Abstract
Background
Prior studies of aortic valve replacement (AVR) in patients with normal-flow, low-gradient aortic stenosis (NF-LG AS) have demonstrated conflicting results regarding the survival benefit of AVR. Changes in quality of life (QoL) after transcatheter AVR (TAVR) have not been reported in this population.
Objectives
The purpose of this study was to compare changes in QoL after TAVR for patients with NF-LG AS to patients with high-gradient aortic stenosis (HG-AS).
Methods
Patients who underwent TAVR for severe aortic stenosis (AS) were divided into 4 hemodynamic profiles of AS, including NF-LG AS. Changes in Kansas City Cardiomyopathy Questionnaire-12 score from baseline to 1 year were compared between AS groups. The primary composite outcome indicating clinical improvement consisted of survival to 1 year and improved Kansas City Cardiomyopathy Questionnaire overall summary score of ≥5 points while adjusting for relevant baseline factors.
Results
Out of 860 patients who underwent TAVR, high gradient AS was present in 368 (42.8%) patients and NF-LG AS in 245 (28.5%). HG-AS and NF-LG AS groups had a similar proportion of patients who met the primary unadjusted outcome of clinical improvement (70.4% vs 63.9%, respectively; P = 0.189). One-year Kaplan-Meier mortality estimates were higher for NF-LG AS patients than HG-AS patients (12.9% vs 5.8%, P < 0.001). In the primary adjusted analysis, there was no significant difference in the composite outcome between HG and NF-LG AS groups (adjusted OR: 0.72, 95% CI: 0.47-1.11).
Conclusions
Selected patients with NF-LG AS experienced similar improvement in QoL after TAVR compared with HG-AS. Further investigation of patients with NF-LG AS will help to inform optimal selection for treatment with TAVR.
Key words: health-related quality of life (QoL), low gradient aortic stenosis, normal-flow, transcatheter aortic valve replacement (TAVR)
Central Illustration
Multiple hemodynamic profiles of aortic stenosis (AS) exist and are defined by the flow and gradient across the aortic valve. Current valve guidelines recommend aortic valve replacement (AVR) in symptomatic patients with severe AS in the following hemodynamic profiles: high-gradient AS (HG-AS); low-flow, low-gradient AS (LF-LG AS) with reduced left ventricular ejection fraction (LVEF) (classical LF-LG AS); and LF-LG AS with preserved LVEF (paradoxical LF-LG AS).1,2 Of patients with preserved LVEF and aortic valve area (AVA) <1.0 cm2, there is a sizable subset (15-40%) with normal-flow, low-gradient severe AS (NF-LG AS), characterized by an AVA <1.0 cm2, mean aortic valve gradient <40 mm Hg, peak velocity <4.0 m/s, and stroke volume index (SVi) >35 mL/m2.3 Despite the prevalence of this patient population, there are currently no guidelines to inform the timing of AVR in this group.
Data are conflicting regarding the benefit of AVR in patients with NF-LG AS. Several observational studies have demonstrated that patients with NF-LG AS treated medically have better outcomes than patients with HG-AS, have similar all-cause mortality to patients with moderate AS, and do not have a survival benefit from AVR.4, 5, 6, 7, 8 On the other hand, a meta-analysis by Dayan et al9 demonstrated that patients with NF-LG AS had increased mortality compared with moderate AS and demonstrated similar survival benefit with AVR compared with HG-AS. Intervention for symptomatic patients with NF-LG AS is considered appropriate by expert consensus and has been used as inclusion criteria for randomized trials but has not been specifically included in valve guidelines.1,2,10,11 NF-LG AS likely represents a heterogeneous group of patients. The estimation of AVA by echocardiography is prone to error due to the dependence on multiple measurements; inaccuracy is especially introduced by left ventricular outflow tract area measurement.3 Therefore, a subset of patients may truly have moderate AS and be less likely to benefit from AVR. However, after accounting for measurement error, there is a known discrepancy between thresholds for severe AS. On average, an AVA of 1.0 cm2 by echocardiography corresponds to a mean gradient of 28 mmHg rather than 40 mmHg in normal flow conditions.12 Although gradients fall within the moderate range, patients with NF-LG AS may represent a more severe degree of stenosis.7 Such heterogeneity likely contributes to the conflicting association between NF-LG AS and survival.
The Valve Academic Research Consortium-3 outlines several end points deemed critical in the evaluation of TAVR outcomes.13 Of these, patient-reported outcomes and health status are important in informing clinicians of symptomatic relief or progression following intervention. Given the relatively high prevalence of NF-LG AS among patients with severe AS, conflicting data regarding mortality benefit, and lack of data regarding symptomatic benefit in this group, an analysis of health-related quality of life (QoL) outcomes after TAVR could help inform management in this patient population. Therefore, the objective of this study was to compare changes in QoL and clinical improvement (1-year survival plus clinically significant increase in Kansas City Cardiomyopathy Questionnaire [KCCQ]) after TAVR for patients with NF-LG AS to patients with HG-AS. We hypothesized that patients who underwent TAVR for NF-LG AS would derive less symptomatic improvement compared with patients who underwent TAVR for HG-AS.
Methods
Population
We included consecutive patients who underwent commercial TAVR for severe, native valve AS during the period of 2013 to 2021 at the University of Michigan. The study was approved by the University of Michigan institutional review board with a waiver of consent. Patients with moderate or severe aortic regurgitation (based on multiparametric assessment per American Society of Echocardiography guidelines)14 were excluded. Patients were then stratified into 4 mutually exclusive AS hemodynamic profiles according to flow state and aortic valve gradient using baseline echocardiographic data.3 The 4 groups included: 1) HG-AS (peak velocity ≥4.0 m/s OR mean gradient ≥40 mmHg regardless of LVEF or flow state); 2) classical LF-LG AS (peak velocity <4.0 m/s AND mean gradient <40 mm Hg, AVA <1.0 cm2, LVEF <50%); 3) paradoxical LF-LG AS (peak velocity <4.0 m/s AND mean gradient <40 mm Hg, AVA <1.0 cm2, LVEF ≥50%, SVi <35 mL/m2); and 4) NF-LG AS (peak velocity <4.0 m/s AND mean gradient <40 mm Hg, AVA <1.0 cm2, LVEF ≥50%, and SVi ≥35 mL/m2). Patients were only included for analysis if they had complete baseline KCCQ-12 data (88 patients were excluded for missing baseline KCCQ data).
Data Collection
Baseline patient data including demographics, comorbidities, and baseline echocardiographic data were obtained from patient preprocedural Transcatheter Valve Therapy (TVT) registry forms. Data for calculation of stroke volume is not available in versions 1.0 and 2.0 of TVT data collection forms; therefore, necessary data were obtained from the University of Michigan echocardiographic database for calculation of baseline flow state based on stroke volume indexed to body size (SVi) (SVi = π·[LVOT radius]2·LVOT VTI/body surface area).
Study Outcomes
The assessment of health-related QoL using the KCCQ has been well-validated in patients with AS.15 An increase in the KCCQ score by 5 points or more is associated with clinically significant improvement.16 Patient QoL was assessed preprocedurally and at 1-year post-TAVR using the KCCQ-12 overall summary score. We used a composite outcome to define clinical improvement, consisting of: 1) survival to 1 year; and 2) clinically significant increase in KCCQ from baseline to 1 year (defined by an increase of 5 or more points. The primary study outcome is an adjusted analysis of the composite outcome.
Adverse events outlined in the TVT registry collection form were collected during the index hospitalization, at 30 days and 1 year postprocedure. Furthermore, adjudicated events (including ischemic/hemorrhagic/undetermined stroke, transient ischemic attack, and aortic valve intervention) were assessed for more complete follow-up. Patient mortality was evaluated at similar intervals with causes of death separated into cardiovascular, noncardiovascular, and unknown.
Statistical Analysis
Categorical variables are reported as frequencies and percentages; comparisons between groups were made using chi-square tests. Continuous variables are reported as mean ± SD. Student’s t-tests were used to compare continuous variables between groups. Patients with NF-LG AS comprised the primary study population; comparisons were made to HG-AS as the primary comparator group. Mean differences in baseline to 1-year KCCQ scores were compared using Student t-tests. Kaplan-Meier analysis was used to compare 1-year all-cause mortality between groups; differences in survival were determined using the log rank test.
The primary adjusted analysis was performed using multivariable logistic regression, which included weighted linear regression adjusting for covariates listed (Supplemental Table 1) and accounting for missingness in the end point using inverse probability weighting.17 Weights were determined using the predicted probabilities from a logistic regression model with the availability of outcome data as the dependent variable and adjusting for all covariates used in adjusted outcome analysis (Supplemental Table 1). Weights were then scaled so that the mean weight was equal to 1 among those patients with available outcome data used for outcome models.
Baseline covariate selection was based on modeling of predictors of QoL after TAVR by Arnold et al.18 Several variables from the original model are not routinely collected in the TVT registry: mean arterial pressure, Mini-Mental Status Examination, and 6-minute walk test distance. The 6-minute walk test distance was replaced by the median 5-meter walk time of up to 3 attempts, which is routinely collected in the registry. If no times were recorded for any attempt, the median value was imputed to 42 minutes, the maximum value recorded in the dataset. Mean aortic valve gradient was used to define study groups and was thus excluded as a baseline covariate. For serum creatinine, an additional interaction term was used for the presence or absence of renal replacement therapy. The original model by Arnold et al was based on the PARTNER trial of high surgical risk, defined by Society of Thoracic Surgeons (STS) risk score of 10% or greater; in this population, the STS mortality risk score was not an independent predictor, likely due to the homogeneous STS risk scores included. We included STS risk score as a baseline covariate due to our study population representing a wide spectrum of STS risk. We also included age due to its well-known prognostic importance.
Results
Baseline and procedural characteristics
A total of 860 patients undergoing commercial TAVR for severe, native AS during years 2013 to 2021 at the University of Michigan met inclusion criteria (Table 1). HG-AS was present in 368 (42.8%) patients and NF-LG AS in 245 (28.5%). The NF-LG AS and HG-AS groups were overall similar with a few key differences: NF-LG AS patients were older, had a higher prevalence of prior myocardial infarction, and had a lower mean preprocedural creatinine. There were no significant differences in type of prosthetic valve used, with the majority of patients undergoing self-expanding TAVR (n = 745, 86.6%). Rates of pacemaker implantation and moderate or greater prosthetic aortic regurgitation at 30-day postprocedure were similar between groups. NF-LG AS patients did have a lower mean aortic valve gradient than HG-AS patients at 30 days postprocedure (7.8 vs 9.9 mm Hg, respectively, P < 0.001) (Table 2).
Table 1.
Baseline Characteristics
| HG-AS (n = 368, 42.8%) | NF-LG AS (n = 245, 28.5%) | Classical LF-LG AS (n = 74, 8.6%) | Paradoxical LF-LG AS (n = 173, 20.1%) |
P Value |
||
|---|---|---|---|---|---|---|
| Overall | HG vs NF-LG | |||||
| Baseline clinical variables | ||||||
| Age (y) | 76.7 ± 9.8 | 80.0 ± 8.0 | 78.0 ± 12.3 | 80.2 ± 8.5 | <0.001 | <0.001 |
| Male | 193 (52.4) | 125 (51.0) | 56 (75.7) | 92 (53.2) | 0.002 | 0.792 |
| Race | ||||||
| White | 334 (90.8) | 230 (93.9) | 68 (91.9) | 168 (97.1) | 0.051 | 0.214 |
| Black/African American | 19 (5.2) | 6 (2.4) | 1 (1.4) | 3 (1.7) | 0.085 | 0.145 |
| Asian | 6 (1.6) | 3 (1.2) | 0 (0.0) | 0 (0.0) | 0.273 | 0.947 |
| American Indian/Alaskan Native | 3 (0.8) | 0 (0.0) | 3 (4.1) | 0 (0.0) | 0.002 | 0.409 |
| STS Risk Score | 5.38 ± 3.69 | 5.23 ± 3.74 | 8.03 ± 4.61 | 5.56 ± 4.10 | <0.001 | 0.574 |
| Diabetes mellitus | 153 (41.6) | 84 (34.3) | 34 (45.9) | 76 (43.9) | 0.119 | 0.083 |
| Prior PCI | 117 (31.8) | 97 (39.6) | 47 (63.5) | 70 (40.7) | <0.001 | 0.058 |
| Prior CABG | 53 (14.4) | 49 (20.0) | 27 (37.0) | 29 (16.8) | <0.001 | 0.087 |
| Prior myocardial infarction | 51 (13.9) | 55 (22.4) | 32 (43.2) | 29 (16.8) | <0.001 | 0.008 |
| Prior stroke | 36 (9.8) | 31 (12.7) | 7 (9.5) | 24 (13.9) | 0.447 | 0.331 |
| Peripheral arterial disease | 150 (40.8) | 102 (41.6) | 38 (51.4) | 65 (37.6) | 0.248 | 0.896 |
| Severe chronic lung disease | 32 (8.7) | 13 (5.3) | 8 (10.8) | 12 (6.9) | 0.299 | 0.156 |
| Home oxygen use | 45 (12.2) | 27 (11.0) | 10 (13.5) | 24 (13.9) | 0.833 | 0.744 |
| Dialysis dependent | 22 (6.0) | 3 (1.2) | 4 (5.4) | 3 (1.7) | 0.007 | 0.007 |
| Mean preprocedure creatinine | 1.33 ± 1.15 | 1.16 ± 0.72 | 1.54 ± 1.33 | 1.15 ± 0.66 | 0.006 | 0.047 |
| Atrial fibrillation/flutter | 126 (34.2) | 86 (35.2) | 35 (47.3) | 100 (57.8) | <0.001 | 0.865 |
| 5-m walk time (s) | 7.58 ± 4.11 | 7.97 ± 3.83 | 6.62 ± 2.27 | 8.17 ± 5.08 | 0.061 | 0.269 |
| Baseline KCCQ | 49.4 ± 26.0 | 49.3 ± 23.1 | 42.4 ± 26.5 | 43.5 ± 21.6 | 0.010 | 0.989 |
| Baseline echocardiographic variables | ||||||
| Aortic valve peak velocity (m/s) | 4.47 ± 0.45 | 3.50 ± 0.35 | 3.22 ± 0.45 | 3.34 ± 0.41 | <0.001 | <0.001 |
| Aortic valve mean gradient (mm Hg) | 48.5 ± 11.1 | 30.7 ± 5.1 | 26.1 ± 7.1 | 27.5 ± 6.5 | <0.001 | <0.001 |
| Aortic valve area (cm2) | 0.71 ± 0.18 | 0.85 ± 0.16 | 0.73 ± 0.18 | 0.74 ± 0.15 | <0.001 | <0.001 |
| LVEF (%) | 61.7 ± 11.5 | 64.0 ± 6.2 | 32.3 ± 8.7 | 62.5 ± 6.7 | <0.001 | 0.004 |
| ≥Moderate mitral regurgitation | 36 (9.8) | 17 (6.9) | 21 (28.4) | 26 (15.0) | <0.001 | 0.28 |
| Mitral stenosis | 45 (13.0) | 17 (7.5) | 6 (8.7) | 15 (9.5) | 0.185 | 0.056 |
| ≥Moderate tricuspid regurgitation | 48 (13.0) | 31 (12.7) | 13 (17.6) | 45 (26.0) | 0.001 | 0.985 |
| Right ventricular systolic pressure (mm Hg) | 47.0 ± 16.2 | 43.9 ± 15.0 | 44.8 ± 12.9 | 47.9 ± 17.4 | 0.073 | 0.034 |
Values are mean ± SD or n (%).
AS = aortic stenosis; LF-LG = low-flow, low-gradient; NF-LG = normal flow-low gradient.
Table 2.
Procedural Characteristics and Outcomes
| Overall (N = 860) | HG-AS (n = 368) | NF-LG AS (n = 245) | Classical LF-LG AS (n = 74) | Paradoxical LF-LG AS (n = 173) |
P Value |
||
|---|---|---|---|---|---|---|---|
| Overall | HG vs NF-LG | ||||||
| Follow-up vital status | |||||||
| Alive | 774 (90.0) | 345 (93.8) | 216 (88.2) | 66 (89.2) | 147 (84.9) | ||
| Deceased | 86 (10.0) | 23 (6.2) | 29 (11.8) | 8 (10.8) | 26 (15.1) | 0.009 | 0.001 |
| Cardiovascular death (% of deceased) | 30 (34.9) | 8 (34.8) | 11 (37.9) | 2 (25) | 9 (34.6) | 0.927 | 0.815 |
| Noncardiovascular death (% of deceased) | 36 (41.9) | 6 (26.1) | 12 (41.4) | 4 (50) | 14 (53.8) | 0.25 | 0.25 |
| Unknown cause of death (% of deceased) | 20 (23.2) | 9 (39.1) | 6 (20.7) | 2 (25) | 3 (11.5) | 0.147 | 0.145 |
| Death during TAVR admission (% of total) | 5 (0.6) | 0 (0) | 4 (1.6) | 0 (0) | 1 (0.6) | 0.0638 | 0.0139 |
| Prosthetic valve type | 0.657 | 0.581 | |||||
| Self-expanding | 745 (86.6) | 313 (85.0) | 213 (86.9) | 68 (91.9) | 151 (87.3) | ||
| Balloon-expandable | 108 (12.6) | 51 (13.9) | 31 (12.7) | 5 (6.8) | 21 (12.1) | ||
| Mechanically-expanding | 7 (0.8) | 4 (1.1) | 1 (0.4) | 1 (1.4) | 1 (0.6) | ||
| Follow-up echocardiographic parameters | |||||||
| ≥Moderate prosthetic regurgitation | 7 (0.8) | 5 (1.4) | 1 (0.4) | 1 (1.4) | 0 (0) | 0.316 | 0.452 |
| Mean prosthetic gradient (mm Hg) | 8.6 ± 4.4 | 9.9 ± 4.4 | 7.8 ± 3.4 | 7.6 ± 3.3 | 7.4 ± 3.7 | <0.001 | <0.001 |
| Follow-up events (%) | |||||||
| Pacemaker implantation within 30 d | 116 (13.5) | 57 (15.7) | 30 (12.9) | 8 (11.0) | 21 (12.9) | 0.604 | 0.404 |
| Aortic valve reintervention | 2 (0.2) | 1 (0.3) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0.173 | 0.414 |
| Hemorrhagic stroke | 3 (0.4) | 3 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.259 | 0.157 |
| Ischemic stroke | 16 (1.9) | 4 (1.1) | 8 (3.4) | 2 (2.7) | 2 (1.2) | 0.203 | 0.0565 |
| Transient ischemic attack | 14 (1.7) | 7 (1.9) | 6 (2.6) | 0 (0.0) | 1 (0.6) | 0.306 | 0.645 |
Values are n (%) or mean ± SD. Death during TAVR admission is included in total number of deaths within the first year.
AS = aortic stenosis; HG = high-gradient; KCCQ = Kansas City Cardiomyopathy Questionnaire; LF-LG = low-flow, low-gradient; NF-LG = normal-flow, low-gradient.
Mortality and adverse events
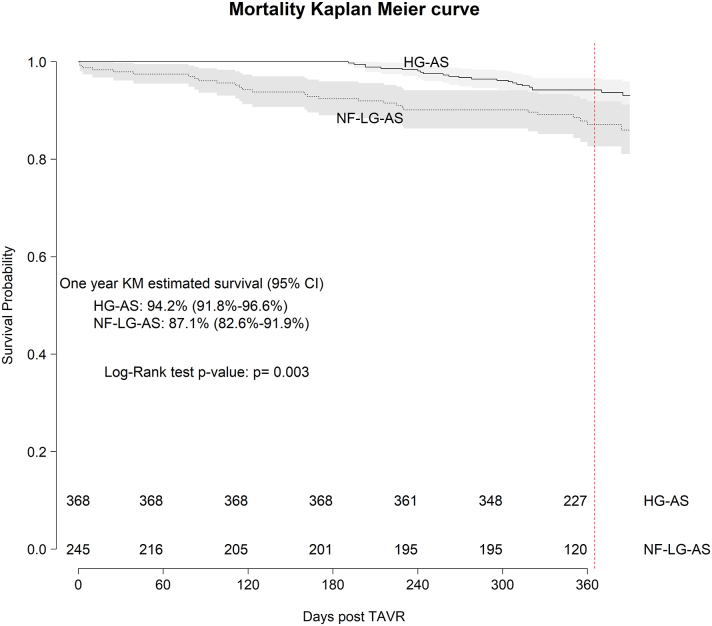
Out of a total of 860 patients, 86 (10.0%) died within 1 year, 244 (28.4%) were alive without 1-year KCCQ scores, 529 (61.5%) were alive with 1-year KCCQ scores, and 1 patient had 1-year KCCQ scores obtained just prior to death within the first year. This resulted in 615 (71.5%) patients with complete end point data and 530 (61.6%) with both baseline and 1-year KCCQ follow-up. Mortality rates at 1-year were higher for NF-LG AS (11.8%) compared with HG-AS (6.2%, P = 0.001) (Table 2). Survival curves for NF-LG AS differed significantly from HG-AS with earlier mortality seen for the former and significant survival differences maintained over the first year of follow-up (log rank P = 0.003) (Figure 1). NF-LG AS had higher mortality during the index TAVR admission compared with HG-AS (1.6% and 0%, respectively), with 4 postprocedural deaths. Although the numbers are small, this does represent 14% of the deaths within the NF-LG AS group. Three deaths were related to procedural complications: left main occlusion, resuscitated cardiac arrest during rapid pacing with refractory hypotension, and type A aortic dissection; one was attributable to nonprocedural-related factors: known cirrhosis with progressive liver dysfunction. Major nonfatal adverse events did not differ significantly between the groups (Table 2). Contrary to other hemodynamic groups, a large proportion of deaths in the NF-LG AS group occurred in patients with the lowest quartile of baseline KCCQ (0-29.2), indicating very poor baseline QoL among many who died in the first year (Supplemental Figure 1).
Figure 1.
Kaplan-Meier 1-Year Survival After TAVR
Kaplan-Meier survival curve comparing survival to 1 year for HG-AS compared to NF-LG AS groups. Estimated 1-year survival rates with 95% CI are reported, with comparisons made by the log-rank test. HG-AS = high-gradient aortic stenosis; NF-LG AS = normal-flow, low-gradient aortic stenosis.
Baseline and Changes in KCCQ Scores Across Hemodynamic Profiles
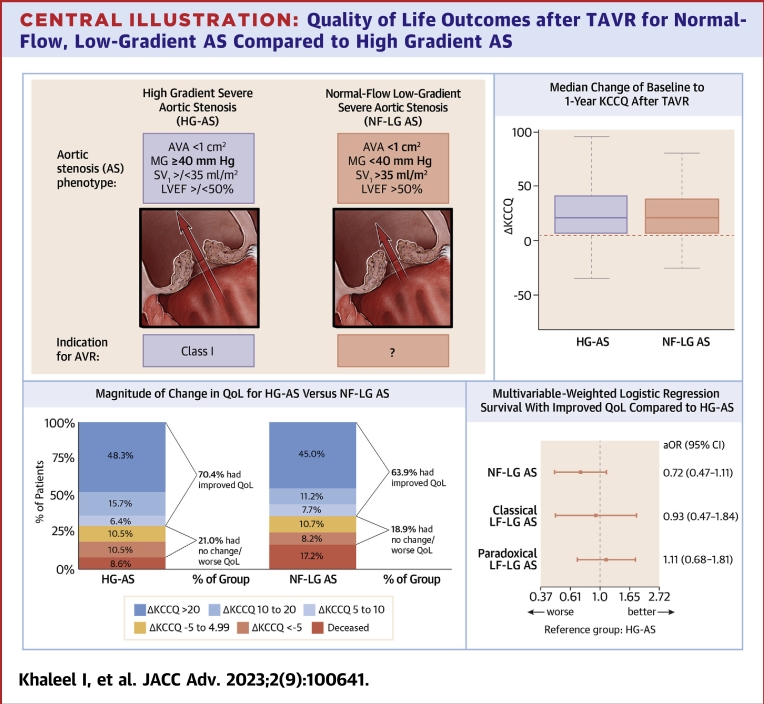
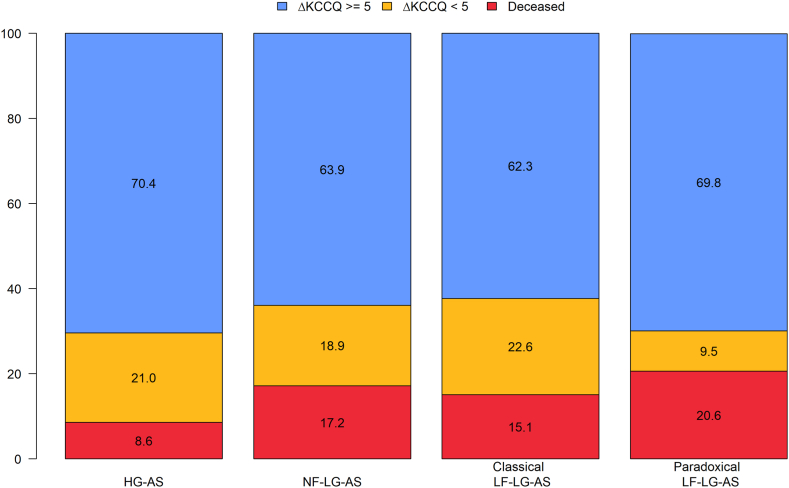
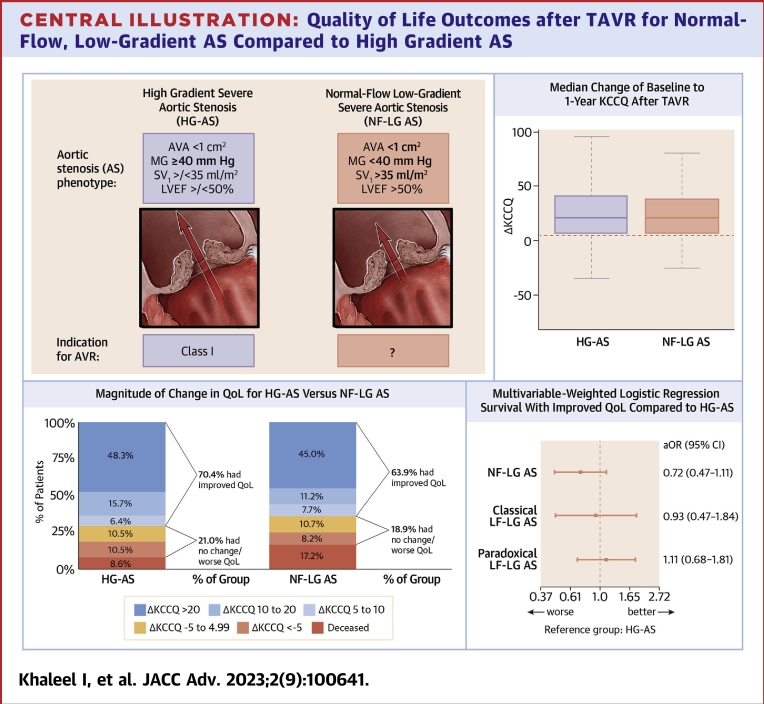
HG-AS and NF-LG AS groups had similar baseline KCCQ scores (Table 1). Baseline and 1-year KCCQ scores for patients with complete KCCQ follow-up data (n = 530) are shown in Table 3. All 4 groups demonstrated significant improvement in baseline to 1-year KCCQ scores with a similar magnitude seen across the groups (Figure 2). The HG-AS and NF-LG AS groups demonstrated a similar median increase in KCCQ (21.4 vs 20.8, respectively, P = 0.44) consistent with large improvements in QoL with similar distribution of ΔKCCQ (Central Illustration). Clinical improvement was achieved in 63.9% of the NF-LG AS group compared with 70.4% in the HG-AS group. Similar proportions of patients achieved large improvements in QoL (ΔKCCQ score ≥20): 45.0% of patients in the NF-LG AS group and 48.3% in the HG-AS group (Central Illustration).
Table 3.
Unadjusted Changes in KCCQ Among Patients With Complete Follow-Up Data
| Overall | HG-AS | NF-LG AS | Classical LF-LG AS | Paradoxical LF-LG AS |
P Value |
||
|---|---|---|---|---|---|---|---|
| Overall | HG vs NF-LG | ||||||
| Complete KCCQ data | 530a (61.6) | 245a (66.6) | 140 (57.1) | 45 (60.8) | 100 (57.8) | ||
| Baseline KCCQ-12 | 49.5 ± 24.8 | 51.3 ± 26.2 | 51.1 ± 22.2 | 45.9 ± 27.9 | 44.3 ± 22.3 | 0.061 | 0.918 |
| Follow-up KCCQ-12 | 73.5 ± 23.3 | 76.2 ± 23.2 | 73.3 ± 22.0 | 68.6 ± 27.1 | 69.3 ± 22.6 | 0.032 | 0.219 |
| ΔKCCQ-12 | 24.0 ± 24.8 | 24.9 ± 27.5 | 22.2 ± 21.6 | 22.7 ± 25.5 | 25.0 ± 21.6 | 0.722 | 0.318 |
| Complete end point data | 615 (71.5) | 267 (72.6) | 169 (69.0) | 53 (71.6) | 126 (72.8) | ||
| Achieving primary endpoint (1 y survival with ΔKCCQ ≥5) | 417 (67.8) | 188 (70.4) | 108 (63.9) | 33 (62.3) | 88 (69.8) | 0.393 | 0.189 |
Values are n (%) or mean ± SD. Baseline, follow-up and ΔKCCQ includes only those with complete KCCQ follow-up. Achieving primary end point includes all patients that had complete endpoint data (death within 1 year or 1-year follow-up KCCQ score); percentages are based on the total number of patients with available end point follow-up data.
AS = aortic stenosis; HG = high-gradient; KCCQ = Kansas City Cardiomyopathy Questionnaire; LF-LG = low-flow, low-gradient; NF-LG = normal-flow, low-gradient.
Follow-up KCCQ data were reported for one patient who died within the 1-year follow-up, with available follow-up KCCQ performed at 310 days post-TAVR.
Figure 2.
1-Year Vital Status and Change in QoL According to Baseline Gradients and Flow State
The percentage of patients with 1-year follow-up data with improved KCCQ score by ≥5 (blue), improvement by <5 points or decrease in KCCQ score (yellow) and death (red) at 1-year follow-up across all 4 hemodynamic profiles of aortic stenosis (AS). HG = high-gradient; KCCQ = Kansas City Cardiomyopathy Questionnaire; LF-LG = low-flow, low-gradient; NF-LG = normal-flow, low-gradient.
Central Illustration.
Quality of Life Outcomes after TAVR for Normal-Flow, Low-Gradient AS Compared to High Gradient AS
(Top left) Hemodynamic criteria for high-gradient severe aortic stenosis (HG-AS) and normal flow-low gradient severe aortic stenosis (NF-LG AS). AHA/ACC guidelines do not provide specific recommendations for timing of intervention for NF-LG AS. (Top right) Change in baseline to 1-year KCCQ shown only for patients alive with KCCQ follow-up for HG-AS and NF-LG AS groups. Boxes represent median with inner quartile range with whiskers representing 95% confidence intervals. (Bottom left) Magnitude of baseline to 1-year KCCQ change in patients with complete follow-up (mortality or KCCQ follow-up data) comparing HG-AS and NF-LG AS Groups. (Bottom right) Primary adjusted end point consisting of: 1) survival to 1 year; and 2) improved KCCQ score of ≥5 for AS groups compared to HG-AS group as reference. aOR = adjusted odds ratio; AV = aortic valve; AVA = aortic valve area; AVR = aortic valve replacement; KCCQ = Kansas City Cardiomyopathy Questionnaire; LF-LG = low-flow, low-gradient; LVEF = left ventricular ejection fraction; MG = mean gradient; SVi = indexed stroke volume; TAVR = transcatheter aortic valve replacement.
Primary adjusted analysis
Baseline characteristics data were relatively complete (Supplemental Table 2), with the exception of 5 m walk time, where 14.2% of patients did not have values collected. For these cases, values were imputed to the maximum observed value of 42 minutes for inclusion in the primary end point analysis. Otherwise, baseline covariate data was complete with no patients excluded from the final analysis due to missing baseline data. After adjusting for baseline characteristics, rates of the primary composite outcome were not significantly different between groups (Central Illustration). There was a trend toward worse QoL outcomes in the NF-LG AS group compared with the HG-AS; however, this did not reach statistical significance (adjusted OR: 0.72, 95% CI: 0.47-1.11; P = 0.13). Outcomes were similar for classical LF-LG AS and paradoxical LF-LG AS compared with HG-AS.
Discussion
In this single-center study of QoL after TAVR according to baseline aortic valve gradients and flow state, patients with NF-LG AS experienced similar rates of clinical improvement compared with other hemodynamic profiles. In unadjusted analyses, all 4 hemodynamic groups demonstrated remarkably similar improvements in QoL after TAVR. The mean increase in baseline to 1-year KCCQ score for NF-LG AS patients was 22.2 points, which corresponds to large improvement in QoL after TAVR and was comparable to the other 3 groups for which specific guidelines-based indications for timing of intervention exist.1,2,16 The primary adjusted analysis demonstrated no significant difference in rates of clinical improvement after TAVR for NF-LG AS compared with HG-AS. There was increased early mortality for patients with NF-LG AS as compared with HG-AS, which requires further investigation.
Clinical implications
Patients with cardiovascular symptoms and echocardiographic evidence of NF-LG AS represent a clinical conundrum. Careful assessment of echocardiographic measurements to exclude measurement error resulting in an inappropriately low AVA and a detailed assessment to rule out other more likely causes of symptoms are critical. In the elderly population of patients with AS, comorbidities are common and symptoms are often multifactorial.19 The extent to which a patient’s symptoms are attributable to their AS is often uncertain, especially in the absence of a severely elevated aortic valve gradient.5 Although aortic valve intervention has demonstrated clear improvements in hard end points such as mortality in patients with HG-AS, classical LF-LG AS, and paradoxical LF-LG AS,1,2 survival benefit is less clear for patients with NF-LG AS based on prior studies with conflicting results. Therefore, an understanding of expected improvements in QoL after aortic valve intervention is critical for informed decision-making for symptomatic patients with NF-LG AS, a subgroup in which QoL data after TAVR are lacking.
This study demonstrates that in highly selected patients with NF-LG AS, improvements in QoL are similar to other hemodynamic profiles of AS with guideline-based indications for AVR. This study supports that selected symptomatic patients with NF-LG AS derive significant symptomatic benefit from TAVR. Although the primary adjusted analysis does suggest a trend toward improved outcomes for HG-AS compared to NF-LG AS, this is consistent with prior studies demonstrating that the degree of benefit is related to baseline aortic valve gradients.2,18 A remarkable finding from this study was the degree of symptomatic benefit achieved by patients with NF-LG AS, with 64% of patients achieving some degree of clinically significant improvement in symptoms after TAVR and 46% of patients achieving large improvements in QoL, both similar to patients with HG-AS.
The differences in mortality between NF-LG AS and HG-AS are notable. There were no significant differences in nonfatal adverse procedure-related events between the hemodynamic groups. Furthermore, deaths in the NF-LG AS group disproportionately occurred in patients with very poor QoL at baseline compared with other groups. Both of these findings strongly support that mortality differences seen between groups were not driven by the TAVR procedure itself but rather were reflective of baseline clinical differences between groups.
Study Limitations
The findings from this study should be considered in the context of several important limitations. Most limitations are related to the observational nature of the study design. Although we adjusted for baseline covariates that have been shown to be associated with QoL outcomes after TAVR, there are likely unmeasured confounding variables that are not collected within the TVT registry dataset. We chose to be inclusive of all patients undergoing TAVR that met our prespecified aortic valve hemodynamic criteria without excluding patients with significant noncardiac medical conditions such as significant kidney or liver disease. Our intent was for this to represent a real-world population of patients deemed appropriate for TAVR by a multidisciplinary group. As a result, it is very likely that significant unmeasured confounding medical conditions are driving differences in mortality between AS groups. The baseline covariates used in our model were selected based on prior analyses by Arnold et al8 identifying prognostic indicators of poor QoL outcome after TAVR. Several variables in the previously described model are not routinely collected in the TVT registry and were replaced by surrogate markers with similar physiologic significance, though these have not been individually validated in this context.
All patients in this study underwent TAVR; therefore, these data cannot be used to draw conclusions about the comparative effects of TAVR vs medical management on survival or symptomatic improvement in NF-LG AS. The placebo effect is important to consider in studies of QoL and has been shown to be significant in studies of invasive cardiology procedures.20 Although we cannot rule out some degree of placebo effect resulting in improved QoL after TAVR, this finding was consistent with all other hemodynamic profiles of AS, making this an unlikely contributor to the majority of the QoL improvements seen. Missingness in the form of incomplete patient data is an important limitation. The completeness of our QoL data is comparable to other studies, with Arnold et al reporting 26.6% of 1-year survivors with missing KCCQ scores in a real-world population, compared to 28.4% in this study.17 We attempted to minimize the effect of missingness by performing inverse probability weighting to account for patients with missing follow-up scores—a methodology that is consistent with prior studies. Finally, our study’s single-center design with resultant limited number of patients does increase the risk of type 2 statistical error from inadequate power. This may be responsible for the lack of statistically significant difference between HG-AS and NF-LG AS groups in the primary adjusted analysis. Larger, multicenter studies will help to provide further information to guide management in patients with NF-LG AS.
Conclusions
Contemporary valve guidelines have not included recommendations for management of patients with symptomatic NF-LG AS, despite this representing a sizeable subset of patients with AS. In this single-center study comparing changes in QoL after TAVR according to baseline aortic valve gradients and flow states, significant improvement in QoL was seen in patients with NF-LG AS. A similar magnitude of QoL improvement was seen in NF-LG AS as compared to patients with HG-AS. Increased early mortality was observed in the NF-LG AS group, which requires further investigation. Multicenter investigation of QoL after TAVR will help to inform management of patients with NF-LG AS and aid in shared decision-making with patients.
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Select symptomatic patients with NF-LG AS demonstrated improved health-related QoL after TAVR with similar rates of clinical improvement compared with HG-AS patients.
TRANSLATIONAL OUTLOOK: Further research is needed to identify which patients with NF-LG AS are most likely to benefit from aortic valve intervention.
Funding support and author disclosures
Dr Fukuhara is a consultant for Terumo Aortic. Dr Chetcuti has received grant support and sponsorship from Edwards Lifesciences, Boston Scientific, and Medtronic has received research sponsorship from St. Jude Medical; and has received proctoring fees from and served as a consultant for Medtronic. Dr Grossman has received grant support from Edwards Lifesciences, Boston Scientific, and Medtronic; and has received proctoring fees from Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgment
The authors thank Brittany Powell, BS, for her tireless work as our TVT registry coordinator.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX
For supplemental tables and figures, please see the online version of this paper.
Supplementary data
References
- 1.Vahanian A., Beyersdorf F., Praz F., et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Rev Esp Cardiol. 2022;75(6):524. doi: 10.1016/j.rec.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Clavel M.A., Magne J., Pibarot P. Low-gradient aortic stenosis. Eur Heart J. 2016;37(34):2645–2657. doi: 10.1093/eurheartj/ehw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tribouilloy C., Rusinaru D., Maréchaux S., et al. Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. J Am Coll Cardiol. 2015;65(1):55–66. doi: 10.1016/j.jacc.2014.09.080. [DOI] [PubMed] [Google Scholar]
- 5.Jander N., Minners J., Holme I., et al. Outcome of patients with low-gradient severe aortic stenosis and preserved ejection fraction. Circulation. 2011;123(8):887–895. doi: 10.1161/CIRCULATIONAHA.110.983510. [DOI] [PubMed] [Google Scholar]
- 6.Eleid M.F., Sorajja P., Michelena H.I., Malouf J.F., Scott C.G., Pellikka P.A. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation. 2013;128(16):1781–1789. doi: 10.1161/CIRCULATIONAHA.113.003695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maes F., Boulif J., Piérard S., et al. Natural history of paradoxical low-gradient severe aortic stenosis. Circ Cardiovasc Imaging. 2014;7(4):714–722. doi: 10.1161/CIRCIMAGING.113.001695. [DOI] [PubMed] [Google Scholar]
- 8.Chadha G., Bohbot Y., Rusinaru D., Maréchaux S., Tribouilloy C. Outcome of normal-flow low-gradient severe aortic stenosis with preserved left ventricular ejection fraction: a propensity-matched study. J Am Heart Assoc. 2019;8(19):11–14. doi: 10.1161/JAHA.119.012301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dayan V., Vignolo G., Magne J., Clavel M.A., Mohty D., Pibarot P. Outcome and impact of aortic valve replacement in patients with preserved LVEF and low-gradient aortic stenosis. J Am Coll Cardiol. 2015;66(23):2594–2603. doi: 10.1016/j.jacc.2015.09.076. [DOI] [PubMed] [Google Scholar]
- 10.Bonow R.O., Brown A.S., Gillam L.D., et al. ACC/AATS/AHA/ASE/EACTS/HVS/SCA/SCAI/SCCT/SCMR/STS 2017 appropriate use criteria for the treatment of patients with severe aortic stenosis: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Valve Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;70(20):2566–2598. doi: 10.1016/j.jacc.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Popma J.J., Deeb G.M., Yakubov S.J., et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706–1715. doi: 10.1056/nejmoa1816885. [DOI] [PubMed] [Google Scholar]
- 12.Minners J., Allgeier M., Gohlke-Baerwolf C., Kienzle R.P., Neumann F.J., Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: Haemodynamic studies in patients with apparently normal left ventricular function. Heart. 2010;96(18):1463–1468. doi: 10.1136/hrt.2009.181982. [DOI] [PubMed] [Google Scholar]
- 13.Généreux P., Piazza N., Alu M.C., et al. Valve Academic Research Consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. 2021;42(19):1825–1857. doi: 10.1093/eurheartj/ehaa799. [DOI] [PubMed] [Google Scholar]
- 14.Zoghbi W.A., Adams D., Bonow R.O., et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Arnold S.V., Spertus J.A., Lei Y., et al. Use of the Kansas city cardiomyopathy questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6(1):61–67. doi: 10.1161/CIRCHEARTFAILURE.112.970053. [DOI] [PubMed] [Google Scholar]
- 16.Spertus J.A., Jones P.G., Sandhu A.T., Arnold S.V. Interpreting the Kansas city cardiomyopathy questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(20):2379–2390. doi: 10.1016/j.jacc.2020.09.542. [DOI] [PubMed] [Google Scholar]
- 17.Arnold S.V., Cohen D.J., Dai D., et al. Predicting quality of life at 1 year after transcatheter aortic valve replacement in a real-world population. Circ Cardiovasc Qual Outcomes. 2018;11(10) doi: 10.1161/CIRCOUTCOMES.118.004693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold S.V., Reynolds M.R., Lei Y., et al. Predictors of poor outcomes after transcatheter aortic valve replacement results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129(25):2682–2690. doi: 10.1161/CIRCULATIONAHA.113.007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzzetti E., Poulin A., Annabi M.S., et al. Transvalvular flow, sex, and survival after valve replacement surgery in patients with severe aortic stenosis. J Am Coll Cardiol. 2020;75(16):1897–1909. doi: 10.1016/j.jacc.2020.02.065. [DOI] [PubMed] [Google Scholar]
- 20.Al-Lamee R., Thompson D., Dehbi H.M., Sen S., Tang K., Davies J. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomized controlled trial. J Vasc Surg. 2018;67(2):673. doi: 10.1016/j.jvs.2017.11.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.