Abstract
Background
Patients with likely pathogenic/pathogenic desmoplakin (DSP) variants are poorly characterized. Some of them meet diagnostic criteria for arrhythmogenic right ventricular cardiomyopathy (ARVC), but it is unclear how risk stratification strategies for ARVC perform in this setting.
Objectives
The purpose of this study was to characterize arrhythmic outcomes and to test the performance of the recently validated ARVC risk calculator in patients with DSP likely pathogenic/pathogenic variants fulfilling definite 2010 ARVC Task Force Criteria (DSP-TFC+).
Methods
DSP-TFC+ patients were enrolled from 20 institutions across 3 continents. Ventricular arrhythmias (VA), defined as a composite of sustained ventricular tachycardia (VT), appropriate implantable cardioverter defibrillator therapies, and ventricular fibrillation/sudden cardiac death events in follow-up, were reported as the primary outcome. We tested the performance of the ARVC risk calculator for VA prediction, reporting c-statistics.
Results
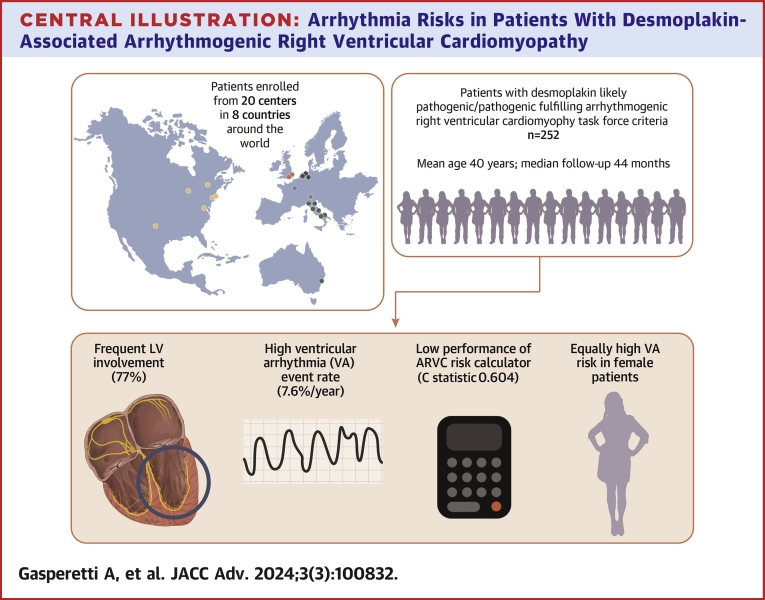
Among 252 DSP-TFC+ patients (age 39.6 ± 16.9 years, 35.3% male), 94 (37.3%) experienced VA over 44.5 [IQR: 19.6-78.3] months. Patients with left ventricle involvement (n = 194) were at higher VA risk (log-rank P = 0.0239). History of nonsustained VT (aHR 2.097; P = 0.004) showed the strongest association with VA occurrence during the first 5-year follow-up. Neither age (P = 0.723) nor male sex (P = 0.200) was associated with VAs at follow-up. In 204 patients without VA at diagnosis, incident VA rate was high (32.8%; 7.37%/y). The ARVC risk calculator performed poorly overall (c-statistic 0.604 [0.594-0.614]) and very poorly in patients with left ventricular disease (c-statistic 0.558 [0.556-0.560]).
Conclusions
DSP-TFC+ patients are at substantial risk for VAs. The ARVC risk calculator performs poorly in DSP-TFC+ patients suggesting need for a gene-specific risk algorithm. Meanwhile, DSP-TFC+ patients with nonsustained VT should be considered as high-risk.
Key words: ACM, ARVC, desmoplakin, risk stratification
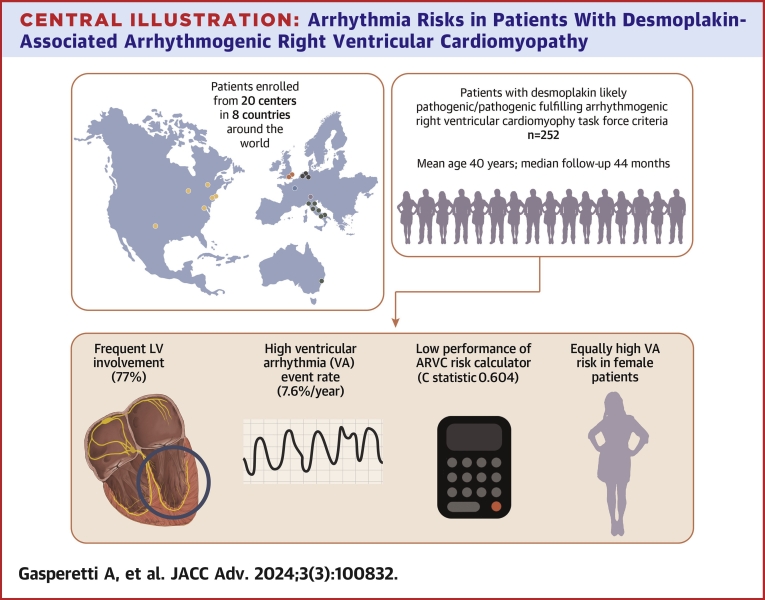
Central Illustration
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a heterogeneous genetic disease associated with pathogenic variants in genes encoding the cardiac desmosome associated with the development of potentially lethal ventricular arrhythmias (VA).1 In light of this elevated risk for sudden cardiac death, the usual next step following a patient’s ARVC diagnosis is an individualized assessment of arrhythmic risk and a decision regarding the placement of an implantable cardioverter defibrillator (ICD).2,3 However, the indications for primary prevention ICD use in patients with ARVC have historically been less clear.
In 2019, a novel risk stratification tool for aiding in ICD decision-making for patients with a definite diagnosis of ARVC, as per the 2010 Task Force Criteria (TFC), and no previous sustained VA events was proposed.4 Since then, the ARVC risk calculator has been found to be reliable in multiple external validation cohorts.5, 6, 7, 8, 9, 10, 11 This risk stratification tool, however, was derived from an ARVC patient cohort primarily composed of plakophilin-2 (PKP2) variant carriers and gene-elusive patients.4 Studies have suggested suboptimal performance of the ARVC risk calculator in left dominant forms of ARVC, although the relatively low patient sample size and event rate of those studies precluded definite conclusions.5,7,10 Foremost among these underrepresented ARVC patients are those with likely pathogenic or pathogenic (LP/P) desmoplakin (DSP) variants.
DSP variants are associated with both ARVC and dilated cardiomyopathy pathogenesis.12,13 Consistent with this, patients with LP/P DSP variants often have a phenotype in which the left ventricle (LV) is extensively affected even at early stages of disease, and studies suggest an aggressive arrhythmic course.14, 15, 16 However, long-term outcome characterization and optimal approaches to arrhythmia risk stratification have been limited by the relatively small sample sized patient cohorts,14,15 around half of whom did not fulfill TFC. Even when the TFC are fulfilled, it is unclear how ARVC-based risk stratification strategies perform in patients with LP/P DSP variants and thus whether they should be applied.5,7,10 This study therefore aims to characterize arrhythmic outcomes over long-term follow-up and to test the performance of a recently validated risk stratification algorithm (ARVC risk calculator) in patients with DSP LP/P variants who fulfill the definite 2010 ARVC TFC (DSP-TFC+).
Methods
Study cohort
Patients were ascertained from ARVC and genetic cardiomyopathy registries of 20 academic institutions from 8 different countries (United States of America, United Kingdom, France, Italy, the Netherlands, Canada, Australia, and Switzerland). Each registry is, in itself, a longitudinal cohort study.
From each registry, patients were included in the study if they: 1) harbored a pathogenic (P) or likely pathogenic (LP) genetic variant in DSP per the American College of Medical Genetics and Genomics criteria17; 2) fulfilled a definite diagnosis of ARVC in accordance with the 2010 TFC1; 3) had at least one cardiac imaging test available (cardiac magnetic resonance [CMR] or echocardiography) at the time of TFC fulfillment; and 4) had at least 1 day of follow-up available for outcome ascertainment.
Ethical review board approval and written patient consent were obtained in accordance with local regulations. The study was performed in accordance with the Declaration of Helsinki.
Data collection
Available demographics, patient medical history, genetic test results, baseline cardiac instrumental exams (12-lead electrocardiogram, echocardiography, CMR, 24-hour Holter-electrocardiogram monitor) were retrieved for each patient. All DSP genetic variants initially considered P or LP locally underwent expert review by core lab from specialists in cardiac genetics (B.M., C.A.J.). A list of all genetic variants included in the study has been reported in Supplemental Table 1. Nonsustained ventricular tachycardia (NSVT) has been defined as 3 or more sequential premature ventricular complexes at a frequency >120 beats/min. Heart failure (HF) episodes were defined as a clinical presentation consistent with acute or decompensated HF requiring hospitalization. LV involvement was defined as the presence of late gadolinium enhancement (LGE) in the LV on CMR and/or the presence of an LV ejection fraction (LVEF) <50% on any cardiac imaging test.
Study outcomes
Consistent with the published ARVC risk calculator, the primary outcome was first sustained VA following confirmed ARVC diagnosis.10,11,18 Sustained VA was defined as a composite of the occurrence of sudden cardiac arrest, spontaneous sustained ventricular tachycardia lasting ≥30 s with a frequency of at least 100 beats/min or with hemodynamic compromise, ventricular fibrillation/flutter, or appropriate ICD intervention.4, 5, 6, 7, 8, 9, 10, 11,18 Fast VA was defined as sustained VA events with a rate >250 beats/min. The primary prevention cohort was composed of those patients with no history of sustained VA at the time of TFC fulfillment.
Statistical analysis
Analyses were performed in PyCharm software version 2021.2.2 (JetBrains Inc) and the open-source Pandas, Lifelines, and Statsmodels statistical code libraries. Categorical variables were summarized as frequencies (%) and compared using proportional z-tests. Continuous variables were presented as mean ± SD or median (IQR) and compared using independent sample Student t-tests or the Mann-Whitney U-tests, as appropriate. The overall probability of freedom from sustained VA was estimated using the Kaplan-Meier method. Rates of incident VA are reported as averages over the 5-year period following initial diagnosis, both within the overall cohort and stratified by both: 1) presence/absence of sustained VA prior to ARVC diagnosis (primary vs secondary prevention cohort); and 2) presence/absence of LV involvement. Log-rank (LR) testing was used to assess differences in VA event rates between subgroups. Associations between individual risk factors included within the ARVC risk calculator or the presence of LV involvement as well as its individual components (LGE in the LV on CMR, LVEF <50%) and sustained VA events were assessed using Cox proportional hazards regression models; those risk factors for whom the P value was <0.10 were included in a subsequent multivariable Cox proportional hazard regression model. Competing-risk sensitivity analysis for nonarrhythmic death and heart transplants were performed using Fine and Gray’s proportional subhazards models. Supplemental Methods details the methods used for assessing and testing the performance of the ARVC risk calculator in this patient cohort.
Results
Patient cohort
A cohort of 252 DSP-TFC+ patients was included in the study. Probands made up 59.9% of the cohort. The mean age at TFC fulfillment was 39.6 ± 16.9 years (n = 7, age <14 years), at which time most patients (204, 81.0%) had no history of sustained VA events (primary prevention cohort). The vast majority (84.9%) of patients had >500 PVCs/24 h, with a median 24-hour PVC burden of 2000 [650-5,000]. Mean LVEF and right ventricular ejection fraction (RVEF) of the study cohort were mildly reduced, 45.0% ± 13.3% and 46.4% ± 11.2%, respectively. LV involvement was observed in 194 (77.0%) patients (n = 140 with LVEF reduction; n = 131 with LGE). A total of 165 (65.5%) patients were on a beta-blocker and 37 (14.7%) on an antiarrhythmic drug at time of TFC fulfillment. Table 1 summarizes the baseline characteristics of the overall, primary, and secondary prevention cohorts. Specifics regarding TFC fulfillment of the overall cohort have been reported in Supplemental Table 2. Supplemental Table 3 reports cohort characteristics stratified by proband status.
Table 1.
Baseline Characteristics
| Overall Cohort (N = 252) | Primary Prevention (n = 204) | Secondary Prevention (n = 48) | |
|---|---|---|---|
| Age at TFC fulfillment, y | 39.6 ± 16.9 | 39.1 ± 17.4 | 42.2 ± 14.3 |
| Male | 85 (33.7) | 65 (31.9) | 20 (41.7) |
| Ethnicity | |||
| White Caucasian | 238 (94.4) | 194 (95.1) | 44 (91.7) |
| South Asian | 6 (2.4) | 5 (2.5) | 1 (2.1) |
| African American/Black | 5 (2.0) | 3 (1.5) | 2 (4.1) |
| Hispanic | 2 (0.8) | 2 (0.9) | 0 |
| East Asian | 1 (0.4) | 0 | 1 (2.1) |
| Probands | 151 (59.9) | 113 (55.4) | 38 (79.2) |
| Sustained VA at/prior to TFC fulfillment | 48 (19.0) | - | 48 (100) |
| ECG | |||
| Overall n of TWI | 3 [1-4] | 3 [1-4] | 3 [1-5] |
| TWI in ≥3 precordial leads | 124 (49.2) | 95 (46.6) | 29 (60.4) |
| TWI in ≥2 inferior leads | 45 (17.9) | 36 (17.6) | 9 (20.0) |
| 24-h PVC burden | 2,000 (650-5,000) | 1,920 (612-5,000) | 2,438 (1,180-6,124) |
| 24-h PVC burden ≥500 | 155 (84.9) | 133 (65.2) | 22 (45.8) |
| LVEF at TFC fulfillment | 45.0 ± 13.3 | 46.6 ± 10.5 | 41.5 ± 12.5 |
| RVEF at TFC fulfillment | 46.4 ± 11.2 | 45.8 ± 13.4 | 45.4 ± 13.7 |
| LGE at TFC fulfillment (assessed n = 193/166/33) | 131 (68.2) | 111 (66.9) | 20 (60.6) |
| LV disease involvement | 194 (77.0) | 156 (76.5) | 38 (79.1) |
| Medical therapy at baseline | |||
| BB | 165 (65.5) | 136 (66.7) | 29 (60.4) |
| AAD | 37 (14.7) | 24 (11.8) | 13 (27.1) |
| ACEI/ARB | 113 (44.8) | 89 (43.6) | 24 (50.0) |
| MRA | 38 (15.1) | 29 (14.2) | 9 (18.8) |
| ICD at TFC fulfillment | 118 (46.8) | 81 (39.7) | 37 (77.1) |
Values are mean ± SD, n (%), or median (IQR).
ACEI = angiotensin converting enzyme inhibitor; AAD = anti-arrhythmic drug; ARB = angiotensin receptor blocker; BB = beta blocker; ICD = implantable cardioverter defibrillator; LGE = late gadolinium enhancement; LV = left ventricular; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist; PVC = premature ventricular contraction; RVEF = right ventricular ejection fraction; TFC = Task Force Criteria; TWI = T-wave inversion; VA = ventricular arrhythmias.
Outcomes
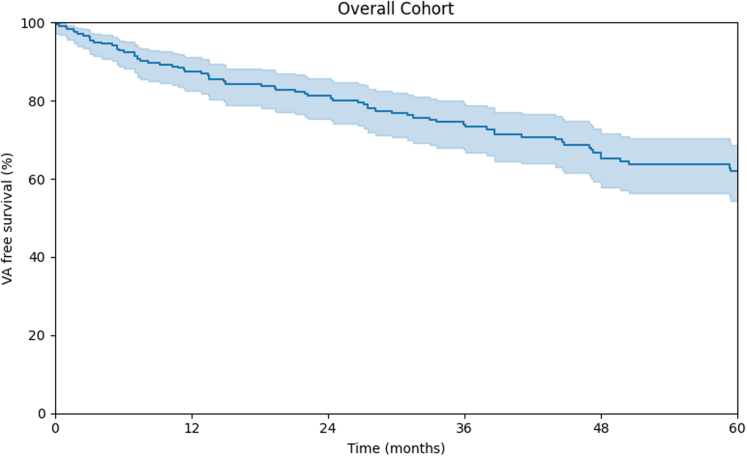
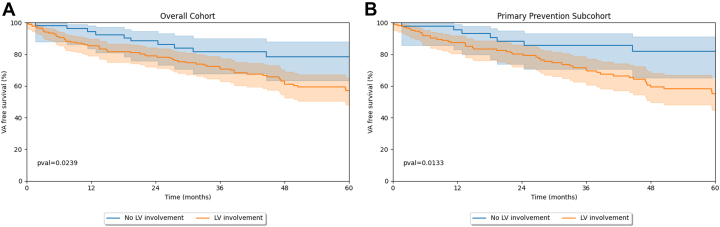
Table 2 summarizes study outcomes for the overall and primary prevention cohorts stratified by LV involvement. Over a median follow-up of 44.5 (IQR: 19.6-78.3) months, 94 (37.3%) patients experienced a sustained VA event (VA event rate annualized over 5-year 7.6% [IQR: 6.2%-9.2%]; fast VA event rate annualized over 5-year 3.2 [IQR: 2.2%-4.6%]). Figure 1 reports the KM curve for the entire cohort. Patients with a prior sustained VA event at TFC fulfillment experienced a higher arrhythmic event rate in follow-up compared to the primary prevention cohort (LR P = 0.034). Overall, a higher rate of VA events was observed in patients with LV involvement, both in the overall (Figure 2A) (LR, P = 0.0239) and in the primary patient cohort (LR, P = 0.0133) (Figure 2B). As per Table 2, during follow-up, 47 (18.6%) patients experienced congestive HF episodes, with 22 (8.7%) patients undergoing heart transplantation (n = 12 terminal HF; n = 6 intractable VA; n = 2 unknown). Overall patient mortality at last follow-up was 2.8%. At the last available follow-up, 175 (69.4%) patients were implanted with an ICD. Competing-risk sensitivity analysis was performed for nonarrhythmic death and transplant but did not impact the results.
Table 2.
Outcomes Stratified by Presence or Absence of Left Ventricular Involvement
| Follow-Up Data |
||||||
|---|---|---|---|---|---|---|
| Overall Cohort |
Primary Prevention Cohort |
|||||
| Overall (N = 252) | LV Involvement (n = 194) | No LV Involvement (n = 58) | Overall (N = 204) | LV Involvement (n = 156) | No LV Involvement (n = 48) | |
| Length of follow-up (mo) | 44.5 (19.6-78.3) | 42.2 (16.4-74.2) | 51.0 (26.0-114.2) | 44.5 (20.1-78.3) | 41.6 (17.6-73.4) | 53.8 (26.7-122.0) |
| Patient with VA events | 94 (37.3) | 77 (39.7) | 17 (29.3) | 67 (32.8) | 56 (35.9) | 11 (22.9) |
| Sustained VT | 30 (11.9) | 23 (11.9) | 7 (12.1) | 26 (12.7) | 21 (13.4) | 5 (10.4) |
| ICD shocks | 57 (22.6) | 49 (25.3) | 8 (13.8) | 36 (17.6) | 31 (19.9) | 5 (10.4) |
| VF/SCA | 7 (2.8) | 5 (2.6) | 2 (3.4) | 5 (2.5) | 4 (2.6) | 1 (2.0) |
| HF episodes | 47 (18.7) | 41 (21.1) | 6 (10.3) | 37 (18.1) | 33 (21.2) | 4 (8.3) |
| Transplant | 22 (8.7) | 22 (11.3) | 0 | 18 (8.8) | 18 (11.5) | 0 |
| Death | 7 (2.8) | 6 (3.1) | 1 (1.7) | 6 (2.9) | 5 (3.2) | 1 (2.0) |
| ICD at last follow-up | 175 (69.4) | 139 (71.6) | 36 (62.1) | 133 (65.2) | 105 (67.3) | 28 (58.3) |
Values are median (IQR) or n (%).
HF = heart failure; ICD = implantable cardioverter defibrillator; LV = left ventricular; SCA = sudden cardiac arrest; VA = ventricular arrhythmias; VF = ventricular fibrillation; VT = ventricular tachycardia.
Figure 1.
Freedom From Ventricular Arrhythmia Events in the Overall Study Cohort Kaplan-Meier Curve Displaying Overall Cohort Freedom From Ventricular Arrhythmias OverFollow-Up
Figure 2.
Differences in Arrhythmic Outcomes Depending on LV Involvement
Freedom from ventricular arrhythmia in the overall (right panel) and primary prevention (left panel) cohorts, stratified by LV involvement both panels show a higher occurrence of ventricular arrhythmia events in those patients in which LV involvement is present. Shaded area around the KM curve represents the 95% freedom from ventricular arrhythmia CI. Numbers reported below represents patients at risk (top row: no LV involvement; bottom row: LV involvement); KM = Kaplan Meier; LV = left ventricular.
Predictors of sustained ventricular arrhythmias in the overall cohort
Table 3 reports an association between sustained VA events during follow-up and baseline clinical characteristics of the overall cohort. In univariable analysis, LV involvement (HR: 2.121 [95% CI: 1.088-4.138], P = 0.027), PVC burden (HR: 1.189 [95% CI: 1.034-1.368], P = 0.015) and a history of NSVT episodes (HR: 2.629 [95% CI: 1.655-4.176], P < 0.001) were positively associated with arrhythmic events, while a negative association with RVEF% was observed (HR: 0.978 [95% CI: 0.960-0.998], P = 0.027). In multivariable Cox regression, NSVT episodes remained associated with arrhythmic outcomes (aHR: 2.097 [95% CI: 1.274-3.450], P = 0.004). Supplemental Table 5 shows influence of each component of LV involvement (LVEF, LGE). As can be appreciated, LVEF but not LV LGE was associated with sustained VA in univariable analysis when these aspects of LV involvement were considered separately. In multivariable Cox regression, lower LVEF (aHR: 0.977 [95% CI: 0.956-0.998], P = 0.028) and NSVT episodes (aHR: 2.236 [95% CI: 1.364-3.663], P = 0.001) were associated with VA in follow-up.
Table 3.
Variables Associated With Freedom From VA Events During Follow-Up in the Overall Patient Cohort
| 5-y Arrhythmic Risk Predictors |
||||||
|---|---|---|---|---|---|---|
| Univariable |
Multivariable |
|||||
| HR | 95% CI | P Value | aHR | 95% CI | P Value | |
| Age (/y) | 0.998 | 0.984-1.011 | 0.723 | |||
| Male | 0.710 | 0.420-1.199 | 0.200 | |||
| TWI tot (/lead with TWI) | 0.960 | 0.868-1.061 | 0.423 | |||
| PVC burden (log) | 1.189 | 1.034-1.368 | 0.015 | 1.102 | 0.950-1.279 | 0.200 |
| Cardiac syncope | 1.038 | 0.532-2.026 | 0.912 | |||
| History of NSVT | 2.629 | 1.655-4.176 | <0.001 | 2.097 | 1.274-3.450 | 0.004 |
| RVEF (/%) | 0.978 | 0.960-0.998 | 0.027 | 0.989 | 0.97-1.009 | 0.288 |
| LV involvement | 2.121 | 1.088-4.138 | 0.027 | 1.488 | 0.717-2.924 | 0.302 |
Bold values indicate P < 0.050.
LV = left ventricular; NSVT = nonsustained ventricular tachycardia; RVEF = right ventricular ejection fraction; VA = ventricular arrhythmias.
Risk stratification and performance of the ARVC risk calculator in the primary prevention cohort
Performance of the ARVC risk calculator was tested in the primary prevention patient cohort (n = 204). Over a 5-year follow-up period, 57 (27.9%) primary prevention patients experienced a sustained VA event (VA event rate annualized over 5 years: 7.7% [IQR: 6.1%-9.4%]; fast VA event rate annualized over 5 years: 2.8% [IQR: 1.8%-4.4%]). Among variables of the ARVC risk calculator plus LV involvement, a previous episode of NSVT, PVC burden, and the presence of LV involvement were associated with a higher risk of sustained VA events during 5-year follow-up (HR: 2.506 [95% CI: 1.491-4.231], P = 0.001; HR: 1.243 [95% CI: 1.061-1.455], P = 0.007; HR: 2.618 [95% CI: 1.187-5.777], P = 0.017, respectively), but none of them retained significance in multivariable Cox regression analysis. As shown in Supplemental Table 6, while lower LVEF and presence of LGE were associated with sustained VA in univariable analysis when considered as individual risk factors, neither was retained in the multivariable model.
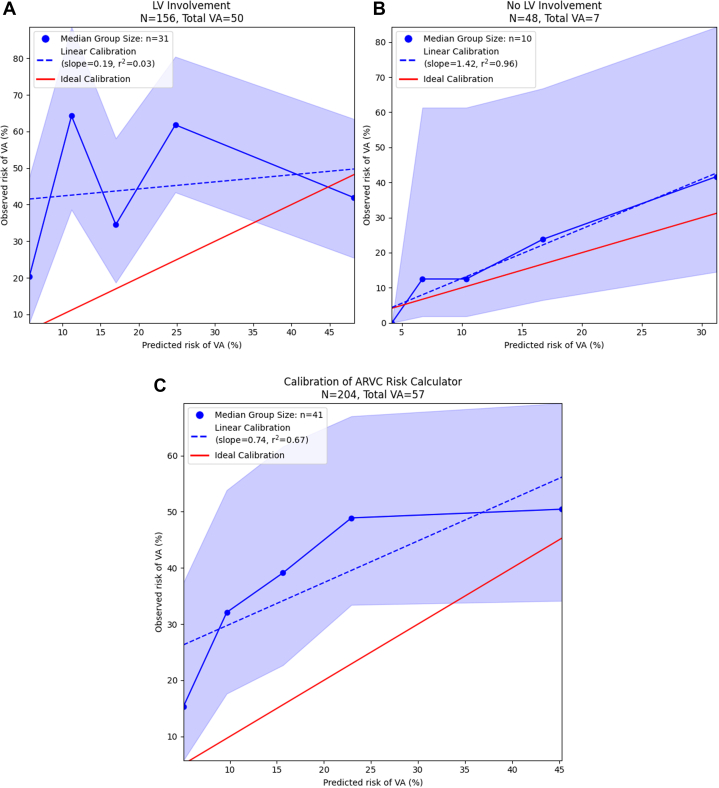
In this primary prevention cohort, the overall median ARVC risk calculator predicted risk of VA at 5 years was 15.4% [IQR: 8.3%-25.0%] and was significantly higher in patients with LV involvement than in those without (16.9% [IQR: 9.2%-27.6%] vs 10.1% [IQR: 6.2%-18.0%], P ≤ 0.001). In the entire primary prevention cohort of patients with DSP-TFC+, discrimination of sustained VA risk by the ARVC risk calculator was poor (c-statistic 0.604 [0.594-0.614]) as was calibration of predicted risks with observed incidence of VA (Figure 3A). Discriminative performance of the ARVC risk calculator was very poor in patients with LV involvement (c-statistic 0.558 [0.556-0.560]) (Figure 3B), but was good in those without (0.756 [0.702-0.810]) LV involvement (Figure 3C). The relationship between risk of VA predicted by the ARVC risk calculator and the observed incidence of VA in patients with LV involvement was highly nonlinear (Figure 3B), while risk was consistently underestimated in patients without LV involvement (Figure 3C). Per-risk bracket performance of the ARVC risk calculator in arrhythmic risk stratification for the primary prevention cohort has been graphically displayed in Supplemental Figure 1 (overall) and Supplemental Figures 2 and 3 (stratification by LV involvement).
Figure 3.
Performance of the ARVC Risk Score in DSP-TFC+ Patients
Performance of the ARVC risk calculator in DSP-TFC+ patients calibration plot for the performance of the ARVC risk calculator in the primary prevention cohort overall (A) and stratified on the presence (B) or absence (C) of left ventricular involvement. Average risk predicted by the ARVC risk calculator is plotted against the observed VA risk and associated 95% CI as estimated by the Kaplan-Meier method for each quintile (blue line). The linearity of this relationship is assessed using linear regression, and the coefficient of determination (r2) reflects the degree to which this relationship is appropriately characterized by a linear model. The red line reflects perfect, linear risk-prediction; risk predictions falling to the upper left of this line represent model underprediction, while risk prediction falling to the lower right of this line represent model overpredictions. ARVC = arrhythmogenic right ventricular cardiomyopathy; LV = left ventricular; VA = ventricular arrhythmias.
Discussion
This multinational study enrolled the largest cohort of patients with a LP/P DSP variant fulfilling the 2010 TFC that has been published to date.
The main findings of this study are summarized as follows (Central Illustration). First, during a median follow-up of almost 4 years, a substantial rate of VA events (overall 37.3%; annualized over 5-year 7.6% [IQR: 6.2%-9.2%]) was observed in DSP-TFC+ patients. The observed VA rate was nearly as high (overall 32.8%; annualized over 5-year 7.7% [IQR: 6.1%-9.4%]) among those patients without previous VA event at diagnosis (“primary prevention patients”). Second, a history of NSVT, a high PVC burden, and LV involvement were associated with an increased risk of VA events in primary prevention patients. Interestingly, in contrast to PKP2 or gene-elusive ARVC, female patients were at appreciable risk. Finally, the ARVC risk calculator did not perform well overall in VA risk discrimination (overall C statistic 0.604 [0.594-0.614]). When stratifying patients by presence or absence of LV involvement, the ARVC risk score performance was fair in DSP-TFC+ patients without LV involvement (C statistic 0.756 [0.702-0.810]), but very poor in patients with LV involvement (C statistic 0.558 [0.556-0.560]).
Central Illustration.
Arrhythmia Risks in Patients With Desmoplakin-Associated Arrhythmogenic Right VentricularCardiomyopathy
Long-term arrhythmic outcomes of DSP-TFC+ patients
Data regarding the long-term outcomes of the different phenotypes of DSP+ patients are limited mostly due to the lack of patient-level data. As a result, when these patients fulfill the 2010 TFC, there is no clear consensus on the relative arrhythmic risk compared to the other ARVC phenotypes (ie, PKP2-ARVC, gene-elusive ARVC) or on appropriate risk stratification approaches. In comparison to classical ARVC phenotypes, higher, similar, or lower VA rates have all been reported for patients with DSP variants.10,14,15,19,20 Prior studies have been hampered by small patient sample sizes, and a definitive answer regarding arrhythmic risk in these patients remains elusive.
More than a third of DSP-TFC+ patients experienced sustained VA during the median 4-year follow up. A previous VA event is known to be strongly associated with additional VA events during follow-up in patients with ARVC,21,22 so the event rate observed in patients with a previous history of VAs is not surprising. In our cohort, however, VA events were frequent even among those “primary prevention” patients without a history of sustained VA events at the time of diagnosis (annual event rate 7.7% [IQR: 6.1%-9.4%]). No direct outcome comparisons between different genotypes were performed in this study, but studies of primary prevention ARVC cohorts primarily comprised of gene-elusive and PKP2 patients available in literature, primarily from our centers, reported substantially lower VA rates (annualized VA rates 2.6%-5.6%4,10,11). These findings strongly point towards DSP-TFC+ being a particularly high arrhythmic risk phenotype, especially when LV involvement is present.
Arrhythmic risk stratification
Given the rate of sustained VA events observed in our study, an appropriate and specific arrhythmic risk stratification strategy for DSP-TFC+ patients is imperative. However, most of the current ARVC risk stratification strategies are based upon data derived from predominantly right-sided PKP2-associated or gene-elusive ARVC cohorts.2, 3, 4 Thus, whether established ARVC arrhythmic risk factors remain important for DSP-TFC+ patients was previously unknown. In our study, some risk factors for sustained VA were similar to those of classical right-sided ARVC phenotypes. For instance, a high burden of PVCs, the presence of NSVT, and a lower RVEF were associated with an increased risk for sustained VAs. In contrast, other arrhythmic risk factors for classical right-sided ARVC, such as younger age, was not identified as risk factors in this population. Of particular interest, male DSP-TFC+ patients did not have an increased risk of VA. This is in stark contrast to what has been commonly observed in most classical ARVC phenotypes.10,18,19 These findings are unexpected and represent an important clinical message. Therapeutic intervention in ARVC patients has historically been more aggressive in male patients due to their increased arrhythmic risk. Based on the results of our study, female DSP-TFC+ patients should be considered as having at least as high an arrhythmic risk as their male counterparts. Finally, there was evidence of worse arrhythmic outcomes in patients with LV involvement, both in the overall and primary prevention patient population. When LVEF and LGE were considered separately, lower LVEF was associated with sustained VA in the overall cohort, but not the primary prevention cohort, in multivariable analysis. Larger studies are needed to definitively assess the individual roles of LGE and LVEF in VA risk stratification for patients with LP/P DSP variants, particularly in the setting of primary prevention.
The ARVC risk calculator is reliable in discriminating the risk of sustained VA events in primary prevention patients with ARVC.10,11 Comparisons between the available risk stratification algorithms (ie, ITFC 2015,3 HRS 20192) have been performed in multiple independent studies.23,24 These studies favored the ARVC risk calculator, which achieved greater arrhythmic protection despite a lower total number of implanted ICDs. However, the possibility of underperformance by the ARVC risk calculator in patients with extensive or exclusive LV involvement,5,7 as well as its potential inadequacy for use in specific genotypes, has recently been postulated.10 In our large cohort of DSP-TFC+ patients, the ARVC risk calculator’s performance was poor overall (C statistic 0.604 [0.594-0.614]). Consistent with previous small reports from Casella et al and Aquaro et al,5,7 the ARVC risk calculator’s discrimination was worst in patients with LV involvement. A better discrimination was observed in patients without LV involvement (C statistic 0.756 [0.702-0.810]), although with a trend toward underpredicting likelihood of sustained VA events (Figure 3C). These findings partially contradict the report from Protonotarios et al, where the ARVC risk score was found to overpredict the arrhythmic risk in ARVC patients with DSP variants. The observed differences can potentially be explained by the predominance of patients being enrolled in cardiomyopathy centers, resulting in a cohort with lower arrhythmic risk/higher HF risk. In our assessment, we included a large number of patients from both cardiomyopathy and arrhythmia clinics in order to capture the whole clinical spectrum of DSP-TFC+ patients and minimize center-specific patient characteristics and differences. Finally, in contrast to studies of right-sided ARVC phenotypes, the ARVC risk calculator did not perform better than current expert recommendations and clinical consensus guidelines in DSP-TFC+ patients. Regardless of the potential threshold of predicted 5-year VA risk, this tool either led to a lower protection rate from arrhythmias or to a similar protection rate but with a higher number of ICD placements needed to prevent an arrhythmic event (a lower “net benefit” ratio).
Future perspectives
This study clearly demonstrates that DSP-TFC+ patients are at high arrhythmic risk. The best modality to perform risk stratification assessment in this population, however, remains unclear. From our data, it seems reasonable to discuss ICD implantation for patients with episodes of NSVT, high PVC burden, or LV involvement, given their strong association with complex VA events during follow-up. Additionally, patients harboring a DSP variant have also been reported as frequently fulfilling DCM criteria.14 In these patients, considering DSP variants as high-risk genetic variants as per the recently released 2022 ESC guidelines for the management of patients with VA (as with variants in phospholamban (PLN), filamin C, and RBM20), seems appropriate.25 Finally, a genotype tailored risk stratification strategy for ARVC has recently been advocated.10,26,27 A similar approach has been implemented in other genetically-based cardiomyopathies. For example, Verstraelen et al28 showed that a PLN-tailored risk stratification algorithm was more effective than other available risk stratification scores (ie, dilated cardiomyopathy guidelines or ARVC risk calculator) for patients with PLN-associated cardiomyopathy, suggesting that such genotype-first strategies may be reasonable. Considering that the ARVC risk calculator did not perform well even in patients with DSP variants who meet TFC, it is likely these patients would benefit from the development of DSP-specific risk stratification tools for the prediction of arrhythmic events.
Study Limitations
This was a retrospective cohort study, potentially prone to all the biases associated with retrospective studies. To reduce those biases (and in particular selection bias), patients from both arrhythmia clinics and cardiomyopathy/HF clinics across the world were enrolled. Additionally, while this study found an association between the presence of NSVT and the occurrence of VA events in this patient population, data regarding the NSVT burden were unfortunately not available. Furthermore, our study addressed the presence of LGE in DSP-TFC+ patients as a categorical variable. While no association between VA and presence of LGE was observed in our study, the possibility that a quantitative assessment of LGE (ie, % of LGE per LV segment) would be associated with VA should not be excluded. Dedicated imaging studies addressing this topic in the future will be of help to further clarify the prognostic role of LGE in DSP patients. Finally, while a commonly observed disease phenotype in patients with DSP P/LP variants is one that fulfills the 2010 TFC, such phenotype does not encompass the complete range of DSP-associated disease. To fully understand this disease, further research should be conducted that includes DSP+ patients enrolled based on genotype rather than the specific disease phenotype they exhibit.
Conclusions
Patients with a DSP LP/P variant who fulfill TFC are at substantial risk for VA events. While arrhythmic risk markers partially overlap with those of classical ARVC, female DSP-TFC+ patients were at similar, if not higher, VA risk as males, and age was not an informative predictor. The ARVC risk calculator had poor performance in DSP-TFC+ patients, albeit better in patients with isolated right-sided disease. DSP-TFC+ patients may benefit from the development of gene-specific risk stratification tools. In the meanwhile, the ARVC risk calculator can only be used in DSP-ARVC patients who have no LV involvement. In the remaining patients, evidence of VA (ie, high PVC burden or NSVT) is a salient marker of VA risk.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients harboring DSP pathogenic/likely pathogenic variants and fulfilling ARVC diagnostic criteria (DSP-TFC+) have a substantial risk of ventricular arrhythmia events during follow-up. Female sex was not associated with a lower arrhythmic risk.
COMPETENCY IN PATIENT CARE: DSP-TFC+ patients are poorly stratified by the ARVC risk calculator. DSP-TFC+ patients with NSVT should be considered as high-risk and have an informed discussion about ICD placement.
Funding support and author disclosures
The Johns Hopkins ARVC program (Dr James, Gasperetti, Calkins, Carrick, Murray, and Tondo) is supported by the Leonie-Wild Foundation, the Leyla Erkan Family Fund for ARVD Research, the Hugh Calkins, Marvin H. Weiner, and Jacqueline J. Bernstein Cardiac Arrhythmia Center, the Dr Francis P. Chiramonte Private Foundation, the Dr Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Bogle Foundation, the Campanella family, the Patrick J. Harrison family, the Peter French Memorial Foundation, and the Wilmerding Endowments and NIH/NCATS UL1 TR003098. ASJMtR and PVT acknowledge support from the Netherlands Cardiovascular Research Initiative, an initiative with support of the Netherlands Heart Foundation, grant nos. CVON2018-30 PREDICT2, CVON2015-12 eDETECT, 2020B005 Double Dose. ASJMtR is supported by the ZonMW Off-Road Grant 2021. The Zurich ARVC Program is supported by the Georg und Bertha Schwyzer-Winiker Foundation, Baugarten Foundation, Wild Foundation, Swiss National Science Foundation (SNF), and the Swiss Heart Foundation. The Bologna Cardiomyopathy Registry has received funding support from the Italian Ministry of Health, RC-2022-2773270 project AAMW received funding from CVON Predict-2. Dr Mestroni is supported by NIH R01HL69071, R01HL116906, R01HL147064, NIH/NCATS UL1 TR002535 and UL1 TR001082. Dr Yazdani is supported by British Heart Foundation FS/CRTF/23/24448 and Alexander Jansons Myocarditis UK. Dr Prasad has received funding from Alexander Jansons Myocarditis UK, Rosetrees Trust, and British Heart Foundation. Dr Medo is supported by the Rose Foundation. This work was supported by the Medical Research Council (UK), British Heart Foundation [RE/18/4/34215], the NIHR Imperial College Biomedical Research Centre, the NIHR Royal Brompton Biomedical Research Centre, and the Sir Jules Thorn Charitable Trust [21JTA]. Dr Saguner has received educational grants through his institution from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, BMS/Pfizer, and Medtronic; and speaker/advisory board/consulting fees from Abbott, Bayer Healthcare, Daiichi-Sankyo, Medtronic, Novartis, and Pfizer. Dr Lakdawala has received unrestricted research support from Pfizer and the O’Hare and Steggall Family Foundations and consulting fees from BMS, Pfizer, Cytokinetics, Sarepta, and Tenaya. Dr Mestroni received educational grant from Greenstone; and advisory board/consulting fees from Tenaya, StrideBio, and Unicure. Dr Gasperetti received advisory board/consulting fees from Lexeo, and has served as an unpaid consultant for StrideBio. Dr James has received research grants from StrideBio Inc and Lexeo Inc; has received advisory board/consulting fees from Pfizer and Lexeo; and has served as an unpaid consultant for StrideBio and Tenaya. Dr Ware has received research support from Bristol-Myers Squibb; and advisory board/consultancy fees from Bristol-Myers Squibb, Foresite Labs, and Pfizer. Dr Calkins is a consultant for Medtronic Inc, Biosense Webster, Pfizer, StrideBio, Rocket, and Abbott. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank the Netherlands Heart Institute for their support of the Netherlands ACM Registry (project 06901). The authors are grateful to all the patients and families who make this work possible.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables, and figures, please see the online version of this paper.
Supplementary data
References
- 1.Marcus F.I., McKenna W.J., Sherrill D., et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J. 2010;31(7):806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Towbin J.A., McKenna W.J., Abrams D.J., et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm. 2019;16(11):e301–e372. doi: 10.1016/j.hrthm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Corrado D., Wichter T., Link M.S., et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J. 2015;36(46):3227–3237. doi: 10.1093/eurheartj/ehv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadrin-Tourigny J., Bosman L.P., Nozza A., et al. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2022;43(32):e1–e9. doi: 10.1093/eurheartj/ehac180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casella M., Gasperetti A., Gaetano F., et al. Long-term follow-up analysis of a highly characterized arrhythmogenic cardiomyopathy cohort with classical and non-classical phenotypes–a real-world assessment of a novel prediction model: does the subtype really matter. Europace. 2020;22(5):797–805. doi: 10.1093/europace/euz352. [DOI] [PubMed] [Google Scholar]
- 6.Gasperetti A., Russo A.D., Busana M., et al. Novel risk calculator performance in athletes with arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2020;17(8):1251–1259. doi: 10.1016/j.hrthm.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Aquaro G.D., De Luca A., Cappelletto C., et al. Prognostic value of magnetic resonance phenotype in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol. 2020;75(22):2753–2765. doi: 10.1016/j.jacc.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Baudinaud P., Laredo M., Badenco N., et al. External validation of a risk prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Can J Cardiol. 2021;37(8):1263–1266. doi: 10.1016/j.cjca.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Zhang N., Wang C., Gasperetti A., et al. Validation of an arrhythmogenic right ventricular cardiomyopathy risk-prediction model in a Chinese cohort. J Clin Med. 2022;11(7):1973. doi: 10.3390/jcm11071973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Protonotarios A., Bariani R., Cappelletto C., et al. Importance of genotype for risk stratification in arrhythmogenic right ventricular cardiomyopathy using the 2019 ARVC risk calculator. Eur Heart J. 2022;43(32):3053–3067. doi: 10.1093/eurheartj/ehac235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordà P., Bosman L.P., Gasperetti A., et al. Arrhythmic risk prediction in arrhythmogenic right ventricular cardiomyopathy: external validation of the arrhythmogenic right ventricular cardiomyopathy risk calculator. Eur Heart J. 2022;43(32):3041–3052. doi: 10.1093/eurheartj/ehac289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James C.A., Jongbloed J.D.H., Hershberger R.E., et al. An international evidence based reappraisal of genes associated with arrhythmogenic right ventricular cardiomyopathy (ARVC) using the ClinGen framework. Circ Genom Precis Med. 2021;14(3) doi: 10.1161/CIRCGEN.120.003273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan E., Peterson L., Ai T., et al. Evidence-based assessment of genes in dilated cardiomyopathy. Circulation. 2021;144(1):7–19. doi: 10.1161/CIRCULATIONAHA.120.053033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith E.D., Lakdawala N.K., Papoutsidakis N., et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation. 2020;141(23):1872–1884. doi: 10.1161/CIRCULATIONAHA.119.044934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W., Murray B., Tichnell C., et al. Clinical characteristics and risk stratification of desmoplakin cardiomyopathy. EP Europace. 2022;24(2):268–277. doi: 10.1093/europace/euab183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhonsale A., Groeneweg J.A., James C.A., et al. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J. 2015;36(14):847–855. doi: 10.1093/eurheartj/ehu509. [DOI] [PubMed] [Google Scholar]
- 17.Richards S., Aziz N., Bale S., et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cadrin-Tourigny J., Bosman L.P., Wang W., et al. Sudden cardiac death prediction in arrhythmogenic right ventricular cardiomyopathy: a multinational collaboration. Circ Arrhythm Electrophysiol. 2021;14(1) doi: 10.1161/CIRCEP.120.008509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bariani R., Cason M., Rigato I., et al. Clinical profile and long-term follow-up of a cohort of patients with desmoplakin cardiomyopathy. Heart Rhythm. 2022;19(8):1315–1324. doi: 10.1016/j.hrthm.2022.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Sen-Chowdhry S., Syrris P., Prasad S.K., et al. Left-dominant arrhythmogenic cardiomyopathy. J Am Coll Cardiol. 2008;52(25):2175–2187. doi: 10.1016/j.jacc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Bosman L.P., Sammani A., James C.A., et al. Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: a systematic review and meta-analysis. Heart Rhythm. 2018;15(7):1097–1107. doi: 10.1016/j.hrthm.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Calkins H., Corrado D., Marcus F. Risk stratification in arrhythmogenic right ventricular cardiomyopathy. Circulation. 2017;136(21):2068–2082. doi: 10.1161/CIRCULATIONAHA.117.030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aquaro G.D., De Luca A., Cappelletto C., et al. Comparison of different prediction models for the indication of implanted cardioverter defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy. ESC Heart Fail. 2020;7(6):4080–4088. doi: 10.1002/ehf2.13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosman L.P., Nielsen Gerlach C.L., Cadrin-Tourigny J., et al. Comparing clinical performance of current implantable cardioverter-defibrillator implantation recommendations in arrhythmogenic right ventricular cardiomyopathy. EP Europace. 2022;24(2):296–305. doi: 10.1093/europace/euab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeppenfeld K., Tfelt-Hansen J., de Riva M., et al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43(40):3997–4126. doi: 10.1093/eurheartj/ehac262. [DOI] [PubMed] [Google Scholar]
- 26.Paldino A., Dal Ferro M., Stolfo D., et al. Prognostic prediction of genotype vs phenotype in genetic cardiomyopathies. J Am Coll Cardiol. 2022;80(21):1981–1994. doi: 10.1016/j.jacc.2022.08.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James C.A., Gasperetti A. Genotype-based risk stratification can outperform phenotype-based practice for inherited cardiomyopathies. J Am Coll Cardiol. 2022;80(21):1995–1997. doi: 10.1016/j.jacc.2022.09.018. [DOI] [PubMed] [Google Scholar]
- 28.Verstraelen T.E., van Lint F.H.M., Bosman L.P., et al. Prediction of ventricular arrhythmia in phospholamban p.Arg14del mutation carriers–reaching the frontiers of individual risk prediction. Eur Heart J. 2021;42(29):2842–2850. doi: 10.1093/eurheartj/ehab294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.