Abstract
Background:
Dengue is one of the neglected tropical diseases, with a wide spectrum of diseases, ranging from acute febrile illness dengue fever to life-threatening dengue hemorrhagic fever or dengue shock syndrome. In recent years, it has become a major public health concern in many nonendemic areas as well.
Materials and Methods:
A secondary data analysis of records available with district Integrated Disease Surveillance Programme cell was conducted to study distribution (time, place, and person) of dengue from 2017 to 2022 in Kangra, a sub-Himalayan district of Himachal Pradesh (HP).
Results:
In the evaluated period (2017–2022), a total of 6008 cases suspected of dengue were tested and test positivity of 7% (441) with male gender predominance was found. Mean age of the diagnosed cases was 37.7 ± 16.8 years. A seasonal trend was observed starting from late August to November in all study years.
Conclusion:
Dengue is still a neglected disease, but it has shown its presence especially in this part of HP, indicating the need for better preparation and sensitization of vector-borne disease control program activities, especially in post-monsoon, to prevent future epidemics.
Keywords: Aedes agypti, Aedes albopictus, dengue, Himachal Pradesh, India, Integrated Disease Surveillance Program, National Vector-Borne Disease Control Program
INTRODUCTION
Dengue is a rapidly spreading mosquito-borne viral disease and is transmitted by Aedes aegypti, a day-biting mosquito that prefers to rest in dark areas. A. aegypti is the principal vector; however, in recent years Aedes albopictus has also been reported in southern and northeastern states. It is considered as a major public health concern as well as one of the 17 neglected tropical diseases.[1,2] The global incidence of dengue has grown drastically, with about half of the world’s population at risk. The number of dengue cases increased over eight-fold in the last two decades, from 505,430 cases in 2000 to over 2.4 million in 2010 and 5.2 million in 2019.[3]
In the last decade, a regular occurrence with periodic upsurges has been noted in many parts of India.[4] India has been identified as one of the several countries affected by dengue in 2020. In the 20th century, this disease spread mainly in the tropical regions of India. However, recently, some cases have also been reported in the subtropical Himalayan regions of the country. Due to urbanization, climate change, human and vector mobility, dengue outbreaks are occurring even in spared hilly regions of the country every year. In India, a total of 1,93,245 cases and 346 deaths due to dengue were reported in 2021 and 1,10,473 cases and 86 deaths were reported in 2022. In Himachal Pradesh, a total of 349 and 2563 new cases of dengue were detected in 2021 and 2022, respectively, without any mortality.[5]
In addition, the risk of dengue outbreaks has also been predicted in the northernmost states such as Himachal Pradesh and Jammu and Kashmir. An investigation into recent outbreaks in a neighbouring district of Himachal Pradesh has verified the existence of both A. aegypti and A. albopictus mosquitoes.[6,7]
Dengue surveillance in the country is conducted through a network of more than 600 sentinel hospitals under the National Vector-Borne Disease Control Program (NVBDCP) and the Integrated Disease Surveillance Program (IDSP).[8]
Analysis of trends of disease over a period provides useful information on how the disease has evolved over the years. To assess the current situation of dengue and to address the implementation gaps in the running program, data from health-care facilities is an essential prerequisite. The present study was conducted in Kangra district, which is one of the 12 districts of Himachal Pradesh, spread out over the western region of the state and also by far the largest region of Himachal Pradesh. The objectives of this study were to analyze the trends and distribution of dengue cases from 2017 to 2022.
MATERIALS AND METHODS
Study setting
This study was carried out in Kangra district of Himachal Pradesh, India. A secondary data analysis of all the dengue cases for the last 6 years from 2017 to 2022 was conducted.
Data source
Data was gathered from the records of district IDSP cell. All the data records that were complete as per the predefined variables were included, and records with incomplete information were excluded from the study. This was the only source of lab-confirmed data at a district level.
Data variables and analysis
The extracted data underwent thorough analysis aimed at elucidating the disease burden, with categorization based on age, gender, education level, time, and residential area. No statistical sample size calculation was performed, and the sample size was equal to the number of patients diagnosed and treated during the mentioned study period. The data collected was tabulated and analyzed using Microsoft Office Excel 2016, and analysis was conducted using Epi Info 7 software. Descriptive statistical tools like mean and standard deviation were used for continuous data, while frequencies and percentages were used for categorical data.
RESULTS
Data from 2017 to 2022 was collected from the district IDSP cell. A total of 6008 suspected cases of dengue were tested. Dengue infection was confirmed in 441 (7%) patients, based on detection of Immunoglobulin M (IgM), Non Structural Protein 1 (NS1) Antigen or both. Serotyping was not done. Table 1 summarizes the sociodemographic and clinical characteristics of the participants. Majority of cases (41%) were middle aged (16–30 years). Mean age of the cases was 37.7 ± 16.8 years, with the youngest being 3 years old and the oldest being 82 years old. Dengue positivity was higher among males (69%) than females (31%), with the M:F ratio being 2.2:0.4. It was also found that males living in rural localities were affected more in comparison to females of the same localities. Year-wise evaluation [Figure 1] showed that maximum number of cases were reported in 2018 with a male predominance [Table 2].
Table 1.
Sociodemographic and clinical characteristics
| Variables | Female (n, %) | Male (n, %) | Total (n, %) | |||
|---|---|---|---|---|---|---|
| Total cases | 136 (31) | 305 (69) | 441 (100) | |||
| Age groups (mean age±SD, 95% CI): 37.75±16.82, 36.17–39.32 | ||||||
| 01–15 | 10 (7.3) | 9 (2.9) | 19 (4.3) | |||
| 16–30 | 51 (37.5) | 132 (43.3) | 183 (41.5) | |||
| 31–45 | 22 (16.2) | 85 (27.9) | 107 (24.3) | |||
| 46–60 | 31 (22.8) | 46 (15.1) | 77 (17.5) | |||
| 61–75 | 17 (12) | 30 (9.8) | 47 (10.7) | |||
| 76–90 | 5 (3.7) | 3 (0.1) | 8 (1.8) | |||
| Place of residence | ||||||
| Rural | 117 (86) | 283 (92.8) | 400 (90.7) | |||
| Urban | 19 (14) | 22 (7.2) | 41 (9.3) | |||
| Education | ||||||
| Illiterate | 84 (61.8) | 73 (23.9) | 157 (35.6) | |||
| Literate | 52 (38.2) | 232 (76.1) | 284 (64.4) | |||
| Clinical characteristics | ||||||
| IPD | 120 (88.2) | 220 (72.1) | 340 (77) | |||
| OPD | 16 (11.8) | 85 (27.9) | 101 (23) | |||
| Test positives | ||||||
| IgM | 75 (55.1) | 157 (51.5) | 232 (52) | |||
| NS1 | 46 (33.8) | 98 (32.1) | 144 (33) | |||
| Both IgM and NS1 | 15 (11) | 50 (16.4) | 65 (15) | |||
CI=confidence interval, IgM=immunoglobulin M, IPD=inpatient department, OPD=outpatient department, SD=standard deviation
Figure 1.
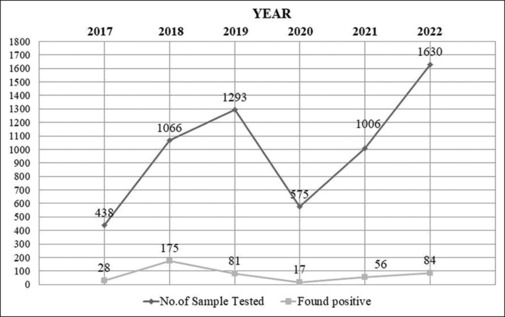
Year-wise distribution of dengue cases tested positive
Table 2.
Gender-wise distribution of cases, 2017–2022
| Year | Female (n, %) | Male (n, %) | Total (n, %) | |||
|---|---|---|---|---|---|---|
| 2017 | 12 (8.8) | 16 (5.3) | 28 (6.3) | |||
| 2018 | 54 (39.7) | 121 (39.7) | 175 (39.7) | |||
| 2019 | 27 (19.8) | 54 (17.7) | 81 (18.4) | |||
| 2020 | 6 (4.4) | 11 (3.6) | 17 (3.9) | |||
| 2021 | 12 (8.8) | 44 (14.4) | 56 (12.7) | |||
| 2022 | 25 (18.4) | 59 (19.3) | 84 (19) | |||
| Total | 136 (31) | 305 (69.2) | 441 (100) |
Figure 2 shows the health block-wise distribution of cases; maximum number of cases (18%) were found in Tiara block of the district. Though this block has not much geographic variation, the maximum number obtained could be because of more testing in this area. In all years, cases gradually started increasing from the month of August with a peak in October and then started to decline till early December, which clearly indicates the vector breeding environment [Figure 3].
Figure 2.
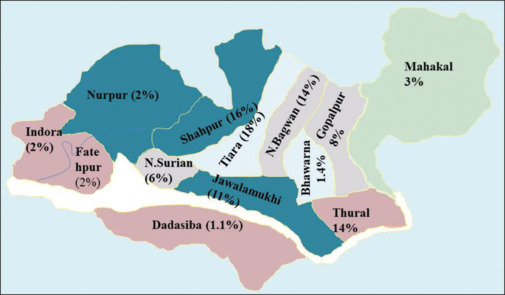
Health block-wise distribution of cases (in %)
Figure 3.
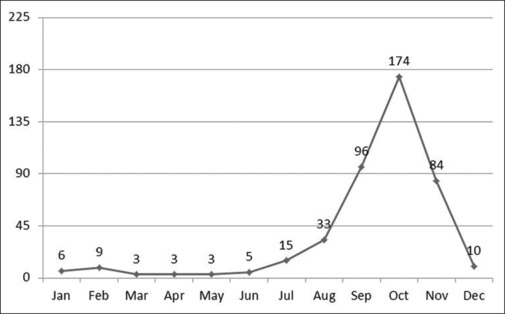
Cumulative month-wise trends of dengue cases, 2017–2022
DISCUSSION
Dengue virus (DV) is a positive-stranded encapsulated RNA virus belonging to the family Flaviviridae.[9] It is transmitted mainly by A. aegypti mosquitoes and also by A. albopictus.[10] Globally, a perceptible upward trend has been reported in recent years, and the disease is associated with increasing frequency of outbreaks.[11] The first virologically confirmed epidemic in India occurred in Kolkata and in the eastern coast of India in 1963–1964.[12,13]
It has been found that ecological and climatic factors influence the seasonal prevalence of the vector A. aegypti and DV.[14] We also found that the largest proportion of cases occurring in the post-monsoon period is consistent with previous studies concluding that the post-monsoon period is the most affected period.[15,16]
In the current study, gender-wise analysis revealed a male predominance, which is consistent with previous observations concluding that males are associated with more outdoor activities leading to increased risk of mosquito bite during daytime. Our study revealed that only 4.3% of children below 15 years of age were positive and the majority of cases were present in the age group of 16–45 years. These findings are consistent with other studies concluding that the age group of 12–45 years is the most affected age group.[17,18,19] However, one of the studies reported dengue as mainly a pediatric public health problem.[20]
A month-wise distribution of dengue cases in the current study shows an increase from August onward, which concurs with other outbreaks in India.[21] We also analyzed the month-wise data to correlate the seasonal variation of the disease. During the study, a gradual increase in cases was noticed from August with a peak in October. The correlation between occurrence of dengue and monsoon season is clearly evident in this study and is further supported by similar findings.[22,23,24]
It was also found in our study that the number of dengue positive cases was higher in rural areas than urban areas. Furthermore, the rising trend observed in 2018 was due to A. albopictus, which was reported from one of the health blocks of the district. This may be partially attributed to the rapid unplanned urbanization with unchecked construction activities and poor sanitation facilities contributing fertile breeding grounds for mosquitoes. It is also true that an increase in alertness among the medical fraternity following the initial epidemics in North India and the availability of diagnostic tools in the hospital have contributed to increased detection of cases.
To the best of our knowledge, this was the first study of its kind in this hilly district. The records of patients of the whole district were analyzed, and the study population is likely to be representative of the general population. Hence, the findings are applicable to the whole district. This, however, needs to be assessed at the population level at periodical intervals. Identification of A. albopictus signifies an effective implementation of NVBDCP in identification of vector-borne diseases.
Despite the clarity of findings, this study has a few limitations. This was a retrospective data analysis based on departmental records; hence, bias in reporting cannot be ruled out and it also makes generalization of our study findings difficult. As only registered records were analyzed, limited variables based on records were included.
CONCLUSION
Our findings highlight the need for implementation of sustained effective measures for early diagnosis while improving awareness to ensure appropriate health-seeking behavior. Trend analysis of dengue incidences helps in improving vector control and surveillance. Surveillance of the seasonal outbreak of disease transmission is very important at the local level for effective control measures, and preventive measures against dengue infection should be fully implemented during water stagnation periods after the initial rainfall as well as at the end of monsoon. Intersectoral coordination can play a vital role in generating awareness and in regular monitoring for further controlling the spread of the disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank the district IDSP cell for providing the requisite database and all the hospital staff members for their efforts in collecting all the relevant information from the records of patients involved in the study.
REFERENCES
- 1.Department of Health and Family Welfare Ministry of Health and Family Welfare Government of India Annual Report (2021-22) Available from: https://main.mohfw.gov.in/sites/default/files/FinalforNetEnglishMoHFW040222.pdf . [Last accessed on 2022 Dec 12] [Google Scholar]
- 2.Operational Guidelines for Prevention and control of Aedes mosquito in hospital settings, National Vector borne disease control programme Ministry of Health and Family Welfare Government of India. 2022 Available from: https://ncvbdc.mohfw.gov.in/Doc/Guidelines/Operational-Guidelines-Hospitals-to-Conrol-Aedes-Breeding-2022.pdf. [Last accessed on 2022 Nov 30] [Google Scholar]
- 3.Dengue and severe dengue. World Health Organization fact sheets. Available from: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue . [Last accessed on 2022 Nov 30] [Google Scholar]
- 4.Maula AW, Fuad A, Utarini A. Ten-years trend of dengue research in Indonesia and South-east Asian countries: A bibliometric analysis. Glob Health Action. 2018;11:1504398. doi: 10.1080/16549716.2018.1504398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dengue situation in India. Ministry of Health and Family Welfare Government of India. National Center for vector borne disease control. 2022 Available from: https://ncvbdc.mohfw.gov.in/index4.php?lang=1&level=0&linkid=431&lid=3715. [Last accessed on 2022 Dec 27] [Google Scholar]
- 6.Murhekar MV, Kamaraj P, Kumar MS, Khan SA, Allam RR, Barde P, et al. Burden of dengue infection in India, 2017: A cross-sectional population based serosurvey. Lancet Glob Health. 2019;7:e1065–73. doi: 10.1016/S2214-109X(19)30250-5. [DOI] [PubMed] [Google Scholar]
- 7.Singh AK, Chawla S, Chawla B, Bhaglani DK, Sharma KC. Role of a surveillance system in the management of an outbreak of dengue in the mid hills of Himachal Pradesh, India. J Clin Diagn Res. 2017;11:LC01–5. [Google Scholar]
- 8.Gubler DJ. Dengue and dengue haemorrhagic fever. Clin Microbiol Rev. 1998;11:480–96. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kar NP, Kumar G, Pasi S, Ojha VP, Negi SS, Dhiman RC. Entomological Investigation during an Outbreak of Dengue in 2018 in Bilaspur District, Himachal Pradesh. J Commun Dis. 2022;54:29–35. [Google Scholar]
- 10.Martina BE, Koraka P, Osterhaus A. Dengue virus pathogenesis, an integrated view. Clin Microbiol Rev. 2009;22:564–81. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta E, Dar L, Kapoor G, Broor S. The changing epidemiology of dengue in Delhi, India. Virol J. 2006;3:92. doi: 10.1186/1743-422X-3-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramakrishanan SP, Gelfand HM, Bose PN, Sehgal PN, Mukharjee RN. The epidemic of acute haemorrhagic fever, Calcutta, 1963: Epidemiological Inquiry. Indian J Med Res. 1964;52:633–50. [PubMed] [Google Scholar]
- 13.Bharaj P, Chaher HS, Pandey A, Diddi K, Lalit D, Guleria R, et al. Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol J. 2008;5:1. doi: 10.1186/1743-422X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh B. Dengue outbreak in 2006: Failure of public health system? Indian J Community Med. 2007;32:99–100. [Google Scholar]
- 15.Sukri NC, Laras K, Wandra T, Didi S, Larasati RP, Rachdyatmaka JR. Transmission of epidemic dengue hemorrhagic fever in easternmost Indonesia. Am J Trop Med Hyg. 2003;68:529–35. doi: 10.4269/ajtmh.2003.68.529. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarti A, Kumaria R. Eco-epidemiological analysis of dengue infection during an outbreak of dengue fever, India. Virol J. 2005;2:32–8. doi: 10.1186/1743-422X-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin MMM, Hussain AMZ, Murshed M, Chowdhury IA, Mannan S, Chowdhuri SA, et al. Sero-Diagnosis of dengue infection by haemagglutination inhibition test (HI) in suspected cases in Chittagong, Bangladesh. Dengue Bull. 1999;23:34–8. [Google Scholar]
- 18.Ukey PM, Bondade SA, Paunipagar PV, Powar RM, Akulwar SL. Study of seroprevalence of dengue fever in central India. Indian J Community Med. 2010;35:517–9. doi: 10.4103/0970-0218.74366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta E, Dar L, Narang P, Srivastava VK, Broor S. Serodiagnosis of dengue during an outbreak at a tertiary care hospital in Delhi. Indian J Med Res. 2005;121:36–8. [PubMed] [Google Scholar]
- 20.Kumar A, Rao R, Pandit V, Shetty S, Bamigatti C, Samaraging CM. Clinical manifestation and trend of dengue cases admitted in tertiary care hospital, Udupi, Karnataka. Indian J Community Med. 2010;35:386–91. doi: 10.4103/0970-0218.69253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chour YM, Ruble G, Hong R, Minn K, Kdan Y, Sok T, et al. Hospital based diagnosis of hemorrhagic fever, encephalitis, and hepatitis in Cambodian children. Emerg Infect Dis. 2002;8:485–9. doi: 10.3201/eid0805.010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lal M, Aggarwal A, Oberoi M. Dengue fever, an emerging viral problem in Ludhiana, North India. Indian J Community Med. 2007;51:198–9. [PubMed] [Google Scholar]
- 23.Ratho RK, Mishra B, Kaur J, Kakkar N, Sharma K. An outbreak of dengue fever in Peri Urban slums of Chandigarh, India, with special reference to entomological and climatic factors. Indian J Med Sci. 2005;59:519–27. [PubMed] [Google Scholar]
- 24.Ahmed S, Arif F, Yahya Y, Rehman A, Abbas K, Ashraf S, et al. Dengue fever outbreak in Karachi 2006 - A study of profile and outcome of children under 15 years of age. J Pakistan Med Assoc. 2008;58:4–8. [PubMed] [Google Scholar]


