Abstract
We provide here new insights into rotavirus (RRV) pathogenicity by showing that RRV infection promotes structural and functional injuries localized at the tight junctions (TJ) in the cell-cell junctional complex of cultured polarized human intestinal Caco-2 cells forming monolayers. RRV infection resulted in a progressive increase in the paracellular permeability to [3H]mannitol as a function of the time postinfection. We observed a disorganization of the TJ-associated protein occludin as a function of the time postinfection, whereas distribution of the zonula adherens associated E-cadherin was not affected. These structural and functional RRV-induced TJ injuries were not accompanied by alteration in cell and monolayer integrity, as assessed by the lack of change in transepithelial membrane resistance and lactate dehydrogenase release. Finally, using the stabilizer of actin filaments Jasplakinolide, we demonstrated that the RRV-induced structural and functional alterations in TJ are independent of the RRV-induced apical F-actin rearrangements.
Rotaviruses (RRV), nonenveloped double-stranded RNA viruses, are recognized as the most important worldwide cause of viral gastroenteritis in infants (for reviews see references 7 and 16). There is increasing information on their replication and maturation processes. Currently, the pathophysiological mechanisms by which RRV induce diarrhea remain unclear. RRV diarrhea might be due to enterocyte destruction from the top of intestinal villi. In agreement with this, extensive studies of animal models have reported the presence of histopathologic changes and functional abnormalities in infected intestinal mucosae that varied from mild to severe depending on the RRV strain virulence. However, this mechanism cannot explain situations in which mild histopathologic changes without enterocyte destruction are associated with low disaccharidase level. Indeed, whatever the severity of histopathologic changes, the activity of functional intestinal proteins is frequently decreased by RRV infection. Recently, we have gained consistent data which lead us to propose a alternate RRV pathophysiological model in which alteration of enterocytic functions depends on perturbation in protein trafficking and the cytoskeleton (3, 13).
In the present study we have investigated the mechanisms by which RRV impair the structural and functional organization of the intestinal epithelial cell monolayers. In epithelia, the permeability barrier between different environments results from the assembly and maintenance of different junctional domains in the polarized cells; that the regulation of the paracellular pathway by its well-defined structures is a complex process is now apparent (for reviews see references 1, 4, and 18). In order to approach in vitro the situation in vivo and to gain further insights into the pathophysiological mechanisms of RRV infection, we have used the human polarized intestinal epithelial Caco-2 cells (23). These cells, established from a human colon adenocarcinoma, spontaneously differentiate after confluency and display many of the morphological and biochemical properties of mature enterocytes (32). Interestingly, the human polarized intestinal Caco-2 cells which form monolayers mimicking an epithelial barrier are known to allow the transcellular passage of water (11). In relation to our previous observation that RRV infection in Caco-2 cells is accompanied by brush border-associated cytoskeletal rearrangements (3, 13), we decided to examine whether or not RRV infection produces structural and functional changes in the junctional domains of polarized Caco-2 cells. We provide here new insights into the pathophysiological events following RRV infection consisting of structural and functional injuries localized at the tight junctions (TJ) in the cell-cell junctional complexes of polarized intestinal cell monolayers mimicking epithelia.
MATERIALS AND METHODS
Reagents.
[2-3H]mannitol (15 to 30 Ci/mM) was from Amersham (Les Ulis, France). [1,2-3H]polyethylene glycol (PEG) 900 and 4000 (2 Ci/mM) were from NEN (Paris, France). Fluorescein-5 and -6 sulfonic acid (FS) and Jasplakinolide (JAS) were from Molecular Probes (Eugene, Oreg.).
Cell lines and culture.
The cultured human colonic adenocarcinoma Caco-2 cells (23) were routinely grown in Dulbecco modified Eagle's minimal essential medium (DMEM) (25 mM glucose) (Eurobio, Paris, France), supplemented with 20% fetal calf serum (Boehringer, Mannheim, Germany) and 1% nonessential amino acids as previously described (13–15). For maintenance purposes, cells were passaged weekly using 0.25% trypsin in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 3 mM EDTA. Maintenance of the cells and all experiments were carried out at 37°C in a 10% CO2–90% air atmosphere.
All experiments were carried out at late confluency, i.e., after 15 days in culture (fully differentiated cells) (3, 13–15). For paracellular flux studies, the cells were seeded at a density of 10,000 cells per cm2 on tissue culture-treated polycarbonate Transwell filters containing pores 0.4 mm in diameter. Apical and/or basal media were replaced at 2-day intervals from day 2. In other experiments, cells were seeded in 24-well tissue culture plates (Corning Glass Works, Corning, N.Y.) at a concentration of 2.5 × 104 cells per well.
MA104 cells were cultured in MEM supplemented with 10% fetal bovine serum FBS, 1% glutamine, antibiotics (20 U of penicillin and 40 U of streptomycin/ml), and 1% nonessential amino acids (100×) in a 5% CO2 incubator. Cells (105/cm2) were seeded in 150-cm2 tissue culture flasks (Falcon; Becton Dickinson, Le Pont-de-Claix, France) and were used for virus stock production after 48 h of culture.
Cell infection.
The method used for Caco-2 cell infection has been described elsewhere (13–15). Briefly, the virus inoculum was activated for 30 min by treatment with 0.5 mg of trypsin/ml. Caco-2 cells (cultured without FBS for 24 h) were apically infected with an inoculum of activated RRV at a multiplicity of infection of 1 or 10 PFU for 1 h at room temperature. The inoculum was then removed, and fresh medium containing 0.5 mg of trypsin/ml was added. Infected cells were incubated at 37°C in a 10% CO2–90% air atmosphere and were processed for experiments at various times postinfection. Each assay was conducted in triplicate with three successive passages of Caco-2 cells.
Antibodies.
The monoclonal antibody (MAb) against E-cadherin was from Biogenesis (Interchim, Montluçon, France). The MAb directed against occludin was obtained from Zymed Laboratories (ICN, San Francisco, Calif.). The ascites fluid containing antibody HBB 2/614/88 against human sucrase-isomaltase (SI) was a gift from H. P. Hauri (Biocenter of the University of Basel, Basel, Switzerland). The polyclonal anti-group A RRV antibody 8148 was a gift from J. Cohen (Institut National de la Recherche Agronomique, Jouy-en-Josas, France). Fluorescein isothiocyanate-phalloidin was from Molecular Probes Inc. Fluorescein-coupled goat anti-mouse immunoglobulins were from Institut Pasteur Productions (Paris, France).
Immunofluorescence.
Monolayers of Caco-2 cells were prepared on glass coverslips, which were placed in 24-well tissue culture plates (Corning Glass Works). Control and RRV-infected cell monolayers were fixed for 15 min at room temperature in 3.5% paraformaldehyde in PBS, washed three times, and then treated with 50 mM NH4Cl for 10 min. When occludin and E-cadherin were to be visualized, the cells grown on coverslips were permeabilized by incubation with 0.2% Triton X-100 in PBS for 4 min, and the coverslips were then rewashed three times with PBS. Permeabilized cell monolayers were incubated with a specific primary antibody (diluted 1:20 to 1:100 in PBS in 0.2% bovine serum albumin [BSA]-PBS) for 45 min at room temperature, washed, and then incubated with their respective secondary fluorescein-conjugated antibodies (Institut Pasteur Productions) (diluted 1:50 in 0.2% BSA-PBS).
SI was revealed by indirect immunofluorescence labeling on unpermeabilized cell monolayers as previously described (13). Preparations were fixed for 10 min at room temperature in 3.5% paraformaldehyde in PBS. Cell monolayers were incubated with MAb anti-SI (diluted 1:100 in 2% BSA-PBS) for 30 min at room temperature, washed, and then incubated with the respective secondary fluorescein-conjugated antibodies. Appropriate secondary antibodies were used at a dilution of 1:20 to 1:100 in 0.2% BSA-PBS. No fluorescence staining was observed when nonimmune serum was used or when the primary antibody was omitted.
RRV proteins were revealed by indirect immunofluorescence in cells fixed postinfection with 3% paraformaldehyde, permeabilized with 0.1% Triton X-100, and labeled with polyclonal anti-group A RRV antibody 8148 (diluted 1:100 in PBS in 0.2% BSA-PBS) and fluorescein isothiocyanate-labeled anti-rabbit immunoglobulin G antibody (Institut Pasteur Productions) (diluted 1:50 in 0.2% BSA-PBS), as previously described (13–15). No fluorescence staining was observed when nonimmune serum was used or when the primary antibody was omitted.
When F-actin was to be visualized, cells grown on coverslips were permeabilized by incubation with 0.2% Triton X-100 in PBS for 4 min at room temperature before incubation with fluorescein-phalloidin for 45 min at 22°C. The coverslips were then rewashed three times with PBS.
Specimens were examined using a Leitz Aristoplan microscope with epifluorescence coupled to a Visiolab 1000 image analyzer (Biocom, Les Ulis, France). More than 200 individual cells were examined for each assay, and assays were conducted in triplicate with three successive passages of Caco-2 cells. All photographs were taken on T-MAX 400 black-and-white film (Eastman Kodak Co., Rochester, N.Y.).
LDH release.
Cell integrity was determined by measuring the release of lactate dehydrogenase (LDH) into the culture medium postinfection using a commercially available kit (Enzyline LDH; Biomérieux, Dardilly, France). The results are expressed as the units per liter of LDH released.
TER measurements.
Monolayers of Caco-2 cells were grown in filters mounted in culture chambers (Costar culture plate inserts; 0.4-μm pore size; 4.7 cm2; 3 × 104 cells per cm2), an arrangement which delineates an apical (luminal) and a basolateral (serosal) reservoir. After RRV infection, the integrity of the confluent polarized monolayers was checked by measuring transepithelial membrane resistance (TER) with a volt-ohmmeter (Millicel ERS; Millipore, Saint Quentin, France). TER (in units of ohms times centimeters squared) was calculated as the measured electrical resistance times the surface area of a filter. The background reading of a free control filter was subtracted.
Permeability measurements.
The permeability of Caco-2 cell monolayers was determined by measuring the paracellular passage of water-soluble radioactive or fluorescent compounds, having various sizes, from the apical to the basolateral compartments of the culture chamber (Costar culture plate inserts; 0.4-μm pore size; 4.7 cm2; 3 × 104 cells per filter).
[3H]mannitol, [3H]PEG, or FS was dissolved in the culture medium. To measure the flux in the apical-to-basolateral direction, the tracer solution (2.5 μCi/ml for radioactive compounds and 20 μg/ml for FS) was loaded into the apical side of the monolayer and the cells were incubated for 1 h at 37°C. After the incubation period, the tracer concentrations in the apical and basolateral compartments were assayed. The concentrations of [3H]mannitol and [3H]PEG were determined by measurement in a β-scintillation counter. The values were corrected for the background radioactivity of the media or PBS, as appropriate. The fluorescence due to FS was determined using a Jobin-Yvon JY3C spectrofluorimeter at an excitation wavelength of 410 nm (slit width, 2 nm) and an emission wavelength of 530 nm (slit width, 10 nm).
JAS treatment.
JAS (1 μM) was added to the culture medium 30 min before RRV infection, and this concentration was maintained during the infection time course. In a preliminary experiment, an examination of LDH release and TER in uninfected cells treated with JAS showed no modification in the cell and monolayer integrities.
Statistics.
Data are expressed as means ± standard errors of the means of several experiments, with at least three monolayers from three successive passages of cells per experiment. The statistical significance was assessed by Student's t test.
RESULTS
RRV infection induces an increase in paracellular permeability.
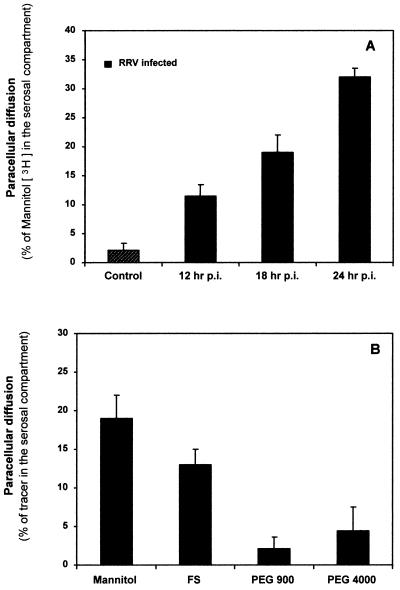
The effect of RRV infection on paracellular permeability in Caco-2 intestinal monolayers was examined using [3H]mannitol (182 Da) (Fig. 1A). The rate of unidirectional flux of [3H]mannitol was negligibly low in control monolayers. RRV infection resulted in a progressive increase in the paracellular permeability to [3H]mannitol as a function of the time postinfection. We investigated monolayer integrity and cell viability at 12, 18, and 24 h postinfection (Table 1). As assessed by TER measurement, there was no significant change in monolayer integrity within the first 24 h postinfection. In contrast, a significant increase in LDH release and a decrease in TER were observed at 36 h postinfection, revealing that RRV-induced cell lysis had commenced. Altogether, these results demonstrated that the RRV-induced increase in paracellular permeability develops before the RRV-induced cell lysis.
FIG. 1.
RRV infection promotes an increase in paracellular fluxes of Caco-2 cell monolayers. Markers were loaded into the apical side of the Caco-2 cell monolayer (1 h at 37°C), and the tracer concentration in the basolateral compartment was assayed. (A) Paracellular permeability to [3H]mannitol as a function of the time postinfection. (B) Levels of RRV-induced paracellular permeability determined using defined paracellular markers having different sizes: [3H]mannitol (182 Da), FS (478 Da), [3H]PEG 900 (900 Da), and [3H]PEG 4000 (4,000 Da).
TABLE 1.
LDH release and TER in uninfected control and RRV-infected Caco-2 cellsa
| Cells (h postinfection) | LDH release (U/liter) | TER (Ω · cm2) |
|---|---|---|
| Control | 200 ± 15 | 900 ± 9 |
| H2O lysed | 3,000 ± 150 | |
| RRV-infected (12) | 175 ± 10 | 920 ± 10 |
| RRV-infected (18) | 178 ± 12 | 920 ± 8 |
| RRV-infected (24) | 180 ± 15 | 890 ± 11 |
| RRV-infected (36) | 410 ± 14∗ | 390 ± 19∗ |
Statistical differences between control and RRV-infected cells were determined by Student's t test. ∗, P < 0.01. Values are means ± standard errors of the means.
The extent of the RRV-induced paracellular permeability was determined using defined paracellular markers having different sizes: FS (478 Da), [3H]PEG 900 (900 Da), and [3H]PEG 4000 (4,000 Da) (Fig. 1B). The mucosal-to-serosal flux rate of markers across the filter-grown Caco-2 cell monolayers was determined at 18 h postinfection. The rate of unidirectional flux of markers was negligibly low in control monolayers. RRV infection resulted in a highly significant increase in the paracellular permeability to FS, which was lower than that to [3H]mannitol. In contrast, the paracellular permeability to PEG 900 and PEG 4000 in RRV-infected monolayers did not change compared with that in control monolayers.
RRV infection induces selective alterations in the distribution of proteins associated with the TJ.
The above results showing an RRV-induced increase in the paracellular permeability to [3H]mannitol and FS suggest a mechanism involving an alteration in the distribution of functional junctional complex-associated proteins. For polarized epithelial cells forming monolayers, the intercellular junctional complexes include well-defined structures including TJ or zonula occludens, zonula adherens (ZA), and desmosomes (for reviews see references 1and 4). The most apical structure of the junctional complex is the TJ, in which functional proteins such as occludin (2, 9, 20) have been identified. Just next to the TJ lies the ZA, also named adherens junction, in which the classical cadherins (e.g., E-, N-, and P-cadherins) act as adhesion receptors (10).
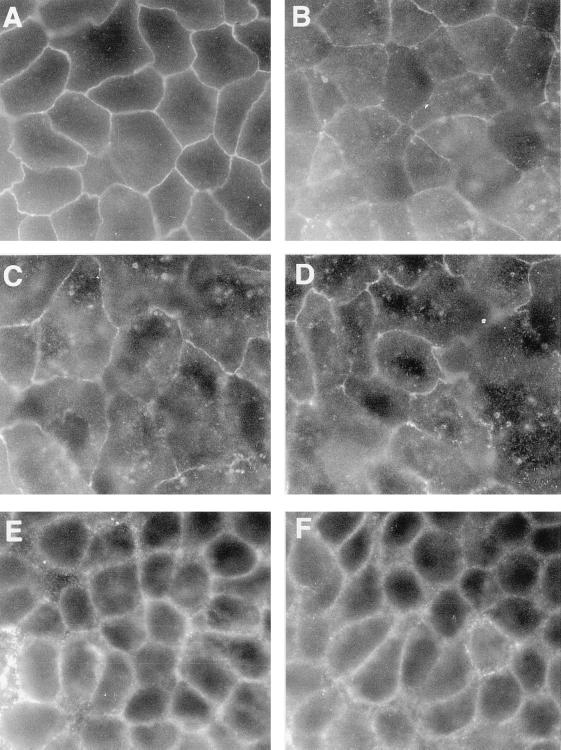
We examined whether or not the distribution of the TJ-associated occludin and ZA-associated E-cadherin was modified in Caco-2 cells upon RRV infection. Figure 2A shows that occludin staining was localized to sites of cell-cell boundaries in control uninfected cells. Occludin distribution was characterized by a brightly stained, continuous band with a sharp honeycomb-like organization. In RRV-infected cells (Fig. 2B to D), the occludin distribution appeared disorganized as a function of the time postinfection. Indeed, at 12 h postinfection randomly distributed cells showed that alteration of the occludin distribution had begun (Fig. 2B). At 18 and 24 h postinfection (Fig. 2C and D, respectively), occludin distribution appeared dramatically altered and was characterized by the formation of large gaps and the appearance of a fluffy disorganized pattern. As disclosed in Fig. 2E the E-cadherin distribution in uninfected control cells results in a continuous large band with a honeycomb-like organization. In contrast, with occludin, no obvious change in E-cadherin distribution in RRV-infected Caco-2 cells was found (Fig. 2F).
FIG. 2.
Alteration in the distribution of junction-associated proteins upon RRV infection in Caco-2 cells. (A) Occludin in control cells. (B to D) Occludin in RRV-infected cells at 12, 18, and 24 h postinfection, respectively. (E and F) E-cadherin in control cells and RRV-infected cells, respectively, at 24 h postinfection. Magnification, ×100.
Altogether, these results demonstrated that infection by RRV in polarized epithelial intestinal cells forming monolayers is followed by the opening of the cell barrier localized just in the upper part of the junctional domain, involving redistribution of the TJ-associated occludin, whereas the ZA domain appeared unmodified.
RRV-induced structural and functional alterations in TJ are independent of apical cytoskeletal rearrangements.
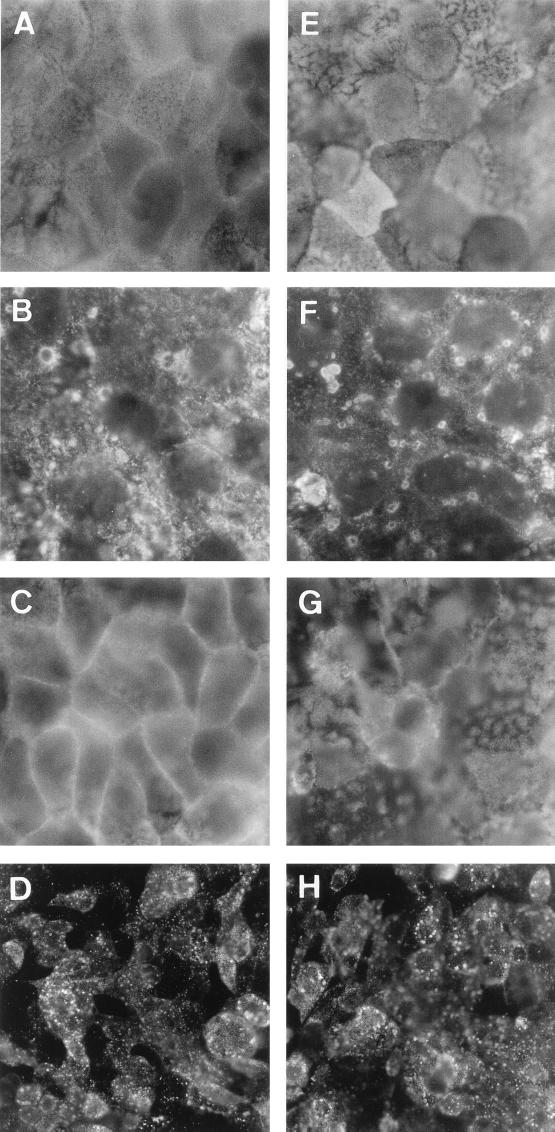
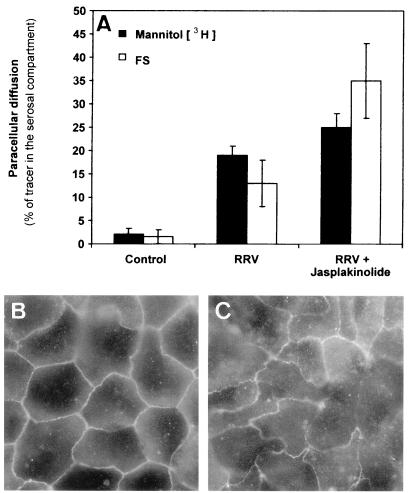
We have previously reported that RRV infection in Caco-2 cells is followed by apical cytoskeleton rearrangements (3, 13). Moreover, alteration in the junctional domain could result from centrifugal traction of the TJ membrane in polarized epithelial cells in which alteration of the apical cytoskeleton develops. In order to assess whether RRV-induced structural and functional alterations in TJ are dependent or not on the RRV-induced apical F-actin rearrangements, we used JAS, a monocyclic peptide isolated from the sea sponge Jaspis johnstoni, known to be a stabilizer of actin filaments (30). As a control, we verified that JAS at the concentration (1 μM) used had no effect on RRV infection (Fig. 3D and H). As disclosed in Fig. 3, JAS treatment of the RRV-infected Caco-2 cells resulted in inhibition of the RRV-induced apical F-actin disassembly (Fig. 3A to C), whereas the RRV-induced decrease in SI expression was partially inhibited (Fig. 3E to G). In contrast, JAS treatment of RRV-infected Caco-2 cells did not modify the increase in the paracellular permeability to [3H]mannitol (Fig. 4A). In parallel, immunolabeling of occludin in RRV-infected cells treated with JAS shows that the TJ-associated protein remains redistributed (Fig. 4C) compared with what is found for untreated RRV-infected cells (Fig. 2C, respectively). Taken together, these results demonstrate that the RRV-induced functional alteration in the TJ of the Caco-2 cell monolayers does not result from the RRV-induced apical cytoskeleton rearrangements. The fact that JAS does not inhibit the RRV-induced increase in mannitol fluxes could be the consequence of occludin not being directly linked to the cytoskeleton. Indeed, occludin is directly linked to the zonula occludens protein ZO-1, which is directly linked to the F-actin cytoskeleton (for a review see reference 4). Results showing that the occludin distribution remains modified in RRV-infected cells treated with JAS support the hypothesis that JAS could reassemble F-actin and ZO-1 together but does not reassemble ZO-1 with occludin, leading to the persistence of the increase in mannitol fluxes.
FIG. 3.
Effect of JAS treatment on RRV-induced apical F-actin and SI rearrangements. (A and E) Uninfected cells. (B, D, F, G, and H) RRV-infected cells at 18 h postinfection. (C, G, and H) Cells treated with JAS. (A to C) F-actin labeling. Control cells show the fine and homogeneous labeling centrally, representing microvillus-associated F-actin (A). RRV-infected cells show the disassembly of F-actin characterized by the disappearance of the fine and homogeneous labeling centrally in the cells and the appearance of clumped F-actin (B). RRV-infected cells treated with JAS (1 μM) show the protection of the apical F-actin characterized by the reappearance of the fine and homogeneous labeling centrally in the cells (C). (E to G) SI labeling. Control cells show the characteristic high expression of SI in a mosaic pattern (E). RRV-infected cells show the disappearance of the SI mosaic pattern and the appearance of clumped SI (F). RRV-infected cells treated with JAS show the incomplete reappearance of the SI mosaic pattern (G). (D and H) RRV protein labeling in infected cells without and with JAS treatment, respectively. Note that JAS treatment does not modify the level of RRV-infected cells.
FIG. 4.
Effect of JAS treatment on the RRV-induced increase in paracellular fluxes and occludin distribution. (A) Paracellular fluxes of [3H]mannitol and FS measured in the mucosal-to-serosal direction (1 h at 37°C) without or with RRV infection (18 h postinfection). (B) Occludin in control cells treated with JAS (1 μM). (C) Occludin in RRV-infected cells treated with JAS (1 μM) (18 h postinfection).
DISCUSSION
We have recently revisited the pathophysiological mechanisms by which RRV infection promotes cell injuries in polarized human intestinal cells. In particular, we have investigated the mechanism by which RRV impairs some intestinal functional proteins without apparent cell destruction. Data demonstrated that RRV infection induced the blockade of the direct transport of SI from the trans-Golgi network to the brush border (13). In parallel, RRV infection induces an important alteration of the brush border-associated cytoskeleton that correlates with decreased SI apical surface expression. Finally, we have demonstrated the ability of one or several intracellular or released viral proteins from RRV-infected human intestinal epithelial cells to induce an alteration in the microvillar cytoskeleton by a Ca2+-dependent mechanism (3).
It is well established that several gastrointestinal epithelial functions are influenced by the establishment and the maintenance of the polarized organization of the epithelial intestinal cells. Organization of polarized epithelial cells in monolayers provides a permeability barrier between different environments (for reviews see references 1, 4, and 18). The junctional domains in polarized epithelial cells function as a “fence” separating apical and basolateral domains, thereby segregating cell surface proteins and lipids into each domain. They also function as a “gate” to provide a permeability barrier between the mucosal and serosal environments. Several diarrheagenic bacteria alter the junctional complexes in polarized epithelial cells to develop pathogenicity (for reviews see references 8 and 25). For example, infection of the polarized epithelial cell monolayers with Salmonella enterica serovar Typhimurium, Helicobacter pylori, or enteropathogenic and enterohemorrhagic Escherichia coli results in alteration in the junctional complexes.
Reports have described the activity of viruses in TJ of the human polarized epithelial cells. The herpes simplex virus (HSV) interacts through its glycoprotein complex gE-gI with the adherent junction, but not the TJ, to mediate the spread of viruses between adjacent cells (5). This interaction occurs without HSV-mediated change of either ZO-1 or β-catenin and without alteration in TJ functions. Wild-type retinal cytomegalovirus strain AD169 infecting the retinal pigment epithelium promotes epithelial permeabilization and ZO-1 disassembly, whereas deletion mutants RV35, RV80, and AD169 do not (21). The results presented here show that the alterations observed in the junctional domain upon RRV infection differed from the alterations induced by the nonenveloped poliovirus in monolayers of Caco-2 cells (27). Indeed, poliovirus infection results in a decrease in TER accompanied by an increase in paracellular permeability to inulin prior to visible cytopathic effect. The results presented here show that RRV infection results in an increase in paracellular permeability to low-molecular-weight markers accompanied by rearrangement in TJ-associated occludin without an associated decrease in TER of monolayers. Dissociation of paracellular permeability from electrical resistance upon RRV infection is surprising since these two parameters have generally been considered to evolve in parallel. The only other example of identically paradoxical results was the result of a recently reported examination of the role of occludin in TJ functionality (2). Indeed, in MDCK II cells transfection with C-terminally truncated occludin increased the paracellular flux without affecting the TER of monolayers. In parallel, the distribution of the mutant occludin exhibited a discontinuous junctional staining pattern accompanied by a disruption of the continuous junctional ring formed by the endogenous TJ-associated protein occludin.
The observation that the RRV-induced selective opening of the TJ allowing passage of low-molecular-weight molecules results from a redistribution of functional TJ-associated proteins independent from the RRV-induced apical cytoskeleton disassembly is of interest. Indeed, the observed RRV-induced alterations in the TJ domain could be related to the alteration in the apical expression of the brush border-associated hydrolase SI previously reported by us (13). This phenomenon results from the blockade of SI transport to the brush border without affecting SI biosynthesis, maturation, or stability. Several cytoskeleton-associated proteins and junction-associated proteins play a pivotal role in the architectural organization of the polarized cells (for reviews see references 12, 17, and 22). Moreover, it is well established that intracellular trafficking of functional intestinal proteins is influenced by the establishment and maintenance of the polarized organization of the epithelial intestinal cells (19). Several intriguing peripheral membrane proteins are concentrated at the TJ and are thought to be involved not only in cross-linking TJ strands with underlying actin-based cytoskeletons but also in vesicle targeting for cellular polarization. For example, rab3B (29) and rab13 (31), belonging to the family of rab GTPases involved in vesicular transport, have been localized at the apical pole near the junctional complexes. A current opinion is that, in association with the subapical compartment in polarized intestinal cells (24, 28), the junctional domain may function as a novel sorting center for the docking and fusion of some apical vesicles transporting brush border-associated functional molecules. In consequence, alterations in TJ induced by RRV infection could explain the previously reported modification of the vectorial delivery of functional components to the apical domain (13).
ACKNOWLEDGMENTS
We thank J. Cohen (INRA, Jouy-en-Josas, France) for kindly providing anti-RRV serum and the RRV strain. We thank J. Cotte-Laffitte for helpful advice.
This work was supported by a grant from the French Ministère de l'Education Nationale, de la Recherche et de la Technologie (MENRT-PRFMMIP [Réseau de Recherche sur les Gastro-entérites à Rotavirus: épidémiologie, structure et interaction avec l'hôte]).
REFERENCES
- 1.Anderson J M, van Itallie C M. Tight junctions and the molecular basis for regulation of paracellular pathway. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 2.Balda M S, Whitney J A, Flores C, Gonzales S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of mutant tight junction membrane protein. J Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunet J-P, Cotte-Laffitte J, Linxe C, Quero A-M, Géniteau-Legendre M, Servin A. Rotavirus infection induces intracellular calcium concentration increase in human intestinal epithelial cells: role in microvillar actin alteration. J Virol. 2000;74:2323–2332. doi: 10.1128/jvi.74.5.2323-2332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denker B M, Nigam S K. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 5.Dingwell K S, Johnson D C. The herpes simplex virus gE-gI complex facilitates cell-to-cell spread and binds to components of cell junctions. J Virol. 1998;72:8933–8942. doi: 10.1128/jvi.72.11.8933-8942.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Zeng C Q Y, Ball J M, Estes M K, Morris A P. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc Natl Acad Sci USA. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estes M K. Rotaviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1329–1352. [Google Scholar]
- 8.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microb Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- 11.Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux J-F. Epithelial properties of the human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol. 1984;247:C260–C267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- 12.Heintzelman M B, Mooseker M S. Assembly of the intestinal brush border cytoskeleton. Curr Top Dev Biol. 1992;26:93–122. doi: 10.1016/s0070-2153(08)60442-1. [DOI] [PubMed] [Google Scholar]
- 13.Jourdan N, Brunet J-P, Sapin C, Blais A, Cotte-Laffitte J, Forestier F, Quero A-M, Trugnan G, Servin A. Rotavirus infection reduces sucrase-isomaltase expression in human intestinal epithelial cells by perturbing protein targeting and organization of microvillar cytoskeleton. J Virol. 1998;72:7228–7236. doi: 10.1128/jvi.72.9.7228-7236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jourdan N, Cotte-Laffitte J, Forestier F, Servin A L, Quero A M. Infection of cultured human intestinal cells by monkey RRV and human Wa rotavirus as a function of intestinal epithelial cell differentiation. Res Virol. 1995;146:325–331. doi: 10.1016/0923-2516(96)80595-4. [DOI] [PubMed] [Google Scholar]
- 15.Jourdan N, Maurice M, Delautier D, Quero A M, Servin A L, Trugnan G. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J Virol. 1997;71:8268–8278. doi: 10.1128/jvi.71.11.8268-8278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapikian A Z, Shanock R M. Rotaviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1657–1708. [Google Scholar]
- 17.Louvard D, Kedinger M, Hauri H P. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu Rev Cell Biol. 1992;8:157–195. doi: 10.1146/annurev.cb.08.110192.001105. [DOI] [PubMed] [Google Scholar]
- 18.Madara J L. Tight junction dynamics: is paracellular transport regulated? Cell. 1988;53:497–498. doi: 10.1016/0092-8674(88)90562-4. [DOI] [PubMed] [Google Scholar]
- 19.Matter K, Hauri H P. Intracellular transport and conformational maturation of intestinal brush border hydrolases. Biochemistry. 1991;30:1916–1923. doi: 10.1021/bi00221a026. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy K M, Skare I B, Stankewich M C, Furuse M, Tsukita S, Rogers R A, Lynch R D, Schneeberger E E. Occludin is a functional component of the tight junction. J Cell Sci. 1996;109:2287–2298. doi: 10.1242/jcs.109.9.2287. [DOI] [PubMed] [Google Scholar]
- 21.Pereira L, Maidji E, Tugisov S, Jones T. Deletion mutants in human cytomegalovirus glycoprotein US9 are impaired in cell-cell transmission and in altering tight junctions of polarized human retinal pigment epithelial cells. Scand J Infect Dis. 1995;99:82–87. [PubMed] [Google Scholar]
- 22.Peterson M D, Mooseker M S. An in vitro model for the analysis of intestinal brush border assembly. I. Ultrastructural analysis of cell contact-induced brush border assembly in Caco-2BBe cells. J Cell Sci. 1993;105:445–460. doi: 10.1242/jcs.105.2.445. [DOI] [PubMed] [Google Scholar]
- 23.Pinto M, Robine-Leon S, Appay M D, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Aussman P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 24.Salas P J I, Rodriguez M L, Viciana A L, Vegas-Salas D E, Hauri H P. The apical submembrane cytoskeleton participates in the organization of the apical pole in epithelial cells. J Cell Biol. 1997;137:359–375. doi: 10.1083/jcb.137.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sears C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian P, Estes M K, Hu Y, Ball J M, Zeng C Q Y, Schilling W P. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J Virol. 1995;69:5763–5772. doi: 10.1128/jvi.69.9.5763-5772.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucker S P, Thornton C L, Wimmer E, Compans R W. Bidirectional entry of poliovirus into polarized epithelial cells. J Virol. 1993;67:29–38. doi: 10.1128/jvi.67.1.29-38.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Ijzendoorn S C D, Hoekstra D. The subapical compartment: A novel sorting centre? Trends Cell Biol. 1999;9:144–149. doi: 10.1016/s0962-8924(99)01512-3. [DOI] [PubMed] [Google Scholar]
- 29.Weber E, Berta G, Tousson A, St. John P, Green M W, Gopalokrishnan U, Jilling T, Sorscher E J, Elton T S, Abrahamson D R, Kirk K L. Expression and polarized targeting of Rab3 isoform in epithelial cells. J Cell Biol. 1994;125:583–594. doi: 10.1083/jcb.125.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zabriskie T M, Klocke J A, Ireland C M, Marcus A H, Molinski T F, Faulkner D J, Xu C, Clardy J C. Jaspamide, a modified peptide from Jaspis sponge, with insecticidal and antifungal activity. J Am Chem Soc. 1986;108:3123–3124. [Google Scholar]
- 31.Zahraoui A, Joberty G, Arpin M, Fontaine J J, Hellio R, Tavitian A, Louvard D. A small rab GTPase is distributed in cytoplasmic vesicles in non polarized cells but colocalizes with the tight junction marker ZO-1 in polarized epithelial cells. J Cell Biol. 1994;124:101–115. doi: 10.1083/jcb.124.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zweibaum A, Laburthe M, Grasset E, Louvard D. Use of cultured cell lines in studies of intestinal cell differentiation and function. In: Schultz S J, Field M, Frizell R A, editors. Handbook of physiology. The gastrointestinal system. Vol. 4. Bethesda, Md: American Physiological Society; 1991. pp. 223–255. [Google Scholar]