Abstract
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide. Over the past 50 years, there has been a substantial decline in the incidence of CVD and related mortality in high-income countries, largely due to the mitigation of modifiable risk factors such as smoking, hypertension, and diabetes. However, a significant burden of CVD remains in low- to middle-income countries, despite their lower prevalence of traditional risk factors; other environmental factors, particularly pollution, play a significant role in this attributable risk. Mounting evidence underscores a strong association between pollution and adverse health effects, including CVD. This article is part 1 of a 2-part state-of-the-art review and discusses air pollution and its adverse effects on CVD, highlighting pathophysiological mechanisms and methods to reduce air pollution and exposure to these pollutants.
Key words: air pollution, global burden of disease, particulate matter
Central Illustration
Highlights
-
•
Air pollution is the most important environmental cardiovascular risk factor, with PM2.5 being the most studied air pollutant.
-
•
Both short-term and long-term exposure to air pollutants can increase CVD-related morbidity and mortality, often acting synergistically with other CVD risk factors.
-
•
A deeper understanding of air pollution’s impact on CVD can guide anticipatory clinical guidelines.
-
•
Clinicians should incorporate air quality considerations when assessing cardiovascular risk factors.
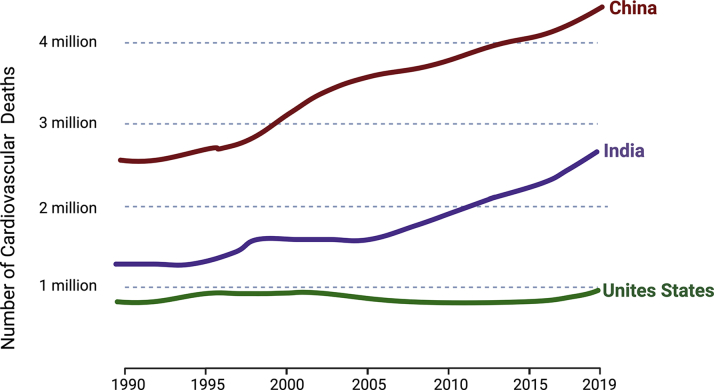
Cardiovascular disease (CVD) is the leading cause of mortality globally, accounting for 17.9 million deaths annually.1 Although CVD is the major cause of death in most developed countries, more than three-quarters of CVD deaths occur in low- and middle-income countries (Figure 1). A complex interplay between lifestyle, genetic susceptibility, and environmental factors contributes to CVD. Modifiable risk factors play an essential role in the development of CVD, with emerging evidence that environmental factors may also serve as key contributors to the pathogenesis of CVD.2 The steady decrease in CVD mortality in high-income countries during the last 50 years has been due to better control of modifiable CVD risk factors. With regard to improvements in environmental factors contributing to CVD risk, decreases in air pollution in many parts of the United States have also contributed to some of this decline3 (Figure 2A). A recent meta-analysis of 22 studies found that a 48% decrease in the incidence of coronary artery disease (CAD) was seen with population-based strategies that reduced both environmental pollution and unhealthy lifestyles.4
Figure 1.
Mortality From Cardiovascular Disease
Cardiovascular disease (CVD) rates are higher in low- and middle-income countries (such as India and China) than in high-income countries (eg, the United States), with a significant surge in CVD rates in developing countries over the past 2 decades relative to the United States (data from Global Burden of Disease 20199).
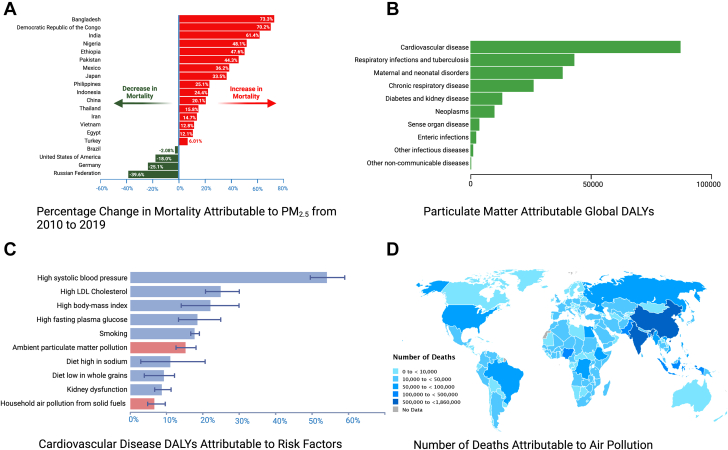
Figure 2.
Air Pollution and Cardiovascular Disease
(A) The percentage shift in overall mortality from 2010 to 2019 caused by PM2.5 in the 20 most populous countries worldwide, with the increase in mortality mainly being seen in developing countries, whereas most developed countries show a decrease in PM2.5-attributable mortality. (B) The distribution of disability-adjusted life years (DALYs) from noncommunicable diseases linked to PM pollution globally in 2019, with the majority of PM-attributable DALYs being due to CVD. (C) The proportion of cardiovascular disease DALYs caused by air pollution compared to other risk factors in 2019. Shown in red bars is the contribution of air pollution due to both ambient air PM pollution as well as household air pollution arising from the burning of solid fuels. Finally, (D) exhibits the global distribution of number of deaths in 2019 attributed to PM2.5 air pollution per country. Data in all panels are adapted from Global Burden of Disease 2019.7 PM = particulate matter.
Pollution is defined as the presence of substances in the environment that have adverse effects on both human health and the overall ecosystem. It is a grave concern, leading to an estimated 9 million deaths annually.5 Furthermore, pollution incurs staggering economic losses, which in 2015 amounted to U.S. $4.6 trillion.6 While there is ample awareness and guidelines available for the control of established traditional risk factors for CVD, such as hypertension (HTN), hyperlipidemia (HLD), and smoking, pollution has not received adequate recognition as a preventable risk factor for CVD. Moreover, evidence-based guidelines specifically targeting pollution-related CVD risk are lacking. Thus, there is an imperative need to not only better understand the impact of pollution on CVD but also to increase awareness and delineate effective and pragmatic methods to reduce this risk.
In the first part of this 2-part review, our focus is on the principal 6 air pollutants for which the United States Environmental Protection Agency has set National Ambient Air Quality Standards. In Part 2, we will discuss key environmental pollutants other than air pollution, namely water, soil, light, noise, pesticides, and metallic pollutants.
Air Pollution
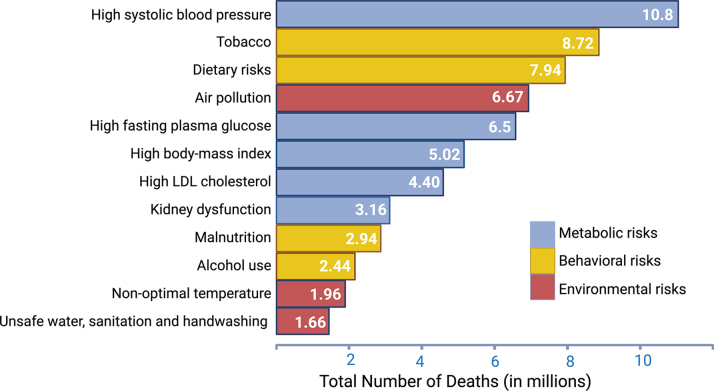
Air pollution, a harmful mix of solid particles and gases, endangers human health worldwide. The World Health Organization estimates that 99% of the world’s population resides in places where the World Health Organization’s air quality standards are not being met.2 The Global Burden of Disease (GBD) 2019 study attributes 6.67 million global deaths to air pollution making it the fourth leading risk factor for global mortality7 (Figure 3). Nearly 45% of air pollution-associated deaths are due to CVD; in contrast, only 8% are due to respiratory disease8 (Figure 2B).
Figure 3.
Global Ranking of Risk Factors Contributing to All-Cause Mortality in 2019
Global estimates of attributable deaths due to various risk factors indicate that environmental risk factors (shown in the red bars), especially air and water pollution and climate change, contributed significantly to global mortality. Data sourced from Global Burden of Disease 2019.7
Air pollution includes household air (indoor) pollution (HAP) and ambient (outdoor) pollution. HAP predominantly stems from inefficient combustion of solid fuels, often in poorly ventilated homes, and resulted in 2.3 million deaths in 2019.7 HAP-related mortality has been declining steadily with sociodemographic development.7 Ambient air pollution, primarily from fossil fuel combustion, caused 4.2 million deaths worldwide in 2019, with a disproportionate burden of 89% of deaths occurring in low- to middle-income countries7 (Figure 2A). This burden tends to increase with industrialization, and pollution-attributable deaths have increased by 51% since 1990.7 Another problem that is gaining in frequency and intensity and that contributes to air pollution is wildfires. As compared to 20 years ago, forest fires currently burn twice as much tree cover. In 2020, it is estimated that 1 in 7 Americans experienced dangerous air quality due to wildfires. Although prescribed burns are a commonly used method to keep forest ecosystems in balance, uncontrolled wildfires are occurring more frequently, as evidenced by the Quebec wildfires in June 2023 that affected air quality for many in Canada and more than 75 million people in the United States. Furthermore, these wildfires are becoming a major source of atmospheric carbon dioxide (CO2) emissions; 1.7 billion tons of CO2 were released in 2021 alone.9 The accompanying loss of forest cover, which normally absorbs 7.6 billion tons of CO2 annually further compounds this problem. The forest canopy also preserves drinking water by reducing soil erosion after rainfalls, reducing the chance of floods, and recharging the groundwater. Forests aid in evapotranspiration and regulate precipitation; all of these regulatory functions of forests are hampered by unchecked wildfires.9
Air pollutants may be classified as primary and secondary pollutants. Primary pollutants are emitted directly into the atmosphere, including particulate matter (PM), carbon monoxide (CO), nitrogen dioxide (NO2), and sulfur dioxide (SO2).10 Secondary pollutants, formed through interactions with other components, include secondary PM and ozone (O3)2 (Table 1). Many pollutants coexist and vary spatially and temporally, so it is challenging to separate their effects.
Table 1.
Air Pollution and Cardiovascular Disease
| Air Pollutant Component | Major Sources of Exposure | Putative Mechanism of CVD Effects |
|---|---|---|
| Particulate matter (PM) | Natural sources: wildfires and sand dust storms Anthropogenic sources: combustion of fossil fuel, transportation, and industrial processes |
Localized pulmonary and systemic inflammation Activation of prothrombic pathways Autonomic dysfunction Hypothalamic-pituitary-adrenal axis activation Endothelial dysfunction |
| Ozone (O3) | Natural sources: lightening Anthropogenic sources: photochemical reactions involving sunlight, NOx, and VOCs emitted by human activities such as transportation and industrial processes |
Activation of inflammatory pathways Endothelial dysfunction Autonomic dysfunction Oxidative stress |
| Carbon monoxide (CO) | Natural sources: wildfires and volcanic activity Anthropogenic sources: incomplete combustion of fossil fuel, industrial processes, and motor vehicles |
Systemic inflammation Hypoxic injury Free radical generation mitochondrial inhibition Platelet activation |
| Nitrogen dioxide (NO2) | Natural sources: wildfires Anthropogenic sources: motorized road traffic and fossil fuel-fired power generation |
Activation of inflammatory pathways Endothelial dysfunction autonomic dysfunction Oxidative stress |
| Sulfur dioxide (SO2) | Natural sources: volcanic activity Anthropogenic sources: coal-fired power plants, burning of heavy fuel oil in shipping, and petroleum processing |
Impaired respiratory function Activation of inflammatory pathways Endothelial dysfunction Autonomic dysfunction Oxidative stress |
| Lead (Pb) | Natural sources: geochemical weathering and volcanic activity Anthropogenic sources: lead-acid battery manufacturing, combustion of leaded gasoline, and lead-based paint | Activation of inflammatory pathways Endothelial dysfunction Oxidative stress Disruption of calcium signaling Interference with heme synthesis |
This table illustrates the most common air pollutants, processes that generate them, and mechanisms of cardiovascular injury.
CVD = cardiovascular disease; NOx = nitrogen oxides; VOC = volatile organic compound.
Particulate matter
PM is a complex mixture of inhaled solid particles and gaseous matter, with the composition varying by time, location, environmental conditions, and emission sources.11 PM is categorized by aerodynamic diameter: coarse particles (PM10) with a diameter of 2.6 to 10 μm, fine particles (PM2.5) with a diameter of 0.1 to 2.5 μm, and ultrafine particles (UFP, or PM0.1) with a diameter <0.1 μm.11 PM2.5 and PM10 are the most studied PM relevant to adverse health effects, with PM2.5 being the most relevant to CVD11 (Figure 2C).
Mechanism of action of particulate matter in cardiovascular disease
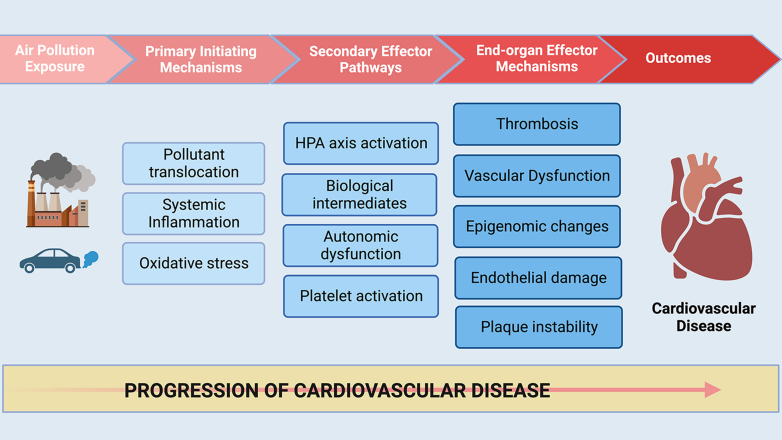
The cardiovascular (CV) effects of PM are from oxidative stress, localized endothelial dysfunction, and systemic inflammation10,12 (Figure 4). PM deposition in the lungs induces localized inflammation that then elicits a systemic inflammatory response and results in the activation of prothrombic pathways, the autonomic nervous system, and the hypothalamic-pituitary-adrenal axis.2 PM2.5 can also cross the alveolar-capillary barrier and induce vascular endothelial dysfunction. These effects lead to the development of HLD, HTN, and diabetes mellitus (DM) and are thus proatherogenic (Central Illustration).10 Although studies are not entirely consistent, some recent studies have shown that higher PM2.5 exposure is linked to increased arterial inflammation, leukopoiesis, higher levels of circulating inflammatory cytokines, and stress hormones.13 PM exposure leads to epigenetic changes in various organ systems and affects various biological pathways leading to adverse downstream CV effects.12
Figure 4.
Mechanisms Underlying PM-Induced Cardiovascular Disease
Inhaled PM localizes within pulmonary tissues, eliciting oxidative stress and systemic inflammation. This in turn triggers secondary effector pathways including the activation of the HPA axis, platelet activation, autonomic dysfunction, and the generation of biological intermediates such as modified phospholipids. These pathways result in endothelial and vascular injury, atherothrombosis, inflammation, epigenomic changes, and ultimately lead to the development of cardiovascular disease. HPA = hypothalamic-pituitary-adrenal.
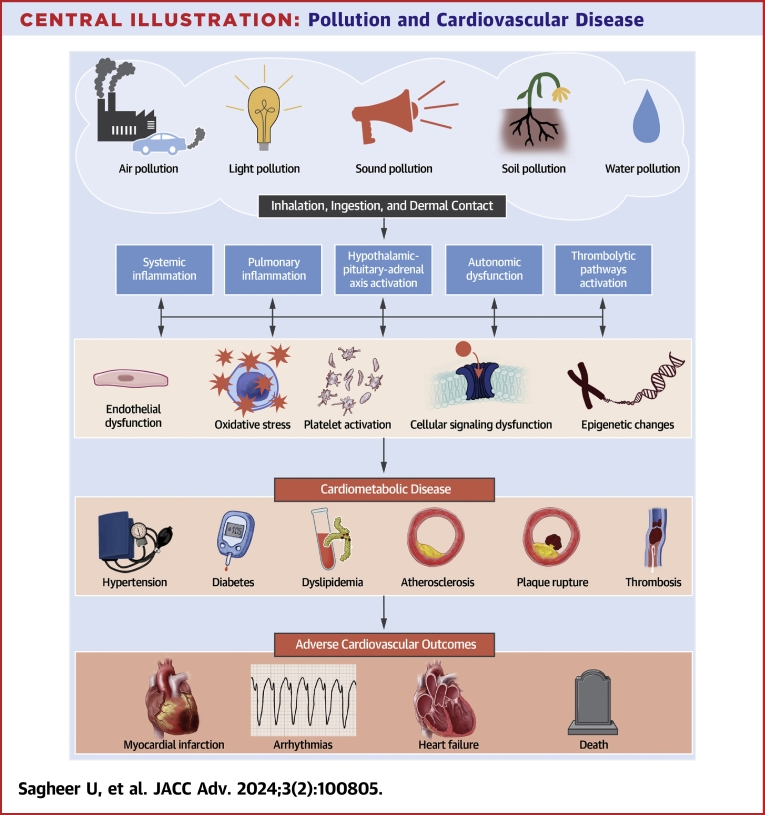
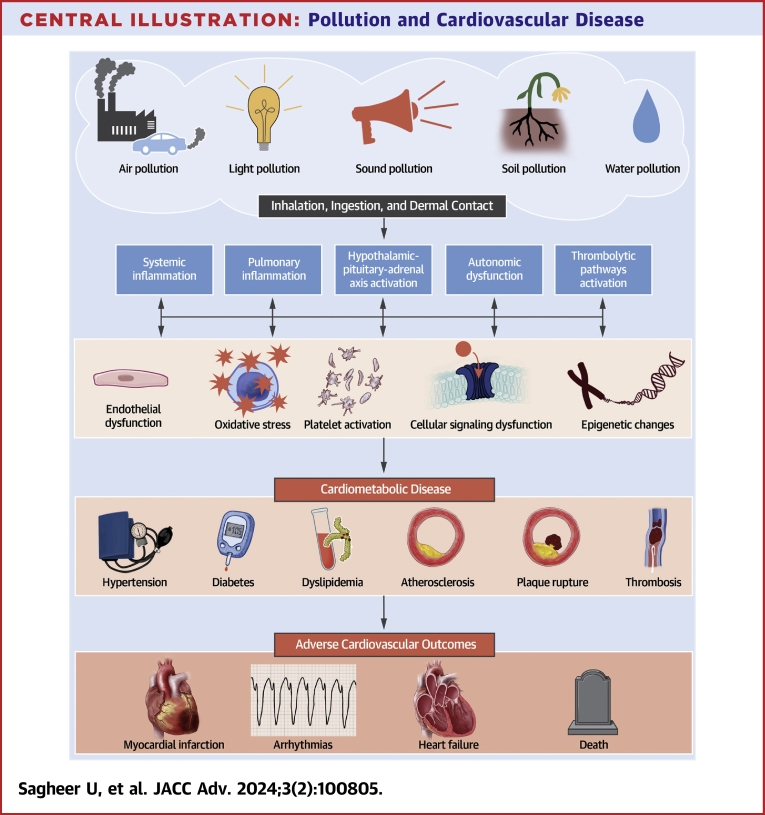
Central Illustration.
Pollution and Cardiovascular Disease
Exposure to environmental pollutants occurs through various routes, leading to localized endothelial dysfunction and systemic inflammation. This inflammation activates prothrombotic pathways, the hypothalamic-pituitary-adrenal axis, and causes autonomic dysfunction, which in turn leads to oxidative stress, platelet activation, increased arterial inflammation, and cellular signaling abnormalities. Collectively, these insults result in the development of hypertension, atherothrombosis, altered glucose metabolism, and plaque instability, leading to adverse cardiovascular outcomes such as myocardial infarction, arrhythmias, and heart failure.
Particulate matter and cardiovascular disease
In 2019, PM accounted for 26% of age-standardized CVD deaths in the eastern Mediterranean region, primarily due to CAD.14 Even in developed countries with lower PM exposure, long-term exposure is linked to increased CVD mortality.15
Observational studies consistently link PM2.5 exposure with subclinical atherosclerosis, increased coronary artery calcium score, high-risk plaque formation, and faster plaque growth.16,17 Long-term exposure is also associated with increased carotid intima-media thickness, a marker of subclinical atherosclerosis burden, and coronary vasomotor abnormalities.18 A meta-analysis of 11 European cohort studies showed a 13% increase in acute coronary syndrome events with a 5 μg/m3 increase in the estimated annual mean PM2.5 exposure and a 12% increase in acute coronary syndrome events with a 10 μg/m3 increase in the estimated annual mean PM10.19 Short-term exposure to PM is also linked to an increased incidence of acute myocardial infarction (MI), mainly ST-segment elevation MI, and related mortality, particularly in elderly patients with preexisting CAD and significant risk factors for CVD.20,21 Supplemental Table 1 summarizes the major studies on the association between PM and CAD.
Like CAD, there is now widespread recognition of the association between PM exposure and the occurrence of stroke. According to a recent GBD 2019 analysis, the global ambient PM2.5-related stroke mortality was reported to be 1.14 million.22 A recent study found that the HR for all cerebrovascular events was 2.14 (95% CI: 1.87-2.44) when comparing the top vs bottom quartiles of PM2.5.23
Exposure to PM, both short-term and long-term, increases the risk of heart failure (HF), related hospitalizations, and mortality. A meta-analysis of 35 studies found that a short-term increase of 10 μg/m3 in PM2.5 and PM10 was associated with 2.12% and 1.63% increased risk of HF hospitalization and mortality, respectively.24 Even in areas with very low air pollution, such as the Australian state of Tasmania, acute PM exposure has been associated with an increased incidence of HF.17 Supplemental Table 2 summarizes major studies on the association between PM and HF.
PM exposure increases the risk of arrhythmias, especially atrial fibrillation (AF). Studies among patients with implantable cardioverter defibrillators have found that higher PM2.5 and PM10 concentrations correspond to an increased risk of AF and ventricular arrhythmias.15,25 A large South Korean study demonstrated a strong association between long-term PM exposure and various cardiac arrhythmias, with increased risks observed for incrementally higher levels of exposure to PM10 and PM2.5.26
Evidence also indicates that PM exposure leads to an increased risk of developing CV risk factors such as HTN, DM, and HLD. A recent meta-analysis showed that each 10 μg/m3 increase in PM2.5 environmental exposure was associated with an increase of 0.63- and 0.31-mm Hg in systolic blood pressure (SBP) and diastolic blood pressure, respectively.27 Randomized trials comparing air filtration to sham filtration also support the link, with a meta-analysis of 10 trials showing that the use of personal air cleaners significantly reduced mean SBP by nearly 4 mmHg (95% CI: −7.00 to −0.89) over a median of 13.5 days.28 Studies have demonstrated a strong correlation between increased exposure to PM with an increase in total cholesterol, triglyceride, and low-density lipoprotein cholesterol levels.29 In the GBD 2019 study, PM2.5 was identified as the third leading risk factor for DM.30 About one-fifth of the global DM burden was attributable to PM2.5 exposure, with approximately 13.4% of deaths due to DM resulting from PM2.5.30
Ozone
Increased ground-level O3 contributes to global warming, and a warming climate facilitates O3 formation and accumulation.31 While high O3 levels have been linked to increased total mortality, the association with CVD is not consistently supported. According to GBD 2019, 0.4 million deaths worldwide were attributed to O3 air pollution.7 Some epidemiological studies and meta-analyses have provided evidence that exposure to high levels of O3 increases CVD risk, including that from CAD, MI, and HF. A nearly decade-long study assessing the association between long-term O3 exposure and subclinical arterial disease found that a 3-parts per billion increase in long-term O3 exposure was linked to a ∼6 μm greater increase in the carotid intima-media thickness over 10 years along with new carotid plaque formation.32 A large Canadian cohort study reported a 4% and 3% increase in the incidence of acute MI and HF admissions, respectively, with each interquartile range increase in long-term exposure to O3.33 These associations have been observed in high-income countries like the United States and Canada, as well as in low- to middle-income populations in China, indicating the widespread impact of O3 exposure on cardiovascular health.34
Carbon monoxide
CO is a colorless gas and has ∼250 times higher affinity for hemoglobin than oxygen. Its toxic effects mainly stem from hypoxic injury, free radical generation, mitochondrial inhibition, platelet activation, and inflammation.35 A large study from China found that for every 1 mg/m³ increase in CO levels in the environment, there was a 2.08%, 2.35%, and 2.28% increase in years of life lost from all causes, CVD, and CAD, respectively.36 Short-term exposure to elevated CO levels has been linked to an increased risk of CVD admissions and HF hospitalizations or death. A U.S. study reported that a short-term (1 hour) 1-ppm (part per million) same-day increase in CO was associated with a 0.96% increase in the risk of CVD admissions.37,38 A meta-analysis of 35 studies revealed that a 1 ppm increase in CO was associated with a 3.52% increase in the risk of HF hospitalization or death.24
Nitrogen dioxide
NO2 is a commonly used surrogate for traffic-related air pollutants. Although studies have shown negative CV effects from exposure to elevated NO2 levels, it is unclear whether NO2 independently contributes to CVD or if the compounding effect of other traffic-related co-pollutants plays a role. Some recent studies suggest the possibility of independent effects of NO2 on CVD, even after adjustment for co-pollutants. A recent analysis using a large multi-city, multi-country database revealed 0.46% and 0.37% increases in total and CV mortality associated with a 10 μg/m3 increase in NO2 concentration on the previous day, respectively.39 These associations remained significant even after adjusting for co-pollutants including PM, O3, SO2, and CO.39 In one meta-analysis, a 13% increase in CV mortality was seen with a 10 μg/m3 increase in annual NO2 long-term exposure.40 Short-term exposure to elevated NO2 levels also has negative CV effects. Increased NO2 exposure has also been linked to higher risks of CAD and HF.41 A Belgian study showed a 5.1% increased risk of ST-segment elevation MI for every 10 μg/m3 increase in NO2 exposure, and younger patients were more susceptible due to their greater exposure to traffic-related NO2.41 A meta-analysis reported that a 10-parts per billion increase in NO2 was associated with a 1.7% increase in the risk of HF hospitalization or death.24
Sulfur dioxide
Understanding the independent impact of SO2 on CVD is challenging due to its co-emission with other co-pollutants and involvement in secondary PM formation. A multicenter European study found a 0.7% increase in CV hospital admissions on the same day and the next day following a 10 μg/m3 increase in the daily average SO2.42 SO2 emissions have substantially reduced in Europe and North America in the 21st century, but developing countries like India and China remain major global emitters. A Chinese study found that a 10 μg/m3 increase in the 2-day average concentration of SO2 was associated with a rise of 1.38%, 1.58%, and 1.69% in hospital admissions for total CVD, CAD, and AF, respectively.43 The evidence linking SO2 exposure to HF is less clear than for other pollutants like PM2.5. A case-crossover Chinese study showed that short-term exposure to 23.3 μg/m3 ambient SO2 significantly increased the risk of HF readmissions by ∼15% among elderly patients with HTN.44
Lead
Despite a decrease in lead exposure due to the phasing out of leaded gasoline in many countries, significant amounts remain in the environment, with over 50% of children still having detectable blood lead levels (BLLs).45 Increased lead exposure is associated with higher CVD risk factors and mortality. A systematic review of National Health and Nutrition Examination Survey data estimated that a decrease in environmental lead exposure from 1999 to 2014 has led to an estimated 34,000 to 99,000 avoided deaths, with approximately 16% to 46% of decreased CVD-related mortality being attributable to a reduction in BLLs.46 A U.S. population-based cohort study found that an increase in BLLs from 1.0 to 6.7 μg/dL was associated with higher CVD and CAD mortality with HRs of 1.70 (95% CI: 1.30-2.22) and 2.08 (95% CI: 1.52-2.85), respectively.47 The association between HTN and lead exposure is one of the most well-established CV effects of lead exposure. A meta-analysis found that a 2-fold increase in BLLs led to a 1.0 to 1.25 mmHg increase in SBP and a 0.6 mmHg increase in diastolic blood pressure.48 A recent study found a positive correlation between the risk of subclinical myocardial injury, assessed with a 12-lead electrocardiogram-based cardiac infarction/injury score, and BLLs higher than 3.8 ug/dL.49 A Mexican study also reported a significant association between lead exposure and an increase in body mass index, with BLLs averaging 0.051 ± 0.035 μg/dL, 0.107 ± 0.067 μg/dL, and 0.151 ± 0.063 μg/dL in individuals with normal weight, overweight, and obesity, respectively.50 Supplemental Table 3 summarizes the major studies on the association between lead and CVD.
Conclusions
Over the past decade, compelling evidence has emerged for the association between air pollution and CVD. However, some knowledge gaps persist: the precise biological mechanisms causing CVD for various pollutants, variations in susceptibility to adverse CV effects, thresholds of toxic exposure and reversibility, disparities based on sex, race, social determinants of health, and the isolated vs synergistic effects of various common pollutants remain unclear. These are all potential avenues for research, along with which strategies can be most effective in combating or reversing such CV effects. While much of the research has focused on a few primary pollutants, notably PM2,5 and O3, a broader approach is required to comprehend the roles and interactions of various pollutants. It is also essential to evaluate how other environmental determinants, particularly the recent escalation in extreme weather patterns attributed to global climate change, may intensify the CV risk associated with air pollution because they often coexist. Observational environmental studies aiming to assess the impact of various pollutants face several challenges; some of these include the geospatial and temporal variability in pollutant concentrations, teasing out the individual effects of specific pollutants, underrepresentation of susceptible populations, challenges in establishing clear dose-response correlations, confounding, and other biases.
While multiple observational studies have identified associations between air pollution and CVD, there is an urgent need for interventional studies that inform us on the best, targeted pollution-reducing public health measures that will reduce CVD from air pollution. Ideally, such strategies would be easy to deploy even in countries with limited resources and would result in rapid and sustained reductions in air pollutants. Several cardiovascular societies such as the World Heart Federation, the American College of Cardiology, the American Heart Association, and the European Society of Cardiology have jointly emphasized the urgency of such measures in a recent call-to-action statement that urges both the medical community and health regulators to work together to mitigate the impact of air pollution on global CV health.51
Prioritizing the development of health guidelines, endorsing environmental government programs, raising public awareness about the health advantages of clean air, and collaborating with global policymakers are crucial strategies for efficaciously addressing this significant concern. It is paramount that health care providers recognize the significant impact of air pollution on CV health, incorporate this knowledge into clinical practice, and for educators to train future generations of providers about this important emerging CV topic. Clinicians must ask patients about their living environment, both indoor and outdoor, and their workplace, as this information is important in evaluating CV risk and providing advice on how to reduce exposure to pollutants, as well as personal choices that can reduce the impact on air pollution and climate change.
Funding support and author disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methodology, tables, and references, please see the online version of this paper.
Supplementary data
References
- 1.Collaborators G 2017 C of D Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajagopalan S., Landrigan P.J. Pollution and the heart. N Engl J Med. 2021;385:1881–1892. doi: 10.1056/NEJMra2030281. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro J.S., Walker R. Why is pollution from US manufacturing declining? the roles of environmental regulation, productivity, and trade. Am Econ Rev. 2018;108:3814–3854. [Google Scholar]
- 4.Ahmadi M., Lanphear B. The impact of clinical and population strategies on coronary heart disease mortality: an assessment of Rose’s big idea. BMC Public Health. 2022;22:14. doi: 10.1186/s12889-021-12421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller R., Landrigan P.J., Balakrishnan K., et al. Pollution and health: a progress update. Lancet Planet Health. 2022;6:e535–e547. doi: 10.1016/S2542-5196(22)00090-0. [DOI] [PubMed] [Google Scholar]
- 6.Landrigan P.J., Fuller R., Acosta N.J.R., et al. The Lancet commission on pollution and health. Lancet. 2018;391:462–512. doi: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- 7.Collaborators G 2019 RF Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pope C.A., Burnett R.T., Thurston G.D., et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 9.Alexander S., Anderson J. World Resources Institute; 2023. Five Ways Wildfires Affect People Near and Far. [Google Scholar]
- 10.Rajagopalan S., Al-Kindi S.G., Brook R.D. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 11.Pryor J.T., Cowley L.O., Simonds S.E. The physiological effects of air pollution: particulate matter, physiology and disease. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.882569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bevan G.H., Al-Kindi S.G., Brook R.D., Münzel T., Rajagopalan S. Ambient air pollution and atherosclerosis: insights into dose, time, and mechanisms. Arterioscler Thromb Vasc Biol. 2021;41:628–637. doi: 10.1161/ATVBAHA.120.315219. [DOI] [PubMed] [Google Scholar]
- 13.Abohashem S., Osborne M.T., Dar T., et al. A leucopoietic-arterial axis underlying the link between ambient air pollution and cardiovascular disease in humans. Eur Heart J. 2021;42:761–772. doi: 10.1093/eurheartj/ehaa982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motairek I., Ajluni S., Khraishah H., et al. Burden of cardiovascular disease attributable to particulate matter pollution in the eastern mediterranean region: analysis of the 1990-2019 global burden of disease. Eur J Prev Cardiol. 2022;30(3):256–263. doi: 10.1093/eurjpc/zwac256. [DOI] [PubMed] [Google Scholar]
- 15.Ljungman P.L., Berglind N., Holmgren C., et al. Rapid effects of air pollution on ventricular arrhythmias. Eur Heart J. 2008;29:2894–2901. doi: 10.1093/eurheartj/ehn463. [DOI] [PubMed] [Google Scholar]
- 16.Jilani M.H., Simon-Friedt B., Yahya T., et al. Associations between particulate matter air pollution, presence and progression of subclinical coronary and carotid atherosclerosis: a systematic review. Atherosclerosis. 2020;306:22–32. doi: 10.1016/j.atherosclerosis.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Huynh Q.L., Blizzard C.L., Marwick T.H., Negishi K. Association of ambient particulate matter with heart failure incidence and all-cause readmissions in Tasmania: an observational study. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-021798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilli M., Russo M., Rinaldi R., et al. Air pollution and coronary vasomotor disorders in patients with myocardial ischemia and unobstructed coronary arteries. J Am Coll Cardiol. 2022;80:1818–1828. doi: 10.1016/j.jacc.2022.08.744. [DOI] [PubMed] [Google Scholar]
- 19.Cesaroni G., Forastiere F., Stafoggia M., et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE project. BMJ. 2014;348:f7412. doi: 10.1136/bmj.f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mustafic H., Jabre P., Caussin C., et al. Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA. 2012;307:713–721. doi: 10.1001/jama.2012.126. [DOI] [PubMed] [Google Scholar]
- 21.Cai X., Li Z., Scott E.M., Li X., Tang M. Short-term effects of atmospheric particulate matter on myocardial infarction: a cumulative meta-analysis. Environ Sci Pollut Res Int. 2016;23:6139–6148. doi: 10.1007/s11356-016-6186-3. [DOI] [PubMed] [Google Scholar]
- 22.Bo Y., Zhu Y., Zhang X., et al. Spatiotemporal trends of stroke burden attributable to ambient PM2.5 in 204 countries and territories, 1990-2019: a global analysis. Neurology. 2023;101(7):e764–e776. doi: 10.1212/WNL.0000000000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulick E.R., Eliot M.N., Szpiro A.A., et al. Long-term exposure to ambient particulate matter and stroke etiology: results from the Women’s Health Initiative. Environ Res. 2023;224 doi: 10.1016/j.envres.2023.115519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah A.S., Langrish J.P., Nair H., et al. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382:1039–1048. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Link M.S., Luttmann-Gibson H., Schwartz J., et al. Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol. 2013;62:816–825. doi: 10.1016/j.jacc.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z., Kang J., Hong Y.S., et al. Long-term particulate matter exposure and incidence of arrhythmias: a cohort study. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niu Z., Duan Z., Yu H., et al. Association between long-term exposure to ambient particulate matter and blood pressure, hypertension: an updated systematic review and meta-analysis. Int J Environ Health Res. 2023;33:268–283. doi: 10.1080/09603123.2021.2022106. [DOI] [PubMed] [Google Scholar]
- 28.Walzer D., Gordon T., Thorpe L., et al. Effects of home particulate air filtration on blood pressure: a systematic review. Hypertension. 2020;76:44–50. doi: 10.1161/HYPERTENSIONAHA.119.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Z.Z., Guo P.Y., Xu S.L., et al. Associations of particulate matter sizes and chemical constituents with blood lipids: a panel study in Guangzhou, China. Environ Sci Technol. 2021;55:5065–5075. doi: 10.1021/acs.est.0c06974. [DOI] [PubMed] [Google Scholar]
- 30.Collaborators G 2019 D and AP Estimates, trends, and drivers of the global burden of type 2 diabetes attributable to PM. Lancet Planet Health. 2022;6:e586–e600. doi: 10.1016/S2542-5196(22)00122-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J.J., Wei Y., Fang Z. Ozone pollution: a major health hazard worldwide. Front Immunol. 2019;10:2518. doi: 10.3389/fimmu.2019.02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang M., Sampson P.D., Sheppard L.E., Stein J.H., Vedal S., Kaufman J.D. Long-term exposure to ambient ozone and progression of subclinical arterial disease: the multi-ethnic study of atherosclerosis and air pollution. Environ Health Perspect. 2019;127 doi: 10.1289/EHP3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bai L., Shin S., Burnett R.T., et al. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: a population-based study of 5.1 million Canadian adults living in Ontario. Environ Int. 2019;132 doi: 10.1016/j.envint.2019.105004. [DOI] [PubMed] [Google Scholar]
- 34.Niu Y., Zhou Y., Chen R., et al. Long-term exposure to ozone and cardiovascular mortality in China: a nationwide cohort study. Lancet Planet Health. 2022;6:e496–e503. doi: 10.1016/S2542-5196(22)00093-6. [DOI] [PubMed] [Google Scholar]
- 35.Rose J.J., Wang L., Xu Q., et al. Carbon monoxide poisoning: pathogenesis, management, and future directions of therapy. Am J Respir Crit Care Med. 2017;195:596–606. doi: 10.1164/rccm.201606-1275CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Li J., Wang L., et al. The impact of carbon monoxide on years of life lost and modified effect by individual- and city-level characteristics: evidence from a nationwide time-series study in China. Ecotoxicol Environ Saf. 2021;210 doi: 10.1016/j.ecoenv.2020.111884. [DOI] [PubMed] [Google Scholar]
- 37.Samoli E., Touloumi G., Schwartz J., et al. Short-term effects of carbon monoxide on mortality: an analysis within the APHEA project. Environ Health Perspect. 2007;115:1578–1583. doi: 10.1289/ehp.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bell M.L., Peng R.D., Dominici F., Samet J.M. Emergency hospital admissions for cardiovascular diseases and ambient levels of carbon monoxide: results for 126 United States urban counties, 1999-2005. Circulation. 2009;120:949–955. doi: 10.1161/CIRCULATIONAHA.109.851113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meng X., Liu C., Chen R., et al. Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: multilocation analysis in 398 cities. BMJ. 2021;372:n534. doi: 10.1136/bmj.n534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faustini A., Rapp R., Forastiere F. Nitrogen dioxide and mortality: review and meta-analysis of long-term studies. Eur Respir J. 2014;44:744–753. doi: 10.1183/09031936.00114713. [DOI] [PubMed] [Google Scholar]
- 41.Argacha J.F., Collart P., Wauters A., et al. Air pollution and ST-elevation myocardial infarction: a case-crossover study of the Belgian STEMI registry 2009-2013. Int J Cardiol. 2016;223:300–305. doi: 10.1016/j.ijcard.2016.07.191. [DOI] [PubMed] [Google Scholar]
- 42.Sunyer J., Ballester F., Tertre A.L., et al. The association of daily sulfur dioxide air pollution levels with hospital admissions for cardiovascular diseases in Europe (The Aphea-II study) Eur Heart J. 2003;24:752–760. doi: 10.1016/s0195-668x(02)00808-4. [DOI] [PubMed] [Google Scholar]
- 43.Chen L., Wang X., Qian Z.M., et al. Ambient gaseous pollutants and emergency ambulance calls for all-cause and cause-specific diseases in China: a multicity time-series study. Environ Sci Pollut Res Int. 2022;29:28527–28537. doi: 10.1007/s11356-021-18337-x. [DOI] [PubMed] [Google Scholar]
- 44.Xu R., Tian Q., Wei J., et al. Short-term exposure to ambient air pollution and readmissions for heart failure among 3660 post-discharge patients with hypertension in older Chinese adults. J Epidemiol Community Health. 2022;76:984–990. doi: 10.1136/jech-2022-219676. [DOI] [PubMed] [Google Scholar]
- 45.Hauptman M., Niles J.K., Gudin J., Kaufman H.W. Individual- and community-level factors associated with detectable and elevated blood lead levels in US children: results from a national clinical laboratory. JAMA Pediatr. 2021;175:1252–1260. doi: 10.1001/jamapediatrics.2021.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown L., Lynch M., Belova A., Klein R., Chiger A. Developing a health impact Model for Adult lead exposure and cardiovascular disease mortality. Environ Health Perspect. 2020;128 doi: 10.1289/EHP6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanphear B.P., Rauch S., Auinger P., Allen R.W., Hornung R.W. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health. 2018;3:e177–e184. doi: 10.1016/S2468-2667(18)30025-2. [DOI] [PubMed] [Google Scholar]
- 48.Pirkle J.L., Schwartz J., Landis J.R., Harlan W.R. The relationship between blood lead levels and blood pressure and its cardiovascular risk implications. Am J Epidemiol. 1985;121:246–258. doi: 10.1093/oxfordjournals.aje.a113995. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z., Huang X., Li J., Liu N., Wei Q. Lead exposure is non-linearly associated with subclinical myocardial injury in the general population without cardiovascular disease. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.975413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernández-Mendoza H., Rios-Lugo M.J., Álvarez-Loredo H.E., et al. Serum lead levels and its association with overweight and obesity. J Trace Elem Med Biol. 2022;72 doi: 10.1016/j.jtemb.2022.126984. [DOI] [PubMed] [Google Scholar]
- 51.Brauer M., Casadei B., Harrington R.A., et al. Taking a stand against air pollution—the impact on cardiovascular disease: a joint opinion from the World Heart Federation, American College of Cardiology, American Heart Association, and the European Society of Cardiology. J Am Coll Cardiol. 2021;77:1684–1688. doi: 10.1016/j.jacc.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.