Abstract
Background
Clinical trials suggest that therapeutic-dose heparin may prevent critical illness and vascular complications due to COVID-19, but knowledge gaps exist regarding the efficacy of therapeutic heparin including its comparative effect relative to intermediate-dose anticoagulation.
Objectives
The authors performed 2 complementary secondary analyses of a completed randomized clinical trial: 1) a prespecified per-protocol analysis; and 2) an exploratory dose-based analysis to compare the effect of therapeutic-dose heparin with low- and intermediate-dose heparin.
Methods
Patients who received initial anticoagulation dosed consistently with randomization were included. The primary outcome was organ support-free days (OSFDs), a combination of in-hospital death and days free of organ support through day 21.
Results
Among 2,860 participants, 1,761 (92.8%) noncritically ill and 857 (89.1%) critically ill patients were treated per-protocol. Among noncritically ill per-protocol patients, the posterior probability that therapeutic-dose heparin improved OSFDs as compared with usual care was 99.3% (median adjusted OR: 1.36; 95% credible interval [CrI]: 1.07-1.74). Therapeutic heparin had a high posterior probability of efficacy relative to both low- (94.6%; adjusted OR: 1.26; 95% CrI: 0.95-1.64) and intermediate- (99.8%; adjusted OR: 1.80; 95% CrI: 1.22-2.62) dose thromboprophylaxis. Among critically ill per-protocol patients, the posterior probability that therapeutic heparin improved outcomes was low.
Conclusions
Among noncritically ill patients hospitalized for COVID-19 who were randomized to and initially received therapeutic-dose anticoagulation, heparin, compared with usual care, was associated with improved OSFDs, a combination of in-hospital death and days free of organ support. Therapeutic heparin appeared superior to both low- and intermediate-dose thromboprophylaxis.
Key words: anticoagulation, clinical trial, COVID-19, heparin, thrombosis
Central Illustration
COVID-19 pneumonia, the syndrome caused by SARS-CoV-2 infection, is characterized by systemic inflammation, hypercoagulability, and vascular thrombosis.1, 2, 3, 4 Two randomized clinical trials (RCTs) demonstrated that therapeutic-dose anticoagulation with heparin improves composite outcomes including survival and organ support receipt or thrombosis in noncritically ill, but not in critically ill, patients hospitalized for COVID-19,5, 6, 7 and 2 other RCTs observed a consistent trend.8,9 Nevertheless, several important knowledge gaps exist. First, randomized trials have been pragmatic and open label, and the effectiveness based on assigned study dose is uncertain. In the multiplatform randomized clinical trial (mpRCT) involving the ATTACC (Antithrombotic Therapy To Ameliorate Complications of Covid-19), ACTIV-4a (Accelerating Covid-19 Therapeutic Interventions and Vaccines-4 Antithrombotics Inpatient platform trial), and REMAP-CAP (Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia) platforms, patients hospitalized for COVID-19 were randomized to a pragmatic strategy of therapeutic-dose anticoagulation with heparin or usual-care thromboprophylaxis.5,6 Approximately 12% of participants randomized to therapeutic-dose heparin received initial dosing that was below the therapeutic range. The effect of therapeutic heparin among those receiving it as per protocol is uncertain. Second, a recent clinical trial reported that, among noncritically ill patients hospitalized for COVID-19, intermediate-dose heparin conferred an 86% probability of reducing the composite of death and critical care organ support, leaving uncertainty as to which dose intensity may be optimal in this population.10 This uncertainty regarding the comparative effectiveness of low-, intermediate-, and therapeutic-dose anticoagulation with heparin in patients hospitalized for COVID-19 may challenge clinical care and practice guidelines.
The current study was designed to evaluate 2 corresponding dose-related questions. First, what is the effectiveness of therapeutic heparin among patients randomized to and subsequently receiving this dose relative to those receiving usual care? To address this question, we performed a prespecified analysis of the mpRCT per-protocol population. Second, what is the comparative effectiveness of therapeutic-dose heparin compared with low- vs intermediate-dose thromboprophylaxis? To address this question, we performed a secondary analysis of the mpRCT by stratifying the usual care arm based on whether they received low- or intermediate-dose heparin and compared outcomes with those randomized to and receiving therapeutic-dose heparin.
Methods
The multiplatform clinical trial
The ATTACC/ACTIV-4a/REMAP-CAP mpRCT was a randomized, open-label clinical trial that investigated anticoagulation strategies in noncritically ill and critically ill patients hospitalized for COVID-19.5,6,11 Eligibility criteria, interventions, outcome measures, and data collection procedures were prospectively harmonized across the 3 federated participating platforms to form 1 trial.12 Patients were prospectively analyzed in 2 groups on the basis of baseline illness severity. Critically ill patients were defined by receipt of intensive care unit (ICU)-level organ support (defined as the use of oxygen delivered by high-flow nasal cannula, noninvasive or invasive respiratory support, extracorporeal life support, vasopressors, or inotropes); all others were considered noncritically ill. REMAP-CAP additionally required that such organ support be administered in an ICU for patients to be considered critically ill. Noncritically ill patients were further categorized according to their baseline D-dimer level, using the threshold of 2 times the upper limit of normal according to local laboratory criteria. Patients were eligible to be included in REMAP-CAP within the first 48 hours of ICU admission/initiation of ICU level of care if critically ill or within 14 days since hospital admission if noncritically ill, and in ATTACC and ACTIV-4a within 72 hours from hospital admission or confirmation of COVID-19. Patients were ineligible for the trial if they were at high bleeding risk, or had concomitant use of dual antiplatelet therapy, an independent clinical indication for anticoagulation, a history of heparin-induced thrombocytopenia or heparin allergy (Supplemental Methods).11 The trial was approved by the relevant ethics boards and all patients or surrogates provided informed consent in accordance with regional regulations.
Patients were randomized to receive therapeutic-dose anticoagulation with heparin or usual-care thromboprophylaxis according to local standards of practice, with general guidance provided by the trial protocol. Therapeutic-dose anticoagulation could be administered with unfractionated heparin by monitored intravenous infusion or subcutaneous low-molecular-weight heparin for 14 days or until recovery (defined as hospital discharge or more than 24 hours without receiving supplemental oxygen). Usual-care thromboprophylaxis could include low-dose or intermediate-dose thromboprophylaxis in ATTACC and REMAP-CAP, as per clinical judgment (Supplemental Methods). A 1:1 randomized allocation ratio was used initially, with the implementation of response-adaptive randomization toward the end of recruitment, as prespecified in the protocol (ATTACC and REMAP-CAP).12 Triggers for adaptive stopping of key patient groups were selected a priori based on pretrial simulations including control for type I error. The primary outcome was a composite of hospital survival and freedom from ICU-level organ support through 21 days in survivors (organ support-free days [OSFDs]). This outcome was evaluated on an ordinal scale, starting at −1 (death during index hospitalization, censored at 90 days) and then progressing from 0 to 22 to indicate the number of days free of respiratory or cardiovascular organ support among survivors (use of oxygen delivered by high-flow nasal cannula, noninvasive or invasive respiratory support, extracorporeal life support, vasopressors, or inotropes). Higher values of this scale represent better outcomes. The primary analyses were performed using a Bayesian framework in a modified intention-to-treat population of patients with laboratory-confirmed SARS-CoV-2 (approximately 99% of noncritically ill randomized participants and 91% of critically ill participants). Within the Bayesian framework, results are expressed with probabilities that a certain therapy might benefit a patient (posterior probabilities).13,14 Importantly, there is not a single value above which one would consider a treatment to be beneficial, since decision-making would largely depend on the beliefs of patients and clinicians within a specific clinical context, taking into account disease severity, availability of other therapies, etc.13
The primary results of the mpRCT were published previously.5,6 Briefly, enrollment of critically ill patients was stopped in December 2020, when the trial reached a prespecified trigger for futility in this group, and in January 2021, when it reached superiority triggers in both of the noncritically ill D-dimer-stratified groups. Among 2,231 noncritically ill patients overall, the posterior probability that therapeutic-dose anticoagulation with heparin improved OSFDs compared to usual-care thromboprophylaxis was 98.6% (median adjusted OR: 1.27; 95% credible interval [CrI]: 1.03-1.58). The probability of superiority was 97.3% among those with high D-dimer, 97.3% among those with unknown D-dimer, and 92.9% among those with low D-dimer. Among 1,103 critically ill patients, the probability that therapeutic-dose anticoagulation with heparin was superior to usual-care thromboprophylaxis was 5.0% (median adjusted OR: 0.83; 95% CrI: 0.67-1.03).
Per-protocol analysis
The trial statistical analysis plan prespecified a per-protocol analysis based on participants’ initial post-randomization anticoagulant dose received. Participants’ baseline initial stable anticoagulant dose was determined centrally by either a computerized algorithm or by investigator review using a prespecified consensus dose-intensity framework that reflected patients’ renal function, body size, and weight. Anticoagulant doses were categorized into 4 groups: low, intermediate, subtherapeutic, and therapeutic (Supplemental Methods). Dose thresholds were informed by guidance from the American Society of Hematology and the National Institute for Health and Care Excellence (NICE) (United Kingdom).15,16 To determine an initial stable dose, doses prescribed within the first 48 hours after randomization were reviewed.
The per-protocol population consisted of: 1) patients randomized to therapeutic-dose anticoagulation who initially received either therapeutic or subtherapeutic doses of heparin (ie, a dose greater than intermediate); and 2) patients randomized to usual care who initially received a low- or intermediate-intensity dose of anticoagulant. Patients without information on initial dosing or without confirmed SARS-CoV-2 infection were excluded. The primary outcome in the mpRCT and in this per-protocol analysis was OSFDs. Key secondary outcomes included survival to hospital discharge, a composite of thrombotic events or death, the composite of macrovascular arterial or venous thrombotic events or death within 28 days, and major bleeding (as per the International Society on Thrombosis and Haemostasis definition) within 14 days.17 Thrombotic events included pulmonary embolism, myocardial infarction, ischemic cerebrovascular event, systemic arterial thromboembolism, and deep vein thrombosis. The mpRCT did not employ systematic screening for venous thrombotic events. All thrombotic and bleeding events were independently adjudicated by blinded assessors using consensus definitions (Supplemental Methods).
Secondary heparin dose intensity analysis
In a post hoc analysis of the per-protocol population aimed at determining if therapeutic-dose anticoagulation with heparin is superior to both low- and intermediate-dose thromboprophylaxis, noncritically ill patients randomized to usual-care thromboprophylaxis were further categorized as those receiving low-dose or intermediate-dose thromboprophylaxis. Outcomes of these 2 subgroups were separately compared to those who were randomized to and received therapeutic anticoagulation.
Statistical analysis
Baseline characteristics were summarized with mean, median, or proportions as appropriate. OSFDs were analyzed using a hierarchical Bayesian cumulative ordinal logistic regression model that calculated the posterior distribution of the proportional OR comparing therapeutic-dose anticoagulation versus usual-care thromboprophylaxis. The primary model generated separate treatment estimates based on illness severity and baseline D-dimer level (within noncritically ill patients), permitting dynamic borrowing of information on treatment effect between patient groups. When similar effects were observed between groups, the posterior distribution for each intervention group effect was shrunk toward the overall estimate.18 Dynamic borrowing for the primary outcome was employed to accelerate trial conclusions where treatment effects were similar and to mitigate outlying treatment estimates. In a sensitivity analysis, independent treatment effects of the primary outcome were modeled without dynamic borrowing. A single treatment effect was also estimated for all noncritically ill patients. As in the overall trial analysis, the secondary endpoints were modeled as dichotomous outcomes, without dynamic borrowing, using Bayesian logistic regression. The Bayesian models used weakly informative prior distributions and were fitted using a Markov chain Monte Carlo simulation algorithm with 10,000 samples drawn from the joint posterior distribution. The models were adjusted for age, sex, trial site, enrollment time interval (2-week epoch) and, among noncritically ill patients, baseline D-dimer group. No modeling was performed for the outcome of major bleeding due to the low number of events. In the post hoc heparin dose intensity analysis, noncritically ill patients from the per-protocol population were categorized as those treated with therapeutic-dose anticoagulation (including the subtherapeutic dose category), intermediate-dose thromboprophylaxis, or low-dose thromboprophylaxis and outcomes were compared between these groups. All statistical analyses were conducted using R project.
Results
Characteristics of the patients
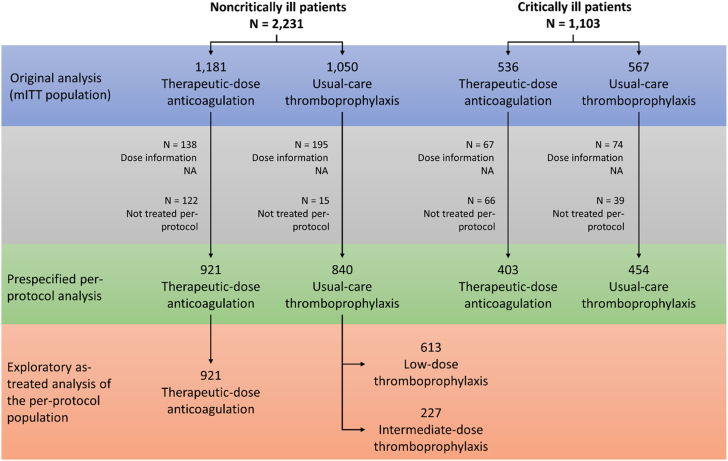
Among 2,231 noncritically ill patients, dose information was available for 1,898 patients, of whom 1,761 (92.8%) were included in the per-protocol analysis; among 1,103 critically ill patients, dose information was available for 962 patients, of whom 857 (89.1%) were included (Figure 1). More patients with dose information randomized to usual-care thromboprophylaxis than to therapeutic-dose anticoagulation received treatment per-protocol (98.2% vs 88.3%, respectively, among noncritically ill patients, and 92.1% vs 85.9% among critically ill patients).
Figure 1.
Diagram Illustrating the Composition of the Per-Protocol Cohorts
From the initial mITT population used in the previously reported primary analyses,5,6 patients without dose information available or those with dose information not as per the protocol recommendations were excluded. In the prespecified per-protocol analysis, patients who received low-dose thromboprophylaxis and intermediate-dose thromboprophylaxis were grouped as usual-care thromboprophylaxis. In the post hoc analysis of the noncritically ill patients, low-dose and intermediate-dose thromboprophylaxis were analyzed separately. mITT = modified intention-to-treat; NA = not available.
Baseline characteristics between treatment groups were similar in the noncritically ill (Table 1) and the critically ill (Table 2) patients. Comorbidity burden and D-dimer levels appeared lower in patients included in the per-protocol population compared to those not in the per-protocol population (Supplemental Tables 1 and 2), while oxygen requirements appeared higher in noncritically ill patient included in the per-protocol population compared to those not included. Otherwise, the included and excluded patients appeared similar regarding most baseline characteristics.
Table 1.
Demographic and Clinical Characteristics of the Per-Protocol Population at Baseline: Noncritically Ill Patients
| Therapeutic-Dose Anticoagulation (n = 921) | Usual-Care Thromboprophylaxis (n = 840) | |
|---|---|---|
| Age, y | 58.3 ± 14.2 | 58.6 ± 13.9 |
| Male | 567 (61.6) | 471 (56.1) |
| Race | ||
| White | 471/776 (60.7) | 455/688 (66.1) |
| Asian | 37/776 (4.8) | 37/688 (5.4) |
| Black | 190/776 (24.5) | 137/688 (19.9) |
| First Nations/American Indian | 91/761 (12.0) | 68/667 (10.2) |
| Other | 5/862 (0.6) | 9/778 (1.2) |
| Hispanic or Latino ethnicity | 445/801 (55.6) | 418/715 (58.5) |
| Body mass index, kg/m2 | 29.7 (26.4-34.6) | 30.4 (26.7-35.0) |
| Pre-existing conditions | ||
| Hypertension | 425/820 (51.8) | 352/729 (48.3) |
| Diabetes mellitus | 272/921 (29.5) | 254/840 (30.2) |
| Severe cardiovascular disease | 87/919 (9.5) | 96/840 (11.4) |
| Chronic kidney disease | 60/914 (6.6) | 58/829 (7.0) |
| Chronic respiratory disease | 189/908 (20.8) | 171/813 (21.0) |
| Immunosuppressive disease | 90/912 (9.9) | 84/830 (10.1) |
| Baseline treatments | ||
| Antiplatelet agent | 117/893 (13.1) | 94/815 (11.5) |
| Remdesivir | 343/921 (37.2) | 325/840 (38.7) |
| Corticosteroids | 400/651 (61.4) | 372/574 (64.8) |
| Tocilizumab | 5/921 (0.5) | 5/840 (0.6) |
| Baseline respiratory support | ||
| None | 124 (13.5) | 98 (11.7) |
| Low-flow nasal cannula/face mask | 628 (68.2) | 570 (67.9) |
| High-flow nasal cannula | 19 (2.1) | 22 (2.6) |
| Noninvasive mechanical ventilation | 15 (1.6) | 19 (2.3) |
| Unspecified | 135 (14.7) | 131 (15.6) |
| Laboratory values | ||
| D-dimer level relative to site ULN | 1.5 (0.9-2.5) | 1.5 (1.0-2.6) |
| Platelets, per mm3 | 223,000 (175,000-291,000) | 216,000 (170,000-283,800) |
| Lymphocytes, per mm3 | 900 (700-1,300) | 1,000 (700-1,400) |
| Creatinine, mg/dL | 0.9 (0.7-1.1) | 0.9 (0.7-1.1) |
| Platform of enrollment | ||
| ATTACC | 550 (59.7) | 463 (55.1) |
| ACTIV-4a | 270 (29.3) | 266 (31.7) |
| REMAP-CAP | 101 (11.0) | 111 (13.2) |
| Country of enrollment | ||
| United Kingdom | 69 (7.5) | 75 (8.9) |
| United States | 440 (47.8) | 400 (47.6) |
| Canada | 82 (8.9) | 77 (9.2) |
| Brazil | 202 (21.9) | 192 (22.9) |
| Other | 128 (13.9) | 96 (11.4) |
| Anticoagulant drug | ||
| Enoxaparin | 767/904 (84.8) | 578/732 (79.0) |
| Dalteparin | 78/904 (8.6) | 74/732 (10.1) |
| Tinzaparin | 26/904 (2.9) | 25/732 (3.4) |
| Subcutaneous unfractionated heparin | 0/904 (0.0) | 44/732 (6.0) |
| Intravenous unfractionated heparin | 33/904 (3.7) | 4/732 (0.5) |
| Direct oral anticoagulant | 0/904 (0.0) | 7/732 (1.0) |
| Dose equivalents | ||
| Low-dose thromboprophylaxis | - | 613 (73.0) |
| Intermediate-dose thromboprophylaxis | - | 227 (27.0) |
| Therapeutic-/subtherapeutic-dose anticoagulation | 921 (100.0) | - |
Values are mean ± SD, n (%), n/N (%), or median (IQR). Patients in this table were those with a laboratory confirmed COVID-19 infection, noncritically ill, who received an anticoagulant dose in the first 48 hours concordant with their randomized group (per-protocol population). The total number of patients in the therapeutic-dose anticoagulation and usual-care thromboprophylaxis groups is unequal owning to the response-adaptive randomization and differences in protocol compliance between the groups. Race or ethnic group was self-reported. Severe cardiovascular disease defined as a baseline history of heart failure, myocardial infarction, coronary artery disease, peripheral arterial disease, or cerebrovascular disease (stroke or transient ischemic attack) in the ATTACC and ACTIV-4a platforms and as a baseline history of NYHA class IV symptoms in the REMAP-CAP platform. Chronic respiratory disease defined as a baseline history of asthma, chronic obstructive pulmonary disease, bronchiectasis, interstitial lung disease, primary lung cancer, pulmonary hypertension, active tuberculosis, or the receipt of home oxygen therapy.
ACTIV-4a = Accelerating Covid-19 Therapeutic Interventions and Vaccines-4 Antithrombotics Inpatient platform trial; ATTACC = Antithrombotic Therapy to Ameliorate Complications of Covid-19; REMAP-CAP = Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia; ULN = upper limit of normal.
Table 2.
Demographic and Clinical Characteristics of the Per-Protocol Population at Baseline: Critically Ill Patients
| Therapeutic-Dose Anticoagulation (n = 403) | Usual-Care Thromboprophylaxis (n = 454) | |
|---|---|---|
| Age, y | 59.8 ± 13.1 | 61.7 ± 12.5 |
| Male | 298 (73.9) | 313 (68.9) |
| Race | ||
| White | 242/320 (75.6) | 272/361 (75.3) |
| Asian | 52/320 (16.2) | 60/361 (16.6) |
| Black | 16/320 (5.0) | 13/361 (3.6) |
| Other | 10/320 (3.1) | 16/361 (4.4) |
| Body mass index, kg/m2 | 30.1 (26.9-35.4) | 30.2 (26.3-34.5) |
| APACHE II score | 13 (8-20) | 13 (8.2-19.0) |
| Pre-existing conditions | ||
| Diabetes mellitus | 128/403 (31.8) | 156/454 (34.4) |
| Severe cardiovascular disease | 30/398 (7.5) | 34/449 (7.6) |
| Chronic kidney disease | 42/382 (11.0) | 33/419 (7.9) |
| Chronic respiratory disease | 92/397 (23.2) | 105/436 (24.1) |
| Chronic liver disease | 5/397 (1.3) | 2/446 (0.4) |
| Baseline treatments | ||
| Antiplatelet agent | 27 (6.7) | 32 (7.0) |
| Remdesivir | 143 (35.5) | 136 (30.0) |
| Corticosteroids | 336 (83.4) | 374 (82.4) |
| Tocilizumab | 9 (2.2) | 7 (1.5) |
| Baseline organ support | ||
| Low-flow nasal cannula/face mask/no supplement | 2 (0.5) | 4 (0.9) |
| High-flow nasal cannula | 131 (32.5) | 156 (34.4) |
| Noninvasive mechanical ventilation | 160 (39.7) | 150 (33.0) |
| Invasive mechanical ventilation | 110 (27.3) | 144 (31.7) |
| Vasopressors or inotropes | 62 (15.4) | 91 (20.0) |
| PaO2: FiO2 ratio | 120 (90.0-162.2) | 120 (91.5-161.5) |
| Laboratory values | ||
| D-dimer level relative to site ULN | 1.8 (1.1-3.3) | 1.8 (1.2-3.6) |
| International normalized ratio | 1.1 (1.0-1.3) | 1.1 (1.0-1.3) |
| Platelets, per mm3 | 247,000 (187,800-318,000) | 243,000 (183,000-311,000) |
| Lymphocytes, per mm3 | 700 (500-1,000) | 700 (500-900) |
| Neutrophil, per mm3 | 8,000 (5,300-10,200) | 8,000 (5,800-10,700) |
| Platform of enrollment | ||
| ATTACC | 16 (4.0) | 19 (4.2) |
| ACTIV-4a | 47 (11.7) | 48 (10.6) |
| REMAP-CAP | 340 (84.4) | 387 (85.2) |
| Country of enrollment | ||
| United Kingdom | 289 (71.7) | 316 (69.6) |
| United States | 56 (13.9) | 70 (15.4) |
| Canada | 37 (9.2) | 54 (11.9) |
| Brazil | 10 (2.5) | 4 (0.9) |
| Other | 11 (2.7) | 10 (2.2) |
| Anticoagulant drug | ||
| Enoxaparin | 200 (49.6) | 242 (53.3) |
| Dalteparin | 131 (32.5) | 157 (34.6) |
| Tinzaparin | 31 (7.7) | 18 (4.0) |
| Subcutaneous unfractionated heparin | 3 (0.7) | 22 (4.8) |
| Intravenous unfractionated heparin | 43 (10.7) | 2 (0.4) |
| Direct oral anticoagulant | 0 (0.0) | 0 (0.0) |
| Other | 1 (0.2) | 1 (0.2) |
| Unknown | 0 (0.0) | 15 (3.3) |
| Dose equivalents | ||
| Low-dose thromboprophylaxis | - | 199 (43.8) |
| Intermediate-dose thromboprophylaxis | - | 255 (56.2) |
| Therapeutic-/subtherapeutic-dose anticoagulation | 403 (100.0) | - |
Values are mean ± SD, n (%), n/N (%), or median (IQR). Patients in this table were those with a laboratory confirmed COVID-19 infection, critically ill, who received an anticoagulant dose in the first 48 hours concordant with their randomized group (per-protocol population). The total number of patients in the therapeutic-dose anticoagulation and usual-care thromboprophylaxis groups is unequal owning to the response-adaptive randomization and differences in protocol compliance between the groups. Race or ethnic group was self-reported. APACHE II scores and the PaO2: FiO2 ratio were available only in REMAP-CAP. APACHE II scores range from 0 to 71, with higher scores indicating more severe illness. Please refer to Table 1 for definitions of severe cardiovascular disease and chronic respiratory disease.
ACTIV-4a = Accelerating Covid-19 Therapeutic Interventions and Vaccines-4 Antithrombotics Inpatient platform trial; APACHE = Acute Physiology and Chronic Health Evaluation; ATTACC = Antithrombotic Therapy to Ameliorate Complications of Covid-19; PaO2: FiO2 ratio = ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2); REMAP-CAP = Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia; ULN = upper limit of normal.
Per-protocol analysis: outcomes in noncritically ill patients
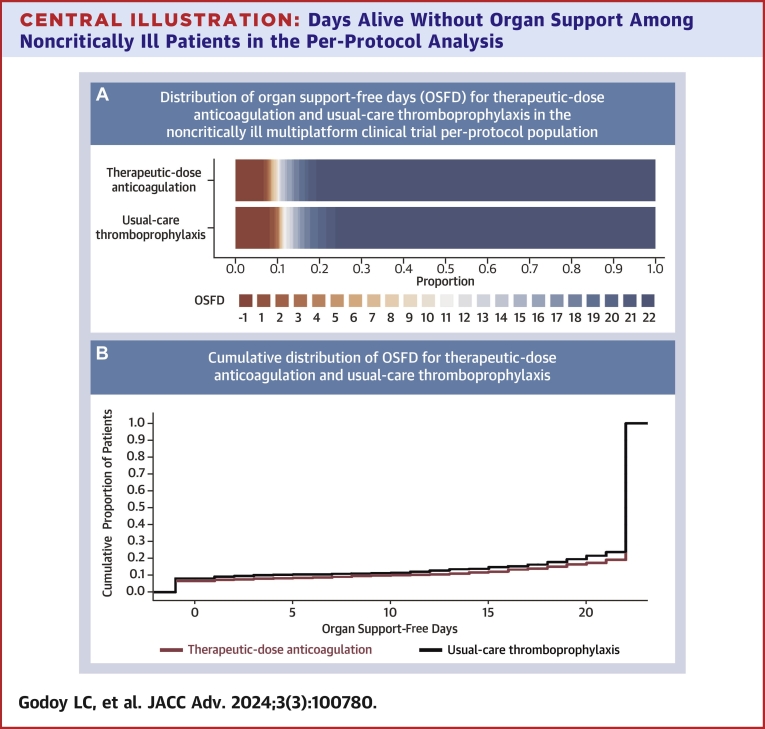
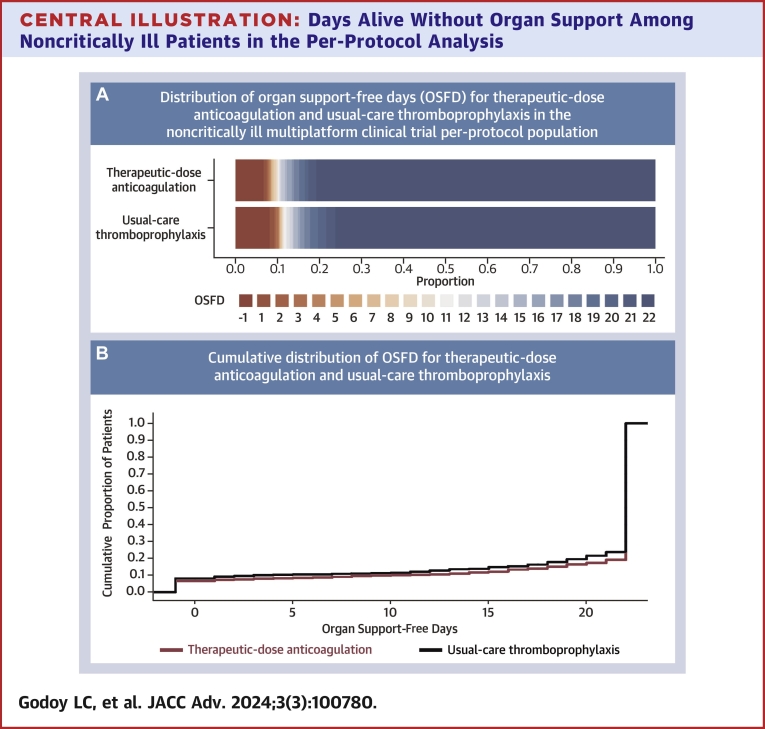
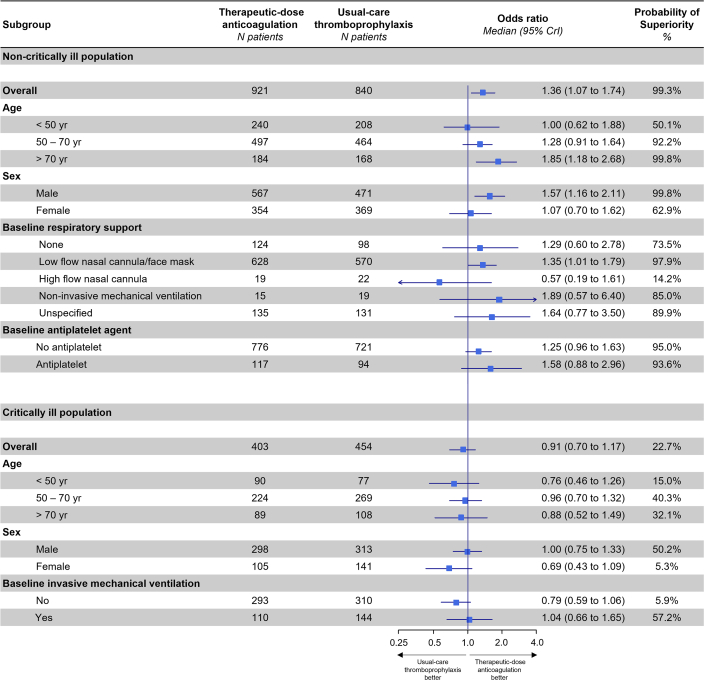
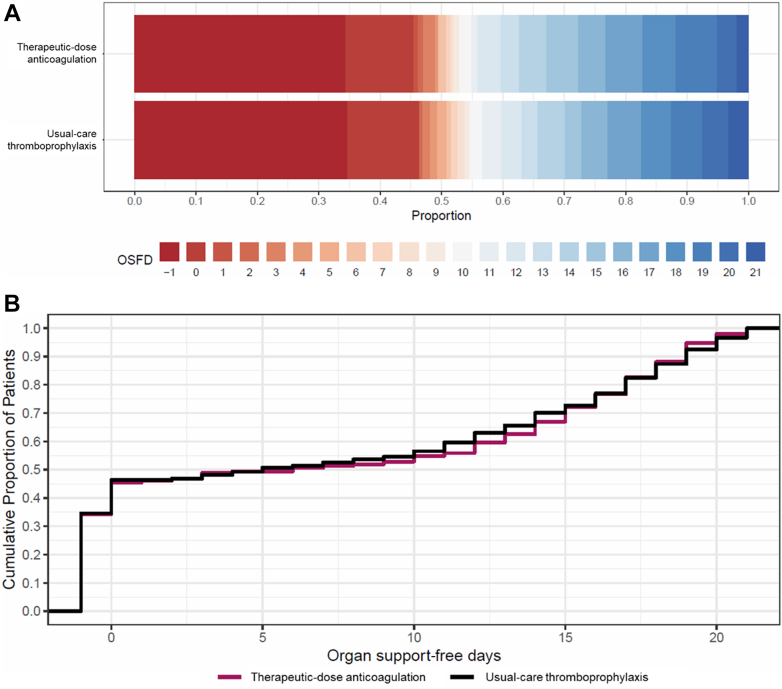
Among all 1,761 noncritically ill patients included in the per-protocol analysis, the posterior probability that therapeutic-dose anticoagulation with heparin improved OSFDs as compared with usual care was 99.3% (median adjusted OR for improved OSFD: 1.36; 95% CrI: 1.07-1.74) (Central Illustration, Table 3). Of 839 noncritically ill patients in the usual-care thromboprophylaxis group, 640 (76.3%) survived until hospital discharge without receipt of organ support during the first 21 days, as compared with 743 of 919 patients (80.8%) in the therapeutic-dose anticoagulation group. The median adjusted absolute difference was 5.1% (95% CrI: 1.2%-8.6%), favoring the therapeutic-dose anticoagulation group. Major bleeding was infrequent in both groups (2.2% [n = 20] in the therapeutic-dose anticoagulation and 1.0% [n = 8] in the usual-care thromboprophylaxis group). In the primary adaptive analysis of D-dimer-defined groups, permitting information on treatment effect from each group to inform estimates in others (dynamic borrowing), the posterior probability of OR >1.0 for improvement in OSFDs in the high D-dimer group was 99.7%; in the unknown D-dimer group was 99.1%; and in the low D-dimer group was 81.7% (Table 3, Supplemental Figure 1). In sensitivity models removing dynamic borrowing, posterior probabilities were similar, except in the low D-dimer group (40.6%, compared to 99.4% in the high D-dimer group; 98.1% probability of a more pronounced effect in the high vs low D-dimer groups) (Supplemental Table 3). In a post hoc analysis, the treatment effect did not vary substantially according to baseline respiratory support or antiplatelet agent use (Figure 2). Beneficial treatment effects appeared greater in men and in patients older than 70 years. The posterior probability of benefit in men was 99.8%, compared to 62.9% in women, while it was 99.8% in patients older than 70 years, compared to 50.1% in those younger than 50 years (Figure 2).
Central Illustration.
Days Alive Without Organ Support Among Noncritically Ill Patients in the Per-Protocol Analysis
(A) Empirical distribution of organ support-free days (OSFD) for therapeutic-dose anticoagulation and usual-care thromboprophylaxis in the noncritically ill multiplatform clinical trial per-protocol population. In-hospital death is represented in dark red and corresponds to a score of −1 on the ordinal scale. Scores from 0 to 21 represent the number of days alive without organ support. A score of 22 (represented as dark blue) corresponds to survival until hospital discharge without the need of organ support and was the most common OSFD outcome among noncritically ill patients. (B) Empirical cumulative distribution of organ support-free days (OSFD) for therapeutic-dose anticoagulation and usual-care thromboprophylaxis in the noncritically ill multiplatform clinical trial per-protocol population. The difference in the height of the 2 curves at any point represents the difference in the cumulative probability of having a value for days without organ support of less than or equal to that point on the x axis.
Table 3.
Primary and Secondary Outcomes in the Noncritically Ill Per-Protocol Population
| Therapeutic-Dose Anticoagulation | Usual-Care Thromboprophylaxis |
Adjusted Difference in Risk (95% CrI), Percentage Points | Adjusted OR (95% CrI) | Probability of Superiority of Therapeutic-Dose Anticoagulation, % | |
|---|---|---|---|---|---|
| Primary outcome, organ support-free daysa | |||||
| Overall groupb | 743/919 (80.8)c | 640/839 (76.3)c | 5.1 (1.2-8.6) | 1.36 (1.07-1.74) | 99.3 |
| D-dimer groupd | |||||
| High level | 208/259 (80.3)c | 159/223 (71.3)c | 7.9 (1.9-13.9) | 1.53 (1.10-2.32) | 99.7 |
| Low level | 384/474 (81.0)c | 341/422 (80.8)c | 2.6 (−3.5 to 6.5) | 1.19 (0.81-1.63) | 81.7 |
| Unknown level | 151/186 (81.2)c | 140/194 (72.2)c | 7.6 (1.3-14.5) | 1.52 (1.07-2.51) | 99.1 |
| Secondary outcomes | |||||
| Survival until hospital dischargea | 858/919 (93.4) | 771/839 (91.9) | 2.0 (−0.7 to 3.0) | 1.35 (0.91-2.00) | 93.7 |
| All thrombotic events or deathe | 67/921 (7.3) | 85/839 (10.1) | −3.0 (−5.0 to −0.4) | 0.68 (0.48-0.96) | 98.6 |
| Major bleedingf | 20/921 (2.2) | 8/839 (1.0) | - | - | - |
Values are n/N (%) unless otherwise indicated. The primary outcome of organ support-free days (OSFDs) consisted of death during index hospitalization, censored at 90 days, and the number of days free of cardiovascular or respiratory organ support among survivors. OSFD was modeled as an ordinal outcome and counted up to day 21 among patients who survived to hospital discharge. Results for this outcome are presented in the entire noncritically ill per-protocol analysis population and in each prespecified D-dimer cohort. The primary adaptive model estimated treatment effects through the use of a Bayesian hierarchical approach in critically and noncritically ill patients, the latter stratified on the basis of baseline D-dimer. Additionally, a model assuming a single treatment effect in all noncritically ill patients in the per-protocol analysis regardless of their baseline D-dimer was evaluated. This primary model for OSFDs permitted dynamic borrowing across illness-severity and D-dimer groups (similar treatment effects are shrunk together on the basis of their degree of similarity). Results from a sensitivity analysis without dynamic borrowing are provided in Supplemental Table 3. Survival until hospital discharge and the composite of thrombotic events or death were modeled as dichotomous outcomes, without borrowing. Models were adjusted for age, sex, trial site, D-dimer group, and enrollment period. The ORs summarize the comparison of therapeutic-dose anticoagulation group vs usual-care thromboprophylaxis.
CrI = credible interval.
For these outcomes, an OR >1.00 indicates benefit with therapeutic-dose anticoagulation.
This model assumes a single treatment effect in all noncritically ill patients in the per-protocol analysis regardless of their baseline D-dimer. Dynamic borrowing of information on treatment effect from critically ill patients was permitted, which occurred to an extent reflective of how similar the treatment effects were. In the context of divergent treatment effects by disease severity, little dynamic borrowing occurred between critically ill and noncritically ill patients, and the results from a sensitivity analysis assuming independent treatment effects were similar. In the overall trial modified intention-to-treat population of noncritically ill patients,5 the OR was 1.27; 95% CrI: 1.03-1.58; probability of superiority of therapeutic-dose anticoagulation: 98.6%.
The median value for OSFD was 22 in both the therapeutic-dose anticoagulation and usual-care thromboprophylaxis arms in all noncritically ill patient groups. Accordingly, the proportion of patients who survived to hospital discharge without receipt of organ support (22 on the ordinal scale–the most common value) is reported.
The noncritically ill primary adaptive stopping groups evaluated the treatment effect based on baseline D-dimer groups, defined as high D-dimer (≥2 times local upper limit of normal for assay), low D-dimer (<2 times local upper limit of normal for assay), and unknown D-dimer.
For this outcome, an OR <1.00 indicates benefit with therapeutic-dose anticoagulation.
No modeling was performed for the outcome of major bleeding because of the low number of events.
Figure 2.
Subgroup Analyses on the Primary Endpoint (Organ Support-Free Days) Among Noncritically Ill and Critically Ill Participants of the Per-Protocol Population
Among noncritically ill patients, the probability that the OR of the primary outcome was higher in men than in women was 92.7% and higher in patients 70 years and older vs those younger than 50 years was 92.1%. Among critically ill patients, the probability that the OR of the primary outcome was higher in men than in women was 91.5%. No meaningful variation of the ORs was observed in other subgroups.
Survival to hospital discharge occurred in 93.4% (858/919) of all noncritically ill patients treated per-protocol with therapeutic-dose anticoagulation and in 91.9% (771/839) treated per-protocol with usual care (Table 3). The posterior probability that therapeutic-dose anticoagulation with heparin increased survival to hospital discharge as compared with usual care was 93.7% (median adjusted OR: 1.35; 95% CrI: 0.91-2.00), corresponding to a median adjusted between-group difference of 2.0% (95% CrI: −0.7 to 3.0). A thrombotic event or in-hospital death occurred in 7.3% (67/921) of noncritically ill patients treated with therapeutic-dose anticoagulation and in 10.1% (85/839) treated with usual care. The posterior probability that therapeutic-dose anticoagulation with heparin reduced thrombotic events or death as compared with usual care was 98.6% (median adjusted OR: 0.68; 95% CrI: 0.48-0.96), corresponding to a median adjusted between-group difference of −3.0% (95% CrI: −5.0 to −0.4). Major bleeding occurred in 2.2% (20/921) noncritically ill patients treated with therapeutic-dose anticoagulation and in 1.0% (8/839) who received usual care. Additional secondary endpoints are shown in the Supplemental Figure 2.
Per-protocol analysis: outcomes in critically ill patients
Among 962 critically ill patients included in the per-protocol analysis, the posterior probability that therapeutic-dose anticoagulation with heparin improved OSFDs compared with usual care was 22.7% (median adjusted OR: 0.91; 95% CrI: 0.70-1.17) (Figure 3). In a post hoc analysis, there was a 91.5% probability that therapeutic-dose anticoagulation was more harmful with respect to its effect on OSFDs in women than in men, without meaningful variation in effect estimate according to age or baseline receipt of invasive mechanical ventilation (Figure 2). There was a low probability that therapeutic-dose anticoagulation improved hospital survival or reduced composite thrombotic events and death (Table 4).
Figure 3.
Days Alive Without Organ Support Among Critically Ill Patients in the Per-Protocol Analysis
(A) Empirical distribution of organ support-free days (OSFD) for therapeutic-dose anticoagulation and usual-care thromboprophylaxis in the critically ill multiplatform clinical trial per-protocol population. In-hospital death is represented in dark red and corresponds to a score of −1 on the ordinal scale. Scores from 0 to 21 represent the number of days alive without organ support. A score of 21 on the ordinal scale (represented as dark blue) corresponds to survival until hospital discharge with at least 21 days free of organ support (best possible outcome in the scale). (B) Empirical cumulative distribution of organ support-free days (OSFD) for therapeutic-dose anticoagulation and usual-care thromboprophylaxis in the critically ill multiplatform clinical trial per-protocol population. The difference in the height of the 2 curves at any point represents the difference in the cumulative probability of having a value for days without organ support of less than or equal to that point on the x axis.
Table 4.
Primary and Secondary Outcomes in the Critically Ill Per-Protocol Population
| Therapeutic-Dose Anticoagulation | Usual-Care Thromboprophylaxis |
Adjusted Difference in Risk (95% CrI),Percentage Points | Adjusted OR (95% CrI) | Probability of Superiority, % | Probability of Inferiority (Harm), % | |
|---|---|---|---|---|---|---|
| Organ support-free days up to day 21a,b | 6 (−1 to 16) | 5 (−1 to 16) | - | 0.91 (0.70-1.17) | 22.7 | 77.3 |
| Survival until hospital dischargea | 265/403 (65.8) | 295/451 (65.4) | −2.2 (−9.9 to 4.9) | 0.91 (0.66-1.25) | 28.3 | 71.7 |
| All thrombotic events or deathc | 153/399 (38.3) | 182/448 (40.6) | −0.5 (−7.9 to 7.4) | 0.98 (0.71-1.35) | 55.2 | 44.8 |
| Major bleedingd | 13/397 (3.3) | 10/450 (2.2) | - | - | - | - |
Values are median (IQR) or n/N (%) unless otherwise indicated. The primary outcome of organ support-free days was modeled as an ordinal outcome. This model estimated treatment effects with the use of a Bayesian hierarchical approach, permitting dynamic borrowing of patients from the high, low, and unknown D-dimer level subgroups of noncritically ill patients. Results from a sensitivity analysis without dynamic borrowing are also provided in Supplemental Table 3. Survival until hospital discharge and the composite of thrombotic events or death were modeled as dichotomous outcomes, without borrowing. Models were adjusted for age, sex, trial site, and enrollment period. The ORs summarize the comparison of the therapeutic-dose anticoagulation group vs usual-care thromboprophylaxis.
CrI = credible interval.
For these outcomes, an OR >1.00 indicates benefit with therapeutic-dose anticoagulation.
Dynamic borrowing of information on treatment effect from noncritically ill patients was permitted, which occurred to an extent reflective of how similar the treatment effects were. In the context of divergent treatment effects by disease severity, little dynamic borrowing occurred between critically ill and noncritically ill patients, and the results from a sensitivity analysis assuming independent treatment effects were similar. In the overall trial modified intention-to-treat population of critically ill patients,6 the OR was 0.83; 95% CrI: 0.67-1.03; probability of superiority of therapeutic-dose anticoagulation: 5.0%; probability of inferiority of therapeutic-dose anticoagulation: 95.0%.
For this outcome, an OR <1.00 indicates benefit with therapeutic-dose anticoagulation.
No modeling was performed for the outcome of major bleeding because of low number of events.
Secondary analysis of outcomes in the noncritically ill per-protocol population according to heparin dose intensity
Among the 840 noncritically ill patients in the per-protocol population who were assigned to usual care, 613 (73.0%) were treated with low-dose thromboprophylaxis and 227 (27.0%) were treated with intermediate-dose thromboprophylaxis. Compared with patients treated with low-dose thromboprophylaxis, patients treated with intermediate-dose thromboprophylaxis appeared slightly younger (mean age: 56.3 vs 59.5 years) and had a similar comorbidity burden, although they were more often receiving higher level respiratory support at enrollment and were more often enrolled from the ATTACC and REMAP-CAP platforms (notably, often from the United Kingdom and Brazil), as well as less often treated with corticosteroids and remdesivir (Supplemental Table 4).
In the usual-care group, there was a 95.7% posterior probability that OSFDs were lower among patients treated with intermediate-dose thromboprophylaxis than in those treated with low-dose thromboprophylaxis (Table 5). Therapeutic-dose anticoagulation with heparin had a high probability of benefit relative to both usual care dose categories: the posterior probability that therapeutic-dose anticoagulation was associated with improved OSFDs as compared with low-dose thromboprophylaxis was 94.6% (median adjusted OR: 1.26; 95% CrI: 0.95-1.64), and as compared with intermediate-dose thromboprophylaxis was 99.8% (median adjusted OR: 1.80; 95% CrI: 1.22-2.62).
Table 5.
Primary and Secondary Outcomes in the Per-Protocol Population According to the Heparin Intensity Dose Received:Noncritically Ill Stratum
| Low-Dose Thromboprophylaxis (n = 613) | Intermediate-Dose Thromboprophylaxis (n = 227) | Therapeutic-Dose Anticoagulation (n = 921) | |
|---|---|---|---|
| Organ support-free days up to day 21 (primary outcome)a | |||
| n/N (%) | 478/613 (78.0) | 162/226 (71.7) | 743/919 (80.8) |
| Adjusted difference in risk (95% CrI) | Ref | −6.7 (−15.5 to 0.8) | 3.7 (−0.9 to 7.3) |
| Adjusted OR (95% CrI) | Ref | 0.70 (0.47-1.05) | 1.26 (0.95-1.64) |
| Probability of superiority, % | Ref | 4.3 | 94.6 |
| Probability of inferiority, % | Ref | 95.7 | 5.4 |
| Survival until hospital dischargea | |||
| n/N (%) | 567/613 (92.5) | 204/226 (90.3) | 858/919 (93.4) |
| Adjusted difference in risk (95% CrI) | Ref | −0.8 (−6.7 to 2.9) | 1.7 (−1.2 to 3.7) |
| Adjusted OR (95% CrI) | Ref | 0.90 (0.49-1.70) | 1.32 (0.85-2.04) |
| Probability of superiority, % | Ref | 37.0 | 90.0 |
| Probability of inferiority, % | Ref | 63.0 | 10.0 |
| All thrombotic events or deathb | |||
| n/N (%) | 62/612 (10.1) | 23/227 (10.1) | 67/921 (7.3) |
| Adjusted difference in risk (95% CrI) | Ref | −0.9 (−4.9 to 6.0) | −3.7 (−5.7 to −0.9) |
| Adjusted OR (95% CrI) | Ref | 0.89 (0.50-1.60) | 0.61 (0.41-0.90) |
| Probability of superiority, % | Ref | 64.0 | 99.2 |
| Probability of inferiority, % | Ref | 36.0 | 0.8 |
| Major bleedingc | |||
| n/N (%) | 8/612 (1.3) | 0/227 (0.0) | 20/921 (2.2) |
Organ support-free days were modeled as an ordinal outcome, while survival until hospital discharge and all thrombotic events of death were modeled as a dichotomous outcome. The ORs summarize the comparison of the therapeutic-dose anticoagulation group or intermediate-dose thromboprophylaxis vs low-dose thromboprophylaxis. Models were adjusted for age, sex, trial site, D-dimer group, and enrollment period.
CrI = credible interval.
A benefit of therapeutic-dose anticoagulation or intermediate-dose thromboprophylaxis is expressed by an OR >1.00.
A benefit of therapeutic-dose anticoagulation or intermediate-dose thromboprophylaxis is expressed by an OR <1.00.
No modeling was performed for the outcome “major bleeding” because of low number of events.
Discussion
This study reports the results from 2 dose-related analyses of a completed RCT in patients hospitalized for COVID-19. First, in a prespecified per-protocol analysis, among all 1,761 noncritically ill patients initially treated as per-protocol, there was a 99.3% probability that therapeutic-dose anticoagulation with heparin improved OSFDs as compared with usual care, with an OR for improved OSFDs of 1.36, as compared to 1.27 in the overall trial modified intention-to-treat population. For every 1,000 noncritically ill patients hospitalized for COVID-19, receipt of therapeutic-dose anticoagulation, compared to usual-care thromboprophylaxis, would be expected to result in the survival of 51 additional patients until hospital discharge without organ support. This benefit translates into a number needed to treat of 20, as compared to 25 in the modified intention-to-treat analysis.5,6 Therapeutic heparin also reduced the composite of death and vascular events, while leading only to very low absolute increases in bleeding. Major bleeding occurred in 2.2% and 1.0% of patients in the therapeutic anticoagulation and usual-care arms, respectively. These observations affirm a high probability of benefit, and the tendency toward potentially greater treatment effects in subjects treated per protocol may attest to the validity of the overall trial conclusions. Second, in a secondary analysis of the trial based on dose intensity, therapeutic-dose anticoagulation had a high probability of being superior to both low- and intermediate-dose thromboprophylaxis. Therapeutic-dose anticoagulation was nonbeneficial in critically ill patients.
Activation of systemic inflammation and intravascular coagulation occurs in patients with severe infection including COVID-19,2 related to upregulation of inflammatory cytokines, neutrophil extracellular traps, monocyte presentation of tissue factor, platelet activation, and complement activation.19 Although there is evidence that severe respiratory infections broadly may cause early and late cardiovascular and other organ failure complications,20, 21, 22 the association was readily perceptible in COVID-19 which may represent an extreme phenotype for this process. Although immunothrombosis may have evolved as an innate immune defense to protect hosts from nonself by sequestering pathogens for killing in the vascular compartment (potentially limiting tissue injury),23 this response in COVID-19 may be excessive and become maladaptive. Microvascular and macrovascular thrombosis may also contribute to organ failure and death.3,4 RCTs suggest that preventive therapeutic-dose heparin—but possibly not other classes of anticoagulants—improves clinical outcomes in noncritically ill patients hospitalized for COVID-19.5,7, 8, 9,24, 25, 26 In this prespecified analysis of the multiplatform trial,5,6 the treatment effect in subjects treated per-protocol was consistent with the primary result, with the improvement in OSFDs and reduction in the composite of death and thrombotic events appearing possibly more favorable than in the overall trial cohort. Among noncritically ill patients, the unadjusted frequency of major bleedings was 2.2% in the therapeutic-dose anticoagulation group and 1.0% in the usual-care thromboprophylaxis group. Among critically ill patients, the probability that therapeutic-dose anticoagulation improved OSFDs was low, consistent with other trials in this population.7,27 Although major bleeding numerically occurred more frequently with therapeutic-dose anticoagulation, it was infrequent, raising the possibility that therapeutic-dose anticoagulation may worsen OSFDs by additional mechanisms. These results support the overall trial conclusions.5
In the FREEDOM COVID Anticoagulation Trial, among noncritically ill patients hospitalized for COVID-19, the primary composite outcome of all-cause mortality, ICU level-of-care, systemic thromboembolism, or ischemic stroke at 30 days was not significantly different between patients receiving prophylactic-dose enoxaparin vs therapeutic-dose anticoagulation, either with enoxaparin or apixaban (the primary outcome occurred in 13.2% of the patients in the prophylactic-dose group and 11.3% in the combined anticoagulation group; HR: 0.85; 95% CI: 0.69-1.04; P = 0.11).9 Lower than anticipated event rates and particularities of the outcome distributions might have contributed to the lack of statistical significance, even considering that FREEDOM COVID enrolled almost 3,400 patients. Of note, in FREEDOM COVID, therapeutic-dose anticoagulation led to a significant 30% reduction in all-cause mortality at 30 days compared to prophylactic-dose enoxaparin (HR: 0.70; 95% CI: 0.52-0.93; P = 0.01).9 A similar pattern was observed in the RAPID (Therapeutic Anticoagulation vs Standard Care as a Rapid Response to the COVID-19 Pandemic trial—a smaller trial that also had a neutral primary outcome but a significant reduction in mortality.8 Although these trials were neutral, their findings may support the beneficial effects of therapeutic heparin observed in the mpRCT.5,8,9
In the current analysis, a high probability of benefit was present when D-dimer was ≥2-fold elevated (or unknown), whereas it was more modest when D-dimer was <2-fold elevated. In the context of higher baseline risk, absolute risk improvement appeared greatest in the high D-dimer group. This pattern appeared more pronounced than in the overall trial, particularly in the sensitivity analysis without borrowing (where effects in each group are shrunk toward the overall estimate).5 D-dimer is a plasma marker of fibrin degradation which may reflect intravascular thrombosis, although extravascular sources have been described in systemic capillary leak syndromes—possibly including COVID-19.28 It is uncertain whether D-dimer provides predictive enrichment for patients with COVID-19 who may preferentially benefit from escalated anticoagulant dosing, although it clearly provides prognostic enrichment.29 The mpRCT employed an adaptive sequential stopping design to allow group stopping at different times based on the treatment effect in D-dimer-defined noncritically ill patient groups—potentially accelerating trial conclusions if effects differed, as well as mitigating outlying treatment effects due to random chance.5,12,30 Importantly, the mpRCT was not designed or powered to test comparisons of the treatment effects based on D-dimer, and any comparisons of relative treatment effects by D-dimer group are speculative. Irrespective, the results from this per-protocol analysis are consistent with the primary trial observation that absolute treatment benefit is most evident among patients with elevated D-dimer.5 Given that absolute clinical benefits may be of greater relevance than relative benefits to patients and practitioners, D-dimer may have value in informing individualized clinical decision-making. Some clinical practice guidelines groups, including the National Institutes of Health COVID-19 Treatment Guidelines, recommend considering noncritically ill hospitalized patients for empiric therapeutic-dose heparin in part on the basis of an elevated D-dimer.31
A recent large RCT identified an 86% probability that therapeutic anticoagulation improved the composite of death and critical care organ support receipt.10 To assess whether a beneficial treatment effect of therapeutic-dose heparin may exist relative to both conventional low- and intermediate-dose thromboprophylaxis among noncritically ill patients, we performed an analysis within the per-protocol population that compared OSFDs among patients receiving conventional low-dose thromboprophylaxis, intermediate-dose thromboprophylaxis, and therapeutic-dose anticoagulation with heparin. We observed that patients who initially received intermediate-dose thromboprophylaxis had higher initial respiratory support requirements, possibly reflecting a higher level of illness severity. The use of corticosteroids and remdesivir was also different between thromboprophylaxis dosing groups, which may be a consequence of different patterns of practice across various countries and timepoints during the pandemic. Accordingly, comparisons of treatment effect between low- and intermediate-dose thromboprophylaxis were at risk for confounding despite adjustment.32 Nevertheless, therapeutic-dose anticoagulation had a high probability of benefit compared with both of these thromboprophylaxis dosing groups.
Several potential limitations bear mention. First, per-protocol analyses by nature exclude patients from the analytical population based on post-randomization factors, which might lead to imbalances between treatment groups and potentially introduce bias.33 The direction of such bias impacting results is not certain in this analysis. Our results should be seen either as a corroboration of the main multiplatform trial analyses or, particularly regarding the low D-dimer subgroup of noncritically ill patients, as hypothesis-generating. Second, some patients were excluded due to incomplete dose information. Nevertheless, baseline characteristics of patients with vs without dose information appeared similar. Third, we defined eligibility for the per-protocol analysis based on the initial stable post-randomization anticoagulant dose equivalent, administered within the first 48 hours following randomization, while outcomes were assessed during the entire hospitalization. Following this initial post-randomization period, the influence of post-randomization clinical events and related crossover might increase. Some clinical events that could have appropriately led to treatment crossover were not study outcomes and were not collected (eg, atrial arrhythmias, clinically relevant nonmajor bleeding, invasive procedures, etc). The mpRCT was designed as a pragmatic initial strategy trial; the results suggest that an initial strategy of empiric therapeutic-dose anticoagulation with heparin improves OSFDs and possibly hospital survival free of macrovascular thrombosis. Fourth, the comparisons between low- and intermediate-dose thromboprophylaxis were not prespecified and should be regarded as exploratory, particularly in view of imbalances in baseline characteristics. Most patients in the mpRCT received enoxaparin at doses consistent with their randomization. The greatest variability in dose was observed in the control group, where doses ranged from low- to intermediate-intensity. Still, consistent treatment benefit was observed for therapeutic heparin when comparted to both low- and intermediate-thromboprophylaxis doses. Finally, it is uncertain how the results from this trial, which enrolled patients from April 2020 through January 2021, apply to patients with COVID-19 caused by later emerging SARS-CoV-2 variants and in the presence of broader vaccination rates.
Conclusions
In noncritically ill hospitalized patients with COVID-19, treatment with therapeutic-dose anticoagulation with heparin, as compared with usual-care thromboprophylaxis, is associated with improved OSFD, a combination of in-hospital death and days free of ICU-level organ support. This benefit was consistent, and possibly strengthened, in the per-protocol population, as compared to the overall noncritically ill modified intention-to-treat population. Therapeutic-dose anticoagulation appeared superior to both low and intermediate usual-care thromboprophylaxis dose ranges. In critically ill patients with COVID-19, treatment with therapeutic-dose anticoagulation with heparin is not associated with a greater probability of OSFDs, a result that is consistent with the overall critically ill modified intention-to-treat population.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: The effect of therapeutic-dose anticoagulation as compared with usual-care thromboprophylaxis on organ support-free days appeared stronger in patients initially treated per-protocol in comparison to that in the overall trial modified intention-to treat population, potentially biologically supporting a beneficial effect of heparin in patients hospitalized for COVID-19.
COMPETENCY IN PATIENT CARE: Noncritically ill patients hospitalized for COVID-19 should receive therapeutic-dose anticoagulation with heparin.
TRANSLATIONAL OUTLOOK 1: Absolute risk improvement appeared greatest in the prespecified high D-dimer group of noncritically ill patients, a difference that was more pronounced in the per-protocol population than in the overall modified intention-to-treat trial population. Dedicated prospective studies are needed to confirm the potential role of D-dimer in guiding selection of an anticoagulant strategy in noncritically ill patients with COVID-19.
TRANSLATIONAL OUTLOOK 2: Therapeutic-dose anticoagulation appeared superior to both low and intermediate usual-care thromboprophylaxis dose categories, which is contrary to a previous smaller study. A dedicated trial might be needed to evaluate this question prospectively.
Funding support and author disclosures
The ACTIV-4a platform was sponsored by the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda and administered through OTA-20 to 011. The research was, in part, funded by the National Institutes of Health (NIH) Agreement 1OT2HL156812 through the National Heart, Lung, and Blood Institute (NHLBI) CONNECTS program. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH. The ATTACC platform was supported by grants from the Canadian Institutes of Health Research, LifeArc, Thistledown Foundation, Peter Munk Cardiac Centre, Research Manitoba, CancerCare Manitoba Foundation, Victoria General Hospital Foundation, and the Ontario Ministry of Health. The REMAP-CAP platform was supported by the European Union—through FP7-HEALTH-2013-INNOVATION: the Platform for European Preparedness Against (Re)emerging Epidemics (PREPARE) consortium (602525), and Horizon 2020 research and innovation program: the Rapid European Covid-19 Emergency Research response (RECOVER) consortium (101003589)—and by the Australian National Health and Medical Research Council (APP1101719), the Health Research Council of New Zealand (16/631), the Canadian Institutes of Health Research (Strategy for Patient-Oriented Research Innovative Clinical Trials Program Grant - 158584, and COVID-19 Rapid Research Operating Grant - 447335), the U.K. NIHR and the NIHR Imperial Biomedical Research Centre, the Health Research Board of Ireland (CTN 2014-012), the UPMC Learning While Doing Program, the Translational Breast Cancer Research Consortium, the French Ministry of Health (PHRC-20-0147), the Minderoo Foundation, Amgen, Eisai, the Global Coalition for Adaptive Research, and the Wellcome Trust Innovations Project (215522). Dr Godoy is supported by the Frederick Banting and Charles Best Canada Graduate Scholarship (Doctoral Research Award) from the Canadian Institutes of Health Research. Dr Neal has received grants from the National Institutes of Health and United States Department of Defense; has received research support from Haemonetics, Janssen and Instrumentation Laboratories; has received honoraria from Haemonetics, Janssen, and CSL Behring; and serves on the Scientific Advisory Board of Haima Therapeutics. Dr Goligher has received personal fees and nonfinancial support from Getinge, nonfinancial support from Timpel, outside the submitted work. Dr Cushman has received personal fees from the National Institutes of Health, during the conduct of the study. Dr Bradbury has received personal fees from BMS Pfizer; nonfinancial support from Amgen; personal fees and nonfinancial support from Bayer; personal fees and nonfinancial support from Novartis; personal fees from Janssen; personal fees from Portola; and personal fees from Ablynx, outside the submitted work. Dr Tritschler is a member of the Canadian Venous Thromboembolism Research Network (CanVECTOR); the network receives grant funding from the Canadian Institutes of Health Research (CDT-142654). Dr Kahn is supported by a Tier 1 Canada Research Chair. Dr L. Berry has received grants from PREPARE Network, European Commission through University Antwerp, grants from OPTIMISE CAP. She reports Australia funding through Monash University; grants from REMAP-CAP; New Zealand funding through Medical Research Institute of New Zealand; grants from Global Coalition for Adaptive Research (GCAR); United States funding through grants from ATTACC; Canada funding through University Health Network, grants from ACTIV-4; and reports IP funding, University of Pittsburgh, during the conduct of the study. Dr Lorenzi has received grants from PREPARE in EU (University Antwerp), grants from OPTIMISE-CAP in Australia (Monash University), grants from REMAP-CAP in New Zealand (Medical Research Institute of New Zealand (MRINZ)), grants from REMAP-COVID in the US (GCAR), grants from ATTACC in Canada (University Health Network), grants from ACTIV-4 IP in the U.S. (University of Pittsburgh), during the conduct of the study. Dr Higgins has received grants from National Health and Medical Research Council, grants from Minderoo Foundation, during the conduct of the study. Dr Berger has received grants from National Institutes of Health - NHLBI, during the conduct of the study; personal fees from Astra Zeneca, personal fees from Janssen, personal fees from Amgen, outside the submitted work. Dr Gong has received grants from NHLBI, during the conduct of the study. Dr Castellucci has received unrelated honoraria received from Bayer, BMS-Pfizer Alliance, The Academy, LEO Pharma, Sanofi, Servier, and Valeo; and holds a Heart and Stroke Foundation of Canada National New Investigator Award, and a Tier 2 research Chair in Thrombosis and Anticoagulation Safety from the University of Ottawa. Dr Le Gal holds a Heart and Stroke Foundation of Canada National Clinician-Scientist Award, and the Chair on Diagnosis of Venous Thromboembolism from the University of Ottawa. Dr Rosenson has a patent EFSID 40934007 pending. Dr Derde has received grants from EU FP7-HEALTH-2013-INNOVATION-1, grant number 602525, grants from H2020 RECOVER grant agreement No 101003589, during the conduct of the study; COVID-19 guideline committee SCCM/ESICM/ SSC, ESICM COVID-19 taskforce, Dutch intensivists (NVIC) taskforce infectious threats, outside the submitted work. Dr Kumar has received grants from Merck, outside the submitted work. Dr McVerry has received grants from NIH - National Heart, Lung and Blood Institute, during the conduct of the study; grants from Bayer Pharmaceuticals, Inc, outside the submitted work. Dr Nicolau has received personal fees from AMGEN, grants from AstraZeneca, grants and personal fees from Bayer, grants from Esperion, grants from CLS Behring, personal fees from Daiichi-Sankyo, grants from Dalcor, grants from Janssen, grants and personal fees from Novartis, grants from NovoNordisk, grants and personal fees from Sanofi, personal fees from Servier, grants from Vifor, outside the submitted work. Dr Huang has received grants from the NIH, during the conduct of the study. Dr Reynolds has received grants from National Heart, Lung and Blood Institute, during the conduct of the study; nonfinancial support from Abbott Vascular, nonfinancial support from BioTelemetry Inc, nonfinancial support from Siemens, outside the submitted work. Dr Carrier has received grants from Pfizer, grants from Canadian Institutes of Health Research, grants from BMS, during the conduct of the study; grants and personal fees from Leo Pharma, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Bayer, personal fees from Sanofi Aventis, personal fees from Pfizer, outside the submitted work. Dr S. Berry reports grants from PREPARE Network, grants from Optimise-CAP, grants from REMAP-CAP, grants from GCAR, grants from University Health Network, grants from University of Pittsburgh, during the conduct of the study. Dr Webb reports grants from National Health and Medical Research Council, grants from Minderoo Foundation, during the conduct of the study. Dr Turgeon reports grants from Canadian Institutes of Health Research, during the conduct of the study. Dr McArthur has received grants from Health Research Council of New Zealand, during the conduct of the study. Dr Farkouh has received grants from Amgen, grants from Novo Nordisk, grants from Novartis, outside the submitted work. Dr Hochman is a principal investigator for the ISCHEMIA trial which, in addition to funding by NIH, received support in the form of devices and medications provided by: Medtronic, Inc; Abbott Vascular, Inc (formerly St. Jude Medical, Inc); Royal Philips NV (formerly Volcano Corporation); Arbor Pharmaceuticals, LLC; AstraZeneca Pharmaceuticals, LP; Merck Sharp & Dohme Corp; Omron Healthcare, Inc; Sunovion Pharmaceuticals, Inc; Espero BioPharma; and Amgen Inc; and financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. Dr Zarychanski has received unrelated grant funding from the Canadian Institutes of Health Research, CancerCare Manitoba Foundation, and Research Manitoba and receives operating support as the Lyonel G. Israels Research Chair in Hematology at the University of Manitoba. Dr Lawler has received unrelated consulting fees from Novartis, CorEvitas, and Brigham and Women’s Hospital, and unrelated royalties from McGraw-Hill Publishing; and is supported by a Heart and Stroke Foundation of Canada National New Investigator career award. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors are grateful to the patients who participated in this trial and their families.
Footnotes
James L. Januzzi, MD, served as Guest Associate Editor for this paper. Michael Landzberg, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, results, tables, and figures, please see the online version of this paper.
Supplementary data
References
- 1.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, Antithrombotic therapy, and Follow-up: JACC State-of-the-Art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godoy L.C., Goligher E.C., Lawler P.R., Slutsky A.S., Zarychanski R. Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19. CMAJ. 2020;192:E1156–E1161. doi: 10.1503/cmaj.201240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFadyen J.D., Stevens H., Peter K. The emerging threat of (Micro)Thrombosis in COVID-19 and its therapeutic Implications. Circ Res. 2020;127:571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawler P.R., Goligher E.C., Berger J.S., et al. Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goligher E.C., Bradbury C.A., McVerry B.J., et al. Therapeutic anticoagulation with heparin in critically ill patients with covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spyropoulos A.C., Goldin M., Giannis D., et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181:1612–1620. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sholzberg M., Tang G.H., Rahhal H., et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ. 2021;375:n2400. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone G.W., Farkouh M.E., Lala A., et al. Randomized trial of anticoagulation strategies for noncritically ill patients hospitalized with COVID-19. J Am Coll Cardiol. 2023;81:1747–1762. doi: 10.1016/j.jacc.2023.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McQuilten Z.K., Venkatesh B., Jha V., et al. Anticoagulation strategies in non-critically ill patients with covid-19. NEJM Evid. 2022;2 doi: 10.1056/EVIDoa2200293. [DOI] [PubMed] [Google Scholar]
- 11.Houston B.L., Lawler P.R., Goligher E.C., et al. Anti-thrombotic therapy to ameliorate complications of COVID-19 (ATTACC): study design and methodology for an international, adaptive Bayesian randomized controlled trial. Clin Trials. 2020;17:491–500. doi: 10.1177/1740774520943846. [DOI] [PubMed] [Google Scholar]
- 12.Lawler P.R., Hochman J.S., Zarychanski R. What are adaptive platform clinical trials and what role may they have in cardiovascular medicine? Circulation. 2022;145:629–632. doi: 10.1161/CIRCULATIONAHA.121.058113. [DOI] [PubMed] [Google Scholar]
- 13.Yarnell C.J., Abrams D., Baldwin M.R., et al. Clinical trials in critical care: can a Bayesian approach enhance clinical and scientific decision making? Lancet Respir Med. 2021;9:207–216. doi: 10.1016/S2213-2600(20)30471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Schoot R., Depaoli S., King R., et al. Bayesian statistics and modelling. Nat Rev Methods Prim. 2021;1:1. [Google Scholar]
- 15.Cuker A., Tseng E.K., Nieuwlaat R., et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021;5:872–888. doi: 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The National Institute for Health and Care Excellence (NICE) COVID-19 rapid guideline: managing COVID-19, v 15.0. https://app.magicapp.org/#/guideline/L4Qb5n [PubMed]
- 17.Schulman S., Kearon C. Subcommittee on control of anticoagulation of the scientific and Standardization committee of the international Society on thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 18.McGlothlin A.E., Viele K. Bayesian hierarchical models. JAMA. 2018;320:2365–2366. doi: 10.1001/jama.2018.17977. [DOI] [PubMed] [Google Scholar]
- 19.Bonaventura A., Vecchié A., Dagna L., et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musher D.M., Abers M.S., Corrales-Medina V.F. Acute infection and myocardial infarction. N Engl J Med. 2019;380:171–176. doi: 10.1056/NEJMra1808137. [DOI] [PubMed] [Google Scholar]
- 21.Kosyakovsky L.B., Angriman F., Katz E., et al. Association between sepsis survivorship and long-term cardiovascular outcomes in adults: a systematic review and meta-analysis. Intensive Care Med. 2021;47:931–942. doi: 10.1007/s00134-021-06479-y. [DOI] [PubMed] [Google Scholar]
- 22.Lawler P.R., Bhatt D.L., Godoy L.C., et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. 2020;42:113–131. doi: 10.1093/eurheartj/ehaa099. [DOI] [PubMed] [Google Scholar]
- 23.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 24.Lopes R.D., de Barros e Silva P.G.M., Furtado R.H.M., et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger J.S., Kornblith L.Z., Gong M.N., et al. Effect of P2Y12 Inhibitors on survival free of organ support among non–critically ill hospitalized patients with COVID-19: a randomized clinical trial. JAMA. 2022;327:227–236. doi: 10.1001/jama.2021.23605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sholzberg M., da Costa B.R., Tang G.H., et al. Randomized trials of therapeutic heparin for COVID-19: a meta-analysis. Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohula E.A., Berg D.D., Lopes M.S., et al. Anticoagulation and antiplatelet therapy for prevention of venous and arterial thrombotic events in critically ill patients with COVID-19: covid-PACT. Circulation. 2022;146:1344–1356. doi: 10.1161/CIRCULATIONAHA.122.061533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung P.C., Eisch A.R., Maleque N., Polly D.M., Auld S.C., Druey K.M. Fatal Exacerbations of systemic capillary leak syndrome complicating Coronavirus disease. Emerg Infect Dis. 2021;27:2529–2534. doi: 10.3201/eid2710.211155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger J.S., Kunichoff D., Adhikari S., et al. Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19. Arterioscler Thromb Vasc Biol. 2020;40:2539–2547. doi: 10.1161/ATVBAHA.120.314872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawler P.R., Fan E. Heterogeneity and phenotypic stratification in acute respiratory distress syndrome. Lancet Respir Med. 2018;6:651–653. doi: 10.1016/S2213-2600(18)30287-X. [DOI] [PubMed] [Google Scholar]
- 31.National Institutes of Health Coronavirus disease 2019 (COVID-19) treatment guidelines. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 32.INSPIRATION Investigators Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care Unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith V.A., Coffman C.J., Hudgens M.G. Interpreting the results of intention-to-treat, per-protocol, and as-treated analyses of clinical trials. JAMA. 2021;326:433–434. doi: 10.1001/jama.2021.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.