Corresponding Author

Key words: artificial intelligence, deep learning, diagnosis, echocardiography, HFpEF
The proportion of patients with heart failure (HF) presenting with preserved ejection fraction (HFpEF) is still increasing globally, and outcomes remain poor despite recent advances in treatment.1 This may partially be explained by a lack of consensus on how to diagnose HFpEF.2,3 Diagnosis is straightforward in patients presenting late, decompensated stage with congestion and elevated left ventricular filling pressures (LVFPs). However, many patients present earlier with exercise intolerance and exertional dyspnea, with high LVFP only during exercise.4 Resting examinations, including echocardiography, are often normal at this stage. The ‘gold standard’ method to diagnose early HFpEF is exercise right heart catheterization, however this is an invasive procedure that is not widely available and requires specific expertise.5 Scoring systems for HFpEF diagnosis based on resting examinations have been validated, including the H2FPEF and Heart Failure Association PEFF (HFA-PEFF) scores. However, many patients are labeled as having an ‘intermediate’ risk, mandating further testing.6
Artificial intelligence (AI) applications are increasingly used to extract patterns from medical images that humans cannot reliably observe.7 These applications promise to enhance the precision of the noninvasive estimation of LVFP. Broadly defined, AI refers to computational software capable of making autonomous decisions by analyzing and interpreting collected data, such as those obtained from pattern identification. Machine learning is a subset of AI that allows an algorithm to adapt and learn without being explicitly programmed. Deep learning (DL) is a class of machine learning algorithms that utilize multiple layers for the extraction of progressively higher-level features from a raw input (eg, convolutional neural networks).7 With the growth in size and complexity of echocardiographic data, AI has promising applications in clinical research and routine practice for diagnosing and assessing HFpEF and other cardiac conditions. Recent examples of AI applications in echocardiography include fully automated annotation of echocardiograms,8 detection of subclinical reductions in cardiac function,9 and quantification of valvular stenosis and regurgitation.10 These advancements have consistently demonstrated a level of precision that surpasses human interpretation.11
In this issue of JACC: Advances, Akerman et al12 used AI algorithms to identify HFpEF from a single 4-chamber echocardiographic video. The authors found the algorithm could discriminate well between HFpEF and non-HFpEF patients in holdout and independent validation cohorts. Subsequently, the diagnostic performance of the AI algorithm was compared to scoring systems, demonstrating that the AI algorithm correctly reclassified many patients that were designated as ‘indeterminate’ using the scores. Additionally, patients identified as ‘HFpEF’ by the algorithm had higher mortality.
Previously, Chiou et al13 also trained a DL model for diagnosing HFpEF on a smaller data set of 4-chamber echocardiography videos, achieving high accuracy, sensitivity, and specificity. Other AI applications for detecting HFpEF have been limited to analysis of human-labeled Doppler measurements or still images of left ventricular functional measurements, limiting their rapid online use in clinical practice.14,15 Akerman et al12 need to be commended for using a large and granular data set spanning several centers and countries, carefully developing and testing their algorithm even in relevant subgroups, and, importantly, executing validation ‘by the book’ in separate holdout and independent data sets, which is rare in AI studies. Furthermore, the authors used heatmaps to identify the most important echocardiographic features the algorithm used to identify a patient as HFpEF or non-HFpEF. This effectively ‘opened the black box’ of DL algorithms and can give physicians insights into the algorithm's decision-making process.
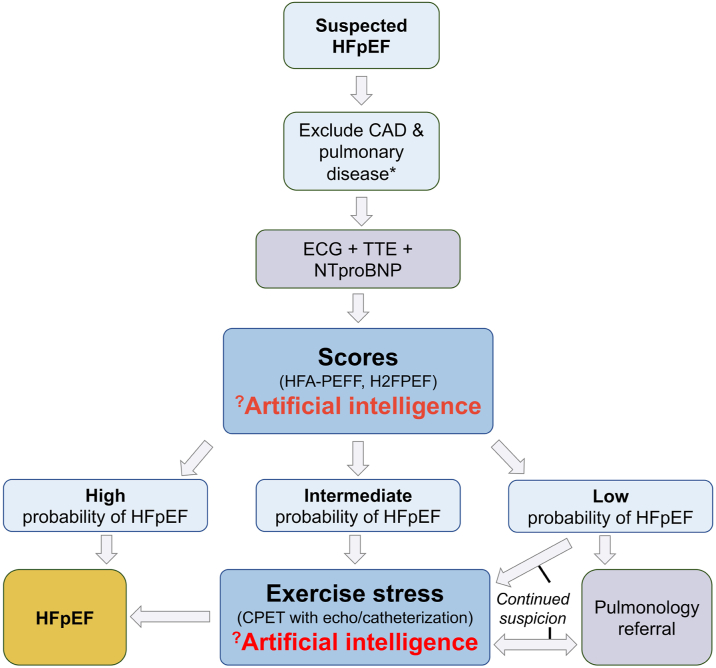
However, several questions and areas for future study remain. Methods to confirm a definite HFpEF diagnosis remain suboptimal in all the studies mentioned above. Akerman et al12 relied on International Classification of Disease codes to verify and exclude a diagnosis of HFpEF. Unfortunately, codes for systolic HF were included, and a wide timeframe of 1 year was used, enabling labeling of HF patients with recovered or declining left ventricular ejection fraction as ‘HFpEF.’ Also, the absence of International Classification of Disease codes related to HF and elevated LVFP at rest do not exclude a diagnosis of (early) HFpEF, limiting the validity of the control sample. Ideally, the algorithm will need to be recalibrated using resting echocardiograms of patients with HFpEF confirmed by exercise right heart catheterization, but samples of these patients are limited and entail significant referral bias. Second, the better performance of the model compared to the scoring systems needs to be interpreted with caution. Natriuretic peptide levels are an essential part of the HFA-PEFF score.2 However, these were only available in 12% of the validation population, implying that a diagnosis of HFpEF could not be established anyway in the majority of patients using this score. Both scoring systems mandate further (exercise) testing in patients labeled as ‘intermediate’ risk, which was not accounted for in the current study. Finally, all patients labeled as HFpEF in the present study already had signs of elevated LVFP on echocardiography. While matching the accuracy of traditional echocardiographic indicators of elevated LVFP by using only a 4-chamber view is certainly impressive, the accuracy of these indicators in HFpEF is heavily debated.16 It remains unclear whether the AI model is also useful in more challenging clinical scenarios without evident signs of elevated LVFP at rest (Figure 1).
Figure 1.
Potential Application of Artificial Intelligence in the Diagnosis of HFpEF
Depending on the training population (prevalence of HFpEF, rule-in, and rule-out method), artificial intelligence models could serve as ‘decision maker’ in intermediate probability and/or a ‘gatekeeper’ for advanced exercise testing. ∗Based on history and clinical examination. CPET = cardiopulmonary exercise test; ECG = electrocardiogram; H2FPEF = HFpEF score according to Reddy et al; HFA-PEFF = Heart Failure Association PEFF score according to Pieske et al; HFpEF = heart failure with preserved ejection fraction; TTE = transthoracic echocardiography.
As the authors imply in their paper, the ideal use for this algorithm (or similar AI applications) would be as a ‘second read’ when other clinical or echocardiographic metrics remain uncertain regarding a diagnosis of HFpEF. AI could serve as a ‘decision maker’ in intermediate or indeterminate cases and/or a ‘gatekeeper’ for advanced exercise testing to enhance early HFpEF diagnosis and enable timely treatment (Figure 1).
Funding support and author disclosures
Dr Gevaert has received lecture/advisory board fees paid to his institution by Abbott, AstraZeneca, Boehringer Ingelheim, Novartis, and Menarini outside the submitted work. Dr Van De Heyning has received personal fees from Daiichi-Sankyo, Bayer, Pfizer, and Edwards Lifesciences (lectures) outside the submitted work. Dr Tromp is supported by the National University of Singapore Start-up grant, the tier 1 grant from the Ministry of Education, and the CS-IRG New Investigator Grant from the National Medical Research Council; has received consulting or speaker fees from Daiichi-Sankyo, Boehringer Ingelheim, Roche diagnostics and Us2.ai; and owns patent US-10702247-B2 unrelated to the present work.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Tromp J., Ferreira J.P., Janwanishstaporn S., et al. Heart failure around the world. Eur J Heart Fail. 2019;21:1187–1196. doi: 10.1002/ejhf.1585. [DOI] [PubMed] [Google Scholar]
- 2.Pieske B., Tschöpe C., de Boer R.A., et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Am Coll Cardiol. 2022;79:e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Gevaert A.B., Kataria R., Zannad F., et al. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart. 2022;108:1342–1350. doi: 10.1136/heartjnl-2021-319605. [DOI] [PubMed] [Google Scholar]
- 5.Borlaug B.A., Sharma K., Shah S.J., Ho J.E. Heart failure with preserved ejection fraction. J Am Coll Cardiol. 2023;81:1810–1834. doi: 10.1016/j.jacc.2023.01.049. [DOI] [PubMed] [Google Scholar]
- 6.Verbrugge F.H., Reddy Y.N.V., Sorimachi H., Omote K., Carter R.E., Borlaug B.A. Diagnostic scores predict morbidity and mortality in patients hospitalized for heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23(6):954–963. doi: 10.1002/ejhf.2142. [DOI] [PubMed] [Google Scholar]
- 7.Dey D., Slomka P.J., Leeson P., et al. Artificial intelligence in cardiovascular imaging. J Am Coll Cardiol. 2019;73:1317–1335. doi: 10.1016/j.jacc.2018.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tromp J., Seekings P.J., Hung C.-L., et al. Automated interpretation of systolic and diastolic function on the echocardiogram: a multicohort study. Lancet Digit Health. 2022;4:e46–e54. doi: 10.1016/S2589-7500(21)00235-1. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang D., He B., Ghorbani A., et al. Video-based AI for beat-to-beat assessment of cardiac function. Nature. 2020;580:252–256. doi: 10.1038/s41586-020-2145-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang F., Chen X., Lin X., et al. Automated analysis of Doppler echocardiographic videos as a screening tool for valvular heart diseases. J Am Coll Cardiol Img. 2022;15:551–563. doi: 10.1016/j.jcmg.2021.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Tromp J., Bauer D., Claggett B.L., et al. A formal validation of a deep learning-based automated workflow for the interpretation of the echocardiogram. Nat Commun. 2022;13:6776. doi: 10.1038/s41467-022-34245-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akerman A.P., Porumb M., Scott C.G., et al. Automated echocardiographic detection of heart failure with preserved ejection fraction using artificial intelligence. JACC: Adv. 2023;2(6) [Google Scholar]
- 13.Chiou Y.-A., Hung C.-L., Lin S.-F. AI-assisted echocardiographic prescreening of heart failure with preserved ejection fraction on the basis of intrabeat dynamics. J Am Coll Cardiol Img. 2021;14:2091–2104. doi: 10.1016/j.jcmg.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Martinez S., Duchateau N., Erdei T., et al. Machine learning analysis of left ventricular function to characterize heart failure with preserved ejection fraction. Circ Cardiovasc Imaging. 2018;11 doi: 10.1161/CIRCIMAGING.117.007138. [DOI] [PubMed] [Google Scholar]
- 15.Pandey A., Kagiyama N., Yanamala N., et al. Deep-learning models for the echocardiographic assessment of diastolic dysfunction. J Am Coll Cardiol Img. 2021;14(10):1887–1900. doi: 10.1016/j.jcmg.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Nauta J.F., Hummel Y.M., Van Der Meer P., Lam C.S.P., Voors A.A., Van Melle J.P. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2018;20(9):1303–1311. doi: 10.1002/ejhf.1220. [DOI] [PubMed] [Google Scholar]