Abstract
Background
Emerging evidence suggests a pathophysiological link between obesity and atrial fibrillation (AF). However, the contribution of body fat distribution to left atrial (LA) remodeling and its reversibility remain unclear in nonobese AF patients.
Objectives
The purpose of this study was to investigate the association of body fat distribution with LA size and reverse remodeling (LARR).
Methods
In total, 116 nonobese patients with AF (88 men, age 63 ± 11 years) who underwent first catheter ablation (CA) were included. Body fat distribution was assessed with bioelectrical impedance, and body fat percentage (BF%) and central fat percentage (CF%) were calculated. Patients were categorized by body size metrics (body mass index [BMI] and waist-to-hip [W/H] ratio) and fat parameters (BF% and CF%). Echocardiography was performed before and 6 months after CA. Multivariable logistic regression was used to examine the association between the 4 metrics (ie, BMI, W/H ratio, BF%, and CF%) and a lack of LARR (<15% reduction or increase in the LA volume index).
Results
Body size metrics and adiposity measures were not independently associated with baseline LA size. Six months after CA, the higher W/H ratio and CF% groups exhibited persistent LA enlargement compared to their counterparts (both P < 0.01). In the multivariable analysis, W/H ratio and CF% were associated with a lack of LARR (adjusted ORs of 3.86 and 2.81 per 0.10 and 10% increase, respectively, both P < 0.01). The combined assessment of CF% with W/H ratio provided complementary risk stratification for persistent LA enlargement.
Conclusions
Central adiposity was associated with a lack of LARR after CA, highlighting the importance of assessing body fat distribution even in nonobese patients.
Key words: atrial fibrillation, bioelectrical impedance analysis, body fat distribution, catheter ablation, left atrial reverse remodeling
Central Illustration
Atrial fibrillation (AF), the most prevalent sustained arrhythmia in daily clinical practice, has doubled in incidence in the past 3 decades, affecting approximately 60 million people worldwide.1 AF promotes left atrial (LA) structural and electrical remodeling, which in turn enhances the perpetuation of AF.2 Recently, catheter ablation (CA) has been confirmed to bring about a more favorable effect on sinus rhythm maintenance as well as cardiovascular outcomes compared with antiarrhythmic drugs.3,4 The restoration of sinus rhythm contributes to the amelioration of LA structural changes, namely LA-reverse remodeling, which is an important surrogate marker for AF-free survival.5, 6, 7, 8 However, LA reverse remodeling after CA varies substantially among individuals.6,7
Obesity is an established risk factor for AF,9,10 and obese patients with AF exhibit more advanced LA remodeling and are at higher risk for AF recurrence after CA compared with nonobese patients.11,12 However, nonobese patients with AF also experience approximately 40% of AF recurrence within 1 year after CA, highlighting a need for an alternative to BMI measurement to identify patients prone to AF relapse.12,13 Recently, body fat distribution has garnered attention as a potential pathophysiological mechanism in nonobese patients with AF.10 Bioelectrical impedance analysis (BIA) has emerged as a noninvasive method for evaluating body fat content showing excellent correlations with conventional measurements by dual-energy x-ray absorptiometry, computed tomography (CT), or magnetic resonance imaging (MRI).14, 15, 16 Nonetheless, the association of body fat distribution with LA structural remodeling and its reversibility after CA in nonobese patients with AF is unknown. Elucidation of these associations might enhance our therapeutic strategies in terms of periprocedural management for AF ablation. Therefore, the aim of the present study was to investigate the impact of body size metrics and BIA-assessed fat measures on LA remodeling before and after CA in nonobese patients with AF.
Methods
Study sample
We prospectively enrolled 116 consecutive nonobese patients with AF who underwent their first CA from May 2020 to March 2022 at the University of Tokyo Hospital. The exclusion criteria were as follows: 1) congenital heart disease; 2) moderate or severe valvular disease; 3) dilated or hypertrophic cardiomyopathy; 4) history of cardiothoracic surgery within 3 months; 5) history of cardiac implantable electrical device implantation; and 6) renal insufficiency on hemodialysis. AF was defined as paroxysmal if it terminated spontaneously within 7 days of onset and as persistent if it continued for more than 7 days.17 All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, and the institutional ethics committee of the University of Tokyo approved the study protocol (2018120NI).
Risk factor assessment and laboratory testing
Hypertension was defined as systolic blood pressure ≥130 mm Hg, diastolic blood pressure ≥80 mm Hg, or receiving antihypertensive medications. Diabetes mellitus was determined by fasting glucose ≥126 mg/dl or current use of insulin or hypoglycemic agents. Dyslipidemia was defined as total serum cholesterol >240 mg/dl or the use of lipid-lowering drugs. Laboratory examination included fasting serum glucose, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, C-reactive protein, and B-type natriuretic peptide (BNP). The estimated glomerular filtration rate was computed using the abbreviated Modification of Diet in Renal Disease equation: estimated glomerular filtration rate (ml/min/1.73 m2) = 194 × (serum creatinine)−1.094 × (age)−0.287 × (0.739 if woman). Interleukin (IL)-6 and carboxy-terminal telopeptide of procollagen type I (ICTP) levels in all patients were measured at a commercial laboratory (IL-6, electrochemiluminescence immunoassay; ICTP, radioimmunoassay, SRL, Inc).
Body size metrics and fat distribution measurements
Body mass index (BMI) was calculated using height and weight (kg/m2). BMI <25 kg/m2 and ≥25 kg/m2 were defined as normal weight and overweight, respectively. Patients with obesity, defined as BMI ≥30 kg/m2, were excluded, as mentioned above. Waist circumference was measured at the level of the umbilicus, and hip circumference was measured over the widest part of the buttocks. The waist-to-hip (W/H) ratio was then calculated. BIA was performed to assess body fat parameters with the Tanita Body Composition Analyzer, TANITA MC 780 MA-N (TANITA), a well-validated modality showing excellent correlation with dual-energy x-ray absorptiometry, CT, or MRI14,15 at the time of preprocedural echocardiography 1 to 4 days before CA. Body fat percentage (BF%) was calculated as: fat mass (kg) × 100/body weight (kg), and central fat percentage (CF%) was determined as: (total fat mass [kg] − arm fat mass [kg] − leg fat mass [kg]) × 100/total fat mass (kg).
Echocardiographic measurements
Transthoracic echocardiography was performed with a commercially available system, EPIQ 7 (Koninklijke Philips N.V.) or Vivid E95 (GE Vingmed Ultrasound), 1 to 4 days before and 6 months after CA. All images were recorded by experienced and registered cardiologists. The dimensions of the cardiac chamber were measured according to the guidelines of the American Society of Echocardiography and the European Association of Cardiovascular Imaging.18 Left ventricular (LV) mass was calculated as: 0.8×1.04 × [(LV end-diastolic dimension + posterior wall thickness + interventricular septum thickness)³-(LV end-diastolic dimension)³]+0.6.18 LV ejection fraction and LA volume were assessed using the biplane Simpson’s rule.18 LV mass and LA volume were indexed for body surface area. Peak early (E) diastolic velocity was evaluated by transmitral inflow signals. Peak early diastolic velocity (e’) of the septal and lateral mitral annulus was derived from tissue Doppler imaging and averaged. E/e’ ratio was then calculated.19
Catheter ablation procedures
CA was performed under sedation. All patients underwent pulmonary vein isolation with point-by-point radiofrequency energy or the balloon technique to restore sinus rhythm, with an endpoint of bidirectional block between the LA and the inside of the circumferential pulmonary vein isolation area. Additional procedures, including cavotricuspid isthmus ablation, superior vena cava isolation, roof line, and mitral isthmus line ablation, were performed at the physician’s discretion.
Follow-up and assessment of LA reverse remodeling
After the CA procedure, patients were evaluated every 1 to 2 months at the outpatient clinic. A 12-lead electrocardiogram was carried out at the follow-up visit, and 24-hour Holter monitoring was performed 3 to 6 months after the procedure. Recurrence of arrhythmia was defined as any episode of atrial arrhythmia that lasted longer than 30 seconds on a 12-lead electrocardiogram or Holter monitoring after a 2-month blanking period from CA.7,20 LA reverse remodeling was defined as ≥15% reduction in the LA volume index at the 6-month follow-up echocardiography after the procedure, according to previous studies.7,21,22 An excellent correlation was observed for the analysis of the interobserver variability of the LA volume index in 20 randomly selected patients (r = 0.96). In the Bland-Altman analysis, agreement between the interobserver measurements was −1.5 ± 6.1 ml/m2 (mean ± 1.96 standard deviation).
Statistical analysis
Continuous variables were expressed as mean ± SD or median (IQR) and compared with an unpaired t test or Wilcoxon rank sum test as appropriate. Categorical variables were described as numbers and proportions and analyzed with the chi-square test or Fisher’s exact test. Baseline characteristics and pre-/post-procedural echocardiographic parameters were compared according to body size metrics and fat parameters, including BMI (normal weight vs overweight), W/H ratio, BF%, and CF% (above vs below the sex-specific median values, respectively). Univariable and multivariable linear regression models were constructed to investigate the relationships between body size metrics/adiposity measures as continuous variables and baseline LA size. We also investigated the association between the 4 metrics (ie, BMI, W/H ratio, BF%, and CF%) and a lack of LA reverse remodeling (<15% reduction or increase in the LA volume index after CA) by univariable and multivariable logistic regression analysis. Multivariable analyses were performed with body size metrics and fat measures tested separately as possibly associated with baseline LA size and lack of LA reverse remodeling, adjusting for the effect of clinical, laboratory, and echocardiographic variables identified by univariable analyses (P < 0.10). Statistical analyses were performed with JMP 16 software (SAS Institute).
Results
Baseline characteristics
Among the 116 study participants, the mean age was 63 ± 11 years, and 88 (75.9%) were men. The mean BMI was 24.2 ± 2.9 kg/m2, and the median (25th-75th percentile) W/H ratio was 0.92 (0.89-0.97) for men and 0.86 (0.83-0.91) for women. Baseline characteristics stratified by body size metrics and fat parameters are summarized in Table 1. Systolic blood pressure, diastolic blood pressure, and heart rate were comparable among groups. BNP levels were similar in the BMI- and W/H ratio-categorized groups, whereas the higher BF% (≥23.5% for men and ≥35.1% for women) and CF% (≥60.5% for men and ≥52.3% for women) groups exhibited an elevated BNP concentration compared with their counterparts. Inflammatory markers, such as C-reactive protein and IL-6 levels, were higher in the groups with a larger W/H ratio, BF%, and CF%. In addition, ICTP-1, which reflects collagen degradation, was significantly elevated in the higher BF% and CF% groups. Supplemental Figure 1 shows the relationship between body size metrics and adiposity measures. BMI demonstrated a positive correlation with W/H ratio (r = 0.51, P < 0.001) and BF% (r = 0.62, P < 0.001), while its association was modest with CF% (r = 0.28, P = 0.003). Interestingly, BF% was not correlated with CF% (r = 0.17, P = 0.075).
Table 1.
Baseline Characteristics Stratified by Body Size Metrics and Adiposity Profiles
| BMI |
W/H Ratio |
BF% |
CF% |
|||||
|---|---|---|---|---|---|---|---|---|
| Normal Weight (n = 68) | Overweight (n = 48) | <Median (n = 55) | ≥Median (n = 61) | <Median (n = 58) | ≥Median (n = 58) | <Median (n = 58) | ≥Median (n = 58) | |
| Age, y | 64 ± 11 | 63 ± 10 | 60 ± 11 | 67 ± 9b | 61 ± 11 | 66 ± 10a | 60 ± 11 | 67 ± 10b |
| Men | 52 (76.5) | 36 (75.0) | 42 (76.4) | 46 (75.4) | 44 (75.9) | 44 (75.9) | 44 (75.9) | 44 (75.9) |
| AF types | ||||||||
| Paroxysmal | 37 (54.4) | 24 (50.0) | 25 (45.5) | 36 (59.0) | 36 (62.1) | 25 (43.1)a | 32 (55.2) | 29 (50.0) |
| Persistent | 31 (45.6) | 24 (50.0) | 30 (54.5) | 25 (41.0) | 22 (37.9) | 33 (56.9)a | 26 (44.8) | 29 (50.0) |
| BMI, kg/m2 | 22.5 ± 2.1 | 26.9 ± 1.4b | 23.3 ± 2.9 | 25.3 ± 2.4b | 22.5 ± 2.4 | 26.2 ± 1.9b | 23.5 ± 2.7 | 25.3 ± 2.7b |
| Systolic blood pressure, mm Hg | 119 ± 18 | 122 ± 14 | 118 ± 16 | 122 ± 16 | 119 ± 18 | 121 ± 15 | 118 ± 15 | 122 ± 17 |
| Diastolic blood pressure, mm Hg | 66 ± 11 | 69 ± 10 | 66 ± 12 | 67 ± 10 | 66 ± 11 | 68 ± 11 | 66 ± 12 | 68 ± 10 |
| Heart rate, beats/min | 76 ± 15 | 77 ± 13 | 78 ± 13 | 75 ± 15 | 76 ± 14 | 77 ± 14 | 76 ± 16 | 76 ± 12 |
| Current smoking | 4 (5.9) | 6 (12.5) | 3 (5.5) | 7 (11.5) | 3 (5.2) | 7 (12.1) | 2 (3.4) | 8 (13.8) |
| Hypertension | 30 (44.1) | 35 (72.9)b | 24 (43.6) | 41 (67.2)a | 25 (43.1) | 40 (69.0)b | 22 (37.9) | 43 (74.1)b |
| Diabetes mellitus | 16 (23.5) | 14 (29.2) | 12 (21.8) | 18 (29.5) | 11 (19.0) | 19 (32.8) | 8 (13.8) | 22 (37.9)b |
| Dyslipidemia | 23 (33.8) | 23 (47.9) | 16 (29.1) | 30 (49.2)a | 21 (36.2) | 25 (43.1) | 19 (32.8) | 27 (46.6) |
| CHA2DS2-VASc score | 2 (0-3) | 2 (1-3) | 1 (0-2) | 2 (1-3)b | 1 (0-2) | 2 (1-3)b | 1 (0-2) | 2 (1-4)b |
| Medications | ||||||||
| Beta-blocker | 27 (39.7) | 23 (47.9) | 22 (40.0) | 28 (45.9) | 21 (36.2) | 29 (50.0) | 20 (34.5) | 30 (51.7) |
| RAAS blockers | 13 (19.1) | 19 (39.6)a | 13 (23.6) | 19 (31.1) | 7 (12.1) | 25 (43.1)b | 7 (12.1) | 25 (43.1)b |
| Calcium channel blocker | 17 (25.0) | 23 (47.9)a | 11 (20.0) | 29 (47.5)b | 13 (22.4) | 27 (46.6)b | 10 (17.2) | 30 (51.7)b |
| Statin | 14 (20.6) | 15 (31.3) | 10 (18.2) | 19 (31.1) | 10 (17.2) | 19 (32.8) | 10 (17.2) | 19 (32.8) |
| Oral antidiabetic drug | 11 (16.2) | 10 (20.8) | 10 (18.2) | 11 (18.0) | 7 (12.1) | 14 (24.1) | 6 (10.3) | 15 (25.9)a |
| Antiarrhythmic drug | 21 (30.9) | 9 (18.8) | 15 (27.3) | 15 (24.6) | 16 (27.6) | 14 (24.1) | 17 (29.3) | 13 (22.4) |
| Laboratory data | ||||||||
| Total cholesterol, mg/dl | 196 ± 30 | 191 ± 40 | 194 ± 31 | 194 ± 37 | 199 ± 34 | 189 ± 34 | 196 ± 31 | 193 ± 38 |
| LDL cholesterol, mg/dl | 113 ± 27 | 112 ± 34 | 113 ± 29 | 112 ± 32 | 114 ± 31 | 111 ± 30 | 114 ± 29 | 111 ± 31 |
| HDL cholesterol, mg/dl | 64 ± 16 | 55 ± 13b | 62 ± 17 | 59 ± 14 | 64 ± 16 | 56 ± 14b | 61 ± 16 | 60 ± 15 |
| Fasting blood glucose, mg/dl | 108 ± 43 | 106 ± 29 | 105 ± 42 | 109 ± 33 | 101 ± 29 | 113 ± 44a | 96 ± 18 | 118 ± 48b |
| HbA1c, % | 6.0 ± 0.7 | 6.0 ± 0.7 | 5.9 ± 0.7 | 6.1 ± 0.7 | 5.8 ± 0.6 | 6.1 ± 0.8b | 5.7 ± 0.4 | 6.2 ± 0.8b |
| eGFR, ml/min/1.73 m2 | 67 ± 14 | 64 ± 14 | 66 ± 15 | 65 ± 14 | 68 ± 14 | 64 ± 15 | 66 ± 14 | 65 ± 15 |
| BNP, pg/ml | 59 (24-130) | 74 (21-165) | 62 (24-126) | 60 (24-169) | 40 (22-100) | 92 (29-177)a | 52 (20-94) | 95 (29-177)a |
| C-Reactive protein, mg/dl | 0.05 (0.03-0.09) | 0.06 (0.03-0.13) | 0.03 (0.03-0.08) | 0.06 (0.03-0.16)b | 0.03 (0.03-0.07) | 0.08 (0.04-0.17)b | 0.03 (0.03-0.06) | 0.08 (0.04-0.17)b |
| IL-6, pg/ml | 1.1 (0.8-1.8) | 1.3 (0.9-2.2) | 1.1 (0.7-1.4) | 1.3 (1.0-2.3)b | 1.0 (0.7-1.3) | 1.6 (1.1-2.7)b | 1.0 (0.7-1.3) | 1.6 (0.9-2.7)b |
| ICTP-1, ng/ml | 3.9 (3.1-4.8) | 4.3 (3.3-5.5) | 3.9 (3.3-5.0) | 4.1 (3.3-5.3) | 3.6 (3.1-4.5) | 4.4 (3.4-5.4)b | 3.6 (3.1-4.5) | 4.4 (3.4-5.4)a |
Values are mean ± SD, n (%), or median (IQR).
AF = atrial fibrillation; BF% = body fat percent; BMI = body mass index; BNP = B-type natriuretic peptide; CF% = central fat percent; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; ICTP-1 = carboxy-terminal telopeptide of procollagen type I; IL = interleukin; LDL = low-density lipoprotein; RAAS = renin-angiotensin-aldosterone system; W/H = waist-to-hip.
P < 0.05.
P < 0.01 compared with corresponding counterpart.
Association of body size metrics and adiposity measures with LA size
Baseline echocardiographic parameters according to body size and fat profile are displayed in Table 2. Although LV mass index was greater in the overweight, larger W/H ratio, BF%, and CF% groups, LV size and ejection fraction were similar among the groups. Patients with higher BF% had a significantly larger LA volume index than those with lower BF% (40.6 ± 14.7 ml/m2 vs 34.2 ± 11.7 ml/m2, P = 0.017), but this was not true for the BMI, W/H ratio, and CF% categories. Univariable linear regression analysis was constructed to evaluate the possible association of 4 body size metrics/adiposity measures (BMI, W/H ratio, BF%, and CF%), patient demographics (ie, age, sex, hypertension, diabetes mellitus, dyslipidemia, and smoking status), and AF type with LA volume index before CA. W/H ratio (standardized β = 0.21 per 0.10 increase, P = 0.027) and BF% (standardized β = 0.21 per 10% increase, P = 0.025) as well as older age (standardized β = 0.27, P = 0.003), hypertension (standardized β = 0.17, P = 0.065), and persistent AF (standardized β = 0.51, P < 0.001) were potentially associated with larger LA size. When we adjusted for these variables in the multivariable linear regression analysis, the association of W/H ratio and BF% with preprocedural LA volume index became insignificant (standardized β = 0.08 and 0.14, both P > 0.10, respectively).
Table 2.
Baseline Echocardiographic Parameters According to Each Body Size and Adiposity Measures
| BMI |
W/H Ratio |
BF% |
CF% |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Weight | Overweight | P Value | <Median | ≥Median | P Value | <Median | ≥Median | P Value | <Median | ≥Median | P Value | |
| LVEDVI, ml/m2 | 39.7 ± 9.4 | 40.8 ± 12.7 | 0.940 | 41.1 ± 11.5 | 39.3 ± 10.3 | 0.388 | 40.4 ± 9.0 | 40.0 ± 12.5 | 0.535 | 39.9 ± 9.1 | 40.4 ± 12.4 | 0.836 |
| LVESVI, ml/m2 | 15.3 ± 5.8 | 16.8 ± 7.4 | 0.573 | 16.3 ± 6.2 | 15.6 ± 6.9 | 0.328 | 15.4 ± 5.8 | 16.5 ± 7.2 | 0.406 | 15.5 ± 6.1 | 16.4 ± 6.9 | 0.280 |
| LVEF, % | 61.5 ± 9.6 | 59.3 ± 10.1 | 0.164 | 60.1 ± 10.0 | 61.0 ± 9.7 | 0.514 | 61.8 ± 9.7 | 59.3 ± 9.8 | 0.105 | 61.3 ± 10.4 | 59.8 ± 9.2 | 0.116 |
| LVMI, g/m2 | 81.0 ± 17.6 | 94.2 ± 24.0 | 0.002 | 79.1 ± 18.4 | 93.1 ± 22.0 | <0.001 | 80.3 ± 17.4 | 92.6 ± 23.4 | 0.002 | 80.5 ± 18.0 | 92.4 ± 23.0 | 0.002 |
| E-wave, cm/s | 70.4 ± 20.0 | 74.2 ± 18.2 | 0.171 | 73.9 ± 17.9 | 70.2 ± 20.5 | 0.332 | 71.4 ± 20.1 | 72.5 ± 18.7 | 0.681 | 72.2 ± 18.6 | 71.7 ± 20.2 | 0.800 |
| e’, cm/s | 9.0 ± 2.6 | 8.4 ± 2.2 | 0.266 | 9.7 ± 2.6 | 7.9 ± 2.0 | <0.001 | 9.4 ± 2.6 | 8.1 ± 2.1 | 0.012 | 9.6 ± 2.5 | 8.0 ± 2.2 | <0.001 |
| E/e’ ratio | 8.2 ± 2.7 | 9.3 ± 3.0 | 0.035 | 8.0 ± 2.2 | 9.3 ± 3.2 | 0.059 | 8.0 ± 2.8 | 9.3 ± 2.8 | 0.003 | 7.9 ± 2.4 | 9.5 ± 3.1 | 0.002 |
| LAVI, ml/m2 | 35.5 ± 12.3 | 40.1 ± 15.0 | 0.091 | 34.9 ± 10.9 | 39.7 ± 15.4 | 0.198 | 34.2 ± 11.7 | 40.6 ± 14.7 | 0.017 | 34.8 ± 10.1 | 40.0 ± 16.1 | 0.119 |
Values are mean ± SD.
BF% = body fat percent; BMI = body mass index; CF% = central fat percent; E = early diastolic transmitral flow velocity; e’ = early diastolic mitral annular velocity; LAVI = left atrial volume index; LVEDVI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESVI = left ventricular end-systolic volume index; LVMI = left ventricular mass index; W/H = waist-to-hip.
Ablation procedure
All patients underwent pulmonary vein isolation, accounting for radiofrequency ablation in 72 (62.1%) patients and cryoballoon ablation in 44 (37.9%) patients. Additional ablation was conducted in 31 (26.7%) patients with cavotricuspid isthmus ablation, 2 (1.7%) patients with superior vena cava isolation, and 3 (2.6%) patients with LA line ablation, respectively. 3D mapping was performed using the Carto3 system (Biosense Webster) in 85 (73.3%) patients, followed by EnSite NavX system (Abbot, formerly St. Jude Medical) in 29 (25.0%) patients, and the Rhythmia system (Boston Scientific) in 2 (1.7%) patients. The median (25th-75th percentile) procedure time was 166 (145-202) minutes. There was no significant difference in procedural characteristics among body fat categories except for the higher prevalence of radiofrequency ablation in the larger BF% group (Supplemental Table 1).
Association of body size metrics and adiposity measures with LA reverse remodeling
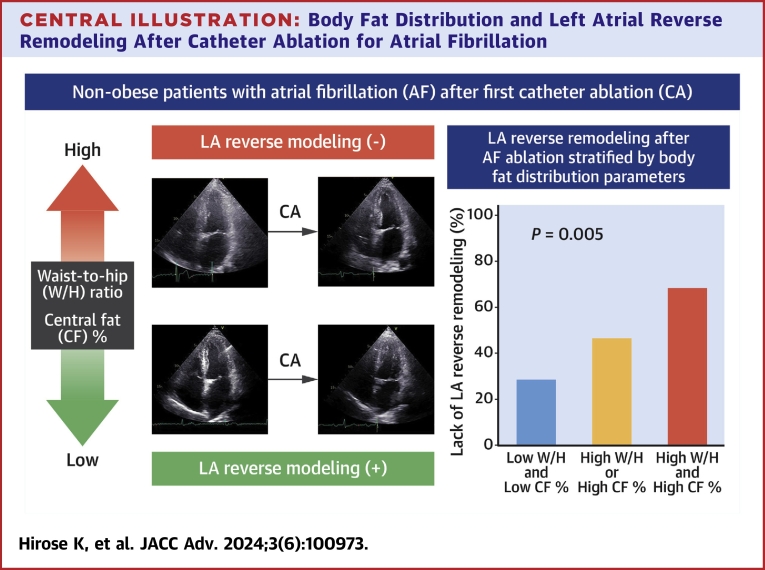
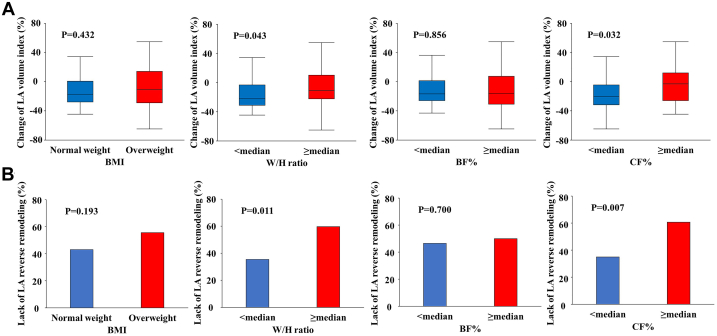
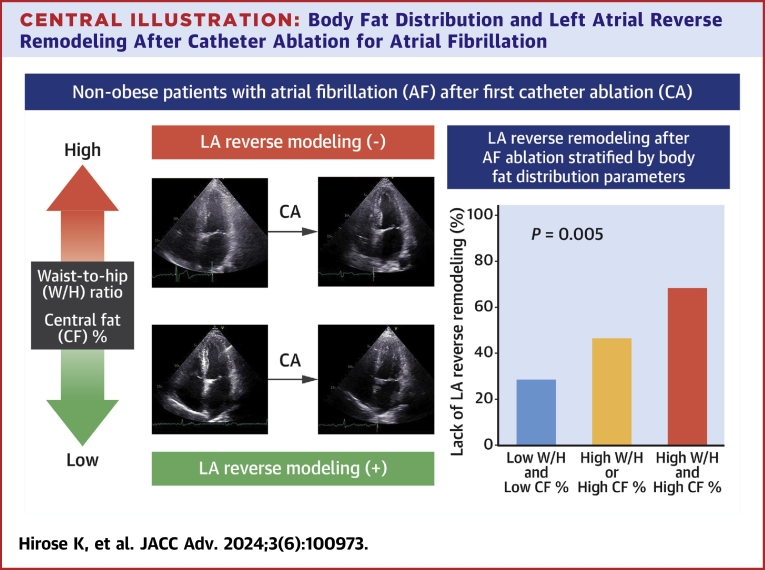
Among the 116 study participants, follow-up echocardiography data at 6 months after CA were available for 108 patients. Table 3 shows the postprocedural echocardiographic parameters. LA volume at 6 months after CA was significantly larger in patients with a higher W/H ratio and CF% compared with their counterparts (W/H ratio: 35.2 ± 11.6 ml/m2 vs 29.4 ± 8.4 ml/m2, P = 0.005; CF%: 35.2 ± 10.6 ml/m2 vs 29.5 ± 9.8 ml/m2, P = 0.003). During 6 months of follow-up, 16 (14.8%) patients experienced recurrent AF. There was no statistical difference in AF recurrence rate between the groups stratified by body size metrics and fat measures (all P > 0.10). As shown in Figure 1A, the reduction in LA volume after CA was significantly smaller in the higher W/H ratio and CF% groups, while there were no significant differences in BMI and BF% categorized groups. In the overall study population, 56 (51.9%) patients experienced LA reverse remodeling. The frequency of the lack of LA reverse remodeling was greater in the higher W/H ratio and CF% groups as compared with their counterparts (W/H ratio: 59.7% vs 35.3%, P = 0.011; CF%: 60.7% vs 34.6%, P = 0.007; Figure 1B). In univariable logistic models, a higher W/H ratio and CF% were likely to have an association with a lack of LA reverse remodeling (both P < 0.10) (Table 4), while BMI and BF% were not. Multivariable analyses adjusted for BNP, LV end-systolic volume index, LV ejection fraction, baseline LA size, and AF type found that a higher W/H ratio (adjusted OR: 3.86 per 0.10 increase, 95% CI 1.60 to 9.31, P = 0.003) and CF% (adjusted OR: 2.81 per 10% increase, 95% CI 1.33-5.92, P = 0.007) were independently associated with unfavorable LA reverse remodeling (also Table 4). The combined assessment of the W/H ratio with CF% provided better risk stratification for persistent LA enlargement, accounting for the highest prevalence in the high W/H ratio and CF% group (67.6%), followed by the high W/H ratio or CF% group (46.2%) and the low W/H ratio and CF% group (28.1%, overall P = 0.005) (Central Illustration).
Table 3.
Echocardiographic Parameters at Follow-Up According to Each Body Size and Adiposity Measure
| BMI |
W/H Ratio |
BF% |
CF% |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Weight | Overweight | P Value | <Median | ≥Median | P Value | <Median | ≥Median | P Value | <Median | ≥Median | P Value | |
| LVEDVI, ml/m2 | 41.6 ± 10.8 | 42.8 ± 11.7 | 0.709 | 42.9 ± 10.4 | 41.5 ± 11.8 | 0.268 | 41.5 ± 10.7 | 42.7 ± 11.6 | 0.924 | 41.6 ± 10.8 | 42.6 ± 11.5 | 0.895 |
| LVESVI, ml/m2 | 15.4 ± 5.2 | 16.3 ± 5.6 | 0.414 | 16.2 ± 5.3 | 15.4 ± 5.5 | 0.319 | 15.5 ± 5.3 | 16.0 ± 5.4 | 0.665 | 15.8 ± 5.3 | 15.8 ± 5.4 | 0.929 |
| LVEF, % | 63.2 ± 6.8 | 61.7 ± 5.9 | 0.156 | 62.3 ± 6.9 | 62.8 ± 6.0 | 0.844 | 62.9 ± 6.9 | 62.3 ± 6.0 | 0.527 | 62.0 ± 6.9 | 63.1 ± 5.9 | 0.523 |
| LVMI, g/m2 | 80.5 ± 17.9 | 91.4 ± 28.3 | 0.026 | 77.4 ± 16.0 | 91.9 ± 26.6 | <0.001 | 79.6 ± 18.1 | 90.5 ± 26.6 | 0.018 | 77.6 ± 15.8 | 91.9 ± 26.9 | 0.003 |
| E-wave, cm/s | 57.1 ± 13.8 | 57.5 ± 18.5 | 0.718 | 58.6 ± 12.7 | 56.1 ± 18.3 | 0.122 | 58.8 ± 15.1 | 55.8 ± 16.5 | 0.197 | 58.0 ± 14.0 | 56.6 ± 17.5 | 0.310 |
| e’, cm/s | 7.7 ± 2.1 | 6.9 ± 2.1 | 0.041 | 8.3 ± 2.2 | 6.6 ± 1.7 | <0.001 | 7.9 ± 2.2 | 6.9 ± 1.9 | 0.009 | 7.7 ± 2.2 | 7.1 ± 2.0 | 0.121 |
| E/e’ ratio | 7.8 ± 2.3 | 8.6 ± 2.6 | 0.089 | 7.4 ± 2.0 | 8.8 ± 2.7 | 0.007 | 7.8 ± 2.4 | 8.5 ± 2.5 | 0.130 | 7.9 ± 2.0 | 8.4 ± 2.8 | 0.578 |
| LAVI, ml/m2 | 30.8 ± 9.6 | 34.8 ± 11.5 | 0.063 | 29.4 ± 8.4 | 35.2 ± 11.6 | 0.005 | 30.5 ± 9.6 | 34.4 ± 11.1 | 0.102 | 29.5 ± 9.8 | 35.2 ± 10.6 | 0.003 |
Values are mean ± SD.
BF% = body fat percent; BMI = body mass index; CF% = central fat percent; E = early diastolic transmitral flow velocity; e’ = early diastolic mitral annular velocity; LAVI = left atrial volume index; LVEDVI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESVI = left ventricular end-systolic volume index; LVMI = left ventricular mass index; W/H = waist-to-hip.
Figure 1.
LA Structural Change After Catheter Ablation According to Body Size Metrics and Fat Profile
(A) Change in LA volume index after catheter ablation according to body size and fat category. (B) The frequency of lack of LA reverse remodeling stratified by BMI, W/H ratio, and adiposity profile. BF% = body fat percent; CF% = central fat percent; LA = left atrium; BMI = body mass index; W/H = waist-to-hip.
Table 4.
Univariable and Multivariable Logistic Regression Models for the Lack of LA Reverse Remodeling
| Univariable Model |
W/H Ratio Model |
CF% Model |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| BMI, kg/m2 | 1.10 (0.96-1.25) | 0.184 | ||||
| W/H ratio, per 0.10-increase | 1.74 (0.93-3.26) | 0.084 | 3.86 (1.60-9.31) | 0.003 | ||
| BF%, per 10%-increase | 1.45 (0.85-2.46) | 0.171 | ||||
| CF%, per 10%-increase | 1.68 (0.95-2.94) | 0.072 | 2.81 (1.33-5.92) | 0.007 | ||
| Age, y | 1.03 (0.99-1.07) | 0.166 | ||||
| Men | 1.22 (0.51-2.93) | 0.657 | ||||
| Current smoking | 0.85 (0.22-3.35) | 0.817 | ||||
| Hypertension | 1.49 (0.69-3.20) | 0.308 | ||||
| Diabetes mellitus | 1.47 (0.62-3.46) | 0.377 | ||||
| Dyslipidemia | 1.32 (0.61-2.85) | 0.477 | ||||
| Beta-blocker | 1.32 (0.62-2.85) | 0.471 | ||||
| RAAS blockers | 1.33 (0.57-3.10) | 0.504 | ||||
| Antiarrhythmic drug | 0.75 (0.32-1.79) | 0.516 | ||||
| Total cholesterol, mg/dl | 1.00 (0.99-1.01) | 0.879 | ||||
| LDL cholesterol, mg/dl | 1.00 (0.99-1.02) | 0.610 | ||||
| HDL cholesterol, mg/dl | 0.99 (0.96-1.01) | 0.269 | ||||
| Fasting blood glucose, mg/dl | 1.00 (0.99-1.01) | 0.555 | ||||
| HbA1c, % | 1.00 (0.59-1.71) | 1.000 | ||||
| eGFR, ml/min/1.73 m2 | 1.02 (0.99-1.05) | 0.187 | ||||
| Log BNP, pg/mL | 0.21 (0.09-0.52) | <0.001 | 1.16 (0.30-4.58) | 0.828 | 0.66 (0.16-2.70) | 0.565 |
| Log CRP, mg/dl | 1.86 (0.82-4.24) | 0.137 | ||||
| Log IL-6, pg/mL | 2.35 (0.71-7.76) | 0.161 | ||||
| Log ICTP-1, ng/mL | 1.85 (0.13-26.65) | 0.652 | ||||
| LVEDVI, ml/m2 | 1.00 (0.96-1.03) | 0.870 | ||||
| LVESVI, ml/m2 | 0.93 (0.86-0.99) | 0.035 | 1.07 (0.95-1.21) | 0.254 | 1.07 (0.94-1.20) | 0.307 |
| LVEF, % | 1.09 (1.03-1.14) | 0.002 | 1.12 (1.03-1.22) | 0.012 | 1.11 (1.02-1.21) | 0.016 |
| LVMI, g/m2 | 1.00 (0.98-1.02) | 0.946 | ||||
| E/e’ ratio | 0.92 (0.79-1.06) | 0.239 | ||||
| LAVI, ml/m2 | 0.93 (0.89-0.97) | <0.001 | 0.91 (0.85-0.97) | 0.003 | 0.93 (0.88-0.99) | 0.019 |
| PeAF (vs PAF) | 0.25 (0.11-0.55) | <0.001 | 0.75 (0.24-2.30) | 0.617 | 0.68 (0.22-2.10) | 0.504 |
| AF recurrence | 1.98 (0.67-5.91) | 0.219 | ||||
Multivariable analysis to evaluate the association of body size metrics and fat measures, one at a time, with LA reverse remodeling adjusting for variables at P < 0.10 in the univariable analysis.
AF = atrial fibrillation; BF% = body fat percent; BMI = body mass index; BNP = B-type natriuretic peptide; CF% = central fat percent; CRP = C-reactive protein; E = early diastolic transmitral flow velocity; e’ = early diastolic mitral annular velocity; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; ICTP-1 = carboxy-terminal telopeptide of procollagen type I; IL = interleukin; LA = left atrium; LAVI = left atrial volume index; LDL = low-density lipoprotein; LVEDVI = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESVI = left ventricular end-systolic volume index; LVMI = left ventricular mass index; PAF = paroxysmal atrial fibrillation; PeAF = persistent atrial fibrillation; RAAS = renin-angiotensin-aldosterone system; W/H = waist-to-hip.
Central Illustration.
Body Fat Distribution and Left Atrial Reverse Remodeling After Catheter Ablation for Atrial Fibrillation
Representative images of patients with and without LA reverse remodeling (left) and the frequency of lack of LA reverse remodeling stratified by the combination of W/H ratio and CF% (right). CF% = central fat percent; LA = left atrium; W/H = waist-to-hip.
When we applied the different cut-off values proposed by World Health Organization (ie, W/H ratio: ≥0.90 in men and ≥0.85 in women, and BF%: >25% in men or >35% in women), preprocedural LA size was similar between higher and lower W/H ratio groups (38.8 ± 14.6 ml/m2 vs 34.7 ± 11.3 ml/m2, P = 0.296), while patients with higher BF% tended to have a larger LA volume index than those with lower BF% (41.3 ± 16.0 ml/m2 vs 34.8 ± 11.2 ml/m2, P = 0.049). At 6 months after CA, both higher W/H ratio and BF% groups showed relatively larger LA size compared with their counterparts, but it did not reach statistical significance in BF% groups (W/H ratio: 34.2 ± 11.1 ml/m2 vs 29.4 ± 8.7 ml/m2, P = 0.018, and BF%: 34.9 ± 10.8 ml/m2 vs 30.8 ± 10.2 ml/m2, P = 0.060).
Discussion
The present study showed that body size metrics and adiposity measures were not independently associated with preprocedural LA size. On the other hand, a higher W/H ratio and CF% were related to persistent LA enlargement at 6 months after CA, independent of baseline LA size and AF type. Our observations suggest that measurement of body fat distribution may provide valuable information for the LA reverse remodeling after CA in nonobese AF patients, which is an important surrogate marker for ablation outcomes.
Several studies have examined the association between body fat distribution and LA remodeling in the general population.23, 24, 25 Fox et al23 demonstrated that CT-assessed visceral fat was correlated with LA size in the Framingham Heart Study. Bello et al24 also showed a positive relationship between BF% and LA volume in the Atherosclerosis Risk in Communities Study. However, limited data are available regarding the association between body fat distribution and LA remodeling in the setting of AF. The present study showed that a high W/H ratio and BF% were associated with LA enlargement in patients with AF who underwent CA, whereas this relationship was attenuated after multivariable adjustment. This might be explained in part by the fact that we excluded patients with obesity, and other factors, such as age and AF severity, rather than body fat, may have a more pronounced impact on preprocedural LA remodeling.
At 6 months after CA, the higher W/H ratio and CF% groups displayed a significantly lower prevalence of LA reverse remodeling, which is a crucial surrogate marker for AF recurrence.6,7,26 Several possible mechanisms might explain the independent association between higher CF% and a lack of LA reverse remodeling. One possible mechanism is a direct effect of central body fat accumulation on LA remodeling. As compared with peripheral fat, body trunk fat is more likely to secrete proinflammatory and profibrotic adipocytokines, which could lead to adverse LA structural alterations.27,28 Our observation of elevated inflammatory and fibrotic markers in patients with a higher CF% may support this hypothesis. In contrast, peripheral fat depots (ie, leg fat) may serve as a “metabolic sink,” storing excessive free fatty acids and preventing ectopic fat accumulation, and may have a less inflammatory and fibrotic effect on the atrial myocardium than central fat depots.29 Indeed, Oliver et al25 found that visceral fat rather than peripheral body fat was an independent determinant of advanced LA remodeling over an 8-year follow-up period in the general population. In addition, higher CF% partly reflects ectopic fat accumulation, such as in the pericardium/epicardium and liver, which also promotes unfavorable LA structural changes.30,31 Another possible mechanism might be an indirect effect of central adiposity as a consequence of LV remodeling. Adipokines released from visceral fat contribute to LV hypertrophy and LV diastolic dysfunction, which in turn lead to LA remodeling.24,32,33 In fact, Kosmala et al33 identified more reduced LV diastolic function with advanced LA remodeling in individuals with central adiposity free from cardiovascular disease than those without central adiposity. We further demonstrated that the predictive value of unfavorable LA reverse remodeling was significantly improved with the combined CF% and W/H ratio. Our findings suggest the utility of body fat distribution assessment beyond body anthropometrics alone for better risk stratification of patients with persistent LA enlargement after CA and also raise the hypothesis that nonobese patients with AF with coexisting abdominal and trunk fat accumulation might represent a phenotype less susceptible to LA structural reversal.
In the present study, we used sex-specific cut-off values because there were substantial differences in body anthropometrics and adiposity measurements between men and women (ie, a larger W/H ratio and CF% in men and a larger BF% in women). Furthermore, recent studies highlight the impact of epicardial fat and its sex-differences on AF.34, 35, 36 Future studies are warranted to investigate the sex-specific differences in the association of fat distribution with LA structural change and its reversibility in patients with AF.
Clinical implications
We comprehensively evaluated body size metrics and fat profiles of nonobese patients with AF using simple, noninvasive, and cost-effective methods with BIA and revealed that a higher W/H ratio and CF% were associated with unfavorable LA reverse remodeling in response to CA. Given that approximately 40% of nonobese patients with AF experience AF recurrence within a year after AF ablation,12 identifying patients less susceptible to LA reverse remodeling, which is a promising surrogate marker of AF recurrence, is of great importance. A previous experimental study showed that fat reduction could ameliorate atrial fibrosis and inflammation, further lowering AF inducibility.37 Recent clinical studies also revealed that intensive lifestyle intervention and pharmacotherapy with sodium-glucose co-transporter-2 inhibitors had beneficial effects on visceral fat reduction and could promote body fat redistribution.38, 39, 40 These approaches targeting body fat distribution may enhance therapeutic strategies before and after CA in nonobese patients with AF, which should be explored in future studies.
Study Limitations
Several limitations of this study should be noted. First, although we assessed central fat accumulation using BIA, we could not measure individual components of adiposity, including subcutaneous, visceral, and ectopic fat. However, evaluation of individual adiposity phenotypes requires imaging modalities, such as CT or MRI, which are somewhat limited in widespread use among all patients with AF because of their high cost, requirement of radiation exposure, and technical issues. BIA is a more cost-effective and feasible method that has an excellent correlation with these imaging modalities.14, 15, 16 Second, the information on preprocedural AF burden is not uniformly available in the present study, which can affect LA remodeling. Third, our 24-hour Holter monitoring method was less able to detect recurrence of AF than other longer-term monitoring methods (ie, 7-day ambulatory monitoring or implantable loop recorders). Fourth, the impact of underweight (BMI <18.5 kg/m2) on LA remodeling and its reversibility cannot be assessed because only 6 (5.2%) patients were classified as underweight in the present study. Finally, the short-term follow-up period limit concluding the association of body size metrics and fat measurements with LA reverse remodeling. Future studies with longer follow-up are needed to confirm our observations.
Conclusions
In nonobese patients with AF, W/H ratio and BIA-assessed central fat adiposity (CF%) were independently associated with a lack of LA reverse remodeling after CA. Future studies are required to investigate whether therapeutic interventions for trunk fat accumulation might improve the reversibility of LA remodeling after CA and possibly reduce the risk of AF recurrence.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: In nonobese AF patients, none of the body size metrics and adiposity measures had an independent relationship with preprocedural LA size. On the other hand, larger CF% and W/H ratio carried a significant risk for lack of LA reverse remodeling, independent of baseline LA size and AF type. The combined assessment of CF% with W/H ratio aided in better risk stratification for persistent LA enlargement.
TRANSLATIONAL OUTLOOK: Future studies are warranted to investigate whether therapeutic interventions on trunk fat accumulation may enhance LA reverse remodeling and reduce AF recurrence in nonobese patients with AF.
Funding support and author disclosures
This work was supported in part by Kaken 22K12859. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors wish to thank Jumpei Ishiwata, MD, and Naoko Sawada, MD, for their general support.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Supplementary data
References
- 1.Roth G.A., Mensah G.A., Johnson C.O., et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung M.K., Refaat M., Shen W.K., et al. Atrial fibrillation: JACC council perspectives. J Am Coll Cardiol. 2020;75:1689–1713. doi: 10.1016/j.jacc.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Marrouche N.F., Brachmann J., Andresen D., et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 4.Eckardt L., Sehner S., Suling A., et al. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST-AFNET 4 trial. Eur Heart J. 2022;43:4127–4144. doi: 10.1093/eurheartj/ehac471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reant P., Lafitte S., Jais P., et al. Reverse remodeling of the left cardiac chambers after catheter ablation after 1 year in a series of patients with isolated atrial fibrillation. Circulation. 2005;112:2896–2903. doi: 10.1161/CIRCULATIONAHA.104.523928. [DOI] [PubMed] [Google Scholar]
- 6.Tops L.F., Delgado V., Bertini M., et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2011;57:324–331. doi: 10.1016/j.jacc.2010.05.063. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi K., Fukuda S., Yamashita H., et al. Pre-procedural serum atrial natriuretic peptide levels predict left atrial reverse remodeling after catheter ablation in patients with atrial fibrillation. J Am Coll Cardiol EP. 2016;2:151–158. doi: 10.1016/j.jacep.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Soulat-Dufour L., Lang S., Addetia K., et al. Restoring sinus rhythm reverses cardiac remodeling and reduces valvular regurgitation in patients with atrial fibrillation. J Am Coll Cardiol. 2022;79:951–961. doi: 10.1016/j.jacc.2021.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Murphy N.F., MacIntyre K., Stewart S., Hart C.L., Hole D., McMurray J.J. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15 000 middle-aged men and women (the Renfrew-Paisley study) Eur Heart J. 2006;27:96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 10.Baek Y.S., Yang P.S., Kim T.H., et al. Associations of abdominal obesity and new-onset atrial fibrillation in the general population. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abed H.S., Samuel C.S., Lau D.H., et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. doi: 10.1016/j.hrthm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 12.Tonnesen J., Pallisgaard J., Ruwald M.H., et al. Short- and long-term risk of atrial fibrillation recurrence after first time ablation according to body mass index: a nationwide Danish cohort study. Europace. 2023;25:425–432. doi: 10.1093/europace/euac225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivasambu B., Balouch M.A., Zghaib T., et al. Increased rates of atrial fibrillation recurrence following pulmonary vein isolation in overweight and obese patients. J Cardiovasc Electrophysiol. 2018;29:239–245. doi: 10.1111/jce.13388. [DOI] [PubMed] [Google Scholar]
- 14.Boneva-Asiova Z., Boyanov M.A. Body composition analysis by leg-to-leg bioelectrical impedance and dual-energy X-ray absorptiometry in non-obese and obese individuals. Diabetes Obes Metabol. 2008;10:1012–1018. doi: 10.1111/j.1463-1326.2008.00851.x. [DOI] [PubMed] [Google Scholar]
- 15.Browning L.M., Mugridge O., Chatfield M.D., et al. Validity of a new abdominal bioelectrical impedance device to measure abdominal and visceral fat: comparison with MRI. Obesity. 2010;18:2385–2391. doi: 10.1038/oby.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee D.H., Park K.S., Ahn S., et al. Comparison of abdominal visceral adipose tissue area measured by computed tomography with that estimated by bioelectrical impedance analysis method in Korean subjects. Nutrients. 2015;7:10513–10524. doi: 10.3390/nu7125548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calkins H., Hindricks G., Cappato R., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace. 2018;20:157–208. doi: 10.1093/europace/eux275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Nagueh S.F., Smiseth O.A., Appleton C.P., et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Gianni C., Mohanty S., Di Biase L., et al. Acute and early outcomes of focal impulse and rotor modulation (FIRM)-guided rotors-only ablation in patients with nonparoxysmal atrial fibrillation. Heart Rhythm. 2016;13:830–835. doi: 10.1016/j.hrthm.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 21.Hanazawa K., Kaitani K., Hayama Y., et al. Effect of radiofrequency catheter ablation of persistent atrial fibrillation on the left atrial function: assessment by 320-row multislice computed tomography. Int J Cardiol. 2015;179:449–454. doi: 10.1016/j.ijcard.2014.11.103. [DOI] [PubMed] [Google Scholar]
- 22.Li Y.G., Gong C.Q., Zhao M.Z., et al. Determinants of postoperative left atrial structural reverse remodeling in patients undergoing combined catheter ablation of atrial fibrillation and left atrial appendage closure procedure. J Cardiovasc Electrophysiol. 2019;30:1868–1876. doi: 10.1111/jce.14094. [DOI] [PubMed] [Google Scholar]
- 23.Fox C.S., Gona P., Hoffmann U., et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–1591. doi: 10.1161/CIRCULATIONAHA.108.828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bello N.A., Cheng S., Claggett B., et al. Association of weight and body composition on cardiac structure and function in the ARIC study (Atherosclerosis Risk in Communities) Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliver W., Matthews G., Ayers C.R., et al. Factors associated with left atrial remodeling in the general population. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas L., Abhayaratna W.P. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. J Am Coll Cardiol Img. 2017;10:65–77. doi: 10.1016/j.jcmg.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Venteclef N., Guglielmi V., Balse E., et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J. 2015;36:795–805. doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 28.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol. 2018;71:2360–2372. doi: 10.1016/j.jacc.2018.03.509. [DOI] [PubMed] [Google Scholar]
- 29.Mikami T., Furuhashi M., Sakai A., et al. Antiatherosclerotic phenotype of perivascular adipose tissue surrounding the saphenous vein in coronary artery bypass grafting. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iacobellis G., Leonetti F., Singh N., A M.S. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2007;115:272–273. doi: 10.1016/j.ijcard.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 31.VanWagner L.B., Wilcox J.E., Ning H., et al. Longitudinal association of non-alcoholic fatty liver disease with changes in myocardial structure and function: the CARDIA study. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell-Wiley T.M., Poirier P., Burke L.E., et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosmala W., Jedrzejuk D., Derzhko R., Przewlocka-Kosmala M., Mysiak A., Bednarek-Tupikowska G. Left ventricular function impairment in patients with normal-weight obesity: contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circ Cardiovasc Imaging. 2012;5:349–356. doi: 10.1161/CIRCIMAGING.111.969956. [DOI] [PubMed] [Google Scholar]
- 34.Kim J.S., Shin S.Y., Kang J.H., et al. Influence of sex on the association between epicardial adipose tissue and left atrial transport function in patients with atrial fibrillation: a multislice computed tomography study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciuffo L., Nguyen H., Marques M.D., et al. Periatrial fat quality predicts atrial fibrillation ablation outcome. Circ Cardiovasc Imaging. 2019;12 doi: 10.1161/CIRCIMAGING.118.008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sang C., Hu X., Zhang D., et al. The predictive value of left atrium epicardial adipose tissue on recurrence after catheter ablation in patients with different types of atrial fibrillation. Int J Cardiol. 2023;379:33–39. doi: 10.1016/j.ijcard.2023.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Mahajan R., Lau D.H., Brooks A.G., et al. Atrial fibrillation and obesity: reverse remodeling of atrial substrate with weight reduction. J Am Coll Cardiol EP. 2021;7:630–641. doi: 10.1016/j.jacep.2020.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Gepner Y., Shelef I., Schwarzfuchs D., et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: CENTRAL magnetic resonance imaging randomized controlled trial. Circulation. 2018;137:1143–1157. doi: 10.1161/CIRCULATIONAHA.117.030501. [DOI] [PubMed] [Google Scholar]
- 39.Ito D., Shimizu S., Inoue K., et al. Comparison of ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: a randomized, 24-week, open-label, active-controlled trial. Diabetes Care. 2017;40:1364–1372. doi: 10.2337/dc17-0518. [DOI] [PubMed] [Google Scholar]
- 40.Sato T., Aizawa Y., Yuasa S., et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17:6. doi: 10.1186/s12933-017-0658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.