007232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang L., Schilling C., Dalziel K.M., et al. Hospital inpatient costs for single ventricle patients surviving the Fontan procedure. Am J Cardiol. 2017;120:467–472. doi: 10.1016/j.amjcard.2017.04.049. [DOI] [PubMed] [Google Scholar]
- 53.Tweddell J.S., Sleeper L.A., Ohye R.G., et al. Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144:152–159. doi: 10.1016/j.jtcvs.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasquali S.K., Ohye R.G., Lu M., et al. Variation in perioperative care across centers for infants undergoing the Norwood procedure. J Thorac Cardiovasc Surg. 2012;144:915–921. doi: 10.1016/j.jtcvs.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galantowicz M., Cheatham J.P., Phillips A., et al. Hybrid approach for hypoplastic left heart syndrome: intermediate results after the learning curve. Ann Thorac Surg. 2008;85:2063–2071. doi: 10.1016/j.athoracsur.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 56.Schranz D., Bauer A., Reich B., et al. Fifteen-year single center experience with the “Giessen Hybrid” approach for hypoplastic left heart and variants: current strategies and outcomes. Pediatr Cardiol. 2015;36:365–373. doi: 10.1007/s00246-014-1015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schranz D., Esmaeili A., Schrewe R., Kerst G., Akintuerk H. Hypoplastic left heart stage I: no Norwood, no hybrid. Circulation. 2020;142:1402–1404. doi: 10.1161/CIRCULATIONAHA.120.047668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holzer R., Marshall A., Kreutzer J., et al. Hybrid procedures: adverse events and procedural characteristics--results of a multi-institutional registry. Congenit Heart Dis. 2010;5:233–242. doi: 10.1111/j.1747-0803.2010.00416.x. [DOI] [PubMed] [Google Scholar]
- 59.Wilder T.J., McCrindle B.W., Hickey E.J., et al. Is a hybrid strategy a lower-risk alternative to stage 1 Norwood operation? J Thorac Cardiovasc Surg. 2017;153:163–172.e6. doi: 10.1016/j.jtcvs.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Freud L.R., McElhinney D.B., Marshall A.C., et al. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: postnatal outcomes of the first 100 patients. Circulation. 2014;130:638–645. doi: 10.1161/CIRCULATIONAHA.114.009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pickard S.S., Wong J.B., Bucholz E., et al. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: a decision analysis. Circ Cardiovasc Qual Outcomes. 2020;13(4) doi: 10.1161/CIRCOUTCOMES.119.006127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.