Abstract
Background
Hypercholesterolemia is a common condition characterized by elevated levels of low-density lipoprotein cholesterol (LDL-C) and increased risk of atherosclerotic cardiovascular disease (ASCVD). Indigenous populations experience disproportionate rates of ASCVD, however, the extent to which hypercholesterolemia contributes to this burden is unknown.
Objectives
This study aimed to estimate the prevalence of hypercholesterolemia, severe hypercholesterolemia, and familial hypercholesterolemia (FH) in Indigenous populations in Canada, the United States, Australia, and New Zealand.
Methods
We searched MEDLINE, EMBASE, Web of Science, Native Health Database, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews for peer-reviewed studies reporting on hypercholesterolemia and elevated LDL-C in Indigenous populations. All diagnostic criteria used to classify hypercholesterolemia were included. Pooled prevalence and 95% CIs were calculated using a random-effects model.
Results
There were no studies reporting the prevalence of FH and one study reporting the prevalence of severe hypercholesterolemia in Indigenous populations. The pooled prevalence of hypercholesterolemia was 28.9% or ∼1 in 3 to 1 in 4 individuals (95% CI: 22.4%-36.4%) and 12.6% (95% CI: 7.7%-19.9%) using an LDL-C cutoff of ≥3.5 mmol/L (135 mg/dL). The pooled prevalence in Indigenous populations in North America was 24.3% (95% CI: 17.1%-33.3%) compared with 40.0% (95% CI: 31.3%-49.3%) in Australia. Meta-regression showed diabetes had a significant effect on prevalence (P = 0.022).
Conclusions
Hypercholesterolemia is prevalent in Indigenous communities and may contribute to the high burden of ASCVD these populations face. There is insufficient research on FH and severe hypercholesterolemia in Indigenous populations worldwide.
Key words: cardiovascular disease, familial hypercholesterolemia, indigenous, LDL-cholesterol, severe hypercholesterolemia
Central Illustration
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death worldwide and disproportionately affects people of Indigenous ancestry.1,2 Hypercholesterolemia is a major risk factor for ASCVD and interventions to reduce cholesterol levels effectively reduce cardiovascular risk.3,4 While there is no universal definition for hypercholesterolemia, commonly used cutoffs include a low-density lipoprotein cholesterol (LDL-C) level ≥3.5 mmol/L (135 mg/dL) (high) and a LDL-C ≥4.0 mmol/L (155 mg/dL) (very high).5, 6, 7 Severe hypercholesterolemia, often defined as a LDL-C ≥5.0 mmol/L (193 mg/dL), confers ∼5-fold increased risk of developing ASCVD and is considered an indication for statin therapy in many national and international guidelines.5,8,9 Severe hypercholesterolemia affects ∼5% of the global population.10,11 A subset of patients with severe hypercholesterolemia have an underlying monogenic cause, called familial hypercholesterolemia (FH), which is the most common inherited lipid disorder, affecting 1 in 310 individuals globally.11,12 FH is characterized by lifelong elevated LDL-C levels and increased cardiovascular risk.12 A recent meta-analysis demonstrated differences in the prevalence of FH between ethnic groups, ranging from 1 in 192 in Black populations, to 1 in 400 in Asian populations.13 However, the prevalence of FH in Indigenous populations is not well established.
Worldwide, Indigenous peoples have an increased burden of chronic disease, including cardiovascular disease.14 While Indigenous populations in Canada, the United States, Australia, and New Zealand have unique genetic, geographic, and cultural backgrounds, they share similar colonial histories,15,16 which, in turn, have profound effects on their health. Emerging guidelines for addressing cardiovascular risk in Indigenous populations discuss the need for inclusion of hypercholesterolemia, FH, and Indigenous-specific risk factors that take into account different groups unique histories.17,18 The goal of this study was to estimate the prevalence of hypercholesterolemia, severe hypercholesterolemia, and FH in Indigenous populations in Canada, the United States, Australia, and New Zealand.
Methods
Population selection
We recognize that “Indigenous” is a broad term used to describe many diverse populations. Throughout this study we use Indigenous as a categorical term, as described by the United Nations as “inheritors and practitioners of unique cultures and ways of relating to people and the environment. They have retained social, cultural, economic, and political characteristics that are distinct from those of the dominant societies in which they live.” According to the United Nations global census data, there are over 476 million Indigenous peoples in over 90 countries.19 This study focuses on Indigenous peoples in Canada, the United States, Australia, and New Zealand as these are all high-income countries with Indigenous minority groups that share similar patterns of colonization and history of forced assimilation into Western-European culture.20, 21, 22 Indigenous populations in these countries have been compared in previous reviews,23, 24, 25 as western colonization has shown to disrupt Indigenous peoples traditional ways of life and result in loss of land, natural resources, and economic opportunities.26
Search strategy
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis consensus statement (Supplemental Table 1). We conducted a systematic review of the literature from inception to May 2021 that included a search for hypercholesterolemia, severe hypercholesterolemia, FH, and LDL-C in global Indigenous populations (Supplemental Table 2). The systematic review was created in consultation with 2 medical librarians and authors (R.K.M., L.R.B., A.I.K., J.G.) with all terms tested to ensure relevant studies were not missed. Searches were conducted in MEDLINE, EMBASE, Web of Science, Native Health Database, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews. The list of global Indigenous populations used for the systematic review search was derived from the University of Alberta’s Native Studies website and contained over 500 distinct groups.27 This included traditional names that communities use to refer to themselves, as well as Eurocentric and westernized terms non-Indigenous people use to reference Indigenous communities. Titles, abstracts, and full texts were screened by a single reviewer (R.K.M.) to ensure inclusion criteria were met and verified by 2 additional reviewers (L.R.B. and A.I.K). Citations were managed using the systematic review software Covidence.28 The Native Health Database prohibits importation of citations into Covidence and therefore titles and abstracts were separately reviewed on the Native Health Database website.
Eligibility and study selection
Studies included in this systematic review met the following prespecified inclusion criteria: 1) published in English; 2) reported data on prevalence of hypercholesterolemia or values of elevated LDL-C; 3) study population included Indigenous adults; 4) participants were from Canada, the United States, Australia, or New Zealand; and 5) the study population was not a duplicate of another included study. To facilitate estimations of prevalence, studies were also required to report the number of hypercholesterolemia or elevated LDL-C cases along with the total study population size or number of Indigenous peoples in the study (if the study population was not entirely composed of Indigenous peoples). Studies were excluded if Indigenous participants could not be distinguished from non-Indigenous participants. All studies that met the above criteria were included regardless of study design.
Data extraction
Following full-text review, we extracted data from studies that included date of publication, name of the Indigenous group, geographic location of the study, number of participants, size of Indigenous population, reported prevalence of hypercholesterolemia or elevated LDL-C, the age range of participants, the mean age of participants, percent of the population that were reported as female, percent of the participants with diabetes, and definition of hypercholesterolemia or LDL-C cutoff used to classify “elevated LDL-C.” One author (R.K.M.) performed the data extraction and conflicts arising throughout were resolved by discussion to consensus with additional reviewers (A.I.K, L.R.B.). In cases where multiple prevalence rates were reported over time, the baseline prevalence was used. In cases where multiple studies reported on the same patient cohort, the study reporting on the largest or most recent sample population was included, with duplicate publications excluded from the analysis. We included studies in which participants were taking lipid lowering medications or had comorbid diseases, as there were limited studies to select overall.
Data analysis
Meta-analysis of single proportions was performed to determine the overall prevalence of hypercholesterolemia using a DerSimonian-Laird random effects model. A random effects model was chosen given high heterogeneity expected between studies. Logit transformation was applied to study-level proportions. Study weights were determined using the inverse-variance method. CIs for individual studies were calculated using the Clopper-Pearson method to provide conservative estimates, and the Jackson method was used for CIs of τ2 and τ.29 We performed subgroup analyses by publication date, geographic location, and hypercholesterolemia and LDL-C definitions. Univariate meta-regression was used to identify possible sources of heterogeneity in the prevalence of hypercholesterolemia. These were conducted using a mixed-effects model of the logit-transformed proportions with DerSimonian-Laird estimator for τ2 and Jackson method for CIs. Variables that were assessed included sample size, proportion of females, the mean age of study population, year of publication, and prevalence of diabetes in the sample population. Assessment of other prominent ASCVD risk factors, including the prevalence of smoking, hypertension, and body mass index, were not possible due to limited data. Publication bias was assessed formally using Egger’s test and by visual inspection of the funnel plot of logit transformed prevalence plotted against SE. All analyses were conducted using the meta package for R (version 4.1.2) and RStudio (2021.09.2) for Windows.30,31
Sensitivity analyses
Robustness of the results was evaluated by a sensitivity analysis which omitted 1 study at a time. We also performed a trim-and-fill analysis to estimate the influence of potentially missing studies on our overall results.32 Finally, we assessed the influence of studies approaching the limits of prevalence (0% or 100%) on our pooled prevalence estimate by repeating our primary analysis using a Freeman-Tukey double arcsine transformation for proportions under inverse-variance weighting.
Results
Search results and study characteristics
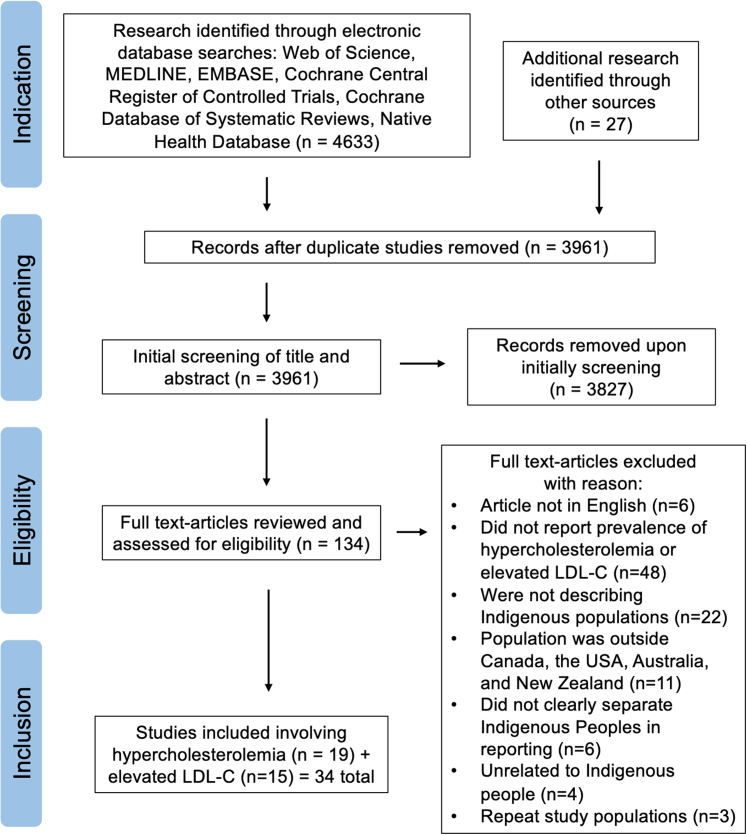
Our search strategy identified 3,961 unique studies (Figure 1). Initial screening of title and abstract revealed 134 studies meeting search criteria. After full text review, 34 studies were identified as meeting full inclusion criteria. We found no studies that reported the prevalence of FH in Indigenous populations in Canada, the United States, Australia, and New Zealand. One study reported a prevalence of severe hypercholesterolemia, defined as a LDL-C level ≥5.0 mmol/L (193 mg/dL), of 6.6% in Indigenous peoples.33 Nineteen studies representing 8,662 participants reported on prevalence of hypercholesterolemia (using any definition of elevated cholesterol), and 15 studies representing 40,399 participants reported on elevated levels of LDL-C (using a defined LDL-C cutoff). The 19 studies on hypercholesterolemia used various classifications to define the disorder, including elevated total blood or serum cholesterol, prior physicians’ diagnosis, and patient self-reports (Supplemental Table 3). The 15 studies reporting on elevated LDL-C used various cutoff points as classifications for elevated LDL-C; the most common being LDL-C ≥4.1 mmol/L (160 mg/dL) (Supplemental Table 4).
Figure 1.
PRISMA Flowchart of the Inclusion of Studies for Meta-analysis
Systematic review process and exclusion criteria with reasoning. Inclusion and exclusion criteria were determined by reviews and applied using Covidence software. Studies found on the Native Health Database were screened independently. Arrows indicate the flow to which the systematic review was conducted. Indication, screening, eligibility, and inclusion sections provide information on each stage of the review and the total number of studies analyzed at each stage. LDL-C = low-density lipoprotein cholesterol; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analysis.
Prevalence of hypercholesterolemia
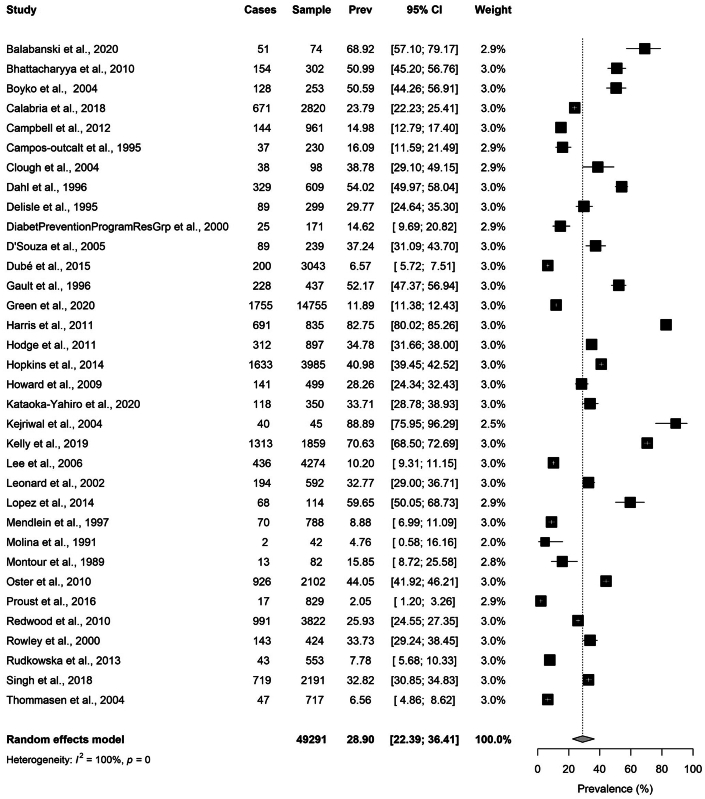
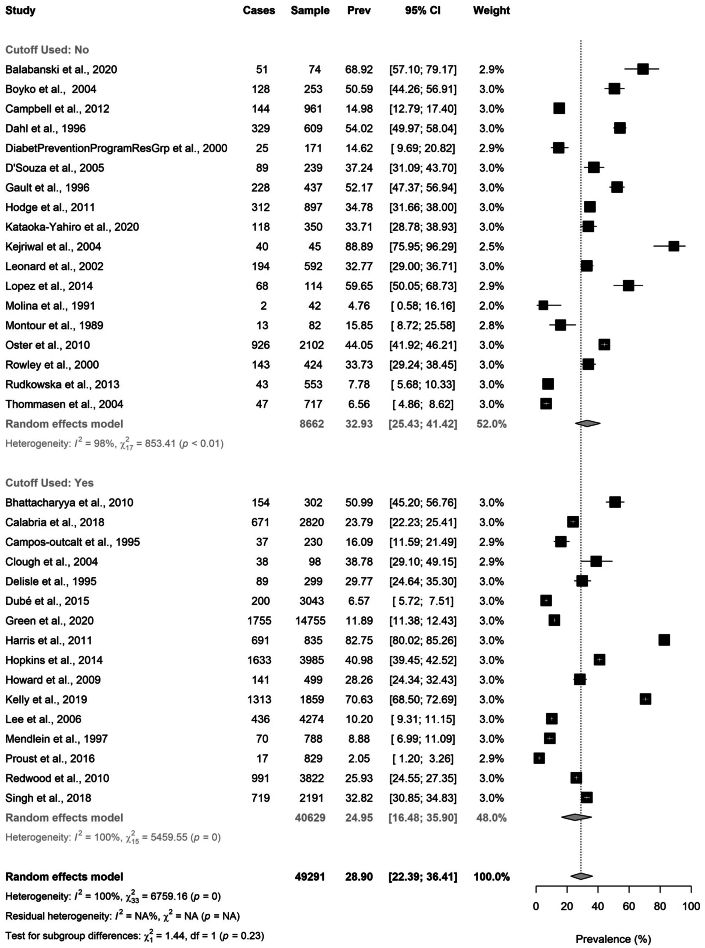
Using a random effects model, we estimated a pooled prevalence of hypercholesterolemia in all included Indigenous populations of 28.9% (95% CI: 22.4%-36.4%; range 2.1%-88.9%) (Figure 2), with high between-study heterogeneity (I2 = 100%, P <0.001). Limiting the analysis to included studies using LDL-C-based criteria, the pooled prevalence of hypercholesterolemia was 25.0% (95% CI: 16.5%-35.9%; range 2.1%-82.8%) with extreme heterogeneity (I2 = 100%, P <0.001) (Figure 3). There were 25 studies conducted in 2001 or later and 9 studies conducted during the year 2000 or prior (Figure 4). Studies published in 2001 or later tended towards a higher prevalence of hypercholesterolemia of 31.3% (95% CI: 23.4%-40.4%; range 2.1%-88.9%), compared to 22.8% (95% CI: 13.4%-36.1%; range 4.8%-54.0%) in the pre-2000 studies, but this was not statistically significant.
Figure 2.
Pooled Prevalence of Hypercholesterolemia in Indigenous Populations
Hypercholesterolemia is classified using any study reported definition of the disorder. Studies are listed individually by first author’s last name with year the study was published. Number of hypercholesterolemia cases, sample size, prevalence rate with 95% CI, and study weight along with a forest plot is included for each study analyzed. Pooled prevalence is reported using a random effects model with 95% CI and total sample size. The black squares indicate the prevalence, and the size of the squares reflect the relative weighting of each study. The horizontal lines represent the 95% confidence intervals. Prev = prevalence.
Figure 3.
Pooled Prevalence of Hypercholesterolemia Grouped by LDL-C Based Cutoff
Studies are separated into 2 groups: 1) “cutoff used: no”: designating all studies which did not report using an LDL-C cut-off to define hypercholesterolemia; and 2) “cutoff used: yes”: designating all studies using any LDL-C cutoff to define hypercholesterolemia. Studies are listed individually by first author’s last name and year of publication. Hypercholesterolemia cases, sample size, prevalence rate, 95% CI, and study weight with a forest plot is included for each study. Pooled prevalence is reported using a random effects model for each group with 95% CI and total sample size. The black squares indicate the prevalence, and the size of the squares reflect the relative weighting of each study. The horizontal lines represent the 95% confidence intervals. LDL-C = low-density lipoprotein cholesterol; Prev = prevalence.
Figure 4.
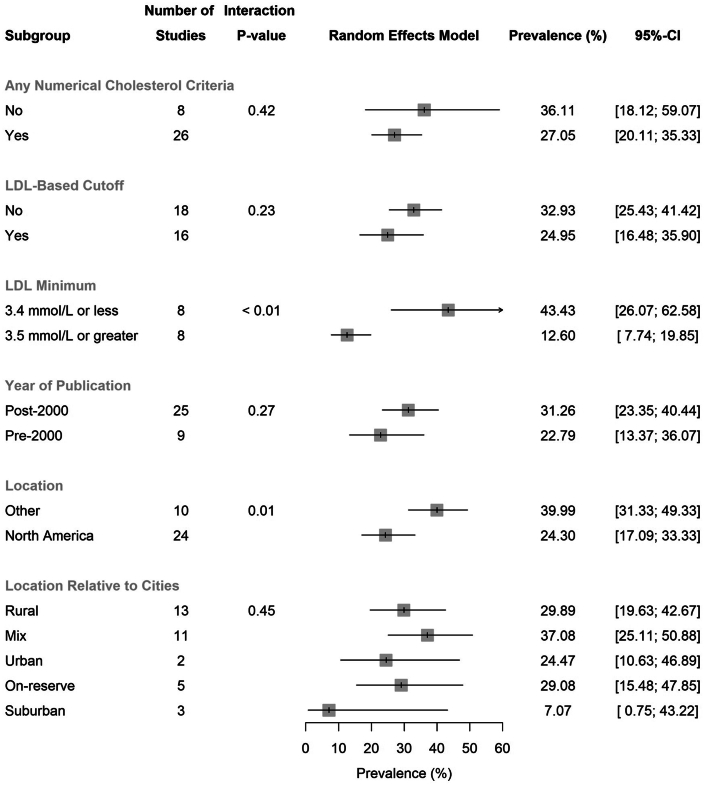
Subgroup Analysis for Prevalence of Hypercholesterolemia
The year of publication subgroups were pre-2000 (including the year 2000) and post-2000. The location subgroup consisted of whether the population was in North America (Canada and the United States) or outside of North America (Australia and New Zealand). Mix location relative to the city includes studies found to be reporting on both rural and urban populations. Low-density lipoprotein cholesterol levels of 3.4 mmol/L and 3.5 mmol/L are equivalent to 131 mg/dL and 135 mg/dL, respectively. The gray squares represent the prevalence. The lines represent the 95% confidence intervals. The arrows indicate that the upper margin of the 95% CI exceeds a value of 60% prevalence.
Elevated LDL-cholesterol
We identified 15 studies reporting on elevated LDL-C, with a specific LDL-C cutoff, in Indigenous populations in Canada, the United States, Australia, and New Zealand. This included 8 studies with a LDL-C level ≥3.5 mmol/L (135 mg/dL) (Figure 4). The pooled prevalence of hypercholesterolemia based on a LDL-C ≥3.5 mmol/L (135 mg/dL) was 12.6% (95% CI: 7.7%-19.9%; range 2.0%-29.8%) (Figure 4).
Geographic reporting in indigenous populations
Twenty-four of the 34 studies were from Indigenous groups from North America (Canada and the United States, exclusively) (Figure 5). The pooled prevalence of hypercholesterolemia in Indigenous populations in North America was 24.3% (95% CI: 17.1%-33.3%; range 2.0%-82.8%) (Figure 4). In comparison, the prevalence in Indigenous Australian (Aboriginal and Torres Strait Islander) populations was higher at 40.0% (95% CI: 31.3%-49.3%; range 15.0%-88.9%). The search did not yield results for Indigenous groups in New Zealand. Results were also stratified according to the location of Indigenous populations relative to cities. This demonstrated similar prevalence values regardless of whether the groups were based in urban, suburban, rural, on-reserve, or mixed localities (Figure 4).
Figure 5.
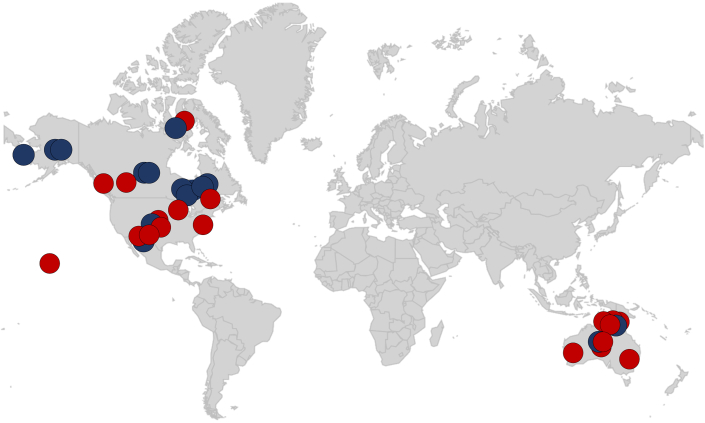
World Map of Included Indigenous Populations
Studies that reported rates of hypercholesterolemia are depicted in red circles and studies that reported elevated low-density lipoprotein cholesterol are depicted in dark blue circles. Circles are found in the general geographic location that is representative of where the study was conducted.
Meta-regression
We evaluated potential explanatory variables affecting the prevalence of hypercholesterolemia in the Indigenous populations using linear regression analyses. Variables that were assessed include study sample size, the mean population age, sex, year of publication, and prevalence of diabetes (Supplemental Table 5). Regression plots depict the effect of these variables on treatment (Supplemental Figure 1). The prevalence of diabetes had a significant influence (P = 0.022) on the prevalence of hypercholesterolemia despite being a poor predictor of the variance (R2 = 0.00%), which may be a consequence of the high variability in the data around the mean. Study sample size (P = 0.23; R2 = 19.61%), the mean population age (P = 0.15; R2 = 0.00%), and proportion of females in the population (P = 0.18; R2 = 1.57%) did not significantly affect the prevalence of hypercholesterolemia in this analysis.
Publication bias
Publication bias was evaluated by Egger’s test and by visual inspection of the funnel plot of logit transformed prevalence plotted against SE (Supplemental Figure 2). Visual inspection demonstrates possible bias toward larger, higher-powered studies with no associated asymmetry of logit transformed prevalence. The wide variation among higher-powered studies in this plot is consistent with the substantial between-study heterogeneity (I2 = 99.5%, P <0.001). Egger’s test suggested against the presence of asymmetry in the funnel plot (bias estimate = 3.16 ± 4.19; P = 0.45).
Sensitivity analyses
Sensitivity analyses (Supplemental Figure 3) demonstrated the robustness of our results, with no overtly influential single-studies. Pooled prevalence estimates, when studies were omitted, fell within the 95% confidence limits of our primary analysis, and ranged from 27.4% (95% CI: 21.1%-34.8%) to 30.7% (95% CI: 23.9%-38.5%). Trim-and-fill analysis (Supplemental Figure 4) yielded 1 imputed study; inclusion resulted in a pooled prevalence of 27.3% (95% CI: 21.1%-34.6%) under the random-effects model. Prevalence estimates derived under the Freeman-Tukey double arcsine transformation did not materially differ from our primary analysis (Supplemental Figure 5).
Discussion
We conducted a systematic review and meta-analysis of hypercholesterolemia in Indigenous populations in Canada, the United States, Australia, and New Zealand. We identified no publication that reported the prevalence of FH and only 1 that reported the prevalence of severe hypercholesterolemia in Indigenous populations. We found that hypercholesterolemia, using any definition, was common, affecting ∼1 in 3 to 1 in 4 or 28.9% of individuals in Indigenous populations. The prevalence of hypercholesterolemia was significantly higher in Indigenous populations in Australia (40.0%) compared to Indigenous populations in North America (24.3%). Through including studies reporting on elevated LDL-C, we found the prevalence of LDL-C ≥3.5 mmol/L (135 mg/dL) to be 12.6%. These findings point to a major knowledge gap and identify that studies of the prevalence of FH and severe hypercholesterolemia should be a priority for future research in collaboration with Indigenous communities.
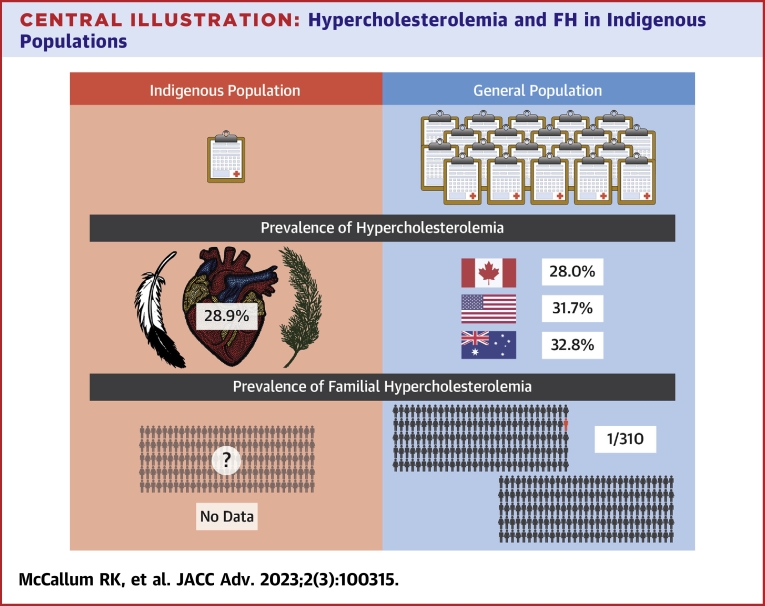
The prevalence of hypercholesterolemia in the general population differs between countries with reports showing 28.0% in Canada, 31.7% in the United States, and 32.8% in Australia.6,34,35 The prevalence of hypercholesterolemia in Indigenous populations of 28.9%, albeit with significant heterogeneity between studies, is at least as common as in the general population in Canada, the United States, or Australia (Central Illustration).6,34,35 A limitation is the differing definitions used, which precludes direct comparison. The significantly higher rates of hypercholesterolemia in Indigenous populations in Australia (40.0%) compared to Indigenous populations in North America (24.3%) further contribute to the heterogeneity in overall prevalence. The search did not yield results for Indigenous groups in New Zealand; however, the New Zealand Ministry of Health reports 9% of the Indigenous Māori population have received a diagnosis of high cholesterol and/or are prescribed cholesterol-lowering medication, as compared to 11% in New Zealand’s European population.36 Rates of treated dyslipidemia are also significantly higher in both rural Māori (15.7%) and urban Māori (7.1%) populations when compared with the general New Zealand population (2.8%), as well as rates of other ASCVD risk factors including obesity, diabetes, and hypertension.37
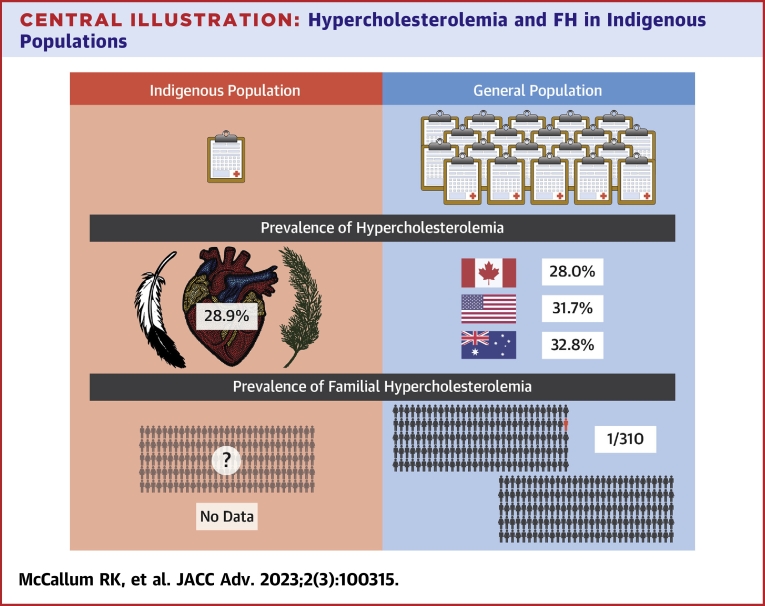
Central Illustration.
Hypercholesterolemia and FH in Indigenous Populations
Comparison of hypercholesterolemia data between Indigenous populations and the general population. There is a clear lack of studies with Indigenous populations compared to the general population for all types of hypercholesterolemia. Using the limited data available our study found a prevalence of 28.9% for Indigenous peoples. This compares to hypercholesterolemia rates of 28% in Canada, 31.7% in the United States, and 32.8% in Australia. There was no familial hypercholesterolemia data found for the Indigenous population. Familial hypercholesterolemia prevalence in the general population is approximately 1 in 310 people. FH = familial hypercholesterolemia.
Despite the fact LDL-C is an important and pragmatic marker of hypercholesterolemia and cardiovascular risk,38 our results suggest that increased levels of LDL-C are not adequately reported for Indigenous peoples. This may negatively impact risk assessment and reduction of ASCVD risk in Indigenous populations. Our results identified one study reporting the prevalence of severe hypercholesterolemia, defined as a LDL-C ≥5.0 mmol/L (193 mg/dL),5,39 in Inuit populations in Canada, the United States, and Greenland and found the prevalence of severe hypercholesterolemia to be ∼1 in 15 people or 6.57%.33 The prevalence of severe hypercholesterolemia in the global population using the same definition is estimated to be ∼1 in 20 or 5.0%.39,40 This suggests that severe hypercholesterolemia is as common in Indigenous populations as in the general population. We also examined studies that reported the prevalence of LDL-C ≥3.5 mmol/L (135 mg/dL), as this is a clinically relevant level, corresponding to the threshold to initiate statin therapy in intermediate risk individuals.5 We found the prevalence of LDL-C ≥3.5 mmol/L (135 mg/dL) to be 12.6% in Indigenous populations. This is comparable to the prevalence of a LDL-C ≥3.5 mmol/L (135 mg/dL) of 14% in non-Indigenous populations,6 suggesting Indigenous peoples experience elevated LDL-C to a similar degree.
Indigenous populations in Canada, the United States, Australia, and New Zealand experience significantly higher rates of chronic diseases, including cardiovascular diseases, compared to the countries non-Indigenous populations.36,41, 42, 43 As a result, guidelines on cardiovascular disease prevention are beginning to recognize Indigenous patients as a high-risk group and suggest earlier screening for hypercholesterolemia compared with other cohorts.5,9 Our findings of a lack of studies reporting on the prevalence of severe hypercholesterolemia and FH in Indigenous patients suggest that lipid screening in these populations is likely to be underused. There are also significant disparities in the rates of lipid-lowering therapy use between ethnic groups, with ethnic minority populations receiving fewer prescriptions and being less likely to achieve cholesterol targets.44
Clinical practice guidelines recommend earlier screening for dyslipidemia in Indigenous individuals because of the higher risk of ASCVD.5 Clinicians should be aware hypercholesterolemia impacts Indigenous patients to the same extent it does non-Indigenous patients, and Indigenous patients should be screened for hypercholesterolemia, FH, and severe hypercholesterolemia. At the same time, our research identifies marked disparities in the inclusion of Indigenous populations in research related to FH. Our findings highlight the need for additional research, in partnership with Indigenous communities, to better understand the burden of hypercholesterolemia in Indigenous populations and how it contributes to the disproportionate burden of ASCVD. Including Indigenous patients in registries and clinical trials related to hypercholesterolemia may help to improve awareness and optimize screening and treatment of dyslipidemia in Indigenous populations.
Study limitations
There are limitations to this study that merit consideration. First, despite our expansive search efforts, it remains possible through restrictions in our inclusion criteria (eg, English language) or screening practices, we may have failed to identify some relevant studies. This study focused on Indigenous populations from Canada, the United States, Australia, and New Zealand, and therefore our results cannot be applied globally, as all Indigenous groups are diverse with their own history, culture, and experiences which contribute to the health of their community. This systematic review was not published on an international prospective systematic review register; however, this review followed a strict prespecified protocol and was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines. A major limitation is the lack of a standardized definition for hypercholesterolemia. We attempted to address this by using numerical cutoff values, including LDL-C ≥3.5 mmol/L (135 mg/dL) as a clinically relevant threshold. Given the paucity of relevant data, and to maximize the sample size for analysis, we decided to include studies containing participants with cardiovascular disease, chronic kidney disease, taking cholesterol lowering medication, or any other administered treatment; further impairing the generalizability of the results. There was extreme between-study heterogeneity likely due to the wide variation in definitions and methodology. This underscores the important observation that global Indigenous communities are under-represented in lipid-related research.
Conclusions
In summary, we estimate the pooled prevalence of hypercholesterolemia to be 28.9% in Indigenous communities in Canada, the United States, Australia, and New Zealand, making it at least as common as in the general population. This study represents the first systematic review and meta-analysis on hypercholesterolemia in Indigenous populations. Future research is needed, in collaboration with Indigenous communities, to study the prevalence of hypercholesterolemia, severe hypercholesterolemia, and FH in Indigenous populations and to optimize the use of lipid screening and treatment in these groups to address the disproportionate burden of ASCVD that affects these communities.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Clinicians need to be aware hypercholesterolemia impacts Indigenous patients to the same extent it does non-Indigenous patients and that Indigenous patients may be less informed as Indigenous-inclusive lipid research data sets are extremely limited.
TRANSLATIONAL OUTLOOK: Indigenous patients should receive screening for hypercholesterolemia, FH, and severe hypercholesterolemia and additional research, in partnership with Indigenous communities, is needed to improve clinical outcomes. Including Indigenous patients in health care registries will improve awareness and decrease the burden of untreated hypercholesterolemia, which will address the disproportionate burden of ASCVD in Indigenous populations.
Funding support and author disclosures
Dr McCallum is supported by a National Science and Engineering Research Council Undergraduate Student Research Award, Canadian Institute for Health Research Canadian Graduate Scholarship, and the Métis Nation of British Columbia. Dr Genest is funded by Canadian Institutes of Health Research Project Grant #PJT-168886JG; is co-Chair of FHCanada (www.FHCanada.net) a non-profit registry of familial hypercholesterolemia in Canada; receives honoraria from Sanofi Canada, Amgen, and Novartis for advisory boards. Dr Brunham is co-chair of FHCanada; has received honoraria from Sanofi Canada, Amgen, and Novartis for advisory boards; and is a Michael Smith Foundation for Health Research Scholar and a Canada Research Chair in Precision Cardiovascular Disease Prevention. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors would like to acknowledge that this research study was created on traditional, ancestral, and unceded lands of the xwməθkwəyəm (Musqueam), Skwxwú7mesh (Squamish), Stó:lō, and Səlílwətaʔ/Selilwitulh (Tsleil-Waututh), as well as the Syilx (Okanagan) Peoples and the Haudenosaunee and Anishinaabeg Nations. There were additional Indigenous scholars consulted in the making of this study including Justin Turner and Janelle Kasperski, who provided guidance with terminology and language. University of British Columbia librarians Vanessa Kitchin and Saeyong Kim helped develop the systematic review and search criteria.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Supplementary data
References
- 1.Smylie J., O'Brien K., Xavier C.G., et al. Primary care intervention to address cardiovascular disease medication health literacy among Indigenous peoples: Canadian results of a pre-post-design study. Can J Public Health. 2018;109:117–127. doi: 10.17269/s41997-018-0034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garner R., Statistics Canada, Health Analysis Division . Stat Canada, Health Analysis Division; 2010. The Health of First Nations Living Off-Reserve, Inuit, and Métis Adults in Canada: The Impact of Socio-Economic Status on Inequalities in Health. [Google Scholar]
- 3.Perez-Calahorra S., Laclaustra M., Marco-Benedí V., et al. Effect of lipid-lowering treatment in cardiovascular disease prevalence in familial hypercholesterolemia. Atherosclerosis. 2019;284:245–252. doi: 10.1016/j.atherosclerosis.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Miname M.H., Santos R.D. Reducing cardiovascular risk in patients with familial hypercholesterolemia: risk prediction and lipid management. Prog Cardiovasc Dis. 2019;62:414–422. doi: 10.1016/j.pcad.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Pearson G.J., Thanassoulis G., Anderson T.J., et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. CJC. 2021;37:1129–1150. doi: 10.1016/j.cjca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Government of Canada SC . 2021. Cholesterol levels of adults, 2016-2019.https://www150.statcan.gc.ca/n1/pub/82-625-x/2021001/article/00003-eng.htm [Google Scholar]
- 7.Nantsupawat N., Booncharoen A., Wisetborisut A., et al. Appropriate total cholesterol cut-offs for detection of abnormal LDL cholesterol and non-HDL cholesterol among low cardiovascular risk population. Lipids Health Dis. 2019;18:28. doi: 10.1186/s12944-019-0975-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mach F., Baigent C., Catapano A.L., et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 9.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Eid W.E., Sapp E.H., McCreless T., Nolan J.R., Flerlage E. Prevalence and characteristics of patients with primary severe hypercholesterolemia in a multidisciplinary healthcare system. Am J Cardiol. 2020;132:59–65. doi: 10.1016/j.amjcard.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Akioyamen L.E., Chu A., Genest J., et al. Prevalence and treatment of familial hypercholesterolemia and severe hypercholesterolemia in older adults in Ontario, Canada. CJC Open. 2022;4(9):739–747. doi: 10.1016/j.cjco.2022.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beheshti S.O., Madsen C.M., Varbo A., Nordestgaard B.G. Worldwide prevalence of familial hypercholesterolemia. J Am Coll Cardiol. 2020;75:2553–2566. doi: 10.1016/j.jacc.2020.03.057. [DOI] [PubMed] [Google Scholar]
- 13.Toft-Nielsen F., Emanuelsson F., Benn M. Familial hypercholesterolemia prevalence among ethnicities—systematic review and meta-analysis. Front Genet. 2022;13 doi: 10.3389/fgene.2022.840797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Work Group for Indigenous Affairs (IWGIA) S.L.: IWGIA; 2006. The Indigenous World 2006. [Google Scholar]
- 15.Schultz A., Nguyen T., Sinclaire M., Fransoo R., McGibbon E. Historical and continued colonial impacts on heart health of indigenous peoples in Canada: what’s reconciliation got to do with it? CJC Open. 2021;3:S149–S164. doi: 10.1016/j.cjco.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand S.S., Abonyi S., Arbour L., et al. Explaining the variability in cardiovascular risk factors among First Nations communities in Canada: a population-based study. Lancet Planet Health. 2019;3:e511–e520. doi: 10.1016/S2542-5196(19)30237-2. [DOI] [PubMed] [Google Scholar]
- 17.Agostino J.W., Wong D., Paige E., et al. Cardiovascular disease risk assessment for Aboriginal and Torres Strait Islander adults aged under 35 years: a consensus statement. Med J Aust. 2020;212(9):422–427. doi: 10.5694/mja2.50529. [DOI] [PubMed] [Google Scholar]
- 18.Pace R., Harris S., Parry M., Zaran H. Primary and secondary cardiovascular prevention among First Nations peoples with type 2 diabetes in Canada: findings from the FORGE AHEAD program. CJC Open. 2020;2:547–554. doi: 10.1016/j.cjco.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United Nations Indigenous peoples at the United Nations | United Nations for indigenous peoples. https://www.un.org/development/desa/indigenouspeoples/about-us.html
- 20.Mitrou F., Cooke M., Lawrence D., et al. Gaps in Indigenous disadvantage not closing: a census cohort study of social determinants of health in Australia, Canada, and New Zealand from 1981–2006. BMC Public Health. 2014;14:201. doi: 10.1186/1471-2458-14-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smallwood R., Woods C., Power T., Usher K. Understanding the impact of historical trauma due to colonization on the health and well-being of indigenous young peoples: a systematic scoping review. J Transcult Nurs. 2021;32:59–68. doi: 10.1177/1043659620935955. [DOI] [PubMed] [Google Scholar]
- 22.Lithopoulos S. Pub Safety Canada; 2007. International Comparison of Indigenous Policing Models. [Google Scholar]
- 23.Camp P., Girt M., Wells A., et al. Virtual care for indigenous populations in Canada, the United States, Australia, and New Zealand: protocol for a scoping review. JMIR Res Protoc. 2020;9 doi: 10.2196/21860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voaklander B., Rowe S., Sanni O., Campbell S., Eurich D., Ospina M.B. Prevalence of diabetes in pregnancy among indigenous women in Australia, Canada, New Zealand, and the USA: a systematic review and meta-analysis. Lancet Glob Health. 2020;8:e681–e698. doi: 10.1016/S2214-109X(20)30046-2. [DOI] [PubMed] [Google Scholar]
- 25.Shahid S., Taylor E.V., Cheetham S., Woods J.A., Aoun S.M., Thompson S.C. Key features of palliative care service delivery to indigenous peoples in Australia, New Zealand, Canada and the United States: a comprehensive review. BMC Palliat Care. 2018;17:72. doi: 10.1186/s12904-018-0325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dennis M.K., Robin T. Healthy on our own terms: indigenous wellbeing and the colonized food system. Crit Diet. 2020;5:4–11. [Google Scholar]
- 27.University of Alberta University of Alberta, library health sciences search filters. 2022. https://guides.library.ualberta.ca/health-sciences-search-filters/indigenous-peoples
- 28.Covidence Covidence systematic review software, veritas health innovation, Melbourne, Australia. www.covidence.org
- 29.Jackson D., Law M., Rücker G., Schwarzer G. The Hartung-Knapp modification for random-effects meta-analysis: a useful refinement but are there any residual concerns? Stat Med. 2017;36:3923–3934. doi: 10.1002/sim.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Pub Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwarzer G. Meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 32.Duval S., Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 33.Dubé J.B., Wang J., Cao H., et al. Common low-density lipoprotein receptor p.G116S variant has a large effect on plasma low-density lipoprotein cholesterol in circumpolar Inuit populations. Circ Cardiovasc Genet. 2015;8:100–105. doi: 10.1161/CIRCGENETICS.114.000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centre for Disease Control . 2021. Cholesterol information | cdc.gov.https://www.cdc.gov/cholesterol/index.htm [Google Scholar]
- 35.Australian Bureau of Statistics. Kalisch D. Australian Bureau of Statistics; 2018. National Health Survey First Results Australia 2017-18; pp. 1–97. [Google Scholar]
- 36.Ministry of Health Manatū Hauora, New Zealand Health Survey New Zealand Health Survey. 2021. https://minhealthnz.shinyapps.io/nz-health-survey-2020-21-annual-data-explorer/_w_a874f673/#!/home
- 37.Cameron V.A., Faatoese A.F., Gillies M.W., et al. A cohort study comparing cardiovascular risk factors in rural Māori, urban Māori and non-Māori communities in New Zealand. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2011-000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller M. Dyslipidemia and cardiovascular risk: the importance of early prevention. QJM. 2009;102:657–667. doi: 10.1093/qjmed/hcp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruel I., Brisson D., Aljenedil S., et al. Simplified Canadian definition for familial hypercholesterolemia. CJC. 2018;34:1210–1214. doi: 10.1016/j.cjca.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Khera A.V., Won H.-H., Peloso G.M., et al. Diagnostic yield of sequencing familial hypercholesterolemia genes in severe hypercholesterolemia. J Am Coll Cardiol. 2016;67:2578–2589. doi: 10.1016/j.jacc.2016.03.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruce S.G., Riediger N.D., Lix L.M. Chronic disease and chronic disease risk factors among First Nations, Inuit and Métis populations of northern Canada. Chronic Dis Inj Can. 2014;34:210–217. [PubMed] [Google Scholar]
- 42.Breathett K., Sims M., Gross M., et al. Cardiovascular health in American Indians and Alaska natives: a scientific statement from the American Heart Association. Circulation. 2020;141(25):e948–e959. doi: 10.1161/CIR.0000000000000773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calabria B., Korda R.J., Lovett R.W., et al. Absolute cardiovascular disease risk and lipid-lowering therapy among Aboriginal and Torres Strait Islander Australians. Med J Aust. 2018;209:35–41. doi: 10.5694/mja17.00897. [DOI] [PubMed] [Google Scholar]
- 44.Kalra D.K. Bridging the racial disparity gap in lipid-lowering therapy. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.019533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.