Abstract
Hypertrophic cardiomyopathy—both obstructive hypertrophic cardiomyopathy (oHCM) and nonobstructive hypertrophic cardiomyopathy (nHCM) subtypes—is the most common monogenic cardiomyopathy. Its structural hallmarks are abnormal thickening of the myocardium and hyperdynamic contractility, while its hemodynamic consequences are left ventricular outflow tract or intracavitary obstruction (in oHCM) and diastolic dysfunction (in both oHCM and nHCM). Several medical therapies are routinely used to improve these abnormalities with the goal to decrease symptom burden in patients with HCM. Current guidelines recommend nonvasodilating beta blockers as first-line and nondihydropyridine calcium channel blockers followed by disopyramide as second- and third-line medical therapies for symptomatic oHCM and give weaker recommendations for beta blockers and calcium channel blockers in nHCM. These recommendations are based on small studies—mostly nonrandomized—and expert opinion. Our review will summarize the available data on the effectiveness of commonly prescribed medications used in oHCM and nHCM to uncover knowledge gaps, but also new data on cardiac myosin inhibitors.
Key words: beta-blockers, cardiac myosin inhibitors, diastolic dysfunction, hypertrophic cardiomyopathy, obstructive hypertrophic cardiomyopathy
Central Illustration
Highlights
-
•
Until recently, medical therapies for obstructive hypertrophic cardiomyopathy have been suboptimal to eliminate outflow obstruction and reduce symptoms.
-
•
Current guideline recommendations on the pharmacologic management of hypertrophic cardiomyopathy are based on small, underpowered studies, most of which were not randomized.
-
•
Cardiac myosin inhibitors are a novel, highly effective medication class that promises to improve symptoms and quality of life and reduce the need for invasive therapies in obstructive hypertrophic cardiomyopathy.
-
•
Evidence on the effectiveness of beta blockers or calcium-channel blockers for improving diastolic function is incomplete but may favor calcium-channel blockers.
Obstructive hypertrophic cardiomyopathy (oHCM) and nonobstructive hypertrophic cardiomyopathy (nHCM) are usually inherited cardiomyopathies due to gene mutations of the sarcomere leading to abnormal thickening of the myocardium, myocyte disarray, and hyperdynamic contractility. The definition of hypertrophic cardiomyopathy (HCM) is usually a maximal left ventricular (LV) wall thickness ≥15 mm (or ≥13 mm in individuals with pathogenic HCM gene mutations or a family history of HCM) in the absence of identifiable causes of hypertrophy (eg, LV pressure overload, infiltrative cardiomyopathies). The hemodynamic consequences of these myocardial abnormalities are left ventricular outflow tract (LVOT) or intracavitary obstruction (in oHCM, defined as a resting or provocable gradient ≥30 mm Hg) and diastolic dysfunction (both oHCM and nHCM), all of which are putative mechanisms for the typical clinical symptoms. While medical therapies addressing underlying pathomechanisms are currently limited, some therapies have been shown to improve hemodynamic abnormalities and symptoms and potentially disease trajectory in patients with HCM. While invasive therapies are available and effective in resolving outflow obstruction, they may not completely normalize underlying structural and functional derangements and associated symptoms.
Medical therapy is currently the mainstay for symptom management for patients with HCM, as it can resolve or improve symptoms sufficiently in the majority of patients. Recent guidelines recommend nonvasodilating beta-blockers (BBs) as first-line and nondihydropyridine calcium-channel blockers (CCBs) followed by disopyramide as second- and third-line medical therapies for symptomatic oHCM (Central Illustration). These recommendations are based on small studies—most of them are nonrandomized and underpowered (Table 1)—and expert opinion. To give practitioners who treat patients with HCM a more complete picture of the available evidence and point out crucial knowledge gaps, this review will summarize the available data on the effectiveness of recommended and commonly prescribed pharmacotherapies.
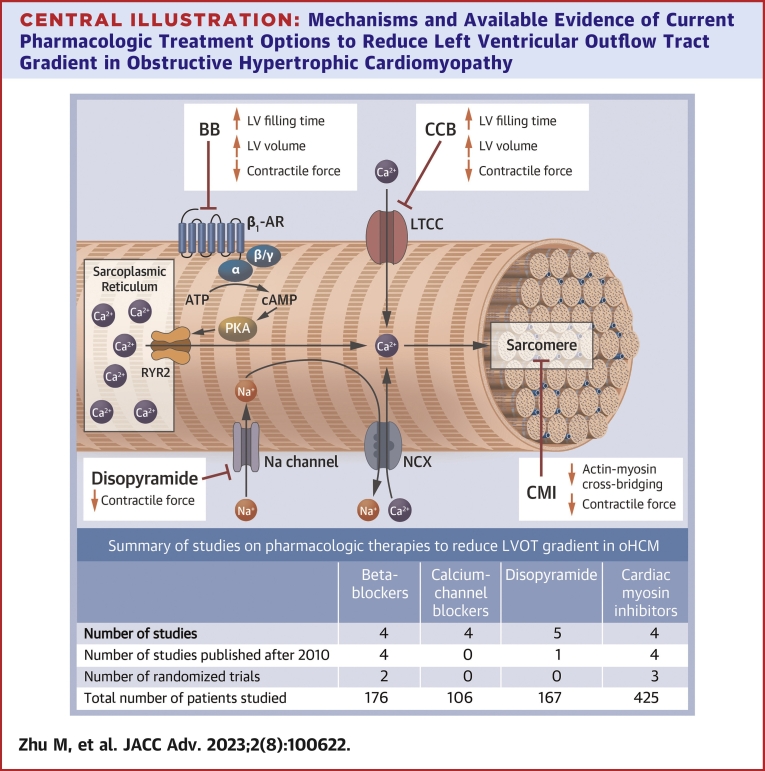
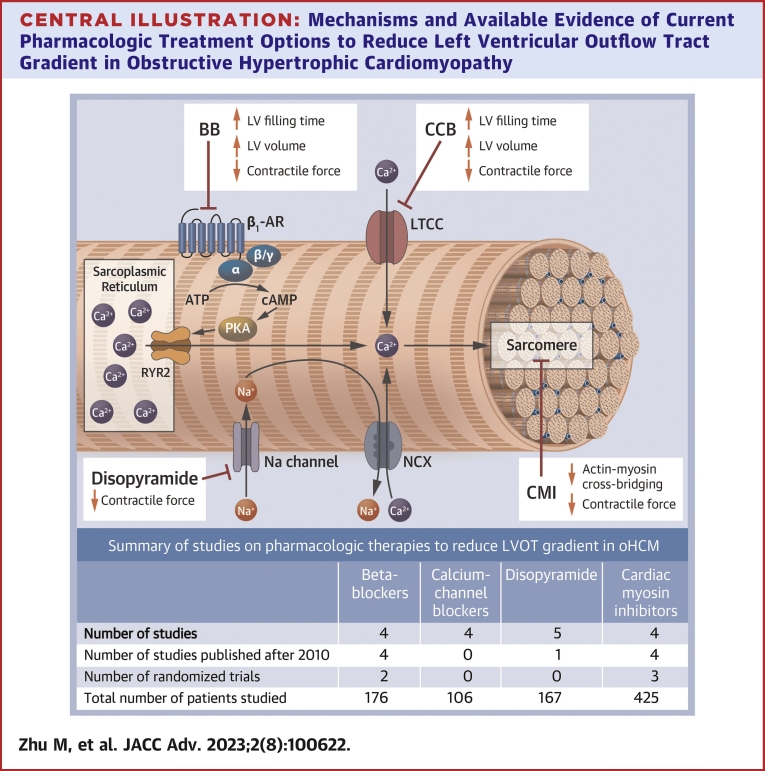
Central Illustration.
Mechanisms and Available Evidence of Current Pharmacologic Treatment Options to Reduce Left Ventricular Outflow Tract Gradient in Obstructive Hypertrophic Cardiomyopathy
Beta-blockers and calcium-channel blockers reduce left ventricular contractile force while increasing left ventricular filling time and volume via beta 1 receptor and L-type calcium channel modulation, respectively. Disopyramide exerts a negative inotropic effect by modulating sodium channels and intracellular calcium. Cardiac myosin inhibitors directly decrease myosin-actin interactions with a negative inotropic and lusitropic effect. ATP = adenosine triphosphate; BB = beta-blockers; CA = calcium; cAMP = cyclic adenosine monophosphate; CCB = calcium-channel blocker; CMI = cardiac myosin inhibitors; LTCC = L-type calcium channel; LV = left ventricular; NCX = Na(+)/Ca(2+) exchanger; PKA = protein kinase; RYR2 = cardiac ryanodine receptor 2.
Table 1.
Summary of the Current Evidence Base for the Effectiveness of Pharmacologic HCM Therapies
| LVOT Gradient Reduction |
Diastolic Dysfunction |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| BB | CCB | Disopyramide | Myosin Inhibitors | BB | CCB | BB Vs CCB | Disopyramide | Myosin Inhibitors | |
| No. of studies | 4 | 4 | 5 | 4 | 4 | 6 | 4 | 5 | 3 |
| No. of randomized trials | 2 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 3 |
| No. of studies published after 2010 | 4 | 0 | 1 | 4 | 3 | 0 | 0 | 1 | 3 |
| Total number of patients studied | 176 | 106 | 167 | 425 | 99 | 130 | 134 | 115 | 408 |
BB = beta-blocker; CCB = calcium-channel blocker; HCM = hypertrophic cardiomyopathy; LVOT = left ventricular outflow tract.
Brief summary of the pathomechanism of outflow obstruction and diastolic dysfunction in HCM
The multifactorial nature of underlying mechanisms for LV hypertrophy, diastolic dysfunction, and LVOT obstruction in HCM poses significant management challenges.1 Sarcomeric dysfunction, secondary to missense gene mutations, appears to be the incepting mechanism.2,3 These genetic alterations lead to increased calcium sensitivity and an increase in myosin ATPase activity. The result is an abundance of myosin heads shifting from the energy-efficient super-relaxed state to the energy-consumptive active state.4, 5, 6, 7 Excess myosin ATPase activation causes an increase in myosin-actin cross-bridging, hypercontractility, and abnormal relaxation,8, 9, 10, 11 as well as the pathognomonic hypertrophic changes in HCM.12,13 In addition, myocardial ischemia due to insufficient and abnormal microvascular blood flow not only causes anginal symptoms but also leads to ischemic injury, myocardial remodeling with myocyte disarray, inflammation, and fibrosis.8,10,11 The increase in cardiac workload from outflow tract obstruction and elevated LV filling pressure renders the heart unable to maintain normal left atrial pressure13 and cardiac output, resulting in heart failure symptoms due to diastolic dysfunction and angina.
Pharmacologic therapies to relieve outflow obstruction
Studies investigating improvements in outflow obstruction are listed in Supplemental Table 1.
Beta-blockers
BBs were first developed in the 1960s, mainly as blood pressure-reducing agents14 and are broadly categorized into nonselective (with affinity to both beta-1 and beta-2 receptors) and selective beta receptor blockers (with no or minimal beta-2 receptor affinity). Commonly used examples are propranolol, labetalol, and carvedilol; and metoprolol, bisoprolol, atenolol, and nebivolol, respectively. Some BBs have more pronounced vasodilating properties such as carvedilol, labetalol, or nebivolol, and while they may be more effective in the treatment of hypertension, they can (at least in theory) worsen LVOT gradients due to afterload reduction.15,16 Generally, BBs’ beneficial effect in oHCM stems from a decrease in contractility (negative inotropic effect) and slowing of the heart rate, thereby increasing LV filling time and presystolic cavity size (Figure 1).
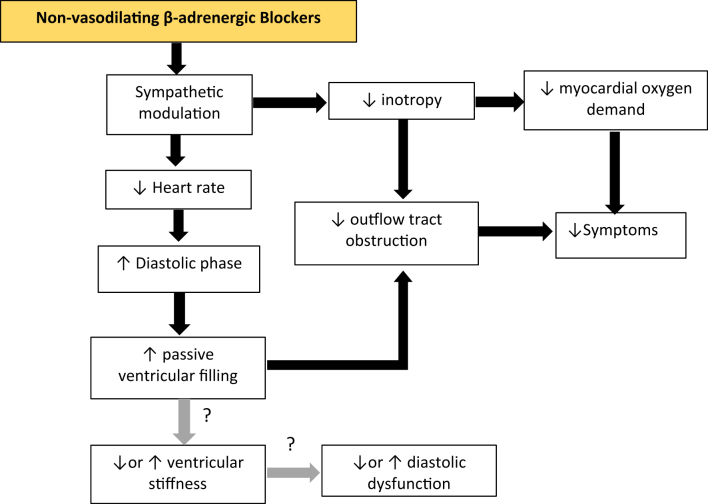
Figure 1.
Pharmacologic Effects of Nonvasodilating Beta-Adrenergic Blockers on the Pathomechanisms of Obstructive Hypertrophic Cardiomyopathy
This drug class modulates the sympathetic tone leading to the following effects: 1) decrease in heart rate that causes prolongation of the diastolic phase, ultimately mitigating the obstructive effects on the LVOT; and 2) decrease in inotropy causing a decrease in myocardial oxygen demand and LVOT obstruction, both ultimately leading to the decrease of patient-felt symptoms. LVOT = left ventricular outflow tract.
Although nonvasodilating BB—specifically metoprolol—are currently recommended as first-line treatment in oHCM, the evidence for its effects on LVOT obstruction has been limited to small studies and anecdotal experience.17
In a nonrandomized study of 27 oHCM patients with a postexercise LVOT gradient >50 mm Hg, longer-term nadolol (40-80 mg/day) or bisoprolol (5-10 mg/day) decreased postexercise gradient from 87 ± 29 mm Hg to 36 ± 22 mm Hg (P < 0.001), with 14 patients (52%) achieving a nonobstructive range (LVOT gradient of <30 mm Hg), 9 patients (33%) with a reduction of ≥20 mm Hg but residual gradient ≥30 mm Hg, and 4 patients with minimal gradient reduction.18 Six patients (22%) did not respond to therapy with persistent severe postexercise obstruction (range 58-80 mm Hg). Of note, these nonresponders all shared similar characteristics of a high body mass index, which could have caused a relative underdosing of BB therapy.
The first randomized placebo-controlled trial of metoprolol vs placebo enrolled 29 patients with oHCM with 2 consecutive 2-week treatment periods in a cross-over design. Dybro et al17 showed that compared with those assigned to placebo, patients randomized to metoprolol (up-titrated to a maximum daily tolerated dose of 150 mg) had a significantly lower LVOT gradient at rest (25 mm Hg [IQR: 15 to 58 mm Hg] vs 72 mm Hg [IQR: 28-87 mm Hg]; P = 0.007), at peak exercise (28 mm Hg [IQR: 18-40 mm Hg] vs 62 mm Hg [IQR: 31-113 mm Hg]; P < 0.001), and postexercise (45 mm Hg [IQR: 24-100 mm Hg] vs 115 mm Hg [IQR: 55-171 mm Hg]; P < 0.0001). Overall, there was an improvement in NYHA dyspnea class; however, 90% of patients on metoprolol reported residual exertional symptoms (ie, Class II or greater), and the patient-reported Kansas City Cardiomyopathy Questionnaire score improved only marginally. Furthermore, peak VO2 during cardiopulmonary exercise testing did not improve, and neither did pulmonary capillary wedge pressure from invasive assessment (see description of therapies to improve diastolic function for a possible explanation). These findings suggest that although metoprolol decreases LVOT obstruction, the effect on wall stress and filling pressures is lacking, and symptom improvement is limited.
In a third trial by Monda et al, 92 oHCM patients with LVOT gradient ≥50 mm Hg were assigned increasing doses of bisoprolol until the primary endpoint of LVOT gradient <30 mm Hg and ≥1 NYHA class improvement (or the maximally tolerated dose) was achieved.19 Bisoprolol decreased the LVOT gradient to <30 mm Hg in 33 (36%) patients and to <50 mm Hg in 57 (62%) patients. However, 35 (38%) patients were considered nonresponders. There were no significant differences in clinical or echocardiographic characteristics between responders and nonresponders except for a higher number of patients with NYHA functional class III in nonresponders (n = 10 [29%] vs n = 7 [12%], P = 0.051).19
Findings from these small studies established the basis of current guideline recommendations (Dybro’s study is not included). All studies demonstrated significant variability in LVOT gradient reduction, limited symptom improvement, and a significant proportion of nonresponders.17 Data from these studies underscore the need for larger randomized trials and alternatively, more effective pharmaceutical options, especially for nonresponders. Side effects and their unknown direct effects on the underlying pathogenesis or disease trajectory pose additional problems for the use of this medication class in oHCM.
Calcium-channel blockers
CCBs can be categorized into dihydropyridine CCB, which exert a predominant effect on vasomotor tone (eg, amlodipine, nifedipine, nicardipine), and nondihydropyridine CCB (eg, verapamil, diltiazem), which predominantly affect intracellular myocyte calcium, exerting a negative inotropic effect, as well as atrioventricular conduction slowing.20 While the effects of the latter group appear to be comparable to BBs, they do differ in their mechanism, their effect on peripheral afterload, and other smooth muscle cells (eg, gastrointestinal tract) (Figure 2). These differences raise concern for a potential deleterious effect on LVOT obstruction (from vasodilation) and their side effect profile (constipation).
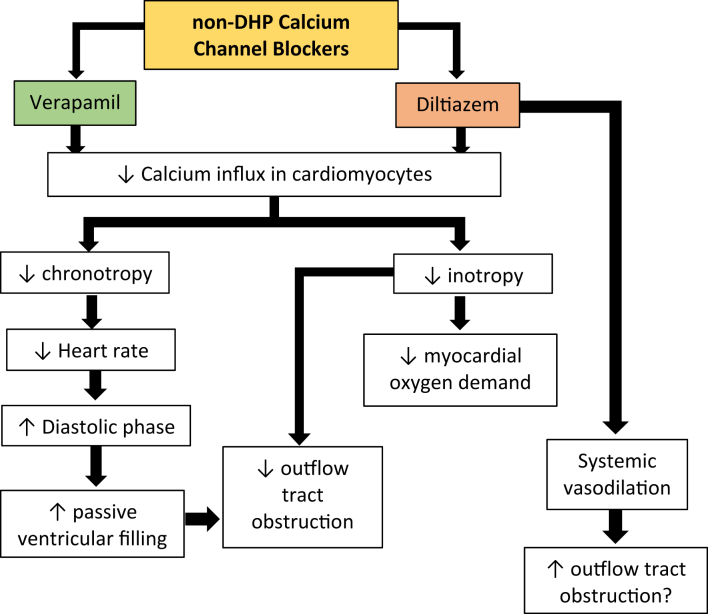
Figure 2.
Pharmacologic Effects of Nondihydropyridine Calcium-Channel Blockers on the Pathomechanisms of Obstructive Hypertrophic Cardiomyopathy
This drug class blocks the calcium influx into the SA and AV nodes resulting to negative effects on: 1) chronotropy leading a decrease in heart rate that causes prolongation of the diastolic phase, ultimately mitigating the obstructive effects on the LVOT; and 2) inotropy causing a decrease in myocardial oxygen demand and LVOT obstruction, both ultimately leading to the decrease of patient-felt symptoms. However, diltiazem is known to have a systemic vasodilating effect that may lead to a paradoxical increase in LVOT obstruction. AV = atrioventricular; LVOT = left ventricular outflow tract; SA = sinoatrial.
In a small study of 13 HCM patients, IV verapamil followed by an invasive assessment of its hemodynamic effects showed a significant LVOT gradient reduction in 10 of the 13 patients, with 2 of the other 3 patients who did not experience a gradient reduction reporting better exercise tolerance during subsequent treadmill exercise tests. Limited by the small sample size, they could not detect significant differences in clinical characteristics between responders and nonresponders. There was also no significant correlation between improvement in exercise capacity with resting (r = 0.27) or provoked (r = 0.50) gradients following oral verapamil. These data suggest that verapamil may affect exertional symptoms not only by improving LVOT gradients but also by other mechanisms (see diastolic function section below).21
Anderson et al22 examined the effect of IV and chronic oral verapamil on LVOT obstruction in 15 patients. With IV verapamil, 6 of the 15 patients experienced a decrease in LVOT gradient, while the other 9 either had an increase or no change in gradient; the entire group had on average no significant change in mean resting or provoked LVOT gradient. With orally administered verapamil, 5 of the 11 patients experienced a decrease in LVOT gradient, while the other 6 had no significant change. Like IV verapamil, chronic oral verapamil did not reduce the overall mean resting or provoke an LVOT gradient, nor was it associated with an improvement in symptoms in this small study.
The largest study of IV verapamil by Rosing et al found that among 62 oHCM patients, mean resting LVOT gradient decreased from 63 ± 34 mm Hg to 29 ± 34 mm Hg (P < 0.05). They also reported an improvement in diastolic function and exercise tolerance in a subgroup of patients, and this benefit may have been greater in patients receiving verapamil vs propranolol. A larger observational portion of this study reported some complications, including 9 deaths, although it is unclear if any of these were related to the treatment with verapamil. Clearly, interpretation of this study is difficult given the observational design.23
Betocchi et al24 studied IV diltiazem (0.25 mg/kg for 2 minutes followed by 0.014 mg/kg/min for 10 minutes) in 16 oHCM patients. Diltiazem increased the outflow gradient in 7 of the 11 patients (range +4 to 68 mm Hg) and decreased the gradient in 4 (range −7 to 10 mm Hg). Overall mean LVOT gradient remained the same, while cardiac index (likely due to a lower systemic vascular resistance) and pulmonary wedge pressure increased. It is unclear how these acute effects translate into chronic diltiazem use. This study may, however, explain differential responses to diltiazem in clinical practice but also raise concern for the potential of worsening obstruction.24
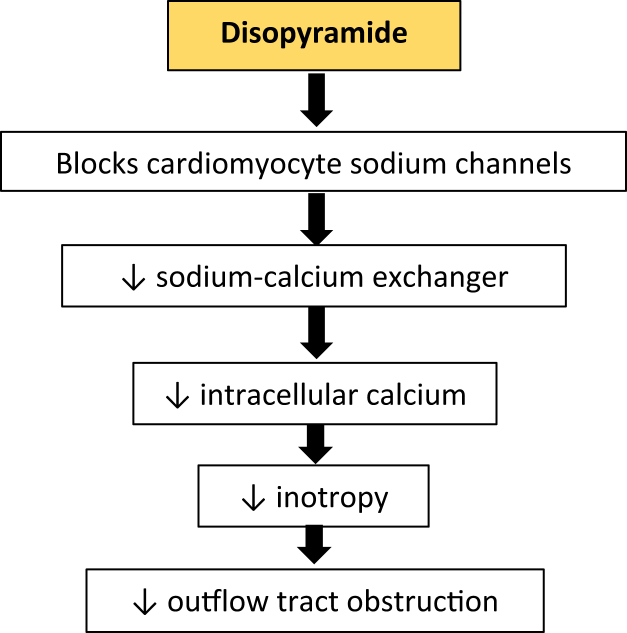
Disopyramide
Disopyramide is a type 1a antiarrhythmic, a sodium channel blocker that lengthens action potential duration and lowers the rate of diastolic depolarization, thus decreasing myocardial excitability (Figure 3). The negative inotropic property, which decreases LVOT obstruction, was a clinical afterthought long after its initial studies and approval in France in 1969 and in the United States in 1982, but it forms the basis for its use in oHCM.25
Figure 3.
Pharmacologic Effects of Disopyramide on the Pathomechanism of Obstructive Hypertrophic Cardiomyopathy
This drug decreases LVOT obstruction by mitigating the effects of energy-wasting hypercontractility in HCM by blocking the sodium channels on cardiomyocytes, leading to a decrease in sodium-calcium exchanger activity, causing a fall in intracardiomyocyte calcium levels. HCM = hypertrophic cardiomyopathy; LVOT = left ventricular outflow tract.
Dr Charles Pollick26 led the groundbreaking studies of intravenous and oral disopyramide in 5 oHCM patients with invasive hemodynamic assessment. Following 10 minutes of intravenous disopyramide (100 mg) administration, the baseline LVOT gradient was virtually abolished in all patients, with minimal effects on cardiac output. The LV end-diastolic pressure was unchanged in 2 patients and reduced in 2 others. Oral disopyramide improved symptom-limited exercise duration.26 In a follow-up study of 43 oHCM patients, Pollick et al27 confirmed the beneficial effects of disopyramide: resting gradient was reduced by a mean of 61 mm Hg (range 16-123 mm Hg), with 35 (78%) achieving a resting gradient of <20 mm Hg. Invasive hemodynamic assessment confirmed a decrease in pre-ejection period and ejection time, ie, a negative inotropic effect; however, cardiac output did not change, likely due to improvement in mitral regurgitation (MR).27
Kimball et al28 confirmed Pollick’s findings in 25 patients with oHCM, in whom baseline resting LVOT gradient was reduced from 86 ± 34 mm Hg to 27 ± 20 mm Hg (P < 0.001). Electrocardiographic changes from disopyramide were minimal, along with a slight increase in heart rate.28 Similarly, Sherrid et al29 evaluated the effects of oral disopyramide in 7 oHCM patients. An initial single dose reduced the average peak gradient from 64 to 14 mm Hg (P < 0.0001) with sustained reductions during the 23-day treatment period. After disopyramide washout, gradients returned to their pretreatment values (79 mm Hg). Finally, after rechallenging patients with disopyramide, the gradient once again declined, to an average of 30 mm Hg (P < 0.001). The investigators also showed that higher disopyramide serum levels correlated with lower outflow tract gradients (r = −0.77, P < 0.0001).29
In the largest observational study of disopyramide to date, Sherrid et al30 compared 118 patients with oHCM who were treated with disopyramide (mean dose 432 ± 181 mg/day) for a mean of 3.1 ± 2.6 years in 4 HCM centers with 373 oHCM control patients; 97% received a beta-blocker as background therapy. In patients who were treated with disopyramide, resting LVOT gradient decreased from 75 ± 33 mm Hg to 40 ± 32 mm Hg (P < 0.0001), and 66% did not undergo septal reduction therapy. In the 34% who ended up with septal reduction therapy, resting LVOT gradient decreased only from 73 ± 35 mm Hg to 63 ± 31 mm Hg (P = 0.05).30 There was a statistically nonsignificant trend towards lower mortality and sudden cardiac death in disopyramide-treated patients. This real-world study suggests that a significant portion of patients appear to derive benefit from disopyramide, but with some variability in resolution of LVOT obstruction. The observational nature of this study, however, limits its validity and interpretation.
Aside from disopyramide, other sodium channel blockers with similar properties have been tested in oHCM. Kajimato et al compared the decrease in invasively measured LV pressure gradient at rest in oHCM patients after administration of a variety of sodium channel blockers (disopyramide, cibenzoline, pilsicainide), verapamil, and propranolol. In group A (n = 12, average baseline resting LVOT gradient 90 ± 24 mm Hg), LVOT gradient was reduced by 7.7% ± 9.9% with verapamil, 19.0% ± 20.2% with propranolol, and 58.6% ± 15.0% with disopyramide, suggesting that disopyramide was more effective than either verapamil or propranolol. In group B (n = 12, average baseline resting LVOT gradient 98 ± 34 mm Hg), gradients were reduced by 55.3% ± 26.6% with disopyramide, 55.3% ± 20.6% with cibenzoline, and 54.7% ± 15.4% with pilsicainide, suggesting an equivalent effect from these 3 agents.31
Cardiac myosin inhibitors
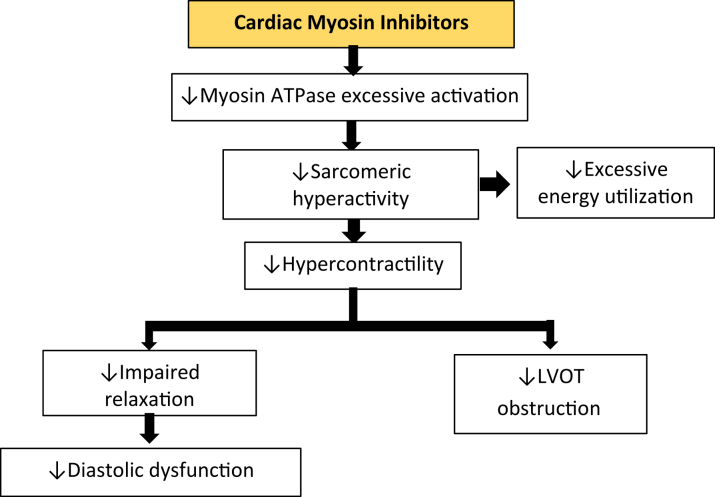
Cardiac myosin inhibitors—Mavacamten and Aficamten—are a new drug class, targeting one underlying cause for the development of hypertrophy, inefficient cellular energetics, and LVOT obstruction: excessive actin-myosin cross-bridging leading to hypercontractility. Reduction of actin-myosin interactions decreases contractile force and thereby LVOT gradient, but also improves cellular oxygen demand and myocardial relaxation (Figure 4).
Figure 4.
Pharmacologic Effects of Cardiac Myosin Inhibitors on the Pathomechanism of Obstructive Hypertrophic Cardiomyopathy
This novel drug class selectively and reversibly binds the allosteric binding site of the cardiac myosin ATPase, leading to a decrease in excessive myosin ATPase activity, which causes pathologic hypercontractility in HCM. Hence, in a downstream of events, causes a decrease in LVOT obstruction as well as diastolic dysfunction by mitigating the impaired LV relaxation in HCM. HCM = hypertrophic cardiomyopathy; LV = left ventricle; LVOT = left ventricular outflow tract.
Mavacamten was first assessed in the PIONEER-HCM, a phase 2 open label study including 21 patients with oHCM assigned to 2 dosing levels (cohorts A and B). Primary efficacy outcome was reduction of LVOT gradient at 12 weeks. In cohort A, the resting gradient was decreased by 48 mm Hg (95% CI: −72 to −23 mm Hg, P = 0.006) and Valsalva-provoked gradient was decreased by 85 mm Hg (95% CI: −114 to −56 mm Hg, P = 0.002). In cohort B, in which a lower dosing regimen compared to cohort A was assigned, smaller reductions in LVOT gradient were seen: −49 mm Hg reduction in resting gradient (95% CI: −83 to −14 mm Hg, P = 0.004) and −47 mm Hg in Valsalva-provoked gradient (95% CI: −82 to −12 mm Hg, P = 0.002).32 In the PIONEER-OLE (open label extension) study, those reductions in LVOT gradient were sustained up until 48 weeks.33
The pivotal EXPLORER-HCM multicenter, double-blind, placebo-controlled, randomized, phase 3 study enrolled 251 patients with obstructive symptomatic HCM randomizing 123 patients to mavacamten and 128 to placebo. EXPLORER-HCM was a landmark trial—the largest adequately powered randomized trial conducted in patients with oHCM to date. Although the primary endpoint consisted of improvement in functional class and/or exercise capacity during cardiopulmonary exercise testing, the secondary outcome of postexercise LVOT gradient was 4-fold lower at 30 weeks compared to placebo with a group difference of −36 mm Hg (95% CI: −43 to −28 mm Hg; P < 0.0001).34 Preliminary data from the EXPLORER LTE, an ongoing 5-year active treatment extension study, demonstrated durable reductions in resting and Valsalva LVOT gradient, as well as improvements in functional class and NT-proBNP levels.35
A third clinical trial of Mavacamten in oHCM—VALOR-HCM—was a double-blind, multicenter, placebo-controlled, randomized study evaluating the eligibility for septal reduction therapy at 16 weeks. Along with a marked difference between randomization arms in SRT eligibility (only 18% of patients assigned to mavacamten met SRT eligibility criteria), there was also a marked improvement in postexercise LVOT gradient among the 112 patients with oHCM randomized to mavacamten compared to those randomized to placebo [−37 mm Hg vs −1.8 mm Hg (P < 0.001)].36
Aficamten is a next-in-class cardiac myosin inhibitor that was evaluated for its efficacy and safety by the REDWOOD-HCM (NCT04219826) and REDWOOD-HCM open label extension, now FOREST-HCM (NCT04848506) clinical trials.37 REDWOOD-HCM enrolled symptomatic oHCM patients in 2 cohorts. In cohort 1, a lower dose (5 to 15 mg) lowered resting LVOT gradients from 54 ± 25 mm Hg to 13 ± 4 mm Hg, and Valsalva LVOT gradients decreased from 74 ± 25 mm Hg to 38 ± 14 mm Hg at 10 weeks (P < 0.0001 for both). In cohort 2, patients received a higher dose range (10 to 30 mg), resulting in an even greater reduction in resting and Valsalva-provoked gradients (58 ± 36 mm Hg to 15 ± 22 mm Hg and 82 ± 37 mm Hg to 30 ± 30 mm Hg, respectively, at 10 weeks). As expected, after the 2-week wash-out period, both treatment cohorts demonstrated an increase in both the resting and Valsalva LVOT gradients near baseline measurements.38 An interim analysis of the FOREST-HCM extension study showed sustained reductions in resting (−33 mm Hg, P = 0.0003) and Valsalva LVOT gradient (−43 mm Hg, P < 0.0001 at 24 weeks).37
These and ongoing pivotal and extension studies will further assess long-term efficacy and safety of cardiac myosin inhibitors. To date, only mavacamten is commercially available in the United States. This new medication class appears to be not only highly effective in reducing LVOT gradient and improving symptoms but also safe with a small portion of patients, however, being at risk for reversible systolic heart failure, which currently requires close clinical and echocardiographic monitoring of treated patients.
Pharmacotherapies to improve diastolic function in HCM
Many studies evaluating medication effects on diastolic function in HCM (Supplemental Table 2) are confounded by the presence of outflow obstruction. Although a systolic rather than diastolic phenomenon, outflow obstruction undoubtedly affects intracardiac pressure throughout the cardiac cycle, and therefore separating the effects on obstruction vs diastology can be challenging.39
Beta-blockers
It is common practice to prescribe BB in patients with diastolic dysfunction, both in patients with HCM and in other forms of cardiomyopathies, including patients with heart failure with preserved ejection fraction. The hypothesis behind this strategy is to allow for prolonged filling time of the left ventricle from a reduced heart rate to overcome abnormal myocardial relaxation and improve filling pressure—a mantra that unfortunately is not grounded in convincing evidence.
Studies on diastolic filling pressures were conducted as early as the 1970s; one such study by Hubner et al40 demonstrated a potential benefit of high-dose propranolol (320 mg/day) or practolol (800 mg/day) compared to placebo in 16 patients with HCM over the course of 4 weeks. A reduced ‘A’ wave of an apex cardiogram as well as reduced isovolumic relaxation time suggested improved LV relaxation and compliance with propranolol and practolol. Another explanation for a shortening of the isovolumic relaxation time, however, could also stem from an increase in left atrial pressure (more rapid initiation of transmitral flow). Furthermore, the methodology of diastolic function assessment in this study is anything but contemporary, and thus this study leaves many questions unanswered.40
Speiser et al41 could not show improved LV diastolic distensibility, LV chamber stiffness, or the pressure intercept measured by biplane LV cineangiography after 15 minutes of IV propranolol administration. These results also indirectly confirm the notion that beta agonists (ie, dobutamine) improve diastolic filling and reduce LV wall stress.42,43
In the aforementioned randomized, double-blind, placebo-controlled crossover trial by Dybro et al,44 metoprolol treatment led to a reduced heart rate at rest (57 ± 11 beats/min vs 77 ± 12 beats/min, P < 0.0001) and during peak exercise (107 ± 9 beats/min vs 139 ± 23 beats/min, P < 0.0001) among the 29 enrolled symptomatic oHCM patients. Such pulse reduction clearly increases end-diastolic volume and possibly stroke volume, but not necessarily cardiac output. In addition, improvement of MR from a reduction of systolic anterior motion of the mitral valve could also contribute to improved cardiac forward output. Therefore, it came as a surprise that treatment with metoprolol did not improve E/e’ nor invasive measurement of pulmonary capillary wedge pressure, with the latter showing a detrimental trend after metoprolol treatment (15 ± 6 mm Hg vs 13 ± 5 mm Hg, P = 0.06)—puzzling given documented improvements of LVOT gradient and MR. In addition, peak VO2 did not improve with metoprolol, nor did left atrial volume, which may not be unexpected after such a short treatment period. Taken together, there is concern that BB—specifically metoprolol in this study—despite their positive effects on LVOT gradient and MR, do not lower filling pressures, which suggests worsening of diastolic function or increased filling pressure from chronotropic incompetence.
As mentioned, E/e’ ratio is a well-established noninvasive measure of diastolic function and LV filling pressure. In a separate randomized, crossover trial of 52 patients with HFpEF, Palau et al45 demonstrated that withdrawal of BB, predominantly bisoprolol (n = 46, 88.5%), improved peak VO2 on cardiopulmonary exercise testing by 2.1 ± 1.29 mL/kg/min (P < 0.001). Echocardiography results point to a decreased septal E/e’ after BB withdrawal, suggesting that the improved functional capacity may have been related to improved diastolic function or a reduction in chronotropic incompetence. Although this study interrogates the effects of BB withdrawal on functional capacity and diastolic function in patients with HFpEF (not HCM), results could possibly be extrapolated to patients with diastolic dysfunction related to HCM, especially those without obstruction. These results may also explain the absence of a positive effect of metoprolol on functional capacity during cardiopulmonary exercise testing seen in the study by Dybro et al.44 Those findings also agree with Efthimiadis et al,46 who showed that chronotropic incompetence was present in 50% of patients and correlated independently with worse peak VO2 and BB therapy in patients with HCM (both obstructive and nonobstructive subgroups). A heart rate reserve of <62 beats/min predicted peak VO2 <80%.
In summary, evidence from small and some noncontemporary studies leave many questions unanswered about the effects of BBs on diastolic function in patients with HCM. The most rigorous recent studies suggest that they may be detrimental with potential for worsening diastolic function in HCM. This effect along with the potential for creating chronotropic incompetence must be weighed against positive effects on LVOT obstruction. In patients with nonobstructive HCM with a “heart failure with preserved ejection fraction” phenotype, BBs should be used with caution, if at all, and as described below, CCBs may be preferable.45
Non-dihydropyridine calcium-channel blockers
The effects of IV and chronic oral administration of verapamil on diastolic function have been tested in multiple (mostly noncontemporary) studies.
The effect of IV verapamil on diastolic function was further examined by several studies: First, Rosing et al administered intravenous verapamil in 62 patients with oHCM, and although it significantly decreased systolic blood pressure from 118 ± 17 mm Hg to 102 ± 17 mm Hg (P < 0.001), they found no significant effect on heart rate, LV end-diastolic pressure, or cardiac output.23 The LV outflow gradient significantly decreased from 62 ± 34 mm Hg to 29 ± 34 mm Hg (P < 0.05), and although overall LV end-diastolic pressure did not change, in 10 of 12 patients with elevated baseline pressure, LV end-diastolic pressure indeed decreased, suggesting a possible benefit in patients with evident diastolic dysfunction.
Second, Hanrath et al measured the effect of IV verapamil on LV relaxation and filling in 11 patients with oHCM or nHCM. A prolonged baseline isovolumic relaxation time significantly decreased from 93 ± 10 ms to 67 ± 15 ms (P < 0.001). The peak rate of posterior wall thinning increased from 64 ± 30 mm/s to 89 ± 38 mm/s (P < 0.001), LV dimension during LV filling period increased from 14.4 ± 2.4 mm to 16.4 ± 2.4 mm (P < 0.01), and duration of the LV relative filling period increased from 47.2% ± 4.6% to 49% ± 5.3% (P < 0.01), all indicating an improvement in LV filling properties.47
Third, in a study of acute IV verapamil in 16 patients with oHCM and nHCM by Tendera et al, LV end-diastolic pressure decreased from 20 ± 6 mm Hg to 17 ± 5 mm Hg (P < 0.001), while LV end-diastolic volume index increased from 82 ± 22 mL/m2 to 91 ± 23 mL/m2 (P < 0.01).48 In 13 (81%) of the 16 patients, IV verapamil led to an improvement of the LV end-diastolic pressure-volume relationship, pointing again to an improvement in diastolic filling.
In 1984, Anderson et al22 enrolled 15 oHCM patients with severe symptoms despite beta blockade. Initially, all 15 patients were given either a 10 mg bolus or received oral verapamil over a 5-day period with subsequent exercise testing according to the protocol previously described by Rosing et al.21 Subsequently, 11 of 15 patients received oral verapamil for 6 months (average 690 mg/day) and were available for re-evaluation. After acute verapamil, there was no significant change in end-diastolic volume or end-diastolic pressure. However, after chronic treatment, there was a significant increase in end-diastolic volume from 86 ± 18 mL to 110 ± 13 mL (P < 0.02) and stroke volume index from 69 ± 19 mL/m2 to 81 ± 11 mL/m2 (P = 0.02) with no significant change in mean LV end-diastolic pressure. All but 2 patients reported improvements in symptoms, which may have been from improved diastolic function.
In contrast, TenCate et al were unable to demonstrate an improvement in isovolumic relaxation time, peak rate of LV lengthening, and rapid ventricular filling duration from IV verapamil, while LV systolic pressure and contractility were reduced.49
Diltiazem, another nondihydropyramide CCB, was studied in 16 HCM patients during atrial pacing at baseline and after IV diltiazem.24 The time constant for isovolumic relaxation during diltiazem administration decreased from 74 ± 40 ms to 59 ± 38 ms (P = 0.045), and the peak filling rate increased from 4.1 ± 1.3 to 6.0 ± 2.4 stroke counts/s (P = 0.004). Together, these demonstrate an improvement in active LV diastolic function, although contributions of changes in left atrial pressure were not evaluated.
Beta-blockers vs calcium-channel blockers
Several head-to-head comparisons between BB and CCBs have been done and although most are also noncontemporary, their results suggest an advantage of CCBs regarding effects on diastolic function.
In 1981, Bonow et al treated 40 patients with oral verapamil (320-480 mg/day), 16 of whom were also treated sequentially with propranolol (80-960 mg/day).50 Following verapamil administration, peak filling rate increased and time to peak filling rate decreased. In contrast, propranolol did not significantly change either parameter. The comparison of these 2 treatment options showed a greater increase in peak filling rate and a shorter time to peak filling rate following verapamil compared to propranolol, strongly suggesting superior effects on diastolic function from verapamil compared to propranolol.
Another study by Hess et al reinforces this notion, 15 patients with either oHCM or nHCM, LV diastolic stiffness was studied before and 15 minutes after administration of propranolol (n = 9) and verapamil (n = 5).51 LV chamber and myocardial stiffness were not significantly changed after propranolol or verapamil. However, the time constant of LV pressure decay significantly worsened following propranolol from 45 to 66 ms (P < 0.05) and improved following verapamil from 53 to 43 ms (P < 0.05). In addition, mean LV diastolic filling rate worsened with propranolol, and the LV midwall lengthening rate improved with verapamil (P < 0.05). These data point towards an advantage of CCBs over BBs in the treatment of diastolic dysfunction.
The effects of acute IV diltiazem (10 mg), chronic oral diltiazem (180 mg/day), and chronic oral propranolol (60-120 mg/day) on LV diastolic function for 2 weeks were investigated by Suwa et al in 13 HCM patients.52 IV and oral diltiazem reduced isovolumic relaxation time from 114 ± 26 ms to 99 ± 21 ms (P < 0.01) and from 105 ± 26 ms to 77 ± 23 ms (P < 0.01), respectively. IV and oral diltiazem also decreased the time to peak rate of LV dimensional lengthening similarly without changes in fractional shortening. On the other hand, propranolol caused no remarkable changes in these parameters. These findings point to diltiazem as a superior treatment for improving LV relaxation and diastolic filling in patients with HCM.
Doiuchi et al compared the effects of CCBs (verapamil 120 mg/day [n = 5] and diltiazem 90 to 180 mg/day [n = 21]) with those of BBs (propranolol 30-60 mg/day [n = 32]) on diastolic function. The IIA-O time and mean MVO-O time, both measures of diastolic function, were significantly decreased (ie, improved) from 235 ± 77 ms to 205 ± 39 ms (P < 0.01) and 133 ± 66 ms to 100 ± 34 ms (P < 0.01), respectively, in CCBs but not propranolol. Both CCBs and BBs significantly decreased the mean A-wave ratio from 18.0% ± 9.8% to 13.7% ± 7.3% (P < 0.01) and from 21.7% ± 12.6% to 17.2% ± 8.0% (P < 0.01), respectively. However, in 5 of 7 patients with obstructive HCM, the A-wave ratio increased following CCB administration, which may suggest increased left atrial pressure. The authors argue that in this obstructive subgroup, CCBs should be used with caution and that BBs may be preferable, which may be an oversimplification.53
Disopyramide
In current guidelines, disopyramide is recommended for oHCM patients with residual obstruction and symptoms despite BB or CCB use.54 Limited data on the effects of disopyramide on diastolic function in patients with HCM suggest minimal improvements that may only be related to afterload reduction.55,56
In 1 study, Sumimoto et al56 studied the effect of disopyramide on the LV diastolic function compared to diltiazem. Here, 10 patients with nHCM were given either disopyramide 100 mg or diltiazem 30 mg. Diastolic function was assessed by using Doppler echocardiography 3 hours after administration. Results reveal that disopyramide significantly increased both early passive mitral filling velocity (E peak velocity went from 43.8 ± 15.0 cm/s to 51.3 ± 16.1 cm/s, P < 0.01) while decreasing the active atrial filling velocity, E/A ratio was therefore increased (E/A went from 0.71 ± 0.2 to 1.00 ± 0.24, P < 0.01). Similar changes were observed with diltiazem. Interpretation of this study is difficult, however, as an increase in E-wave velocity could indicate an improvement in LV relaxation, but it could also indicate an increase in left atrial filling pressure. Tissue velocities (ie, E/e’) were not evaluated to better understand these results.
Matsubara et al studied 13 patients with HCM (6 had oHCM, the rest had nHCM). The participants were given either 50 or 100 mg of disopyramide intravenously at a rate of 10 mg/min after at least a 48-hour washout of all negative inotropic medications. LV function was assessed with ventriculography and invasive hemodynamic measurements before and after disopyramide infusion. Several indices suggesting improvement in diastolic function were found: 1) the time constant of LV pressure decay (tau) shortened from 56 ± 10 ms to 44 ± 8 ms (P < 0.01); 2) the constant of LV chamber stiffness (kc) decreased from 0.049 ± 0.017 m2/mL to 0.038 ± 0.014 m2/mL (P < 0.01); however, the latter only in patients with outflow obstruction; 3) shortening in tau correlated best with decrease in LV systolic pressure (r = 0.84, P < 0.01). In contrast, tau was prolonged from 52 ± 10 ms to 64 ± 11 ms (P < 0.01), and kc was unchanged in patients without outflow obstruction. These findings suggest that improvement in diastolic function was mostly related to the reduction in the afterload (ie, obstruction) and suggest that disopyramide does not improve diastolic function in nHCM.57
Fifer et al58 also evaluated the effects of disopyramide on LV diastolic function in HCM. They recruited 10 HCM patients (6 with oHCM, 1 with mid-cavitary gradient, and 3 with nHCM). Right and left cardiac catheterizations were performed after other concomitant cardiac medications were stopped. Baseline measurements at steady state were followed by disopyramide administration with repeat measurements 5 minutes after completing drug administration. Diastolic properties (peak rate of decrease in the LV pressure, time constant of relaxation by derivative method, and logarithmic method) did not improve in HCM patients without obstruction.58 On the contrary, Pollick et al27 demonstrated that disopyramide significantly decreased LV end-diastolic pressure from 19 mm Hg to 16 mm Hg in the setting of significant LVOT gradient reductions in oHCM. Both attributed the discrepancies in their results to the methods employed by each investigator.27,58
Coppini et al studied the effects of disopyramide (mean dose of 497 mg/day) in 39 symptomatic oHCM patients using more contemporary methods to assess diastolic function with echocardiography prior to and after 96 days of daily disopyramide use. There was no detectable change in septal E/e’ or lateral E/e’.55
In conclusion, improvements in diastolic function following disopyramide are seen in oHCM patients only and are likely related to reductions in LVOT gradients and LV pressure. At present, there is insufficient data to support the use of disopyramide to improve diastolic function in nHCM.
Ranolazine
Although the late-sodium channel inhibitor ranolazine has theoretical benefits on intracellular calcium load,59 this has not translated into a measurable benefit on diastolic function in clinical studies. The only small, randomized trial of ranolazine in 80 nHCM patients failed to demonstrate an improvement in E/e’ on echocardiography and did not improve natriuretic peptide levels after 5 months of treatment.60
Cardiac myosin atpase inhibitors
Mavacamten and aficamten were primarily studied in oHCM but also in smaller nHCM cohorts to assess their effect on diastolic function as secondary or exploratory endpoints.
MAVERICK-HCM was a phase II multicenter, dose-ranging, double-blind, randomized, placebo-controlled study to assess the safety and tolerability of mavacamten in patients with nHCM. The patients were placed in 2 different dosing groups at week 6 based on drug concentrations. Group 1 (n = 19) target serum drug concentrations were approximately 200 mg/ml, group 2 (n = 21) target drug concentrations were approximately 500 mg/ml, and group 3 (n = 19) received placebo. E/e’ ratio in the pooled groups 1 and 2 was compared with that of the placebo arm at 16 weeks. Although there were reductions in NTproBNP values, changes in E/e’ and left atrial size were similar between groups. This proof-of-concept study, however, was limited by a small sample size.61
In oHCM patients, EXPLORER-HCM demonstrated improvements in the ratio between early mitral inflow velocity and lateral early diastolic tissue velocity (E/e’lat) at week 30 by −3.8 (95% CI: −4.7 to −2.8, P < 0.0001) despite a small reduction in left ventricular ejection fraction. These changes demonstrate that the hypercontractility also led to improved diastolic relaxation—presumably from reduced actin-myosin interactions.62 EXPLORER-HCM also included a cardiac magnetic resonance substudy with 35 participants (17 in the mavacamten arm and 18 in the placebo arm) in which left atrial volume index, a surrogate of chronic diastolic dysfunction, decreased from 61 ml/m2 to 42 ml/m2 in the mavacamten group at the end of 30 weeks, while it increased from 57 to 59 ml/m2 in the placebo group (group difference −10.3, P = 0.0004).63
In VALOR-HCM (oHCM patients, NCT04349072), mavacamten led to an improvement of E/e’ at 32 weeks (in the first 16 weeks, no change) by −3.3 (mean, 95% CI: −4.9 to −1.8) and a decrease of left atrial volume index by −6.8 (mean, 95% CI -9.4 to −4.3) along with significant reductions in LVOT gradient, NTproBNP levels, and the primary endpoint of septal reduction therapy eligibility (Table 2).60 29.4% of patients treated with mavacamaten demonstrated improvement in diastolic dysfunction compared to 12.8% in the placebo group.64
Table 2.
Positive Effects of Mavacamten on Biomarkers, Diastolic Function and Left Ventricular Hypertrophy Observed in the VALOR-HCM Trial
| NT-proBNP | Troponin I | E/e Ratio | LV Mass Index | Left Atrial Volume Index | |
|---|---|---|---|---|---|
| Placebo vs mavacamten group (week 16-32) | −451 ng/L (95% CI: −581 to −298) | −6.8 ng/L (95% CI: −8.5 to −4.3) | −2.6 (95% CI: −4.6 to −0.7) | −10.8 g/m2 (95% CI: −16.1 to −5.5) | −5.5 ml/m2 (95% CI: −8.3 to −2.7) |
| Mavacamten group (baseline to week 32) | −417 ng/L (95% CI: −706 to −186) | −7.4 ng/L (95% CI: −11.1 to −4.8) | −3.3 (95% CI: −4.9 to −1.8) | −13.0 g/m2 (95% CI: −18.5 to −7.5) | −6.8 ml/m2 (95% CI: −9.4 to −4.3) |
LV = left ventricular.
In REDWOOD-HCM, Aficamten significantly reduced cardiac biomarkers—cTnI and NT-pro-BNP—which may be attributed to an improvement in diastolic function, but further studies are needed and underway for confirmation.38 A list of the effects of various drug classes in the forementioned studies is provided in Supplemental Table 3.
Conclusions
Symptomatic improvement is the main treatment goal in the management of patients with oHCM and nHCM. In oHCM, current guidelines make strong (Class I) recommendations to use BBs (and CCBs), recommendations that are based on small, underpowered studies with mixed results and short follow-up. Although recommendations for nHCM patients are less strong, our review suggests that BBs may worsen diastolic function and exercise capacity, while CCBs may improve diastolic function and are thus preferable. More data on the effectiveness and safety of current first-line medications used to improve cardiac outflow obstruction and diastolic function are urgently needed, especially in nHCM. Given the much stronger and evolving evidence for the effectiveness of cardiac myosin inhibitors, both for improvement of relief of obstruction and improvement of diastolic function, pending planned head-to-head comparisons with metoprolol, future guidelines will consider them as a first-line option for oHCM.
Funding support and author disclosures
Dr Willeford has received consultant fees from Bristol Myer Squibb and Cytokinetics. Dr Rader has received consultant and speaker fees from Bristol Myer Squibb, Medtronic, Recor Medical and Cytokinetics. Dr Masri has received research grants from Pfizer, Ionis, Attralus, Cytokinetics, and Ultromics; and consulting fees from Cytokinetics, BMS, Eidos, Pfizer, Ionis, Lexicon, Alnylam, Attralus, Haya, Intellia, BioMarin, and Tenaya. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Supplementary data
References
- 1.Sequeira V., Bertero E., Maack C. Energetic drain driving hypertrophic cardiomyopathy. FEBS Lett. 2019;593(13):1616–1626. doi: 10.1002/1873-3468.13496. [DOI] [PubMed] [Google Scholar]
- 2.Nishi H., Kimura A., Harada H., et al. A myosin missense mutation, not a null allele, causes familial hypertrophic cardiomyopathy. Circulation. 1995;91(12):2911–2915. doi: 10.1161/01.cir.91.12.2911. [DOI] [PubMed] [Google Scholar]
- 3.Wolf C.M. Hypertrophic cardiomyopathy: genetics and clinical perspectives. Cardiovasc Diagn Ther. 2019;9(Suppl 2):S388–S415. doi: 10.21037/cdt.2019.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alamo L., Ware J.S., Pinto A., et al. Effects of myosin variants on interacting-heads motif explain distinct hypertrophic and dilated cardiomyopathy phenotypes. Elife. 2017;6 doi: 10.7554/eLife.24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson R.L., Trivedi D.V., Sarkar S.S., et al. Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A. 2018;115(35):E8143–E8152. doi: 10.1073/pnas.1809540115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trivedi D.V., Adhikari A.S., Sarkar S.S., Ruppel K.M., Spudich J.A. Hypertrophic cardiomyopathy and the myosin mesa: viewing an old disease in a new light. Biophys Rev. 2018;10(1):27–48. doi: 10.1007/s12551-017-0274-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Velden J., Tocchetti C.G., Varricchi G., et al. Metabolic changes in hypertrophic cardiomyopathies: scientific update from the working group of myocardial function of the European Society of Cardiology. Cardiovasc Res. 2018;114(10):1273–1280. doi: 10.1093/cvr/cvy147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonne G., Carrier L., Richard P., Hainque B., Schwartz K. Familial hypertrophic cardiomyopathy: from mutations to functional defects. Circ Res. 1998;83(6):580–593. doi: 10.1161/01.res.83.6.580. [DOI] [PubMed] [Google Scholar]
- 9.Daniels M.J., Fusi L., Semsarian C., Naidu S.S. Myosin modulation in hypertrophic cardiomyopathy and systolic heart failure: getting inside the engine. Circulation. 2021;144(10):759–762. doi: 10.1161/CIRCULATIONAHA.121.056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maron B.J. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med. 2018;379(7):655–668. doi: 10.1056/NEJMra1710575. [DOI] [PubMed] [Google Scholar]
- 11.Spudich J.A. Three perspectives on the molecular basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Pflugers Arch. 2019;471(5):701–717. doi: 10.1007/s00424-019-02259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edelberg J.M., Sehnert A.J., Mealiffe M.E., Del Rio C.L., McDowell R. The Impact of mavacamten on the Pathophysiology of hypertrophic cardiomyopathy: a narrative review. Am J Cardiovasc Drugs. 2022;22(5):497–510. doi: 10.1007/s40256-022-00532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijnker P.J.M., Sequeira V., Kuster D.W.D., Velden J.V. Hypertrophic cardiomyopathy: a vicious cycle triggered by sarcomere mutations and secondary disease hits. Antioxid Redox Signal. 2019;31(4):318–358. doi: 10.1089/ars.2017.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker J.G., Hill S.J., Summers R.J. Evolution of beta-blockers: from anti-anginal drugs to ligand-directed signalling. Trends Pharmacol Sci. 2011;32(4):227–234. doi: 10.1016/j.tips.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason D.T. Afterload reduction and cardiac performance. Physiologic basis of systemic vasodilators as a new approach in treatment of congestive heart failure. Am J Med. 1978;65(1):106–125. doi: 10.1016/0002-9343(78)90700-3. [DOI] [PubMed] [Google Scholar]
- 16.Slama M., Tribouilloy C., Maizel J. Left ventricular outflow tract obstruction in ICU patients. Curr Opin Crit Care. 2016;22(3):260–266. doi: 10.1097/MCC.0000000000000304. [DOI] [PubMed] [Google Scholar]
- 17.Dybro A.M., Rasmussen T.B., Nielsen R.R., Andersen M.J., Jensen M.K., Poulsen S.H. Randomized trial of metoprolol in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2021;78(25):2505–2517. doi: 10.1016/j.jacc.2021.07.065. [DOI] [PubMed] [Google Scholar]
- 18.Nistri S., Olivotto I., Maron M.S., et al. Beta blockers for prevention of exercise-induced left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2012;110(5):715–719. doi: 10.1016/j.amjcard.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 19.Monda E., Lioncino M., Palmiero G., et al. Bisoprolol for treatment of symptomatic patients with obstructive hypertrophic cardiomyopathy. The BASIC (bisoprolol AS therapy in hypertrophic cardiomyopathy) study. Int J Cardiol. 2022;354:22–28. doi: 10.1016/j.ijcard.2022.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Eisenberg M.J., Brox A., Bestawros A.N. Calcium channel blockers: an update. Am J Med. 2004;116(1):35–43. doi: 10.1016/j.amjmed.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Rosing D.R., Kent K.M., Maron B.J., Epstein S.E. Verapamil therapy: a new approach to the pharmacologic treatment of hypertrophic cardiomyopathy. II. Effects on exercise capacity and symptomatic status. Circulation. 1979;60(6):1208–1213. doi: 10.1161/01.cir.60.6.1208. [DOI] [PubMed] [Google Scholar]
- 22.Anderson D.M., Raff G.L., Ports T.A., Brundage B.H., Parmley W.W., Chatterjee K. Hypertrophic obstructive cardiomyopathy. Effects of acute and chronic verapamil treatment on left ventricular systolic and diastolic function. Br Heart J. 1984;51(5):523–529. doi: 10.1136/hrt.51.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosing D.R., Idänpään-Heikkilä U., Maron B.J., Bonow R.O., Epstein S.E. Use of calcium-channel blocking drugs in hypertrophic cardiomyopathy. Am J Cardiol. 1985;55(3):185B–195B. doi: 10.1016/0002-9149(85)90630-7. [DOI] [PubMed] [Google Scholar]
- 24.Betocchi S., Piscione F., Losi M A., et al. Effects of diltiazem on left ventricular systolic and diastolic function in hypertrophic cardiomyopathy. Am J Cardiol. 1996;78(4):451–457. doi: 10.1016/s0002-9149(96)00336-0. [DOI] [PubMed] [Google Scholar]
- 25.Sundjaja J.H., Makaryus A.N. StatPearls. StatPearls Publishing; Treasure Island, FL: 2023. Disopyramide. [Google Scholar]
- 26.Pollick C. Muscular subaortic stenosis: hemodynamic and clinical improvement after disopyramide. N Engl J Med. 1982;307(16):997–999. doi: 10.1056/NEJM198210143071607. [DOI] [PubMed] [Google Scholar]
- 27.Pollick C., Kimball B., Henderson M., Wigle E.D. Disopyramide in hypertrophic cardiomyopathy. I. Hemodynamic assessment after intravenous administration. Am J Cardiol. 1988;62(17):1248–1251. doi: 10.1016/0002-9149(88)90268-8. [DOI] [PubMed] [Google Scholar]
- 28.Kimball B.P., Bui S., Wigle E.D. Acute dose-response effects of intravenous disopyramide in hypertrophic obstructive cardiomyopathy. Am Heart J. 1993;125(6):1691–1697. doi: 10.1016/0002-8703(93)90760-7. [DOI] [PubMed] [Google Scholar]
- 29.Sherrid M., Delia E., Dwyer E. Oral disopyramide therapy for obstructive hypertrophic cardiomyopathy. Am J Cardiol. 1988;62(16):1085–1088. doi: 10.1016/0002-9149(88)90553-x. [DOI] [PubMed] [Google Scholar]
- 30.Sherrid M.V., Barac I., McKenna W.J., et al. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;45(8):1251–1258. doi: 10.1016/j.jacc.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Kajimoto K., Imai T., Minami Y., Kasanuki H. Comparison of acute reduction in left ventricular outflow tract pressure gradient in obstructive hypertrophic cardiomyopathy by disopyramide versus pilsicainide versus cibenzoline. Am J Cardiol. 2010;106(9):1307–1312. doi: 10.1016/j.amjcard.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 32.Heitner S.B., Jacoby D., Lester S.J., et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med. 2019;170(11):741–748. doi: 10.7326/M18-3016. [DOI] [PubMed] [Google Scholar]
- 33.GlobeNewswire MyoKardia Announces 48-week data from PIONEER-OLE study of mavacamten. https://www.globenewswire.com/news-release/2019/11/11/1944654/37418/en/MyoKardia-Announces-48-week-Data-from-PIONEER-OLE-Study-of-Mavacamten.html
- 34.Olivotto I., Oreziak A., Barriales-Villa R., et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;396(10253):759–769. doi: 10.1016/S0140-6736(20)31792-X. [DOI] [PubMed] [Google Scholar]
- 35.Efficacy, safety of mavacamten in treating obstructive HCM Holds up in EXPLORER-LTE cohort of MAVA-LTE study. https://www.acc.org/latest-in-cardiology/articles/2022/04/02/13/22/sun-945am-treatment-mavacamten-acc-2022
- 36.Desai M.Y., Owens A., Geske J.B., et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy Referred for septal reduction therapy. J Am Coll Cardiol. 2022;80(2):95–108. doi: 10.1016/j.jacc.2022.04.048. [DOI] [PubMed] [Google Scholar]
- 37.Cytokinetics Cytokinetics announces data from REDWOOD-HCM OLE and GALACTIC-HF PRESENTED as late breaking science presentations at the European Society of Cardiology Heart Failure 2022 Congress. https://ir.cytokinetics.com/news-releases/news-release-details/cytokinetics-announces-data-redwood-hcm-ole-and-galactic-hf
- 38.Maron M.S., Masri A., Choudhury L., et al. Phase 2 study of aficamten in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2023;81(1):34–45. doi: 10.1016/j.jacc.2022.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Menon S.C., Ackerman M.J., Ommen S.R., et al. Impact of septal myectomy on left atrial volume and left ventricular diastolic filling patterns: an echocardiographic study of young patients with obstructive hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2008;21(6):684–688. doi: 10.1016/j.echo.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 40.Hubner P.J., Ziady G.M., Lane G.K., et al. Double-blind trial of propranolol and practolol in hypertrophic cardiomyopathy. Br Heart J. 1973;35(11):1116–1123. doi: 10.1136/hrt.35.11.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Speiser K.W., Krayenbuehl H.P. Reappraisal of the effect of acute betablockade on left ventricular filling dynamics in hypertrophic obstructive cardiomyopathy. Eur Heart J. 1981;2(1):21–29. doi: 10.1093/oxfordjournals.eurheartj.a061160. [DOI] [PubMed] [Google Scholar]
- 42.Harada K., Tamura M., Ito T., Suzuki T., Takada G. Effects of low-dose dobutamine on left ventricular diastolic filling in children. Pediatr Cardiol. 1996;17(4):220–225. doi: 10.1007/BF02524797. [DOI] [PubMed] [Google Scholar]
- 43.Zeppellini R., Bolognesi R., Javernaro A., et al. Effect of dobutamine on left ventricular relaxation and filling phase in patients with ischemic heart disease and preserved systolic function. Cardiovasc Drugs Ther. 1993;7(3):325–331. doi: 10.1007/BF00880155. [DOI] [PubMed] [Google Scholar]
- 44.Dybro A.M., Rasmussen T.B., Nielsen R.R., et al. Effects of metoprolol on exercise hemodynamics in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2022;79(16):1565–1575. doi: 10.1016/j.jacc.2022.02.024. [DOI] [PubMed] [Google Scholar]
- 45.Palau P., Seller J., Domínguez E., et al. Effect of beta-blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. 2021;78(21):2042–2056. doi: 10.1016/j.jacc.2021.08.073. [DOI] [PubMed] [Google Scholar]
- 46.Efthimiadis G.K., Giannakoulas G., Parcharidou D.G., et al. Chronotropic incompetence and its relation to exercise intolerance in hypertrophic cardiomyopathy. Int J Cardiol. 2011;153(2):179–184. doi: 10.1016/j.ijcard.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 47.Hanrath P., Mathey D.G., Kremer P., Sonntag F., Bleifeld W. Effect of verapamil on left ventricular isovolumic relaxation time and regional left ventricular filling in hypertrophic cardiomyopathy. Am J Cardiol. 1980;45(6):1258–1264. doi: 10.1016/0002-9149(80)90487-7. [DOI] [PubMed] [Google Scholar]
- 48.Tendera M., Polonski L., Kozielska E. Left ventricular end-diastolic pressure-volume relationships in hypertrophic cardiomyopathy. Changes induced by verapamil. Chest. 1983;84(1):54–57. doi: 10.1378/chest.84.1.54. [DOI] [PubMed] [Google Scholar]
- 49.TenCate F.J., Serruys P.W., Mey S., Roelandt J. Effects of short-term administration of verapamil on left ventricular relaxation and filling dynamics measured by a combined hemodynamic-ultrasonic technique in patients with hypertrophic cardiomyopathy. Circulation. 1983;68(6):1274–1279. doi: 10.1161/01.cir.68.6.1274. [DOI] [PubMed] [Google Scholar]
- 50.Bonow R.O., Rosing D.R., Bacharach S.L., et al. Effects of verapamil on left ventricular systolic function and diastolic filling in patients with hypertrophic cardiomyopathy. Circulation. 1981;64(4):787–796. doi: 10.1161/01.cir.64.4.787. [DOI] [PubMed] [Google Scholar]
- 51.Hess O.M., Grimm J., Krayenbuehl H.P. Diastolic function in hypertrophic cardiomyopathy: effects of propranolol and verapamil on diastolic stiffness. Eur Heart J. 1983;4(Suppl F):47–56. doi: 10.1093/eurheartj/4.suppl_f.47. [DOI] [PubMed] [Google Scholar]
- 52.Suwa M., Hirota Y., Kawamura K. Improvement in left ventricular diastolic function during intravenous and oral diltiazem therapy in patients with hypertrophic cardiomyopathy: an echocardiographic study. Am J Cardiol. 1984;54(8):1047–1053. doi: 10.1016/s0002-9149(84)80142-3. [DOI] [PubMed] [Google Scholar]
- 53.Doiuchi J., Hamada M., Ito T., Kokubu T. Comparative effects of calcium-channel blockers and beta-adrenergic blocker on early diastolic time intervals and A-wave ratio in patients with hypertrophic cardiomyopathy. Clin Cardiol. 1987;10(1):26–30. doi: 10.1002/clc.4960100106. [DOI] [PubMed] [Google Scholar]
- 54.Verlinden N.J., Coons J.C. Disopyramide for hypertrophic cardiomyopathy: a pragmatic reappraisal of an old drug. Pharmacotherapy. 2015;35(12):1164–1172. doi: 10.1002/phar.1664. [DOI] [PubMed] [Google Scholar]
- 55.Coppini R., Ferrantini C., Pioner J.M., et al. Electrophysiological and contractile effects of disopyramide in patients with obstructive hypertrophic cardiomyopathy: a translational study. J Am Coll Cardiol Basic Trans Science. 2019;4(7):795–813. doi: 10.1016/j.jacbts.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sumimoto T., Hamada M., Ohtani T., et al. Effect of disopyramide on left ventricular diastolic function in patients with hypertrophic cardiomyopathy: comparison with diltiazem. Cardiovasc Drugs Ther. 1992;6(4):425–428. doi: 10.1007/BF00054192. [DOI] [PubMed] [Google Scholar]
- 57.Matsubara H., Nakatani S., Nagata S., et al. Salutary effect of disopyramide on left ventricular diastolic function in hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol. 1995;26(3):768–775. doi: 10.1016/0735-1097(95)00229-W. [DOI] [PubMed] [Google Scholar]
- 58.Fifer M.A., O'Gara P.T., McGovern B.A., Semigran M.J. Effects of disopyramide on left ventricular diastolic function in hypertrophic cardiomyopathy. Am J Cardiol. 1994;74(4):405–408. doi: 10.1016/0002-9149(94)90416-2. [DOI] [PubMed] [Google Scholar]
- 59.Coppini R., Ferrantini C., Yao L., et al. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation. 2013;127(5):575–584. doi: 10.1161/CIRCULATIONAHA.112.134932. [DOI] [PubMed] [Google Scholar]
- 60.Desai M.Y., Owens A., Geske J.B., et al. Dose-blinded myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy: Outcomes through 32 weeks. Circulation. 2023;147(11):850–863. doi: 10.1161/CIRCULATIONAHA.122.062534. [DOI] [PubMed] [Google Scholar]
- 61.Ho C.Y., Mealiffe M.E., Bach R.G., et al. Evaluation of mavacamten in symptomatic patients with Nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2020;75(21):2649–2660. doi: 10.1016/j.jacc.2020.03.064. [DOI] [PubMed] [Google Scholar]
- 62.Hegde S.M., Lester S.J., Solomon S.D., et al. Effect of mavacamten on echocardiographic Features in symptomatic patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2021;78(25):2518–2532. doi: 10.1016/j.jacc.2021.09.1381. [DOI] [PubMed] [Google Scholar]
- 63.Saberi S., Cardim N., Yamani M., et al. Mavacamten Favorably Impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER-HCM cardiac magnetic resonance Substudy analysis. Circulation. 2021;143(6):606–608. doi: 10.1161/CIRCULATIONAHA.120.052359. [DOI] [PubMed] [Google Scholar]
- 64.Cremer P.C., Geske J.B., Owens A., et al. Myosin inhibition and left ventricular diastolic function in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy: insights from the VALOR-HCM study. Circ Cardiovasc Imaging. 2022;15(12):e014986. doi: 10.1161/CIRCIMAGING.122.014986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.