Abstract
Human immunodeficiency virus (HIV)-positive individuals express elevated levels of interleukin-8 (IL-8), which is believed to be responsible for some of the clinical manifestations occurring during AIDS. We report here that virion-derived HIV type 1 (HIV-1) protein R (Vpr) increased IL-8 expression in primary T cells and macrophages, as well as in the T-cell line Jurkat, the monocytic cell line U937, and the epithelial cell line A549. Vpr appeared to increase IL-8 expression and IL-8 promoter activity by activating transcription factors NF-κB and NF-IL-6. Elevated Vpr was also shown to increase transcription of the NF-κB and NF-IL-6 enhancer-containing viral promoters for HIV, cytomegalovirus, and simian virus 40, as well as increase the expression of IL-6 and IL-10 in primary macrophages and in A549 cells, tumor necrosis factor alpha expression in primary T cells, and IL-6 and gamma interferon expression in U937 cells. These results suggest a new role for Vpr in the pathogenesis of HIV infection, namely, the activation of transcription factors NF-IL-6 and NF-κB.
Interleukin-8 (IL-8) is one of several proinflammatory cytokines expressed during the inflammatory response to viral and bacterial infections (53). IL-8 was first identified as a 72-amino-acid neutrophil chemotactic polypeptide (37, 61). Subsequent studies have found that IL-8 stimulates chemotaxis in basophils and T lymphocytes (20, 62), induces neutrophils to release lysosomal enzymes (61), increases neutrophil expression of CD11b/CD18 and CR-1 (14), and promotes adherence of neutrophils to endothelial cells by increasing the expression of VCAM-1 and selectins (25).
IL-8 serum levels are significantly increased in human immunodeficiency virus (HIV)-infected individuals (34-fold [35]) and may contribute to some of the clinical manifestations that occur during AIDS. Studies indicate that HIV-positive individuals infected with Pneumocystis carinii express higher levels of IL-8 as compared to HIV-negative individuals presenting this same bacterial infection (7). In vitro, IL-8, along with IL-6 and tumor necrosis factor alpha (TNF-α), are induced early after HIV monocytic infection, followed by their continued increased expression (15). Interestingly, these studies on the in vitro expression of IL-8 in HIV-infected monocytes showed a close correlation between extracellular IL-8 levels and the levels of HIV p24 antigen (15), suggesting that an HIV lytic-cycle protein or proteins may be involved in the observed increase in IL-8.
The HIV protein R (Vpr) is a highly conserved viral auxiliary protein encoded by open reading frame R and is found in both human and simian immunodeficiency viruses (11, 60). The vpr gene encodes a 14-kDa, 96-amino-acid protein which is expressed primarily from a singly spliced Rev-dependent mRNA during the late phase of the virus life cycle (63). Vpr is assembled in the virus particle and, upon virus entry and uncoating, participates with the viral matrix proteins in targeting the HIV preintegration complex to the cell nucleus (8, 23). When expressed alone, Vpr has also been shown to cause cell cycle arrest in the G2/M phase of the cell cycle by suppressing cyclin B-associated kinase activity (22, 47), to induce cellular differentiation (32), and to promote cell apoptosis (58). Finally, Vpr has also been found associated with several cellular proteins (4, 32), one of which has been tentatively identified as belonging to the glucocorticoid receptor family (48).
Given the ability of HIV to increase the synthesis of proinflammatory cytokines at a time concomitant with virus replication (15), we undertook experiments to determine whether Vpr could alter the expression of the proinflammatory molecule IL-8. We chose IL-8 as our prototypic proinflammatory cytokine based on its increased expression following HIV infection and the presence of enhancer elements within the IL-8 promoter that are common to several other HIV-dysregulated proinflammatory cytokines (12).
MATERIALS AND METHODS
Culture reagents, antiserum, and cell culture.
Recombinant IL-1α was obtained from Boehringer Mannheim (Laval, Quebec, Canada). Rabbit anti-Vpr serum was obtained following injection of bacterially derived Vpr recombinant protein (HIV strain ELI) as previously described (31). The monocytic cell line U937 (CRL-1593; American Type Culture Collection, Manassas, Va.) and the T-lymphoid cell line Jurkat (TIB-152; American Type Culture Collection) were maintained in RPMI 1640 (Gibco BRL, Burlington, Ontario, Canada) supplemented with 10% heat-inactivated fetal calf serum (FCS; Gibco BRL), penicillin (100 U/ml), and gentamicin (80 μg/ml). The human pulmonary epithelial cell line A549 (kindly provided by B. Massie, National Research Council of Canada, Montreal, Quebec) was maintained in Dulbecco's modified Eagle medium (DMEM; Gibco BRL) supplemented with 10% FCS, penicillin (100 U/ml), and gentamicin (80 μg/ml).
Peripheral blood T cells and macrophages were obtained from healthy HIV-negative blood donors following separation on Ficoll-Hypaque density gradients. T cells were purified by CD4- and CD8-Dynabead affinity purification (Dynal, Success Lake, N.Y.). Macrophages were purified by adherence to FCS-coated tissue culture dishes. T-cell and macrophage preparations were 97% ± 1.5% (SE) (mean ± standard error) CD3+ (T cells) and 86% ± 5.4% CD14+ (monocytes/macrophages), respectively, as determined by immunostaining with phycoerythrin-coupled anti-human CD3 mouse monoclonal antibody (clone Leu-4; Becton Dickinson, San Jose, Calif.) or phycoerythrin-coupled anti-human CD14 mouse monoclonal antibody (clone Leu Mϕp9; Becton Dickinson) and flow cytometric analysis.
Plasmid construction.
The various IL-8 promoter constructs used to map Vpr-responsive promoter elements were constructed with PCR amplification products using synthetic primers within the IL-8 promoter region (−1481 to −1462, 5′-GAATTCAGTAACCCAGGCAT; −55 to −35, 5′-GATGAGGGTGCATAAGTTCTC; and +79 to +98, 5′-CCTTCCGGTGGTTTCTTCCT) or unique internal restriction sites within the IL-8 promoter (XbaI site at position −273) or were chemically synthesized (2× NF-κB −80/−70, 5′-gggaagcttGGAATTTCCTGGGAATTTCCTggatccggg; 2× NF-IL-6 −92/−80, 5′-gggaagcttCAGTTGCAAATCGTGGCAGTTGCAAATCGTgggatccgg; or NF-IL-6/NF-κB −92/70, 5′-gggaagcttCAGTTGCAAATCGTGGAATTTCCTggatccggg), using an Applied Biosystems 394 DNA/RNA nucleotide synthesizer. The resulting PCR fragments or synthetic DNAs were either subcloned into the EcoRI restriction site of PCR cloning vector pCR2.1, using the TA cloning kit (Invitrogen, Carlsbad, Calif.), or cloned as a HindIII/BamHI restriction fragment into pUC19, followed by subsequent insertion into the chloramphenicol acetyltransferase (CAT) reporter plasmid pBLCAT3 or pBLCAT2, respectively (33). All IL-8 promoter DNA sequences constructed by PCR amplification were verified for sequence fidelity by DNA sequencing and comparison with the previously reported IL-8 promoter sequence (41; J. Tanner, unpublished data).
The parental plasmid pSVCMVER (31) and its derivative plasmid pHook-3:VPR are HIV Vpr (HIV strain EL1) eukaryotic expression plasmids for which the vpr open reading frame was inserted either into the HindIII restriction site of the pHook-3 expression plasmid (Invitrogen) or into the SalI/SacI restriction sites of pSVCMVexPA (31). HIV EL1 Vpr has previously been shown to exhibit properties comparable to Vpr found in other HIV strains, such as virion incorporation and nuclear targeting, G2/M cell cycle arrest, and induction of cellular apoptosis (65). Viral promoter-linked CAT plasmids pHIVCAT (43, 54), pCMVCAT (17), pSV2CAT and pA10CAT2 (20), and pTKCAT (38) were used to determine additional Vpr-responsive promoters.
Production of pseudotyped HIV and infection of cells.
Vesicular stomatis virus G protein (VSV-G)-pseudotyped HIV-1 stocks were generated by cotransfection of 293T cells with envelope-defective HIV-1 proviral DNA and VSV-G expression plasmid as described elsewhere (25). Briefly, VSV-G-pseudotyped Vpr+ and Vpr− viral stocks were produced by transfection of 293T cells with HxBRUR+/Env− or HxBRUR−/Env− HIV-1 provirus plasmid (59, 64) and pSVCMV-VSV-G expression plasmid (65). Vpr or Vpr mutant R80A was trans -incorporated using the pSVCMV-Vpr expression plasmids (64). Following 72 h of culture, virus was pelleted from the culture supernatant by ultracentrifugation (24), and virus titers were determined by MAGI (multinuclear activation of galactosidase indicator) assay (27).
Primary lymphocytes (105/ml) or Jurkat, U937, or A549 cells (5 × 105/ml) were infected with Vpr+ or Vpr− VSV-G-pseudotyped viruses at multiplicities of infection of 10 for 8 h in the presence of Polybrene (10 μg/ml). Two hours prior to infection, 5 μM 3′-azido-3-deoxythymidine (AZT; Sigma Chemical Inc.) was added to inhibit virus transcription (24). Cell supernatants were collected at 24 h postinfection, treated with 0.01% Tween 20 to inactivate residual virus, and stored at −80°C until use.
Transient transfection.
Transfection of A549 cells was performed using DMRIE-C (Gibco BRL) as recommended by the manufacturer. Briefly, cells that had been seeded at 3 × 105 cells per 35-mm-diameter well and cultured for 18 h were then transfected with a total of 2 or 4 μg of plasmid DNA using 6 μg of DMRIE-C per ml of Opti-MEM I (Gibco BRL). Transfections were stopped after 6 h by adding an equal volume of DMEM containing 20% FCS. For the transfection of U937 and Jurkat cell lines, a protocol similar to that used for A549 cells was followed except that 106 cells were used along with 12 μg of DMRIE-C and 6 μg of plasmid DNA. Twelve hours later, the culture medium was replaced and the cells were incubated for an additional 24 h. For experiments involving the measurement of CAT enzyme or extracellular cytokines, the culture medium was replaced with serum-free medium supplemented with or without mitogens IL-1α, phorbol myristate acetate (PMA), phytohemagglutin in (PHA), or lipopolysaccharide (LPS) during the final 24 h of culture.
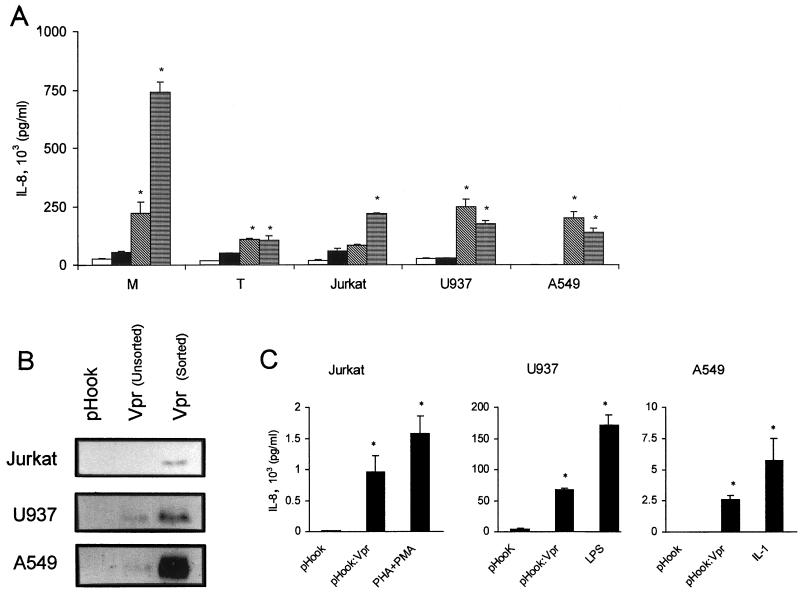
In several experiments, Vpr-expressing cells were enriched by affinity purification following transfection with pHook-3:VPR. The pHook-3 vector expresses and displays a single-chain antibody (sFv) which allows for the rapid isolation of transfected cells following incubation with hapten-coated magnetic beads (10). Twenty-four hours after the start of the transfection, adherent or nonadherent cells were harvested and washed with phosphate-buffered saline containing 3 mM EDTA followed by a 60-min incubation at 37°C on a slow rotator in complete medium containing hapten-coupled magnetic beads. The magnetic bead-bound cells were purified with a magnetic particle concentrator (Dynal), counted, and seeded at 104 cells/well in 96-well plates, using 200 μl of serum-free DMEM per well. Following this protocol, Vpr-containing A549, Jurkat, or U937 cells were enriched to more than 85% of the total cell population, as measured by the hemagglutinin A epitope immunofluorescence from cell surface sFv antibody chain (P. Roux, unpublished data). The enriched cells were incubated for an additional 24 h before assaying the culture media for cytokines.
CAT assay.
Levels of CAT enzyme were determined from 50 μg of cytoplasmic protein lysate using a CAT-specific enzyme-linked immunosorbent assay (ELISA) as recommended by the manufacturer (Boehringer Mannheim). CAT enzyme levels from different transfection experiments were normalized by cotransfection of pCMV-β-galactosidase and measurement of β-galactosidase enzyme (Invitrogen).
EMSA.
Electrophoretic mobility shift assay (EMSA) was performed using nuclear protein extracts from Vpr-transfected or Vpr-untransfected A549 cells as outlined by Scheinman et al. (52). Briefly, nuclei were isolated after 24 h from cells transfected with pSVCMVER or from control transfectants. Following gentle lysis of cell pellets in buffer containing 0.1% Nonidet P-40 and protease inhibitors phenylmethylsulfonyl fluoride, leupeptin, and pepstatin A, nuclear proteins were isolated from nuclei by high-salt (0.4 M) buffer extraction. Nuclear protein concentrations were determined colorimetrically with Bradford dye (Bio-Rad, Mississauga, Ontario, Canada) and stored at −80°C until use. Nuclear protein extract (10 μg) was incubated at 7°C for 20 min with anti-human NF-κB/p50 or anti-human NF-IL-6 antibody and Nu-Shift buffers and reagents (Geneka Inc., Montreal, Quebec, Canada) followed by continued incubation for 20 min with 32P-labeled DNA oligonucleotide probe (10,000 cpm). Protein-bound DNA probe was resolved in a 5% polyacrylamide–Tris-glycine-buffered gel (51). Oligonucleotide probes (Geneka) used in our EMSA were based on both the published DNA sequence data for the IL-8 NF-κB and NF-IL-6 enhancer sequences and our positive CAT results.
Immunoblotting analysis.
Total cellular protein from Vpr-transfected cells was prepared in sodium dodecyl sulfate sample buffer 48 h posttransfection and resolved in a sodium dodecyl sulfate–15% polyacrylamide gel. The proteins were transferred onto a nitrocellulose membrane (Gelman Sciences, Ann Arbor, Mich.) and probed with a rabbit anti-Vpr serum (1:500). Bound antibody was detected with a horseradish peroxidase-labeled donkey anti-rabbit serum (1:3,000; Amersham, Oakville, Ontario, Canada) and developed using an enhanced chemiluminescence immunoblotting detection kit (Amersham).
Cytokine assays.
Extracellular IL-6, IL-8, IL-10, TNF-α, and gamma interferon (IFN-γ) protein concentrations were determined by ELISA (R&D Systems, Minneapolis, Minn.).
Statistical analysis.
Statistical comparison of Vpr-transfected cells with matched control cell populations was performed using the Student t test.
RESULTS
Vpr expression induces IL-8.
IL-8 serum levels are significantly increased in HIV-infected individuals (35). In vitro, IL-8, along with IL-6 and TNF-α, are induced early after HIV monocytic infection, followed by their continued increased expression (15). We determined whether Vpr was capable of inducing IL-8 in HIV-susceptible cells by infecting primary T cells or macrophages, the T-cell line Jurkat, the monocytic cell line U937, or the human pulmonary cell line A549 with pseudotyped HIV-1 virus incorporated with either a wild-type or a mutant Vpr. We chose to test the A549 cell line because this cell line expresses IL-8 following cytokine stimulation and has been shown to faithfully reflect the use of transcription control elements used within the IL-8 promoter for several cell types, including monocytes (36, 42, 56). Results indicated that Vpr+ virus, but not Vpr− virus, increased IL-8 expression in all the cell types tested. As shown in Fig. 1A, Vpr+ virus induced the expression of IL-8 in primary macrophages and T cells four- and twofold, respectively. Vpr+ virus also increased the expression of IL-8 in Jurkat, U937, and A549 cells 1.4-, 9-, and 20-fold, respectively.
FIG. 1.
Induction of IL-8 by Vpr. (A) Extracellular IL-8 protein concentrations from primary T (T) cells, macrophages (M), and Jurkat, U937, and A549 cells following culture for 24 h in medium (open box), infection and culture with Vpr− pseudotyped HIV-1 (black box), Vpr+ pseudotyped HIV-1 (diagonally hatched box), or culture with mitogens (LPS, [1 μg/ml], macrophages and U937; PMA [20 ng/ml]/1:200 [vol/vol] PHA, T cells and Jurkat; IL-1α [4 ng/ml], A549) (horizontally hatched box); extracellular IL-8 protein concentrations from pHook3-transfected cells, pHook3:VPR-transfected cells, or pHook3-transfected cells treated with mitogen IL-1α (4 ng/ml; A549), LPS; (1 μg/ml U937) or PMA (20 ng/ml) 1:200 (vol/vol) PHA (Jurkat). Cells were affinity purified by magnetic beads 24 h posttransfection and processed for anti-Vpr immunoblot (B) or cultured for an additional 24 h at 104 cells/200 μl in serum-free DMEM in the presence or absence of mitogen (C). Results are expressed as the mean IL-8 concentration ± SE and based on samples from two and three independent infections or transfections, respectively. P values of ≤0.05 are designated by asterisks.
To exclude the possibility that Vpr acted in conjunction with another virion protein, we transfected Jurkat, U937, and A549 cells with expression plasmid pHook-3:VPR. Following an initial cell sorting with hapten-coated magnetic beads to enrich the number of cells expressing Vpr in our cultures (Fig. 1B), we observed that Jurkat, U937, and A549 cells transfected with pHook-3:VPR expression plasmid demonstrated 83-, 16-, and 500-fold, respectively, increases in the level of IL-8 protein 24 h postselection (Fig. 1C). This compares with 130-, 40-, and 1,140-fold increases in IL-8 protein level following mitogen stimulation (Fig. 1C). The induction levels for IL-8 following Vpr expression in Jurkat and U937 cells are comparable to previous reports for Jurkat and monocytes using PMA and ionomycin (60-fold) or gp120 (9-fold) as stimulants (9, 45). The induction levels of IL-8 following Vpr expression in A549 cells (500- to 1,140-fold) was based on the minimum detection limit of our ELISA (10 pg/ml), since no IL-8 was detectable in our vector-transfected A549 cells. We do note, however, that the observed 2,550 pg of IL-8 per ml induced by Vpr is in agreement with that previously reported for TNF-α- or IL-1-stimulated A549 cells (2,200 or 1,800 pg/ml, respectively) (49).
Identification of Vpr-responsive elements in the IL-8 promoter.
To determine whether Vpr increased IL-8 expression in part by inducing IL-8 gene transcription, the full-length IL-8 promoter (41), along with various truncated forms of the IL-8 promoter fused to the reporter gene, CAT, were cotransfected with the Vpr expression plasmid pSVCMVER. In initial experiments, cotransfection of A549 cells with increasing amounts of Vpr expression plasmid and a constant amount of full-length IL-8 promoter reporter plasmid resulted in a Vpr dose-dependent increase in IL-8 promoter-driven CAT levels (expressed as stimulation indices [SIs]) (Fig. 2). The IL-8 promoter-driven CAT enzyme levels obtained with optimal amounts of Vpr expression plasmid were comparable to those obtained with exogenously added IL-1α (Fig. 2).
FIG. 2.
Effect of Vpr expression on IL-8 promoter activity in A549 cells. A549 cells transfected with 2 μg of full-length IL-8 promoter-CAT construct (pIL-8CAT-1481) and increasing amounts of Vpr expression plasmid pSVCMVER or A549 cells transfected with pIL-8CAT-1481 and treated with IL-1α (4 ng/ml) were assayed for IL-8 promoter activity by CAT ELISA. To eliminate variable transfection efficiencies due to differing amounts of plasmid DNA, final amounts of plasmid DNA for each transfection were adjusted to 4 μg using pSVCMV.
Fine mapping of the IL-8 promoter indicated that the DNA sequences most responsive to Vpr resided within nucleotide sequences spanning −273 to −55 (Fig. 3). Upstream promoter sequences spanning −1481 to −273 appeared dispensable for maximal Vpr induction (Fig. 3B). Conversely, the deletion of promoter sequences containing the NF-IL-6/NF-κB enhancer sequence (−94 to −70) significantly abolished Vpr responsiveness. Transfection of pIL-8CATΔ(−273/+79) or pIL-8CAT-55 with a Vpr expression plasmid resulted in no marked increase in CAT activity (Fig. 3B). The slight (0.3-fold) increase in CAT activity observed when IL-8 DNA sequences −1481 to −273 were linked to the CAT gene may be due to the presence of a glucocorticoid-responsive element, since this same reporter plasmid was also found to be responsive to dexamethasone (2, 40;Roux, unpublished data).
FIG. 3.
Identification of Vpr-responsive elements in the IL-8 promoter. (A) Schematic representation of the 5′-regulatory region of the IL-8 gene and the various IL-8 promoter truncation constructs fused to the CAT gene used to map Vpr-responsive elements in the IL-8 promoter. (B) CAT induction levels in A549 cells following cotransfection with the various IL-8 promoter-CAT constructs (shown above) and Vpr expression vector pSVCMVER (open bar). A549 cells were also cotransfected with the various IL-8 promoter-CAT constructs and pSVCMV followed by treatment with IL-1α (5 ng/ml) (black bars). CAT SIs were determined following subtraction of pSVCMV-transfected CAT values, which were arbitrarily given a value of 1. Results were then expressed as the mean SI ± SE. Results were based on three independent transfections.
To determine whether NF-IL-6 and NF-κB enhancer sequences, either individually or together as a core IL-8-responsive element, were responsive to Vpr expression, CAT reporter constructs containing each of these elements were cotransfected with the Vpr expression plasmid into A549 cells. As seen in Fig. 3B, NF-κB and NF-IL-6, either together or individually, were responsive to Vpr. The observed increase in IL-8 promoter activity stemming from NF-IL-6 and NF-κB enhancer sequences following Vpr stimulation is in agreement with results previously observed for IL-1α (57).
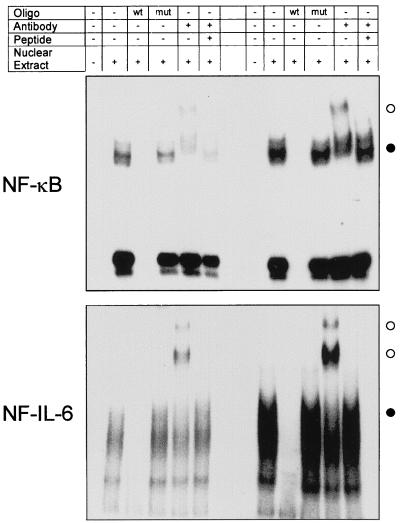
To verify that NF-κB and NF-IL-6 transcription factors bound to our CAT-linked IL-8 promoter, EMSA was performed. Results indicated that Vpr-transfected cells increased NF-κB and NF-IL-6 DNA binding activity three- and fivefold, respectively, relative to that of mock-transfected cells (Fig. 4, lane 2). Binding activity was specific for the NF-κB and NF-IL-6 probe since labeled oligonucleotides could be blocked from binding to nuclear protein with excess cold oligonucleotides but not with excess mutant oligonucleotides (lanes 3 and 4), thus reconfirming that NF-κB and NF-IL-6 activity was increased following Vpr expression. Addition of anti-NF-κB/p50 or anti-NF-IL-6 antiserum to the EMSA mixture also revealed the presence of unique and intensely strong higher-molecular-weight bands in the Vpr-expressing cell nuclear extract versus mock-transfected cell extract (lane 5). These higher-molecular-weight bands could also be competitively inhibited with NF-κB/p50- or NF-IL-6-specific peptides (lane 6), indicating that NF-κB/p50 and NF-IL-6 transcription factors formed part of the transcription complex involved in Vpr activation of the IL-8 promoter.
FIG. 4.
EMSA analysis of Vpr-transfected and untransfected A549 cells. Nuclear protein extracts were prepared from A549 cells harvested 24 h after transfection with either pcDNA3 (left) or pSVCMVER (Vpr; right). 32P-labeled NF-κB (top) and NF-IL-6 oligonucleotide probe (oligo) (bottom) were incubated in the absence (−) or presence (+) of nuclear extract, 10-fold-excess wild-type (wt) or mutant (mut) cold oligonucleotide probe, anti-NF-κB/p50 or NF-IL-6 anti-transcription factor antibody, or excess antibody-specific transcription factor immune peptide, as outlined at the top. NF-κB or NF-IL-6 bands which increased in intensity following Vpr expression (●) or are unique following Vpr expression and anti-transcription factor antibody addition (○) are indicated.
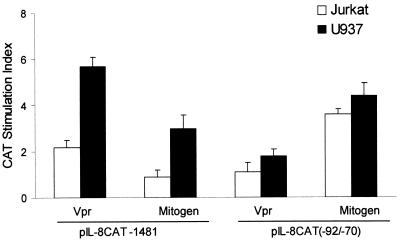
Vpr stimulation of the IL-8 promoter core was also seen in the T-cell line Jurkat and in the monocytic cell line U937. Cotransfection of Vpr expression plasmid pSVCMVER with the full-length IL-8 promoter or the core NF-IL-6/NF-κB enhancer elements (Fig. 5) resulted in a two- to sixfold and a one- to twofold, respectively, increase in CAT activity. Vpr did not increase CAT activity in either Jurkat or U937 cells when tested with individual NF-κB or NF-IL-6 enhancer elements, indicating the need to maintain an intact IL-8 core enhancer structure for optimum promoter activity (Tanner, unpublished data). These results agree well with those seen with A549 cells (Fig. 3) or those previously observed for LPS-stimulated U937 cells (28, 36).
FIG. 5.
IL-8 promoter response following Vpr expression in Jurkat and U937 cells. Jurkat (□) or U937 (■) cells were cotransfected with pIL-8CAT-1481 or pIL-8CAT (−92/−70) and pSVCMVER (Vpr) or pSVCMV. pSVCMV transfected cells were also cultured in the presence or absence of LPS (1 μg/ml; U937) or PMA (20 ng/ml)/1:200 (vol/vol) PHA (Jurkat) (Mitogen). CAT SIs were determined following subtraction of pSVCMV-transfected CAT values, arbitrarily given a value of 1. Results were then expressed as the mean SI ± SE. Results were based on three independent transfections.
In addition to the IL-8 promoter, Vpr was found to increase the transcription of other NF-κB or κB-like enhancer containing promoters. Vpr expression increased the transcription of the HIV long terminal repeat (LTR) promoter, the cytomegalovirus (CMV) immediate-early gene promoter, and the simian virus (SV40) T-antigen promoter two- to fourfold (Table 1). The induction of these viral promoters appeared to be κB specific, since the herpes simplex virus thymidine kinase (HSV TK) promoter, which does not contain κB-like enhancer elements, did not significantly increase transcription following Vpr expression (Table 1).
TABLE 1.
Heterologous viral promoter activity following Vpr stimulation
| Promoter | Avg CAT enzyme concentration (pg/50 μg of cell protein) ± SDa
|
SI | |
|---|---|---|---|
| Mock | Vpr | ||
| IL-8 | 343 ± 67 | 903 ± 24 | 2.6 |
| CMV | 467 ± 8 | 1,869 ± 201 | 4.0 |
| HIV LTR | 163 ± 22 | 491 ± 74 | 3.0 |
| SV40 | 310 ± 8 | 882 ± 5 | 2.8 |
| HSV TK | 19 ± 0.5 | 21 ± 2 | 1.1 |
Based on samples obtained from three separate transfections.
Induction of proinflammatory cytokines by Vpr.
Since Vpr was capable of increasing the expression of IL-8 in primary cells, as well as in Jurkat, U937, and A549 cell lines, we also analyzed whether culture supernatants from Vpr-treated cells contained the proinflammatory cytokines TNF-α, IL-6, IFN-γ, and IL-10. We chose these cytokines since they too have been reported to be controlled by NF-κB or NF-IL-6 enhancer elements and are expressed during HIV infection.
Results revealed that Vpr increased the level of IL-10 15- and 3-fold and increased the level of IL-6 4- and 2-fold in macrophage and A549 cells, respectively (Tables 2 and 3). Primary T cells were found to express a 10-fold increase in the level of TNF-α following Vpr+ virus infection (Table 2). U937 cells were found to increase IL-6 and IFN-γ 3-fold following Vpr expression but showed only a slight increase (0.2-fold) in TNF-α (Table 3). Although we did detect IL-8 expression in Jurkat cells (Fig. 2), we did not detect measurable amounts of TNF-α, IL-6, IL-10, or IFN-γ expression following either Vpr or PHA-PMA stimulation (Tanner, unpublished data). The induction by IL-8, IL-10, and IL-6 in primary macrophages, or the induction of TNF-α in T cells by Vpr+ but not Vpr− infected cells, also suggests that Vpr is capable of overcoming the immunosuppressive properties of AZT (Table 2 and reference 44).
TABLE 2.
Induction of proinflammatory cytokines by virion-transduced Vpra
| Cytokine | Mean cytokine concn (pg/ml) ± SD
|
Vpr SIb | Mitogen (mean cytokine concn ± SD) | Mitogen SI | ||
|---|---|---|---|---|---|---|
| Medium | Vpr mutant | Vpr | ||||
| Macrophages | ||||||
| TNF-α | 32 ± 18 | NDc | ND | 0 | 256 ± 71 | 8 |
| IL-6 | 70 ± 52 | ND | 266 ± 33 | 4 | 810 ± 100 | 12 |
| IFN-γ | 32 ± 8 | ND | ND | 0 | 168 ± 48 | 5 |
| IL-10 | 24 ± 5 | ND | 370 ± 130 | 15 | 171 ± 32 | 7 |
| T cells | ||||||
| TNF-α | 22 ± 2 | ND | 209 ± 140 | 10 | 398 ± 78 | 18 |
| IL-6 | 10 ± 10 | ND | ND | 0 | 570 ± 10 | 57 |
| IFN-γ | 33 ± 9 | ND | ND | 0 | 257 ± 78 | 8 |
| IL-10 | 29 ± 12 | ND | ND | 0 | 148 ± 31 | 5 |
Lymphocytes (105) were infected with Vpr or Vpr mutant containing VSV-G-pseudotyped HIV viruses at a multiplicity of infection of 10 in the presence of 5 μM AZT. Cell supernatants were collected at 24 h postinfection.
Based on medium values.
ND, not detected.
TABLE 3.
Induction of proinflammatory cytokines by plasmid-based Vpra
| Cytokine | Mean cytokine concn (pg/ml) ± SD
|
Vpr SI | Mitogen mean cytokine concn (pg/ml) ± SD | Mitogen SI | |
|---|---|---|---|---|---|
| Mock | Vpr | ||||
| A549 | |||||
| TNF-α | NDc | ND | ND | 0 | |
| IL-6 | 156 ± 23 | 256 ± 18 | 2 | 640 ± 126 | 4 |
| IFN-γ | ND | ND | 0 | ND | |
| IL-10 | 156 ± 42 | 446 ± 72 | 3 | 227 ± 84 | 2 |
| U937 | |||||
| TNF-αb | 26,100 ± 101 | 31,200 ± 304 | 1 | 32,780 ± 135 | 1 |
| IL-6 | 92 ± 15 | 278 ± 34 | 3 | 4,320 ± 143 | 47 |
| IFN-γ | 32 ± 11 | 89 ± 12 | 3 | 110 ± 28 | 3 |
| IL-10 | ND | ND | ND | ||
Cells transfected with pHook-3 (mock) or pHook-3:VPR (Vpr) were sorted and seeded at 104 cells/200 μl in serum-free medium 24 h posttransfection in the presence or absence of mitogens (4 ng of IL-1α per ml for A549 cells; 1 μg of LPS per ml for U937 cells).
Induction of TNF-α in U937 by Vpr or LPS stimulation following an 18-h pretreatment with PMA (20 ng/ml) (50). Untreated U937 cells failed to induce detectable TNF-α following either Vpr transfection or LPS treatment.
ND, not detected.
DISCUSSION
Our results demonstrate that Vpr is capable of increasing the expression of IL-8 through the activation of transcription factors NF-κB and NF-IL-6. This is the first report of Vpr acting as a stimulator of NF-κB or NF-IL-6 for the induction of a proinflammatory cytokine. In addition to IL-8, we observed that other cytokines controlled by NF-κB or NF-IL-6, and several viral promoters containing κB-like or NF-IL-6 enhancer sequences, responded positively to Vpr (Tables 1 to 3).
Vpr appears essential for HIV replication in vivo, as seen by the high rate of mutant Vpr reversion to wild type following mutant virus injection into macaques (29), poor virus replication in primary T cells and monocytes when Vpr was expressed in mutated form (5, 13, 19), and blockage of virus replication when cells were grown in the presence of antisense Vpr oligodeoxynucleotide (6). HIV is also known to replicate more efficiently in blast cells than in quiescent cells (53). Since typical HIV targets, such as monocytes or T cells, would be quiescent and unlikely to contain sufficient amounts of active transcription factor to support initial HIV replication (1), Vpr, in addition to transporting the preintegration virus RNA complex, also may serve to activate transcription factors such as NF-κB and NF-IL-6 and allow for an initial burst of HIV transcription.
Vpr has been shown by a number of laboratories to inhibit cell growth at the G2/M phase of the cell cycle (22, 45), resulting in increased HIV LTR transcription and viral gene expression (59). The exact cause of increased HIV LTR transcription during G2/M arrest is still controversial. While there is a report that G2/M arrest alone is sufficient to increase basal levels of the transcription machinery and thus augment HIV LTR transcription (21), others believe that increased transcription is related to the dephosphorylation of the kinase for p34cdc2-cyclin B and actions of p300 coactivator (16, 22, 46, 47). Our observed increase in NF-κB/p50 and NF-IL-6 activities and HIV LTR promoter transcription with no increase in HSV TK promoter transcription (Fig. 4 and Table 1), coupled with the fact that p300 binds NF-κB and members of the C/EBP family in normal cells (39, 46), favors the latter view.
In vitro, newly HIV-infected monocytes exhibited a 36-fold increase in the levels of IL-8 expression which correlated with lytic virus replication antigen p24 (15). In vivo, IL-8 levels are differentially elevated in HIV-infected individuals compared to their uninfected counterparts (7). Thus, our observed induction of IL-8 by Vpr in vitro suggests that Vpr could contribute to some of the HIV-related pathologies seen in vivo. Elevated expression of IL-8 in HIV-infected monocytes or antigen-presenting cells would be advantageous for the dissemination of HIV. T cells are 2 to 10 times more sensitive than neutrophils (40) to low levels of IL-8 in terms of chemotaxis and may promote T-cell or monocyte interaction through the expression of IL-8-induced adhesion molecules (55). The overall result would be to potentiate additional target cells to the site of infection by chemotaxis, attachment of target cells via induced adhesion molecules, and subsequent virus production as a result of elevated virus-promoting cytokines in the microenvironment. These infected cells would then have the potential to traffic to the draining lymph node and ultimately spread to peripheral blood.
During the completion of our studies a report was published which suggested that Vpr inhibited the expression of several cytokines by inhibiting NF-κB (3). We did not observe an inhibition in the expression of several NF-κB-controlled cytokines, including IL-10 as reported by Ayyavoo et al. (3). Rather, Vpr demonstrated 15- and 3-fold increases in the level of IL-10, and 4- and 2-fold increases in the level of IL-6, in macrophage and A549 cells, respectively (Tables 2 and 3). We also observed a 10-fold increase in the level of TNF-α in T cells and a 2-fold increase in the level of IFN-γ in U937 cells following Vpr stimulation (Tables 2 and 3). Further, we confirmed that Vpr increased the expression of κB-containing promoters, namely, HIV, CMV, and SV40 (Table 1 and references 11, 18, 21, and 59), which further supports the contention that Vpr is not a universal inhibitor of NF-κB-driven promoters. Whether use of alternative transcription factor family members for NF-κB or NF-IL-6 (1) or differences in the proliferative state or cell type used during NF-κB analysis (lymphoid versus the myoblast cell line RD) (3) introduced experimental variations, thereby altering the outcome of cytokine expression experiments, remains to be explored. We do note, however, that high levels of Vpr are reported to be toxic to cells and could result in the appearance of a cytokine-repressed cell phenotype (21).
In conclusion, our results indicate that Vpr expression and subsequent activation of NF-κB or NF-IL-6 can lead to increased expression of IL-8. These observations should prove extremely important in elucidating the initial phase of HIV infection and providing possible mechanisms for AIDS-related pathologies. This work also suggests that IL-8 may represent an important new target for the control of HIV replication.
ACKNOWLEDGMENTS
This work was supported by a grant from the Canadian Foundation for AIDS Research and the Ontario HIV Treatment Network. P.R. was supported by a studentship from the Fondation du Conseil des Clubs de Services Sociaux. C.A. was a scholar of the J. A. DeSève Foundation.
REFERENCES
- 1.Akira S, Kishimoto T. NF-IL-6 and NF-κB in cytokine gene regulation. Adv Immunol. 1997;65:1–46. [PubMed] [Google Scholar]
- 2.Almaui W Y, Beyhum H N, Rohme A A, Rieder M J. Regulation of cytokine and cytokine receptors by glucocorticoids. J Leukoc Biol. 1997;60:563–575. doi: 10.1002/jlb.60.5.563. [DOI] [PubMed] [Google Scholar]
- 3.Ayyavoo V, Mahboubi A, Ramalingam R, Kudschdkar S, Williams W V, Greene D R, Wiener D B. HIV-1 vpr suppressed immune activation and apoptosis through regulation of nuclear factor κB. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 4.Ayyavoo V, Mahalingam S, Rafaeli Y, Kudchodkar S, Chang D, Nagashumugam T, Williams W V, Wienee D B. HIV-1 viral protein R (Vpr) regulated viral replication and cellular proliferation in T cells and monocytoid cells in vitro. J Leukoc Biol. 1997;62:93–99. doi: 10.1002/jlb.62.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 6.Balotta C, Lusso P, Crowley R, Gallo R C, Franchini G. Antisense phosphorothioate oligodeoxynucleotides targeted to the vpr gene inhibit human immunodeficiency virus type 1 replication in primary human macrophages. J Virol. 1993;67:4409–4414. doi: 10.1128/jvi.67.7.4409-4414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benfield T L, Kharazmi A, Larsen C G, Lundgren J D. Neutrophil chemotactic activity in bronchoalveolar lavage fluid of patients with AIDS-associated pneumocystis carinii pneumonia. Scand J Infect Dis. 1997;29:367–371. doi: 10.3109/00365549709011832. [DOI] [PubMed] [Google Scholar]
- 8.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capobianchi M R, Barresi C, Borghi P, Gessani S, Fantuzzi L, Ameglio F, Belardelli F, Papadia S, Dianzani F. Human immunodeficiency virus type 1 gp120 stimulates cytomegalovirus replication in monocytes: possible role of endogenous interleukin-8. J Virol. 1997;71:1591–1597. doi: 10.1128/jvi.71.2.1591-1597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesnut J D, Bayton A R, Russel M, Chang M P, Bernard A, Maxwell I H, Hoeffler J P. Selective isolation of transiently transfected cells from a mammalian cell population with vectors expressing a membrane anchored single-chain antibody. J Immunol Methods. 1996;193:17–27. doi: 10.1016/0022-1759(96)00032-4. [DOI] [PubMed] [Google Scholar]
- 11.Cohen E A, Terwilliger E F, Jalinoos Y, Proulx J, Sodroski J G, Haseltine W A. Identification of HIV-1 vpr product and function. J Acquir Immun Defic Syndr. 1990;3:311–318. [PubMed] [Google Scholar]
- 12.Cohen O J, Kintner A, Fauci A S. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997;159:31–48. doi: 10.1111/j.1600-065x.1997.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 13.Connor R I, Chen B K, Choe S, Landau N. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 14.Detmers P A, Lo S K, Olsen E E, Walz A, Baggiolini M, Cohn Z A. Neutrophil-activating protein 1/interleukin 8 stimulates the binding activity of the leukocyte adhesion receptor CD11b/CD18 on human neutrophils. J Exp Med. 1990;171:1155–1162. doi: 10.1084/jem.171.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esser R, Wolfgang W, Briesen H V, Rubsamen-Waigmann H, Andreesen R. Differential regulation of proinflammatory and hematopoietic cytokines in human macrophages after infection with human immunodeficiency virus. Blood. 1996;88:3474–3481. [PubMed] [Google Scholar]
- 16.Felzien L K, Woffendin C, Hottinger M O, Subbramanian R A, Cohen E A, Nabel G J. HIV transcriptional activation by the accessory protein, VPR is mediated by the p300 co-activator. Proc Natl Acad Sci USA. 1998;95:5281–5286. doi: 10.1073/pnas.95.9.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foecking M K, Hofstetter H. Powerful and versatile enhancer-promoter unit for mammalian expression vectors. Gene. 1986;45:101–105. doi: 10.1016/0378-1119(86)90137-x. [DOI] [PubMed] [Google Scholar]
- 18.Forget J, Yao X J, Mercier J, Cohen E A. Human immunodeficiency virus type I Vpr protein transactivation function mechanism and identification of domains involved. J Mol Biol. 1998;784:915–923. doi: 10.1006/jmbi.1998.2206. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs J S, Lackner A A, Lang S M, Simon M A, Sehgal P K, Daniel M D, Desrosiers R C. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995;69:2378–2383. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorman C M, Moffat L F, Howard B H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gummuluru S, Emerman M. Cell cycle- and Vpr-mediated regulation of human immunodeficiency virus type 1 expression in primary and transformed T-cell lines. J Virol. 1999;73:544–5430. doi: 10.1128/jvi.73.7.5422-5430.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hrimech M, Yao X-J, Bachand F, Rougeau N, Cohen E A. Human immunodeficiency virus type 1 (HIV-1) Vpr functions as an immediate-early protein during HIV-1 infection. J Virol. 1999;73:4101–4109. doi: 10.1128/jvi.73.5.4101-4109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber A R, Kunkel S L, Todd R F, Weiss S J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 26.Jowett J B, Planelles V, Poon B, Shah N P, Chen M L, Chen I S. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunsch C, Rosen C A. NF-κB subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6237–6244. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lang S M, Weeger M, Stahl-Hennig C, Coulibaly C, Hunsmann G, Muller J, Muller-Hermelink H, Fuchs D, Wachter H, Daniel M M, Desrosiers R C, Fleckenstein B. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J Virol. 1993;67:902–912. doi: 10.1128/jvi.67.2.902-912.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen C G, Anderson A O, Appella E, Oppenheim J J, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 31.Lavallée C, Yao X J, Ladha A, Gottlinger H G, Haseltine W A, Cohen E A. Requirement of the pr55gag precursor for incorporation of the vpr product into human immunodeficiency virus type 1 viral particles. J Virol. 1994;68:1926–1934. doi: 10.1128/jvi.68.3.1926-1934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levy D N, Fernandes L S, Williams W V, Weiner D B. Induction of cell differentiation by human immunodeficiency virus 1 vpr. Cell. 1993;72:541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- 33.Levy D N, Refaeli Y, Weiner D B. Extracellular Vpr protein increases cellular permissiveness to human immunodeficiency virus replication and reactivates virus from latency. J Virol. 1995;69:1243–1252. doi: 10.1128/jvi.69.2.1243-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luckow B, Schutz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987;15:5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumoto T, Mike T, Nelson R P, Trudeau W L, Lockey R F, Yodoi J. Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol. 1993;93:149–151. doi: 10.1111/j.1365-2249.1993.tb07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsusaka, Fujikawa T K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL-6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung H F, Leonard E J, Oppenheim J J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDCNF) and the induction of MDCNF mRNA by interleukin-1 and tumor necrosis factor. J Exp Med. 1988;167:1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKnight S L, Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982;217:316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- 39.Mink S, Haenig B, Klempnauer K-H. Interaction and functional collaboration of p300 and C/EBPβ. Mol Cell Biol. 1997;17:6609–6617. doi: 10.1128/mcb.17.11.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukaida N, Morita M, Ishikawa W, Rice N, Okamoto S, Kasahara T, Matsushima K. Novel mechanism of glucocorticoid-mediated gene repression: nuclear factor-κB is a target for glucocorticoid-mediated interleukin 8 gene expression. J Biol Chem. 1994;269:13289–13292. [PubMed] [Google Scholar]
- 41.Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143:1366–1371. [PubMed] [Google Scholar]
- 42.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554–558. [PubMed] [Google Scholar]
- 43.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 44.Nokta M A, Pollard R B. Differential reconstitution of zidovudine-induced inhibition of mitogenic responses by interleukin-2 in peripheral blood mononuclear cells from patients with human immunodeficiency virus infection. Antiviral Res. 1989;11:191–202. doi: 10.1016/0166-3542(89)90004-1. [DOI] [PubMed] [Google Scholar]
- 45.Ott M, Lovett J L, Mueller L, Verdin E. Superinduction of IL-8 in T cells by HIV Tat protein is mediated through NF-κB factors. J Immunol. 1998;160:2872–2880. [PubMed] [Google Scholar]
- 46.Perkins N D, Felzien L K, Betts J C, Leung K, Beach D H, Nabel G J. Regulation of NF-κB by cyclin-dependent kinase associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 47.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2 cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Refaeli Y, Levy D N, Weiner D B. The glucocorticoid receptor type II complex is a target of the HIV-1 vpr gene product. Proc Natl Acad Sci USA. 1995;92:3621–3625. doi: 10.1073/pnas.92.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenthal G J, Germolec D R, Blazka M E, Corsini E, Simenova P, Pollock P, Kong L-Y, Kwon J, Luster M I. Asbestos stimulates IL-8 production from human epithelial cells. J Immunol. 1994;153:3237–3244. [PubMed] [Google Scholar]
- 50.Sajjadi F G, Tabayashi K, Foster A C, Domingo R C, Firestein G F. Inhibition of TNFα expression by adenosine: role of A3 adenosine receptors. J Immunol. 1996;156:3435–3442. [PubMed] [Google Scholar]
- 51.Scheinman R I, Beg A A, Baldwin A S., Jr NF-κB p100 (Lyt-10) is a component of H2TF1 and can function as an IκB-like molecule. Mol Cell Biol. 1993;13:6089–6101. doi: 10.1128/mcb.13.10.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheinman R I, Gualberto A, Jewell C M, Cidlowski J A, Baldwin A S., Jr Characterization of mechanisms involved in transrepression of NF-κB by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schluger N W, Rom W N. Early responses to infection: chemokines as mediators of inflammation. Curr Opin Immunol. 1997;9:504–508. doi: 10.1016/s0952-7915(97)80102-1. [DOI] [PubMed] [Google Scholar]
- 54.Siekevitz M, Josephs S F, Dukovich M, Peffer N, Wong-Staal F, Greene W C. Activation of the HIV-1 LTR by T cell mitogens and transactivator protein of HTLV-1. Science. 1989;238:1575–1578. doi: 10.1126/science.2825351. [DOI] [PubMed] [Google Scholar]
- 55.Springer T A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradign. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 56.Standiford T J, Kunkel S L, Basha M A, Chensue S W, Lynch J P, Toews G B, Westwick J, Strieter R M. Interleukin-8 gene expression by a pulmonary cell line. A model for cytokine networks in the lung. J Clin Investig. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein B, Baldwin A S. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-κB. Mol Cell Biol. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart S A, Poon B, Jowett J B, Chen I S Y. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Subbramanian R A, Kessouselbaz A, Lodge R, Forget J, Yao X S, Bergeron D, Cohen E A. Human immunodeficiency virus type 1 Vpr is a positive regulator of viral transcription and infectivity in primary human macrophage. J Exp Med. 1998;187:1103–1111. doi: 10.1084/jem.187.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tristem M, Marshall C, Karpas A, Hill F. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 1992;11:3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- 62.White M V, Yoshimura T, Hook W, Kaliner M A, Leonard E J. Neutrophil attractant/activation protein-1 (NAP-1) causes human basophil histamine release. Immunol Lett. 1989;22:151–154. doi: 10.1016/0165-2478(89)90182-x. [DOI] [PubMed] [Google Scholar]
- 63.Wong-Staal F, Chanda P K, Ghrayeb J. Human immunodeficiency virus: the eighth gene. AIDS Res Hum Retroviruses. 1987;3:33–39. doi: 10.1089/aid.1987.3.33. [DOI] [PubMed] [Google Scholar]
- 64.Yao X-J, Subbramanian R A, Rougeau N, Boisvert F, Bergeron D, Cohen E A. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao X J, Mouland A J, Subbramian R A, Forget J, Rougeau N, Bergeron D, Cohen E A. Vpr stimulates viral expression and induced cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J Virol. 1998;72:4686–4693. doi: 10.1128/jvi.72.6.4686-4693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]