Abstract
Cardiology shock is a syndrome of low cardiac output resulting in end-organ dysfunction. Few interventions have demonstrated meaningful clinical benefit, and cardiogenic shock continues to carry significant morbidity with mortality rates that have plateaued at upwards of 40% over the past decade. Clinicians must rely on clinical, biochemical, and hemodynamic parameters to guide resuscitation. Several features, including physical examination, renal function, serum lactate metabolism, venous oxygen saturation, and hemodynamic markers of right ventricular function, may be useful both as prognostic markers and to guide therapy. This article aims to review these targets, their utility in the care of patients with cardiology shock, and their association with outcomes.
Key words: cardiogenic shock, lactate, mean arterial pressure, resuscitation, target
Central Illustration
Highlights
-
•
CS carries marked morbidity and mortality, with limited data to guide hemodynamic targets.
-
•
Incorporation of physical exam findings, biochemistry, and invasive hemodynamics best supports adequate end-organ perfusion.
-
•
Randomized clinical trials in CS subsets are necessary to better elucidate optimal perfusion targets.
Clinically assessing systemic tissue perfusion in patients with cardiogenic shock (CS) is critical in both the initial assessment and subsequent resuscitation of patients. However, despite the availability of numerous potential markers, the optimal approach is uncertain. Evidence in CS is limited, but practice may be informed by experience as well as literature in general critical care medicine. In this state-of-the-art review article, we review such available clinical, biochemical, and hemodynamic markers of systemic tissue perfusion with a view toward optimal selection and application in patients with CS. The review highlights the need for more dedicated studies of these markers, and a better understanding of their role as potential resuscitation and decision-making targets, in the management of patients with CS.
Epidemiology of CS
Cardiogenic shock (CS) is a state of low cardiac output resulting in clinical and biochemical manifestations of end-organ hypoperfusion. The most common cause of CS is acute myocardial infarction (AMI), but non–AMI-associated causes include acute decompensation of chronic heart failure, severe valvular disease, arrhythmias, and myocarditis. Following the landmark SHOCK (SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK) trial demonstrating improved survival with urgent revascularization of culprit arteries in AMI-CS,1 mortality rates have remained essentially unchanged over the last 2 decades at upwards of 40%.
Evidence to date and contemporary guidelines on treatment
Three landmark studies have examined vasoactive therapy in CS—in the SOAP (Sepsis Occurrence in the Acutely Ill Patients) II trial, De Backer et al2 examined a subgroup of 280 patients with CS and found that compared to norepinephrine, dopamine was associated with increased arrhythmias and an increased rate of death at 28 days. However, the authors included postcardiotomy, obstructive and valvular shock states, each with unique hemodynamic profiles, failed to explore differing treatment effects across these subgroups, and did not report important AMI and heart failure demographics. More recently, the OptimaCC (Epinephrine vs Norepinephrine for Cardiogenic Shock after Acute Myocardial Infarction) trial examined the safety and efficacy of both agents among 57 patients with AMI-CS and found that patients treated with epinephrine had higher rates of refractory shock and lactic acidosis.3 While the trial is limited by a small sample size and short follow-up duration in an isolated AMI-CS cohort, these findings have tempered the use of epinephrine in CS. Finally, the TRIUMPH (Effect of Tilarginine Acetate in Patients with Acute Myocardial Infarction and Cardiogenic Shock) trial explored the effect of tilarginine acetate, a nitric oxide synthase inhibitor, in a cohort of AMI patients with refractory CS following revascularization—the authors found no difference in 30-day and 6-month all-cause mortality, nor in shock duration or resolution.4 Consequently, norepinephrine has emerged as the first-line vasopressor in CS, but ultimately, selection of vasoactive agents should be individualized to each patient’s shock phenotype. In chronic heart failure, inotrope use has been associated with multiple adverse effects including longer intensive care unit (ICU) and in-hospital length of stay and increased in-hospital mortality.5,6 While vasopressors and inotropes are used to treat end-organ hypoperfusion, potential adverse effects include tachyarrhythmias, increased afterload, myocardial ischemia, and direct myocyte toxicity, which may further compromise an already struggling heart. While mechanical circulatory support (MCS) devices, such as percutaneous left ventricular (LV) assist devices and extracorporeal membrane oxygenation, are being increasingly used in both cases of AMI- and non-AMI-CS, their efficacy has yet to be definitively demonstrated. Further randomized controlled trials are needed to delineate their impact on patient-important outcomes and to better tailor their use.
Current guidelines focus on the treatment of the inciting event and supportive care to restore end-organ perfusion.7,8 Supportive care includes initiation and titration of vasopressors and inotropes to achieve and maintain hemodynamics, usually to a target a mean arterial pressure (MAP) of ≥65 mm Hg. However, the American College of Cardiology/American Heart Association Scientific Statement highlights that no clear blood pressure or MAP recommendations can be made due to limited data, a sentiment echoed in a recent update on the management of CS complicated by AMI (Table 1). Rather, focus is placed on assessing adequacy of serial markers of systemic perfusion, including lactate, venous oxygen saturation, urine output, creatinine, liver function tests, mental status, temperature, and invasive hemodynamic parameters, and individualizing targets accordingly. International guidelines acknowledge that there are little data to guide hemodynamic targets in CS and have leaned on expert opinion, observational and retrospective studies of CS patients along with extrapolation from studies in predominantly vasodilatory shock states to bolster recommendations. Among the most important questions that have yet to be definitively answered are the optimal hemodynamic and perfusion targets in CS. This article aims to review clinical, biochemical, and hemodynamic targets that may be used to guide therapy in CS.
Table 1.
Perfusion Targets From Highlighted Guidelines and Consensus Statements
| Perfusion Targets | 2017 ACC/AHA Scientific Statement on CS8 | 2022 AHA/ACC Guideline for the Management of Heart Failurea | Management of CS Complicated MI: An Update 20197 | 2021 ESC Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failureb |
|---|---|---|---|---|
| Hemodynamic targets | No clear sBP or MAP recommendations | No clear sBP or MAP recommendations | No clear sBP or MAP recommendations. Suggest that MAP >65 mmHg probably not required | No clear sBP or MAP recommendations. In AHF with sBP >110 mmHg, IV vasodilators may be considered as initial therapy to improve symptoms and reduce congestion (Class IIb) |
| Physical exam targets | Use cold/warm and wet/dry descriptors to highlight hemodynamic phenotypes. Longitudinal CVP trends may provide information on trends in fluid status | Severity of congestion and adequacy of perfusion should be assessed to guide triage and initial therapy (Class I) | Not specified | Use wet/dry and warm/cold, as well as mental confusion, dizziness, and narrow pulse pressure. Emphasize that hypoperfusion is not always accompanied by hypotension |
| Renal targets | Suggest serial monitoring of urine output and creatinine. Include KDIGO guidelines that CRRT be considered when “life-threatening changes in fluid, electrolyte, and acid-base balance” exist | Not specified | Suggest serial monitoring of urine output and creatinine RRT initiated with AKI and uremia, refractory volume overload, metabolic acidosis, and/or refractory hyperkalemia (Class IIb) | Suggest serial monitoring of urine output and creatinine |
| Lactate targets | Suggest serial monitoring of arterial lactate q1-4 h | Not specified | Not specified | Suggest serial monitoring and when peripheral hypoperfusion is suspected |
| Additional variables for serial monitoring | Suggest using serial perfusion markers including SvO2 or ScvO2 LFTs, mental status, and other invasive hemodynamic variables | Not specified | Not specified | NT-pro-BNP recommended at admission, predischarge |
| Vasoactive agent selection | Norepinephrine may be vasopressor of choice as associated with fewer arrhythmias Note that optimal first-line vasoactive medication in CS remains unclear Provides pragmatic considerations based on etiology and phenotype of shock |
In patients with CS, intravenous inotrope support should be used to maintain systemic perfusion and preserve end-organ performance (Class I) Choice of inotrope guided by blood pressure, concurrent arrhythmias, and availability |
Norepinephrine is vasoconstrictor of choice when low BP and insufficient tissue perfusion pressure (Class IIb) Inotropes (ie, dobutamine) may be given simultaneously to norepinephrine to improve cardiac contractility (Class IIb) |
Consider inotropes and/or vasopressors for sBP <90 mmHg and hypoperfusion who do not respond to standard treatment, including fluid challenge to improve peripheral perfusion and maintain end-organ function (Class IIb) Inotropic agents not recommended routinely, due to safety concerns, unless patient has symptomatic hypotension and evidence of hypoperfusion (Class III) Vasopressor therapy, preferably norepinephrine, may be considered in patients with CS to increase BP and vital organ perfusion (Class IIb) Consider RRT for persistent hypoperfusion and organ dysfunction (Class IIa) |
ACC = American College of Cardiology; AHA = American Heart Association; AHF = acute heart failure; AKI = acute kidney injury; BP = blood pressure; CRRT = continuous renal replacement therapy; CS = cardiogenic shock; CVP = central venous pressure; ESC = European Society of Cardiology; IV = intravenous; KDIGO = Kidney Disease: Improving Global Outcomes; LFT = liver function test; MAP = mean arterial pressure; MI = myocardial infarction; NT-pro-BNP = N-terminal pro–B-type natriuretic peptide; RRT = renal replacement therapy; sBP = systolic blood pressure; ScvO2 = central venous oxygen saturation; SvO2 = venous oxygen saturation.
Heindenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263-e421.
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599-3726.
The evolution of classification systems for CS
Numerous attempts to classify critically ill cardiac patients have been made over the last several decades. Killip and Kimball’s landmark analysis stratified 250 patients with possible AMI based on core physical exam findings, with mortality rates ranging from 6% to 81%.9 Their work has been validated in a more contemporary cohort, and while mortality rates were markedly reduced, successive stages of heart failure still correlated with increased mortality.10 The Global Registry of Acute Coronary Events (GRACE) score provides discriminatory power for short- and long-term mortality in AMI-CS, but the lack of validation in a non-AMI-CS cohort limits its use in the heterogeneous CS population.11 Other predictive models have been drawn from the general ICU population, including the Acute Physiology and Chronic Health Evaluation (APACHE-3) score and Simplified Acute Physiology Score (SAPS II) systems,12,13 but have limited utility in cardiac patients and suboptimal discrimination power. Two of the most widely used mortality prediction models for CS are the CardShock score and the Intra-aortic Balloon Support for Myocardial Infarction with Cardiogenic Shock (IABP-SHOCK II) score. The CardShock risk score was derived from a mixed population of both AMI and non–AMI-associated CS, whereas the IABP-SHOCK II score was derived from a cohort of AMI-associated CS who all underwent percutaneous coronary intervention. Despite good discrimination in short-term mortality, both require multiple variables, some of which are subjective and related to pre-existing comorbidities, which may not be available at the time of presentation.14,15 Comparison of these scores in a real-world CS cohort has demonstrated modest prognostic accuracy for in-hospital mortality,16 but further research is needed to refine their discriminative capability through inclusion of additional variables and/or identification of novel markers. Finally, there is significant phenotypic variation in CS, often driven by etiology, pathologic mechanisms, hemodynamics, and severity of hypoperfusion. Research is rapidly underway to categorize clinical and biochemical phenotypes of CS. Zweck et al17 have identified 3 clinical phenotypes of CS: noncongested, cardiorenal, and cardiometabolic. While the authors highlight demographic and clinical differences, they emphasize hemodynamic distinctions. Noncongested patients had lower right atrial pressure (RAP) and pulmonary capillary wedge pressure (PCWP) with a higher arterial blood pressure than cardiorenal and cardiometabolic patients. Accordingly, cardiorenal patients had a lower estimated glomerular filtration rate (eGFR) vs cardiometabolic patients who had a higher RAP, along with lower blood pressure, cardiac power output (CPO), and cardiac index (CI).17 These classifications may help characterize distinct subsets of patients and deepen the understanding of how to customize therapeutic interventions.
In 2019, the Society for Cardiovascular Angiography and Interventions (SCAI) published a classification scheme for patients with CS (Table 2).18,19 The 5 stages of shock range from A, those patients at risk of developing CS, to E, denoting those in extremis, using physical exam findings, biochemical markers, and hemodynamic values. The differentiating feature between SCAI classes A-B and C-E is the presence of hypoperfusion, defined by clinical signs such as cool skin, mottled extremities, poor urine output, and mental confusion, as well as biochemical abnormalities, including elevated lactate, renal insufficiency, and increased liver function tests. The most recent 2022 SCAI classification update highlights its impressive prognostic discriminatory power, with progressive SCAI shock stage associated with increased in-hospital mortality consistent across multiple cohorts of patients, including AMI-CS, acute decompensated heart failure, heterogeneous cardiac intensive care unit populations, and those with out-of-hospital cardiac arrest. The authors also highlight additional risk factors that improve mortality risk stratification beyond SCAI shock stage in isolation, including RAP, worsening shock stage over time, and late deterioration—these variables allow for further subgroup discrimination and more nuanced appreciation of a patient’s mortality risk. Finally, the authors presented a novel 3-axis model of mortality predictors, focusing on severity of shock, risk modifiers, and features of hemodynamic phenotype and clinical presentation. This model emphasizes the need to assess the global clinical picture of individual patients, including these high-risk features and nonmodifiable risk factors that portend a poorer prognosis.20 For researchers, the SCAI classification clearly defines CS and is particularly helpful in interpretation of the literature. It allows identification of those studies that truly included a population defined by organ malperfusion. In a recent network meta-analysis, some trials in CS focused primarily on a SCAI A/B population, and while the results are helpful, they need to be interpreted in the context of less severe shock states.21, 22, 23 A more pragmatic application of the SCAI staging criteria was recently applied to a cohort of the Critical Care Cardiology Trials Network registry, focusing on those variables easily attained from clinical trials and registries—the authors reported strong discriminatory power regarding in-hospital mortality, beyond that provided by SOFA (Sequential Organ Failure Assessment) and IABP-SHOCK II scores.24 Finally, the SCAI classification system also has important value for clinicians. With close monitoring of perfusion targets, and progression or improvement through the SCAI classes, clinicians can identify response to therapy, escalate therapy as needed, and appropriately engage patients and families in discussions related to prognosis.
Table 2.
SCAI Classification: Summary of Stages by Clinical, Biochemical, and Hemodynamic Criteria
| Stage | Description | Clinical | Biochemical | Hemodynamic | Short-Term Mortality (%) |
|---|---|---|---|---|---|
| A. “At risk” | No signs or symptoms of CS | Warm, well-perfused, normal JVP, clear lungs, and mentation | Normal lactic acid, renal function | Normotensive CI ≥2.5; CVP <10; PA saturation ≥65% |
0-3.6 |
| B. “Beginning” | Evidence of relative hypotension or tachycardia, without hypoperfusion | Warm, well-perfused, elevated JVP, rales in lungs | Minimal renal function impairment, normal lactate | sBP <90 or MAP <60 or >30 mm Hg drop from baseline HR >100 beats/min CI ≥2.2 and PA saturation ≥65% |
0-33.9 |
| C. “Classic” | Hypoperfusion requiring intervention beyond volume resuscitation to restore perfusion | Unwell, ashen, mottled extremities, cold, clammy, volume overloaded | Any of lactate ≥2; creatinine doubling or >50% drop in eGFR; increased LFTs; elevated BNP | Any of sBP <90 or MAP <60 or >30 mm Hg drop from baseline and drugs/devices used to maintain BP CI <2.2; PCWP >15; RAP/PCWP ≥0.8; PAPi <1.85; CPO ≥0.6 |
12.4-53.9 |
| D. “Deteriorating” | Similar to C, but worse, fails to respond to initial interventions | Any of stage C above | Any of stage C above, and deteriorating | Any of stage C above, and requiring multiple vasopressors or addition of MCS to maintain perfusion | 24.0-66.9 |
| E. “Extremis” | Cardiac arrest with ongoing CPR and/or ECMO, supported by multiple interventions | Cardiac collapse, use of defibrillator, near pulselessness, need for mechanical ventilation | CPR pH ≤7.2, lactate = 5 | No sBP without resuscitation PEA or refractory VT/VF Hypotension despite maximal support |
42.0-77.4 |
BNP = brain natriuretic peptide; BP = blood pressure; CI = cardiac index; CPO = cardiac power output; CPR = cardiopulmonary resuscitation; CS = cardiogenic shock; CVP = central venous pressure; ECMO = extracorporeal membrane oxygenation; eGFR = estimated glomerular filtration rate; HR = heart rate; JVP = jugular venous pressure; LFT = liver function test; MAP = mean arterial pressure; MCS = mechanical circulatory support; PA = pulmonary artery; PAPi = pulmonary artery pulsatility index; PCWP = pulmonary capillary wedge pressure; PEA = pulseless electrical activity; RAP = right atrial pressure; sBP = systolic blood pressure; SCAI = Society for Cardiovascular Angiography and Interventions; VF = ventricular fibrillation; VT = ventricular tachycardia.
SCAI classification and associated in-hospital mortality from Naidu et al.20
Hemodynamic and perfusion targets in CS
Clinicians use multiple variables to select therapies and guide resuscitative strategies in CS. Historically, the classic clinical signs or “windows of perfusion”—mentation, skin quality, and urine output—have served as targets. The introduction of the Swan-Ganz catheter, or pulmonary artery catheter (PAC), in 1970 advanced the care of patients with shock, transporting hemodynamics from the catheterization lab to the bedside. Recent data suggest a possible reduction in in-hospital mortality with the use of PAC values in both AMI- and non-AMI-CS. Hemodynamic data obtained from a PAC may allow for earlier recognition of shock subtype, identification of biventricular dysfunction and better selection of the most-appropriate MCS device, and feedback of hemodynamic response to drug titration.25 While the utility and efficacy of right heart catheterization in the resuscitation and management of CS is beyond the scope of this article, it is important to note that hemodynamic-guided resuscitation, while potentially impactful, has not yet been demonstrated to improve morbidity or mortality in prospective clinical trials.
Physical examination
Despite advances in biomarkers and invasive hemodynamics, the physical examination remains the first indicator of severity of shock. Two components of the bedside assessment are particularly helpful: markers of congestion or elevated filling pressures, and markers of perfusion or cardiac output. Unfortunately, the majority of data to date lie in the decompensated heart failure population, and therefore, caution should be exercised in extrapolating the existing literature. Randomized data have failed to show benefit of invasive hemodynamic monitoring by PAC in addition to clinical assessment alone in the management of patients with New York Heart Association class 4 heart failure. However, a significant proportion of these patients had evidence of congestion with PCWP of >22 mm Hg (64%) and CI of <2.3 L/min/m2 (73%).26 A prespecified secondary analysis comparing history and physical exam findings to hemodynamics found that estimates of RAP from the jugular venous pressure (JVP) correlated with invasive measurements, including PCWP, arguing that JVP can be used to accurately estimate left-sided filling pressures. The RAP and PCWP were also associated with survival at 6 months following hospitalization, even after adjustment for other prognostic variables. Finally, from a perfusion perspective, a global assessment of “cold” was associated with reduced CI, and this classification was associated with a markedly increased risk of death or rehospitalization in a population of patients with severe heart failure.27 Similar phenotype profiles have been described in cohorts of patients with CS,8,20 and the focused physical exam should include those signs that identify both congestion and hypoperfusion, namely normal mentation, JVP elevation, presence and extent of rales in the lungs, and/or mottled extremities. More recently, Thayer et al28 examined 1,414 patients with CS and found that elevated biventricular filling pressures, identified by PCWP ≥18 mm Hg and RAP ≥12 mm Hg, were a significant predictor of in-hospital mortality compared to isolated left-sided congestion or no congestion; furthermore, right-sided congestion was associated with higher SCAI shock stage and greater in-hospital mortality.
Moving beyond specific physical examination findings, the original Forrester classification from 1976 identified 4 hemodynamic profiles of patients following AMI, based on the presence or absence of congestion and presence or absence of adequate perfusion: warm-dry (no congestion or hypoperfusion); wet-warm (congestion but no hypoperfusion); dry-cold (no congestion but hypoperfusion); and wet-cold (congestion and hypoperfusion).29 These profiles correlate with short-term mortality, with higher mortality noted with congestion and further increased with the added insult of hypoperfusion. Stevenson et al applied these hemodynamic profiles to a cohort of 452 patients with advanced heart failure and hypothesized about the utility of titrating therapy in hemodynamic profile—focusing on diuresis on the warm-wet patient—vs accepting the risks of inotrope therapy in the cold-wet patient who needs both augmented diuresis and perhaps, inotropic support.30 For the clinician at the bedside, the findings of an elevated JVP, pulmonary congestion, prolonged capillary refill time, and “cold extremities” may be particularly useful in the initial assessment of the patient with CS. It is difficult to identify clear therapeutic targets in the physical exam, but given the prognostic value of both signs of congestion and hypoperfusion, one could reasonably titrate therapy to target clinical euvolemia and a peripheral exam suggestive of warm, well-perfused extremities. Ultimately, the physical examination in isolation can provide important prognostic information and should be integrated into the initial assessment and management of these patients.
Urine output and renal markers
Acute kidney injury (AKI) is defined by an abrupt decrease in kidney structure and/or function and is seen in 15% to 55% of patients with CS.31,32 Diagnosis is most often made based on the Kidney Disease: Improving Global Outcomes criteria, which include rises in serum creatinine and reductions in eGFR and urine output.31 The eGFR can be difficult to interpret when the plasma creatinine is changing, as it may be in states of shock, and so the concept of kinetic eGFR has gained momentum, as it gives us the power to estimate kidney function in a dynamic and rapidly changing clinical state.33 The relationship between the heart and kidneys is complex, with Ronco et al34 first proposing the 5-part classification of cardiorenal syndrome: type 1 refers to abrupt worsening of cardiac function producing renal injury; type 2 refers to chronic heart failure progressively eroding renal function and causing chronic kidney disease; type 3 is sudden worsening of renal function leading to acute cardiac decompensation; type 4 refers to chronic kidney disease leading to decreased cardiac function/increased risk of cardiovascular events; and type 5 refers to systemic conditions that result in both cardiac and renal dysfunction. AKI among patients with CS is usually due to a type 1 cardiorenal syndrome, a consequence of decreased cardiac output and renal perfusion, with subsequent oliguria.34 AKI may also be mediated by venous congestion, as central venous pressure is a reliable predictor for AKI in CS.35 Several compensatory mechanisms in CS can further worsen renal function: an elevated sympathetic tone can produce severe systemic vasoconstriction, ultimately overcoming renal autoregulation, and activation of the renin-angiotensin-aldosterone system produces sodium and water retention, further increasing cardiac afterload. The development of AKI in CS is associated with poor outcomes, including need for long-term dialysis, prolonged hospitalization, and both short- and long-term mortality.36,37 In fact, the retrospective review of 118 patients with CS by Koreny et al37 found that one-third of patients developed AKI in the first 24 hours of CS, conferring a significant mortality risk of 87% compared to 53% in those patients who did not develop AKI. Beyond strategies to protect the kidneys from further insults during CS, there are limited therapeutic interventions, and it is still unknown if the use of renal replacement therapy (RRT) improves outcomes in patients, and if so, what the optimal timing and strategy is in CS. It is evident from the literature that the need for RRT for CS-associated AKI is associated with a marked increased risk of in-hospital mortality, need for temporary MCS, and bleeding requiring blood transfusion. Further risks from dialysis catheters themselves must also be considered and include vascular complications, acquired infections, catheter-associated thrombus formation, and central venous stenosis, which may impact long-term venous access.36 The STARRT-AKI (Standard vs Accelerated Initiation of Renal Replacement Therapy) trial demonstrated no mortality reduction in the accelerated use of RRT in critically ill patients; the authors found that early dialysis increased the risk of long-term dialysis dependence, evoking concern for dialysis-induced kidney injury.38 As only a small proportion of patients ultimately died from refractory CS (approximately 5% of total deaths), these findings support the need for dedicated large clinical trials examining RRT timing and strategies in CS. RRT can be considered in cases of refractory volume overload, marked disruption in acid-base homeostasis, or electrolyte abnormalities, in line with the Kidney Disease: Improving Global Outcomes guidelines.31 The goal with continuous RRT is to reverse life-threatening biochemical abnormalities and support end-organ function while awaiting evidence of renal recovery. There are no specific creatinine or eGFR thresholds yet established, but decisions around RRT initiation should be tailored to individual patients. Finally, while several novel biomarkers have been studied, the prognostic value of serum creatinine on short- and long-term mortality in CS remains greater than that of cystatin C, kidney injury molecule 1, and neutrophil gelatinase-associated lipocalin.39
Mean arterial pressure
The MAP is defined as the average blood pressure during a single cardiac cycle. It is equated to the “perfusion pressure” of the organs and, accordingly, has been upheld as one of the crucial “targets” of shock resuscitation. Of particular importance is the contribution of diastolic blood pressure, which is a critical variable in establishing adequate coronary perfusion pressure (diastolic blood pressure-LV end-diastolic pressure [LVEDP]), especially for the ischemic myocardium. The vast majority of the evidence for MAP targets in shock is taken from predominantly vasoplegic, septic, or hemorrhagic shock. The classic target MAP of ≥65 mm Hg was first derived from observational studies of patients with septic shock, comparing outcomes with time above and below varying MAP thresholds in the first 24 to 48 hours of resuscitation.40,41 The optimal MAP in septic shock represents a moving target, with tremendous evolution over time. The SEPSISPAM (High vs Low Blood-Pressure Target in Patients with Septic Shock) study randomized septic shock patients to a target MAP of 80 to 85 mm Hg vs 65 to 70 mm Hg and found no significant difference in 28-day mortality. Among patients with chronic hypertension, those in the higher target group had lower requirement for new RRT but greater rates of new atrial fibrillation, highlighting the potential need for tailored therapeutic targets.42 The more recent 65-Trial randomized patients older than 65 years with vasodilatory shock to permissive hypotension, with an MAP target of 60 to 65 mm Hg or usual care at the discretion of the treating physician. Patients in the permissive hypotension group had significantly lower exposure to vasopressors and lower total vasopressor dose, with no difference in 90-day mortality. Contrary to SEPSISPAM, patients with a history of chronic hypertension randomized to the permissive hypotension group did not have higher rates of RRT requirements.43 The most recent Society of Critical Care Medicine Surviving Sepsis guidelines support an MAP goal of 65 mm Hg over higher targets—a revision from previous recommendations.44 MAP targets have been studied in other critically ill populations, including trauma patients, traumatic brain injury patients, and those with spinal cord injuries.45, 46, 47 A systematic review and meta-analysis of RCTs examining hemodynamic targets in adult trauma patients with hemorrhagic shock found that targeting a systolic blood pressure (sBP) of 50 to 70 mm Hg or MAP of ≥50 mm Hg may confer a survival benefit over conventional resuscitation targets of sBP of 65 to 100 mm Hg or MAP ≥65 mm Hg. Patients resuscitated with conservative MAP targets received fewer blood products and had lower estimated blood losses.48 Both the septic shock and hemorrhagic shock literature weigh in favor of more conservative MAP targets; however, ultimately, MAP may be best targeted on a customized basis, as evidenced by a trial in postoperative patients showing reduced organ dysfunction with a target sBP based on the baseline sBP compared to a standard treatment strategy of treating sBP <80 mm Hg or lower than 40% of baseline resting value.49 Customization in MAP targets should also be considered in those patients who have evidence of ongoing organ hypoperfusion despite achieving the initial hemodynamic target.
Although the literature in CS is much less robust, current guidelines similarly reflect a more conservative MAP target at ≥65 mm Hg. Within the CS cohort, the MAP target of 65 mm Hg is weakly supported by a single study by Burstein et al50 retrospectively reviewing 1,001 patients with a diagnosis of CS at the time of admission to a cardiac ICU; the authors found that the average MAP over the first 24 hours was inversely associated with ICU and in-hospital mortality, even after adjustment for SCAI classification. As expected, noncardiovascular organ failure and severe AKI were higher among those patients with lower MAP, suggesting that lower MAP may simply be an indicator of more severe illness. The authors make an interesting observation regarding patients with CS secondary to decompensated heart failure, who seemed to have better clinical outcomes with an MAP above 70 mm Hg.50 This highlights the importance of CS phenotypes and whether or how therapy should be titrated to different subsets of CS patients. In a substudy of the CAPITAL DOREMI (Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock) trial, there were higher event rates of the composite primary outcome in the lower MAP (average MAP < 70 mm Hg over the first 36 hours) group than those in the higher MAP group and increased all-cause mortality. As expected, the vasoactive inotrope score and serum lactate levels were higher in the low-MAP group.51,52 Whether augmentation of MAP in CS truly results in clinically important benefit or if a lower MAP is a marker of poor prognosis remains unclear. However, macrocirculation does not always correlate with microcirculation, namely tissue perfusion. Commonly used macrocirculatory parameters include MAP, central venous pressure, and mixed venous oxygen saturation (SvO2), but microcirculatory dysfunction can persistent even with normalization of these values. Microcirculation can be assessed in several ways including near-infrared spectroscopy (NIRS), total small vessel density, and videomicroscopy, but techniques are limited to specific anatomic areas (most often the sublingual region) and require specialized equipment at bedside and time-consuming data interpretation. Accordingly, surrogates of the microcirculation more easily available to clinicians include serum lactate levels, arterial-venous carbon dioxide (CO2)-gap, and capillary refill time.53 An observational study in 30 patients with AMI-CS compared hemodynamic measurements at baseline and after titration of vasopressor and inotrope agents to achieve a CI of ≥2.5 L/min/m2 or mixed venous oxygen saturation ≥70% and MAP ≥70 mm Hg with measures of tissue perfusion identified via central-peripheral temperature gradient and sublingual perfusion capillary density. Interestingly, while all patients achieved hemodynamic targets with doses of dobutamine or enoximone and norepinephrine, tissue perfusion was not adequately restored in most patients and was associated with marked mortality of 50% at 30 days following admission.54 MAP is simply 1 of numerous potential hemodynamic targets, and it is likely more useful to correlate MAP with improvement in microcirculatory dysfunction and restoration of tissue perfusion, rather than using it as a singular numerical target in isolation. A similar approach could be implemented for sBP, as there are no specific thresholds to target; however, an initial goal of sBP >90 mm Hg and/or MAP of 55 to 75 mm Hg with concurrent monitoring of perfusion markers may be reasonable. The data to date support the use of norepinephrine initially to target the selected MAP target, with close monitoring of possible vasopressor-related adverse effects and all clinical and biochemical markers of systemic perfusion. To date, no trials of MAP target in CS have been performed, and this remains an important avenue for future research.
Lactate and lactate clearance
Lactate production in the human body is greatest in muscle and, under normal conditions, is predominantly hepatically cleared, with a small contribution of renal clearance. In CS, with decreased tissue perfusion, microcirculatory dysfunction, and high levels of endogenous catecholamines, cells switch into anaerobic metabolism, and lactic acid is produced. Lactate rises in situations of beta-2 receptor activation in skeletal muscle, which triggers aerobic glycolysis and lactate production as an alternative source of fuel—this phenomenon is often seen with epinephrine administration. While the absolute levels of lactate are established prognostic markers in multiple shock states, these patients are highly dynamic, and an assessment of lactate over time may be of greater value. In 2018, Fuernau et al55 evaluated serum lactate levels and lactate clearance (LC) in a cohort of the IABP-SHOCK II trial, finding that serum lactate at 8 hours and LC were independent predictors of 30-day mortality. They also found that an 8-hour lactate of ≥3.1 mmol/L and LC of −3.45%/h in the first 8 hours of resuscitation were of highest predictive value.55 In a substudy of the CAPITAL DOREMI trial, LC was shown to be an independent predictor of 30-day survival as early as 8 hours after enrollment. Complete LC, defined as the time between baseline lactate to the first normal lactate (defined as <2.0 mmol/L), was the strongest predictor of 30-day survival of all the definitions of LC. A similar distinguishing LC value at 8 hours for survival was seen in CAPITAL DOREMI: −5.55%/h in survivors and −3.06%/h in nonsurvivors.56 An understanding of LC and a time point of 8 hours could support a short, predefined period of pharmacologic stabilization to monitor those patients who will continue to improve and not require advanced therapies vs avoiding “missing the window” to escalate support in those patients who would continue to fail on first-line support and may benefit the most from the addition of MCS. To date, randomized trials utilizing LC as an endpoint in resuscitation of other shock states have not shown improvements in mortality.57,58 No such trials have been conducted in CS, suggesting another potential area for future study.
Metabolic acidosis caused by lactic acidosis from tissue hypoperfusion and anaerobic metabolism, compounded by AKI and reduced hepatic LC, is the most common acid-base abnormality seen in CS. Acidosis may further impair cardiovascular function and worsen hemodynamics through reduced vasopressor responsiveness, decreased cardiac contractility, and altered systemic vascular resistance. There are a number of ways to quantify metabolic acidosis including base deficit, anion gap, and strong ion gap. Of particular interest in the CS cohort is base deficit or the amount of base required to titrate a liter of arterial blood to a pH of 7.40. Attana et al59 looked at 63 patients with CS following ST-segment elevation myocardial infarction and found that a greater base deficit was seen among nonsurvivors, but there were no detectable differences in anion gap or strong ion gap. A study examining the prognostic value of serum bicarbonate on 28-day mortality in a cohort of AMI-CS found that baseline serum bicarbonate was higher, and base deficit lower, in survivors compared to nonsurvivors. Interestingly, bicarbonate levels were seen to decrease before a significant rise in lactate occurred. Furthermore, incrementally lower baseline bicarbonate levels were associated with progressively higher mortality rates.60 Serum bicarbonate and base deficit may be additional prognostic markers in CS. However, their utility as another potential target of resuscitation remains uncertain.
Central and mixed venous oxygen saturation
The 4 components of venous oxygen saturation are arterial oxygen saturation, hemoglobin concentration, cardiac output, and tissue oxygen consumption. In clinical practice, either the central venous oxygen saturation (ScvO2) or SvO2 is most frequently used. The ScvO2 is obtained from the cavoatrial junction, and the SvO2 is obtained from the main pulmonary artery. Under normal physiologic conditions, the ScvO2 can be used as a surrogate for the SvO2 although usually 2% to 5% lower due to high cerebral oxygen uptake reflected in the superior vena cava inflow and significant renal blood flow from the inferior vena cava. This correlation disintegrates in states of shock due to preferential shunting of blood to vital organs and splanchnic hypoperfusion. Greater discrepancies are noted when SvO2 is <70%, arguing that these values are certainly not interchangeable when managing a critically ill patient.61 In the CS population, a pathologically low venous oxygen saturation is due to increased oxygen extraction as a consequence of inadequate oxygen delivery from low cardiac output. A small study of 60 patients with acute decompensated heart failure found that an increase in ScvO2 to >60% at 24 hours following introduction of a dobutamine infusion was associated with lower rates of in-hospital death, need for cardiac support device, or heart transplant. Improvement in ScvO2 was more strongly associated with markers of venous congestion (reduced vena cava diameter and improved urine output) rather than improvement in cardiac output.62 Given the ease of access, and rapidity of point-of-care testing, ScvO2 may represent a potentially important therapeutic target, both in terms of forward flow and titration of diuretic therapy in congested patients. The data for dedicated SvO2 is predominantly limited to the cardiac surgical population, but there is evidence of low SvO2 correlating with worse outcomes among patients with severe cardiac and respiratory disease.63 Given the renewed interest in PACs to support hemodynamic-guided therapy in CS, further exploration of the prognostic and potentially therapeutic implications of SvO2 may be worthwhile.
LV and right ventricular pressures
LV and right ventricular (RV) filling pressures reflect Arthur Guyton’s seminal work on the venous circulation and its role in cardiac output. Guyton defined the mean circulatory filling pressure as the integrated pressure throughout the circulatory system in a state of no flow—a parameter highly sensitive to changes in volume and vessel wall relaxation.64 A foundational concept of Guyton’s model is that blood flow to the right atrium is driven by the differences between the mean circulatory filling pressure and the RAP and not necessarily the arterial pressure. Guyton’s work raises the question of how filling pressures are predictive of outcomes in CS. From an LV perspective in ST-segment elevation myocardial infarction, a LVEDP >18 mm Hg, sBP to LVEDP ratio of <4, and CPO/Cardiac Power Index are associated with poorer short-term outcomes, including in-hospital mortality.65, 66, 67, 68 Titration of the systolic BP/LVEDP ratio is much more difficult, as beyond diuresis and vasopressors to support systolic BP, there are few evidence-based therapies to be added. Given the uncertain efficacy and concerning safety profile of the inotropic therapy, it may be reserved to those patients who are failing on conventional pharmacologic stabilization with diuresis and vasoactive agents, with close monitoring of perfusion markers. While the efficacy of MCS has not been definitively established, these patients may be the ones most likely to benefit from escalation to device therapy. Finally, CPO has been established as a clear predictor of in-hospital mortality in AMI-CS and may represent another variable to be integrated into decisions around escalation of therapy.69,70
Interest in and understanding of the right ventricle has dramatically increased over the last decade, and there has been renewed interest in the predictive and therapeutic value of right-sided hemodynamics. RV parameters of particular interest include (pulmonary artery pulsatility index, defined as pulmonary artery pulse pressure/RAP), RAP, RAP/PCWP, and CPA (pulmonary artery compliance, defined as RV stroke volume [SV]/PA pulse pressure). The predictive value regarding in-hospital mortality of RV dysfunction has been examined in AMI-CS and acute decompensated heart failure: the retrospective study by Jain et al71 found higher RAP, RA/PCWP, and lower pulmonary artery pulsatility index and RV stroke work index values in nonsurvivors than those in survivors. Furthermore, RV dysfunction was progressively more severe with escalating SCAI stage.71 Regarding CPA, in a study of patients with CS due to primarily LV failure, CPA was significantly associated with mortality, with lower CPA in nonsurvivors associated with more severe RV systolic dysfunction.72 While there is some suggestion of potential benefit, what remains to be seen is if titration of therapy according to invasive hemodynamics truly changes patient-important outcomes—only randomized controlled trials can definitively answer these crucial questions.
Other markers
Thera are a number of additional windows into microcirculatory dysfunction that may represent therapeutic targets in CS. The partial pressure of CO2 in tissues increases in patients with shock.73 This may be due to a higher CO2 production without a parallel increase in blood flow to wash out or anaerobic metabolism and elevated lactate production requiring bicarbonate buffering and CO2 production. Several strategies can be used to measure tissue CO2 levels—the most common is the arteriovenous difference in CO2 from sampling of arterial and mixed venous blood. The delta CO2 is inversely proportional to the CI in circulatory failure, and a persistently elevated delta CO2 may help identify patients who remain inadequately resuscitated.74 An increased P(v-a)CO2 gap is significantly associated with increased risk of mortality in a cohort of CS patients requiring extracorporeal membrane oxygenation, highlighting its utility as a marker of microcirculatory dysfunction.75 End-tidal CO2 (PetCO2) via capnography is part of standard of care in the ongoing management of mechanically ventilated patients. In fact, PetCO2 is already used as a marker of poor outcome in resuscitation of patients suffering from a cardiac arrest: a PetCO2 of <10 mm Hg after 20 minutes of cardiopulmonary resuscitation is predictive of in-hospital mortality.76 These measures of tissue CO2 levels are easily obtained with current bedside equipment and could be studied as potential targets in the care of patients with CS.
The use of novel biomarkers in prognostication of CS is a rapidly growing field. The classic biomarkers, including troponin and natriuretic peptides, have shown variable predictive value77,78 but are readily available at the bedside. Novel biomarkers, including dipeptidyl peptidase 3, adrenomedullin, and angiopoietin-2, have been studied in the CardShock, IABP-SHOCK II, and OptimaCC trial cohorts with promising results.79, 80, 81 Biomarker-based risk-prediction tools are being increasingly explored. The Cardiogenic Shock 4 Proteins score is based on 4 proteins that reflect multiorgan dysfunction, systemic inflammation, and immune activation and has been examined in CS cohorts demonstrating improved predictive values in short-term mortality when used with clinical risk scores.82 The CLIP score, referring to cystatin C, lactate, interleukin-6, and N-terminal pro–B-type natriuretic peptide, has been externally valued and outperformed the SAPS II and IABP-SHOCK II risk scores regarding prognostication of 30-day mortality in AMI-CS.83 It is yet to be determined how these biomarkers will be best integrated into the care of patients from a diagnostic, therapeutic, and prognostic perspective.
Optimization of positive pressure ventilation parameters in CS is a growing area of interest given its use in upwards of 40% of patients in contemporary CS registries. However, there is a lack of prospective, randomized trials to guide specific practices. Positive end-expiratory pressure (PEEP) has a number of beneficial effects on the LV, including improved myocardial contractility, decreased wall tension, decreased afterload, and increased arterial oxygen concentration. While the data do not suggest an optimal PEEP in CS, selection and titration can reasonably be based on clinical assessment of preload sensitivity. In preload-dependent states like RV dysfunction, pulmonary hypertension, or tamponade physiology, initial selection of the lowest possible PEEP that allows for adequate oxygenation is combined with aggressive management of other variables known to impact RV function (ie, pH, PaCO2, hypoxemia, optimization of ventilation-perfusion mismatch, etc). Conversely, in afterload-dependent states like profound LV dysfunction, a higher initial PEEP may be selected and titrated upwards with close monitoring of hemodynamics and ensuring patient-ventilator synchrony to minimize myocardial oxygen demand.84 Of particular use may be higher PEEP titration in those patients with significant mitral regurgitation—as evidenced by the study of Patzelt et al85 demonstrating greater technical feasibility of transcatheter edge-to-edge repair with higher PEEP levels, due to reduced mitral valve anterior-posterior and mediolateral dimensions. Similar physiology may be applied to those patients with CS and significant mitral regurgitation, in that higher PEEP levels may improve CO hemodynamics and, perhaps, clinical outcomes. Certainly, as the cohort of patients cared for in cardiac intensive care units continues to grow in complexity, trials focused on optimal ventilatory parameters are both warranted and necessary to progress their care forward.
Finally, there are emerging technologies that are yet to be proven but are used with varying frequency in CS. Two of these devices are the FloTrac system (Edwards LifeSciences) and NIRS. The FloTrac system uses an arterial catheter and pressure waveform analysis to obtain CO, CI, SV, SV variation, and SV index; with the addition of a central venous catheter, systemic vascular resistance and ScvO2 can also be measured. The value and reliability of the FloTrac system have been most extensively studied in the trauma and postoperative patient populations, with data suggesting decreased utility in a vasoplegic cohort.86 Although intriguing, further research, comparing derived values to gold standard measures of invasive hemodynamics, is needed to clarify its utility in clinical practice. NIRS is a noninvasive method to continuously monitor tissue oxygenation, based on the absorption spectrums of oxygenated and deoxygenated hemoglobin. These values of oxyhemoglobin and deoxyhemoglobin are then used to calculate the tissue hemoglobin oxygen saturation. Similar to the FloTrac, NIRS has most commonly been used during cardiac surgery to identify and treat cerebral desaturation, and data supporting its use in shock states are much more limited. A recent systematic review found that baseline tissue hemoglobin oxygen saturation was associated with other markers of more severe shock state, like SvO2 and serum lactate levels, as well as increased mortality.87 Greater experience with these technologies in the management of patients with CS should provide a better understanding of how to best integrate them into evolving models of care.
Future directions
The mortality of patients with CS remains high, and many questions in the field remain unanswered. The current body of literature supports the use of multimodality assessment, integrating physical exam findings with clinical and biochemical markers of perfusion to guide pharmacologic therapy, and consideration of escalation of therapy (Central Illustration). Of particular utility are measures of LC, venous oxygen saturation, hemodynamic parameters, and markers of right-sided congestion. While many important questions remain, several of the most pressing gaps lie in identification of ideal MAP targets in various phenotypes of CS, efficacy and safety of single and/or combined inotrope therapy, ideal timing of RRT, and safety and efficacy of MCS devices in different etiologies of CS. The development of randomized clinical trials focusing on subsets of CS patients and dedicated to answering these specific questions is key to moving the field forward. There is an urgent need for clinical trials to demonstrate safety and efficacy of current therapies and to comprehensively evaluate forthcoming interventions.
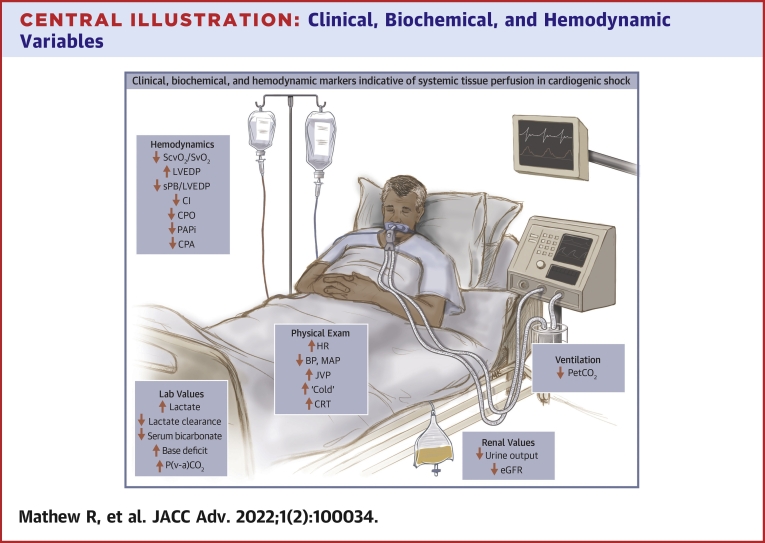
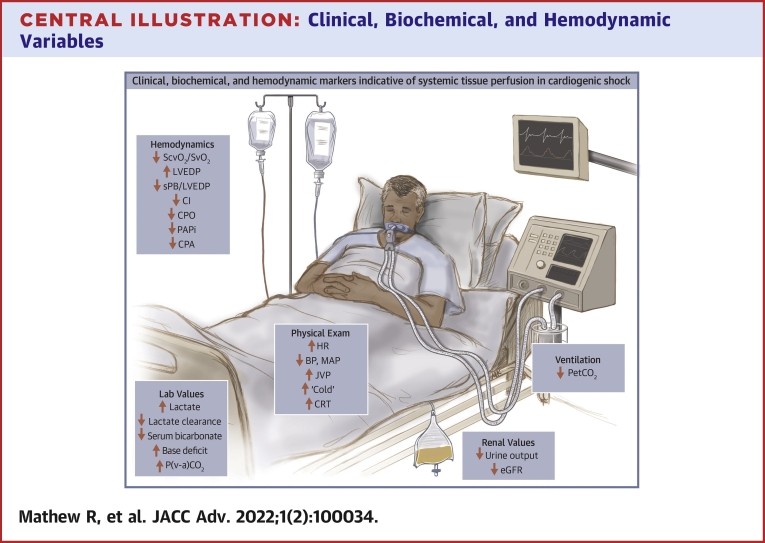
Central Illustration.
Clinical, Biochemical, and Hemodynamic Variables
This figure highlights clinical, biochemical, and hemodynamic parameters that are commonly trending in the ongoing management of patients with cardiogenic shock. Arrows identify the direction of change in states of clinical deterioration. BP = blood pressure; CI = cardiac index; CPA = pulmonary artery compliance; CPO = cardiac power output; CRT = capillary refill time; eGFR = estimated glomerular filtration rate; HR = heart rate; JVP = jugular venous pressure; LC = lactate clearance; LVEDP = left ventricular end-diastolic pressure; MAP = mean arterial pressure; P(v-a)CO2 gap = venous arterial carbon dioxide gap; PAPi = pulmonary artery pulsatility index; PetCO2 = end-tidal CO2; sBP = systolic blood pressure; ScvO2 = central venous oxygen saturation; SvO2 = mixed venous oxygen saturation.
Funding support and author disclosures
Dr Brodie has received research support from ALung Technologies; has been on the medical advisory boards for Abiomed, Xenios, Medtronic, Inspira, and Cellenkos; is the President-elect of the Extracorporeal Life Support Organization (ELSO); and is the Chair of the Executive Committee of the International ECMO Network (ECMONet). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Sarah Visintini, MLIS, (Berkman Library, University of Ottawa Heart Institute) for the literature searches she conducted in support of this paper.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Hochman J.S., Sleeper L.A., Webb J.G., et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341(9):625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 2.De Backer D., Biston P., Devriendt J., et al. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362(9):779–789. doi: 10.1056/NEJMoa0907118. [DOI] [PubMed] [Google Scholar]
- 3.Levy B., Clere-Jehl R., Legras A., et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2018;72(2):173–182. doi: 10.1016/j.jacc.2018.04.051. [DOI] [PubMed] [Google Scholar]
- 4.TRIUMPH Investigators. Alexander J.H., Reynolds H.R., et al. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297(15):1657–1666. doi: 10.1001/jama.297.15.joc70035. [DOI] [PubMed] [Google Scholar]
- 5.Felker G.M., Benza R.L., Chandler A.B., et al. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 2003;41(6):997–1003. doi: 10.1016/s0735-1097(02)02968-6. [DOI] [PubMed] [Google Scholar]
- 6.Abraham W.T., Adams K.F., Fonarow G.C., et al. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Am Coll Cardiol. 2005;46(1):57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 7.Thiele H., Ohman E.M., de Waha-Thiele S., Zeymer U., Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671–2683. doi: 10.1093/eurheartj/ehz363. [DOI] [PubMed] [Google Scholar]
- 8.van Diepen S., Katz J.N., Albert N.M., et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136(16):e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 9.Killip T., Kimball J.T. Treatment of myocardial infarction in a coronary care unit: a two year experience with 250 patients. Am J Cardiol. 1967;20(4):457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 10.de Mello B.H.G., Oliveira G.B.F., Ramos R.F., et al. Validation of the Killip-Kimball classification and late mortality after acute myocardial infarction. Arq Bras Cardiol. 2014;103:107–117. doi: 10.5935/abc.20140091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang E.W., Wong C.K., Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J. 2007;153(1):29–35. doi: 10.1016/j.ahj.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Knaus W.A., Wagner D.P., Draper E.A., et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 13.Le Gall J.R., Lemeshow S., Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 14.Harjola V.P., Lassus J., Sionis A., et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17(5):501–509. doi: 10.1002/ejhf.260. [DOI] [PubMed] [Google Scholar]
- 15.Pöss J., Köster J., Fuernau G., et al. Risk stratification for patients in cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol. 2017;69(15):1913–1920. doi: 10.1016/j.jacc.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Miller R.J.H., Southern D., Wilton S.B., et al. Comparative prognostic accuracy of risk prediction models for cardiogenic shock. J Intensive Care Med. 2020;35(12):1513–1519. doi: 10.1177/0885066619878125. [DOI] [PubMed] [Google Scholar]
- 17.Zweck E., Thayer K.L., Helgestad O.K.L., et al. Phenotyping cardiogenic shock. J Am Heart Assoc. 2021;10(14) doi: 10.1161/JAHA.120.020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baran D.A., Grines C.L., Bailey S., et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv. 2019;94(1):29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 19.Jentzer J.C., van Diepen S., Barsness G.W., et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol. 2019;74(17):2117–2128. doi: 10.1016/j.jacc.2019.07.077. [DOI] [PubMed] [Google Scholar]
- 20.Naidu S.S., Baran D.A., Jentzer J.C., et al. SCAI SHOCK stage classification expert consensus update: a review and incorporation of validation studies. J Am Coll Cardiol. 2022;79(9):933–946. doi: 10.1016/j.jacc.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Caldicott L.D., Hawley K., Heppell R., Woodmansey P.A., Channer K.S. Intravenous enoximone or dobutamine for severe heart failure after acute myocardial infarction: a randomized double-blind trial. Eur Heart J. 1993;14(5):696–700. doi: 10.1093/eurheartj/14.5.696. [DOI] [PubMed] [Google Scholar]
- 22.Jia Z., Guo M., Zhang Y.Q., Liang H.Q., Zhang L.Y., Song Y. Efficacy of intravenous levosimendan in patients with heart failure complicated by acute myocardial infarction. Cardiology. 2014;128(2):195–201. doi: 10.1159/000357864. [DOI] [PubMed] [Google Scholar]
- 23.Moiseyev V.S., Põder P., Andrejevs N., et al. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN) Eur Heart J. 2002;23(18):1422–1432. doi: 10.1053/euhj.2001.3158. [DOI] [PubMed] [Google Scholar]
- 24.Lawler P.R., Berg D.D., Park J.G., et al. The range of cardiogenic shock survival by clinical stage: data from the critical care cardiology trials network registry. Crit Care Med. 2021;49(8):1293–1302. doi: 10.1097/CCM.0000000000004948. [DOI] [PubMed] [Google Scholar]
- 25.Garan A.R., Kanwar M., Thayer K.L., et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in-hospital mortality. J Am Coll Cardiol HF. 2020;8(11):903–913. doi: 10.1016/j.jchf.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Binanay C., Califf R.M., Hasselblad V., et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294(13):1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 27.Drazner M.H., Hellkamp A.S., Leier C.V., et al. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ Heart Fail. 2008;1(3):170–177. doi: 10.1161/CIRCHEARTFAILURE.108.769778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thayer K.L., Zweck E., Ayouty M., et al. Invasive hemodynamic assessment and classification of in-hospital mortality risk among patients with cardiogenic shock. Circ Heart Fail. 2020;13(9) doi: 10.1161/CIRCHEARTFAILURE.120.007099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forrester J.S., Diamond G.A., Swan H.J.C. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol. 1977;39(2):137–145. doi: 10.1016/s0002-9149(77)80182-3. [DOI] [PubMed] [Google Scholar]
- 30.Nohria A., Tsang S.W., Fang J.C., et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41(10):1797–1804. doi: 10.1016/s0735-1097(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 31.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 32.Sheikh O., Nguyen T., Bansal S., Prasad A. Acute kidney injury in cardiogenic shock: a comprehensive review. Catheter Cardiovasc Interv. 2021;98(1):E91–E105. doi: 10.1002/ccd.29141. [DOI] [PubMed] [Google Scholar]
- 33.Chen S. Retooling the creatinine clearance equation to estimate kinetic GFR when the plasma creatinine is changing acutely. J Am Soc Nephrol. 2013;24(6):877–888. doi: 10.1681/ASN.2012070653. [DOI] [PubMed] [Google Scholar]
- 34.Ronco C., McCullough P., Anker S.D., et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J. 2010;31(6):703–711. doi: 10.1093/eurheartj/ehp507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Akker J.P.C., Bakker J., Groeneveld A.B.J., den Uil C.A. Risk indicators for acute kidney injury in cardiogenic shock. J Crit Care. 2019;50:11–16. doi: 10.1016/j.jcrc.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Adegbala O., Inampudi C., Adejumo A., et al. Characteristics and outcomes of patients with cardiogenic shock utilizing hemodialysis for acute kidney injury. Am J Cardiol. 2019;123(11):1816–1821. doi: 10.1016/j.amjcard.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 37.Koreny M., Karth G.D., Geppert A., et al. Prognosis of patients who develop acute renal failure during the first 24 hours of cardiogenic shock after myocardial infarction. Am J Med. 2002;112(2):115–119. doi: 10.1016/s0002-9343(01)01070-1. [DOI] [PubMed] [Google Scholar]
- 38.Barbar S.D., Clere-Jehl R., Bourredjem A., et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med. 2018;379(15):1431–1442. doi: 10.1056/NEJMoa1803213. [DOI] [PubMed] [Google Scholar]
- 39.Fuernau G., Poenisch C., Eitel I., et al. Prognostic impact of established and novel renal function biomarkers in myocardial infarction with cardiogenic shock: a biomarker substudy of the IABP-SHOCK II-trial. Int J Cardiol. 2015;191:159–166. doi: 10.1016/j.ijcard.2015.04.242. [DOI] [PubMed] [Google Scholar]
- 40.Dünser M.W., Takala J., Ulmer H., et al. Arterial blood pressure during early sepsis and outcome. Intensive Care Med. 2009;35(7):1225–1233. doi: 10.1007/s00134-009-1427-2. [DOI] [PubMed] [Google Scholar]
- 41.Varpula M., Tallgren M., Saukkonen K., Voipio-Pulkki L.M., Pettilä V. Hemodynamic variables related to outcome in septic shock. Intensive Care Med. 2005;31(8):1066–1071. doi: 10.1007/s00134-005-2688-z. [DOI] [PubMed] [Google Scholar]
- 42.Asfar P., Meziani F., Hamel J.F., et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370(17):1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 43.Lamontagne F., Richards-Belle A., Thomas K., et al. Effect of reduced exposure to vasopressors on 90-day mortality in older critically ill patients with vasodilatory hypotension: a randomized clinical trial. JAMA. 2020;323(10):938–949. doi: 10.1001/jama.2020.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SCCM | surviving sepsis campaign guidelines 2021 [internet]. Society of Critical Care Medicine (SCCM) https://sccm.org/Clinical-Resources/Guidelines/Guidelines/Surviving-Sepsis-Guidelines-2021
- 45.Carrick M.M., Leonard J., Slone D.S., Mains C.W., Bar-Or D. Hypotensive resuscitation among trauma patients. Biomed Res Int. 2016;2016 doi: 10.1155/2016/8901938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossaint R., Bouillon B., Cerny V., et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Crit Care. 2016;20(1):100. doi: 10.1186/s13054-016-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kong C.Y., Hosseini A.M., Belanger L.M., et al. A prospective evaluation of hemodynamic management in acute spinal cord injury patients. Spinal Cord. 2013;51(6):466–471. doi: 10.1038/sc.2013.32. [DOI] [PubMed] [Google Scholar]
- 48.Tran A., Yates J., Lau A., Lampron J., Matar M. Permissive hypotension versus conventional resuscitation strategies in adult trauma patients with hemorrhagic shock: a systematic review and meta-analysis of randomized controlled trials. J Trauma Acute Care Surg. 2018;84(5):802–808. doi: 10.1097/TA.0000000000001816. [DOI] [PubMed] [Google Scholar]
- 49.Futier E., Lefrant J.Y., Guinot P.G., et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346–1357. doi: 10.1001/jama.2017.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burstein B., Tabi M., Barsness G.W., Bell M.R., Kashani K., Jentzer J.C. Association between mean arterial pressure during the first 24 hours and hospital mortality in patients with cardiogenic shock. Crit Care. 2020;24(1):513. doi: 10.1186/s13054-020-03217-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathew R., Di Santo P., Jung R.G., et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N Engl J Med. 2021;385(6):516–525. doi: 10.1056/NEJMoa2026845. [DOI] [PubMed] [Google Scholar]
- 52.Parlow S., Di Santo P., Mathew R., et al. The association between mean arterial pressure and outcomes in patients with cardiogenic shock: insights from the DOREMI trial. Eur Heart J Acute Cardiovasc Care. 2021;10(7):712–720. doi: 10.1093/ehjacc/zuab052. [DOI] [PubMed] [Google Scholar]
- 53.Yeh Y.C., Chiu C.T. Association and dissociation of microcirculation and macrocirculation in critically ill patients with shock. J Emerg Crit Care Med. 2019;3:60. [Google Scholar]
- 54.den Uil C.A., Lagrand W.K., van der Ent M., et al. Conventional hemodynamic resuscitation may fail to optimize tissue perfusion: an observational study on the effects of dobutamine, enoximone, and norepinephrine in patients with acute myocardial infarction complicated by cardiogenic shock. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0103978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuernau G., Desch S., de Waha-Thiele S., et al. Arterial lactate in cardiogenic shock: prognostic value of clearance versus single values. J Am Coll Cardiol Intv. 2020;13(19):2208–2216. doi: 10.1016/j.jcin.2020.06.037. [DOI] [PubMed] [Google Scholar]
- 56.Marbach J.A., Stone S., Schwartz B., et al. Lactate clearance is associated with improved survival in cardiogenic shock: a systematic review and meta-analysis of prognostic factor studies. J Card Fail. 2021;27(10):1082–1089. doi: 10.1016/j.cardfail.2021.08.012. [DOI] [PubMed] [Google Scholar]
- 57.Jones A.E., Shapiro N.I., Trzeciak S., et al. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(8):739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arnold R.C., Shapiro N.I., Jones A.E., et al. Multicenter study of early lactate clearance as a determinant of survival in patients with presumed sepsis. Shock. 2009;32(1):35–39. doi: 10.1097/shk.0b013e3181971d47. [DOI] [PubMed] [Google Scholar]
- 59.Attanà P., Lazzeri C., Chiostri M., Picariello C., Gensini G.F., Valente S. Strong-ion gap approach in patients with cardiogenic shock following ST-elevation myocardial infarction. Acute Card Care. 2013;15(3):58–62. doi: 10.3109/17482941.2013.776691. [DOI] [PubMed] [Google Scholar]
- 60.Wigger O., Bloechlinger S., Berger D., et al. Baseline serum bicarbonate levels independently predict short-term mortality in critically ill patients with ischaemic cardiogenic shock. Eur Heart J Acute Cardiovasc Care. 2018;7(1):45–52. doi: 10.1177/2048872616683526. [DOI] [PubMed] [Google Scholar]
- 61.Ho K.M., Harding R., Chamberlain J., Bulsara M. A comparison of central and mixed venous oxygen saturation in circulatory failure. J Cardiothorac Vasc Anesth. 2010;24(3):434–439. doi: 10.1053/j.jvca.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Gallet R., Lellouche N., Mitchell-Heggs L., et al. Prognosis value of central venous oxygen saturation in acute decompensated heart failure. Arch Cardiovasc Dis. 2012;105(1):5–12. doi: 10.1016/j.acvd.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Pölönen P., Ruokonen E., Hippeläinen M., Pöyhönen M., Takala J. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg. 2000;90(5):1052–1059. doi: 10.1097/00000539-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Guyton A.C., Polizo D., Armstrong G.G. Mean circulatory filling pressure measured immediately after cessation of heart pumping. Am J Physiol. 1954;179(2):261–267. doi: 10.1152/ajplegacy.1954.179.2.261. [DOI] [PubMed] [Google Scholar]
- 65.Bagai A., Armstrong P.W., Stebbins A., et al. Prognostic implications of left ventricular end-diastolic pressure during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: findings from the Assessment of Pexelizumab in Acute Myocardial Infarction study. Am Heart J. 2013;166(5):913–919. doi: 10.1016/j.ahj.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 66.Planer D., Mehran R., Witzenbichler B., et al. Prognostic utility of left ventricular end-diastolic pressure in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2011;108(8):1068–1074. doi: 10.1016/j.amjcard.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 67.Sola M., Venkatesh K., Caughey M., et al. Ratio of systolic blood pressure to left ventricular end-diastolic pressure at the time of primary percutaneous coronary intervention predicts in-hospital mortality in patients with ST-elevation myocardial infarction. Catheter Cardiovasc Interv. 2017;90(3):389–395. doi: 10.1002/ccd.26963. [DOI] [PubMed] [Google Scholar]
- 68.Tesak M., Kala P., Jarkovsky J., et al. The value of novel invasive hemodynamic parameters added to the TIMI risk score for short-term prognosis assessment in patients with ST segment elevation myocardial infarction. Int J Cardiol. 2016;214:235–240. doi: 10.1016/j.ijcard.2016.03.073. [DOI] [PubMed] [Google Scholar]
- 69.Fincke R., Hochman J.S., Lowe A.M., et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: a report from the SHOCK trial registry. J Am Coll Cardiol. 2004;44(2):340–348. doi: 10.1016/j.jacc.2004.03.060. [DOI] [PubMed] [Google Scholar]
- 70.Mendoza D.D., Cooper H.A., Panza J.A. Cardiac power output predicts mortality across a broad spectrum of patients with acute cardiac disease. Am Heart J. 2007;153(3):366–370. doi: 10.1016/j.ahj.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 71.Jain P., Thayer K.L., Abraham J., et al. Right ventricular dysfunction is common and identifies patients at risk of dying in cardiogenic shock. J Card Fail. 2021;27(10):1061–1072. doi: 10.1016/j.cardfail.2021.07.013. [DOI] [PubMed] [Google Scholar]
- 72.Zorzi M.F., Cancelli E., Rusca M., Kirsch M., Yerly P., Liaudet L. The prognostic value of pulmonary artery compliance in cardiogenic shock. Pulm Circ. 2019;9(3) doi: 10.1177/2045894019877161. 2045894019877161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Danin P.E., Siegenthaler N., Levraut J., Bernardin G., Dellamonica J., Bendjelid K. Monitoring CO2 in shock states. J Clin Monit Comput. 2015;29(5):591–600. doi: 10.1007/s10877-014-9638-7. [DOI] [PubMed] [Google Scholar]
- 74.Teboul J.L., Mercat A., Lenique F., Berton C., Richard C. Value of the venous-arterial PCO2 gradient to reflect the oxygen supply to demand in humans: effects of dobutamine. Crit Care Med. 1998;26(6):1007–1010. doi: 10.1097/00003246-199806000-00017. [DOI] [PubMed] [Google Scholar]
- 75.McDonald C.I., Brodie D., Schmidt M., Hay K., Shekar K. Elevated venous to arterial carbon dioxide gap and anion gap are associated with poor outcome in cardiogenic shock requiring extracorporeal membrane oxygenation support. ASAIO J. 2021;67(3):263–269. doi: 10.1097/MAT.0000000000001215. [DOI] [PubMed] [Google Scholar]
- 76.Levine R.L., Wayne M.A., Miller C.C. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337(5):301–306. doi: 10.1056/NEJM199707313370503. [DOI] [PubMed] [Google Scholar]
- 77.Jolly S.S., Shenkman H., Brieger D., et al. Quantitative troponin and death, cardiogenic shock, cardiac arrest and new heart failure in patients with non-ST-segment elevation acute coronary syndromes (NSTE ACS): insights from the Global Registry of Acute Coronary Events. Heart. 2011;97(3):197–202. doi: 10.1136/hrt.2010.195511. [DOI] [PubMed] [Google Scholar]
- 78.Prondzinsky R., Lemm H., Swyter M., et al. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38(1):152–160. doi: 10.1097/CCM.0b013e3181b78671. [DOI] [PubMed] [Google Scholar]
- 79.Takagi K., Blet A., Levy B., et al. Circulating dipeptidyl peptidase 3 and alteration in haemodynamics in cardiogenic shock: results from the OptimaCC trial. Eur J Heart Fail. 2020;22(2):279–286. doi: 10.1002/ejhf.1600. [DOI] [PubMed] [Google Scholar]
- 80.Tolppanen H., Rivas-Lasarte M., Lassus J., et al. Adrenomedullin: a marker of impaired hemodynamics, organ dysfunction, and poor prognosis in cardiogenic shock. Ann Intensive Care. 2017;7:6. doi: 10.1186/s13613-016-0229-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pöss J., Fuernau G., Denks D., et al. Angiopoietin-2 in acute myocardial infarction complicated by cardiogenic shock--a biomarker substudy of the IABP-SHOCK II-Trial. Eur J Heart Fail. 2015;17(11):1152–1160. doi: 10.1002/ejhf.342. [DOI] [PubMed] [Google Scholar]
- 82.Rueda F., Borràs E., García-García C., et al. Protein-based cardiogenic shock patient classifier. Eur Heart J. 2019;40(32):2684–2694. doi: 10.1093/eurheartj/ehz294. [DOI] [PubMed] [Google Scholar]
- 83.Ceglarek U., Schellong P., Rosolowski M., et al. The novel cystatin C, lactate, interleukin-6, and N-terminal pro-B-type natriuretic peptide (CLIP)-based mortality risk score in cardiogenic shock after acute myocardial infarction. Eur Heart J. 2021;42(24):2344–2352. doi: 10.1093/eurheartj/ehab110. [DOI] [PubMed] [Google Scholar]
- 84.Alviar C.L., Rico-Mesa J.S., Morrow D.A., et al. Positive pressure ventilation in cardiogenic shock: review of the evidence and practical advice for patients with mechanical circulatory support. Can J Cardiol. 2020;36(2):300–312. doi: 10.1016/j.cjca.2019.11.038. [DOI] [PubMed] [Google Scholar]
- 85.Patzelt J., Zhang Y., Seizer P., et al. Effects of mechanical ventilation on heart geometry and mitral valve leaflet coaptation during percutaneous edge-to-edge mitral valve repair. J Am Coll Cardiol Intv. 2016;9(2):151–159. doi: 10.1016/j.jcin.2015.09.038. [DOI] [PubMed] [Google Scholar]
- 86.De Backer D., Marx G., Tan A., et al. Arterial pressure-based cardiac output monitoring: a multicenter validation of the third-generation software in septic patients. Intensive Care Med. 2011;37(2):233–240. doi: 10.1007/s00134-010-2098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Varis E., Pettilä V., Wilkman E. Near-infrared spectroscopy in adult circulatory shock: a systematic review. J Intensive Care Med. 2020;35(10):943–962. doi: 10.1177/0885066620907307. [DOI] [PubMed] [Google Scholar]