Abstract
Background
High-sensitivity cardiac troponin T (hs-cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are cardiac biomarkers commonly detected in adults with type 2 diabetes (T2D) and are associated with heart failure risk.
Objectives
The purpose of this study was to evaluate the effects of exercise training (ET) on hs-cTnT and NT-proBNP and evaluate the associations of these biomarkers with cardiorespiratory fitness among adults with T2D.
Methods
Participants of the HART-D (Health Benefits of Aerobic and Resistance Training in Individuals with Type 2 Diabetes) trial who were randomly assigned to one of 3 ET groups or a non-exercise control group were included. Cardiac biomarkers and cardiorespiratory fitness (evaluated by peak oxygen uptake [VO2peak]) were assessed at baseline and after 9 months. The effects of ET (3 ET groups pooled) vs non-exercise control on hs-cTnT and NT-proBNP were assessed using separate analysis of covariance models. Multivariable-adjusted linear regression was performed to identify factors associated with follow-up biomarkers and ΔVO2peak.
Results
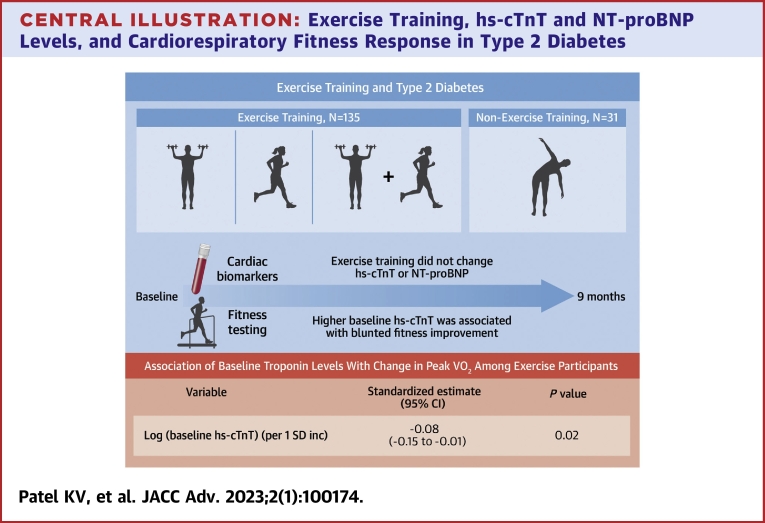
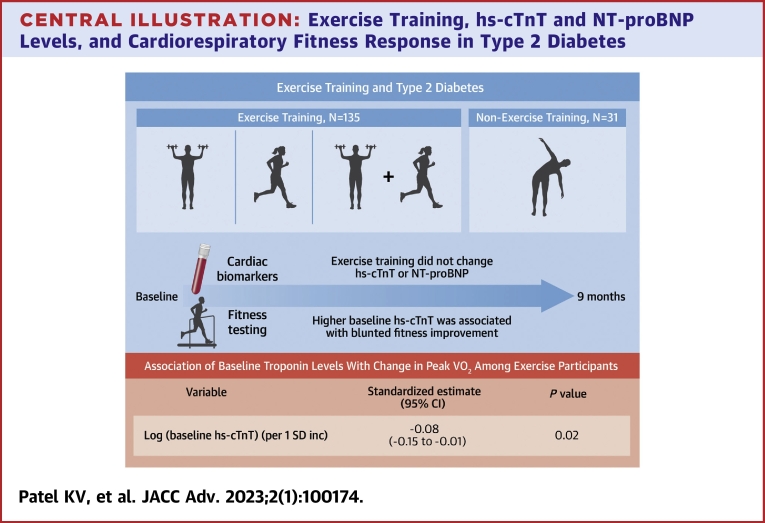
The present study included 166 participants randomized to the ET (n = 135) and non-exercise control (n = 31) groups. Compared with the non-exercise control, ET did not significantly change hs-cTnT or NT-proBNP. In adjusted analysis, each ET group and ΔVO2peak were not significantly associated with hs-cTnT or NT-proBNP levels on follow-up. Among individuals in the ET group, baseline hs-cTnT was inversely associated with ΔVO2peak [per 1 SD higher log (hs-cTnT): β = −0.08 (95% CI = −0.15 to −0.01)].
Conclusions
Among individuals with T2D, ET did not modify cardiac biomarkers. Higher baseline hs-cTnT was associated with blunted cardiorespiratory fitness improvement in response to exercise.
Key words: exercise training, high-sensitivity cardiac troponin T, N-terminal pro-B-type natriuretic peptide, type 2 diabetes
Central Illustration
High-sensitivity cardiac troponin T (hs-cTnT) is a biomarker of myocardial injury that is commonly detectable among community dwelling adults, with a higher prevalence in disease states such as type 2 diabetes (T2D).1 Higher levels of hs-cTnT are independently associated with structural heart disease and increased risk of heart failure (HF).1, 2, 3 Similarly, N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a biomarker of myocardial stress and neurohormonal activation that is associated with HF risk and cardiovascular death in the general population4,5 and among individuals with T2D.3,6 Longitudinal increases in these cardiac biomarkers are also associated with greater risk of HF or all-cause mortality suggesting that biomarker-associated risk of HF may be a modifiable target for preventive interventions.7, 8, 9, 10
Healthy lifestyle practices, such as regular physical activity and exercise, are recommended for adults in part because of the cardioprotective, inverse association between physical activity and risk of HF.11,12 Prior observational studies have also demonstrated that greater physical activity was associated with favorable subclinical phenotypes including superior diastolic and systolic function.13 However, the interrelationships between physical activity, exercise, and hs-cTnT are less clear with a potential U-shaped association. Higher amounts of habitual physical activity, within recommended levels, in community-based cohorts are associated with lower hs-cTnT and NT-proBNP.14,15 In contrast, high-intensity and prolonged duration exercise has been associated with an acute rise in hs-cTnT.16 While exercise training (ET) improves cardiorespiratory fitness (CRF), an important and modifiable HF risk factor, the independent effects of ET on cardiac biomarkers among high-risk individuals with T2D are not well established.17 Furthermore, the associations of baseline and longitudinal changes in cardiac biomarkers and CRF have not been fully evaluated in high-risk populations, such as adults with T2D.
The HART-D (Health Benefits of Aerobic and Resistance Training in Individuals with Type 2 Diabetes) study was a randomized controlled trial that evaluated the effects of different ET interventions on hemoglobin A1c (HbA1c) among adults with T2D and sedentary lifestyle.18 In this secondary analysis of the HART-D study, we investigated the effect of multiple ET interventions on levels of hs-cTnT and NT-proBNP and further explored whether changes in these biomarkers were associated with changes in CRF assessed by peak oxygen consumption (VO2peak). Based on previous studies, we hypothesized that ET would attenuate the expected increase in hs-cTnT and NT-proBNP over time.14,15,19
Methods
Study population
The study design and primary results of the HART-D study have been published previously.18 In brief, HART-D included 262 adults, aged 30 to 75 years of age, with T2D (HbA1c 6.5%-11.0%) and sedentary lifestyle. Sedentary status was defined as exercising fewer than 3 days/week for <20 minutes per session. Exclusion criteria included severe obesity [body mass index (BMI) ≥48 kg/m2], uncontrolled hypertension [blood pressure (BP) >160/100 mm Hg], kidney disease [serum creatinine >1.5 mg/dL or urine protein >100 mg/dL], severe hypertriglyceridemia [fasting triglycerides >500 mg/dL], insulin pump use, history of retinopathy, advanced neuropathy, stroke, or other serious medical condition that would prevent safe exercise or adherence to the study protocol. The majority of participants enrolled in HART-D were free of established cardiovascular disease (3% had a history of myocardial infarction; 5% had a history of coronary artery bypass surgery). The Pennington Biomedical Research Center Institutional Review Board approved the HART-D study protocol. All study participants provided written informed consent. The present study included participants who had available baseline hs-cTnT as well as data to calculate ΔVO2peak (Supplemental Figure 1). Participants in the ET groups with exercise adherence <70% were excluded from the present analysis similar to a separate secondary analysis of the HART-D trial.20 Baseline NT-proBNP data were missing for one participant.
Treatment groups
Participants of the HART-D study were randomly assigned to a non-exercise control group or 1 of the 3 following supervised ET groups: 1) aerobic training only; 2) resistance training only; and 3) combination of aerobic and resistance training. The treatment interventions were designed to achieve similar total weekly exercise times across groups and meet physical activity guideline recommendations over 9 months.18
Non-exercise control
Participants randomly assigned to the non-exercise control group were asked to maintain their baseline physical activity level throughout the study period. Weekly stretching and relaxation sessions were offered but not mandatory. The step count remained constant throughout the study period (4,180-4,376 steps/week).18 Due to an increase in HbA1c among a substantial proportion of participants in the non-exercise control, randomization to this group was terminated early as recommended by the data safety monitoring board. Fewer participants were randomized to the non-exercise control group than originally planned and led to a smaller proportion of participants in the control vs ET groups.
Aerobic training only
Participants were encouraged to perform moderate to vigorous intensity exercise at a heart rate associated with 50% to 80% of VO2peak. The exercise prescription targeted an aerobic dose of 12 kcal/kg of body weight per week. Body weight was measured weekly to calculate updated aerobic dose targets. A recuperation week with a target exercise dose reduction of one-third was implemented during weeks 12 and 24. Participants typically exercised using a treadmill approximately 3 sessions per week for 2 hours per week.18 After accounting for warm-ups and cool-downs, the average treadmill time was approximately 140 minutes per week. Participants in the aerobic training only group achieved 680 MET-minutes per week.
Resistance training only
Participants performed resistance training 3 days/week. Sessions included 2 sets of upper body exercises (shoulder press, pull down, seated row, bench press), abdominal crunches, and back extensions as well as 3 sets of lower body exercises (leg flexion, extension, press). Each set of exercises included 10 to 12 repetitions. Weight was progressively increased for each exercise after a participant demonstrated the ability to complete 12 repetitions per set of exercises on consecutive training sessions. A period of flexibility and stretching was included at the end of the resistance training session such that each ET group had similar total exercise time (approximately 141 minutes per week for the resistance training group).18
Combination of aerobic and resistance training
In the combination training group, the aerobic dose target was 10 kcal/kg/week. Participants were also prescribed 2 sessions per week of the same resistance exercises as those in the resistance training only group but only 1 set per exercise to allow for similar weekly exercise time as the other ET groups. This translated to approximately 110 and 35 min/week of treadmill and resistance training time, respectively. The combined aerobic and resistance training group achieved 570 MET-min/week.18
Clinical covariates
Anthropometric measurements were obtained according to a standardized protocol.18 Height was measured with a stadiometer. Body weight was measured weekly using an electronic scale (GSE 450, GSE Scale Systems, Novi, Michigan). BMI was calculated by dividing the weight in kilograms by the height in meters2. While the participant was at minimal expiration, waist circumference was measured at the level of the iliac crest.21 Participants underwent dual energy X-ray absorptiometry scans using a QDR 4500/A whole-body scanner (Hologic Inc, Bedford, Massachusetts) to assess body composition. HbA1c was measured from blood samples obtained at baseline and after training using a UniCel DxC 600 Pro (Beckman Coulter, Brea, California).
Assessment of cardiorespiratory fitness
CRF was assessed by simultaneously measuring respiratory gases using a True Max 2400 Metabolic Measurement Cart (ParvoMedics) while participants exercised on a treadmill (Trackmaster 425, Carefusion).18 Participants began the exercise test at a self-selected, brisk walking speed. Treadmill grade was increased by 2% every 2 minutes while maintaining a constant speed until volitional exhaustion. CRF was defined as VO2peak during the exercise test. The same treadmill speed and protocol was used to assess VO2peak at baseline and follow-up. Change in VO2peak (ΔVO2peak) was defined as the absolute difference between 9-month and baseline VO2peak.
Biomarker measurements
At baseline and 9-month follow-up, fasting blood samples were collected from participants prior to exercise testing and stored at −80 °C. Serum was thawed just prior to measurement of cardiac biomarkers. Cardiac biomarker levels were measured in a core laboratory (Inova Hospital, Fairfax, Virginia) and the laboratory personnel were blinded to the study group assignment. Commercially available assays were used to measure cardiac biomarkers. Hs-cTnT was measured using the Troponin T Gen 5 STAT assay (Roche Diagnostics).22 The lower limit of detection of the hs-cTnT assay was 6 ng/L. Nondetectable hs-cTnT values were assigned a value half of the lower limit of detection of the assay (3 ng/L).2,23 NT-proBNP (range, 5-35,000 pg/mL) was also measured using the Roche Diagnostics platform (Cobas e602).14
Statistical analysis
Baseline characteristics were compared across non-exercise control vs ET groups (pooled all 3 ET groups) using Kruskal-Wallis test for continuous variables and chi-square tests (or Fisher’s exact test when appropriate) for categorical variables. Baseline and 9-month follow-up levels of hs-cTnT and NT-proBNP were not normally distributed and were natural log-transformed for analyses as necessary.
Within all treatment groups, paired t-tests were used to evaluate changes in cardiac biomarker levels over time. The effects of ET (pooled and individual groups) vs non-exercise control on hs-cTnT and NT-proBNP were assessed in separate analysis of covariance models including the baseline cardiac biomarker. Among all participants (non-exercise control and ET groups), predictors of postintervention cardiac biomarkers of interest [log (follow-up hs-cTnT) and log (follow-up NT-proBNP)] were assessed in separate linear regression models adjusted for the following covariates: demographics (age, sex, race), baseline BMI, systolic BP, baseline HbA1c, log (baseline biomarker of interest), treatment group, and ΔVO2peak.
Participants randomized to an ET group were stratified according to data-derived tertiles of ΔVO2peak with the highest tertile including participants with the greatest increase in VO2peak. Across tertiles of ΔVO2peak, baseline characteristics were compared using Jonckheere-Terpstra test for continuous and categorical data. A multivariable-adjusted linear regression model was constructed to identify association of baseline biomarker levels with ΔVO2peak. The adjusted model included the following covariates selected a priori based on biological plausibility and previous studies18,20,24: demographics (age, sex, race [Black vs non-Black]), baseline lean body mass, waist circumference, systolic BP, HbA1c, baseline VO2peak, baseline cardiac biomarkers of interest [log (baseline hs-cTnT), log (baseline NT-proBNP)], and ET group [aerobic training only, resistance training only, combination of aerobic and resistance training]. The association of ΔVO2peak and change in each cardiac biomarker (Δhs-cTnT and ΔNT-proBNP) was assessed using Spearman correlation.
Statistical analysis was performed using SAS 9.4 (SAS Institute). All relevant statistical tests were 2-sided and P < 0.05 was considered statistically significant.
Results
The present study included 166 participants (61.4% female, 38.6% Black) (Supplemental Figure 1). In the present study, 31 participants (18.7%) were randomized to the non-exercise control group while 42 (25.3%), 47 (28.3%), and 46 (27.7%) participants were assigned to the aerobic training only, resistance training only, and combination training group, respectively. Baseline characteristics of participants in the non-exercise control and pooled ET groups were similar (Table 1). Baseline levels of VO2peak were comparable between groups. The non-exercise control and pooled ET groups had similar hs-cTnT (3.0 [IQR: 3.0-9.8] ng/L and 3.0 [IQR: 3.0-6.8] ng/L, respectively) and NT-proBNP levels (22.1 [IQR: 10.0-58.4] pg/mL and 18.0 [IQR: 7.9-52.3] pg/mL, respectively) at baseline.
Table 1.
Baseline Characteristics Across the Non-Exercise Control and Combined Exercise Training Groups
| Non-Exercise Control Group (n = 31) | Pooled Exercise Training Groups (n = 135) | P Value | |
|---|---|---|---|
| Age, y | 59 (52-64) | 57 (51-63) | 0.54 |
| Female | 21 (67.7) | 81 (60.0) | 0.42 |
| Race/ethnicity | |||
| White | 16 (51.6) | 77 (57.0) | 0.58 |
| Black | 13 (41.9) | 51 (37.8) | 0.67 |
| Hispanic | 0 (0) | 1 (0.7) | >0.99 |
| Other | 2 (6.5) | 6 (4.4) | 0.64 |
| BMI, kg/m2 | 36.5 (31.9–39.5) | 33.7 (30.3–38.2) | 0.22 |
| Waist circumference, cm | 107.3 (100.8–121.2) | 110.5 (102.8–120.3) | 0.85 |
| Body fat, % | 41.4 (35.9–42.9) | 38.0 (32.0–43.3) | 0.34 |
| Lean mass, kg | 55.9 (48.7–64.3) | 55.1 (49.6–65.1) | 0.84 |
| Duration of diabetes, y | 7 (4–10) | 6 (3–11) | 0.90 |
| Insulin use | 5 (16.1) | 26 (19.3) | 0.69 |
| Antihypertensive medication use | 23 (74.2) | 109 (80.7) | 0.42 |
| Resting heart rate, bpm | 85 (72–94) | 83 (71–90) | 0.41 |
| Mean systolic BP, mm Hg | 127 (116–136) | 124 (117–135) | 0.78 |
| Mean diastolic BP, mm Hg | 76 (70–80) | 74 (70–80) | 0.18 |
| Abnormal ECG | 4 (12.9) | 26 (19.3) | 0.41 |
| Resting RER | 0.90 (0.85–0.92) | 0.91 (0.87–0.97) | 0.13 |
| Maximum systolic BP, mm Hg | 192 (172–212) | 199 (176–212) | 0.97 |
| VO2peak, L/min | 1.66 (1.45–2.05) | 1.81 (1.53–2.22) | 0.47 |
| HbA1c, % | 7.2 (6.5–8.7) | 6.8 (6.4–7.8) | 0.14 |
| hs-CRP, mg/L | 2.5 (0.8–7.5) | 3.1 (1.30–5.9) | 0.51 |
| hs-cTnT, ng/L | 3.0 (3.0–9.8) | 3.0 (3.0–6.8) | 0.29 |
| hs-cTnT > 6, ng/L | 11 (35.5) | 41 (30.4) | 0.67 |
| NT-proBNP, pg/mL | 22.1 (10.0–58.4) | 18.0 (7.9–52.3) | 0.41 |
| Study group | |||
| Non-exercise control | 31 (100) | 0 (0) | <0.001 |
| Aerobic training | 0 (0) | 42 (31.1) | |
| Resistance training | 0 (0) | 47 (34.8) | |
| Combination training | 0 (0) | 46 (34.1) |
Values are median (IQR) and compared across groups using Kruskal-Wallis test; or n (%) and compared across groups using chi-square (or Fisher’s exact test when appropriate).
BMI = body mass index; BP = blood pressure; ECG = electrocardiogram; HbA1c = hemoglobin A1c; hs-CRP = high-sensitivity C-reactive protein; hs-cTnT = high-sensitivity cardiac troponin T; NT-proBNP = N-terminal pro-B-type natriuretic peptide; RER = respiratory exchange ratio; VO2peak = peak oxygen consumption.
Exercise training and cardiac biomarker levels
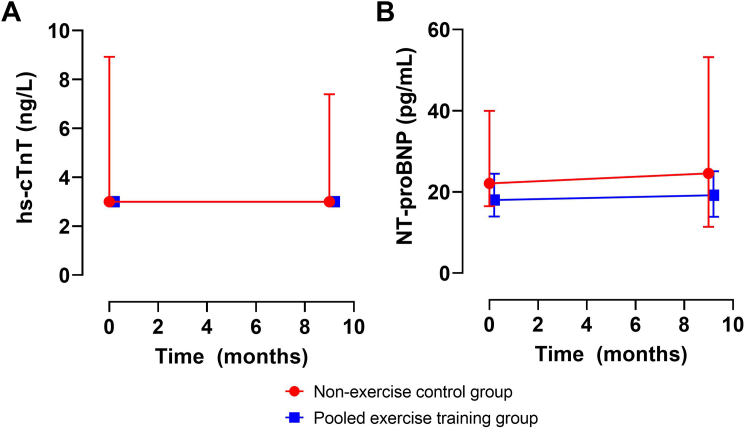
Baseline and 9-month follow-up levels of hs-cTnT and NT-proBNP across the non-exercise control and pooled ET groups are shown in Figure 1. Among participants in the non-exercise control and pooled ET groups, hs-cTnT and NT-proBNP levels did not change significantly from baseline to 9-month follow-up. Compared with the non-exercise control group, ET did not significantly change hs-cTnT (P = 0.45) or NT-proBNP (P = 0.26). Aerobic training, resistance training, and combined aerobic and resistance training did not significantly change hs-cTnT or NT-proBNP compared with the non-exercise control group (P > 0.05 for all) (Supplemental Figure 2).
Figure 1.
Effect of Exercise Training on Cardiac Biomarkers Over 9 Months
The data are presented as median (95% CI) for hs-cTnT and NT-proBNP in A and B, respectively. Separate ANCOVA models including the baseline cardiac biomarker demonstrated no significant difference in follow-up hs-cTnT (P = 0.45) or NT-proBNP (P = 0.26) between pooled exercise and non-exercise control training groups. ANCOVA = analysis of covariance; hs-cTnT = high-sensitivity cardiac troponin T; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
In adjusted analysis, there was no significant association of any of the ET groups (vs the non-exercise control group) or ΔVO2peak with hs-cTnT or NT-proBNP levels on follow-up (Table 2). The baseline biomarker level was associated with each postintervention biomarker after adjustment for other variables.
Table 2.
Multivariable Adjusted Association Between Exercise Training Groups and Longitudinal Change in Cardiorespiratory Fitness With Follow-Up Cardiac Biomarkers Among All Participants
| Follow-Up hs-cTnT |
Follow-Up NT-proBNP |
|||
|---|---|---|---|---|
| Standardized Estimate (95% CI) | P Value | Standardized Estimate (95% CI) | P Value | |
| Aerobic training (vs non-exercise control) | −0.01 (−0.17 to 0.16) | 0.92 | −0.15 (−0.53 to 0.23) | 0.43 |
| Resistance training (vs non-exercise control) | −0.06 (−0.22 to 0.10) | 0.45 | −0.08 (−0.45 to 0.28) | 0.66 |
| Combination training (vs non-exercise control) | −0.02 (−0.19 to 0.14) | 0.78 | −0.20 (−0.57 to 0.18) | 0.31 |
| ΔVO2peak (per 1 SD inc) | 0.13 (−0.14 to 0.40) | 0.33 | −0.48 (−1.08 to 0.12) | 0.12 |
Separate multivariable adjusted linear regression models were created to assess the independent predictors of each follow-up biomarker of interest [log (follow-up hs-cTnT), log (follow-up NT-proBNP)] with adjustment for the following covariates: demographics (age, sex, race), baseline BMI, systolic BP, HbA1c, log (baseline biomarker of interest), treatment group, and ΔVO2peak. ΔVO2peak was defined as the absolute difference between 9-month and baseline VO2peak.
BMI = body mass index; BP = blood pressure; HbA1c = hemoglobin A1c; hs-cTnT = high-sensitivity cardiac troponin T; NT-proBNP = N-terminal pro-B-type natriuretic peptide; VO2peak = peak oxygen consumption.
Predictors of longitudinal change in VO2peak
Among participants randomized to an ET group, there was a wide gradient in ΔVO2peak in which approximately one-third of individuals experienced a decrease, one-third had minimal change, and one-third had an increase in VO2peak from baseline. Participant characteristics stratified by tertiles of ΔVO2peak are shown in Supplemental Table 1. Individuals who experienced an increase in VO2peak (highest tertile of ΔVO2peak) were younger, more commonly prescribed antihypertensive medication, and had lower systolic BP at baseline compared with those who had a decrease in VO2peak (lowest tertile of ΔVO2peak). Across tertiles of ΔVO2peak, baseline levels of VO2peak, hs-cTnT, and NT-proBNP were similar. The proportion of participants in the combination aerobic and resistance training group was greater among individuals with an increase in VO2peak (highest tertile of ΔVO2peak) vs those with a decrease in VO2peak (lowest tertile of ΔVO2peak).
In adjusted analysis, higher baseline hs-cTnT was inversely associated with ΔVO2peak (β, −0.08 [95% CI: −0.15 to −0.01], P = 0.02) (Central Illustration). No significant association was observed between baseline NT-proBNP and ΔVO2peak in adjusted analysis (β, 0.02 [95% CI: −0.02 to 0.06], P = 0.30). There was no significant correlation between ΔVO2peak and change in either hs-cTnT (Spearman correlation = 0.14 [95% CI: −0.01 to 0.29], P = 0.07) or NT-proBNP (Spearman correlation = −0.12 [95% CI: −0.27 to 0.03], P = 0.11).
Central Illustration.
Exercise Training, hs-cTnT and NT-proBNP Levels, and Cardiorespiratory Fitness Response in Type 2 Diabetes
Linear regression model was adjusted for demographics, cardiovascular risk factors, NT-proBNP, and exercise training modality. hs-cTnT = high-sensitivity cardiac troponin T; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
Discussion
In this secondary analysis of adults with T2D from the HART-D study, we observed that ET and changes in VO2peak were not associated with changes in hs-cTnT and NT-proBNP over 9-month follow-up (Central Illustration). Furthermore, higher levels of hs-cTnT at baseline were significantly associated with a blunted CRF improvement in response to ET.
A previous study examined the relationship between myocardial injury and CRF and demonstrated associations between higher exercise capacity and lower hs-cTnT.25 However, this prior study included predominantly White men who had few comorbidities. Furthermore, this previous study only examined the cross-sectional association and reverse causation may have contributed to the observed relationships. Among studies with longitudinal follow-up, higher physical activity has been associated with less temporal increase in hs-cTnT and NT-proBNP among community dwelling adults suggesting potential benefits of lifestyle interventions on subclinical cardiac disease.14,15 Moreover, findings from these observational studies were supported by a small pilot randomized trial that demonstrated a physical activity intervention attenuated the rise in hs-cTnT among elderly adults.19 In contrast, prior studies that evaluated resistance training in older adults did not demonstrate favorable effects on hs-cTnT.26,27 In the present study, we build on these prior observations by evaluating the effects of different supervised ET regimens over a 9-month period on changes in levels of hs-cTnT and NT-proBNP among adults with T2D who did not have prevalent HF. Here, we observed that none of the ET modalities evaluated in HART-D were significantly associated with longitudinal changes in hs-cTnT or NT-proBNP. This null effect was observed despite achieving recommended physical activity goals of at least 500 MET-minutes per week.18 These findings differ from some of the previous studies for several possible reasons. First, there were marked differences in the study populations. Participants in the CHS (Cardiovascular Health Study)14 and LIFE-P (Lifestyle Interventions and Independence for Elders pilot) trial19 were approximately 15 years older and had higher levels of hs-cTnT (∼8-10 ng/L) and NT-proBNP (∼110 pg/mL) at baseline compared with those evaluated in the present study. Additionally, T2D prevalence was <25% in these prior studies whereas all participants in the present study had T2D. Second, the follow-up interval over which cardiac biomarkers was evaluated across studies differed. Hs-cTnT and NT-proBNP were assessed over 2 to 3 years from baseline in CHS14 whereas the interval was 12 and 9 months in LIFE-P19 and HART-D,18 respectively, and shorter in other studies.26,27 Shorter duration follow-up may not provide sufficient time for the exercise intervention evaluated in HART-D to modify cardiac biomarkers or the subclinical cardiac structural phenotypes they reflect. Third, exercise may also transiently increase hs-cTnT and NT-proBNP in the short-term, especially in younger individuals which may confound the ability to use serial biomarkers to interrogate subclinical HF phenotypes.28,29 Prolonged exercise may lead to myocardial stretch and detection of cardiac biomarkers in serum.16 Additionally, exercise may impact other factors that influence cardiac biomarker levels. For example, in the Look AHEAD trial that enrolled adults with overweight and obesity who had T2D, an intensive lifestyle intervention led to reductions in weight, waist circumference, HbA1c, and systolic BP but increased NT-proBNP, likely due to reduction in body mass.30,31 Future studies with longer-term follow-up are needed to determine the prognostic implications of the changes in biomarker levels with ET.
In secondary analyses, we observed that elevated levels of hs-cTnT at baseline were associated with a blunted response to ET, as reflected by less improvement in CRF. If replicated in additional studies, these findings suggest that cardiac biomarkers such as hs-cTnT may be used to identify individuals with T2D who are more likely to benefit from specific preventive interventions such as ET. This is particularly relevant because CRF response to ET in T2D is heterogeneous. A prior analysis from randomized controlled trials have demonstrated approximately 30 to 40% of participants undergoing supervised ET have no improvement in CRF.20,32 Consistent with our observations, in the DREW (Dose-Response to Exercise in postmenopausal Women) trial, abnormal remodeling patterns identified by left ventricular (LV) hypertrophy and increased relative wall thickness were each associated with lower CRF responsiveness to ET.32 It is plausible that participants with a more favorable cardiac substrate without abnormal remodeling or myocardial injury may be better suited to adapt to exercise with physiological remodeling as compared with those with abnormal cardiac remodeling and subclinical myocardial injury. Elevated levels of hs-cTnT may identify patients with adverse LV remodeling, a less modifiable substrate, who are less likely to improve their CRF in response to ET. Strategies to better predict ET response, such as assessment of cardiac biomarkers and echocardiography, may help in allocating these effective but expensive and logistically cumbersome ET interventions among at-risk participants who are most likely to benefit from the same. A report from the American Diabetes Association recommends measurement of hs-cTnT or NT-proBNP at least annually among adults with T2D to guide initiation of preventive therapies.33 Future studies are also needed to determine the optimal exercise dose and modality for individuals with T2D and subclinical myocardial injury or abnormal LV remodeling who are at a greater risk of non-response to exercise. For example, a prolonged high-intensity interval training program improved CRF among adults with subclinical myocardial injury.34 This exercise intervention included a tailored training plan for each individual consisting of 4 different heart rate training zones determined from a maximal exercise test and ventilatory and lactate thresholds. High-risk subgroups may require more targeted, personalized interventions with higher intensity and/or dose of ET or using specific modalities to improve CRF.
The ET interventions evaluated in the HART-D trial did not meaningfully impact cardiac biomarkers, neither decrease or increase, suggesting exercise is safe in patients with T2D. In addition, ET has beneficial effects on several cardiometabolic parameters. Combination aerobic and resistance training improved glycemic control, cardiorespiratory fitness, and body composition with reduction in overall body mass driven primarily by decrease fat mass.18 Optimization of each of these cardiometabolic health parameters is associated with a lower risk of downstream HF, suggesting these are important modifiable risk factors.17,35,36
Study Strengths and limitations
Our study has several notable strengths. In the present study, we examined the effects of ET on multiple cardiac biomarkers in the setting of a randomized trial that included a non-exercise control group. The ET interventions were tailored to the individual and included high-intensity and supervised exercise sessions. Furthermore, CRF was assessed objectively during an exercise test by direct VO2peak assessment using a standardized protocol at baseline and follow-up.
However, there are several noteworthy limitations in the present analysis. First, there is potential for selection bias in this secondary analysis. Participants without baseline hs-cTnT plus baseline and follow-up measurements of CRF were excluded from this analysis. This is notable as individuals lost to follow-up or unable to repeat ET may be systematically different than those who were included in the present study. Furthermore, study participants included in this analysis had relatively well-controlled T2D, had at least 70% adherence to ET, and findings from this study may not be generalizable to all individuals with T2D. HART-D enrolled mostly younger adults free of established cardiovascular disease with mostly normal biomarker levels of cardiac injury and stress providing supportive evidence for ET as part of a comprehensive risk reduction strategy for primary prevention. Second, limited follow-up duration may bias study findings toward the null as there may have not been adequate time for cardiac biomarkers and CRF to meaningfully change in response to the 9-month study intervention. Prior studies that have demonstrated associations between longitudinal changes in cardiac biomarkers or CRF with the risk of HF were performed over several years.14,17 Third, baseline hs-cTnT levels were below the limit of detection in more than half of participants, which may also have reduced the power to detect change over serial measurements. Fourth, the present study is small and may have not been adequately powered to evaluate longitudinal changes in cardiac biomarkers across the non-exercise control and ET groups. Finally, echocardiography was not performed and clinical endpoints such as HF or other cardiovascular disease events were not captured as part of the study protocol.
Conclusions
Among adults with T2D and mostly normal baseline measures of subclinical myocardial injury and stress enrolled in the HART-D study, ET had no effect on hs-cTnT or NT-proBNP. Higher level of hs-cTnT at baseline identifies individuals with T2D who may have less improvement in CRF in response to ET. Future studies are needed to evaluate the effects of alternative lifestyle interventions on subclinical cardiac phenotypes.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Among adults with type 2 diabetes, exercise training did not reduce levels of high-sensitivity cardiac troponin T or N-terminal pro-B-type natriuretic peptide which represent cardiac injury and stress, respectively. Subclinical myocardial injury, assessed by elevated levels of high-sensitivity cardiac troponin T, was associated with a blunted fitness improvement in response to exercise training.
TRANSLATIONAL OUTLOOK: High-sensitivity cardiac troponin T levels can identify adults with type 2 diabetes who are more likely to benefit from exercise training. Future studies are needed to evaluate the implications of changes in cardiac biomarkers with exercise training on cardiovascular disease.
Funding support and author disclosures
The HARTD Study was supported by grant DK-068298 from the National Institutes of Health. Funding for biomarker assays was provided by Roche Diagnostics. Dr Patel has served as a consultant to Novo Nordisk. Mr Ayers has received statistical consulting fees from the National Institutes of Health outside the submitted work. Dr Rohatgi is supported by NIH/NHLBI R01HL136724, NIH/NHLBI K24HL146838, and NHLBI R01HL146462. Disclosures include Merck research grant (significant), CSL Limited consultant (modest), HDL Diagnostics Advisory Board (modest). Dr Berry has received grant support from the NIH, Roche Diagnostics and Abbott Diagnostics; consulting fees from Roche Diagnostics, AstraZeneca, and the Cooper Institute. Dr deFilippi has received research grants from Roche Diagnostics; has received consulting fees from Abbott Diagnostics, FujiRebio, Metabolomics, Ortho Diagnostics, Roche Diagnostics, and Siemens Healthcare; has received honoraria from WebMD; and has received royalties from UpToDate. Dr Church serves as the Chief Medical Officer at Wondr Health, Dallas, TX, USA. Dr de Lemos has received grant support from Roche Diagnostics and Abbott Diagnostics; consulting fees from Roche Diagnostics, Abbott Diagnostics, Ortho Clinical Diagnostics, Quidel Cardiovascular, Inc, and Siemen’s Health Care Diagnostics; and has been named a co-owner on a patent awarded to the University of Maryland (US Patent Application Number: 15/309,754) entitled: “Methods for Assessing Differential Risk for Developing Heart Failure.” Dr Pandey has served on the advisory board of Roche Diagnostics; has received nonfinancial support from Pfizer and Merck; and has received research support from the Texas Health Resources Clinical Scholarship, the Gilead Sciences Research Scholar Program, the National Institute on Aging GEMSSTAR Grant (1R03AG067960-01), Myovista, and Applied Therapeutics.
Acknowledgments
The authors thank the participants, staff, and investigators of the HART-D study.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table and figures, please see the online version of this paper.
Supplementary data
References
- 1.de Lemos J.A., Drazner M.H., Omland T., et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders J.T., Nambi V., de Lemos J.A., et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandey A., Vaduganathan M., Patel K.V., et al. Biomarker-based risk prediction of incident heart failure in pre-diabetes and diabetes. J Am Coll Cardiol HF. 2021;9:215–223. doi: 10.1016/j.jchf.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang T.J., Larson M.G., Levy D., et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 5.Kistorp C., Raymond I., Pedersen F., Gustafsson F., Faber J., Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293:1609–1616. doi: 10.1001/jama.293.13.1609. [DOI] [PubMed] [Google Scholar]
- 6.Januzzi J.L., Jr., Xu J., Li J., et al. Effects of canagliflozin on amino-terminal pro-B-type natriuretic peptide: implications for cardiovascular risk reduction. J Am Coll Cardiol. 2020;76:2076–2085. doi: 10.1016/j.jacc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 7.deFilippi C.R., de Lemos J.A., Christenson R.H., et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.deFilippi C.R., Christenson R.H., Gottdiener J.S., Kop W.J., Seliger S.L. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett B.M., Brooks M.M., Vlachos H.E., et al. Troponin and cardiac events in stable ischemic heart disease and diabetes. N Engl J Med. 2015;373:610–620. doi: 10.1056/NEJMoa1415921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEvoy J.W., Chen Y., Ndumele C.E., et al. Six-year change in high-sensitivity cardiac troponin T and risk of subsequent coronary heart disease, heart failure, and death. JAMA Cardiol. 2016;1:519–528. doi: 10.1001/jamacardio.2016.0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey A., Garg S., Khunger M., et al. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation. 2015;132:1786–1794. doi: 10.1161/CIRCULATIONAHA.115.015853. [DOI] [PubMed] [Google Scholar]
- 12.Patel K.V., Simek S., Ayers C., et al. Physical activity, subclinical myocardial injury, and risk of heart failure subtypes in Black adults. J Am Coll Cardiol HF. 2021;9:484–493. doi: 10.1016/j.jchf.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegde S.M., Goncalves A., Claggett B., et al. Cardiac structure and function and leisure-time physical activity in the elderly: the Atherosclerosis Risk in Communities Study. Eur Heart J. 2016;37:2544–2551. doi: 10.1093/eurheartj/ehw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.deFilippi C.R., de Lemos J.A., Tkaczuk A.T., et al. Physical activity, change in biomarkers of myocardial stress and injury, and subsequent heart failure risk in older adults. J Am Coll Cardiol. 2012;60:2539–2547. doi: 10.1016/j.jacc.2012.08.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fretz A., McEvoy J.W., Rebholz C.M., et al. Relation of lifestyle factors and life's simple 7 score to temporal reduction in troponin levels measured by a high-sensitivity assay (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2018;121:430–436. doi: 10.1016/j.amjcard.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shave R., Baggish A., George K., et al. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol. 2010;56:169–176. doi: 10.1016/j.jacc.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Pandey A., Patel K.V., Bahnson J.L., et al. Association of intensive lifestyle intervention, fitness, and body mass index with risk of heart failure in overweight or obese adults with type 2 diabetes mellitus: an analysis from the look AHEAD trial. Circulation. 2020;141:1295–1306. doi: 10.1161/CIRCULATIONAHA.119.044865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Church T.S., Blair S.N., Cocreham S., et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253–2262. doi: 10.1001/jama.2010.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.deFilippi C.R., de Lemos J.A., Newman A.B., et al. Impact of moderate physical activity on the longitudinal trajectory of a cardiac specific biomarker of injury: results from a randomized pilot study of exercise intervention. Am Heart J. 2016;179:151–156. doi: 10.1016/j.ahj.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Pandey A., Swift D.L., McGuire D.K., et al. Metabolic effects of exercise training among fitness-nonresponsive patients with type 2 diabetes: the HART-D study. Diabetes Care. 2015;38:1494–1501. doi: 10.2337/dc14-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senechal M., Swift D.L., Johannsen N.M., et al. Changes in body fat distribution and fitness are associated with changes in hemoglobin A1c after 9 months of exercise training: results from the HART-D study. Diabetes Care. 2013;36:2843–2849. doi: 10.2337/dc12-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giannitsis E., Kurz K., Hallermayer K., Jarausch J., Jaffe A.S., Katus H.A. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 23.D'Angelo G., Weissfeld L., and on behalf of GenIMS Investigators An index approach for the Cox model with left censored covariates. Stat Med. 2008;27:4502–4514. doi: 10.1002/sim.3285. [DOI] [PubMed] [Google Scholar]
- 24.Pandey A., Park B.D., Ayers C., et al. Determinants of racial/ethnic differences in cardiorespiratory fitness (from the Dallas heart study) Am J Cardiol. 2016;118:499–503. doi: 10.1016/j.amjcard.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 25.DeFina L.F., Willis B.L., Radford N.B., Christenson R.H., deFilippi C.R., de Lemos J.A. Cardiorespiratory fitness and highly sensitive cardiac troponin levels in a preventive medicine cohort. J Am Heart Assoc. 2016;5(12) doi: 10.1161/JAHA.116.003781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Linden N., Tieland M., Klinkenberg L.J., et al. The effect of a six-month resistance-type exercise training program on the course of high sensitive cardiac troponin T levels in (pre)frail elderly. Int J Cardiol. 2014;175:374–375. doi: 10.1016/j.ijcard.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 27.van der Linden N., Klinkenberg L.J., Leenders M., et al. The effect of exercise training on the course of cardiac troponin T and I levels: three independent training studies. Sci Rep. 2015;5:18320. doi: 10.1038/srep18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neumayr G., Pfister R., Mitterbauer G., Eibl G., Hoertnagl H. Effect of competitive marathon cycling on plasma N-terminal pro-brain natriuretic peptide and cardiac troponin T in healthy recreational cyclists. Am J Cardiol. 2005;96:732–735. doi: 10.1016/j.amjcard.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 29.Leers M.P., Schepers R., Baumgarten R. Effects of a long-distance run on cardiac markers in healthy athletes. Clin Chem Lab Med. 2006;44:999–1003. doi: 10.1515/CCLM.2006.179. [DOI] [PubMed] [Google Scholar]
- 30.Look A.R.G., Wing R.R., Bolin P., et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertoni A.G., Wagenknecht L.E., Kitzman D.W., et al. Impact of the look AHEAD intervention on NT-pro brain natriuretic peptide in overweight and obese adults with diabetes. Obesity (Silver Spring) 2012;20:1511–1518. doi: 10.1038/oby.2011.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandey A., Ayers C., Blair S.N., et al. Cardiac determinants of heterogeneity in fitness change in response to moderate intensity aerobic exercise training: the DREW study. J Am Coll Cardiol. 2015;65:1057–1058. doi: 10.1016/j.jacc.2014.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pop-Busui R., Januzzi J.L., Bruemmer D., et al. Heart failure: an underappreciated complication of diabetes. A consensus report of the American Diabetes Association. Diabetes Care. 2022;45:1670–1690. doi: 10.2337/dci22-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hearon C.M., MacNamara J.P., Hieda M., et al. 1 Year of HIIT and omega-3 fatty acid supplementation to improve cardiometabolic risk in stage-A heart failure. J Am Coll Cardiol HF. 2022;10:238–249. doi: 10.1016/j.jchf.2022.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Patel K.V., Bahnson J.L., Gaussoin S.A., et al. Association of baseline and longitudinal changes in body composition measures with risk of heart failure and myocardial infarction in type 2 diabetes: findings from the look AHEAD trial. Circulation. 2020;142:2420–2430. doi: 10.1161/CIRCULATIONAHA.120.050941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segar M.W., Patel K.V., Vaduganathan M., et al. Association of long-term change and variability in glycemia with risk of incident heart failure among patients with type 2 diabetes: a secondary analysis of the ACCORD trial. Diabetes Care. 2020;43:1920–1928. doi: 10.2337/dc19-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.