Abstract
Background
Heart failure with preserved ejection fraction (HFpEF) has been observed to have a twice as high prevalence in women compared to men with similar predisposing risk factors between both sexes.
Objectives
This study aimed to identify sex-specific pathophysiological features in HFpEF using rest and exercise stress right heart catheterization (RHC), echocardiography and cardiovascular magnetic resonance imaging (CMR).
Methods
Seventy-five patients with exertional dyspnea, preserved ejection fraction (EF) (≥50%), and signs of diastolic dysfunction on echocardiography were prospectively recruited in the HFpEF Stress Trial. Patients underwent RHC, echocardiography and CMR at rest and during exercise stress. Patients were diagnosed with HFpEF and noncardiac dyspnea according to RHC measurements.
Results
After exclusion, the final study cohort comprised 68 patients (females n = 44, males n = 24) with a mean age of 66.9 ± 9.7 years. Compared to men, women with HFpEF revealed lower right ventricular stroke volumes during exercise stress (females 38.1 vs males 50.4 mL/m2 BSA; P = 0.011). This was accompanied by a decreasing left atrial EF in women but not men comparing resting to exercise conditions (females −2.7% vs males 2.5%, P = 0.020) and impaired left ventricular filling (females 35.5 vs males 44.2 mL/m2 BSA, P = 0.017) in women with HFpEF during exercise stress. These sex-specific differences were not present in noncardiac dyspnea.
Conclusions
Women with HFpEF demonstrate sex-specific functional alterations of right ventricular, left atrial, and left ventricular function during exercise stress. This unique pathophysiology represents a sex-specific diagnostic target, which may allow early identification of women with HFpEF for future individualized therapeutic approaches.
Key words: cardiac magnetic resonance imaging, exercise stress testing, heart failure with preserved ejection fraction, right heart catheterization, sex-specific diagnostic
Central Illustration
The incidence of heart failure with preserved ejection fraction (HFpEF) is on the rise, while HFpEF patients account for up to half of the heart failure population, already.1,2
Multiple risk factors have been described influencing pleiotropic cardiac remodeling resulting in HFpEF.3, 4, 5 Despite a quite similar distribution of those risk factors in between both sexes, studies have observed an approximately twice as high prevalence of HFpEF in women than in men.6, 7, 8 Indeed, women are at higher risk of developing hypertrophic remodeling compared to men, who tend to suffer from eccentric remodeling.9 Although women and men share the same risk for adverse outcomes in heart failure with reduced ejection fraction, inconsistent evidence exists on differing mortality rates in between both sexes in HFpEF.10, 11, 12 Varying onset of symptomatic burden initiating clinical consultation and differences in disease progression within both sexes could be a reason to heterogeneous mortality rates.13 Female patients tend to be more prone to metabolic stress than men14 and have an increased risk of congestive heart failure as a response to hypertension.15 Those pathophysiological findings are supported by therapy studies, showing that women with HFpEF are rather likely to benefit from pharmacological and device-based therapeutic interventions.16,17
Data on imaging biomarkers elucidating sex-specific pathophysiological differences in HFpEF are scarce. Patients with HFpEF are characterized by an inadequate cardiac response to physical exercise, which significantly impacts the diagnostic workup.18,19 Therefore, we hypothesized that specific and subtle hemodynamic changes could be detected using multimodal state-of-the-art diagnostic approaches. The present study employs rest and exercise stress right heart catheterization (RHC), echocardiographic and cardiovascular magnetic resonance imaging (CMR) to identify potential sex-specific differences in the hemodynamic response to exercise in patients with HFpEF. Identification of sex-specific pathophysiological features may promote the implementation of sex-specific diagnostic targets as well as thresholds in functional quantifications. This in turn may enable earlier diagnosis and more efficient therapeutic intervention in HFpEF.
Methods
Study population
The HFpEF Stress Trial prospectively recruited 75 patients referred for echocardiographic evaluation of exertional dyspnea between August 2017 and September 2019.20 Patients with exertional dyspnea (NYHA functional class ≥II), preserved ejection fraction (EF) (≥50%), and signs of diastolic dysfunction (E/e’ ≥8) were eligible for participation. Exclusion criteria were defined as typical contraindications for CMR,21 the presence of a pacemaker as well as other causes of dyspnea including pulmonary disease identified on spirometry (FEV1 <80%), coronary artery disease or cardiovascular disease including cardiomyopathies, ischemic, nonischemic, or valvular heart diseases.20 Patients had to be in sinus rhythm at the point of examination.
Patients were diagnosed with HFpEF based on invasive RHC thresholds of pulmonary capillary wedge pressure (PCWP) ≥15 mm Hg at rest or ≥25 mm Hg during exercise stress RHC according to current guideline recommendations.22 Simultaneously to RHC, a stress echocardiography was performed and followed by CMR within 24 hours.20
Patients without HFpEF were classified as patients with noncardiac dyspnea (NCD) in the absence of other pulmonary and cardiovascular diseases.
All echocardiographic, RHC and CMR surveys were performed by an individual experienced observer for the respective technique. All patients within the study were scanned by the same modality-specific operator. An independent experienced observer performed the final analysis of echocardiographic and CMR assessments while being blinded to the results of RHC.
The study was approved by the local ethics committee at the University Medical Center Göttingen, and all patients gave written informed consent prior to participation. The study was conducted according to the principles of the Helsinki Declaration and was funded by the German Center for Cardiovascular Research (DZHK-17) (NCT03260621).
Right heart catheterization and echocardiographic assessment
RHC was performed using a standardized protocol as previously described. Briefly, the exercise protocol was performed using a bicycle ergometer in the supine position with a 5-W increasing ramp protocol to achieve and maintain heart rates between 100 and 110 beats/min.20,23 Measurements included pulmonary artery pressure, PCWP, cardiac output indexed to the body surface area (BSA) (cardiac index), and the pulmonary vascular resistance. Effective arterial elastance was approximated as the quotient of systolic blood pressure and stroke volume at rest and during exercise stress as described previously.24
Echocardiographic assessments were conducted simultaneously to RHC at rest and during exercise stress.20 Measurements were performed in apical long-axis and parasternal short-axis views. Morphological parameters (ventricular and atrial dimensions) as well as functional parameters for diastolic and systolic phases were obtained using appropriate techniques including M-mode, pulse-wave and continuous-wave Doppler, respectively.20 Echocardiographic diastolic function was quantified using dedicated techniques as suggested by current guidelines.22 Speckle tracking of the left ventricle was conducted in longitudinal 2-, 3-, and 4-chamber views for the calculation of global longitudinal strain at rest and during exercise stress.20
Cardiovascular magnetic resonance imaging
CMR was performed on a 3.0-T Magnetom Skyra (Siemens Healthcare) using a 32-channel cardiac surface receiver coil. Exercise stress was conducted using the identical protocol compared to RHC on a dedicated CMR-compatible supine ergometer (Lode).
At rest, balanced steady-state free precession cine sequences were acquired in 2-, 3-, and 4-chamber long-axis views, as well as a short-axis stack with full coverage of the atria and ventricles. Volumetric parameters were derived from the short-axis stack. In addition to conventional functional imaging, feature-tracking deformation assessment was conducted for quantification of global longitudinal strain and global circumferential strain using commercially available software (2D CPA MR, Cardiac Performance Analysis, TomTec Imaging Systems).25 Feature-tracking results were based on the average of 3 consecutive measurements.
For postprocessing, a commercially available software was used (Medis, QMass, Medical Imaging Systems, Leiden, Netherlands).26
At rest and during exercise stress, real-time (RT) imaging was conducted using heavily under sampled balanced steady-state free precession sequences and iterative reconstruction as described by Uecker et al27 in 2- and 4-chamber views as well as a short-axis stack. Time-volume curves were generated for volumetric analysis of all 4 chambers (left atrium [LA]/left ventricle [LV]/right atrium [RA]/right ventricle [RV]) for the duration of a single heartbeat using the RT short-axis stack. Additionally, manual long axis strains for all 4 chambers of the heart were assessed using OsiriX MD (Pixmeo SARL).20 A RT phase-contrast acquisition28 of the pulmonary artery distal to the pulmonary valve allowed for the assessment of RV stroke and peak flow volumes at rest and during exercise stress.
The RA/RV/LA/LV filling reserve was defined as the increment of filling volumes from rest to stress. The LA filling volume equals the absolute value of the LA ejection volume as the calculation of both is based on the difference of the LA maximum volume and LA minimum volume. The ΔLA EF is calculated as the difference of LA EF between measurements at rest and under exercise stress.
Statistical analysis
Statistical analysis for sex differences were performed using SPSS version 27.0 (IBM) and GraphPad Prism 9 (GraphPad Software). Results were normalized to the BSA. Normal distribution was tested with a Shapiro-Wilk test. Results are plotted as median (IQR) for continuous variables and as frequencies with corresponding percentages for categorical variables, respectively. Using a nonparametric Mann-Whitney U test for continuous variables and chi-square test for categorical variables, significance levels were assessed for individual parameters.
Results
Study population
Of the initial study population (n = 75), 7 patients were excluded due to novel diagnosis of disease as outlined in the exclusion criteria in echocardiography or CMR (n = 4 coronary artery disease, n = 1 amyloidosis, n = 1 hypertrophic cardiomyopathy, n = 1 moderate aortic stenosis). The final study population of the HFpEF Stress Trial comprised 68 patients (n = 34 with HFpEF and n = 34 with NCD).20 Within the final cohort, 44 patients were female, 24 patients were male (HFpEF: female n = 25, male n = 9; NCD: female n = 19, male n = 15). Baseline characteristics, cardiovascular risk factors, and laboratory test results for HFpEF and NCD patients are reported in Table 1 (HFpEF) and Supplemental Table 1 (NCD). Baseline characteristics were distributed similarly comparing women and men with HFpEF (P ≥ 0.201) and NCD (P ≥ 0.074). Baseline characteristics and measurements according to sex within the overall cohort are reported in the Supplemental Tables 5 to 8.
Table 1.
Baseline Characteristics in Women and Men With Heart Failure With Preserved Ejection Fraction
| Women (n = 25) | Men (n = 9) | P Value | |
|---|---|---|---|
| Baseline characteristics | |||
| Age (y) | 69.0 (65.5;77.5) | 69.0 (67.0;73.5) | 0.848 |
| Atrial fibrillation | 11 (44) | 5 (55) | 0.551 |
| HFA-PEFF22 score | 5 (2, 6) | 6 (4.5, 6) | 0.335 |
| H2FPEF29 score | 5 (1.5, 8.5) | 6 (3.5, 8.5) | 0.280 |
| NYHA functional class | II (n = 14)III (n =11) | II (n = 7)III (n = 2) | 0.355 |
| Patients with antiarrhythmic drugs | 4 (16) | 1 (11) | 0.723 |
| Cardiovascular risk factors | |||
| Hypertension | 19 (76) | 8 (89) | 0.412 |
| BMI (kg/m2 BSA) | 29.3 (26.7-33.2) | 27.8 (25.7-35.6) | 0.759 |
| Elevated LDL | 16 (64) | 5 (55) | 0.655 |
| Diabetes | 3 (12) | 2 (22) | 0.458 |
| Active smoking | 3 (12) | 0 (0) | 0.201 |
| Laboratory testing | |||
| NT-pro-BNP (ng/L) | 267.9 (92.3-734.6) | 242.1 (141.0-395.5) | 0.939 |
| Creatinine (mg/dL) | 0.8 (0.7-1.0) | 1.0 (0.9-1.1) | 0.041 |
| eGFR (mL/min/1.73 m2) | 73.3 (56.9-87.8) | 75.0 (71.9-86.4) | 0.565 |
| Hemoglobin (g/dL) | 13.3 (12.9-13.9) | 15.0 (14.5-16.6) | <0.001 |
Values are median (IQR) (and were compared using the Mann-Whitney U test), or n (%) (and were compared using the chi-square test). Patient characteristics of patients with HFpEF as defined by right heart catheterization. The data are presented according to the sex. Bold values indicate statistical significance below 0.05.
BMI = body mass index; BSA = body surface area; eGFR = estimated glomerular filtration rate; NT-pro-BNP = N-terminal prohormone of brain natriuretic peptide; NYHA = New York Heart Association class.
Echocardiography and right heart catheterization
Echocardiographic and RHC measurements are reported in Table 2 (HFpEF) and Supplemental Table 2 (NCD). Echocardiography revealed no differences comparing women and men in HFpEF.
Table 2.
Echocardiography and Right Heart Catheterization Measurements in Patients With Heart Failure With Preserved Ejection Fraction
| Women (n = 25) | Men (n = 9) | P Value | |
|---|---|---|---|
| Echocardiography | |||
| LAVI (mL/m2) | 41.7 (34.9 to 52.0) | 49.7 (40.3 to 63.0) | 0.263 |
| E/e’ rest | 12.7 (10.3 to 14.1) | 10.8 (8.6 to 12.6) | 0.086 |
| E/e’ stress | 14.9 (11.4 to 16.9) | 10.9 (9.6 to 14.1) | 0.094 |
| PAPsys (mm Hg) | 27.2 (23.4 to 31.1) | 33.0 (22.6 to 39.1) | 0.263 |
| LV-GLS rest (%) | −15.3 (−17.4 to −11.9) | −11.6 (−14.8 to −9.1) | 0.110 |
| LV-GLS stress (%) | −13.9 (−15.8 to −12.4) | −15.9 (−17.1 to −9.3) | 0.685 |
| LV-GLS reserve (%) | 0.9 (−3.5 to −3.9) | −1.5 (−4.6 to 2.8) | 0.570 |
| LA strain rest (%) | 23.2 (12.7 to 30.9) | 20.3 (7.7 to 23.3) | 0.381 |
| LA strain stress (%) | 20.7 (13.3 to 24.5) | 21.4 (9.6 to 31.6) | 0.808 |
| Right heart catheterization | |||
| Heartrate rest (beats/min) | 73 (65 to 78) | 70 (58 to 77) | 0.514 |
| Heartrate stress (beats/min) | 108 (97 to 110) | 102 (96 to 113) | 0.759 |
| Blood pressure rest (mm Hg) | 148/81 (134/75 to 156/90) | 152/96 (140/76 to 168/104) | 0.414 |
| Blood pressure stress (mm Hg) | 173/94 (150/82 to 199/101) | 196/104 (157/92 to 207/126) | 0.409 |
| Maximum workload (W) | 40 (27 to 55) | 75 (60 to 82) | <0.001 |
| PCWP rest (mm Hg) | 13.0 (10.5 to 18.5) | 15.0 (11.0 to 17.5) | 0.818 |
| PCWP/CO rest (mm Hg·min/mL) | 2.7 (1.8 to 3.7) | 1.9 (1.4 to 2.5) | 0.120 |
| PCWP stress (mm Hg) | 27.0 (26.0 to 31.0) | 26.0 (24.5 to 30.0) | 0.280 |
| PCWP/CO stress (mm Hg·min/mL) | 2.1 (2.7 to 4.2) | 1.9 (1.7 to 2.4) | <0.001 |
| PCWP/workload (mm Hg/W) | 0.7 (0.5 to 1.1) | 0.3 (0.3 to 0.4) | <0.001 |
| PA pressure rest (mm Hg) | 21.0 (20.0 to 26.5) | 26.0 (19.0 to 30.0) | 0.730 |
| PA pressure stress (mm Hg) | 43.0 (39.0 to 52.0) | 47.0 (38.0 to 53.0) | 0.818 |
| CO rest (mL/min) | 5.2 (4.5 to 5.9) | 7.2 (6.2 to 8.3) | <0.001 |
| CI rest (L/m2 BSA) | 2.7 (2.4 to 3.0) | 3.1 (2.9 to 3.8) | 0.022 |
| CO stress (mL/min) | 8.7 (6.6 to 10.3) | 13.0 (9.9 to 15.0) | 0.002 |
| CI stress (L/m2 BSA) | 4.9 (3.6 to 5.3) | 6.2 (4.7 to 6.9) | 0.015 |
| CI reserve (L/m2 BSA) | 1.9 (1.3 to 2.4); 67 ± 36 | 2.8 (1.7 to 3.2); 81 ± 49 | 0.066 |
| PVR rest (WU) | 1.7 (±1.4 to 2.4) | 1.2 (1.1 to 1.5) | 0.033 |
| PVR stress (WU) | 1.6 (1.3 to 2.7) | 1.4 (1.0 to 1.6) | 0.318 |
| PVR difference (WU) | −0.1 (−0.4 to 0.1) | 0.1 (−0.2 to 0.3) | 0.514 |
| Arterial elastance rest (mm Hg/mL) | 2.1 (1.6 to 2.6) | 1.3 (1.1 to 1.7) | 0.004 |
| Arterial elastance stress (mm Hg/mL) | 1.9 (1.6 to 2.4) | 1.4 (1.1 to 1.7) | 0.015 |
Values are median (IQR) (and were compared using the Mann-Whitney U test) or mean ± SD. Echocardiographic and right heart catheterization measurements in patients with HFpEF as defined by right heart catheterization. Data are presented according to patients’ sex. Bold values indicate statistical significance below 0.05.
BSA = body surface area; CO = cardiac output; CI = cardiac index; E = passive mitral inflow; e’ = septal and lateral mitral annulus velocity; GLS = global longitudinal strain; LA = left atrium; LAVI = left atrial volume index; LV/RV = left/right ventricle; PA = pulmonary artery; PAPsys = pulmonary artery systolic pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance.
For RHC, there were no differences in pulmonary artery pressures and PCWP at rest and under exercise stress between women and men in HFpEF (pulmonary artery pressure: P ≥ 0.730; PCWP: P ≥ 0.280) and NCD (pulmonary artery pressure: P ≥ 0.537; PCWP: P ≥ 0.242). However, in HFpEF, the cardiac index was smaller in women compared to men both, at rest (female vs male −0.4 L/m2 BSA; P = 0.022) and during exercise stress (female vs male −1.3 L/m2 BSA P = 0.015) while in NCD a significant difference emerged during exercise stress only (female vs male −1.7 L/m2 BSA; P < 0.001). Despite a lower workload in women compared to men in general (P < 0.001), women had a higher PCWP per Watt compared to men in both, HFpEF (female vs male 0.4 mm Hg/W, P < 0.001) and NCD (female vs male 0.2 mm Hg/W; P < 0.003).
Female patients with HFpEF had the numerically lowest increment of cardiac index from rest to stress compared to men with HFpEF (female HFpEF vs male HFpEF −0.9 L/m2 BSA (−14%); P = 0.066) and female patients with NCD (female HFpEF vs female NCD −0.3 L/m2 BSA (−10%); P = 0.591), even though not reaching statistical significance.
At rest, arterial elastance was higher in women compared to men with HFpEF (female vs male 0.8 mm Hg/mL, P = 0.004), while there was no difference in patients with NCD (female vs male −0.3 mm Hg/mL, P = 0.105). Meanwhile during exercise stress, arterial elastance was higher in female patients with HFpEF (female vs male 0.5 mm Hg/mL; P = 0.015) and NCD (female vs male 0.6 mm Hg/mL; P < 0.001).
Comprehensive analysis in cardiac magnetic resonance imaging
Conventional CMR-derived parameters are displayed in Table 3 (HFpEF) and Supplemental Table 3 (NCD), RT CMR assessments at rest and during exercise stress are shown in Table 4 (HFpEF) and Supplemental Table 4 (NCD).
Table 3.
Conventional Cardiac Magnetic Resonance Imaging Measurements in Patients With Heart Failure With Preserved Ejection Fraction
| Women (n = 25) | Men (n = 9) | P Value | |
|---|---|---|---|
| CMR – right ventricle | |||
| RV EDV (mL) | 110.5 (97.8 to 134.8) | 164.3 (145.4 to 209.8) | <0.001 |
| RV EDV (mL/m2 BSA) | 59.9 (53.3 to 69.6) | 77.1 (69.9 to 89.5) | <0.001 |
| RV ESV (mL) | 33.7 (28.6 to 42.6) | 60.7 (46.5 to 74.6) | <0.001 |
| RV ESV (mL/m2 BSA) | 19.2 (15.8 to 21.6) | 27.6 (22.2 to 33.7) | <0.001 |
| RV SV (mL) | 73.0 (67.4 to 98.7) | 101.0 (89.9 to 137.8) | 0.005 |
| RV SV (mL/m2 BSA) | 40.5 (37.4 to 49.2) | 48.1 (45.1 to 57.2) | 0.072 |
| RV EF (%) | 68.4 (63.8 to 74.6) | 65.1 (58.0 to 68.3) | 0.050 |
| CMR – left ventricle | |||
| LV Mass (g/m2 BSA) | 53.9 (50.7 to 60.2) | 67.7 (65.5 to 77.2) | 0.001 |
| LV EDV (mL) | 120.5 (105.4 to 144.6) | 160.3 (139.7 to 204.4) | <0.001 |
| LV EDV (mL/m2 BSA) | 64.9 (56.2 to 73.9) | 75.2 (68.9 to 93.6) | 0.008 |
| LV ESV (mL) | 34.1 (26.7 to 45.7) | 58.6 (44.0 to 67.3) | <0.001 |
| LV ESV (mL/m2 BSA) | 17.0 (14.2 to 24.4) | 25.8 (20.4 to 32.0) | 0.009 |
| LV SV (mL) | 89.2 (77.4 to 101.3) | 114.2 (97.9 to 133.8) | 0.005 |
| LV SV (mL/m2 BSA) | 48.5 (42.0 to 53.8) | 51.9 (45.8 to 60.9) | 0.14 |
| LV EF (%) | 73.4 (67.1 to 76.9) | 66.8 (62.0 to 74.6) | 0.12 |
| GLS (%) | −17.3 (−19.1 to −16.3) | −17.9 (−19.2 to −15.0) | 0.818 |
| CMR – left atrium | |||
| LAVI (mL/m2) | 46.7 (36.1 to 55.1) | 51.0 (39.7 to 69.1) | 0.316 |
| LA min volume rest (mL) | 42.3 (33.9 to 53.4) | 56.1 (45.0 to 76.7) | 0.048 |
| LA min volume stress (mL) | 49.0 (38.6 to 65.8) | 56.3 (49.6 to 90.4) | 0.224 |
| LA min volume reserve (mL) | 6.7 (2.4 to 12.6) | 4.4 (−4.3 to 11.3) | 0.281 |
| LA max volume rest (mL) | 62.8 (52.7 to 77.9) | 88.8 (72.7 to 103.6) | 0.013 |
| LA max volume stress (mL) | 72.1 (56.8 to 90.7) | 100.0 (77.5 to 111.0) | 0.023 |
| LA max volume reserve (mL) | 8.5 (2.8 to 13.0) | 8.4 (−1.1 to 13.8) | 0.929 |
Values are median (IQR) (and were compared using the Mann-Whitney U test). Conventional CMR measurements in patients with HFpEF as defined by right heart catheterization. Data are presented according to patients’ sex. Bold values indicate statistical significance below 0.05.
BSA = body surface area; EDV/ESV = end-diastolic volume/end-systolic volume; EF = ejection fraction; GLS = global longitudinal strain; LA = left atrium; LAVI = left atrial volume index; LV/RV = left/right ventricle; SV = stroke volume.
Table 4.
Cardiac Magnetic Resonance Imaging Real-Time Functional Parameters in Patients With Heart Failure With Preserved Ejection Fraction
| Women (n = 25) | Men (n = 9) | P Value | |
|---|---|---|---|
| Right atrium | |||
| RA LAS rest (%) | 28.3 (23.4 to 33.3) | 26.3 (23.3 to 33.3) | 0.848 |
| RA LAS stress (%) | 32.0 (25.1 to 38.0) | 32.7 (26.9 to 36.9) | 0.815 |
| RA filling rest (mL) | 30.9 (23.4 to 36.9) | 42.4 (32.8 to 54.2) | 0.005 |
| RA filling rest (mL/m2 BSA) | 15.9 (12.3 to 19.2) | 21.6 (15.5 to 23.4) | 0.041 |
| RA filling stress (mL) | 39.7 (33.1 to 47.7) | 55.1 (49.8 to 59.8) | 0.005 |
| RA filling stress (mL/m2 BSA) | 21.8 (18.5 to 24.8) | 26.2 (23.3 to 27.2) | 0.048 |
| RA filling reserve (mL/m2 BSA) | 5.2 (2.7 to 8.2) | 4.1 (1.7 to 8.5) | 0.929 |
| Right ventricle | |||
| RV LAS rest (%) | 26.9 (24.1 to 30.4) | 21.9 (20.8 to 25.4) | 0.029 |
| RV LAS stress (%) | 27.1 (20.8 to 30.1) | 26.3 (22.7 to 29.6) | 0.815 |
| Flow: stroke volume rest (mL) | 74.4 (57.8 to 88.7) | 109.6 (92.9 to 126.2) | <0.001 |
| Flow: stroke volume rest (mL/m2 BSA) | 39.2 (31.4 to 45.7) | 50.3 (43.9 to 57.3) | 0.004 |
| Flow: stroke volume stress (mL) | 74.1 (60.9 to 88.6) | 110.8 (104.5 to 128.3) | 0.002 |
| Flow: stroke volume stress (mL/m2 BSA) | 38.1 (35.6 to 49.3) | 50.4 (48.6 to 58.8) | 0.011 |
| RV filling rest (mL) | 32.2 (25.2 to 42.2) | 42.7 (26.2 to 69.6) | 0.015 |
| RV filling rest (mL/m2 BSA) | 18.4 (13.9 to 21.3) | 22.8 (16.4 to 29.7) | 0.120 |
| RV filling stress (mL) | 32.7 (26.1 to 38.9) | 47.3 (28.2 to 80.5) | 0.098 |
| RV filling stress (mL/m2 BSA) | 17.7 (14.3 to 20.9) | 24.1 (13.7 to 34.1) | 0.352 |
| RV filling reserve (mL/m2 BSA) | −0.1 (−2.9 to 2.9) | 1.0 (−3.3 to 4.8) | 0.696 |
| Left atrium | |||
| LA EF rest (%) | 34.1 (27.3 to 38.6) | 36.8 (21.1 to 38.7) | 0.789 |
| LA EF stress (%) | 31.9 (22.6 to 36.3) | 39.6 (18.5 to 45.4) | 0.242 |
| ΔLA EF (%) | −2.7 (−7.5 to 0.8) | 2.5 (−1.5 to 6.4) | 0.020 |
| LA LAS rest (%) | 15.3 (10.8 to 19.8) | 16.1 (10.8 to 19.8) | 0.730 |
| LA LAS stress (%) | 15.6 (11.9 to 18.6) | 17.5 (10.1 to 23.2) | 0.591 |
| LA filling rest (mL) | 20.9 (17.9 to 25.3) | 26.3 (20.5 to 32.7) | 0.148 |
| LA filling rest (mL/m2 BSA) | 11.8 (8.8 to 13.8) | 11.5 (9.7 to 16.8) | 0.688 |
| LA filling stress (mL) | 23.1 (17.3 to 26.7) | 37.0 (20.5 to 43.2) | 0.038 |
| LA filling stress (mL/m2 BSA) | 11.6 (9.3 to 14.3) | 16.6 (9.8 to 18.1) | 0.112 |
| LA filling reserve (mL) | 0.8 (−3.0 to 3.75) | 4.0 (1.6 to 10.5) | 0.023 |
| LA filling reserve (mL/m2 BSA) | −0.4 (−1.9 to 1.6) | 2.1 (5.6 to 0.7) | 0.030 |
| Left ventricle | |||
| LV LAS rest (%) | 13.2 (10.6 to 15.4) | 13.7 (11.9 to 15.2) | 0.565 |
| LV LAS stress (%) | 13.1 (11.9 to 17.5) | 16.4 (16.0 to 18.7) | 0.011 |
| LV filling rest (mL) | 53.2 (45.2 to 64.5) | 74.4 (64.6 to 89.9) | 0.010 |
| LV filling rest (mL/m2 BSA) | 29.1 (24.7 to 34.2) | 34.5 (26.4 to 42.9) | 0.107 |
| LV filling stress (mL) | 68.8 (55.9 to 75.0) | 101.4 (84.4 to 118.4) | <0.001 |
| LV filling stress (mL/m2 BSA) | 35.5 (30.7 to 40.3) | 48.0 (34.9 to 56.8) | 0.017 |
| LV filling reserve (mL/m2 BSA) | 5.3 (3.4 to 12.2) | 10.8 (7.4 to 18.3) | 0.061 |
Values are median (IQR) (and were compared using the Mann-Whitney U test). Functional real-time CMR derived parameters in patients with HFpEF as defined by right heart catheterization. Data are presented according to the patients’ sex. BoldP values indicate statistical significance below 0.05.
BSA = body surface area; EF = ejection fraction; LA/RA = left/right atrium; LAS = long-axis strain; LV/RV = left/right ventricular.
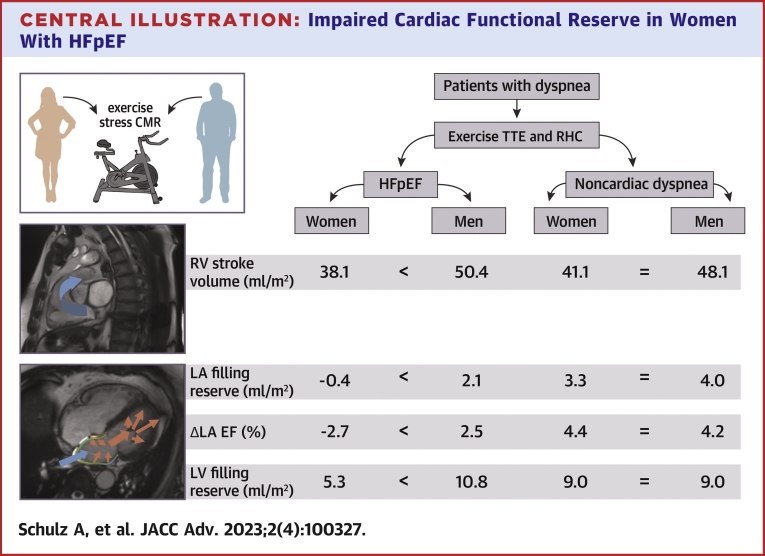
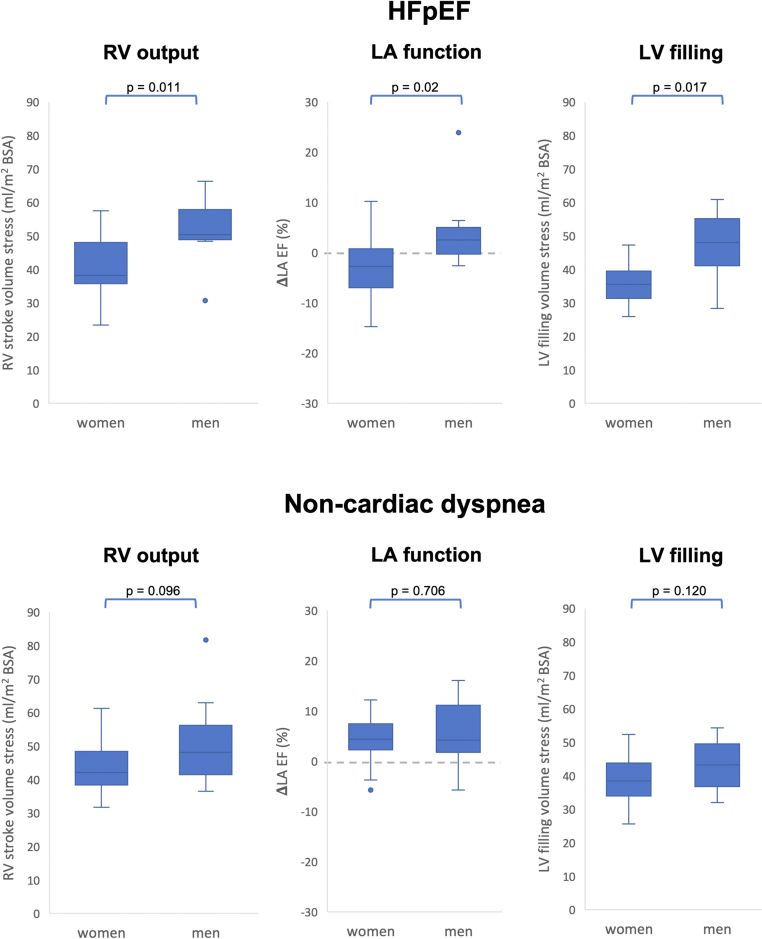
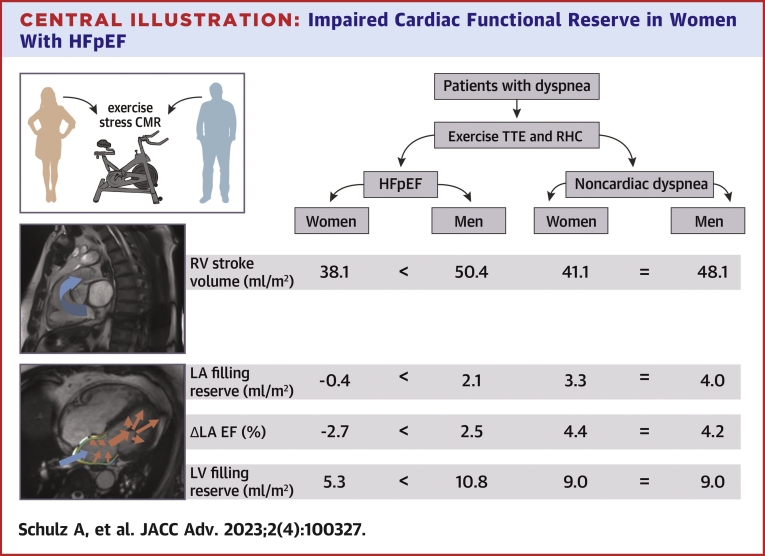
Selected and major CMR-derived functional differences between women in men in HFpEF compared to NCD are demonstrated in Table 5, Figure 1, and the Central Illustration. At rest, both in HFpEF and NCD, female patients had lower RV output as appreciated by lower RV stroke volumes (HFpEF: female vs male −11.1 mL/m2 BSA, P = 0.004; NCD: female vs male −13.4 mL/m2 BSA, P = 0.027). During exercise stress, however, RV strokes volumes were smaller in women with HFpEF (female vs male −12.3 mL/m2 BSA; P = 0.011) while in women with NCD this difference was numerically smaller but not statistically significant (female vs male −6 mL/m2 BSA; P = 0.096).
Table 5.
Differences in Cardiac Magnetic Resonance Imaging Parameters in Patients With Noncardiac Dyspnea Compared to Patients With Heart Failure With Preserved Ejection Fraction
| Noncardiac Dyspnea |
HFpEF |
P Value |
||||||
|---|---|---|---|---|---|---|---|---|
| Women (n = 19) | Men (n = 15) | P Value | Women (n = 25) | Men (n = 9) | P Value | NCD vs HFpEF (Women) | NCD vs HFpEF (Men) | |
| Right ventricle | ||||||||
| Flow: stroke volume rest (mL/m2 BSA) | 36.7 (34.4 to 44.9) | 50.1 (34.6 to 56.1) | 0.027 | 39.2 (31.4 to 45.7) | 50.3 (43.9 to 57.3) | 0.004 | 0.883 | 0.861 |
| Flow: stroke volume stress (mL/m2 BSA) | 42.1 (38.2 to 49.6) | 48.1 (41.3 to 56.3) | 0.096 | 38.1 (35.6 to 49.3) | 50.4 (48.6 to 58.8) | 0.011 | 0.211 | 0.558 |
| Left atrium | ||||||||
| ΔLA EF (%) | 4.4 (2.1 to 7.5) | 4.2 (1.7 to 11.7) | 0.706 | −2.7 (−7.5 to 0.8) | 2.5 (−1.5 to 6.4) | 0.020 | <0.001 | 0.447 |
| LA filling reserve (mL/m2 BSA) | 3.3 (2.1 to 4.4) | 4.0 (1.8 to 5.4) | 0.758 | −0.4 (−1.9 to 1.6) | 2.1 (5.6 to 0.7) | 0.030 | <0.001 | 0.447 |
| Left ventricle | ||||||||
| LV filling rest (mL/m2 BSA) | 28.7 (23.7 to 35.0) | 33.4 (27.5 to 38.2) | 0.157 | 29.1 (24.7 to 34.2) | 34.5 (26.4 to 42.9) | 0.107 | 0.840 | 0.681 |
| LV filling stress (mL/m2 BSA) | 38.4 (32.7 to 44.1) | 43.2 (33.8 to 49.6) | 0.120 | 35.5 (30.7 to 40.3) | 48.0 (34.9 to 56.8) | 0.017 | 0.201 | 0.325 |
| LV filling reserve (mL/m2 BSA) | 9.0 (6.6 to 14.6) | 9.0 (5.1 to 15.9) | 0.973 | 5.3 (3.4 to 12.2) | 10.8 (7.4 to 18.3) | 0.061 | 0.126 | 0.428 |
Values are median (IQR) (and were compared using the Mann-Whitney U test). Main pathophysiological findings derived by real-time CMR in patients with HFpEF and NCD as defined by right heart catheterization. Data are presented according to the patients’ sex. BoldP values indicate statistical significance below 0.05.
BSA = body surface area; EF = ejection fraction; LA = left atrium; LV/RV = left/right ventricular.
Figure 1.
Functional Parameters in Patients With HFpEF and NCD During Exercise Stress
Functional parameters in patients with HFpEF (top row) and NCD (bottom row). Displayed is the right ventricular (RV) stroke volume under exercise stress, the ΔLA EF (left atrial ejection fraction) in % and the left ventricular (LV) filling under exercise stress in women and men suffering from HFpEF and NCD. P values below 0.05 are considered statistically significant. HFpEF = heart failure with preserved ejection fraction; NCD = noncardiac dyspnea.
Central Illustration.
Impaired Cardiac Functional Reserve in Women With HFpEF
Under exercise stress, women with HFpEF, but not men or patients with NCD revealed lower right ventricular (RV) stroke volumes, a lower increase of left atrial (LA) filling, a decrease of the LA ejection fraction (EF) and lower increase of left ventricular (LV) filling volumes. < indicates differences in absolute volumes between the groups while = indicates no statistically significant differences in absolute volumes between the groups. HFpEF = heart failure with preserved ejection fraction; NCD = noncardiac dyspnea.
After dichotomization at the median PCWP of women with HFpEF at rest and during exercise stress, female patients with HFpEF and lower PCWP did not show significant differences of right heart functional parameters at rest (P > 0.311) or during exercise stress (P > 0.208) compared to women with higher PCWP as shown in Supplemental Tables 9 and 10.
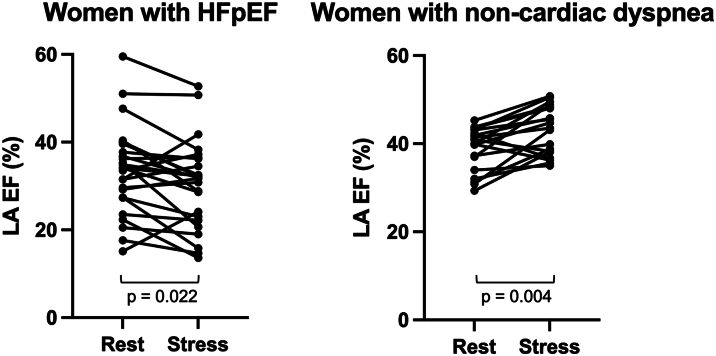
In HFpEF, female patients showed a reduced LA filling from rest to stress compared to men (female vs male −2.5 mL/m2 BSA, P = 0.030) but no difference for the increase of the LA maximum volume from rest to stress (female vs male 0.1 mL, P = 0.929). In contrast, women with NCD had showed no differences for both, the increase of LA filling (female vs male −0.7 mL/m2 BSA, P = 0.758) and LA maximum volume (female vs male −4.6 mL, P = 0.410) from rest to stress compared to men with NCD. This was paralleled by a decrease in LA EF in response to exercise stress in women with HFpEF but not men (female −2.7 vs male 2.5%, P = 0.020) as displayed in Figure 1. In contrast, in NCD, no sex-specific difference in LA EF in response to exercise stress was seen as both women and men with NCD showed an increase of LA EF (NCD: female 4.4% vs male 4.2%, P = 0.706) during exercise stress (Figures 1 and 2).
Figure 2.
Left Atrial Ejection Fraction in Women With HFpEF and NCD
Displayed is the left atrial (LA) ejection fraction (EF, %) at rest and under exercise stress in women with HFpEF and NCD. While female patients with HFpEF showed a trend of decreasing EF under exercise stress, women with NCD were more likely to increase their ejection fraction under exercise stress. P values below 0.05 were considered as significant. HFpEF = heart failure with preserved ejection fraction; NCD = noncardiac dyspnea.
LV filling was not compromised at rest, with similar filling volumes in women with HFpEF compared to men (female vs male −5.1 mL/m2 BSA, P = 0.107) as well as in women with HFpEF compared to women with NCD (female HFpEF vs female NCD 0.4 mL/m2 BSA, P = 0.840). However, during exercise stress women with HFpEF had lower LV filling volumes compared to men (female vs male −12.5 mL/m2 BSA, P = 0.017) (Figure 1). This sex-specific difference of LV filling during exercise stress was not significant in NCD (female vs male −4.8 mL/m2 BSA, P = 0.120).
Discussion
This study demonstrates a noninvasive approach to quantify sex-specific cardiac functional differences in the pathophysiology of HFpEF. Women showed a lower RV cardiac output and an impaired augmentation of LA EF and LA filling during exercise stress compared to men. In addition, women revealed reduced LV filling volumes, specifically women with HFpEF. Importantly, these sex-specific functional differences were not present comparing women to men in the NCD population. Consequently, these findings underline a complex interplay of both, the right and the left side of the heart in HFpEF and especially the female population, which in turn may contribute to higher incidence of symptomatic women in HFpEF. Functional impairment can be unmasked and quantified using comprehensive noninvasive RT exercise stress CMR.
Previous studies outlined the importance of right ventricular dysfunction in patients with HFpEF.30,31 As assessed by conventional volumetric measurement at rest, women showed a similar or higher RV EF compared to men, which is in line with previously published data.32 Notwithstanding, employing state-of-the-art RT exercise stress CMR unmasked impaired RV output in women compared to men as appreciated from RV stroke volume quantification. Beyond restrictive RV physiology, RV remodeling with diffuse fibrosis could be found in the general HFpEF population, which might substantially contribute to right heart failure.33, 34, 35 An additional underlying reason for RV functional failure could be latent pulmonary disease in women which is in line with previous pathophysiological observations in HFpEF,36,37 and the present study population in which women showed increased pulmonary vascular resistance compared to men.
While the underlying reason to this adverse remodeling has yet to be elucidated, a possible explanation could originate in a higher left ventricular and arterial stiffness in women compared to men and with a more pronounced increase of the stiffness with higher age.36,37 Hypothetically, increased LV stiffness in women with subsequently higher pressure in the upstream pulmonary and RV circulation might be the reason to higher rates of postcapillary hypertension in female sex and could be accompanied by RV and pulmonary vascular remodeling. It remains unclear which functional alterations in RV, pulmonary, or LV physiology are the initial trigger to the pathophysiological changes.36
The LA has been reported to show morphological differences between both sexes and female sex was determined as an independent risk factor for atrial fibrosis.38,39 However, left atrial function did only show small or no differences in healthy patient cohorts, which often disappeared as soon as they were indexed to the BSA.39,40 Further assessments of LA stiffness and compliance using proposed methods in the literature41 could aid to identify potential sex-specific causalities leading to the observed worsening of RV function due to an impaired RV-PA coupling. The present data demonstrate that women suffering from HFpEF are prone to LA functional failure during exercise stress as shown by an exercise-induced decrease of LA EF. Atrial functional reserve is an essential part of the diastolic cardiac function including the atrial booster pump and atrial conduit function42 and its impairment during exercise stress results in a direct worsening of LV filling. Apart from established parameters, the LA EF might indicate atrial cardiomyopathy with diminished atrial function and goes beyond conventional LV diastolic dysfunction. Earlier studies already described atrial cardiomyopathy as an independent entity43 which plays an important role for diastolic dysfunction, but may exist out of proportion to LV myopathy in HFpEF.41,44,45
As the augmentation of LA maximum filling during exercise stress revealed no sex-specific differences, the lower increment of the LA ejection volume and EF in women during exercise stress is most likely caused by increased LV pressure during exercise stress as observed in previous studies.37 In addition, higher ventricular stiffness in women46 potentially contributes to an increased strain on the LA with a reduced LA EF during exercise stress and subsequently impaired LV filling. This theory is supported by the increased arterial elastance in women in general, while in particular women with HFpEF showed higher values of arterial elastance at rest and during exercise stress. As there was no difference in the median HR at rest and exercise stress, this proposes a higher systemic vascular resistance in women47 with subsequent higher arterial pulsatile and a close association with atrial dysfunction48 which might impact and aggravate the observed LA and LV dysfunction. This suggests worse LV-LA coupling to trigger increased RV pulsatile loading with a lower RV stroke volume in women with HFpEF and argues against innate RV dysfunction with lower RV stroke volume to primarily cause impaired atrial function. However, women with HFpEF in this study did not show RV functional deterioration with increased PCWP at rest and during exercise stress. This could be subject to the small number of individuals in this study or explained by an additional innate right heart failure in women with HFpEF.
The combination of intrinsic LA functional failure with reduced preload and impaired RV mechanics as well as increased afterload imposed by congestion in LV diastolic dysfunction might result in the inability of particularly female HFpEF patients to cope with increased volume challenges and hemodynamic demands imposed by exercise stress. Indeed, these functional sex-specific differences were not seen in the NCD population. A recent study demonstrated an inability of HFpEF patients with LV EF >60% to augment LV filling and stroke volume during exercise stress which was associated with a diminished preload reserve in this patient group.49 In the present study, both male and female patients with HFpEF had an LV EF >60% while the investigated cohort of Rosch et al49 consisted of 80% female patients, which could explain similarities in the findings compared to the present study population of female HFpEF patients. The female patients in the present study presented with a likewise impaired LV filling reserve and preload reserve as appreciated by the reduced RV stroke volume and LA filling. Due to the coincidental overlap of both study cohorts, there could be a link between the impaired mechanics at an LV EF >60% and female sex. Those failing RV and LA and LV mechanics with a reduction in LV filling and output could expose women to an earlier and more pronounced symptom onset. Consequently, these findings may offer a potential explanation for higher symptomatic burden in women with HFpEF as opposed to men and could be the reason for higher incidence of symptomatic HFpEF in female patients in general. This in turn could result in an earlier and higher rate of definite diagnosis of HFpEF compared to men.
The demonstrated functional impairment in the left and right side of the heart during exercise stress in women suffering from HFpEF offers an explanation for a distinct progression of the disease. In addition, the identification of functional alterations in women might help to determine pathophysiological pathways leading to symptomatic HFpEF in general and aid to define features to discriminate patients at risk for CV events and hospitalization in the future.
STUDY Limitations
The HFpEF Stress trial was a monocentric study conducted in an experienced CMR core laboratory, thus data have not been tested on its reproducibility in other centers. While no dedicated intraobserver reliability assessment was performed within this study, all parameters were assessed following a standardized training50 which guarantees a proven high interobserver and intraobserver agreement for the measured parameters within our core-lab.26,51, 52, 53 Included patients were highly selected in the initial study to ensure a bias-free interpretation of the diastolic dysfunction and might not represent the general population. Observed differences in between men and women with HFpEF and NCD might be subject to further comorbidities or lifestyle habits which have not been investigated within this study but should be considered in future research. Furthermore, this cohort was rather small with an imbalance of men and women in the cohort of patients with HFpEF. This implies that some of the results could just be reported as a trend without being statistically significant. In particular, the cohort of men was small in this study, implying that the reported findings will have to be confirmed in larger and multicentric trials in the future. RT imaging for the assessment RV and LV volumes can be prone to errors by, eg, through the plane motion of free breathing,20 and might have impaired the results by leading to an underestimation of the volumes. While this study just proposes potential pathophysiological mechanisms predisposing women to HFpEF, further dedicated studies will be needed to confirm the results and investigate subsequent diagnostic implications.
Conclusions
Women suffering from HFpEF showed a reduced RV output, an impaired LA function, and a reduced LV filling during exercise stress. Presumably, a worse LV-LA coupling could result in higher RV afterload with subsequent RV dysfunction, however, this mechanism must undergo further validation. The diminished LA function under exercise stress could be a crucial part of diastolic failure in women. This pathophysiological observation in women with HFpEF, with a complex interplay of the right and left side of the heart, should be subject to a more detailed investigation in the future.
Funding support and author disclosures
The study was carried out using the clinical–scientific infrastructure of the German Center for Cardiovascular Research (DZHK-17). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE 1: This manuscript describes sex-specific features of cardiac functional impairment in HFpEF during exercise stress.
COMPETENCY IN MEDICAL KNOWLEDGE 2: The observed pathophysiology might contribute to a more frequent diagnosis of HFpEF in women, as the disease is unmasked by earlier symptoms compared to men. Those distinct features should be considered while diagnosing HFpEF in patients of both sexes suffering from dyspnea.
TRANSLATIONAL OUTLOOK: State-of-the-art diagnostic approaches yield the capabilities for more individualized and sex-specific care in clinical practice. Meanwhile, those mechanisms could enhance therapy studies, even though they require further investigations in multicentric trials.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Supplementary data
References
- 1.Tsao C.W., Lyass A., Enserro D., et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. J Am Coll Cardiol HF. 2018;6:678–685. doi: 10.1016/j.jchf.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owan T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M., et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Schulz A., Schuster A. Visualizing diastolic failure: non-invasive imaging-biomarkers in patients with heart failure with preserved ejection fraction. EBioMedicine. 2022;86 doi: 10.1016/j.ebiom.2022.104369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeWinter M.M., Meyer M. Mechanisms of diastolic dysfunction in heart failure with a preserved ejection fraction. Circ Heart Fail. 2013;6:1112–1115. doi: 10.1161/CIRCHEARTFAILURE.113.000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borlaug B.A., Paulus W.J. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borlaug B.A., Redfield M.M. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–2014. doi: 10.1161/CIRCULATIONAHA.110.954388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masoudi F.A., Havranek E.P., Smith G., et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41:217–223. doi: 10.1016/s0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 8.Lee D.S., Gona P., Vasan R., et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham heart study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piro M., DellaBona R., Abbate A., Biasucci L.M., Crea F. Sex-related differences in myocardial remodeling. J Am Coll Cardiol. 2010;55:1057–1065. doi: 10.1016/j.jacc.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 10.Russo G., Rea F., Barbati G., et al. Sex-related differences in chronic heart failure: a community-based study. J Cardiovasc Med (Hagerstown) 2021;22:36–44. doi: 10.2459/JCM.0000000000001049. [DOI] [PubMed] [Google Scholar]
- 11.Sun J., Tai S., Guo Y., et al. Sex differences in characteristics and outcomes in elderly heart failure patients with preserved ejection fraction: a post-hoc analysis from TOPCAT. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.721850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotomi Y., Hikoso S., Nakatani D., et al. Sex differences in heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10 doi: 10.1161/JAHA.120.018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Regitz-Zagrosek V. Sex and gender differences in heart failure. Int J Heart Fail. 2020;2:157–181. doi: 10.36628/ijhf.2020.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeSimone G., Devereux R.B., Chinali M., et al. Sex differences in obesity-related changes in left ventricular morphology: the Strong Heart Study. J Hypertens. 2011;29:1431–1438. doi: 10.1097/HJH.0b013e328347a093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy D., Larson M.G., Vasan R.S., Kannel W.B., Ho K.K. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 16.Shah S.J., Borlaug B.A., Chung E.S., et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022;399(10330):1130–1140. doi: 10.1016/S0140-6736(22)00016-2. [DOI] [PubMed] [Google Scholar]
- 17.McMurray J.J.V., Jackson A.M., Lam C.S.P., et al. Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction. Circulation. 2020;141:338–351. doi: 10.1161/CIRCULATIONAHA.119.044491. [DOI] [PubMed] [Google Scholar]
- 18.Melenovsky V., Borlaug B.A., Rosen B., et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49:198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 19.Borlaug B.A., Nishimura R.A., Sorajja P., Lam C.S.P., Redfield M.M. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backhaus S.J., Lange T., George E.F., et al. Exercise stress real-time cardiac magnetic resonance imaging for noninvasive characterization of heart failure with preserved ejection fraction. Circulation. 2021;143:1484–1498. doi: 10.1161/CIRCULATIONAHA.120.051542. [DOI] [PubMed] [Google Scholar]
- 21.Kramer C.M., Barkhausen J., Flamm S.D., et al. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. doi: 10.1186/1532-429X-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pieske B., Tschope C., de Boer R.A., et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
- 23.Erdei T., Smiseth O.A., Marino P., Fraser A.G. A systematic review of diastolic stress tests in heart failure with preserved ejection fraction, with proposals from the EU-FP7 MEDIA study group. Eur J Heart Fail. 2014;16:1345–1361. doi: 10.1002/ejhf.184. [DOI] [PubMed] [Google Scholar]
- 24.Kelly R.P., Ting C.T., Yang T.M., et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86(2):513–521. doi: 10.1161/01.CIR.86.2.513. [DOI] [PubMed] [Google Scholar]
- 25.Schuster A., Hor K.N., Kowallick J.T., Beerbaum P., Kutty S. Cardiovascular magnetic resonance myocardial feature tracking: concepts and clinical applications. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.004077. [DOI] [PubMed] [Google Scholar]
- 26.Gertz R.J., Lange T., Kowallick J.T., et al. Inter-vendor reproducibility of left and right ventricular cardiovascular magnetic resonance myocardial feature-tracking. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uecker M., Zhang S., Voit D., Karaus A., Merboldt K.D., Frahm J. Real-time MRI at a resolution of 20 ms. NMR Biomed. 2010;23:986–994. doi: 10.1002/nbm.1585. [DOI] [PubMed] [Google Scholar]
- 28.Joseph A.A., Merboldt K.D., Voit D., et al. Real-time phase-contrast MRI of cardiovascular blood flow using undersampled radial fast low-angle shot and nonlinear inverse reconstruction. NMR Biomed. 2012;25:917–924. doi: 10.1002/nbm.1812. [DOI] [PubMed] [Google Scholar]
- 29.Reddy Y.N.V., Carter R.E., Obokata M., Redfield M.M., Borlaug B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obokata M., Reddy Y.N.V., Melenovsky V., Pislaru S., Borlaug B.A. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J. 2019;40:689–697. doi: 10.1093/eurheartj/ehy809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammed S.F., Hussain I., AbouEzzeddine O.F., et al. Right ventricular function in heart failure with preserved ejection fraction. Circulation. 2014;130:2310–2320. doi: 10.1161/CIRCULATIONAHA.113.008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duca F., Zotter-Tufaro C., Kammerlander A.A., et al. Gender-related differences in heart failure with preserved ejection fraction. Sci Rep. 2018;8:1080. doi: 10.1038/s41598-018-19507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melenovsky V., Hwang S.-J., Lin G., Redfield M.M., Borlaug B.A. Eur Heart J. 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel R.B., Li E., Benefield B., et al. Diffuse right ventricular fibrosis in heart failure with preserved ejection fraction and pulmonary hypertension. ESC Heart Fail. 2020;7:253. doi: 10.1002/ehf2.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Wezenbeek J., Kianzad A., Bovenkamp A.V.D., et al. Right ventricular and right atrial function are less compromised in pulmonary hypertension secondary to heart failure with preserved ejection fraction: a comparison with pulmonary arterial hypertension with similar pressure overload. Circ Heart Fail. 2022;15:e008726. doi: 10.1161/CIRCHEARTFAILURE.121.008726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scantlebury D.C., Borlaug B.A. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26:562–568. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 37.Beale A.L., Nanayakkara S., Segan L., et al. Sex differences in heart failure with preserved ejection fraction pathophysiology: a detailed invasive hemodynamic and echocardiographic analysis. JACC Heart Fail. 2019;7:239–249. doi: 10.1016/j.jchf.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Westerman S., Wenger N. Gender differences in atrial fibrillation: a review of epidemiology, management, and outcomes. Curr Cardiol Rev. 2019;15:136–144. doi: 10.2174/1573403X15666181205110624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikitin N.P., Witte K.K.A., Thackray S.D.R., Goodge L.J., Clark A.L., Cleland J.G.F. Effect of age and sex on left atrial morphology and function. Eur J Echocardiogr. 2003;4:36–42. doi: 10.1053/euje.2002.0611. [DOI] [PubMed] [Google Scholar]
- 40.Maceira A.M., Cosin-Sales J., Prasad S.K., Pennell D.J. Characterization of left and right atrial function in healthy volunteers by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2016;18:64. doi: 10.1186/s12968-016-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy Y.N.V., Obokata M., Verbrugge F.H., Lin G., Borlaug B.A. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. 2020;76:1051–1064. doi: 10.1016/j.jacc.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kowallick J.T. Left atrial physiology and pathophysiology: role of deformation imaging. World J Cardiol. 2015;7:299. doi: 10.4330/wjc.v7.i6.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goette A., Kalman J.M., Aguinaga L., et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterisation, and clinical implication. J Arrhythmia. 2016;32:247–278. doi: 10.1016/j.joa.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel R.B., Lam C.S.P., Svedlund S., et al. Disproportionate left atrial myopathy in heart failure with preserved ejection fraction among participants of the PROMIS-HFpEF study. Sci Rep. 2021;11:4885. doi: 10.1038/s41598-021-84133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Backhaus S.J., Rösel S.F., Schulz A., et al. RT-CMR imaging for noninvasive characterization of HFpEF. J Am Coll Cardiol Img. 2022;15 doi: 10.1016/j.jcmg.2021.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Redfield M.M., Jacobsen S.J., Borlaug B.A., Rodeheffer R.J., Kass D.A. Age- and gender-related ventricular-vascular stiffening. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 47.Segers P., Stergiopulos N., Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol. 2002;282:H1041–H1046. doi: 10.1152/ajpheart.00764.2001. [DOI] [PubMed] [Google Scholar]
- 48.Chirinos J.A., Phan T.S., Syed A.A., et al. Late systolic myocardial loading is associated with left atrial dysfunction in hypertension. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosch S., Kresoja K.P., Besler C., et al. Characteristics of heart failure with preserved ejection fraction across the range of left ventricular ejection fraction. Circulation. 2022;146:506–518. doi: 10.1161/CIRCULATIONAHA.122.059280. [DOI] [PubMed] [Google Scholar]
- 50.Backhaus S.J., Metschies G., Billing M., et al. Cardiovascular magnetic resonance imaging feature tracking: impact of training on observer performance and reproducibility. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kowallick J.T., Morton G., Lamata P., et al. Quantification of atrial dynamics using cardiovascular magnetic resonance: inter-study reproducibility. J Cardiovasc Magn Reson. 2015;17:36. doi: 10.1186/s12968-015-0140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Backhaus S.J., Metschies G., Zieschang V., et al. Head-to-head comparison of cardiovascular MR feature tracking cine versus acquisition-based deformation strain imaging using myocardial tagging and strain encoding. Magn Reson Med. 2021;85:357–368. doi: 10.1002/mrm.28437. [DOI] [PubMed] [Google Scholar]
- 53.Backhaus S.J., Schuster A., Lange T., et al. Impact of fully automated assessment on interstudy reproducibility of biventricular volumes and function in cardiac magnetic resonance imaging. Sci Rep. 2021;11 doi: 10.1038/s41598-021-90702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.