Abstract
Background
Sex-based differences in clinical outcomes among patients with stroke related to left ventricular assist devices (LVADs) are not well described.
Objectives
In this study, the authors examined differences in clinical characteristics and outcomes in men and women who had a stroke during LVAD hospitalization.
Methods
The National Inpatient Sample from 2010 and 2019 was used to identify patients with stroke during LVAD hospitalization. Outcomes of interest include inpatient mortality and clinical complications among men vs women. Weighted logistic regression was used to determine the association of sex and outcomes. Adjustments were made for age and the Elixhauser comorbidity index.
Results
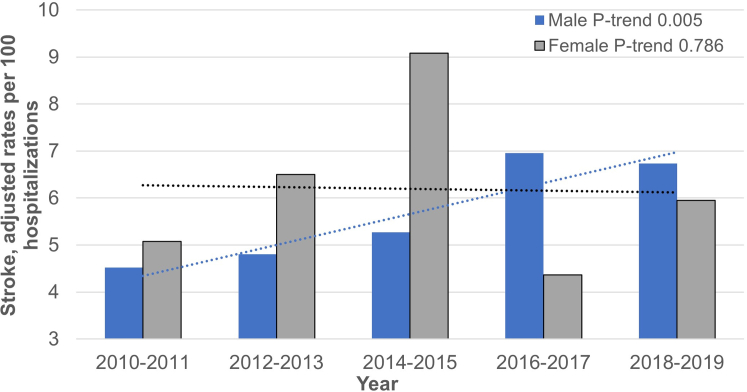
In total, 35,820 patients underwent LVAD implantation (77% men), and 6.12% (n = 2,192) of patients experienced stroke. Women who had stroke were younger than men who had stroke (mean age in women was 51 years vs men 59 years, P < 0.001). Men with strokes had a higher burden of comorbidities than women. While there were no differences in the odds of ischemic stroke, women had higher odds of hemorrhagic stroke compared to men (OR: 1.49 [95% CI: 1.02-2.18]). Mortality in patients with LVAD who had stroke was significantly higher than in those without stroke. Between 2010 and 2019, stroke rates significantly increased among men, while the trend remained variable among women.
Conclusions
In this national cohort, men had a higher comorbidity burden and had worsening stroke trends over the last decade compared to women. Women had fewer LVAD implants and a higher incidence of hemorrhagic stroke. Understanding the factors that contribute to sex-related outcome disparities among LVAD stroke patients is crucial in addressing these diverging trends.
Key words: heart failure, hemorrhagic stroke, left ventricular assisted device, LVAD, sex difference, stroke
Central Illustration
Despite advancements in medicine, there is an overbearing burden on patients with heart failure with a concomitant rise in patients with advanced heart failure requiring either left ventricular assist devices (LVADs) or transplant.1,2 The Society of Thoracic Surgeons Interagency Registry for Mechanically Assisted Circulatory Support (Intermacs) 2020 report indicates a promising future for LVAD with average survival now approaching 5 years. A record number of over 2,400 LVADs were implanted in 2021.2 However, LVAD-related adverse events, particularly stroke, major infections, and multiorgan failure, contribute to over 50% of LVAD-related deaths.1,2 The risk for LVAD-related complications remains highest in the early postoperative phase (<90 days).1 Stroke remains the primary cause of death among patients with LVAD; approximately 13% of individuals will experience a stroke within a year of implantation.3,4 It is imperative to identify patient populations that are at the highest-risk of adverse events to effectively mitigate their risk, particularly in the highest risk period (early postoperative phase). For example, preimplant risk assessments tailored for sex-based differences could inform personalized perioperative care plans. Additionally, targeted pharmacological interventions, like anticoagulant regimens tailored to individual risk profiles, may also be beneficial.
It is known that LVAD-related complications differ among sex. Women sex was found to be an important preimplant predictor of stroke in an Intermacs analysis for patients implanted from 2012 to 2015.5 According to a recent International Society for Heart and Lung Transplantation registry analysis, women have higher odds of death predominantly due to neurological events compared to men in the first 3 months post-implant.6,7 However, there have not been many studies focusing on sex-based differences and clinical outcomes in patients with stroke during the early post-implant phase.
In this study, we used the National Inpatient Sample (NIS) database to compare demographic and clinical characteristics, inpatient outcomes, and measures of health care utilization among patients who experienced a stroke during LVAD hospitalization. We present and explain patient outcomes associated with sex-based disparities related to LVAD.8
Methods
Data source
This was a retrospective analysis of discharge data from the NIS between January 1, 2010 and December 31, 2019. The NIS is an all-payer database that approximates a 20% stratified sample of discharges from U.S. community hospitals participating in the Healthcare Cost and Utilization Project (HCUP).8 It contains unweighted data from more than 7 million yearly hospital stays and, once weighted, estimates approximately 35 million stays. It includes deidentified, clinical, and nonclinical elements such as primary and secondary diagnoses, patient demographics, payment source, length of stay (LOS), and severity and comorbidity measures.9 Given the nature of the database, institutional review board approval is exempted. HCUP redesigned the NIS in 2012 to improve national estimates. It was changed to a sample of discharge records from all HCUP-participating hospitals, rather than a sample of hospitals from which all discharges were retained. To account for the NIS sample redesign, we used the updated trend weights for the period between 2010 and 2011 and the original discharge weights for 2012 to 2019.
Study design and population
The primary focus of the study was identifying sex differences in stroke during hospitalization for LVAD implantation. We identified all LVAD hospitalizations in patients aged 18 years and above in the NIS database from January 1, 2010, through December 31, 2019, as per data availability at the time of analysis, using the International Classification of Diseases-9th Revision-Clinical Modification (ICD-9-CM) procedure code of 3766 and ICD-10-CM code 02HA0QZ.8 In addition, hospitalizations with stroke were identified using ICD-9-CM codes 430, 431, 43301, 43311, 43321, 43331, 43381, 43391, 43401, 43411, 43491, and 436 and ICD-10-CM codes I60xx, I61xx, I63.x, I9782xx, and I9781xx. For each hospitalization, baseline demographic characteristics, hospital characteristics, and clinically relevant comorbidities were identified. Clinical co-morbidities were identified using Elixhauser comorbidities and ICD-9-CM and ICD-10-CM codes provided in Supplemental Tables 1 to 3.
Statistical analysis
Descriptive statistics were used to summarize the continuous and categorical variables. The mean ± SE were used for continuous variables, and the categorical variables were expressed as frequencies (percentages). Univariate analyses for between-group comparisons used the Rao-Scott chi-square test for categorical variables (eg, sex and risk factors) and weighted simple linear regression for continuous variables (eg, age).
Weighted logistic regression was performed to estimate adjusted ORs and 95% CIs to determine the association of sex and various clinical outcomes in hospitalizations with LVAD procedure who had stroke. The logistic regression model was adjusted for age and the Elixhauser comorbidity index.
Hospital total charges were converted to cost estimates using hospital-specific cost-to-charge ratios provided by HCUP. Total charges were inflated to 2019 US dollars using the Consumer Price Index inflation calculator published by the US Bureau of Labor Statistics.10
Trends in unadjusted hospitalization outcomes were examined using linear regression for continuous variables, including LOS and inflation-adjusted cost, and binary logistic regression for a categorical variable (ie, percentage of stroke, mortality, and disposition), with year as the sole predictor. Subsequently, for obtaining trends for adjusted percentage of stroke, mortality, disposition, LOS, and inflation-adjusted cost, we used margins post-estimation commands. Adjustments were made for age, sex, and the Elixhauser comorbidity index.
Using Stata 16.1 (Stata Corp), our analyses took into account survey design complexity by incorporating sampling weights, primary sampling units, and strata. This allowed us to estimate population proportions, means, and regression coefficients using svy commands. Standard errors were computed using Taylor series linearization. P values <0.05 were considered statistically significant.
Results
In the NIS between 2010 and 2019, 35,820 hospital admissions for LVAD were identified, 77% men (n = 27,617) and 23% women (n = 8,203). Men were older compared to women (mean age 57.5 vs 53.6 years, P < 0.001). A higher burden of underlying comorbidities was noted among men. Men showed higher burden of atrial fibrillation, diabetes, hypertension, renal failure, dyslipidemia, smoking, alcohol use, previous myocardial infarction, previous coronary artery bypass graft, and previous percutaneous coronary intervention. On the other hand, women showed a higher burden of hypothyroidism and valvular disease when compared to men. The frequency of chronic pulmonary disease, carotid artery disease, peripheral vascular disease, liver disease, drug abuse, prior cardiovascular disease, and prior pacemaker was not statistically significant between men and women (Table 1). There was a statistically significant difference in household income and residence between sexes. Thirty-one percent of women with LVADs belonged to the lowest income resident category (0-25th), while men constituted a higher percentage of the highest income resident category (75th-100th) compared to women (23% vs 18%, respectively; P < 0.001) (Table 2).
Table 1.
Baseline Demographics in Patients With LVAD Hospital Admissions Between 2010 to 2019: Stratified by Sex
| Male | Female | Total | P Value | |
|---|---|---|---|---|
| (n = 27,617) | (n = 8,203) | (N = 35,820) | ||
| Age, y | 57.52 ± 0.19 | 53.6 ± 0.34 | 56.63 ± 0.17 | <0.001 |
| Race and ethnicity | <0.001 | |||
| White | 16,741 (64.87) | 4,150 (53.98) | 20,890 (62.37) | |
| Black | 5,612 (21.74) | 2,674 (34.79) | 8,286 (24.74) | |
| Hispanics | 1,692 (6.56) | 494 (6.43) | 2,186 (6.53) | |
| Others | 1,764 (6.83) | 369 (4.80) | 2,133 (6.37) | |
| Comorbidities | ||||
| Chronic pulmonary disease | 9,528 (34.50) | 2,920 (35.59) | 12,447 (34.75) | 0.40 |
| Atrial fibrillation | 12,321 (44.61) | 3,027 (36.90) | 15,348 (42.85) | <0.001 |
| Diabetes mellitus | 9,944 (36.01) | 2,604 (31.75) | 12,549 (35.03) | 0.002 |
| Hypertension | 18,376 (66.54) | 4,727 (57.63) | 23,103 (64.50) | <0.001 |
| Obesity | 4,530 (16.40) | 1,696 (20.67) | 6,226 (17.38) | <0.001 |
| Carotid artery disease | 329 (1.19) | 144 (1.76) | 473 (1.32) | 0.086 |
| Peripheral vascular disease | 6,738 (24.40) | 1,906 (23.24) | 8,644 (24.13) | 0.33 |
| Renal failure | 13,731 (49.72) | 2,930 (35.72) | 16,661 (46.51) | <0.001 |
| Liver disease | 5,157 (18.67) | 1,490 (18.16) | 6,646 (18.55) | 0.64 |
| Dyslipidemia | 10,468 (37.90) | 2,536 (30.92) | 13,004 (36.30) | <0.001 |
| Hypothyroidism | 2,776 (10.05) | 1,241 (15.13) | 4,017 (11.22) | <0.001 |
| Valvular disease | 9,647 (34.93) | 3,451 (42.07) | 13,097 (36.56) | <0.001 |
| Smoking | 7,399 (26.79) | 1,863 (22.71) | 9,262 (25.86) | 0.002 |
| Alcohol abuse | 950 (3.44) | 150 (1.82) | 1,100 (3.07) | 0.001 |
| Drug abuse | 1,000 (3.62) | 214 (2.61) | 1,214 (3.39) | 0.044 |
| Previous myocardial infarction | 5,101 (18.47) | 1,047 (12.77) | 6,149 (17.17) | <0.001 |
| Previous CABG | 3,052 (11.05) | 419 (5.10) | 3,471 (9.69) | <0.001 |
| Previous PCI | 3,437 (12.45) | 464 (5.66) | 3,901 (10.89) | <0.001 |
| Prior CVD | 1,974 (7.15) | 614 (7.49) | 2,589 (7.23) | 0.64 |
| Prior PPM or ICD | 9,528 (34.50) | 2,627 (32.02) | 12,155 (33.93) | 0.076 |
Values are mean ± SD or n (%). Significant values (P < 0.05) are indicated in bold.
CABG = coronary artery bypass graft; CVD = coronary vascular disease; ICD = implantable cardioverter-defibrillator; PCI = percutaneous coronary intervention; PPM = permanent pacemaker.
Table 2.
Hospital Characteristics, Geographic Locations, and Household Income in Patients With LVAD Hospital Admissions Between 2010 to 2019: Stratified by Sex
| Male | Female | Total | P Value | |
|---|---|---|---|---|
| (n = 27,617) | (n = 8,203) | (N = 35,820) | ||
| Hospital location | 0.84 | |||
| Rural/urban nonteaching | 403 (1.46) | 125 (1.52) | 528 (1.47) | |
| Urban teaching | 27,214 (98.54) | 8,078 (98.48) | 35,292 (98.53) | |
| Bed size of the hospital | ||||
| Small/medium | 2,839 (10.28) | 804 (9.8) | 3,643 (10.17) | |
| Large | 24,779 (89.72) | 7,399 (90.20) | 32,177 (89.83) | |
| Region | 0.009 | |||
| Northeast | 5,768 (20.88) | 1,529 (18.64) | 7,296 (20.37) | |
| Midwest | 6,892 (24.96) | 2,342 (28.55) | 9,235 (25.78) | |
| South | 11,059 (40.04) | 3,313 (40.39) | 14,372 (40.12) | |
| West | 3,898 (14.11) | 1,019 (12.42) | 4,917 (13.73) | |
| Median household income of patient zip code | <0.001 | |||
| 0-25th | 7,091 (26.13) | 2,511 (31.09) | 9,602 (27.27) | |
| 26th-50th | 6,999 (25.79) | 2,029 (25.12) | 9,029 (25.64) | |
| 50th-75th | 6,814 (25.11) | 2,069 (25.61) | 8,883 (25.23) | |
| 75th-100th | 6,230 (22.96) | 1,469 (18.18) | 7,699 (21.86) | |
| Insurance status | <0.001 | |||
| Medicare | 13,496 (49.16) | 3,908 (47.79) | 17,404 (48.84) | |
| Medicaid | 3,073 (11.19) | 1,248 (15.26) | 4,321 (12.13) | |
| Private insurance | 9,803 (35.71) | 2,731 (33.39) | 12,534 (35.18) | |
| Self-pay, no charge | 274 (1.00) | 115 (1.40) | 388 (1.09) | |
| Other | 809 (2.95) | 176 (2.16) | 985 (2.76) | |
| Elixhauser comorbidity index | 0.022 | |||
| 0-5 | 6,628 (24.00) | 2,188 (26.67) | 8,816 (24.61) | |
| ≥6 | 20,989 (76.00) | 6,015 (73.33) | 27,004 (75.39) |
Values are n (%). Significant values (P < 0.05) are indicated in bold.
Strokes in patients with LVAD hospital admissions
Among all LVAD hospital admissions, 6.12% (n = 2,192) of patients experienced stroke (Table 3). Men who had strokes were older compared to women, with mean age of men and women being 59.3 vs 50.7 years, respectively (P < 0.001). Men who had strokes had a higher incidence of comorbidities than women including hypertension, diabetes, renal failure, dyslipidemia, and previous percutaneous coronary intervention (Table 4).
Table 3.
Stroke and Mortality in Patients With LVAD Hospital Admissions: Sex-Based Differences
| Male(n = 27,617) | Female (n = 8,203) | Total (N = 35,820) | Unadjusted OR/Beta-Coefficient | 95% CI(Lower-Upper) | P Value | Adjusted OR/Beta-Coefficient | 95% CI(Lower-Upper) | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| Stroke | 1,636 (5.93) | 556 (6.77) | 2,192 (6.12) | 1.15 | (0.92-1.45) | 0.23 | 1.17 | (0.93-1.47) | 0.18 |
| Ischemic stroke | 1,248 (4.52) | 368 (4.49) | 1,616 (4.51) | 0.99 | (0.77-1.29) | 0.97 | 1.01 | (0.78-1.31) | 0.92 |
| Hemorrhagic stroke | 488 (1.77) | 217 (2.64) | 705 (1.97) | 1.51 | (1.03-2.21) | 0.036 | 1.49 | (1.02-2.18) | 0.04 |
| Inpatient mortality in ischemic stroke | 438 (35.10) | 114.65 (31.11) | 553 (34.19) | 0.84 | (0.48-1.44) | 0.52 | 0.87 | (0.49-1.52) | 0.62 |
| Inpatient mortality in hemorrhagic stroke | 243 (49.86) | 138 (63.56) | 381 (54.07) | 1.75 | (0.82-3.73) | 0.15 | 1.87 | (0.85-4.11) | 0.12 |
Values are n (%) unless otherwise indicated. Significant values (P < 0.05) are indicated in bold.
Table 4.
Baseline Characteristics in Patients With LVAD Hospital Admission and Stroke: Stratified by Sex
| Male | Female | Total | P Value | |
|---|---|---|---|---|
| (n = 1,636) | (n = 556) | (N = 2,192) | ||
| Age, y | 59.32 ± 0.73 | 50.71 ± 1.62 | 57.13 ± 0.71 | <0.001 |
| Race and ethnicity | 0.008 | |||
| White | 1,034 (65.99) | 254 (51.68) | 1,288 (62.57) | |
| Black | 245 (15.62) | 160 (32.44) | 404 (19.64) | |
| Hispanics | 155 (9.87) | 35 (7.12) | 190 (9.21) | |
| Others | 133 (8.52) | 43 (8.76) | 177 (8.58) | |
| Comorbidities | ||||
| Chronic pulmonary disease | 364 (22.25) | 90 (16.17) | 454 (20.71) | 0.15 |
| Atrial fibrillation | 567 (34.66) | 139 (24.99) | 706 (32.21) | 0.072 |
| Diabetes mellitus | 477 (29.14) | 90 (16.20) | 567 (25.86) | 0.012 |
| Hypertension | 992 (60.62) | 194 (34.90) | 1,186 (54.10) | <0.001 |
| Obesity | 143 (8.75) | 60 (10.80) | 203 (9.27) | 0.51 |
| Peripheral vascular disease | 295 (18.03) | 78 (14.06) | 373 (17.02) | 0.34 |
| Renal failure | 711 (43.42) | 155 (27.82) | 865 (39.47) | 0.004 |
| Liver disease | 443 (27.07) | 198 (35.68) | 641 (29.25) | 0.077 |
| Dyslipidemia | 459 (28.04) | 100 (18.00) | 559 (25.50) | 0.046 |
| Hypothyroidism | 113 (6.93) | 91 (16.30) | 204 (9.31) | 0.004 |
| Valvular disease | 338 (20.63) | 151 (27.10) | 488 (22.27) | 0.20 |
| Smoking | 245 (14.95) | 54 (9.78) | 299 (13.64) | 0.17 |
| Alcohol abuse | 40 (2.44) | <11 cell count | 50 (2.29) | 0.72 |
| Drug Abuse | 20 (1.22) | <11 cell count | 30 (1.37) | 0.65 |
| Previous myocardial infarction | 229 (13.99) | 35 (6.30) | 264 (12.04) | 0.029 |
| Previous CABG | 187 (11.46) | 34 (6.19) | 222 (10.12) | 0.087 |
| Previous PCI | 160 (9.75) | 15 (2.70) | 175 (7.96) | 0.02 |
Values are mean ± SD or n (%) unless otherwise indicated. Significant values (P < 0.05) are indicated in bold.
CABG = coronary artery bypass graft.
The overall rate of stroke was similar in women as compared to men. Despite a lower comorbidity burden and younger age in women with stroke vs men with stroke, women were more likely to experience a hemorrhagic stroke (Table 3). The rate of ischemic stroke remained similar in both groups. Knowing that device type may influence the occurrence of adverse events such as cerebrovascular accidents, we analyzed the data per time period to examine the potential impact of changing pumps on the incidence of cerebrovascular accidents, but no significant difference was observed (Supplemental Table 3).
Outcomes in patients with LVAD hospital admissions and strokes
Mortality in patients who had stroke was significantly higher when compared to those who did not have stroke (39.0% vs 9.62%, respectively; P < 0.001). Of these, hemorrhagic strokes were associated with higher mortality in both men and women when compared to ischemic strokes (men 49.9% vs 35.1%; women 63.6% vs 31.1%), with a trend toward a higher hemorrhagic stroke-related mortality in women (63.6% vs 49.9%, OR: 1.87, 95% CI: 0.85-4.11) (Table 3). There was no significant sex-based difference in other clinical outcomes (pressors use, invasive mechanical ventilation, kidney injury, or cardiac arrest), hospital LOS, or hospital discharge to a facility (Table 5). No regional difference in stroke rates was seen between men and women. However, approximately 37% of total strokes were seen in the South vs the Northeast (22%), Midwest (23%), and West (16%) (P = 0.009).
Table 5.
Clinical Outcomes and Resource Utilization in Patients With LVAD Hospital Admission and Stroke: Sex-Based Differences
| Male (n = 1,636) |
Female (n = 556) |
Total (N = 2,192) |
Unadjusted OR/ Beta-Coefficient |
95% CI (Lower-Upper) |
P Value | Adjusted OR/ Beta-Coefficient |
95% CI (Lower-Upper) |
P Value | |
|---|---|---|---|---|---|---|---|---|---|
| Pressors use | 214 (13.10) | 55 (9.90) | 269 (12.29) | 0.73 | (0.37-1.45) | 0.37 | 0.72 | (0.37-1.43) | 0.35 |
| Invasive mechanical ventilation | 910 (55.60) | 322 (57.99) | 1,232 (56.21) | 1.10 | (0.71-1.71) | 0.66 | 1.12 | (0.71-1.76) | 0.62 |
| Acute kidney injury | 1,176 (71.84) | 357 (64.34) | 1,533 (69.94) | 0.71 | (0.46-1.09) | 0.12 | 0.79 | (0.50-1.25) | 0.32 |
| Acute kidney injury requiring dialysis | 154 (9.38) | 55 (9.90) | 209 (9.52) | 1.06 | (0.54-2.08) | 0.86 | 1.08 | (0.56-2.05) | 0.82 |
| Gastrostomy | 169 (10.33) | 25 (4.50) | 194 (8.86) | 0.41 | (0.16-1.04) | 0.06 | 0.48 | (0.19-1.21) | 0.12 |
| Tracheostomy | 354 (21.65) | 143 (25.80) | 498 (22.70) | 1.26 | (0.77-2.05) | 0.36 | 1.36 | (0.80-2.29) | 0.26 |
| Cardiac arrest | 248 (15.17) | 74 (13.32) | 322 (14.70) | 0.86 | (0.47-1.58) | 0.63 | 0.79 | (0.42-1.49) | 0.46 |
| Inpatient mortality | 626 (38.28) | 228 (41.00) | 854 (38.97) | 1.12 | (0.74-1.7) | 0.59 | 1.13 | (0.73-1.75) | 0.57 |
| LOS, days | 48.67 ± 1.82 | 57.62 ± 4.22 | 59.95 ± 1.74 | 8.95 | (−0.08 to 17.99) | 0.05 | 6.09 | (−2.67 to 14.84) | 0.17 |
| Inflation-adjusted cost, US $ | 343,767.10 ± 10,675.27 | 401,386.10 ± 22,689.21 | 358,414.20 ± 10,020.30 | 57,619.04 | (8,786.31-106,451.80) | 0.02 | 39,779.67 | (−9,010.72 to 88,570.07) | 0.11 |
Values are n (%) or mean ± SD unless otherwise indicated.
LOS = length of stay.
In general, there was a variable trend for strokes in women over the 10-year period and an increasing trend in men (Figure 1).
Figure 1.
Sex-Based Trends in Stroke Among PatientsWith Left Ventricular Assist Device, 2010 to 2019
Discussion
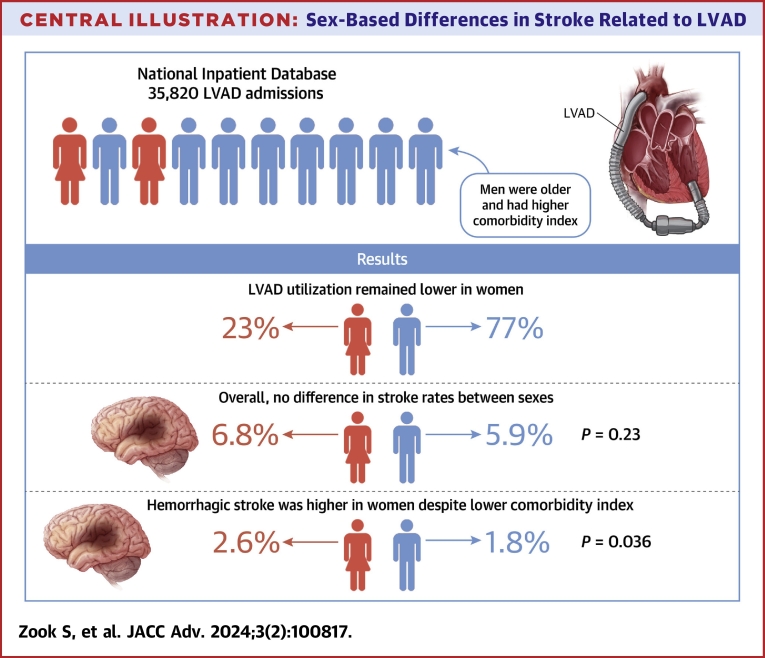
Studies on stroke-related sex disparities in patients with LVADs are limited. In the current study, we used the NIS database and evaluated sex disparity demographics in LVAD-associated strokes during index hospitalization over a 10-year period. Our analysis showed that: 1) fewer women received LVAD implantation than men; and 2) despite lower rates of comorbidities, women had a higher incidence of hemorrhagic stroke and trended toward a higher hemorrhagic stroke-related mortality in the early post-implant phase (Central Illustration).
Central Illustration.
Sex-Based Differences in Stroke Related to LVAD
Figure shows that despite lower utilization of left ventricular assist devices (LVADs) in women as compared to men, the overall stroke rate trended to be higher in women. Of particular concern is the statistically significant increase in hemorrhagic stroke risk observed in women, despite a lower comorbidity index. This highlights the need for a sex-specific approach in the management of heart failure, particularly in the context of LVAD utilization and stroke prevention.
Only 23% of the total participants who received LVAD were women. This finding is consistent with previously published data stating women are less likely to be referred for LVAD compared to men and are largely underrepresented in clinical trials.11, 12, 13 With the advent of continuous-flow LVADs, implantation has almost doubled among women between 2009 and 2014, but there is still a huge gap between men and women. Factors identified in prior studies that contribute to a lower implant rate in women include patient size, poor social support, lower socioeconomic status, higher refusal rates, and religious reasons.3,11Another possible contributing factor could be the difference in the prevalence of heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), with HFrEF being more predominant in men and HFpEF more common in women.14 Our analysis showed that men have a higher comorbidities overall, yet women were found to have higher risk of hemorrhagic stroke. Stroke is one of the major postoperative adverse events in LVAD patients.15 Although ischemic strokes are generally more common, hemorrhagic strokes are associated with higher patient mortality.3,15,16 Previous studies identified predictors of stroke as uncontrolled blood pressure, infection, atrial fibrillation, device type and duration, and female sex, with women showing a significantly higher risk for fatal stroke,17, 18, 19 and a higher proportion experiencing hemorrhagic vs ischemic stroke.5 This is similar to our analysis, which showed that women had a significantly higher risk of hemorrhagic stroke than men. We suggest this sex difference to be attributable to sex-hormone-related influences on hemocompatibility, sex differences in the endothelial activation system, or pharmacological anticoagulation management in women compared to men.
It is postulated that hemodynamic and blood flow velocity changes attributable to LVAD contribute to the development of strokes.20 LVADs cause significant endothelial damage and activation of the coagulation pathway. A decrease in the release of endothelin, a protective factor for hemodynamic stability, has been seen in centrifugal force-left ventricular assisted device (CF-LVAD) patients. This, along with a dysfunction in the synthesis of fibrinolytic substances, has contributed to the pathology of thrombus formation and stroke in CF-LVAD patients.21
There are sex-dependent factors and differences in vascular reactivity. Estrogen influences coagulability and endothelium functions, which are known mechanisms involved in stroke occurrence.22 Estrogen has been shown to increase nitric oxide production from the endothelium by regulating enzyme expression. Nitric oxide production has been linked to reducing vascular resistance and promoting better cerebral tissue perfusion.23 Conversely, estrogen has also been linked to activation of coagulation factors, which increases the incidence of thrombus formation and stroke.23 There is no clarity on the mechanisms through which estrogen mitigates or increases the incidence of stroke risk in women.
Women have different risk factors for stroke depending on whether they are premenopausal or postmenopausal. Menarche and menopause have been proven to be indicators of stroke risk in women.24 Women who have early-onset menopause have an increased risk of hemorrhagic stroke development, and stroke risk continues to increase in postmenopausal women.25 Higher premenopause estrogen levels are suspected to have a protective effect against stroke and cardiovascular disease, so it was theorized that hormone replacement therapy might serve as a protective measure against stroke as well. However, there have been studies suggesting both that hormone replacement therapy has no effect on stroke rate and that hormone replacement therapy causes an increase in stroke rate and severity.22,23 Estrogen appears to play a very nuanced role in stroke incidence, and the topic warrants significant further research.
The discrepancy in anticoagulation management in women compared to men could contribute to the higher incidence of stroke in women as well. Anticoagulant treatment with LVAD includes warfarin therapy managed at a low INR (1.5-2.5) window.26 A study noted that for CF-LVAD recipients’ receiving warfarin, women and prior warfarin usage were predictors of lower time in therapeutic range (TTR).27 TTR evaluates the safety efficacy of warfarin therapy. A low TTR is indicative of poor outcomes, including ischemic and hemorrhagic strokes.28 Women presenting with poor outcomes on warfarin anticoagulant therapy as opposed to men can potentially explain the reason for increase in strokes in women. However, further research is necessary to understand the sex disparities in effectiveness of anticoagulant therapy in CF-LVAD recipients.
In addition, platelet function and response to antiplatelet therapy is now known to be sex-dependent. In a recent study on sex-specific mortality risk score in LVAD recipients, Nayak et al6 demonstrated a sex-specific prognostic impact of a lower platelet count and ischemic cardiomyopathy as the causes of heart failure. This provides valuable insight into the difference in effects of platelet therapy, but further research is necessary to validate these findings.
Study Limitations
We acknowledge the limitations of our study. Although utilization of the NIS provided an expansive database to analyze LVAD utilization across a 10-year life span, only mortality, morbidity, comorbidity, and sex-based differences in LVAD hospitalization could be examined. Some variables that might be important risk factors for stroke, such as medications, were not available. In addition, the device type is not available in the NIS database. Also, the NIS is an administrative discharge database, so there is no longitudinal follow-up of patients after discharge. It is important to note that the small size of the groups in this cohort (male vs female) may limit the statistical significance of some comparisons. While this is the case, data is from a national database and is representative of the United States population. Lastly, we did not exclude the patients who already had LVAD, and some of the current LVAD implantations may represent LVAD replacements.
Conclusions
In this national cohort of patients with hospital admissions for LVAD implantation, men had a higher comorbidity burden and worse stroke trends over the last 10 years when compared to women. Women had fewer LVAD implantations and had a higher incidence of hemorrhagic strokes. Additional studies are needed to understand factors influencing sex-related outcomes among patients with stroke on an LVAD.
PERSPECTIVES.
COMPETENCY IN PATIENT CARE: There is a need to consider the unique care requirements of women around the time of LVAD implantation and postimplantation management. Careful attention to these considerations is necessary to fully elucidate the reasons behind the observed phenomena and to develop effective strategies for reducing the associated risks.
TRANSLATIONAL OUTLOOK: The paucity of women representation in research studies hinders the accurate assessment of pathophysiological mechanisms underlying the development of hemorrhagic stroke post-LVAD. To obtain a better understanding of this phenomenon, it is imperative to conduct prospective studies with a larger sample size of women.
Funding support and author disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Supplementary data
References
- 1.Molina E.J., Shah P., Kiernan M.S., et al. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann Thorac Surg. 2021;111:778–792. doi: 10.1016/j.athoracsur.2020.12.038. [DOI] [PubMed] [Google Scholar]
- 2.Yuzefpolskaya M., Schroeder S.E., Houston B.A., et al. The Society of Thoracic Surgeons Intermacs 2022 Annual Report: focus on the 2018 Heart Transplant Allocation System. Ann Thorac Surg. 2023;115:311–327. doi: 10.1016/j.athoracsur.2022.11.023. [DOI] [PubMed] [Google Scholar]
- 3.Tsiouris A., Heliopoulos I., Mikroulis D., Mitsias P.D. Stroke after implantation of continuous flow left ventricular assist devices. J Card Surg. 2019;34:541–548. doi: 10.1111/jocs.14079. [DOI] [PubMed] [Google Scholar]
- 4.Cornwell W.K., Ambardekar A.V., Tran T., et al. Stroke incidence and impact of continuous-flow left ventricular assist devices on cerebrovascular physiology. Stroke. 2019;50:542–548. doi: 10.1161/STROKEAHA.118.022967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acharya D., Loyaga-Rendon R., Morgan C.J., et al. INTERMACS analysis of stroke during support with continuous-flow left ventricular assist devices: risk factors and outcomes. J Am Coll Cardiol HF. 2017;5:703–711. doi: 10.1016/j.jchf.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nayak A., Hu Y., Ko Y.-A., et al. Creation and validation of a novel sex-specific mortality risk score in LVAD recipients. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nayak A., Hu Y., Ko Y.-A., et al. Gender differences in mortality after left ventricular assist device implant: a causal mediation analysis approach. ASAIO J. 2021;67:614. doi: 10.1097/MAT.0000000000001288. [DOI] [PubMed] [Google Scholar]
- 8.Anon NIS database documentation. https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp
- 9.Anon HCUP-US NIS overview. https://hcup-us.ahrq.gov/nisoverview.jsp
- 10.Anon CPI inflation calculator. https://www.bls.gov/data/inflation_calculator.htm
- 11.Ahmed A., Adegbala O., Akintoye E., et al. Gender differences in outcomes after implantation of left ventricular assist devices. Ann Thorac Surg. 2020;109:780–786. doi: 10.1016/j.athoracsur.2019.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Joyce D.L., Conte J.V., Russell S.D., Joyce L.D., Chang D.C. Disparities in access to left ventricular assist device therapy. J Surg Res. 2009;152:111–117. doi: 10.1016/j.jss.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 13.Radhoe S.P., Jakus N., Veenis J.F., et al. Sex-related differences in left ventricular assist device utilization and outcomes: results from the PCHF-VAD registry. ESC Heart Fail. 2023;10:1054–1065. doi: 10.1002/ehf2.14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmonds S.J., Cuijpers I., Heymans S., Jones E.A.V. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells. 2020;9:242. doi: 10.3390/cells9010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakkar S., Rupareliya C., Doshi R. Incidence and impact of ischemic and hemorrhagic stroke after left ventricular assist device implantation: a nationwide study. Indian Heart J. 2019;71:422–424. doi: 10.1016/j.ihj.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inamullah O., Chiang Y.P., Bishawi M., et al. Characteristics of strokes associated with centrifugal flow left ventricular assist devices. Sci Rep. 2021;11:1645. doi: 10.1038/s41598-021-81445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habal M.V., Axsom K., Farr M. Advanced therapies for advanced heart failure in women. Heart Fail Clin. 2019;15:97–107. doi: 10.1016/j.hfc.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Sherazi S., Kutyifa V., McNitt S., et al. Effect of gender on the risk of neurologic events and subsequent outcomes in patients with left ventricular assist devices. Am J Cardiol. 2017;119:297–301. doi: 10.1016/j.amjcard.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Morris A.A., Pekarek A., Wittersheim K., et al. Gender differences in the risk of stroke during support with continuous-flow left ventricular assist device. J Heart Lung Transplant. 2015;34:1570–1577. doi: 10.1016/j.healun.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Caruso M.V., Gramigna V., Rossi M., Serraino G.F., Renzulli A., Fragomeni G. A computational fluid dynamics comparison between different outflow graft anastomosis locations of Left Ventricular Assist Device (LVAD) in a patient-specific aortic model. Int J Numer Method Biomed Eng. 2015;31(2):e02700. doi: 10.1002/cnm.2700. [DOI] [PubMed] [Google Scholar]
- 21.Poredos P., Jezovnik M.K., Radovancevic R., Gregoric I.D. Endothelial function in patients with continuous-flow left ventricular assist devices. Angiology. 2021;72:9–15. doi: 10.1177/0003319720946977. [DOI] [PubMed] [Google Scholar]
- 22.Billeci A., Paciaroni M., Caso V., Agnelli G. Hormone replacement therapy and stroke. Curr Vasc Pharmacol. 2008;6:112–123. doi: 10.2174/157016108783955338. [DOI] [PubMed] [Google Scholar]
- 23.Roy-O'Reilly M., McCullough L.D. Sex differences in stroke: the contribution of coagulation. Exp Neurol. 2014;259:16. doi: 10.1016/j.expneurol.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisabeth L., Bushnell C. Menopause and stroke: an epidemiologic review. Lancet Neurol. 2012;11:82–91. doi: 10.1016/S1474-4422(11)70269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welten S.J.G.C., Onland-Moret N.C., Boer J.M.A., Verschuren W.M.M., van der Schouw Y.T. Age at menopause and risk of ischemic and hemorrhagic stroke. Stroke. 2021;52:2583–2591. doi: 10.1161/STROKEAHA.120.030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogaev R.C., Pamboukian S.V., Moore S.A., et al. Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant. 2011;30:515–522. doi: 10.1016/j.healun.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Henderson J.B., Iyer P., Coniglio A.C., Katz J.N., Chien C., Hollis I.B. Predictors of warfarin time in therapeutic range after continuous-flow left ventricular assist device. Pharmacotherapy. 2019;39:1030–1035. doi: 10.1002/phar.2324. [DOI] [PubMed] [Google Scholar]
- 28.Fovel L.M., Miller C.D., Seabury R.W., Probst L.A., Horvath L. Evaluation of warfarin patients with low time in therapeutic range (TTR) for transition to non-vitamin-K oral anticoagulant (NOAC) therapy. P T. 2019;44:364. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.