Abstract
A chimeric fusion protein encompassing the CD46 ectodomain linked to the C-terminal part of the C4b binding protein (C4bp) α chain (sCD46-C4bpα) was produced in eukaryotic cells. This protein, secreted as a disulfide-linked homo-octamer, was recognized by a panel of anti-CD46 antibodies with varying avidities. Unlike monomeric sCD46, the octameric sCD46-C4bpα protein was devoid of complement regulatory activity. However, sCD46-C4bpα was able to bind to the measles virus hemagglutinin protein expressed on murine cells with a higher avidity than soluble monomeric sCD46. Moreover, the octameric sCD46-C4bpα protein was significantly more efficient than monomeric sCD46 in inhibiting virus binding to CD46, in blocking virus induced cell-cell fusion, and in neutralizing measles virus in vitro. In addition, the octameric sCD46-C4bpα protein, but not the monomeric sCD46, fully protected CD46 transgenic mice against a lethal intracranial measles virus challenge.
Control of virus infection is currently a major challenge. The identification of cellular receptors used by viruses to enter their host cells has allowed several groups to investigate the potential antivirus properties of recombinant soluble receptors. Whereas recombinant soluble CD4 and Tva exhibited high neutralizing properties, at least in vitro, against human immunodeficiency virus (13, 19, 27, 46) and subgroup A avian sarcoma and leukosis viruses (5, 11), respectively, the anti-measles virus (anti-MV) activity of recombinant soluble monomeric CD46 (sCD46) against MV was very poor (16, 45). Since MV virus binding and fusion to cells likely involves several CD46 receptor molecules (7), we hypothesized that a multimeric form of soluble CD46 could have more potent antivirus activity.
Measles virus, a member of the order Mononegavirales, is responsible for an acute human pulmonary disease with high morbidity and mortality, killing over 1 million young children every year, mainly in developing countries. Infection is associated with a profound but transient cellular immunodepression. In rare cases, MV can induce lethal neuropathological diseases, acute encephalopathy, measles inclusion bodies encephalitis, or subacute sclerosis panencephalitis. MV attenuated by growth in chicken embryonic fibroblasts is currently used as an effective but limited vaccine because of its inefficiency in children less than 9 months old.
Human CD46 (or membrane cofactor protein), which is expressed on all cells except erythrocytes, is used as a cellular receptor by at least a subgroup of laboratory and wild-type MV strains (17, 38; see reference 22 for a review) through the interaction of its ectodomain with that of the MV envelope glycoprotein hemagglutinin (H) (16). This MV H-CD46 interaction induces a multimolecular scaffold in which the MV fusion glycoprotein (F) initiates the fusion between the MV envelope and the plasma cell membrane at a neutral pH (7). These properties explain the occurrence of cell-cell fusion observed after MV infection. CD46 is a transmembrane glycoprotein that belongs to the regulators of complement activation gene family. The dominant structural units of CD46 are the four short consensus repeat (SCR) domains of 60 to 64 amino acids that are responsible for complement binding and regulatory functions. Structurally, the N-terminal four SCRs of CD46 precede a heavily glycosylated serine-threonine-proline (STP)-rich domain, a transmembrane domain, and one of two alternative cytoplasmic tails. CD46 protects all cells but erythrocytes from complement activation by acting as a cofactor for the factor I serine protease, which cleaves C3b (see reference 30 for a review), and also prevents the alternative pathway amplification loop of C3b deposition on the cell surface (15). SCRs II, III, and IV are required for this cofactor activity, with SCRs III and IV being mainly involved in the binding to C3b (1).
The H binding site on CD46 has been mapped to the first two N-terminal SCR domains (7, 28, 34, 40). Modeling of CD46 SCR domains I and II (37), which was recently proved to be largely correct following X-ray diffraction analysis of CD46 SCR I and II crystals (8), together with H, MV, and antibody binding studies on site-directed mutated CD46 protein (6, 26, 32), indicated that the H protein interacts on one face extending from the top of SCR I to the bottom of SCR II. Although dispensable for MV binding, the underlying SCR III and IV domains optimize this interaction (14), with SCR IV playing a major role (9). The STP regions are not directly involved in CD46-mediated MV entry (21, 33).
The poor antivirus activity of a recombinant soluble form of CD46 (16, 45) led to the design of an oligomeric form of the receptor. This was based on C4b binding protein (C4bp), another complement regulatory molecule. This molecule is a multimer associating seven α chains, each consisting of eight SCR domains linked to a C-terminal oligomerization peptide, and one β chain composed of three SCR domains linked to an oligomerization peptide. When adsorbed to thin carbon films and examined under electron microscopy, C4bp has a spider-like structure (12, 47). A fusion protein between the four CD46 SCR domains, STP B region, and the oligomerization site of the C4bp α chain was generated and tested for its MV-neutralizing properties.
MATERIALS AND METHODS
Cloning procedure and isolation of cell lines producing sCD46-C4bpα fusion protein.
A cDNA coding for the signal peptide and the first 269 amino acids of CD46, which encompasses the four SCR domains and the STP B region, was fused to a cDNA encoding the amino-acid 57-C-terminal sequence of the C4bp α chain, which encompasses the C4bp multimerization domain. The cDNA encoding the CD46-C4bpα chimeric protein was subcloned under the simian virus 40 promoter in the pKC3 eukaryotic vector (29). After cotransfection with the pMAMNeo plasmid (coding for neomycin resistance) into CHO cells, stable clones secreting the CD46-C4bpα protein were isolated and amplified as published (29). The cDNA encoding the sCD46-C4bpα protein was also subcloned under the cytomegalovirus promoter into the APEX3 vector (10), which was used to derive human 293EBNA cells expressing the chimeric protein. The secretion of the chimeric protein was determined using a dot blot assay with MCI20.6 anti-CD46 antibody and anti-mouse immunoglobulin (Ig)–alkaline phosphatase conjugate as previously detailed (20). Throughout all the analyses, an immunopurified recombinant monomeric sCD46 previously described (10) was used for comparative studies.
Purification and biochemical characterization of sCD46-C4bpα chimeric protein.
The recombinant sCD46-C4bpα protein was immunopurified from cell supernatant using the anti-CD46 monoclonal antibody (MAb) E4.3 immobilized on activated Sepharose 4B (Pharmacia) according to the manufacturer instructions and was eluted with 0.1 M HCl–glycine buffer (pH 2.8). Alternatively, the sCD46-C4bpα protein was produced in serum-free medium supplemented with the plant-derived growth factor Prolifix (Biomedia), concentrated on 100-kDa exclusion membrane, and purified by exclusion chromatography on Sephacryl 200 (Pharmacia). Metabolic labeling with Tran35S-label (NEN) for 30 min followed by a chase of 2 h and immunoprecipitation of cell extract and cell supernatant using J4-48 antibodies and protein G-Sepharose beads were performed according to published procedures (20, 38).
Matrix-assisted laser desorption mass spectrometry (MALDI MS).
After dialysis and concentration under vacuum, the sample was dissolved in 0.1% trifluoroacetic acid and mixed with matrix (saturated solution of 3,5-dimethoxy-4-hydroxycinnamic acid in 0.1% trifluoroacetic acid in water-acetonitrile [2:1, vol/vol]). One microliter of the mixture with a matrix-to-sample ratio of ca. 10,000 was deposited on a thin layer of matrix crystals prepared on the target. After drying in air at ambient temperature, resulting crystals were analyzed in the mass spectrometer (Bruker Biflex; Bremen, Germany). External calibration was made with carbonic anhydrase (29,024 kDa) or monomeric and dimeric bovine serum albumin (66,430 and 132,858 kDa). Spectra were acquired in linear mode (turbo mode) at an acceleration voltage of 28 kV.
Enzyme-linked immunosorbent assay procedure.
The reactivity of sCD46-C4bpα with anti-CD46 antibodies was tested in an antibody binding competition assay on immobilized recombinant sCD46 revealed by a phosphatase alkaline–anti-mouse Ig conjugate as previously detailed (6, 16). The apparent avidity of antibodies was determined from the representation of Lineweaver and Burk, i.e., 1/bound antibody as a function of 1/antibody concentration (6).
C3b deposition.
Human C3b deposition on CHO and CHO.CD46 cells following activation of the alternative complement pathway was performed essentially as recently described (15). Briefly, CHO cells were incubated with human serum diluted 1:3 in the presence of 20 mM MgCl2, 100 mM EGTA, and serial dilutions of either sCD46-C4bpα or sCD46 for 1 h at 37°C. After being washed, the cells were immunolabeled using anti-C3b(C3c) WM1 antibody and anti-mouse IgG–phycoerythrin conjugate. The level of C3b was measured by flow cytometry.
Cytofluorometry assays.
The binding of sCD46-C4bpα to transmembrane MV H protein was tested after incubation of serially diluted protein with 2 × 105 L cells expressing MV H glycoprotein in a round-bottom 96-well microtiter plate (30 min at 20°C). Following washing, cells were incubated with an appropriate dilution of the anti-CD46 GB24 antibody. Phycoerythrin–anti-mouse IgG (heavy- and light-chain) conjugate was added following additional washing and cytofluorometry analysis performed as detailed previously (16). The results were expressed in mean fluorescence values and used to estimate the apparent avidity of sCD46-C4bp from the representation of Lineweaver and Burk.
Virus binding assay.
The assay was performed essentially as described (16). Serial dilutions of the recombinant sCD46 material were incubated with purified MV (Hallé strain) for 1 h at 20°C, and the mixture was added to CHO.CD46 cells. MV binding was measured after immunolabeling and flow cytometry.
Cell fusion.
A quantitative fusion assay based on the conditional expression of β-galactosidase (β-Gal) under the control of the T7 polymerase promoter was used. Briefly, the first HeLa cell partner was infected with MV (Hallé strain) (at a multiplicity of infection [MOI] of 2) for 8 h at 37°C. After 7 h of incubation a recombinant vaccinia virus encoding the T7-DNA-dependent RNA polymerase (vvT7) (at an MOI of 1) was added; at the end of the 8-h period the cells were washed once and cultured for an additional 16 h in the presence of a fusion peptide inhibitor, z-d-Phe-Phe-Gly, to prevent ongoing cell fusion and cell death. A second cell partner was infected with a recombinant vaccinia virus encoding the T7-driven β-Gal cDNA (vCB21R-lacZ) (at an MOI of 1) (3) for 1 h, washed once, and then incubated for an additional 16 h at 37°C. MV- and vvT7-infected cells were washed three times to eliminate the z-d-Phe-Phe-Gly peptide and resuspended in culture medium supplemented with 40 μM AraC to stop the replication of vaccinia virus and reduce nonspecific induction of β-Gal activity (39). A total of 105 cells were coincubated with a serial dilution of either sCD46, sCD46-C4bpα, monoclonal anti-MV-H 48cl6, anti-MV-F Y503, or anti-human C3b WM1 antibody for 30 min at 4°C prior to the addition of 105 vCB21R-lacZ infected cells. After 6 h of incubation at 37°C, the cells were lysed and the β-Gal activity was determined by colorimetry using o-nitrophenyl-β-d-galactopyranoside substrate (Sigma).
In vitro neutralization assays.
Two different methods were used. In the first one, serial dilutions of sCD46, sCD46-C4bpα, or bovine serum albumin were incubated for 30 min at 37°C with 100 PFU of MV (Edmonston strain) (American Type Culture Collection) in tissue culture medium supplemented with 2% fetal calf serum. The mixture was then layered onto a Vero cell monolayer and incubated for 4 days. Cells were fixed in 10% formalin and stained with methyl blue, and the number of PFU was counted. The second method was devised so as to test the reversibility of the virus neutralization. Briefly, 102, 103, 104, and 105 50% tissue culture infective doses (TCID50) of MV (Hallé strain) was incubated with 300 μg of sCD46-C4bpα, WM1 anti-C3b, 48cl6 anti-MV-H, or Y503 anti-MV-F antibodies per ml in a final volume of 30 μl for 1 h at 37°C. After the addition of 270 μl of culture medium, serial dilutions (1:3) of the mixture were made in 96-well microtiter plates, with each dilution being equally aliquoted into eight wells. Vero cells (104 in 200 μl) were added to each well, and the Microplates were incubated for 10 days at 37°C. Under these conditions, the final concentration of the inhibitor during the Vero cell infection step was inferior or equal to 10 nM at most. Infectious virus was quantified using the TCID50 procedure.
In vivo neutralization assay.
Transgenic mice ubiquitously expressing the human CD46 protein (line MCP-7) have been described previously (25). These mice were shown to be highly sensitive to intracranial infection by MV (18). Before infection, phosphate-buffered saline (PBS), sCD46, or sCD46-C4bpα was mixed with either MV (Edmonston strain) or canine distemper virus (CDV) (Onderstepoort strain) and incubated for 15 min at 37°C. This mixture (30 μl) was then used to intracranially inoculate 2- to 3-day-old suckling CD46 transgenic mice. Animals were observed for clinical symptoms and death daily for 10 weeks.
RESULTS
CD46-C4bpα protein is secreted as a homo-octamer.
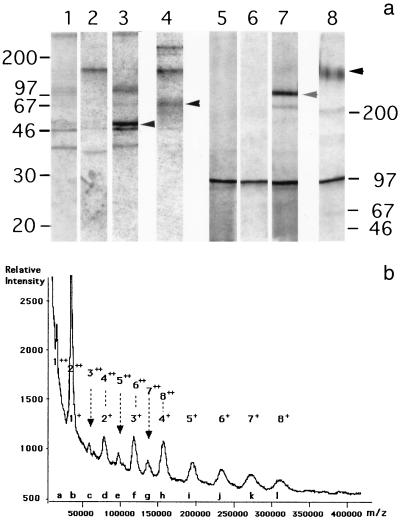
After transfection with CD46-C4bp.pKC3 or APEX-CD46-C4bp eukaryotic vectors and selection in the presence of the appropriate antibiotics, several clones of CHO and 293EBNA cells were found to secrete a protein which reacted with the MCI20.6 anti-CD46 MAb in a dot blot assay. One of the CHO cell clones, 2B5, was metabolically radiolabeled for 30 min and then subjected to a chase of 2 h. From the cell extract, the anti-CD46 MAb J4-48 specifically immunoprecipitated a protein which resolved after polyacrylamide gel electrophoresis into a doublet of ∼50 kDa and a single band of ∼250 kDa under reducing and nonreducing conditions, respectively (Fig. 1a, lanes 3 and 7). From the cell supernatant, the immunoprecipitated material resolved into broad bands of ∼65 and ∼320 kDa under reducing and nonreducing conditions, respectively (Fig. 1a, lanes 4 and 8). In addition, a band with a mass of >200 kDa, which could correspond to unreduced sCD46-C4bpα, was also detected under reducing conditions (Fig. 1a, lane 4). The size increase of the secreted material as well as the broadening of the bands probably reflects heterogeneous glycosylation, which is typically observed with CD46 (30, 38).
FIG. 1.
(a) Polyacrylamide gel electrophoresis autoradiogram of metabolically 35S-radiolabeled CD46-C4bpα immunoprecipitated using J4-48 anti-CD46 antibody under reducing (lanes 1 to 4) and nonreducing (lanes 5 to 8) conditions of cell extract from CHO (lanes 1 and 5), CHO-CD46-C4bpα cells (lanes 3 and 7), and from supernatant of CHO (lanes 2 and 6) or CHO-CD46-C4bpα (lanes 4 and 8) cells. Major bands specifically immunoprecipitated are indicated by arrows. A prominent nonspecific band around 100 kDa was also observed. (b) MALDI MS of sCD46-C4bpα protein. Peaks labeled b, d, f, h, i, j, k, and l are related to the singly charged ion series, and peaks a through h are related to the doubly charged ion series. Peaks b, d, f, and h correspond to ions belonging to both singly and doubly charged series. Ion species of the singly and doubly charged series contributing to each peak are indicated above the curve. The mass errors range between 0.01 (peak b) and 1.7% (peak l).
Sufficient material was immunopurified on an E4.3 MAb column for MALDI MS analysis. The spectrum resolved into 12 peaks labeled as follows: a, 19,086 Da; b, 37,963 Da; c, 42,772 Da; d, 80,735 Da; e, 100,232 Da; f, 120,899 Da; g, 139,233 Da; h, 159,945 Da; i, 199,079 Da; j, 238,363 Da; k, 277,362 Da; and l, 317,421 Da. This series was completely different from those resulting from usual multiply-charged ions or from ion signals corresponding to molecular clusters (dimer [2M + H+], trimer [3M + H+], etc.) which may appear in MALDI MS at high analyte concentration. Indeed, as shown in Fig. 1b, the peak intensities indicate that the twelve peaks actually correspond to eight singly charged ions (peaks b, d, f, h, i, j, k, and l) and eight doubly charged ions (peaks a to h). The ion nomenclature is based on the number (one to eight) of ∼40-kDa subunits and the number of protons bound to the fragment. Four of these peaks (b, d, f, and h) are obviously heterogeneous and reflect the occurrence of one singly and one doubly charged ion. For example, the 4+ and 82+ ions contribute to the intensity of peak h. The molecular masses (MMs) and intensities of the 16 ion species suggest that they are produced by eight fragments, or subunits, of a native molecule differing by an incremental MM of approximately 40 kDa. Furthermore, the spectrum shows that, as expected, (i) the MM of ions producing peaks b, d, f, and h are exactly those that can be expected for the 82+ and 4+, 62+ and 3+, 42+ and 2+, and 22+ and 1+ ions, respectively, of a 317-kDa whole molecule; (ii) peaks a, c, e, and g, which are presumed to be produced by a single ion type, have much lower intensities than their neighbors (peaks b, d, f, and h), corresponding to a mixture of singly and doubly charged ions; (iii) peaks i, j, k, and l exhibit decreasing intensities as expected for a series of ions with increasing masses; and (iv) peak l, the peak with the highest MM of the series, has a MM 16 times greater than peak a and 8 times greater than peak b. The expected nature and the location in the spectrum of the 16 singly and doubly charged ions is given in Fig. 1.
We conclude that the peak series correspond to the monomer, dimer, trimer, tetramer, pentamer, hexamer, heptamer, and octamer of a subunit with a MM of 37,963 Da. Mass values may be slightly underestimated because of possible laser beam-induced carbohydrate loss during MALDI analysis of glycoprotein samples. Thus, the CD46-C4bpα chimerical protein was secreted as homo-octamers linked by disulfide bonds.
Reactivity of sCD46-C4bpα protein with anti-CD46 antibodies.
The sCD46-C4bpα protein was found to react with a panel of anti-CD46 MAbs directed against CD46 SCR-I, SCR-II, SCR-III, or SCR-IV domains (Table 1). However, when compared to monomeric sCD46, the octameric sCD46-C4bpα exhibited an increase in avidity to E4.3 (70-fold), MCI20.6 (3-fold), and GB24 (2-fold) antibodies, whereas a decrease in avidity to M75 antibody (11-fold) was observed. Similar avidity values were observed with other anti-CD46 MAbs, TRA2.10 and 10.88. Taken together this indicates that, upon multimerization, some minor conformational changes of SCR domains of CD46 occurs. This pattern of antibody reactivity was also different from that observed with the natural transmembrane CD46, which exhibits the highest avidity for all antibodies tested.
TABLE 1.
Apparent avidity (KD) of anti-CD46 antibodies for transmembrane CD46 (tmCD46) expressed on CHO cells, as determined by flow cytometry, and for monomeric sCD46 or octameric sCD46-C4bpα proteins, as determined by competition enzyme-linked immunosorbent assay on immobilized sCD46a
| Antibody | Binding site on CD46 |
KD (nM) of anti-CD46 antibodies for:
|
Inhibitory activity against:
|
|||
|---|---|---|---|---|---|---|
| tmCD46 | sCD46 | sCD46-C4bpα | Cofactor activity | MV sH binding | ||
| E4.3 | SCR-I | 0.4 | 47.2 | 0.7 | − | +/− |
| MCI20.6 | SCR-I | 0.3 | 6.1 | 2.1 | − | + |
| TRA2.10 | SCR-I | 0.4 | 36.0 | 29.6 | − | + |
| M75 | SCR-II | 0.2 | 0.3 | 3.3 | +++ | +++ |
| GB24 | SCR-III and IV | 0.3 | 4.6 | 2.0 | +++ | − |
| 10.88 | SCR-III and IV | 0.3 | 1.9 | 2.3 | ? | − |
sCD46-C4bpα has no complement regulatory activity.
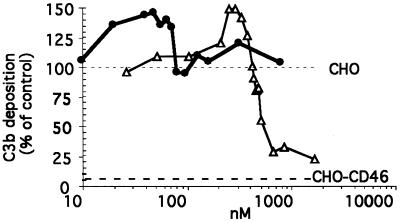
The sCD46-C4bpα was mixed with human serum and incubated with CHO cells to determine its ability to prevent the amplification loop of C3b deposition of the alternative complement pathway (Fig. 2). The sCD46-C4bpα protein was unable to decrease the level of the C3b deposition on CHO cells even at the highest concentration tested (250 μg/ml or 780 nM, equivalent to 6240 nM of monovalent CD46). In contrast, and in agreement with previous work (10), monomeric sCD46 at concentrations of >420 nM (i.e., 25 μg/ml) displayed cofactor activity. At concentrations of >1,000 nM, monomeric sCD46 was almost as efficient as transmembrane CD46, which completely prevented the amplification loop of the C3b deposition, leaving only around 5% of the C3b deposition, corresponding to the primary tick-over phase (Fig. 3 [bottom dotted line]) (15). In addition, with intermediate concentrations of both of the sCD46-C4bpα (10 to 80 nM, equivalent to 80 to 640 nM monovalent CD46) and sCD46 (150 to 400 nM) proteins, the amount of C3b deposition was higher than on untreated CHO cells (Fig. 3 [see values above the upper dotted line]). This enhancement was not observed when the complement activation was performed on CHO.CD46 cells (not shown).
FIG. 2.
C3b deposition after alternative complement activation of human serum on CHO cells in the presence of octameric sCD46-C4bpα (circles) or monomeric sCD46 (triangles) proteins. The results are expressed as a percentage of the C3b deposition level observed on CHO cells in the absence of inhibitor. The level of deposition of C3b on CHO-CD46 cells is indicated by the bottom horizontal dotted line. The results are cumulative data from four different experiments.
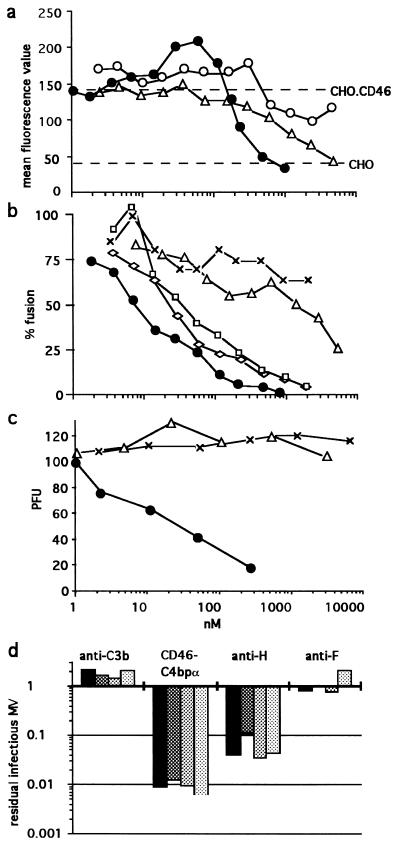
FIG. 3.
Inhibition of virus binding (a), virus-induced cell-cell fusion (b), and virus infectivity (c and d). (a) Purified MV was incubated with either sCD46-C4bpα protein (black circles) or sCD46 protein (triangles) before the addition of CHO-CD46 cells; alternatively MV was incubated with CHO-CD46 cells to which the sCD46-C4bpα protein was added afterwards (open circles). (b) Inhibition of fusion in the presence of sCD46-C4bpα protein (circles), sCD46 protein (triangles), 48Cl6 anti-H (diamonds), Y503 anti-F (squares), or WM1 anti-C3b(C3c) (exes) MAbs. The results are expressed as a percentage of the fusion between HeLa and MV-infected HeLa cells observed in the absence of inhibitor as determined by the level of β-Gal activity. (c) MV (100 PFU) was incubated with sCD46-C4bpα protein (circles), sCD46 protein (triangles), or bovine serum albumin (exes) prior to infection of Vero cells. (d) MV (105, 104, 103, and 102 TCID50 [black to light grey columns, respectively]) was incubated with the indicated reagent, and the remaining virus was titrated using the TCID50 assay. The results are expressed as the MV fraction not neutralized. Note that no infectious MV was recovered from 102 TCID50 MV incubated with sCD46-C4bpα (i.e., recovery fraction = 0:100).
sCD46-C4bpα protein can bind to MV H.
Both the octameric sCD46-C4bpα and monomeric sCD46 were able to bind to L cells expressing the MV H protein but not to the parental murine L cells (not shown). sCD46-C4bpα exhibited a 2.5-fold-higher apparent avidity (48 nM, equivalent to 384 nM monovalent CD46) towards H protein than sCD46 (119 nM).
sCD46-C4bpα is a potent inhibitor of MV binding to CD46.
The preincubation of purified MV with sCD46-C4bpα protein resulted in the abolition of the CD46-mediated specific binding to CHO.CD46 cells at a concentration of >470 nM (equivalent to 3,760 nM monovalent CD46) (Fig. 3a). A similar binding inhibition was observed with 5,000 nM monomeric sCD46. Thus, when their respective valences are taken into account, both proteins have a similar inhibitory binding efficiency. However, while the inhibition curve with the monomeric sCD46 protein shows a regular linear relationship between 625 and 5,000 nM, the corresponding inhibition curve observed with the octameric sCD46-C4bpα protein has a steeper slope. In addition, within the 15 to 120 nM range (equivalent to 120 to 960 nM monovalent CD46), a significant enhancement of MV binding to octameric sCD46-C4bpα was observed, possibly reflecting the binding of virus aggregated in solution by the nonsaturating multimeric protein. When the inhibitor was added after MV binding to CHO-CD46 cells, neither enhancement nor inhibition was observed.
sCD46-C4bpα is a potent inhibitor of MV glycoprotein-induced cell-cell fusion.
A quantitative fusion assay based on the conditional expression of β-Gal was used to assess the functional property of the octameric sCD46-C4bpα protein to inhibit cell-cell fusion. After 30 min of preincubation of MV-infected HeLa cells with this protein at 4°C, an almost linear decrease in fusion with HeLa cells was observed, with 50% inhibition at 7 nM (equivalent to 56 nM monovalent CD46) and complete inhibition at 950 nM (equivalent to 7,600 nM monovalent CD46). Similar data was also observed following preincubation with anti-MV H and anti-MV F antibodies, although these antibodies were not as efficient, with 50% inhibition being observed at 15 and 22 nM, respectively (Fig. 3b). In comparison, the monomeric sCD46 protein was a poor fusion inhibitor, with 50% inhibition activity at 1,100 nM (Fig. 3b). As expected the unrelated WM1 antibody had a minimal level of inhibition even at the highest concentration tested. Similar results were obtained when the hamster CHO cells coinfected with recombinant vaccinia virus coding for MV H and F proteins and CHO.CD46 were used as cell fusion partners. As a control for specificity, every fusion inhibitor was found not to inhibit the cell-cell fusion assay mediated by the closely related CDV.
sCD46-C4bpα is a potent inhibitor of MV infection in vitro.
When the octameric sCD46-C4bpα protein was incubated with 100 PFU of MV in the presence of Vero indicator cells, it was a potent inhibitor of infection, with 50% inhibitory activity at 17 nM (equivalent to 136 nM monovalent CD46) (Fig. 3c), i.e., consistent with the 50% inhibitory activity observed with cell-cell fusion (7 nM). In contrast, and as previously reported (16, 45), the monomeric sCD46 was unable to neutralize the virus, as was the control bovine serum albumin. To test the reversibility of this neutralizing effect, 102, 103, 104, and 105 TCID50 of MV were incubated with a 300-μg/ml concentration (i.e., 950 nM for sCD46-C4bpα) of inhibitor for 1 h and then diluted 100-fold and more (i.e., below the 50% inhibitory activity level observed when sCD46-C4bpα is left throughout the MV neutralization assay [Fig. 3c]). A constant proportion of approximately 99% of MV (i.e., 2 log units) was irreversibly neutralized by the sCD46-C4bpα protein (Fig. 3d). The 48cl6 anti-H MAb was also very efficient at neutralizing MV although not as efficient as sCD46-C4bpα, with between 90 and 95% (i.e., 1-log range) of MV being neutralized. Interestingly, despite being a potent inhibitor of MV-induced cell-cell fusion, the Y503 anti-F MAb was unable to irreversibly neutralize the virus. As a control, incubation of MV with WM1 antibody had no effect.
sCD46-C4bpα is a potent inhibitor of MV infection in vivo.
The neutralizing properties of CD46 reagents were then tested in a transgenic CD46 mouse characterized by (i) high susceptibility to MV infection and productive replication in the brain after intracranial inoculation and (ii) obligatory use of the cellular receptor CD46 by MV (18, 25). When 11 μg (i.e., 35 pmol [equivalent to 280 pmol of monovalent CD46]) of octameric sCD46-C4bpα protein was coinjected intracranially into newborn transgenic CD46 mice with 6,000 PFU of MV (Edmonston strain), all animals survived, whereas mice inoculated with MV alone were all killed, with a mean survival time of 7.6 days (Table 2). In the group of mice inoculated with MV and 24 μg (i.e., 400 pmol) of monomeric sCD46, three out of four mice died, with a mean survival time of 13 days. The protective effect of both octameric sCD46-C4bpα and monomeric sCD46 were specific to MV, since they did not prevent or delay the death induced by the inoculation of transgenic CD46 mice with CDV, which does not use CD46 as a receptor.
TABLE 2.
In vivo neutralizing activity of octameric sCD46-C4bpα and monomeric sCD46 proteinsa
| Inoculated virus | Treatment | Death ratiob | Mean survival time (days) |
|---|---|---|---|
| MV | PBS | 6/6 | 7.6 |
| sCD46 | 3/4 | 13.0 | |
| sCD46-C4bpα | 0/6 | —c | |
| CDV | PBS | 3/3 | 7.3 |
| sCD46 | 3/3 | 7.6 | |
| sCD46-C4bpα | 3/3 | 7.7 |
Suckling transgenic CD46 mice (2 to 3 days old) were inoculated intracranially with either 11 μg (i.e., 35 pmol) of octameric sCD46-C4bpα (equivalent to 280 pmol of monovalent CD46) or 24 μg (i.e., 400 pmol) of monomeric sCD46 or PBS together with 6,000 infectious units of either MV or CDV. Animals were observed for clinical symptoms and death daily during 10 weeks.
Number of mice that died/number of mice inoculated.
—, all mice survived.
DISCUSSION
The fusion of the C4bpα bundle domain to the ectodomain of CD46 resulted in the generation of a chimeric disulfide-bound homo-octameric protein, sCD46-C4bpα. This structure is similar to the homo-octameric C4bp α chains synthesized in the absence of the C4bp β chain (23) and to the homo-octameric chimeric anti-Rh(D) Fv antibody (29). Compared to the natural transmembrane CD46 molecule, the octameric sCD46-C4bpα shows a reduced reactivity with two antibodies, anti-SCR II M75 and anti-SCR III and IV GB24, which have a strong inhibitory activity against the CD46 cofactor activity (1, 44) (Table 1). Accordingly, it lacks any cofactor activity, whereas the monomeric sCD46, which shows a reduced reactivity towards only one antibody (GB24) still exhibits a significant cofactor activity. Noteworthy, the SCR II domain of CD46 does not contain primary binding sites for C3b but is required for the cofactor activity, and the SCR III and IV domains contain the binding site for C3b (1). The lack of cofactor activity of sCD46-C4bpα on C3b deposition following alternative complement activation was surprising because the natural C4bp α chain is structurally and functionally related to CD46. Indeed, the C4bp molecule (seven α chains plus one β chain) is a cofactor of factor I for the cleavage of C4b but not of C3b (24, 43). This activity has been mapped to the first three N-terminal domains of the C4bp α chain (24). A monomeric membrane-anchored C4bp α chain protein displays an additional cofactor activity for the factor I-mediated cleavage of C3b, which maps to both N-terminal and C-terminal SCR domains (35). We suggest that the localization of the CD46 SCR III and IV domains adjacent to the bundle region of C4bp α chain effectively hampers binding to C3b and/or cofactor activity through steric hindrance. We do not have a satisfactory explanation for the unexpected enhancement of C3b deposition at intermediate concentrations of both sCD46 and sCD46-C4bpα, although one can speculate about a transient stabilizing effect on the C3bBb convertase, as previously observed with solubilized transmembrane CD46 in the absence of factor I (42).
The SCR I and II domains of sCD46-C4bpα are accessible for binding to the MV H protein. The 2.5-fold-higher avidity of the chimeric protein, compared to that of monomeric sCD46, could be related to cooperative binding of each of the CD46-C4bpα monomers and/or to subtle conformational changes in the H binding site induced by modified interactions with the underlying SCR III and/or IV domains (9, 14). The sCD46-C4bpα protein exhibits a lower reactivity with three anti-CD46 antibodies able to compete with MV interaction, including M75, a very strong inhibitor of the binding of an MV-soluble H (6) (Table 1). This suggests that the local structure of the sCD46-C4bpα protein subtly differs from that of the natural transmembrane CD46 but can still accommodate efficient interaction with MV. This is in agreement with the relative insensitivity of CD46 to point mutations of amino acids in SCR I and II domains (6, 26, 32). How does the oligomeric structure of the sCD46-C4bpα compare with that of natural transmembrane CD46? From cross-linking experiments, CD46 seems to exist as dimers and possibly trimers (31), and the crystal structure of a CD46 SCR I-SCR II fragment revealed a trimeric arrangement (8).
The octamerization of the CD46 ectodomain resulted in a chimeric protein with an anti-MV activity improved by 2 orders of magnitude, thus far exceeding the modest increase of its avidity for the MV H protein. The mechanism of this antiviral activity could be a competition for binding to the cell surface CD46 receptor and/or an irreversible conformational change of the fusion protein induced by the simultaneous binding of several adjacent H companion molecules to the octameric receptor. In favor of the latter, (i) the octameric and monomeric receptors displayed similar efficiencies in saturating CD46 binding sites at equal valency, (ii) at intermediate concentrations, the octameric protein resulted in an increased amount of virus binding and/or uptake with decreased infectivity (compare Fig. 3a and c), and (iii) unlike the anti-F MAb, its neutralizing ability was irreversible in vitro. Moreover, this would explain the potent neutralizing activity of the octameric soluble receptor in vivo.
sCD46-C4bpα is derived from two human proteins, is devoid of complement regulatory properties, has a high MM which should increase its serum half-life, and displays potent in vitro and in vivo neutralizing properties. Consequently, it is a good candidate for clinical use in the control of MV infection in immunocompromised patients (2, 4, 36), in patients suffering from acute or subacute encephalitis, and in young children infected at the critical transition age between maternally transmitted antibody protection and a successful anti-measles vaccination coverage (41). Further studies using animals which more closely model the human disease are in progress to validate this new therapeutic concept. The C4bpα-based octamerization procedure of a cellular receptor might also prove useful in generating other efficient antivirus reagents.
ACKNOWLEDGMENTS
D.C., P.D., A.E., and B.H. contributed equally to this work.
We thank B. Loveland for providing us with E4.3 MAb, recombinant sCD46 protein, and CHO.CD46 cells; R. Cattaneo for providing TRA2.10 and 10.88; M. Felhman and B. Rossi for providing GB24; and T. Seya for providing M75 antibodies. The reagent vCB21R-lacZ was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, from C. C. Broder, P. E. Kennedy, and E. A. Berger.
This work was supported in part by grants from the Ministère de l'Education nationale et de la Recherche et de la Technologie (FNMIP) and from the Association pour la Recherche contre le Cancer. Dale Christiansen, Patricia Devaux, and Alexey Evlashev were supported by fellowships from the European Union (Marie Curie), Fondation Mérieux, and Fondation pour la Recherche Medicale, respectively.
REFERENCES
- 1.Adams E M, Brown M C, Nunge M, Krych M, Atkinson J P. Contribution of the repeating domains of membrane cofactor protein (CD46) of the complement system to ligand binding and cofactor activity. J Immunol. 1991;147:3005–3011. [PubMed] [Google Scholar]
- 2.Aicardi J, Goutieres F, Arsenio-Nunes M L, Lebon P. Acute measles encephalitis in children with immunosuppression. Pediatrics. 1977;69:232–239. [PubMed] [Google Scholar]
- 3.Alkhatib G, Broder C C, Berger E A. Cell type-specific fusion cofactors determine human immunodeficiency virus type I tropism for T-cell lines versus primary macrophages. J Virol. 1996;70:5487–5494. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angel J B, Walpita P, Lerch R A, Mohinderjit S S, Masurekar M, DeLellis R A, Noble J T, Snydman D R, Udem S A. Vaccine-associated measles pneumonitis in adult with AIDS. Ann Int Med. 1998;129:104–106. doi: 10.7326/0003-4819-129-2-199807150-00007. [DOI] [PubMed] [Google Scholar]
- 5.Balliet J W, Berson J, D'Cruz C M, Huang J, Crane J, Gilbert J M, Bates P. Production and characterization of a soluble, active form of Tva, the subgroup A avian sarcoma and leukosis virus receptor. J Virol. 1999;73:3054–3061. doi: 10.1128/jvi.73.4.3054-3061.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchholz C J, Koller D, Devaux P, Mumenthaler C, Schneider-Schaulies J, Braun W, Gerlier D, Cattaneo R. Mapping of the primary binding site of measles virus to its veceptor CD46. J Biol Chem. 1997;272:22072–22079. doi: 10.1074/jbc.272.35.22072. [DOI] [PubMed] [Google Scholar]
- 7.Buchholz C J, Schneider U, Devaux P, Gerlier D, Cattaneo R. Cell entry by measles virus: long hybrid receptors uncouple binding from membrane fusion. J Virol. 1996;70:3716–3723. doi: 10.1128/jvi.70.6.3716-3723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casanovas J M, Larvie M, Stehle T. Crystal structure of two CD46 domains reveals an extended measles virus-binding surface. EMBO J. 1999;18:2911–2922. doi: 10.1093/emboj/18.11.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christiansen D, Loveland B, Kyriakou P, Lanteri M, Escoffier C, Gerlier D. Interaction of CD46 with measles virus (MV): accessory role of CD46 SCRIV. J Gen Virol. 2000;81:911–917. doi: 10.1099/0022-1317-81-4-911. [DOI] [PubMed] [Google Scholar]
- 10.Christiansen D, Milland J, Thorley B R, Mckenzie I F C, Mottram P L, Purcell L J, Loveland B E. Engineering of recombinant soluble CD46: an inhibitor of complement activation. Immunology. 1996;87:348–354. [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly L, Zingler K, Young J A. A soluble form of a receptor for subgroup A avian leukosis and sarcoma viruses (ALSV-A) blocks infection and binds directly to ALSV-A. J Virol. 1994;68:2760–2764. doi: 10.1128/jvi.68.4.2760-2764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahlbäck B, Smith C A, Muller-Eberhard H J. Visualization of human C4b-binding protein and its complex with vitamin K-dependent protein S and complement protein C4b. Proc Natl Acad Sci USA. 1983;80:3461–3465. doi: 10.1073/pnas.80.11.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deen K C, McDougal J S, Inacker R, Folena-Wasserman G, Arthos J, Rosenberg J, Maddon P J, Axel R, Sweet R W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988;331:82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- 14.Devaux P, Buchholz C J, Schneider U, Escoffier C, Cattaneo R, Gerlier D. CD46 short consensus repeats III and IV enhance measles virus binding but impair soluble hemagglutinin binding. J Virol. 1997;71:4157–4160. doi: 10.1128/jvi.71.5.4157-4160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaux P, Christiansen D, Fontaine M, Gerlier D. Control of C3b and C5b deposition by CD46 (membrane cofactor protein) after alternative but not classical complement activation. Eur J Immunol. 1999;29:815–822. doi: 10.1002/(SICI)1521-4141(199903)29:03<815::AID-IMMU815>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Devaux P, Loveland B, Christiansen D, Milland J, Gerlier D. Interactions between the ectodomains of haemagglutinin and CD46 as a primary step in measles virus entry. J Gen Virol. 1996;77:1477–1481. doi: 10.1099/0022-1317-77-7-1477. [DOI] [PubMed] [Google Scholar]
- 17.Dörig R E, Marcil A, Chopra A, Richardson C D. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 18.Evlashev A, Moyse E, Valentin H, Azocar O, Trescol-Biémont M C, Marie J C, Rabourdin-Combe C, Horvat B. Productive measles virus brain infection and apoptosis in CD46 transgenic mice. J Virol. 2000;74:1373–1382. doi: 10.1128/jvi.74.3.1373-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher R A, Bertonis J M, Meier W, Johnson V A, Costopoulos D S, Liu T, Tizard R, Walker B D, Hirsch M S, Schooley R T, et al. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988;331:76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- 20.Gerlier D, Garnier F, Forquet F. Haemagglutinin of measles virus: purification and storage with preservation of biological and immunological properties. J Gen Virol. 1988;69:2061–2069. doi: 10.1099/0022-1317-69-8-2061. [DOI] [PubMed] [Google Scholar]
- 21.Gerlier D, Loveland B, Varior-Krishnan G, Thorley B, McKenzie I F C, Rabourdin-Combe C. Measles virus receptor properties are shared by several CD46 isoforms differing in extracellular regions and cytoplasmic tails. J Gen Virol. 1994;75:2163–2171. doi: 10.1099/0022-1317-75-9-2163. [DOI] [PubMed] [Google Scholar]
- 22.Gerlier D, Varior-Krishnan V, Devaux P. CD46-mediated measles virus entry: a first key to host-range specificity. Trends Microbiol. 1995;3:338–345. doi: 10.1016/s0966-842x(00)88972-6. [DOI] [PubMed] [Google Scholar]
- 23.Hardig Y, Garcia de Frutos P, Dahlbäck B. Expression and characterization of a recombinant C4b-binding protein lacking the beta-chain. Biochem J. 1995;308:795–800. doi: 10.1042/bj3080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardig Y, Hillarp A, Dahlbäck B. The amino-terminal module of the C4b-binding protein alpha-chain is crucial for C4b-binding and factor I-cofactor function. Biochem J. 1997;323:469–475. doi: 10.1042/bj3230469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horvat B, Rivailler P, Varior-Krishnan G, Cardoso A, Gerlier D, Rabourdin-Combe C. Transgenic mice expressing human measles virus (MV) receptor CD46 provide cells exhibiting different permissivities to MV infections. J Virol. 1996;70:6673–6681. doi: 10.1128/jvi.70.10.6673-6681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu E C, Dorig R E, Sarangi F, Marcil A, Iorio C, Richardson C D. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J Virol. 1997;17:6144–6154. doi: 10.1128/jvi.71.8.6144-6154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussey R E, Richardson N E, Kowalski M, Brown N R, Chang H C, Siliciano R F, Dorfman T, Walker B, Sodroski J, Reinherz E L. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988;331:78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- 28.Iwata K, Seya T, Yanagi Y, Pesando J M, Johnson P M, Okabe M, Ueda S, Ariga H, Nagasawa S. Diversity of sites for measles virus binding and for inactivation of complement C3b and C4b on membrane cofactor protein CD46. J Biol Chem. 1995;270:15148–15152. doi: 10.1074/jbc.270.25.15148. [DOI] [PubMed] [Google Scholar]
- 29.Libyh T, Goosens D, Oudin S, Gupta N, Dervillez X, Juszczak G, Cornillet P, Bougy F, Reveil F, Philbert F, Tabary T, Klatzmann D, Rouger P, Cohen J H M. A recombinant human anti-Rh(D) antibody with multiple valences using a C-terminal fragment of C4-binding protein. Blood. 1997;90:3978–3983. [PubMed] [Google Scholar]
- 30.Liszewski M K, Post T W, Atkinson J P. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–455. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 31.Lozahic S, Christiansen D, Manié S, Gerlier D, Billard M, Boucheix C, Rubinstein E. CD46 associates with multiple β1 integrins and tetraspans. Eur J Immunol. 2000;30:900–907. doi: 10.1002/1521-4141(200003)30:3<900::AID-IMMU900>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 32.Manchester M, Gairin J E, Patterson J B, Alvarez J, Liszewski M K, Eto D S, Atkinson J P, Oldstone M B A. Measles virus recognizes its receptor, CD46, via two distinct binding domains within SCR1-2. Virology. 1997;233:174–184. doi: 10.1006/viro.1997.8581. [DOI] [PubMed] [Google Scholar]
- 33.Manchester M, Liszewski M K, Atkinson J P, Oldstone M B A. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manchester M, Valsamakis A, Kaufman R, Liszewski M K, Alvarez J, Atkinson J P, Lublin D M, Oldstone M B A. Measles virus and C3 binding sites are distinct on membrane cofactor protein (CD46) Proc Natl Acad Sci USA. 1995;92:2030–2307. doi: 10.1073/pnas.92.6.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikata S, Miyagawa S, Fukui A, Murakami Y, Shirakura R, Matsuda H, Hatanaka M, Matsumoto M, Seya T, Suzuki K, Nagasawa S. A monomeric human C4b-binding protein (C4bp) more efficiently inactivates C3b than natural C4bp: participation of C-terminal domains in factor I-cofactor activity. Mol Immunol. 1998;35:537–544. doi: 10.1016/s0161-5890(98)00046-7. [DOI] [PubMed] [Google Scholar]
- 36.Mitus A, Holloway A, Evans A E, Enders J F. Attenuated measles vaccine in children with acute leukemia. Am J Child Dis. 1962;103:243–248. doi: 10.1001/archpedi.1962.02080020425051. [DOI] [PubMed] [Google Scholar]
- 37.Mumenthaler C, Schneider U, Buchholz C J, Koller D, Braun W, Catteneo R. A 3D model for the measles virus receptor CD46 based on homology modeling, Monte Carlo simulations, and hemagglutinin binding studies. Protein Sci. 1997;6:588–597. doi: 10.1002/pro.5560060308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naniche D, Varior-Krishnan G, Cervoni F, Wild T F, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nussbaum O, Broder C C, Berger E A. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activity. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nussbaum O, Broder C C, Moss B, Bar-Lev Stern L, Rozenblatt S, Berger E A. Functional and structural interactions between measles virus hemagglutinin and CD46. J Virol. 1995;69:3341–3349. doi: 10.1128/jvi.69.6.3341-3349.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osterhaus A, van Amerongen G, van Binnendjik R. Vaccine strategies to overcome maternal antibody mediated inhibition of measles vaccine. Vaccine. 1998;16:1479–1481. doi: 10.1016/s0264-410x(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 42.Seya T, Atkinson J P. Functional properties of membrane cofactor protein of complement. Biochem J. 1989;264:581–588. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seya T, Holers V M, Atkinson J P. Purification and functional analysis of the polymorphic variants of the C3b/C4b receptor (CR1), and comparison to H, C4b-binding protein (C4bp), and decay-accelerating factor (DAF) J Immunol. 1985;135:2661–2667. [PubMed] [Google Scholar]
- 44.Seya T, Iwata K, Hara T, Matsumoto M, Nagasawa S. CD46 workshop: a panel of monoclonal antibodies against CD46 that block complement regulatory activity and measles virus receptor function of CD46. In: Kishimoto T, Kikutani H, von dem Borne A E G K, Goyert S M, Mason D Y, Miyasaka M, Moretta L, Okumura K, Shaw S, Springer T A, Sugamura K, Zola H, editors. Leucocyte typing VI. New York, N.Y: Garland Publishing Inc.; 1997. pp. 507–509. [Google Scholar]
- 45.Seya T, Kurita M, Hara T, Iwata K, Sembra T, Hatanaka M, Matsumoto M, Yanagi Y, Ueda S, Nagasawa S. Blocking measles virus infection with a recombinant soluble form of, or monoclonal antibodies against, membrane cofactor protein of complement (CD46) Immunology. 1995;84:619–625. [PMC free article] [PubMed] [Google Scholar]
- 46.Smith D H, Byrn R A, Marsters S A, Gregory T, Groopman J E, Capon D J. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science. 1987;238:1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- 47.Villoutrex B O, Hardig Y, Wallqvist A, Cowell D V, Garcia de Frutos P, Dahlbäck B. Structural investigations of C4b-binding protein by molecular modeling: localization of putative binding sites. Proteins. 1998;31:391–405. doi: 10.1002/(sici)1097-0134(19980601)31:4<391::aid-prot6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]