Abstract
Introduction:
Breast cancer incidence has overtaken that of cervical cancer among women in India according to the Globacon 2020 reports. Cancer management is also being streamlined at the Center and district levels, such that comprehensive integrated management is offered to cases to optimize the best results. In breast cancer, there are two modes of surgery namely Breast Conservation Surgery(BCS) and Modified Radical Mastectomy (MRM) now over 2 decades, with recommended Chemo radiation depending on the extent of the disease. HRQOL (Health-related Quality of Life) studies have been done in these groups of patients, due to their added relevance in terms of the loss of a vital organ like the breast. EORTC 30 and BR23 are standardized and detailed tools that have been seen to estimate QOL, keeping in mind a whole array of domains that are affected by the disease.
Objective:
To evaluate the “Body Image” and “Quality of life” (QOL) in operated breast cancer patients using BR -23 and EORTC – QLQ- questionnaire at 1month (after surgery) and then 3 to 4 months after surgery.
Methods:
This article attempts to draw a comparison among of EORTC30 and BR 23 scores calculated for 46 breast cancer patients operated during the pandemic time in one center and consenting to repeat the measures at pre-decided three time periods during the course of management.
Results:
No significant differences are noted in the mean scores for EORTC 30 and BR23 for the two types of surgeries. Visit 1 scores for both modes of surgery are over 75 and by Visit 3 become less than 55 for EORTC. BR 23 (which measures the symptoms core to Breast cancer) at all 3 visits are between 45 to 55. Friedman’s test shows that the scores are not significant for age groups, the number of living children, or lifestyle factors like alcohol or tobacco chewing, though quadratic graphs depict the distinct variations in the scores at the 3 times reinforcing the need for follow-up of mental health in these subjects at intervals. The study largely brings out a strong need for repeated follow-up and counseling at regular and short intervals, post-surgery in breast cancer patients. EORTC 30 and BR 23 tools are excellent to use to essay information on the mental health of patients with breast cancer.
Keywords: Breast cancer, breast conservation surgery (BCS), EORTC 30 and BR 23 tools, modified radical mastectomy (MRM), psychotherapy
INTRODUCTION
Advances in medical sciences have helped better survival in cases of non-communicable diseases (NCDs) such as cancer. In India, cancer is still a disease that carries a lot of fear and stigma, with a crude incidence of 100.4 per 100,000 cases, summing up to one in every nine people who may report cancer in their lifetime. Lung and breast cancer are the leading sites of cancer in males and females, respectively.[1] However, the silver lining is that breast cancer has good screening, diagnostic, and treatment modalities, thus assuring better survival for the subject.[2] The fear of recurrence, loss of body functions, systemic immediate effects, as well as some long-term effects of the anti-cancer agents on the body, warrant a multidisciplinary approach to managing cancer patients.[3] Typically, in breast cancer, as loss of the breast causes immense psychological trauma, which is now limited by breast conservation surgery (BCS), which is being done for patients as per the extent of the spread of the disease. Thus, the novel term health-related QOL (HR-QOL) has come up for all cancer survivors. HR-QOL attempts to score them on domains that are likely to be affected during the course of cancer management; this definitely helps in improving disease outcomes. In this regard, some common scoring tools are the WHO Brief Questionnaire[4] and the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) used in conjunction with the Quality-of-Life Questionnaire Breast Cancer module 23 (QLQ-BR23),[5,6] which have been widely used to augment treatment outcomes and predict survival. The latter tool is validated in most Indian languages, besides Odia,[7] the local language of the center, where the study was planned, and hence offers a ready-to-use tool to public health specialists to study the variations in the QOL of cancer patients.
The drawback in Middle-income countries is that there is often a solitary focus on offering secondary management to cancer patients, however, the follow-up and documentation on Quality-of-Life post-management is skewed or sparingly done and not taken up by all centres, barring some State of art centres.
In most studies done in India or Asian countries, it has been tried to see the EORTC QLQ30 and QLQ-BR23, post-surgery for a maximum of one or two visits.[8,9,10] This study envisages monitoring the QOL in a breast cancer sample, followed up for 3 visits as a series in the continuum, during the course of the disease management to closely observe the changes in the overall scores of the tool.
The study aimed at assessing the HRQOL of operated breast cancer patients for three postoperative follow-up visits compared among two different surgical approaches (BCS vs. modified radical mastectomy (MRM)).
In addition, it aimed to look for any associations of the HRQOL with sociodemographic variables in the study cohort.
METHODS
A longitudinal single-center, hospital-based study was planned wherein all female patients of operable breast carcinoma attending the state-of-the-art cancer center at Bhubaneswar, Odisha, between the period November 2020 to March 2022 were considered for participation in the study. A convenient (non-probabilistic) sample of operable breast cancer patients was enrolled as per inclusion criteria, that is, female, literate, and consenting for study participation and follow-up protocol. Women with diffuse metastatic disease with obvious poor disease outcomes and failing to turn up for follow-up or opting for changing the parent institute for subsequent treatments were not included.
Since the study period spanned over the two phases of the COVID-19 pandemic, wherein patient attendance in the tertiary care setup was drastically hit due to a series of lockdowns and shutdowns in the State and most of the curative services being diverted for Covid care, only 46 participants completed the follow-up protocol of the study. The study visits were V1, the point of recruitment into the study was to be at least 4 weeks after surgery; V2 was 3–4 months post surgery after the start of the 1st cycle of the adjuvant therapy (which is chemotherapy and radiotherapy included); and V3 was within 2 weeks of the completion of the full course of treatment. The present study was part of a larger longitudinal study on HRQOL of operated breast cancer patients seeking treatment from the comprehensive cancer care center. So this study can be taken as pilot study for which a sample more than 20 is appropriate.[11]
The study tool comprised Section A, which included sociodemographic information of the respondent; Section B, which included information about the disease, diagnosis, and disease characteristics; and Section C, which included documented responses for the QOL tool, that is, EORTC QLQ C-30 and BR23 (European Organization for Research and Treatment of Cancer) questionnaire, validated in the local language (Odia). The tools were used after getting permission from eortc.org, and the validated translated versions were used for the study (7).
EORTC 30, as the name suggests, includes 30 items, grouped into five functional scales (physical, role, cognitive, emotional, and social) as well as three symptom scales (fatigue-FA, nausea and vomiting-NV, and pain-FA) and six single-item scales (dyspnea - DY, insomnia - SL, appetite loss - AP, constipation - CO, diarrhea - DI, and financial difficulties – FI). Thus, this scale was used to measure QOL in all cancer patients. QLQ-BR23 with 23 items is an addendum scale, more specific to breast cancer-relevant functional scales (body image - BRBI, sexual functioning - BRSEF, sexual enjoyment - BRSEE, and future perspective - BRFU) and symptom scales (systemic therapy side effects - BRST, breast symptoms - BRBS, arm symptoms – BRAS, and upset by hair loss - BRHL). The total scores ranged from 0 to 100, using fixed formulas and linear transformations as specified in the EORTC module. In both cases, low scores hinted at a poor QoL.[7]
Data analysis was done using SPSS version 25, and percentages were used to depict the sociodemographic characteristics of the sample. Considering paired follow-up on three time instances of study participants, repeated measures ANALYSIS of variance (ANOVA) within and between subjects was used to calculate the means of the total scores as well as that of the subscales. Means scores were considered to permit comparability with the findings of other studies. Pooled mean score inferences for EORTC30 and BR23 were discussed and not individual items to restrict the conclusions as per objectives and to identify the trends in the mean HRQOL estimates for the three visits.
The data were nonparametric; thus, Friedman’s test was used to see the statistical significance of results at P < 0.05 for correlates such as age and other independent variables. Quadratic graphs were used to depict the changes in QOL scores over the three visits for both EORTC30 as well as BR23.
RESULTS
A total of 46 women completed all three visits during the study period, that is, V1 within 2 weeks of surgery, V2 within 2 weeks of 1 cycle of CT/RT, and V3 within 2 weeks after the completed treatment.
Table 1 presents the sociodemographic, behavioral, and sample characteristics of the women in the study.
Table 1.
General socio-demographic characteristics
| Variables | Categories | No. (%) n=46 | ||
|---|---|---|---|---|
| Age (in years) | <30 | 0 | ||
| 30–60 | 38 (82.7) | |||
| >60 | 8 (17.4) | |||
| Residence | Urban | 25 (54.3) | ||
| Rural | 21 (45.7) | |||
| Marital Status | Married | 46 (100) | ||
| No. of children | 1 2-3 4 or more |
12 (26.1) 31 (67.4) 3 (6.5) |
||
| Age at menarche (in years) | 9–11 12 & above |
23 (50.0) 23 (50.0) |
||
| Tobacco habits (occasional and regular included) | Smokeless Smoking Both Never |
4 (8.7) 8 (17.4) 2 (4.30) 32 (69.6) |
||
| Alcohol consumption (more than once in the last 3 months) | Yes No |
12 (26.1) 34 (73.9) |
||
| Nutrition Non-veg (eggs, animal meat included) | Yes | 23 (50) |
None of the participants were under 30 years of age and most ie 82.7% were from 30-60 years of age. Proportionate Urban: rural distribution was roughly 5.4:4.5 and 67.4% of participants had 2-3 children and more than a quarter had a single child. Age of menarche was equidistributed in the groups 9–12 years and above. Furthermore, 17.7% reported a habit of smoking (quantification was not possible as subjects were reluctant to give detailed history), and 4.3% both smoked and used chewing forms of tobacco. Alcohol consumption history was given by 26.1% of women, of which 89.3% were from urban, which hints at a strong cultural transition among women in urban India, where alcohol consumption is not commonly consumed by women. The team adds a disclaimer regarding the quantity in the case of alcohol and has taken any amount of consumption more than once in the last 3 months as a positive history of alcohol consumption.
Table 2 presents the variations in mean scores for EORTC 30 and BR 23 for the three times’ assessment, simultaneously compared against the two types of surgery done. On review, there was no statistically significant difference observed in the mean QOL scores for all three visits when compared against the two types of surgery. The overall QOL values dipped by 8 points from V1 to V2 and further dipped by 9 points for V3 for both surgeries, which suggests that initially the surgery was well tolerated by all subjects and QOL was not remarkably affected. The adjuvant chemoradiation, given now as a standard treatment plan for all breast cancer cases, affects the QOL to a great extent subsequently during management. V2, which is timed after the 1st cycle of the adjuvant therapy, showed a dip in QOL, and further on, the dip was aggravated by V3, which was timed 2–4 weeks after completion of a full course of adjuvant therapy. Table 2 also depicts BR 23 for three visits. BR 23 is a measure of the functional index of body image and symptoms in the subjects. The scores varied from 45.95 to 44.78 across V1 to V3. This hints that symptoms post surgery are replaced by side effects of adjuvant therapy, and the body image indices continue to remain low even after 6–8 months post surgery. The table also gives a cumulative grand mean scores comparison both of EORTC30 and BR23 for subjects operated for both BCS as well as MRM, and it further reaffirms that both the surgeries report the same trend in scores when measured at three points of time.
Table 2.
Comparisons of Means for EORTC 30 (QOL) & BR 23 during 3 timelines V1, V2, V3 for the two types of surgery (n=46)
| Scores of QOL measures at 3 visits (V1–V3) | Type of Surgery | Mean | Std. Deviation | SE | 95% Confidence Interval |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||||||
| QLQ_Total_V1 | BCS (n1=6) | 75.83 | 8.93 | - | - | - | ||||||
| MRM (n2=40) | 77.15 | 8.24 | - | - | - | |||||||
| Total | 76.98 | 8.24 | 1.82 | 72.82 | 80.16 | |||||||
| QLQ_Total_V2 | BCS | 65.83 | 10.80 | - | - | - | ||||||
| MRM | 68.90 | 9.27 | - | - | - | |||||||
| Total | 68.50 | 9.41 | 2.07 | 63.19 | 71.54 | |||||||
| QLQ_Total_V3 | BCS | 57.17 | 15.22 | - | - | - | ||||||
| MRM | 53.35 | 11.79 | - | - | - | |||||||
| Total | 53.85 | 12.16 | 2.68 | 49.87 | 60.65 | |||||||
| BR_Total_V1 | BCS | 45.50 | 7.06 | - | - | - | ||||||
| MRM | 46.03 | 17.29 | - | - | - | |||||||
| Total | 45.96 | 16.26 | 3.60 | 38.51 | 53.02 | |||||||
| BR_Total_V2 | BCS | 55.33 | 9.35 | - | - | - | ||||||
| MRM | 54.90 | 6.32 | - | - | - | |||||||
| Total | 54.96 | 6.66 | 1.47 | 52.15 | 58.09 | |||||||
| BR_Total_V3 | BCS | 43.17 | 8.28 | - | - | - | ||||||
| MRM | 45.03 | 7.69 | - | - | - | |||||||
| Total | 44.78 | 7.70 | 1.70 | 40.67 | 47.52 | |||||||
| Grand Mean EORTC 30 (all 3 visits) | BCS | 66.278 | 8.92 | 2.294 | 61.655 | 70.901 | ||||||
| MRM | 66.467 | 10.22 | 0.888 | 64.676 | 68.257 | |||||||
| Grand Mean BR 23 | BCS | 48.000 | 7.91 | 2.610 | 42.740 | 53.260 | ||||||
| MRM | 48.650 | 6,88 | 1.011 | 46.613 | 50.687 | |||||||
However, within-subjects repeated measures ANOVA for EORTC 30 over three visits, sphericity assumed univariate analysis F (2,46) =22.188 (P < 0.001), and for BR23 over time, sphericity assumed univariate analysis F (2,46) =5.814 (P < 0.001), suggesting that the variations over the visits for both scores were highly significant in the given sample.
Within-subjects contrasts showed a significant linear association between time F = 37.809 (P < 0.001) for EORTC [Figure 1], while BR23 showed a significant quadratic association with time, F = 16.648 (P < 0.001) [Figure 2].
Figure 1.
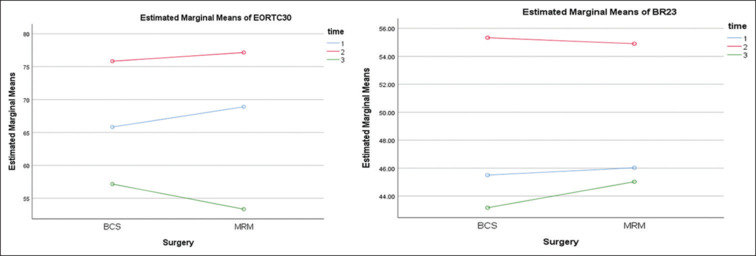
EORTC 30 and BR23 grand means over time
Figure 2.
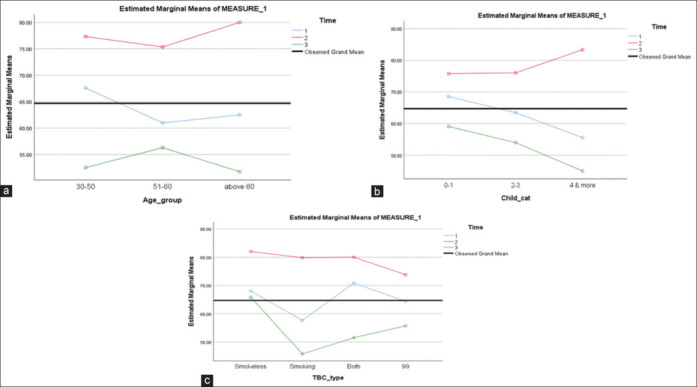
(a-c) show the variations in EORTC 30 mean scores age-wise, number of living children, and type of tobacco addiction in the given sample of n = 46 subjects for the three time assessments, regardless of the type of surgery
Findings in Table 3 further clarify the between-subject variations for the measures at the time points and the significance levels at 95% CI. EORTC 30 was highly significant in terms of deterioration of the scores from V1 to V3, and BR 23 the variations between V2 to V3 are highly significant and not between V1 and V2 or V1 and V3.
Table 3.
Repeated ANOVA pair-wise comparisons to measure the effect of the time factor for QOL scores
| Measure | (I) time | (J) time | Mean Difference (I-J) | Std. Error | Sig.b | 95% Confidence Interval for Differenceb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||||||||||
| EORTC30 | 1 | 2 | 9.125* | 3.067 | 0.014 | 1.491 | 16.759 | |||||||
| 3 | 21.233* | 3.453 | 0.000 | 12.638 | 29.828 | |||||||||
| 2 | 1 | −9.125* | 3.067 | 0.014 | −16.759 | −1.491 | ||||||||
| 3 | 12.108* | 3.057 | 0.001 | 4.499 | 19.718 | |||||||||
| 3 | 1 | −21.233* | 3.453 | 0.000 | −29.828 | −12.638 | ||||||||
| 2 | −12.108* | 3.057 | 0.001 | −19.718 | −4.499 | |||||||||
| BR23 | 1 | 2 | −9.354* | 3.736 | 0.048 | −18.654 | −.055 | |||||||
| 3 | 1.667 | 3.996 | 1.000 | −8.279 | 11.613 | |||||||||
| 2 | 1 | 9.354* | 3.736 | 0.048 | 0.055 | 18.654 | ||||||||
| 3 | 11.021* | 2.548 | 0.000 | 4.679 | 17.363 | |||||||||
| 3 | 1 | −1.667 | 3.996 | 1.000 | −11.613 | 8.279 | ||||||||
| 2 | −11.021* | 2.548 | 0.000 | −17.363 | −4.679 | |||||||||
Based on estimated marginal means. *The mean difference is significant at the 0.05 level. bAdjustment for multiple comparisons: Bonferroni.
Figure 2a-c depict the quadratic variations over the three visits for EORTC 30 against demographic variables such as age, number of children, and tobacco habits. Figure 3a-c give the quadratic variations for the same variables for BR23. The equations show that there were no linear or constant trends in the mean scores for the HRQOL indices across the three time assessments. Though none of the variables were statistically significant for the changes in EORTC30 and BR23 mean scores, what is appreciably evident is that the best scores were immediately after surgery, and over time with the start and completion of the adjuvant therapy, the scores changed dramatically, as can be seen for the current sampled subjects.
Figure 3.
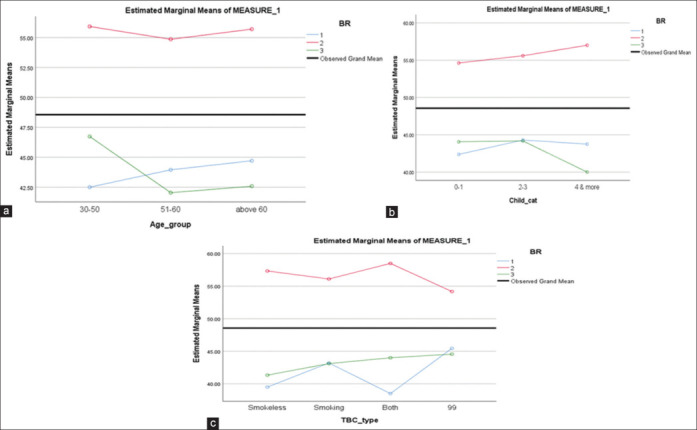
(a–c) depict BR 23 scores compared the number of living children, and type of tobacco addiction in the given sample of n = 46 subjects for three time assessments, regardless of the type of surgery
DISCUSSION
HRQOL studies in treated cancer patients are now gathering momentum as cancer care is becoming more comprehensive and multidisciplinary in approach. Contrary to the last decade, wherein cancer patients were treated by general surgeons and in noncomprehensive facilities, current awareness and accessibility to world-class facilities have seen a welcome change in cancer care. The sample in the study was more in the younger age group as compared to reported studies, where the age was mostly above 60 years,[12] which could be an indication of caution of breast cancer being detected in lower age groups in Asian populations, though the sample cannot be generalizable. Lifestyle factors, such as alcohol consumption being 26.1% in the study group was much higher than the 1.2%–2.7% alcohol use reported among women according to NNMS survey data. The group seeking care in this study were urban to semi-urban women. The changes in their risk factors are clearly visible from the data in Table 1.
In this study, the focus was on the use of HRQOL assessments as subjects of breast cancer are taken through the course of management, including surgery (BCS vs. MRM) as well as the CT and RT protocols. Most studies done in India have done the assessment for one or two visits at an interval of 6 months. Contrary to the study reported by the Munich Cancer Registry in 2004 (wherein a QoL survey using EORTC QLQ-C30 was taken up at regular intervals over 5 years), mastectomy patients had significantly (P < 0.01) lower body image, role, and sexual functioning scores and their lives were more disrupted than BCT patients.[12] However, in this study, both grand means for EORTC 30 and BR 23 calculated over three time visits did not show any difference for the types of surgery, being in the range of 66 and 48 points, respectively, for both surgeries. Despite the nonparametric nature of the data, given the limited sample size, mean scores were reported in results to maintain conformity in the way of reporting QOL scores. In addition, not much difference was seen between the median and the mean scores. Individual item scales were not compared in this study to have a sustained focus on the grand scores in the Indian context due to the paucity of reported literature in this study. The individual scales are discussed in a detailed article from the study. However, the findings may not be generalizable as the sample was only 46 subjects visiting one tertiary care set up in Bhubaneswar, Odisha, for treatment at the time of the COVID-19 pandemic. The Munich study also measured QOL annually for 5 years; thus, the scores indisputably report the annual QOL scores over a definite period. In contrast, in this study, the timings were planned to suit the researcher’s feasibility to report results; hence, three time points of assessment were taken within 1 year of the surgery.
In an Indian study on 534 breast cancer patients doing the assessment one time,[13] body image score and future perspective score were better in patients undergoing BCS compared to patients undergoing MRM. However, in both studies, no relation was reported between age groups and the scores. In the present article, the number of live children and tobacco habits were also accounted for and did not affect the mean scores. From the graphs, it is clearly evident that immediately post surgery, both EORTC 30 and BR23 were high, indicating good acceptance of the surgery as a procedure among the study participants. The beginning of adjuvant therapy caused a dip in the score, and post the completion of the therapy, the score further dipped.
This brings about an important sentinel need for psychotherapy and counseling at all points in cancer therapy, which have been strongly emphasized by several studies.[14,15,16,17] The patient may have been relieved from the burden of the cancer surgically; however, the ongoing treatments of RT and CT inflict greater trauma in terms of side effects and a lingering fear of the disease.
The study admits limitations of small sample size, single-center data, and the disadvantage of excluding severe modes of disease. It also perhaps encompasses the bias of added fear of the pandemic, wherein being able to get treated at a time when most health services were at a standstill may have been the reason for post-surgery high QOL scores and the subsequent fear of lingering pandemic, attributing to low scores. Financial concerns were evident among most of the patients, but discussing them was beyond the scope of this article.
Nevertheless, it does bring out very lucidly that both forms of surgeries are well received, even in this study wherein most women were above 30 years old as compared to other studies, where the age range of participants was even above 60 years. There is a need to dispel fears and concerns and even the symptomatic afflictions of the women treated after surgery, and the emphasis on empathetic follow-up is emphatically coming out in the study. More planned multicentric data in this direction is likely to offer better scientific evidence on HRQOL in breast cancer patients and its benefit in achieving better survival outcomes.
With the increasing awareness for breast cancer detection, there should be an amalgamation of good treatment outcomes as well as achieving an optimum QOL in the patients. This would work a great way to counter the phobia and stigma that continues to plague cancer management, especially among women of developed countries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Sathishkumar K, Chaturvedi M, Das P, Stephen S, Mathur P. Cancer incidence estimates for 2022 and projection for 2025: Result from National Cancer Registry Programme, India. Indian J Med Res. 2022;156:598–607. doi: 10.4103/ijmr.ijmr_1821_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashyap D, Pal D, Sharma R, Garg VK, Goel N, Koundal D, et al. Global increase in breast cancer incidence: Risk factors and preventive measures. Biomed Res Int. 2022;2022:9605439. doi: 10.1155/2022/9605439. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Purushottam AD, Pain SJ, Miles D, Harnett A. Variations in treatment and survival in breast cancer. Lancet Oncol. 2001;2:719–25. doi: 10.1016/s1470-2045(01)00585-x. [DOI] [PubMed] [Google Scholar]
- 4.Wu TY, Chang TW, Chang SM, Lin YY, Wang JD, Kuo YL. Dynamic changes of body image and quality of life in breast cancer patients. Cancer Manag Res. 2019;11:10563–71. doi: 10.2147/CMAR.S223314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salas M, Mordin M, Castro C, Islam Z, Tu N, Hackshaw MD. Health-related quality of life in women with breast cancer: A review of measures. BMC Cancer. 2022;22:66. doi: 10.1186/s12885-021-09157-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra I, Kamal KM. A systematic review of quality of life instruments in long-term breast cancer survivors. Health Qual Life Outcomes. 2012;10:14. doi: 10.1186/1477-7525-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Available from: https://qol.eortc.org/modules/ [Google Scholar]
- 8.Kshirsagar AS, Wani SK. Health-related quality of life in patients with breast cancer surgery and undergoing chemotherapy in Ahmednagar district. J Can Res Ther. 2021;17:1335–8. doi: 10.4103/jcrt.JCRT_154_19. [DOI] [PubMed] [Google Scholar]
- 9.Shandiz FH, Karimi FZ, Anbaran ZK, Abdollahi M, Rahimi N, Ghasemi M. Investigating the quality of life and the related factors in Iranian women with breast cancer. Asian Pac J Cancer Prev. 2017;18:2089–92. doi: 10.22034/APJCP.2017.18.8.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma N, Purkayastha A. Impact of radiotherapy on psychological, financial, and sexual aspects in postmastectomy carcinoma breast patients: A prospective study and management. Asia Pac J Oncol Nurs. 2017;4:69–76. doi: 10.4103/2347-5625.199075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birkett MA, Day SJ. Internal pilot studies for estimating sample size. Stat Med. 1994;13:2455–63. doi: 10.1002/sim.4780132309. [DOI] [PubMed] [Google Scholar]
- 12.Aberaraw R, Boka A, Teshome R, Yeshambel A. Social networks and quality of life among female breast cancer patients at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia 2019. BMC Women’s Health. 2020;20:50. doi: 10.1186/s12905-020-00908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engel J, Kerr J, Schlesinger-Raab A, Sauer H, Hölzel D. Quality of life following breast-conserving therapy or mastectomy: Results of a 5-year prospective study. Breast J. 2004;10:223–31. doi: 10.1111/j.1075-122X.2004.21323.x. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N, Pandey AK, Dimri K, Jyani G, Goyal A, Prinja S. Health-related quality of life among breast cancer patients in India. Support Care Cancer. 2022;30:9983–90. doi: 10.1007/s00520-022-07395-7. [DOI] [PubMed] [Google Scholar]
- 15.Enien MA, Ibrahim N, Makar W, Darwish D, Gaber M. Health-related quality of life: Impact of surgery and treatment modality in breast cancer. J Can Res Ther. 2018;14:957–63. doi: 10.4103/0973-1482.183214. [DOI] [PubMed] [Google Scholar]
- 16.Pakseresht S, Ingle GK, Garg S. Quality of life of women with breast cancer at the time of diagnosis in New Delhi. J Cancer Sci Ther. 2011;3:66–9. [Google Scholar]
- 17.Jyani G, Chauhan AS, Rai B, Ghoshal S, Srinivasan R, Prinja S. Health-related quality of life among cervical cancer patients in India. Int J Gynecol Cancer. 2020;30:1887–92. doi: 10.1136/ijgc-2020-001455. [DOI] [PubMed] [Google Scholar]


