Abstract
Background
Left ventricular noncompaction (LVNC) is characterized by excessive trabeculations of the left ventricular (LV) wall.
Objectives
The authors aimed to examine changes in LV function and morphology in 2 to 4-year-old children with and without LVNC at birth and to describe the prevalence of LVNC in first-degree relatives.
Methods
Echocardiograms in children with and without LVNC (matched 1:4) were performed at 2 to 4 years and in first-degree relatives. LVNC was blindly assessed and defined as a ratio of non-compact to compact myocardium of ≥2 in ≥1 LV segment. Trabeculations were expressed as a percentage of the number of segments with LVNC out of the total number of segments.
Results
In total, 14 (median age 3 years, 71% male) of 16 children with LVNC at birth and 56 children without (median age 4 years, 71% male), 37 first-degree relatives of children with LVNC (median age 31 years, 46% male) and 146 first-degree relatives of children without (median age 33 years, 50% male) were included. In children with LVNC, trabeculation (8% vs 13%, P = 0.81) and LV ejection fraction (50% vs 49%, P = 0.91) were unchanged from birth to follow-up but LV ejection fraction was lower compared to children without LVNC (49% vs 60%, P < 0.001). In relatives of children with LVNC, 11 of 37 (30%) fulfilled LVNC criteria compared to no relatives to children without LVNC (P < 0.001).
Conclusions
At 2 to 4 years, children with LVNC diagnosed at birth had reduced systolic function compared to children without but did not have progression of LV dysfunction or extent of trabeculations. In first-degree relatives to children with LVNC, 30% fulfilled criteria.
Key words: cardiomyopathy, child, echocardiography, myocardium, trabeculation
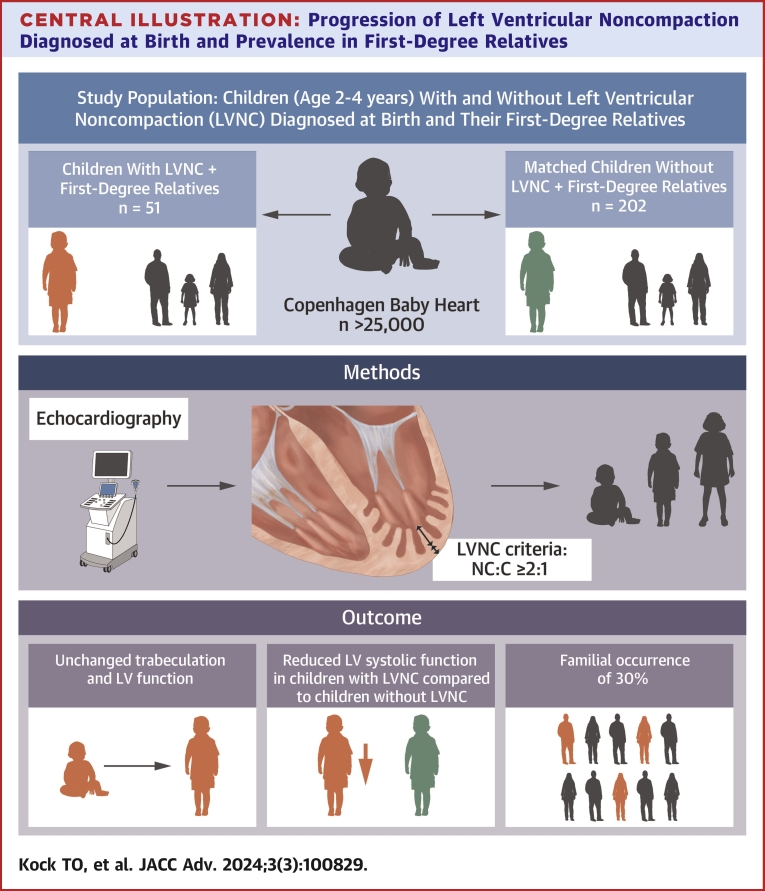
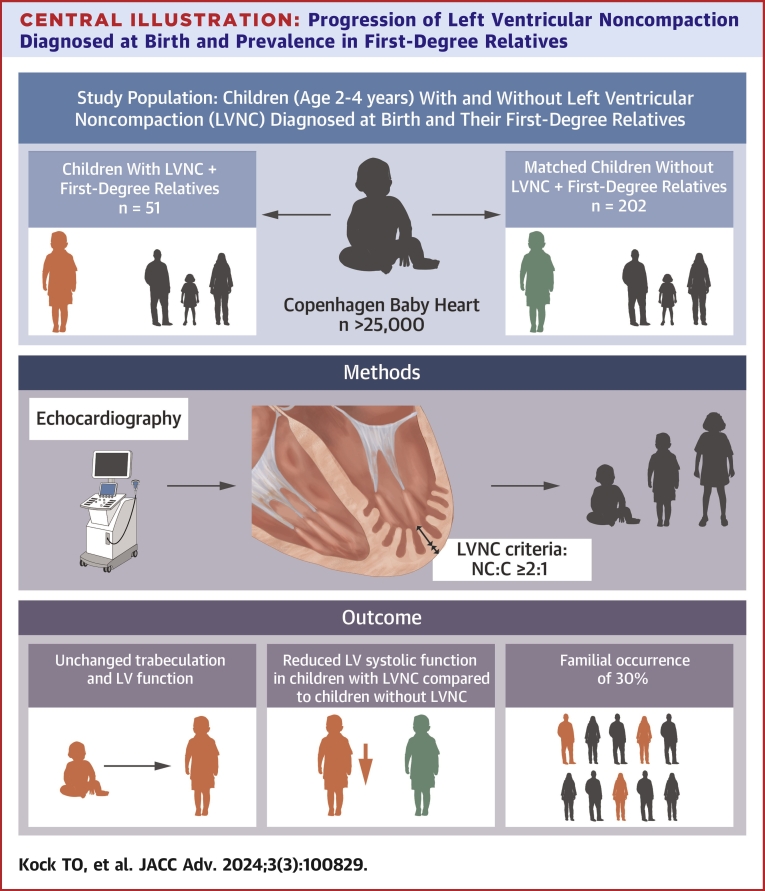
Central Illustration
Left ventricular noncompaction (LVNC) is characterized by a thin epicardial compact layer and a thick endocardial non-compact layer with prominent trabeculations and intertrabecular recesses. When LVNC is associated with left ventricular (LV) dysfunction, it is known as LVNC cardiomyopathy, but the morphologic findings seen in LVNC can also be seen in individuals with normal ventricular function. Complications of LVNC cardiomyopathy include heart failure, arrhythmias, thromboembolic events, and sudden cardiac death.1, 2, 3
There is controversy as to whether LVNC is a distinct trait that occurs as a consequence of an arrest in the normal myocardial compaction during fetal development, or whether LVNC may develop later in life as a morphologic trait associated with other types of cardiomyopathies.2, 3, 4 Approximately 30% of first-degree relatives to patients with LVNC cardiomyopathy are also affected.1,5,6 The familial occurrence of morphologic LVNC only, meaning individuals with normal systolic function, has not yet been established.
In a previous study from the Copenhagen Baby Heart Study (CBHS), a population-based cohort study of newborns (n > 25,000) focusing on cardiac structure and function, 16 newborns were diagnosed with LVNC based on morphologic criteria, corresponding to a prevalence of LVNC of 0.076%. These newborns with morphologic LVNC had a reduced systolic function compared to matched newborns without LVNC, although still within the normal range.7 Previous studies have demonstrated that prominent trabeculations are associated with adverse outcomes regardless of systolic function but that the prognosis of individuals with LVNC and reduced ventricular function is worse than in patients with morphologic LVNC and normal ventricular function.2,3,5,8, 9, 10 The progression of LV dysfunction over time in LVNC remains to be fully uncovered, particularly in pediatric patient groups, and follow-up studies are needed.
This study had 2 aims. The first aim was to assess changes in LV systolic function and morphologic LVNC pattern during early childhood in children with LVNC diagnosed at birth, compared with matched children without LVNC. Secondly, the study aimed to assess the prevalence of LVNC in first-degree relatives to children with LVNC.
Methods
Study population and design
CBHS is a large-scale population-based cohort study with prospective collection of data focusing on cardiac structure and function in newborns born between April 2016 and October 2018 in Copenhagen, Denmark. Transthoracic echocardiography (TTE), electrocardiography, and pulse oximetry were conducted within 60 days of birth on the >25,000 participants of the study. Information on medical and family history was obtained from questionnaires and registries.11,12
The present study is a prospective cohort follow-up study of the 16 children with LVNC identified neonatally in the CBHS, matched children from the CBHS cohort without LVNC and their first-degree relatives. The children without LVNC were matched 1:4 on age of mother at birth, parity at birth, and the age of the child at the follow-up examination, with the aim of matching the family composition as well as the children. Children without LVNC with structural heart diseases including atrial and ventricular septal defects, patent ductus arteriosus, or bicuspid aortic valves were excluded. All first-degree relatives (siblings and parents) of the LVNC children and children without LVNC, including half-siblings, were eligible for inclusion.
Ethical approval
This study was approved by the Regional Ethics Committee of the Capital City Region of Denmark (H-16001518 for primary inclusion and H-19038069 for follow-up) and the Danish Data Protection Agency (Pactius jr.nr.: P-2020-402). Oral and written information was provided to adult and adolescent participants, as well as to parents to included children, before written consent was obtained.
Data collection
Similarly, to the initial examination of the newborns in CBHS, all participants underwent TTE, electrocardiography, and clinical examination. TTE was performed by 1 cardiac sonographer (T.O.K.) using a Vivid E9 machine from GE Healthcare. The M5Sc-D probe was used for examination of adults, whereas the 6S or the 12S probes were used for examination of children and younger siblings. The echocardiographic protocol contained standard subxiphoid, apical, parasternal, and suprasternal views (Supplemental Table 1). TTE images were obtained in accordance with the American Society of Echocardiography’s guidelines for adult and pediatric echocardiography.13,14 Furthermore, blood was drawn from children with LVNC and their first-degree relatives for future genetic investigation.
Analyses
The echocardiograms were analyzed blinded and retrospectively like the previous study from CBHS using the criteria outlined by Paterick et al2 suggesting a proposed ratio of non-compact to compact myocardium thickness (NC:C) ≥2 in any segment, measured at end-diastole. The criteria were applied on the apical 4-chamber, apical 2-chamber, and 3-chamber view as well as the parasternal short axis view on the level of papillary muscles. Each view was divided into 6 segments, resulting in a total of 24 measurements in 18 individual segments to be assessed for possible non-compaction. The contrast between myocardium and blood had to be acceptable and with all segments within the sector in all the apical views. For each segment, it was first noted whether a trabeculated myocardium was present or not. If trabeculation was present, the ratio of NC:C myocardium was measured perpendicular to the LV cavity in all 24 measurements. Since the initial assessments of LVNC at baseline were conducted in 12 segments, the trabeculation in the current study was assessed as a calculated percentage of number of segments fulfilling criteria for LVNC out of the total number of measurable segments in each individual. This calculation allowed for comparison between trabeculation levels at baseline and follow-up. The measurements of LV dimensions, volumes, and systolic function were completed according to guidelines, specifically using the biplane modified Simpson’s method for the measurement of LV ejection fraction (LVEF), LV end-systolic volume (LVESV), and LV end-diastolic volume.15,16 The measurements were conducted in Echopac software (General Electric Viewpoint Echopac plugin v203.82.0).
Statistics
A standard sample size calculation was limited by only 16 individuals being identified with LVNC out of the more than 25,000 included newborns in CBHS. The previously reported prevalence of LVNC in the CBHS cohort of 0.076% was used in the calculation as a proxy of the prevalence in relatives to children without LVNC. Based on previous reports,1,5,6 a prevalence of at least 10% was assumed in relatives to LVNC children. The assumed prevalence required inclusion of at least 26 relatives to LVNC children and at least 130 relatives to children without LVNC to show a statistically significant difference (P < 0.050) in prevalence with a power of 90%.
Median, IQR, and full range were calculated for the continuous variables. Comparisons between those with and without LVNC were performed using the Mann-Whitney U test (Wilcoxon rank sum test) for continuous variables, and Fisher exact test for categorical variables. The prevalence of LVNC in first-degree relatives was presented with 95% CI. Two-sided P values were used for all statistical analyses with significance defined as <0.05. Statistical analyses and graphical illustrations were performed in R version 3.6.0.
Results
Study population
Of the 16 individuals identified with LVNC at birth, 14 (age 3 [IQR: 3-4] years, 71% male) were included in this study along with 37 of their first-degree relatives (age 31 [IQR: 4-38] years, 46% male) consisting of 12 mothers, 13 fathers, and 12 siblings. None of these 16 children were diagnosed with genetic syndromes. Similarly, 56 children without LVNC at birth (age 4 [IQR: 3-4] years, 71% male) and 146 of their first-degree relatives (age 33 [IQR: 11-40] years, 50% male) consisting of 56 mothers, 48 fathers, 37 siblings, and 5 half-siblings were included as those without LVNC, resulting in 253 participants in total. Demographic characteristics of children and relatives are presented in Table 1.
Table 1.
Characteristics of Children With LVNC
| LVNC | No LVNC | P Value | |
|---|---|---|---|
| Maternal data | (n = 14) | (n = 56) | |
| Age at birth, y | 30 (26-36) | 31 (27-37) | 0.85 |
| Prepregnancy BMI, kg/m2 | 23.2 (21.3-25.4) | 21.6 (20.1-24.4) | 0.23 |
| Parity | |||
| Nullipara | 11 (79) | 44 (79) | |
| Primipara | 2 (14) | 8 (14) | |
| Multipara | 1 (7) | 4 (7) | |
| Birth data of newborns | (n = 14) | (n = 56) | |
| Male | 10 (71) | 40 (71) | 1.00 |
| Gestational age, wk | 39 (38-40) | 40 (39-41) | 0.11 |
| Height, cm | 53 (50-55) | 52 (51-53) | 0.98 |
| Weight, g | 3,592 (3,362-3,952) | 3,630 (3,298-3,848) | 0.96 |
| Data of children on follow-up examination day | (n = 14) | (n = 56) | |
| Age, mo | 49 (42-54) | 51 (43-55) | |
| Height, cm | 108 (104-114) | 109 (104-113) | 0.88 |
| Weight, kg | 17.3 (15.9-20.7) | 18.2 (16.4-19.7) | 0.72 |
| Data of first-degree relatives on examination day | (n = 37) | (n = 146) | |
| Male | 17 (46) | 73 (50) | 0.72 |
| Age, y | 31 (4-38) | 33 (11-40) | 0.33 |
| Height, cm | 170 (116-180) | 171 (148-181) | 0.64 |
| Weight, kg | 74.5 (18.4-89.6) | 70.3 (34.4-84.5) | 0.55 |
Values are median (IQR) or n (%).
Maternal, delivery, newborn, and first-degree relative characteristics for children with LVNC (n = 14) and children without LVNC (n = 56).
BMI = body mass index; LVNC = left ventricular non-compaction.
Changes in children with LVNC from birth to the age of 2 to 4 years
In children diagnosed with LVNC in the CBHS, 12.5% (IQR: 8.3%-15.6%) of assessed segments of the LV had a NC:C ratio ≥2 at follow-up. This proportion was statistically unchanged when compared to baseline findings (8.3% [IQR: 8.3%-16.7%], P = 0.81). In accordance with baseline findings, the segments fulfilling the criteria for LVNC were primarily found in the apical part of the ventricle (Supplemental Table 2). The majority of segments with NC:C ratio ≥2 at follow-up were either the same or neighboring to the segments identified with NC:C ratio ≥2 at baseline (74%). The neighboring segments were included in this calculation since the apical 3-chamber and apical 2-chamber views were not included at baseline. The median NC:C of the segments fulfilling criteria for LVNC was 2.2 (IQR: 2.0-2.3). None of the children had developed other types of cardiomyopathies since birth.
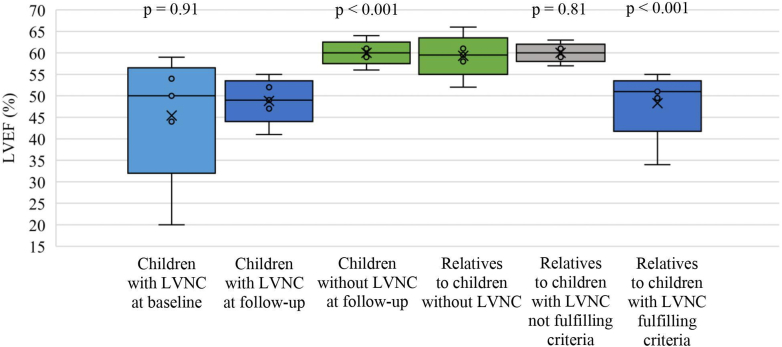
Figure 1 shows the ventricular systolic function, measured as LVEF, in the children with LVNC at follow-up compared to the baseline evaluation. LVEF at follow-up was not different when compared to baseline (49% [IQR: 47%-52%] vs 50% [IQR: 44%-54%], P = 0.91). At baseline, 11 (79%) of the children had a LVEF <55% and 6 (43%) had <50%, whereas at follow-up, 12 (86%) children had a LVEF <55% and 7 (50%) had <50%. Only 1 child with LVNC was on treatment with angiotensin-converting-enzyme inhibitors due to a LVEF <45% which at baseline was severely reduced at 20%. No other children were on any medication. All children were determined as asymptomatic following a clinical assessment and by interviewing parents about signs and symptoms suspicious of heart failure in their children.
Figure 1.
Systolic Function in Children With LVNC and First-Degree Relatives
Systolic function in children with LVNC at follow-up examination (2-4 years of age) compared to baseline (within 60 days of birth) and to children without LVNC at follow-up examination (2-4 years of age), and systolic function in first-degree relatives of children with LVNC compared with first-degree relatives to children without LVNC. LVEF = left ventricular ejection fraction; LVNC = left ventricular non-compaction.
Comparisons of children with and without LVNC at follow-up
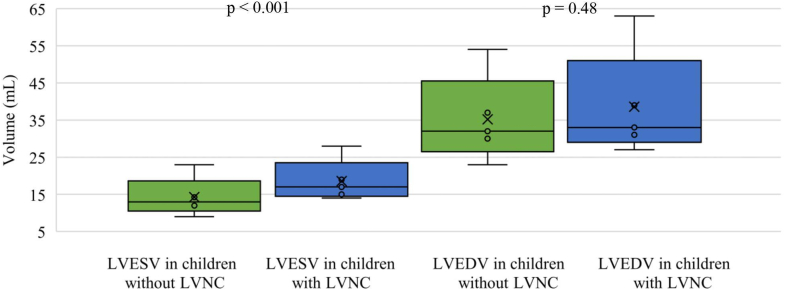
In children with LVNC, 12.5% (IQR: 8.3%-15.6%) of segments fulfilled the NC:C ≥2 criteria compared to no segments in children without LVNC (P < 0.001). The LVEF in children with LVNC was lower than in matched children without LVNC (49% [IQR: 47%-52%] and 60% [IQR: 59%-61%], P < 0.001) (Figure 1). All of the matched children without LVNC had a normal LVEF >55%. LV end-diastolic volume in children with LVNC was similar to children without LVNC (33 mL [IQR: 31-39 mL] vs 32 mL [IQR: 30-37 mL], P = 0.48) (Figure 2) but LVESV was significantly larger in LVNC children (17 mL [IQR: 15-19 mL]) compared to children without LVNC (13 mL [IQR: 12-15 mL], P < 0.001) (Figure 2). None of the children with or without LVNC had a z-score >2 in left ventricular internal diameter in end-diastole (−5.3 [IQR: −5.5 to 4.38] vs −1.47 [IQR: −2.1 to 0.49]). However, 29% (n = 4) of the children with LVNC and 5% (n = 3) of the children without LVNC had a z-score >2 in LVIDs (0.95 [IQR: 0.18-2.1] vs −0.31 [IQR: −0.80 to 0.49]).17
Figure 2.
LVESV and LVEDV in Children
LVESV and LVEDV in children with and without LVNC at follow-up examination (2-4 years of age). LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; LVNC = left ventricular non-compaction.
Echocardiographic LVNC criteria and systolic function in first-degree relatives
Out of 37 first-degree relatives to the children with LVNC, 11 (30% [95% CI: 15%-44%]) had at least 1 segment with NC:C ≥2, fulfilling the set criteria for LVNC. None of these relatives fulfilling the criteria had previously been diagnosed with LVNC. None of the 146 first-degree relatives to matched children without LVNC fulfilled the criteria for LVNC (P < 0.001). Of the 14 children with LVNC, 5 had 1 first-degree relative fulfilling the criteria for LVNC and 3 of the children had 2 first-degree relatives fulfilling the criteria. The remaining 6 children with LVNC did not have any first-degree relatives fulfilling criteria. The 11 first-degree relatives fulfilling the criteria for LVNC had 2 (IQR: 2-3) individual segments with a NC:C >2. As observed in the children with LVNC, the majority of non-compacted segments were found in the apical part of the LV (Supplemental Table 4). The median of the measured NC:C of the segments fulfilling criteria was 2.0 (IQR: 2.0-2.1). LVEF in first-degree relatives fulfilling criteria for LVNC was significantly lower than LVEF in the first-degree relatives to the matched children without LVNC (51% [IQR: 50%-52%] vs 60% [IQR: 58%-61%], P < 0.001). Only 1 of the first-degree relatives with LVNC had a LVEF <45%, considered in the low range. LVEF in first-degree relatives not fulfilling criteria for LVNC was not significantly different from first-degree relatives to the matched children without LVNC (60% [IQR: 59%-61%] vs 60% [IQR: 58%-61%], P = 0.81) (Central Illustration). None of the first-degree relatives fulfilling criteria for LVNC had symptoms or clinical findings consistent with heart failure. They did not fulfill criteria for any other type of cardiomyopathy, such as dilated or hypertrophic cardiomyopathy. Also, no first-degree relatives fulfilling criteria reported a family history of inherited cardiac disease or sudden cardiac death.
Central Illustration.
Progression of Left Ventricular NoncompactionDiagnosed at Birth and Prevalence in First-Degree Relatives
Discussion
In children diagnosed with LVNC at birth in a population-based cohort study, we assessed the development of LV function during early childhood. In addition, we assessed the prevalence of LVNC in first-degree relatives of children with LVNC. The study revealed 3 major findings. 1) There was no significant change in the level of trabeculation or further reduction in LV systolic function in asymptomatic children with LVNC at 2 to 4 years of age compared to levels at baseline. 2) The LV systolic function in children of 2 to 4 years of age with LVNC remained significantly lower compared to matched children without LVNC. 3) The prevalence of LVNC in first-degree relatives to children with LVNC was 30%.
Distribution of segments with NC/C ≥2
Non-compact segments were primarily distributed in the apical segments (anterior septal, lateral, inferior septal, inferior) in the apical views (3-, 4-, and 2-chamber) (Supplemental Table 2). No basal segments fulfilled the criteria of NC:C ≥2. These findings are consistent with previous findings of the distribution of non-compact segments more often being the apical segments.1,3,5,18,19 The mean NC:C of non-compact segments in the children with LVNC and their first-degree relatives with LVNC was 2.2 and 2.1, respectively. This is lower than a previously reported mean of NC:C of 3.41,5 and may be explained by less affected individuals than in previous studies, hence the systolic function being near the normal range. Also, the previously reported mean is from adult studies which may impact the mean ratio as well.
Association with LV systolic function
The association between LVNC and reduced systolic function has been reported in both adult and pediatric patient groups.2, 3, 4,20 Our study add strength to this evidence. Children with LVNC had significantly lower systolic function when compared to matched children without LVNC both at birth and at follow-up at 2 to 4 years. Also, LVESV was significantly larger in children with LVNC indicating a reduced contractile function in these children. Systolic function did not decline further compared to baseline. Nucifora et al21 reported a preserved LVEF in children with LVNC but a significantly reduced LVEF in young adults with LVNC and a similar extent of trabeculation as the children, compared to healthy children without LVNC. We found that the systolic function was, although significantly lower than children without LVNC, still categorized near the normal range, except for 1 individual. The findings of Nucifora et al21 describing a decline in LVEF when comparing children to young adults combined with the fact that our study did not find a further decline in LVEF at 2 to 4 years, indicates that later follow-up of LVNC patients may be necessary to detect progression of systolic function throughout childhood.
Familial occurrence of LVNC
Previous research suggests an estimated frequency of LVNC in first-degree relatives to patients with LVNC and a reduced systolic function to be approximately 30%.1,5,6 Our study assessed LVNC in adult and pediatric first-degree relatives to individuals with LVNC and for the vast majority an LVEF >45%. Despite the contrasting study populations, the current study still found a familial occurrence of LVNC of 30%, like the previously reported.1,5,6 In our group of first-degree relatives fulfilling the criteria for LVNC, the proportion of non-compacted segments was 10%, and the mean of the LVNC segments was 2.1. In relatives (n = 156), Caliskan et al6 found a mean percentage of non-compacted segments of 47%, the mean of LVNC segments to be 2.6 and LV dysfunction in most of the affected relatives from the family screening. The discrepancy indicates that LVNC may progress with age, resulting in more evident findings in adults with LVNC.
Study limitations
The current study has a limited number of individuals with LVNC. It is uncertain whether parents are more or less inclined to participate in CBHS if their child had already been referred clinically for follow-up. Therefore, selection based on clinical referral may contribute to a potential bias. Also, our cohort is biased from being found by systematic cardiac evaluation meaning generalizing the results to other cohorts may be problematic. Not all segments were accessible for assessment due to suboptimal echocardiographic image quality (<1%). No contrast-TTE, cardiac magnetic resonance imaging, or genetic testing was conducted in this study. A diagnostic gold standard has not yet been truly established in relation to assessment of LVNC, meaning results are dependent on which diagnostic criteria are chosen.
Conclusions
Children with LVNC diagnosed at birth as part of a population study persistently had a reduced systolic function when compared to children without LVNC but showed no further progression of LV dysfunction or extent of trabeculation at the age of 2 to 4 years. Of the first-degree relatives to children with LVNC, 30% fulfilled the criteria for LVNC and had reduced systolic function compared to first-degree relatives of children without LVNC. These findings indicate that family screening and clinical follow-up of children with LVNC should be considered.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE 1: This study is the first population study to follow the development of the LV systolic function and morphology in individuals with extensive trabeculations detected by systemic echocardiography at birth.
COMPETENCY IN MEDICAL KNOWLEDGE 2: The results of this study offer novel insights into anticipated echocardiographic findings in early childhood after diagnosis of LVNC at birth in an unselected cohort.
COMPETENCY IN MEDICAL KNOWLEDGE 3: Also, the familial occurrence, even in families with morphologic LVNC and a systolic function near the normal range, is 30% indicating a clear familial pattern of the LVNC phenotype. These results specify that first-degree relatives are at high risk of having LVNC themselves.
TRANSLATIONAL OUTLOOK 1: The findings therefore support that echocardiographic screening be offered when LVNC is diagnosed, to potentially detect cases of LVNC at an earlier stage, plan surveillance and allow early heart failure treatment.
TRANSLATIONAL OUTLOOK 2: Also, since a significantly lower systolic function was found in the children with LVNC compared to children without LVNC, clinical and echocardiographic follow-up should be considered, in order to detect reduced LV systolic function with the aim of providing early treatment.
Funding support and author disclosures
The Copenhagen Baby Heart Study was supported by the Danish Heart Association, Copenhagen, Denmark; the Danish Children’s Heart Foundation, Copenhagen, Denmark; the Toyota Foundation, Copenhagen, Denmark; the Herlev-Gentofte Hospital Research Foundation, Copenhagen, Denmark; and Candy’s Foundation, Vaduz, Lichtenstein. The funders had no part in the design of the study; in the collection, analysis, or interpretation of data or publication. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Suppplementary data
References
- 1.Bhatia N.L., Tajik A.J., Wilansky S., Steidley D.E., Mookadam F. Isolated noncompaction of the left ventricular myocardium in adults: a systematic overview. J Card Fail. 2011;17:771–778. doi: 10.1016/j.cardfail.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Paterick T.E., Umland M.M., Jan M.F., et al. Left ventricular noncompaction: a 25-year odyssey. J Am Soc Echocardiogr. 2012;25(4):363–375. doi: 10.1016/j.echo.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 3.Stanton C., Bruce C., Connolly H., et al. Isolated left ventricular noncompaction syndrome. Am J Cardiol. 2009;104(8):1135–1138. doi: 10.1016/j.amjcard.2009.05.062. [DOI] [PubMed] [Google Scholar]
- 4.Chin T.K., Perloff J.K., Williams R.G., Jue K., Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990;82(2):507–513. doi: 10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 5.Aras D., Tufekcioglu O., Ergun K., et al. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail. 2006;12(9):726–733. doi: 10.1016/j.cardfail.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Caliskan K., Michels M., Geleijnse M.L., et al. Frequency of asymptomatic disease among family members with noncompaction cardiomyopathy. Am J Cardiol. 2012;110(10):1512–1517. doi: 10.1016/j.amjcard.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Børresen M.F., Blixenkrone-Møller E., Kock T.O., et al. Prevalence of left ventricular noncompaction in newborns. Circ Cardiovasc Imaging. 2022;15(6) doi: 10.1161/CIRCIMAGING.121.014159. [DOI] [PubMed] [Google Scholar]
- 8.Brescia S.T., Rossano J.W., Pignatelli R., et al. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation. 2013;127(22):2202–2208. doi: 10.1161/CIRCULATIONAHA.113.002511. [DOI] [PubMed] [Google Scholar]
- 9.De Agustín J.A., Gomez De Diego J.J., Rodrigo J.L., et al. Subclinical systolic dysfunction in left ventricular non-compaction cardiomyopathy unmasked by contrast echocardiography. Int J Cardiol. 2014;172(3) doi: 10.1016/j.ijcard.2013.12.266. [DOI] [PubMed] [Google Scholar]
- 10.Sigvardsen P.E., Fuchs A., Kühl J.T., et al. Left ventricular trabeculation and major adverse cardiovascular events: the Copenhagen general population study. Eur Heart J Cardiovasc Imaging. 2021;22(1):67–74. doi: 10.1093/ehjci/jeaa110. [DOI] [PubMed] [Google Scholar]
- 11.Sillesen A.S., Raja A.A., Pihl C., et al. Copenhagen baby heart study: a population study of newborns with prenatal inclusion. Eur J Epidemiol. 2019;34(1):79–90. doi: 10.1007/s10654-018-0448-y. [DOI] [PubMed] [Google Scholar]
- 12.Vøgg R.O.B., Basit S., Raja A.A., et al. Cohort profile: the Copenhagen baby heart study (CBHS) Int J Epidemiol. 2021;50(6):1778–1779m. doi: 10.1093/ije/dyab147. [DOI] [PubMed] [Google Scholar]
- 13.Orsinelli D.A., Armour A., De Cara J., et al. The American Society of Echocardiography recommendations for cardiac chamber quantification in. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Lopez L., Colan S.D., Frommelt P.C., et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Lai W.W., Geva T., Shirali G.S., et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the task force of the pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19(12):1413–1430. doi: 10.1016/j.echo.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 17.Boston Children’s Hospital. BCH Z-score calculator [internet] 2023. https://zscore.chboston.org/
- 18.Arunamata A., Stringer J., Balasubramanian S., Tacy T.A., Silverman N.H., Punn R. Cardiac segmental strain analysis in pediatric left ventricular noncompaction cardiomyopathy. J Am Soc Echocardiogr. 2019;32(6):763–773.e1. doi: 10.1016/j.echo.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Arunamata A., Punn R., Cuneo B., Bharati S., Silverman N.H. Echocardiographic diagnosis and prognosis of fetal left ventricular noncompaction. J Am Soc Echocardiogr. 2012;25(1):112–120. doi: 10.1016/j.echo.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Pignatelli R.H., McMahon C.J., Dreyer W.J., et al. Clinical characterization of left ventricular noncompaction in children: a relatively common form of cardiomyopathy. Circulation. 2003;108(21):2672–2678. doi: 10.1161/01.CIR.0000100664.10777.B8. [DOI] [PubMed] [Google Scholar]
- 21.Nucifora G., Sree Raman K., Muser D., et al. Cardiac magnetic resonance evaluation of left ventricular functional, morphological, and structural features in children and adolescents vs. young adults with isolated left ventricular non-compaction. Int J Cardiol. 2017;246:68–73. doi: 10.1016/j.ijcard.2017.05.100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.