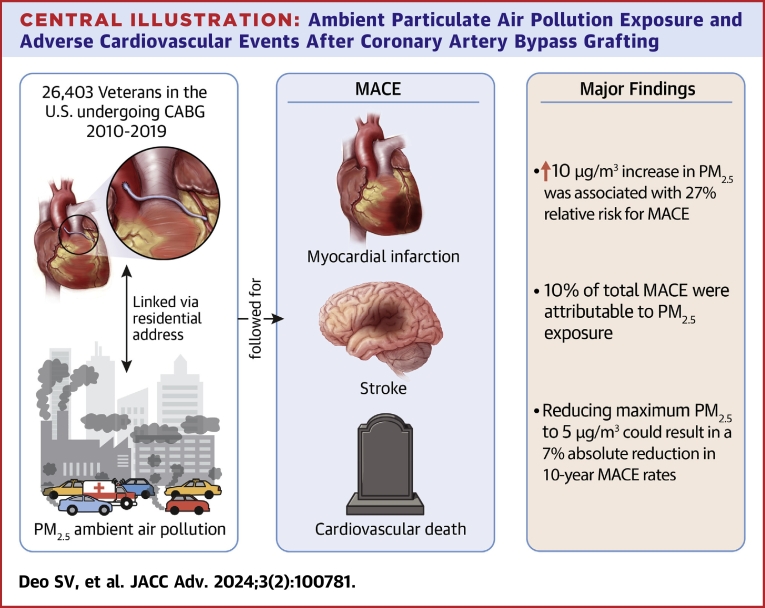
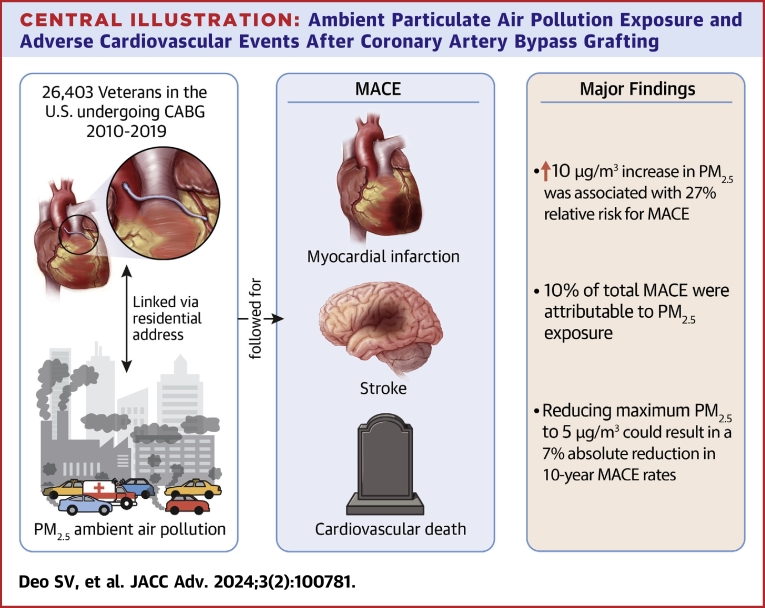
Abstract
Background
Increased particulate matter <2.5 μm (PM2.5) air pollution is associated with adverse cardiovascular outcomes. However, its impact on patients with prior coronary artery bypass grafting (CABG) is unknown.
Objectives
The purpose of this study was to evaluate the association between major adverse cardiovascular events (MACE) (defined as myocardial infarction, stroke, or cardiovascular death) and air pollution after CABG.
Methods
We linked 26,403 U.S. veterans who underwent CABG (2010-2019) nationally with average annual ambient PM2.5 estimates using residential address. Over a 5-year median follow-up period, we identified MACE and fit a multivariable Cox proportional hazard model to determine the risk of MACE as per PM2.5 exposure. We also estimated the absolute potential reduction in PM2.5 attributable MACE simulating a hypothetical PM2.5 lowered to the revised World Health Organization standard of 5 μg/m3.
Results
The observed median PM2.5 exposure was 7.9 μg/m3 (IQR: 7.0-8.9 μg/m3; 95% of patients were exposed to PM2.5 above 5 μg/m3). Increased PM2.5 exposure was associated with a higher 10-year MACE rate (first tertile 38% vs third tertile 45%; P < 0.001). Adjusting for demographic, racial, and clinical characteristics, a 10 μg/m3 increase in PM2.5 resulted in 27% relative risk for MACE (HR: 1.27, 95% CI: 1.10-1.46; P < 0.001). Currently, 10% of total MACE is attributable to PM2.5 exposure. Reducing maximum PM2.5 to 5 μg/m3 could result in a 7% absolute reduction in 10-year MACE rates.
Conclusions
In this large nationwide CABG cohort, ambient PM2.5 air pollution was strongly associated with adverse 10-year cardiovascular outcomes. Reducing levels to World Health Organization-recommended standards would result in a substantial risk reduction at the population level.
Key words: air pollution, major adverse cardiovascular events, coronary artery bypass grafting, population attributable fraction, cardiovascular mortality
Central Illustration
Environmental exposures contribute to over 9 million deaths annually with >50% of these events directly related to atherosclerotic cardiovascular disease.1 Air pollution contributes to the vast preponderance of deaths attributable to environmental exposures. While empirical evidence strongly implicates a gradation of risk with those with underlying risk factors most at risk, the evidence to implicate those at the highest risk, namely individuals with established coronary artery disease (CAD) who have previously also undergone revascularization, is to date lacking.1,2 With more than 400,000 procedures every year, coronary artery bypass grafting (CABG) is among the most commonly performed adult surgical procedures in the United States.2 Patients receiving CABG often have complex multivessel CAD and remain at risk of suffering from recurrent major adverse cardiovascular events (MACE) like myocardial infarction (MI), stroke, and cardiovascular mortality.3, 4, 5 While much of this recurrent risk can be reduced by optimal medical therapy and lifestyle/behavioral improvements, some patients continue to have a high residual risk for suffering such adverse events. Traditionally, the construct to think about residual risk has been overwhelmingly in terms of conventional risk factors, but the role of other factors in the environment that may continually amplify and contribute to residual risk has not been systematically considered. In this context, the incremental contribution of air pollution, particularly fine particulate matter (PM2.5) exposure, to patients who have undergone CABG with careful consideration of the concomitant comorbidities that many of these individuals suffer from is of particular interest.6,7 Mechanistically, animal model studies report that air pollution exposure can lead to immune activation, thrombosis, disruption in lipid metabolism, and consequently atherosclerosis, all factors that continue to play a role in the post-CABG patient.7 This laboratory research has been supported by cohort studies that have reported a strong correlation between increased PM2.5 exposure and higher rates of hypertension, stroke, and ischemic heart disease.6, 7, 8, 9 In an earlier nationwide analysis of patients receiving percutaneous coronary intervention, we reported that patients exposed to higher PM2.5 levels had higher mortality rates and reduced life expectancy after their procedure.10 However, in spite of post-CABG patients being at high risk for adverse cardiovascular events, the impact of PM2.5 exposure has not been examined in this cohort. Hence, to resolve this question, we analyzed data from a large nationwide cohort of CABG patients.
Methods
The VA Surgical Quality Initiative Project (VASQIP), our primary data registry, is managed by the National Surgery Office of the Veterans Affairs Administration. This contains rigorously defined, nurse-adjudicated information on the preoperative, intraoperative, and postoperative periods for all patients receiving cardiac surgery at VA medical centers. The VASQIP data was supplemented with information from the corporate data warehouse, which contains data regarding their nonindex in- and out-patient visits, biochemical test results, and echocardiographic data. For this study, we identified US veterans that underwent isolated CABG (excluding patients that received concomitant valve procedures, ascending aorta replacement, or maze procedures) nationwide from 2010 through 2019.
The main exposure of interest in our study was the annual PM2.5 particulate air pollution estimated at the zip code level. Validated PM2.5 exposure estimates developed by the Atmospheric Composition Analysis Group were utilized.11,12 These hybrid estimates combine information from satellite remote sensing, chemical transport modeling, and calibration with ground-based observations to generate concentration estimates. Data are provided in 1 × 1 km grids throughout North America. Raster files were imported to an open-source geographical information system software, QGIS v. 3.16 (Open-Source Geospatial Foundation), and mapped to the 2018 zip code boundaries from the U.S. Census Bureau. The mean zip PM2.5 exposure for each zip code was calculated as a simple average over the study period using zonal statistics in QGIS and this was assigned as the PM2.5 exposure for each patient.
We obtained demographic, clinical, laboratory, and pharmacy data prior to their procedure for patients from the VASQIP tables; when this information was not directly available in VASQIP, we obtained it from the preoperative outpatient visit (closest to the surgery date) using the International Classification of Disease-9th and-10th editions or Common Procedure Terminology codes. Demographics included age at CABG, sex, and self-reported race and ethnicity. Clinical factors obtained were hypertension, diabetes mellitus, dyslipidemia, obesity (body mass index ≥30 kg/m2), heart failure, chronic kidney disease (estimated glomerular filtration rate <60 mL/min/m2), smoking status, and prior MI. We obtained information regarding whether patients had acute coronary syndrome prior to surgery and the urgency of surgery (elective, urgent [performed within the same hospital admission], or emergent [performed within 48 hours of hospital admission]). We additionally also adjusted for area-level socioeconomic variables. The social deprivation index (SDI) is a validated composite metric derived at the level of the zip code tabulation areas to evaluate the socioeconomic condition of that geographical area. The summary SDI score is created based on the following criteria: household income, house ownership, education level, vehicular access, and family composition.13 The score ranges between 0 and 100, with higher numbers indicative of higher deprivation (lower socioeconomic position).
We studied MACE as our primary endpoint. MACE is a composite of cardiovascular mortality and the first instance of nonfatal MI or nonfatal stroke. Nonfatal MI or nonfatal stroke were defined as being admitted to a VA medical center with these conditions as the primary diagnosis. We obtained the dates for these events and calculated the time-to-event as the duration between the surgery date and the event date.
Statistical analysis
We reported continuous data and categorical data as mean ± SD and count (percentage), respectively. We calculated the cumulative incidence of MACE over the study period for the whole cohort and separately for each tertile of PM2.5. We then compared the MACE incidence for the PM2.5 tertiles by pairwise log-rank tests with the Hochberg correction for multiplicity.14 To study the association between MACE and PM2.5, we used PM2.5 as a continuous variable and fit incrementally more complex. Cox proportional hazard models as follows: model 1–unadjusted- only PM2.5; model 2–model 1+ age at surgery, sex, race, ethnicity, and social deprivation; model 3–model 2+ preoperative prevalence of diabetes, chronic kidney disease, heart failure, prior MI, prior stroke, left ventricular systolic ejection fraction, urgency of surgery, presence of triple vessel disease, use of multiarterial grafting, need for an intra-aortic balloon pump in the perioperative period, smoking status, chronic obstructive pulmonary disease, and baseline discharge medications. In our initial models, we explored whether using an interaction term between PM2.5 and race, a spline term for PM2.5, or an interaction between PM2.5 and social deprivation would provide any additional benefit in our explanatory regression model. However, comparing these nested models using the analysis of variance method, we dropped these terms from our final model as they did not significantly improve the model fit at the 95% confidence level. As prior studies have modeled annual PM2.5 values using a time-varying approach, we performed a sensitivity analysis to confirm our primary findings using this approach. Using the date of surgery and date of death or censoring, we identified the calendar years that each patient was exposed to and provided each patient with the corresponding annual PM2.5 according to their zip code. We then fitted a frailty-type Cox proportional hazards model using this time-varying PM2.5 as our primary exposure and included all the variables reported for model 3 as covariates. We further performed subgroup analyses on our primary model according to sex, race, ethnicity, pre-existing diabetes, chronic kidney disease, and heart failure. As we were interested in evaluating the potential benefit of lowering PM2.5 to the new Environmental Protection Agency recommendations (PM2.5 limit: 8 μg/m3), we grouped patients into 2 groups (PM2.5 <8 μg/m3 and PM2.5 >8 μg/m3). We then reported the relative risk for MACE in those exposed to PM2.5 >8 μg/m3 using a multivariable Cox proportional hazards model (using variables included in model 3 reported above).
To understand the extent to which PM2.5 contributes to the occurrence of MACE, using the adjusted model (M3), we calculated the attributable fraction for PM2.5 over the 10-year study period. We then evaluated the potential reduction in MACE that may occur if PM2.5 exposure were to be limited to the new Environmental Protection Agency recommendations (PM2.5 limit: 8 μg/m3) or the World Health Organization (WHO) 2021 standard (PM2.5 limit: 5 μg/m3). We calculated CIs for these estimates using bootstrapping approaches.
We observed missing values in the following variables: preoperative left ventricular ejection fraction 16%, preoperative atrial fibrillation 10%, VA-PROM 3%, Hispanic ethnicity 3%, chronic kidney disease <1%, SDI <1%, NYHA functional class <1%, current smoker <1%, and prior heart surgery <1%. To handle missing information, we used the multiple imputation by chained equations approach and fitted a classification and regression tree approach to impute the missing information.15 As a sensitivity analysis, we repeated the above model using a complete case method. Patients often have a higher hazard for mortality in the early postoperative period. We therefore repeated our final model with a landmark approach limiting the analysis to only those patients that were MACE-free at 180 days (6 months) after surgery.
Data availability statement
Those credentialed to perform research in the Department of Veteran Affairs can directly obtain the data using the regulatory submission methods. Readers can contact the corresponding authors for code used in the analyses presented in this manuscript. The scripts are also available online at the corresponding authors github repository: svd09. We have reported this study according to the Strengthening the Reporting of Observational Studies (STROBE) guidelines.
Results
Description of the study cohort
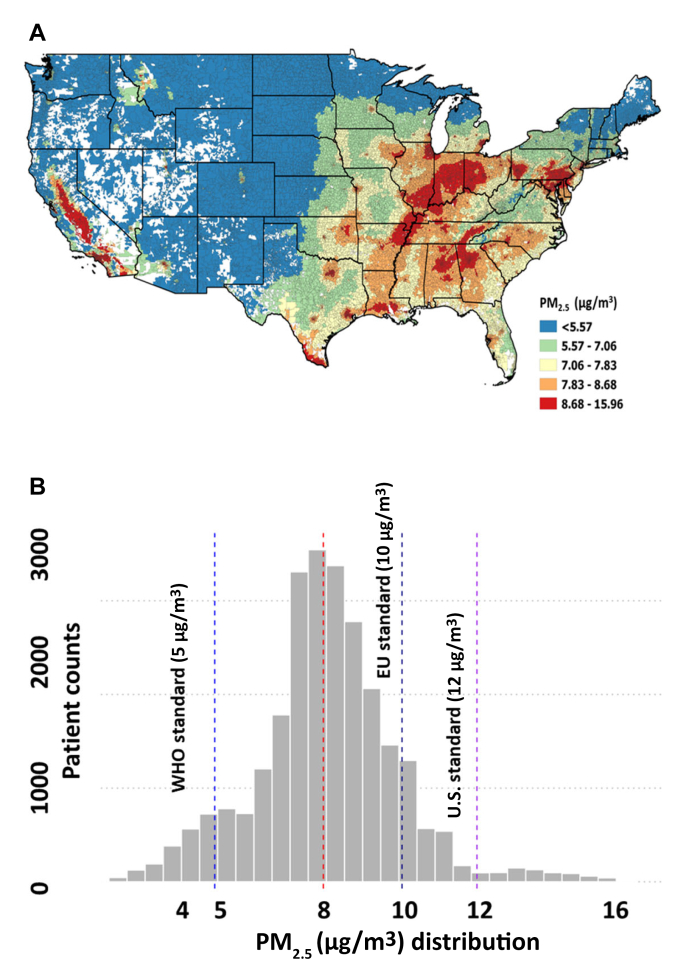
Between 2010 and 2019, 26,403 U.S. veterans (mean age 65 years, female 1%, Black patients 10%, Hispanic patients 5%) underwent CABG at 42 different VA medical centers nationwide. The prevalence of preoperative diabetes, chronic kidney disease, and heart failure was 46%, 21%, and 3%, respectively. The median PM2.5 exposure during the study period was 7.9 μg/m3 [IQR: 7.0-8.9 μg/m3] (Figures 1A and 1B); 5% of patients were exposed to PM2.5 <5 μg/m3 (WHO standards), while 12% and 3% were exposed to PM2.5 values of >10 μg/m3 (EU standard) and >12 μg/m3 (U.S. Environmental Protection Agency standard), respectively. Compared to patients in the lowest tertile (1.93-7.37 μg/m3), those in the highest tertile (8.59-15.96 μg/m3) were slightly younger (mean age 65 vs 66 years), more likely to be Black (17% vs 3%), Hispanic (7% vs 4%), and lived in more socially deprived zip codes (mean SDI 61 vs 43).
Figure 1.
Annual PM2.5 Concentrations Observed in Our Study in the United States
(A) Map of PM2.5 distribution according to zip codes in the U.S./histogram for PM2.5 (lines at median, WHO, EU, U.S.). (B) Histogram of PM2.5 distribution in the study cohort.
PM2.5 and MACE rates
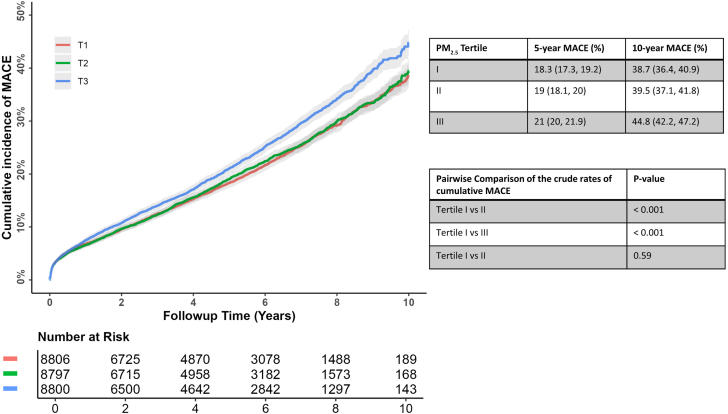
We followed up patients for a median of 4.9 years (maximum 10.4 years). The 5- and 10-year cumulative incidence of MACE was 19.4% (95% CI: 18.9%-19.9%) and 40.9% (95% CI: 39.5%-42.9%), respectively. The cumulative incidence for MACE at 5-years in the lowest and highest PM2.5 tertile was 18.3% (95% CI: 17.8%-19.2%) and 21% (95% CI: 20%-21.9%), respectively; at 10 years, the corresponding values were 38.7% (95% CI: 36.4%-40.9%) and 44.8% (95% CI: 42.4%-47.2%), respectively (pairwise log-rank P < 0.001) (Figure 2). On crude analysis, an increase in 10 μg/m3 of PM2.5 was associated with a significantly higher relative risk for MACE (1.36 [95% CI: 1.18%-1.55%]). This effect persisted in our fully adjusted model, with every 10 μg/m3 increase in PM2.5 exposure being associated with a significant increase in the relative risk for MACE (HR: 1.18 [95% CI: 1.02-1.37]; P < 0.001) (Table 1, Central Illustration). The sensitivity analysis, where the PM2.5 was treated as time-varying value, supported our primary model, and each 10 μg/m3 PM2.5 was associated with a comparable relative MACE risk (HR: 1.34 [95% CI: 1.27-1.42]; P < 0.001). With patients exposed to PM2.5 ≤ 8 μg/m3 as a reference, those exposed to PM2.5 >8 μg/m3 had a significantly higher MACE risk (HR: 1.10 [95% CI: 1.05-1.16]; P < 0.001). We observed that our primary results were supported by the landmark analysis of patients free of MACE for at least 180 days after surgery (HR: 1.30 [95% CI: 1.11-1.54]).
Figure 2.
The Cumulative Incidence for Major Adverse Cardiovascular Events According to the PM2.5Tertiles
We grouped patients based on their PM2.5 into tertiles as follows: tertile I (1.93-7.37 μg/m3), tertile II (7.37-8.59 μg/m3), and tertile III (8.59-15.96 μg/m3). The crude 10-year MACE rate for patients in tertile III for PM2.5 was significantly higher than those observed for tertile I (P < 0.001) and tertile II (P < 0.001). MACE = major adverse cardiovascular events.
Table 1.
Baseline Characteristics of the Study Cohort
| Tertile of Average PM2.5 (μg/m3) During the Study Period |
|||||
|---|---|---|---|---|---|
| Whole Cohort(N = 26,403) | Tertile I 1.93-7.37(n = 8,806) | Tertile II 7.38-8.59(n = 8,797) | Tertile III 8.60-15.96(n = 8,800) | P Value | |
| Sociodemographic data | |||||
| Age, y | 65.67 ± 7.60 | 66.54 ± 7.45 | 65.32 ± 7.64 | 65.17 ± 7.62 | <0.001 |
| Men | 26,122 (98.9) | 8,718 (99.0) | 8,703 (98.9) | 8,701 (98.9) | 0.718 |
| Race | <0.001 | ||||
| Black | 2,714 (10.3) | 264 (3.0) | 881 (10.0) | 1,569 (17.8) | |
| White | 19,923 (75.5) | 7,097 (80.6) | 6,810 (77.4) | 6,016 (68.4) | |
| Others | 3,766 (14.3) | 1,445 (16.4) | 1,106 (12.6) | 1,215 (13.8) | |
| Hispanic ethnicity | 1,305 (5.1) | 344 (4.1) | 337 (3.9) | 624 (7.3) | <0.001 |
| Body mass index (kg/m2) | 30.09 ± 5.36 | 30.24 ± 5.35 | 30.13 ± 5.38 | 29.90 ± 5.34 | <0.001 |
| Social deprivation index | 52.33 ± 26.63 | 43.85 ± 23.94 | 51.80 ± 25.15 | 61.35 ± 27.71 | <0.001 |
| Clinical characteristics | |||||
| Triple vessel coronary stenosis | 20,025 (75.8) | 6,789 (77.1) | 6,594 (75.0) | 6,642 (75.5) | 0.007 |
| Chronic obstructive pulmonary disease | 6,027 (22.8) | 1,980 (22.5) | 2,086 (23.7) | 1,961 (22.3) | 0.05 |
| Diabetes | <0.001 | ||||
| No diabetes | 14,220 (53.9) | 4,992 (56.7) | 4,721 (53.7) | 4,507 (51.2) | |
| Noninsulin-treated diabetes | 5,089 (19.3) | 1,570 (17.8) | 1,739 (19.8) | 1,780 (20.2) | |
| Insulin-treated diabetes | 7,094 (26.9) | 2,244 (25.5) | 2,337 (26.6) | 2,513 (28.6) | |
| Smoking | 6,525 (24.7) | 1,911 (21.7) | 2,308 (26.2) | 2,306 (26.2) | <0.001 |
| Acute coronary syndrome | 3,469 (13.1) | 1,209 (13.7) | 1,086 (12.3) | 1,174 (13.3) | 0.02 |
| Atrial fibrillation | 3,014 (11.4) | 1,129 (12.8) | 1,044 (11.9) | 841 (9.6) | <0.001 |
| Chronic kidney disease | 5,731 (21.7) | 1,851 (21.0) | 1,922 (21.9) | 1,958 (22.3) | 0.133 |
| Prior myocardial infarction | 2,725 (10.3) | 980 (11.1) | 860 (9.8) | 885 (10.1) | 0.008 |
| Peripheral artery disease | 5,048 (19.1) | 1,779 (20.2) | 1,691 (19.2) | 1,578 (17.9) | 0.001 |
| Priority status for surgery (%) | <0.001 | ||||
| Elective | 22,364 (84.7) | 7,301 (82.9) | 7,559 (85.9) | 7,504 (85.3) | |
| Urgent (within the same hospital admission) | 3,420 (13.0) | 1,232 (14.0) | 1,072 (12.2) | 1,116 (12.7) | |
| Emergent (within 48 h of the same hospital admission) | 619 (2.3) | 273 (3.1) | 166 (1.9) | 180 (2.0) | |
| Prior heart surgery | 439 (1.7) | 159 (1.8) | 156 (1.8) | 124 (1.4) | 0.074 |
| Cerebrovascular disease | 6,377 (24.2) | 2,016 (22.9) | 2,203 (25.0) | 2,158 (24.5) | 0.002 |
| Heart failure | 781 (3.0) | 229 (2.6) | 278 (3.2) | 274 (3.1) | 0.052 |
| Recent acute coronary syndrome | 1,597 (6.0) | 541 (6.1) | 504 (5.7) | 552 (6.3) | 0.287 |
| Laboratory and echocardiographic data | |||||
| Serum creatinine | 1.20 ± 1.10 | 1.15 ± 0.79 | 1.18 ± 0.86 | 1.26 ± 1.50 | <0.001 |
| Hemoglobin A1c | 6.80 ± 4.33 | 6.70 ± 1.45 | 6.77 ± 1.47 | 6.95 ± 7.31 | 0.002 |
| LDL cholesterol (mg/dL) | 93.25 ± 37.26 | 94.86 ± 37.31 | 94.10 ± 36.72 | 90.84 ± 37.63 | <0.001 |
| Preoperative LVEF | 48.97 ± 13.90 | 48.83 ± 14.18 | 49.00 ± 13.72 | 49.08 ± 13.80 | 0.53 |
| Ozone 2014 (ppm) | 38.32 ± 3.95 | 38.58 ± 4.31 | 38.11 ± 3.05 | 38.28 ± 4.34 | <0.001 |
Values are mean ± SD or n (%).
LDL = low-density lipoprotein; LVEF = left ventricular ejection fraction.
Central Illustration.
Ambient Particulate Air Pollution Exposure and Adverse Cardiovascular Events After Coronary Artery Bypass Grafting
Subgroup analyses
Overall, the association between PM2.5 and MACE was consistent with the main results in the examined subgroups (Table 2). Specifically, we did not find any statistically significant difference in the point estimates of the main model according to age, race, sex, left ventricular function, and social deprivation status (Table 2).
Table 2.
Association Between PM2.5 and Major Adverse Cardiovascular Events in Subgroups
| Adjusted HR for MACE (95% CI) | P Value for Interaction | |
|---|---|---|
| Overall | 1.18 (1.02-1.37) | |
| Age | 0.52 | |
| <70 y | 1.19 (1.00-1.41) | |
| ≥70 y | 1.11 (0.86-1.44) | |
| Race | 0.70 | |
| White | 1.34 (1.13-1.59) | |
| Black | 1.24 (0.75-2.04) | |
| Nonelective surgery | 0.05 | |
| No | 1.34 (1.13-1.58) | |
| Yes | 0.98 (0.74-1.30) | |
| Chronic kidney disease | ||
| Yes | 1.10 (0.84-1.43) | 0.21 |
| No | 1.40 (1.20-1.65) | |
| Diabetes | ||
| Yes | 1.29 (1.07-1.56) | 0.54 |
| No | 1.23 (1.00-1.50) | |
| LVEF | ||
| <40% | 1.30 (1.09-1.54) | 0.83 |
| ≥40% | 1.25 (0.98-1.60) | |
| Social deprivation index | 0.15 | |
| Quartile 1 | 1.09 (0.75-1.57) | |
| Quartile 2 | 1.15 (0.86-1.55) | |
| Quartile 3 | 1.16 (0.88-1.52) | |
| Quartile 4 | 1.32 (1.02-1.72) |
LVEF = left ventricular ejection fraction; MACE = major adverse cardiovascular events.
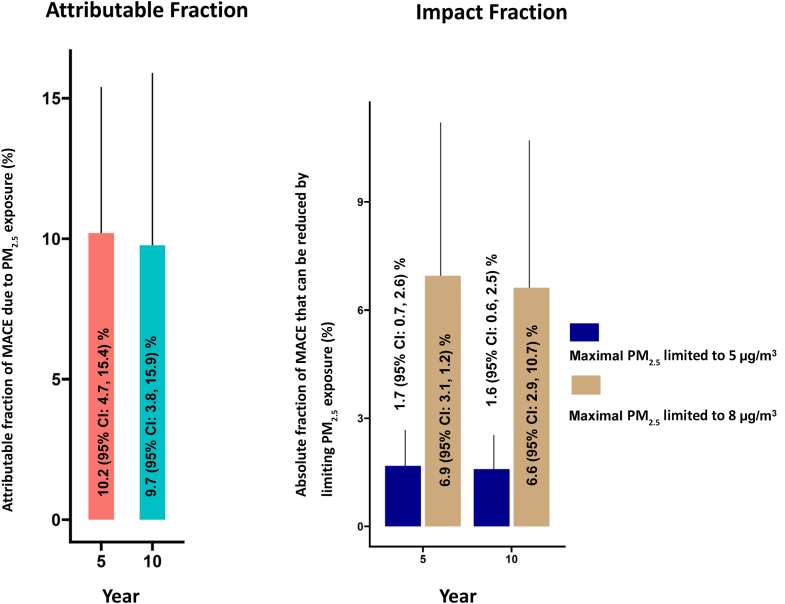
Attributable fraction and simulated impact under lower thresholds of PM2.5
After adjusting for specified covariates, PM2.5 was attributed to 10.2% (95% CI: 4.7%-15.4%) and 9.7% (95% CI: 3.8%-15.9%) of all MACE events at 5 and 10 years, respectively (Figure 3). A hypothetical scenario where maximum PM2.5 exposure is limited to 8 μg/m3 will result in an absolute reduction of 1.7% (95% CI: 0.7%-2.6%) and 1.6% (95% CI: 0.6%-2.5%) in the MACE rates at 5 and 10 years, respectively. If PM2.5 were further limited to 5 μg/m3, this would result in a potential absolute risk reduction in MACE of 6.9 (95% CI: 3.1-11.2) and 6.6 (95% CI: 2.9-10.7) at 5 and 10 years, respectively.
Figure 3.
Percentage of MACE Attributable to PM2.5at 5- and 10-Year Follow-Up Observed in Our Cohort
We further fit 2 hypothetical situations (PM2.5, 5 and 8 μg/m3) and calculated the possible absolute reduction in MACE that we may predict at 5- and 10-year follow-up. MACE = major adverse cardiovascular events.
Discussion
In this national cohort of U.S. veterans undergoing CABG, we showed that 10-year MACE rates were significantly higher among those exposed to high ambient PM2.5 air pollution levels. Adjusting for multiple factors only strengthened these associations with every 10 μg/m3 increasing the relative risk for MACE by 18%. This association was consistent across age, race, social deprivation, and clinically important subgroups. Approximately 10% of the currently observed MACE rates were attributable to PM2.5 exposure confirming the high attributable fraction related to a common environmental factor even at relatively lower levels of the dose response curve.3 A hypothetical scenario of reducing PM2.5 exposure to 5 μg/m3 as recommended in 2021 by the WHO could lead to a 6% absolute reduction in 10-year MACE in patients post-CABG. The evidence in this work is consistent with a large body of empirical evidence, both in animal models and in humans.4,6, 7, 8, 9 In human studies, both acute time series and chronic prospective cohort studies have clearly implicated a role for air pollution exposure in MACE.2,5
The present study, therefore, builds on prior evidence to support the possibility that air pollution may disproportionately affect the more clinically vulnerable subgroups. However, there have been no studies in CABG patients, who often have multiple comorbidities and a high atherosclerotic burden. The best early evidence of a graded response to air pollution based on underlying risk came from Utah (n = 16,314), where concurrent-day PM2.5 was associated with an increase in suffering from acute coronary syndrome.6 The excess risk was observed only among individuals with angiographic CAD, and led to an increase in ST-segment elevation MI. Additional evidence from a variety of cohort studies have also demonstrated that factors such as age, diabetes, obesity, and cardiac transplantation are all additional risk factors that increase the risk of adverse MACE in response to PM2.5.2,4,5,7 Comparing results obtained in our prior study analyzing data from patients receiving coronary percutaneous interventions (PCIs), it may appear that the deleterious impact of PM2.5 exposure is higher after PCI than CABG.10
However, differences in the baseline characteristics of patient cohorts included in both studies may partly explain the observed difference in effect estimates. Hence, to better understand the differential association between procedure and the effect of PM2.5, we need a single study of both CABG and PCI patients with statistical adjustments for cohort differences.
In this study, we observed that as much as 10% of the observed MACE rates may be attributable to PM2.5 exposure. Therefore, this residual risk would not be reduced by addressing traditional risk factors. Furthermore, reducing PM2.5 levels to the Environmental Protection Agency (<8 μg/m3) and WHO (<5 μg/m3) standards may hypothetically result in an absolute reduction in MACE rates of 2% and 7%, respectively.16,17 While the cumulative MACE rates increased between 5 and 10 years, we observed that the PM2.5 attributable fraction for MACE remained constant and the potential impact of reducing the maximal PM2.5 levels decreased over the same time-frame. A possible explanation is that the burden of MACE attributable to traditional endogenous cardiovascular risk factors (like diabetes, hypertension, dyslipidemia, and smoking) may have increased between 5 and 10 years. However, lowering the PM2.5 has also been observed to reduce the incidence of these traditional cardiovascular risk factors. Therefore, the mechanistic pathways between PM2.5 are complex; therefore, our model may have underestimated the true effect of reducing PM2.5 levels. Therefore, in high-risk patients, it may be prudent to consider air pollution mitigation strategies such as portable air cleaners that have high-efficiency filters; studies have already reported improved cardiometabolic parameters with the use of these devices.18, 19, 20, 21 However, randomized trials in this area are lacking, and we feel that future studies should address this knowledge gap.8,9 In fact, applying such strategies to reduce PM2.5 exposure may synchronously also reduce prevalent traditional cardiovascular risk factors among patients.22
Study Limitations and strengths
These findings should be considered on the background of certain study limitations. We have studied a U.S. veteran cohort that often has more comorbidities than the general population. Yet, while the overall event rates may be lower in the civilian population, we believe that the incremental risk associated with increased PM2.5 levels may be similar to what we observed. Our cohort is also predominantly male, and therefore we were unable to study whether any sex-based differences exist. We chose to model PM2.5 exposure based on patients’ residential zip-code at the time of surgery and therefore did not account for address and zipcode changes during the study period. Lastly, we modeled PM2.5 exposure as the simple average at the residential zip-code level, a method that is a validated approach in such situations. Furthermore, we acknowledge that analysis for isolated exposures (ie, PM2.5) is a somewhat simplistic representation of the overall exposome, and thus the effect estimate of PM2.5 on MACE may be overestimated or underestimated. Acute extreme weather events may substantially impact MACE rates over a short time span. However, studies (like ours) that model event rates associated with averaged chronic exposure may not reliably capture such variation. Future studies should incorporate multiexposure models, potentially using evolving data science approaches, to better characterize the impact of air pollution within the context of the total external exposome.23 The strengths of our study, apart from likely being the first to evaluate this issue in a CABG cohort, are the large sample size, long follow-up, and our ability to accurately map the patient’s longitudinal trajectory using data from a single large nationwide healthcare system.
Conclusions
In this large, contemporary, national study of patients undergoing isolated CABG in the United States, ambient PM2.5 air pollution exposure was strongly associated with higher 10-year adverse cardiovascular outcomes. This harmful effect was consistent across age, race, social deprivation, and important clinical subgroups. Future studies should investigate the applicability of scalable mitigation strategies against air pollution, especially in these high-risk cohorts.
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Exposure to particulate matter pollution is associated with higher 10-year cardiovascular events after CABG. In the studied cohort, reaching WHO-recommended limits may result in a 7% reduction in 10-year MACE rates.
TRANSLATIONAL OUTLOOK: To improve long-term health quality for patients post-CABG, we must investigate the impact of nontraditional risk factors like air pollution in greater details.
Funding support and author disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Contributor Information
Salil V. Deo, Email: svd14@case.edu.
Sadeer Al-Kindi, Email: sal-kindi@houstonmethodist.org.
References
- 1.Rajagopalan S., Landrigan P.J. Pollution and the heart. N Engl J Med. 2021;385:1881–1892. doi: 10.1056/NEJMra2030281. [DOI] [PubMed] [Google Scholar]
- 2.Bachar B.J., Manna B. StatPearls. Treasure Island (FL) StatPearls Publishing; 2023. Coronary artery bypass Graft. [PubMed] [Google Scholar]
- 3.Ferguson T.B. Mortality in coronary artery bypass grafting. Circulation. 2012;125:2409–2411. doi: 10.1161/CIRCULATIONAHA.112.106856. [DOI] [PubMed] [Google Scholar]
- 4.Ohri S.K., Benedetto U., Luthra S., et al. Coronary artery bypass surgery in the UK, trends in activity and outcomes from a 15-year complete national series. Eur J Cardio Thorac Surg. 2022;61:449–456. doi: 10.1093/ejcts/ezab391. [DOI] [PubMed] [Google Scholar]
- 5.Dalén M., Ivert T., Holzmann M.J., Sartipy U. Coronary artery bypass grafting in patients 50 Years or younger. Circulation. 2015;131:1748–1754. doi: 10.1161/CIRCULATIONAHA.114.014335. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopalan S., Al-Kindi S.G., Brook R.D. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72:2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 7.Al-Kindi S.G., Brook R.D., Biswal S., Rajagopalan S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol. 2020;17:656–672. doi: 10.1038/s41569-020-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevan G.H., Al-Kindi S.G., Brook R.D., Münzel T., Rajagopalan S. Ambient air pollution and atherosclerosis: insights into dose, time, and mechanisms. Arterioscler Thromb Vasc Biol. 2021;41:628–637. doi: 10.1161/ATVBAHA.120.315219. [DOI] [PubMed] [Google Scholar]
- 9.Bevan G.H., Al-Kindi S.G., Brook R., Rajagopalan S. Ambient air pollution and atherosclerosis: Recent Updates. Curr Atheroscler Rep. 2021;23:1–10. doi: 10.1007/s11883-021-00958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motairek I., Deo S.V., Elgudin Y., et al. Particulate matter air pollution and long-term outcomes in patients undergoing percutaneous coronary intervention. JACC Adv. 2023;2:100285. [Google Scholar]
- 11.van Donkelaar A., Martin R.V., Li C., Burnett R.T. Regional estimates of chemical composition of fine particulate matter using a combined geoscience-statistical method with information from satellites, models, and monitors. Environ Sci Technol. 2019;53:2595–2611. doi: 10.1021/acs.est.8b06392. [DOI] [PubMed] [Google Scholar]
- 12.Hammer M.S., van Donkelaar A., Li C., et al. Global estimates and long-term trends of fine particulate matter concentrations (1998–2018) Environ Sci Technol. 2020;54:7879–7890. doi: 10.1021/acs.est.0c01764. [DOI] [PubMed] [Google Scholar]
- 13.Anon. Social Deprivation Index (SDI) https://www.graham-center.org/content/brand/rgc/maps-data-tools/social-deprivation-index.html
- 14.Chen S.-Y., Feng Z., Yi X. A general introduction to adjustment for multiple comparisons. J Thorac Dis. 2017;9:1725–1729. doi: 10.21037/jtd.2017.05.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayes T., McArdle J.J. Should we impute or should we weight? Examining the performance of two CART-based techniques for addressing missing data in small sample research with nonnormal variables. Comput Stat Data Anal. 2017;115:35–52. [Google Scholar]
- 16.US EPA O EPA Proposes to strengthen air quality standards to Protect the Public from harmful effects of Soot. 2023. https://www.epa.gov/newsreleases/epa-proposes-strengthen-air-quality-standards-protect-public-harmful-effects-soot
- 17.Hoffmann B., Boogaard H., de Nazelle A., et al. WHO air quality guidelines 2021–aiming for healthier air for all: a joint statement by medical, public health, scientific societies and patient representative organisations. Int J Public Health. 2021;66 doi: 10.3389/ijph.2021.1604465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morishita M., Adar S.D., D’Souza J., et al. Effect of portable air filtration systems on personal exposure to fine particulate matter and blood pressure among residents in a low-income senior facility: a randomized clinical trial. JAMA Intern Med. 2018;178:1350–1357. doi: 10.1001/jamainternmed.2018.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajagopalan S., Brauer M., Bhatnagar A., et al. Personal-level protective Actions against particulate matter air pollution exposure: a Scientific Statement from the American heart association. Circulation. 2020;142:e411–e431. doi: 10.1161/CIR.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 20.Shah S., Kim E., Kim K.-N., Ha E. Can individual protective measures safeguard cardiopulmonary health from air pollution? A systematic review and meta-analysis. Environ Res. 2023;229 doi: 10.1016/j.envres.2023.115708. [DOI] [PubMed] [Google Scholar]
- 21.Walzer D., Gordon T., Thorpe L., et al. The effects of home particulate air filtration on blood pressure: a systematic review. Hypertension. 2020;76:44–50. doi: 10.1161/HYPERTENSIONAHA.119.14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Kindi S.G., Brook R.D., Bhatt U., et al. The benefits of intensive versus standard blood pressure treatment according to fine particulate matter air pollution exposure: a post hoc analysis of SPRINT. Hypertension. 2021;77:813–822. doi: 10.1161/HYPERTENSIONAHA.120.15923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motairek I., Makhlouf M.H.E., Rajagopalan S., Al-Kindi S. The exposome and cardiovascular health. Can J Cardiol. 2023;39:1191–1203. doi: 10.1016/j.cjca.2023.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Those credentialed to perform research in the Department of Veteran Affairs can directly obtain the data using the regulatory submission methods. Readers can contact the corresponding authors for code used in the analyses presented in this manuscript. The scripts are also available online at the corresponding authors github repository: svd09. We have reported this study according to the Strengthening the Reporting of Observational Studies (STROBE) guidelines.