Abstract
Background:
Adolescents and young adults may use cannabidiol (CBD) products in an attempt to reduce depression and anxiety symptoms, despite little research examining this use. This systematic review evaluated preclinical and clinical research on the effects of CBD on depressive and anxiety disorders in adolescence and young adulthood. To provide context, we discuss CBD’s mechanism of action and neurodevelopmental effects.
Methods:
PubMed was searched for articles published through June 2022. Preclinical or clinical CBD administration studies with N > 1 that examined depressive and/or anxiety disorders were eligible.
Results:
Initially, 224 publications were identified. After excluding duplicates and applying eligibility criteria, 6 preclinical (depression: n≈133; anxiety: n≈161) and 4 clinical (anxiety: n=113) articles remained. Due to the low number of studies, results were synthesized qualitatively. The Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence were used to rate each study’s evidence. The preclinical effects of CBD on depression-like behavior appear to differ by sex, early life stress, and duration of use. Despite no evidence that CBD exerts anxiolytic effects in preclinical adolescent models, CBD may reduce anxiety symptoms in human adolescents and young adults with anxiety disorders.
Conclusions:
The existing evidence suggests that CBD may reduce symptoms of anxiety in adolescents and young adults. However, the evidence is sparse and limited by variations in samples and CBD dosing duration. Further research is needed to understand the potential benefits and/or harms of CBD for depression and anxiety disorders in this population. Implications for clinical practice and research are discussed.
Keywords: cannabidiol, depression, anxiety, adolescent, young adult
INTRODUCTION
Cannabidiol (CBD) is a cannabinoid compound derived from Cannabis plants (i.e., Cannabis sativa, Cannabis indica; Meissner & Cascella, 2022; Piomelli & Russo, 2016). CBD differs from the most well-known cannabinoid, Δ9-tetrahydrocannabinol (THC), in that it does not produce intoxicating effects (Meissner and Cascella, 2022). Although CBD has typically been described as non-psychoactive, it has also been suggested to improve symptoms of mental health disorders including depressive and anxiety disorders (Oberbarnscheidt & Miller, 2020). Research suggests that the primary reasons for which adults use CBD are to relieve stress (Wheeler et al., 2020) and psychological distress, such as symptoms of depression and anxiety disorders (Corroon & Phillips, 2018; Fedorova et al., 2021; Goodman et al., 2022).
Indeed, CBD is often marketed as a stress-reliever and a product that relieves psychological distress; however, the Food and Drug Administration (FDA) has publicly opposed the marketing of CBD for the treatment of psychological problems. In particular, the FDA has not classified CBD as a dietary supplement, precluding vendors from making claims regarding CBD’s effectiveness for treating any medical concern (Food and Drug Administration, 2021; Mead, 2017). In response to CBD product advertising, the FDA has issued numerous warning letters to companies who claim their CBD product is effective for treating medical concerns (Food and Drug Administration, 2022). In addition to releasing the names of these companies publicly, the FDA has conducted research on several CBD products and identified that advertised concentrations of CBD are rarely accurate (Food and Drug Administration, 2022). Similar inconsistencies between marketed and actual dosages of CBD products have been identified in recent studies (Bonn-Miller et al., 2017; Miller et al., 2022). Thus, CBD is widely available for purchase, but there are concerning dosage inconsistencies and unsupported marketing claims regarding relief of psychological symptoms. Adolescents and young adults may be particularly at-risk for initiating CBD product use to alleviate depressive and anxiety disorder symptoms given high rates of these disorders in adolescence and young adulthood, and due to the elevated risk for substance use initiation or misuse during adolescence and young adulthood (Substance Abuse and Mental Health Services Administration, 2014, 2019). These populations, as well as clinicians, may be unaware of research on CBD’s actual efficacy in treating depressive and anxiety disorders.
Previous systematic reviews (e.g., Black et al., 2019; Khan et al., 2020; Sarris et al., 2020; Stanciu et al., 2021) have evaluated the evidence for CBD’s effects on psychiatric disorders, with inconsistent conclusions. These reviews have primarily examined clinical research and have either excluded adolescents or summarized findings across all age groups. Thus, the present systematic review evaluates both preclinical and clinical studies, with N > 1, in which the efficacy of CBD to reduce symptoms (or behaviors, preclinically) of depressive and anxiety disorders in adolescents and young adults was investigated. The acute (i.e., immediate or a few hours following drug administration) and non-acute (i.e., several hours, days, or weeks after use) effects of CBD are considered separately. This evidence is evaluated in the context of adolescent and young adult brain development, with consideration of the mechanisms of action by which CBD may exert effects on depression and anxiety symptoms. Implications for clinical practice and research based on this review’s conclusions and in light of possible mechanistic and neurodevelopmental effects of CBD are discussed.
Adolescent and Young Adult Depression, Anxiety, and CBD Use
Depressive and anxiety disorders are common in adolescence and young adulthood. In 2020, it was estimated that around 17% of adolescents and 17% of young adults experienced a major depressive episode (U.S. Department of Health and Human Services, 2020). Lifetime prevalence of anxiety disorders is high among adolescents, ranging from 2.2% for generalized anxiety disorder to 20.0% for specific phobia (Kessler et al., 2012). Among young adults, around 15% reported past-month anxiety symptoms in 2018 (Goodwin et al., 2020). Existing treatments for adolescent and young adult depression and anxiety disorders include psychotherapy (e.g., cognitive behavioral therapy) and pharmacotherapy (e.g., selective-serotonin reuptake inhibitors or SSRIs; APA, 2022; Siegel & Dickstein, 2012).
Adolescents and young adults may look to CBD products to alleviate symptoms of depression and anxiety disorders given that CBD is marketed to relieve psychological distress. However, it is difficult to determine how many adolescents and young adults are using CBD products and why they are using these products. To date, there is very limited data on rates of adolescent CBD use. In the International Cannabis Policy Study in 2019, 42% of adults surveyed in the US and Canada reported past year CBD use (Goodman et al., 2022; Hammond et al., 2020). An additional study that surveyed young adults in the US found that approximately half of participants reported lifetime CBD use (Wheeler et al., 2020). Thus, it appears that a significant number of young adults use CBD, although further research is needed to clarify the prevalence of adolescent CBD use and the reasons for use.
Pharmacokinetics and Neurobiological Effects of CBD
Pharmacokinetics of CBD
Despite the popularity of CBD for both FDA-approved and non-approved purposes, only preliminary evidence exists on the pharmacokinetics of CBD in humans. A recent systematic review including 24 pharmacokinetic parameter studies reported that CBD half-life in humans is between 1.4 and 10.9 hours after oromucosal spray administration and 2-5 days after oral administration. This CBD half-life decreases to 24 hours after an intravenous administration and to 31 hours after smoking CBD. The area-under-the-curve and Cmax follow a dose-dependent increase and can be reached more quickly from smoking or inhalation of CBD compared to oral or oromucosal administration routes (Millar et al., 2018). More studies on the bioavailability and half-life of CBD would be beneficial in the field given its growing popularity, especially for the treatment of psychological concerns.
Neurobiological Action of CBD
Endocannabinoids and their putative endocannabinoid or endocannabinoid-like receptors are widely distributed in the rodent and human brain. They reach significantly higher expression in neurocircuitry that encompass neuronal networks involved in the regulation of affective behavior including the hippocampus, the cortex, and subregions of the medial prefrontal cortex, basal ganglia, and amygdala (Mackie, 2005).
The endocannabinoid system is characterized by the cannabinoid receptors 1 (CB1) and 2 (CB2), endogenously-produced cannabinoids, and several other endocannabinoid receptors and channels. These receptors include nuclear receptors, g protein-coupled receptors (GPCRs) and ion channel receptors that are the target of cannabinoids including phytocannabinoids, endocannabinoids, and several synthetic cannabinoid ligands. The first and most well-studied endocannabinoids to be discovered so far are N-arachidonoyl-ethanolamine (AEA; anandamide) and 2-arachidonoylglycerol (2-AG). They potently act at CB1 and CB2. CB1 is ubiquitous and highly expressed in the central nervous system, and is also expressed in the peripheral nervous system and in a tissue-specific manner in several peripheral organs (Matsuda et al., 1990; Munro et al., 1993). CB1 is also highly expressed in the gastrointestinal tract where it also participates in the regulation of neurotransmitter and hormone secretion (Izzo & Sharkey, 2010). CB2 was initially identified in immune cells with moderate expression in other peripheral organ tissues, such as the cardiovascular system and liver (Howlett et al., 2002). Recent studies have found that CB2 is also expressed in the brain, although at much lower expression levels than CB1. In the brain, CB2 plays a role in drug addiction, nociception, and neuroinflammation (Dhopeshwarkar & Mackie, 2014).
CBD is thought to have several therapeutic actions potentially related to its action in the endocannabinoid system. CBD acts as a partial CB2 agonist and shows a low affinity for the orthosteric site at CB1 (McPartland et al., 2007). While CBD may act as a CB1 antagonist (Thomas et al., 2007), studies have also observed that its action at CB1 is consistent with a negative allosteric modulation of the action of THC and 2-AG agonist profiles (Laprairie et al., 2015). While the GPCRs are among the primary targets of endocannabinoids and phytocannabinoids, several other receptors are also involved in the actions of these molecules. For example, CBD may also act at non-classical cannabinoid receptors including peroxisome proliferator activated receptor (PPAR)-α, β, and γ. These are several isoforms of nuclear hormone receptors and epigenetically modifiable transcription factors that are targeted by several cannabinoids (Matrisciano & Pinna, 2021). Studies using selective antagonists and knockout animal models showed that CBD action appears to be mainly mediated by activation of PPAR-γ (O’Sullivan, 2016). Studies have noted that CBD may protect against β-amyloid-induced neurotoxicity and inflammation through a PPAR-γ mediated mechanism (Esposito et al., 2011; Scuderi et al., 2014). While other endocannabinoids and phytocannabinoids appear to activate PPAR-α, and this isoform is the main target for the endocannabinoid-like N-palmitoyl ethanolamide (PEA) to induce analgesic, anti-inflammatory actions, and improve anxiety and depression, CBD action appears to be mainly mediated by activation of PPAR-γ. Through this activity, CBD has been reported to exert several pharmacological actions including improvement of anxiety symptoms (Locci & Pinna, 2019).
CBD and Neurodevelopment
Adolescence is an important period for neurodevelopment, during which synaptic pruning of gray-matter density and significant increases in white matter density occurs. Indeed, white matter neurodevelopment continues through adolescence and young adulthood (Tsuchida et al., 2021). The endocannabinoid system plays an important role in neuromaturation and synaptic pruning (Viveros et al., 2012). Accumulating evidence suggests that endocannabinoid signaling at CB1 receptors may also regulate synapse formation and maintenance of neuronal connectivity, especially during neurodevelopment (Berghuis et al., 2007; Simone et al., 2022). Given the extensive and complex neurodevelopment that takes place during adolescence, the adolescent brain may be particularly sensitive to disruptions in endocannabinoid signaling. Indeed, several studies have documented that changes in endocannabinoid signaling alter neurodevelopment and behavior (Albaugh et al., 2021; Dow-Edwards & Silva, 2017; Meyer et al., 2018; Miller et al., 2019; Rubino & Parolaro, 2016). To date, it is not clear how phytocannabinoids like CBD, that may not act directly at CB1 receptors, can impact endocannabinoid signaling and related neurodevelopmental processes. More research is needed to better understand the potential impact of CBD use during adolescence on neurodevelopment.
METHODS
PubMed was searched for all articles published through June 2022 using combinations of keywords “cannabidiol,” “CBD,” “adolescent,” “adolescence,” “young adult,” “anxiety,” “phobia,” “social phobia,” “agoraphobia,” “social anxiety disorder,” “depression,” “depressive,” “antidepressant,” and “anxiolytic”. Duplicate articles were removed, and the remaining articles’ titles and abstracts were reviewed for eligibility by one reviewer working independently. Full articles were reviewed when eligibility was not clear from the abstract. Eligible studies included those with N > 1 in which CBD was administered and depressive/anxiety disorder symptoms (clinical) or behaviors (preclinical) were an outcome. All Diagnostic and Statistical Manual, Fifth Edition, Text Revision (DSM-5-TR) depressive disorders and anxiety disorders were eligible (American Psychiatric Association, 2022). Clinical samples with other DSM-5-TR diagnoses were permitted only when these diagnoses were comorbid with one or more depressive or anxiety disorder. Study samples were required to be adolescents or young adults (approximately prenatal days 23-60 in rodent models or age 12-33 in humans). Articles were excluded if CBD was never administered in isolation (i.e., without THC or another substance), if participants were outside the specified age range, if protocols without results were described, if the article had an N of 1 (i.e., case study), if the clinical sample of interest did not have depressive or anxiety disorders, or if depressive/anxiety disorder symptoms, diagnoses, or behaviors were not examined as an outcome.
After eligible articles were identified, one reviewer examined all full articles independently. Relevant outcomes included measures of depression-like and anxiety-like behavior in preclinical models and questionnaire or clinician-administered measures of depressive and anxiety disorders in clinical studies. Both acute (i.e., immediate or a few hours following drug administration) and non-acute (i.e., several hours, days, or weeks after use) timepoints for outcome measurement were included in the systematic review. Studies were grouped for qualitative synthesis based on participant characteristics and time course of outcomes measured: preclinical and clinical research was synthesized separately, depressive disorders and anxiety disorders were synthesized separately, and acute and non-acute effects were synthesized separately. The Oxford Centre for Evidence-Based Medicine (OCEBM) 2011 Levels of Evidence Treatment Benefits item was used to rate the quality of evidence for each study; Level 1 represents the strongest category of evidence, and Level 5 represents the lowest quality of evidence (OCEBM Levels of Evidence Working Group, 2011). Given the low number of studies identified (6 preclinical and 4 clinical), a quantitative synthesis or meta-analysis was not appropriate. When available, effect sizes or means are reported in Tables 1, 2, and 3.
Table 1.
Characteristics and results of preclinical adolescent depression studies included in the current review
| Author | Sample | Total N | Paradigm(s) | CBD dose | Acute results | Non-acute results |
|---|---|---|---|---|---|---|
|
| ||||||
| Bis-Humbert et al., 2020 | Adolescent male rats | 44 | FST, sucrose preference | 3, 10, and 30 mg/kg | N/A | Repeated administration of 10 mg/kg CBD reduced depression-like behaviors in FST short-term (mean percent change vs. control = −25, SEM = 9), but not long-term. No effect of CBD on sucrose preference. |
| Bis-Humbert et al., 2021 | Adolescent male rats with maternal deprivation | 25 | FST, NSF, sucrose preference | 10 mg/kg | Single dose of CBD had no effect on FST; repeated administration of CBD reduced depression-like behaviors in FST (mean reduction in immobility vs. control = 47 s). | Repeated administration of CBD had no effect on depression-like behaviors in FST or sucrose preference, but reduced depression-like behaviors in NSF (mean increase in exploratory time vs. control = 27 s). |
| Ledesma-Corvi et al., 2022 | Adolescent male and female rats, maternal deprivation and control groups | 64-72 (exact N is unknown) | FST, NSF, sucrose preference | 10 mg/kg | Single dose of CBD had no effect on MD males or females in FST but reduced depression-like behaviors in FST for control males (mean/effect size not available). | Repeated administration of CBD reduced depression-like behaviors in FST for control and MD males (mean/effect size not available); no effect for females. No effect of CBD on NSF or sucrose preference. |
CBD: cannabidiol, FST: forced swim test, MD: maternal deprivation, NSF: novelty-suppressed feeding test.
Table 2.
Characteristics and results of preclinical adolescent and young adult anxiety studies included in the current review
| Author | Sample | Total N | Paradigm(s) | CBD dose | Acute results | Non-acute results |
|---|---|---|---|---|---|---|
|
| ||||||
| Bis-Humbert et al., 2020 | Adolescent male rats | 44 | Open field test | 3, 10, and 30 mg/kg | N/A | No effect of repeated CBD administration on anxiety-like behaviors. |
| Kaplan et al., 2021 | Adolescent male and female rats | 26 | Open field test, EPM | 5, 10, and 20 mg/kg | N/A | No effect of repeated CBD administration in adolescence on anxiety-like behaviors in early adulthood. |
| Kasten et al., 2019 | Adolescent and adult male and female mice | 60-84 (total N is unknown) | Open field test, EPM | 20 mg/kg | No effect of CBD on anxiety-like behaviors in EPM or open field. | No effect of repeated CBD administration in adolescence on anxiety-like behaviors in adulthood. |
| Murphy et al., 2017 | Adolescent and young adult male mice | 31 | Open field test, EPM | 3 mg/kg | N/A | No effect of repeated CBD administration in adolescence on anxiety-like behaviors in adolescence or early adulthood. |
CBD: cannabidiol, EPM: elevated plus maze.
Table 3.
Characteristics and results of clinical adolescent and young adult anxiety studies included in the current review
| Author | Sample | Total N | Anxiety diagnosis | Anxiety outcome measures | CBD dose | Results |
|---|---|---|---|---|---|---|
|
| ||||||
| Bergamaschi et al., 2011 | Male and female young adults | 36 | SAD | VAMS, SPSS, BSS | 600 mg single dose | CBD reduced anxiety during public speaking compared to placebo in those with SAD (means/effect size not available). |
| Berger et al., 2022 | Male, female, and non-binary adolescents and young adults | 30 | DSM-5 anxiety disorders | OASIS, HARS | Titrated up to 800 mg/day over 12 weeks | CBD reduced anxiety symptoms compared to baseline (mean reduction in OASIS scores = 42.6%, Cohen d = −1.07; mean reduction in HARS scores = 50.2%, Cohen d = −1.00). |
| Crippa et al., 2011 | Male young adults | 10 | SAD | VAMS | 400 mg single dose | CBD reduced anxiety symptoms (VAMS anxiety factor) compared to placebo at 60 (CBD M = 36.9, PBO M = 45.2), 75 (CBD M = 34.5, PBO M = 42.1), and 140 (CBD M = 30.8, PBO M = 42.1) minutes post-administration. |
| Masataka, 2019 | Male and female young adults | 37 | SAD with comorbid avoidant personality disorder | FNE, LSAS | 300 mg daily for 4 weeks | CBD reduced social anxiety symptoms over placebo and compared to baseline (FNE interaction ηp2 = 0.561; LSAS interaction ηp2 = 0.528). |
CBD: cannabidiol, BSS: Bodily Symptoms Scale, DSM-5: Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition, FNE: Fear of Negative Evaluation Questionnaire, HARS: Hamilton Anxiety Rating Scale, LSAS: Liebowitz Social Anxiety Scale, OASIS: Overall Anxiety Severity and Impairment Scale, SAD: Social Anxiety Disorder, SPSS-N: Self-Statements during Public Speaking Scale Negative Self-Evaluation Subscale, VAMS: Visual Analog Mood Scale.
RESULTS
Study Selection
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 checklists were used to structure the systematic review (see Figure 1 for PRISMA flow diagram of study inclusion) (Page et al., 2021). Using PubMed, 224 articles were initially identified. 139 articles were duplicates and were removed prior to screening. Then, 85 articles were screened and assessed for eligibility. Of those, 75 were ineligible: 43 articles did not describe studies in which CBD was directly administered (e.g., the articles described survey studies, retrospective chart reviews, systematic or narrative reviews, etc.); 7 articles were excluded because CBD’s effects were not studied in isolation from other substances (e.g., THC or other substances were also administered); 15 included participants or subjects outside of the defined age range; 2 articles described protocols for which results had not yet been published; 1 reported on a clinical sample that did not have depressive or anxiety disorders; and 1 was excluded due to depressive/anxiety disorder symptoms or behaviors not being measured outcomes (see Figure 1).
Figure 1.
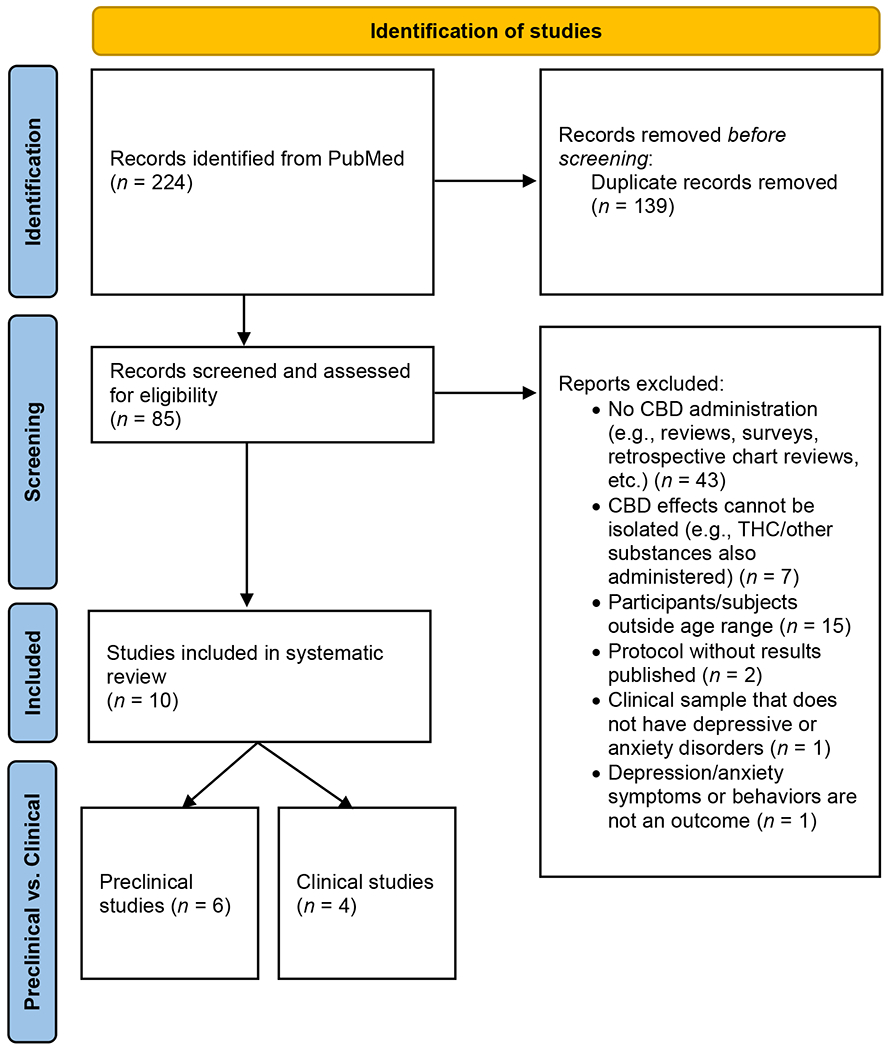
PRISMA 2020 flow diagram of studies identified for inclusion in the systematic review.
After screening and exclusion, 10 studies were included in the systematic review. Of these, 6 reported on preclinical research and 4 described clinical studies. Of the preclinical articles, one measured both depression- and anxiety-like behaviors, 2 measured only depression-like behaviors, and 3 measured only anxiety-like behaviors. All 4 clinical studies examined anxiety disorder symptoms; no clinical studies measuring depressive symptoms were identified.
Effect of CBD on Depression
Preclinical Research
Three preclinical studies were identified that evaluated the effects of CBD on depression-like behaviors in adolescence and young adulthood (Table 1) (Bis-Humbert et al., 2020; Bis-Humbert et al., 2021; Ledesma-Corvi et al., 2022). Two studies evaluated both the acute and non-acute effects of CBD on depression-like behaviors (Bis-Humbert et al., 2021; Ledesma-Corvi et al., 2022) and one evaluated only the non-acute effects of CBD (Bis-Humbert et al., 2020). Depression-like behaviors were measured using the forced swim test (FST) and sucrose preference in all studies (Bis-Humbert et al., 2020; Bis-Humbert et al., 2021; Ledesma-Corvi et al., 2022). Two studies additionally evaluated depression-like behaviors using the novelty-suppressed feeding test (NSF), and included conditions of maternal deprivation as a model of early life stress (Bis-Humbert et al., 2021; Ledesma-Corvi et al., 2022). Bis-Humbert et al. (2020) tested 3, 10, and 30 mg/kg doses of CBD (≥ 98% pure). Bis-Humbert et al. (2021) and Ledesma-Corvi et al. (2022) both tested 10 mg/kg doses of CBD (purities unknown). All studies represent Level 3 evidence by OCEBM standards (OCEBM Levels of Evidence Working Group, 2011).
Results from the FST across studies are mixed, and suggest that both acute and non-acute CBD administration may have differential effects on depression-like behaviors depending on sex, early life stress, and duration of CBD administration. Acute CBD administration had no effect on depression-like behaviors in female rodents, regardless of exposure to early life stress (Ledesma-Corvi et al., 2022). In male rodents without early life stress, a single dose of CBD (10 mg/kg) appears to acutely reduce depression-like behaviors (Ledesma-Corvi et al., 2022). Conversely, results for male rodents exposed to early life stress are mixed. For example, a single dose of 10 mg/kg CBD did not acutely reduce depression-like behaviors in rodents exposed to early life stress (i.e., maternal deprivation; Bis-Humbert et al., 2021; Ledesma-Corvi et al., 2022). However, rodents exposed to early life stress showed reduced depression-like behaviors when measured acutely following four days of repeated administration of CBD (10 mg/kg; Bis-Humbert et al., 2021). Thus, repeated administration of CBD may be necessary to achieve acute reductions in depression-like behavior among male rodents exposed to early life stress.
Findings regarding the non-acute effects of CBD on depression-like behaviors as measured by the FST are also mixed. CBD had no non-acute effect on depression-like behaviors in females, regardless of early life stress (Ledesma-Corvi et al., 2022). In male rodents without early life stress, repeated administration of 10 mg/kg CBD for 7 days reduced depression-like behaviors when measured 1-2 days after administration finished (Bis-Humbert et al., 2020; Ledesma-Corvi et al., 2022). However, when measured 14 days after CBD administration ceased, there was no effect on depression-like behaviors (Bis-Humbert et al., 2020). In male rodents exposed to early life stress, findings are mixed. One study found reductions in depression-like behaviors one day after completing 7 days of CBD repeated administration (Ledesma-Corvi et al., 2022) whereas another found no effect on depression-like behaviors (C. Bis-Humbert et al., 2021).
In addition to the FST, several other preclinical paradigms have examined the non-acute effects of CBD on depression-like behaviors. Three studies found no effect of CBD on sucrose intake, a corollary of anhedonic behavior (Bis-Humbert et al., 2020; Bis-Humbert et al., 2021; Ledesma-Corvi et al., 2022). Studies using the NSF to measure depression-like behaviors were inconsistent; one study found no effect (Ledesma-Corvi et al., 2022) and another reported that CBD reduced depression-like behaviors as measured by increased exploratory time (Bis-Humbert et al., 2021). Thus, the effect of CBD on depression-like behaviors also appears to differ based on the experimental paradigm used.
Clinical Research
No clinical studies that met criteria for the systematic review were identified.
Qualitative Synthesis
Few preclinical studies were identified that examined the effects of CBD on adolescent/young adult depression, and to our knowledge no clinical studies have investigated this topic. The limited preclinical evidence suggests that in male adolescent rodents who have not experienced early life stress, a single dose of CBD may have acute antidepressant effects and repeated administration of CBD may have non-acute antidepressant effects (Bis-Humbert et al., 2020; Ledesma-Corvi et al., 2022). Findings are inconsistent regarding the non-acute effects of CBD on male rodents with early life stress exposure (Bis-Humbert et al., 2021; Ledesma-Corvi et al., 2022). CBD does not appear to impact depressive-like behaviors in adolescent female rats (Ledesma-Corvi et al., 2022). Further research is necessary to clarify these preclinical findings, and to extend preclinical evidence to clinical populations.
Effect of CBD on Anxiety Disorders
Preclinical Research
Four preclinical studies were identified for inclusion in the systematic review (Bis-Humbert et al., 2020; Kaplan et al., 2021; Kasten et al., 2019; Murphy et al., 2017). All four studies used the open field test to measure anxiety-like behaviors; additionally, three articles (Kaplan et al., 2021; Kasten et al., 2019; Murphy et al., 2017) reported on use of the elevated plus maze (EPM) to measure anxiety-like behaviors. All studies examined non-acute effects of CBD, and one (Kasten et al., 2019) additionally investigated acute CBD effects. Bis-Humbert et al. (2020) used ≥ 98% pure CBD, and Kaplan et al. (2021) used > 98% pure CBD; purities were not specified by Kasten et al. (2019) or Murphy et al. (2017). Overall, these studies suggest that adolescent CBD administration has no anxiolytic effects in rodents, as measured in the EPM and the open field test (Table 2; Bis-Humbert et al., 2020; Kaplan et al., 2021; Kasten et al., 2019; Murphy et al., 2017). These studies find no anxiolytic effects of CBD for males or females, for acute or repeated CBD administration, or for anxiety-like behaviors that are measured in adolescence and in adulthood following adolescent CBD exposure (Kaplan et al., 2021; Kasten et al., 2019; Murphy et al., 2017). The majority of these studies represent Level 3 OCEBM evidence for treatment benefits (Bis-Humbert et al., 2020; Kaplan et al., 2021; Murphy et al., 2017) and one presents Level 4 evidence (Kasten et al., 2019).
Clinical Research
Four clinical studies were identified that examined the effect of CBD in treating anxiety disorders among adolescents and young adults (Table 3). Three studies enrolled only young adults with diagnoses of social anxiety disorder (SAD; Bergamaschi et al., 2011; Crippa et al., 2011; Masataka, 2019). The participants in Masataka’s (2019) study additionally had comorbid diagnoses of avoidant personality disorder. One study enrolled both young adults and adolescents who met criteria for any DSM-5 anxiety disorder (Berger et al., 2022). CBD dosages ranged from 300-800 mg, and CBD was administered in either a single dose (Bergamaschi et al., 2011; Crippa et al., 2011) or daily for 4-12 weeks (Berger et al., 2022; Masataka, 2019).
Taken together, findings from these studies suggest that CBD administration may acutely and non-acutely reduce anxiety symptoms in adolescents and young adults, particularly in those with SAD. For example, Crippa et al. (2011) evaluated the acute effect of one dose of CBD (400 mg, approx. 99.9% pure) or placebo in young adults with SAD. Acutely, CBD significantly reduced subjective ratings of anxiety compared to placebo (Level 3 evidence). Additionally, Bergamaschi et al. (2011) examined the acute effect of one dose of CBD (600 mg, approx. 99.9% pure) or placebo administration on anxiety symptoms in young adults with SAD. Those who received CBD reported significantly lower anxiety symptoms during a public speaking paradigm, suggesting that CBD may acutely exert anxiolytic effects in anxiety-inducing situations (Level 3 evidence).
To evaluate the non-acute effects of CBD after repeated administration, Masataka (2019) examined the efficacy of CBD in treating social anxiety symptoms in adolescents and young adults with SAD and avoidant personality disorder. Participants received 300 mg CBD (>99.9% pure) or placebo daily for 4 weeks. Posttreatment, participants who received CBD reported significantly lower symptoms of social anxiety compared to their pretreatment symptoms and compared to the placebo group posttreatment (Level 2 evidence). Preliminary work has also evaluated the non-acute effects of CBD in individuals with anxiety disorders broadly (i.e., including but not limited to SAD). Berger et al. (2022) examined the efficacy of repeated CBD (up to 800 mg/day, >99.9% pure) for 12 weeks on anxiety symptoms among adolescents and young adults diagnosed with a DSM-5 anxiety disorder who had not seen symptom improvement with typical treatment (i.e., CBT, medication). Anxiety symptoms were significantly reduced following CBD treatment (Level 3 evidence). However, the majority of participants reported mild or moderate adverse events (e.g., low mood, fatigue, hot flushes, cold chills). Of note, some of these participants were also taking antidepressant medications. Those who were taking antidepressant medications were more likely to experience an adverse event; thus, some adverse events may have been related to interactions of CBD and antidepressant medication(s) (see Pharmacokinetic Interactions with Pharmacotherapy section).
Qualitative Synthesis
Preliminary clinical evidence suggests that acute and non-acute CBD has beneficial effects on anxiety symptoms in adolescents and young adults with SAD (Bergamaschi et al., 2011; Crippa et al., 2011; Masataka 2019) and other anxiety disorders (Berger et al., 2022), with Level 2-3 evidence. On the contrary, preclinical studies have found no anxiolytic effects of CBD in adolescent and young adult animals with Level 3-4 evidence (Kaplan et al., 2021; Kasten et al., 2019; Murphy et al., 2017). There are several possible explanations for the discrepancies between preclinical and clinical studies of adolescent CBD and anxiety disorders. It is possible that the beneficial effects of CBD observed by Bergamaschi et al. (2011), Crippa et al. (2011), and Masataka (2019) are specific to SAD, which may not be modelled by the elevated plus maze and open field test. Biological differences between humans and rodents may also explain the anxiolytic effects of CBD in clinical, but not preclinical, studies. Further, most of the samples in clinical studies of CBD and anxiety disorders consisted of older adolescents and young adults (Bergamaschi et al., 2011; Crippa et al., 2011; Masataka 2019). It is possible that the anxiolytic effects of CBD observed in these studies are specific to a certain developmental stage. However, this does not explain the anxiolytic effect of CBD observed by Berger et al. (2022) in a broader sample of adolescents and young adults. Further research is needed to determine the effects of CBD on anxiety disorders across developmental stages and to expand these findings in anxiety disorders other than SAD.
DISCUSSION
Few studies were identified that met the criteria for this systematic review of preclinical and clinical effects of CBD on depressive and anxiety disorders and behaviors among adolescents and young adults. Overall, preclinical evidence suggests that CBD may differentially impact depression-like behaviors depending on sex, exposure to early life stress, duration of administration, and acute vs. non-acute effects. Preliminary evidence suggests that depressive behaviors in male rodents may be acutely reduced by a single dose of CBD for rodents with no early life stress and by repeated administration of CBD for those exposed to early life stress (Bis-Humbert et al., 2021; Ledesma-Corvi et al., 2022). Further, repeated administration of CBD may non-acutely reduce depressive-like behaviors in the short-term among rodents not exposed to early life stress (Bis-Humbert et al., 2020). Findings regarding the non-acute effects of CBD in rodents exposed to early life stress are mixed (Bis-Humbert et al., 2021; Ledesma-Corvi et al., 2022). The existing evidence suggests that CBD may not impact depressive-like behaviors in female rodents (Ledesma-Corvi et al., 2022). However, given the low number of existing studies, further research is necessary to replicate and clarify all of these findings.
Further, no clinical studies of CBD administration in adolescents or young adults with depressive disorders were identified for this systematic review. Several case reports have indicated CBD may reduce depression symptoms in adolescents and young adults (Hegazy & Platnick, 2019; Laczkovics et al., 2021; Mansell et al., 2022). However, these studies are limited by very small sample sizes (e.g., N=1), psychiatric and medical comorbidities, and concurrent medication and THC use. Thus, further experimental clinical research is crucial to elucidate the role of CBD in depression among adolescents and young adults.
With regard to anxiety disorders, preclinical and clinical findings are inconsistent. Whereas the preclinical research identified in this review demonstrated no anxiolytic effects of CBD in adolescent and young adult rodents (Bis-Humbert et al., 2020; Kaplan et al., 2021; Kasten et al., 2019; Murphy et al., 2017), clinical studies show some (i.e., Levels 2-3) evidence for acute and long-term anxiolytic effects of CBD (Bergamaschi et al., 2011; Berger et al., 2022; Crippa et al., 2011; Masataka, 2019). These effects have been primarily studied in young adults with SAD (Bergamaschi et al., 2011; Crippa et al., 2011; Masataka, 2019). Berger et al. (2022) was the only identified study that evaluated CBD’s effects in adolescents and young adults with a range of anxiety disorders. Several case studies have also reported on the use of CBD in adolescent and young adult patients with anxiety symptoms (Hegazy & Platnick, 2019; Mansell et al., 2022) and specific phobia, social phobia, and generalized anxiety disorder (Klier et al., 2020; Laczkovics et al., 2021; Mansell et al., 2022; Ponton et al., 2020). These findings further suggest that CBD may reduce anxiety disorder symptoms in some adolescents and young adults, although these case studies are also limited by sample size, psychiatric and medical comorbidities, concurrent psychotherapy/counseling, and concurrent use of psychoactive medications and THC products. The small number of well-controlled studies, as well as differences between methodology of the existing controlled studies (e.g., sample age and diagnoses, measures used to assess anxiety symptoms, varying CBD dosing schedules) makes it difficult to draw conclusions about CBD’s effects on anxiety disorders in adolescents and young adults at this time.
At present, it is unclear how CBD may exert its effects on depressive and anxiety disorders. Existing research on CBD’s mechanism of action in depressive disorders has aimed to evaluate neurological pathways and processes that are disrupted in clinical depression using preclinical models. However, the majority of this research has been conducted with adult rodents. Given differences in the preclinical effects of CBD on depression between adolescents and adults (e.g., Bis-Humbert et al., 2020), these findings may not generalize to adolescents and young adults with depression. Preliminary findings suggest that CBD may decrease depression-like behaviors preclinically by activating serotonin receptors, increasing brain-derived neurotropic factor (BDNF) production, and/or reducing neuroinflammation (Bis-Humbert et al., 2021; Florensa-Zanuy et al., 2021; Melas et al., 2021; Silote et al., 2019). However, further research is needed to extend these findings to adolescents and young adults, both clinically and preclinically.
Similarly, most preclinical and clinical studies of CBD’s mechanism of action in anxiety disorders have been conducted in adults. Preclinical evidence suggests that CBD may reduce anxiety-like behaviors through activation of the 5-HT1A receptor (Melas et al., 2021) or via hippocampal neurogenesis, although these findings are mixed (Bis-Humbert et al., 2020; Melas et al., 2021). CBD may also reduce anxiety associated with phobias by improving extinction of fear conditioning, although the biological mechanisms underlying this process are not well understood (Jurkus et al., 2016). A functional neuroimaging study among young adults with social anxiety disorder (SAD) suggested that CBD’s anxiolytic effects may be due to its action in limbic and paralimbic regions of the brain (Crippa et al., 2011). Taken together, these findings suggest multiple neurological pathways that may underlie the effect of CBD on anxiety disorders. It is possible that CBD may exert different actions in different anxiety disorders (e.g., generalized anxiety disorder vs. specific phobia). Further preclinical and clinical research is needed to determine the extent to which many of these findings generalize to adolescents and young adults, and to explore discrepancies between preclinical and clinical studies of CBD’s effects on anxiety disorders in adolescents and young adults.
Implications for Research and Clinical Practice
Further preclinical and clinical research is needed to clarify the role of CBD in adolescent and young adult depressive and anxiety disorders. The small amount of existing preclinical research on the effects of CBD on depressive-like behaviors in adolescent and young adult animals is mostly inconsistent and varies based on sex, early life stress, duration of CBD use, and experimental paradigm (Bis-Humbert et al., 2020; Bis-Humbert et al., 2021; Ledesma-Corvi et al., 2022). Clinical research on CBD use in adolescent and young adult depression is also lacking. Further research is necessary to clarify the acute and non-acute effects of CBD on depressive-like behaviors among adolescents and young adults, both preclinically and clinically, and to evaluate possible sex differences in CBD’s effects among this population.
Similarly, more preclinical and clinical research is necessary to clarify the role of CBD in adolescent and young adult anxiety disorders. Currently, there are notable discrepancies between preclinical and clinical research on the anxiolytic effects of CBD in adolescence and young adulthood. Thus, future preclinical research may aim to model more types of anxiety disorders and to further elucidate the neurobiological mechanisms of action underlying any observed effects. Preliminary clinical research suggests that CBD is beneficial in reducing symptoms of anxiety disorders, particularly SAD (Bergamaschi et al., 2011; Berger et al., 2022; Crippa et al., 2011; Masataka, 2019). However, more research is needed to replicate these findings in larger samples. Additionally, further research is needed to evaluate the effectiveness of CBD in comparison to (and in conjunction with) existing evidence-based treatments for anxiety disorders, such as pharmacotherapy and cognitive behavioral therapy, among adolescents and young adults. Both preclinical and clinical research on possible sex/gender differences in CBD’s effects among adolescents and young adults is also needed. Thus, although the preliminary evidence for CBD in the treatment of anxiety disorders is promising, additional research is needed to expand these findings and account for discrepancies with preclinical research.
Additionally, there is a notable lack of research on the long-term effects of CBD use in adolescence and young adulthood. The long-term effects of CBD are particularly important to understand given that adolescent cannabis use has been linked to an increased risk for onset of depression and higher depression symptoms (Botsford et al., 2020; Medina et al., 2007; Schoeler et al., 2018). Similarly, cannabis use has been prospectively associated with anxiety symptoms (Kedzior & Laeber, 2014) and increased prevalence of SAD (National Academies of Sciences, 2017). It is unclear what components of cannabis (e.g., CBD, THC) account for these relationships. Further, commercial CBD products may contain THC (Dubrow et al., 2021). Thus, it is unknown if adolescent and young adult CBD use could actually worsen symptoms of anxiety disorders and depression over time.
Furthermore, preliminary evidence suggests that it may be harmful to combine CBD with pharmacotherapy for depression and anxiety disorders. Selective serotonin reuptake inhibitors (SSRIs) are considered first-line pharmacological treatments for depression and anxiety disorders (Dobson et al., 2019; Locher et al., 2017). CBD inhibits some CYP450 enzymes, which metabolize SSRIs including sertraline, fluoxetine, citalopram, escitalopram, and mirtazapine (Anderson et al., 2021; Vaughn et al., 2021). Pharmacokinetic modelling suggests that CBD alters the clearance of these medications, potentially altering efficacy or increasing the risk of side effects. At a low dose of CBD, models estimated increased half-lives and plasma concentrations of sertraline and escitalopram (Vaugn et al., 2021). In vitro, CBD mildly inhibited metabolism of mirtazapine, sertraline, and fluoxetine and strongly inhibited metabolism of escitalopram and citalopram, suggesting that there is a risk of increased side effects or altered efficacy if CBD is used alongside these medications (Anderson et al., 2021). Additionally, trials in which CBD was administered to adolescents and young adults with anxiety disorders have found significant increases in plasma concentrations of SSRIs and higher likelihood of adverse events in those taking SSRIs (Anderson et al., 2021; Berger et al., 2022). These findings suggest that CBD products should not be used by adolescents and young adults receiving SSRIs. Those who do use CBD products while receiving pharmacotherapy should be closely monitored. Further clinical research, with larger samples, is needed to clarify the effect of CBD on SSRI pharmacokinetics.
At this time, there is not sufficient evidence to justify CBD as a treatment for depression or anxiety disorders in adolescence or young adulthood. The concerns regarding pharmacokinetic interactions between CBD and antidepressant/antianxiety medications suggest that CBD use should be discouraged among adolescents and young adults receiving pharmacotherapy for depression or anxiety disorders. Adolescents and young adults reporting CBD use to alleviate depressive or anxious symptoms may benefit from psychoeducation regarding the low evidence for this use. In sum, further research is needed to clarify inconsistent findings regarding CBD use for depression and to expand findings regarding CBD use for anxiety disorders in adolescents and young adults. Clinically, adolescents and young adults with depression and anxiety disorders should be cautioned against CBD use to treat these conditions.
Limitations
This systematic review is limited by several factors. The small number of studies identified prohibited quantitative synthesis across studies, which limited this paper’s ability to draw broad conclusions about CBD’s effects. While the review’s focus on adolescence and young adulthood was intentional with the goal of examining CBD use in a specific developmental stage, this also limited the number of studies that could be included in the present results.
Conclusions
Although CBD products may be marketed for the treatment of depression and anxiety disorders, very little research has examined the efficacy and safety of CBD for these disorders in adolescents and young adults. At this time, there is not sufficient evidence to support the use of CBD to treat depression or anxiety disorders in adolescents and young adults. Preclinical research suggests that acute CBD may reduce depression-like behaviors in male rats not exposed to early life stress (Ledesma-Corvi et al., 2022). However, findings regarding repeated and non-acute CBD administration and rats exposed to early life stress have been mixed (Bis-Humbert et al., 2020, 2021). Further, there is a lack of clinical research examining the use of CBD in adolescents and young adults with depression. With regard to anxiety disorders, preclinical and clinical findings are conflicting. The limited number of preclinical studies examining this topic have found no anxiolytic effects of CBD in adolescents and young adults (Bis-Humbert et al., 2020; Kaplan et al., 2021; Kasten et al., 2019; Murphy et al., 2017). On the contrary, preliminary clinical evidence suggests that CBD may reduce anxiety symptoms in adolescents and young adults with social anxiety disorder and other anxiety disorders (Bergamaschi et al., 2011; Berger et al., 2022; Crippa et al., 2011; Masataka, 2019). Overall, the preclinical effects of CBD on depression-like behaviors appear to differ based on sex, early life stress, and duration of CBD administration; additionally, there is some Level 2-3 evidence that CBD may reduce symptoms of anxiety disorders clinically.
In addition to the relative lack of research examining CBD’s antidepressant and anxiolytic effects in adolescents and young adults, several factors suggest that CBD use in this population may be harmful. Of note, the concentrations of cannabinoids in CBD products sold in the US may be inaccurate (Dubrow et al., 2021). Thus, individuals purchasing these products risk inadvertently ingesting more CBD than intended and/or THC, which may negatively impact depression and anxiety symptoms (Botsford et al., 2020; Medina et al., 2017; Schoeler et al., 2018; Kedzior & Laeber, 2014; National Academies of Sciences, 2017). Further, pharmacokinetic research suggests that concurrent use of CBD and antidepressant/antianxiety medication may lead to increased side effects or reduced efficacy of these medications (Anderson et al., 2021; Berger et al., 2022). Taken together with the low number of existing studies, this suggests that CBD use among adolescents and young adults with depression and anxiety disorders may be harmful.
Further preclinical and clinical research is necessary to elucidate the role of CBD in adolescent and young adult depression and anxiety disorders. Future research should further examine sex and gender differences, the role of early life stress, and differences in acute and non-acute administration with regard to the antidepressant effects of CBD. Additional research examining the effect of CBD in anxiety disorders other than social anxiety disorder is also necessary. Finally, more research is needed to understand the neurobiological mechanisms underlying any observed effects of CBD in these disorders. In sum, additional preclinical and clinical research is needed to determine the effects and mechanisms of action of CBD in adolescents and young adults with depression and anxiety disorders.
Funding:
RKD, NAC, and JB were supported by the National Institute on Drug Abuse (NIDA; K23DA048132, PI: NAC; F31DA057064, PI: JB). JB was also supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA: T32AA026577, PI: Pandey). GP was in part supported by NIAAA grant P50 AA022538 (Pinna, pilot project). Contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA or the National Institutes of Health. RD was supported by grant number T32CA057699 from the National Cancer Institute; contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
CONFLICT OF INTEREST
NAC, RKD, and JB have no conflicts of interest to report. GP is a paid consultant to PureTech Health (Boston, MA, USA), GABA Therapeutics, and NeuroTrauma Sciences (Alpharetta, GA, USA). He has two patent applications, one on N-palmitoylethanolamine (PEA) and peroxisome proliferator-activated receptor alpha (PPAR-α) agonists US20180369171A1, pending, and one on allopregnanolone analogs in the treatment of neuropsychiatric disorders (US11266663B2, granted on March 8, 2022).
LIST OF ABBREVIATIONS
- 2-AG
2-arachidonoylglycerol
- AEA
anandamide; N-arachidonoyl-ethanolamine
- CBD
Cannabidiol
- CB1
Cannabinoid receptor 1
- CB2
Cannabinoid receptor 2
- EPM
Elevated plus maze
- FDA
Food and Drug Administration
- FST
Forced swim test
- GPCR
g protein-coupled receptor
- PPAR
peroxisome proliferator activated receptor
- SAD
Social anxiety disorder
- THC
Δ9-tetrahydrocannabinol
REFERENCES
- Albaugh MD, Ottino-Gonzalez J, Sidwell A, Lepage C, Juliano A, Owens MM, … Garavan H (2021). Association of Cannabis Use During Adolescence With Neurodevelopment. JAMA Psychiatry, 78(9), 1–11. 10.1001/jamapsychiatry.2021.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LL, Doohan PT, Oldfield L, Kevin RC, Arnold JC, Berger M, … McGregor IS (2021). Citalopram and Cannabidiol: In Vitro and In Vivo Evidence of Pharmacokinetic Interactions Relevant to the Treatment of Anxiety Disorders in Young People. J Clin Psychopharmacol, 41(5), 525–533. 10.1097/jcp.0000000000001427 [DOI] [PubMed] [Google Scholar]
- American Psychological Association. (2022). Depression Treatments for Children and Adolescents. American Psychological Association. https://www.apa.org/depression-guideline/children-and-adolescents [Google Scholar]
- American Psychiatric Association. (2022). Diagnostic and statistical manual of mental disorders (5th ed., text rev.). 10.1176/appi.books.9780890425787 [DOI] [Google Scholar]
- Bergamaschi MM, Queiroz RH, Chagas MH, de Oliveira DC, De Martinis BS, Kapczinski F, … Crippa JA (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology, 36(6), 1219–1226. 10.1038/npp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, Li E, Rice S, Davey CG, Ratheesh A, Adams S, … Amminger GP (2022). Cannabidiol for Treatment-Resistant Anxiety Disorders in Young People: An Open-Label Trial. J Clin Psychiatry, 83(5). 10.4088/JCP.21m14130 [DOI] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urbán GM, … Harkany T (2007). Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science, 316(5828), 1212–1216. 10.1126/science.1137406 [DOI] [PubMed] [Google Scholar]
- Bis-Humbert C, García-Cabrerizo R, & García-Fuster MJ (2020). Decreased sensitivity in adolescent versus adult rats to the antidepressant-like effects of cannabidiol. Psychopharmacology (Berl), 237(6), 1621–1631. 10.1007/s00213-020-05481-4 [DOI] [PubMed] [Google Scholar]
- Bis-Humbert C, García-Cabrerizo R, & García-Fuster MJ (2021). Antidepressant-like effects of cannabidiol in a rat model of early-life stress with or without adolescent cocaine exposure. Pharmacological Reports, 73(4), 1195–1202. 10.1007/s43440-021-00285-5 [DOI] [PubMed] [Google Scholar]
- Bis-Humbert C, García-Cabrerizo R, & García-Fuster MJ (2021). Antidepressant-like effects of cannabidiol in a rat model of early-life stress with or without adolescent cocaine exposure. Pharmacol Rep, 73(4), 1195–1202. 10.1007/s43440-021-00285-5 [DOI] [PubMed] [Google Scholar]
- Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, … Degenhardt L (2019). Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry, 6(12), 995–1010. 10.1016/s2215-0366(19)30401-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, & Vandrey R (2017). Labeling Accuracy of Cannabidiol Extracts Sold Online. JAMA, 318(17), 1708–1709. 10.1001/jama.2017.11909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botsford SL, Yang S, & George TP (2020). Cannabis and Cannabinoids in Mood and Anxiety Disorders: Impact on Illness Onset and Course, and Assessment of Therapeutic Potential. Am J Addict, 29(1), 9–26. 10.1111/ajad.12963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corroon J, & Phillips JA (2018). A Cross-Sectional Study of Cannabidiol Users. Cannabis Cannabinoid Res, 3(1), 152–161. 10.1089/can.2018.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, … Hallak JE (2011). Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol, 25(1), 121–130. 10.1177/0269881110379283 [DOI] [PubMed] [Google Scholar]
- Dhopeshwarkar A, & Mackie K (2014). CB2 Cannabinoid receptors as a therapeutic target-what does the future hold? Mol Pharmacol, 86(4), 430–437. 10.1124/mol.114.094649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson ET, Bloch MH, & Strawn JR (2019). Efficacy and Tolerability of Pharmacotherapy for Pediatric Anxiety Disorders: A Network Meta-Analysis. J Clin Psychiatry, 80(1). 10.4088/JCP.17r12064 [DOI] [PubMed] [Google Scholar]
- Dow-Edwards D, & Silva L (2017). Endocannabinoids in brain plasticity: Cortical maturation, HPA axis function and behavior. Brain Res, 1654(Pt B), 157–164. 10.1016/j.brainres.2016.08.037 [DOI] [PubMed] [Google Scholar]
- Dubrow GA, Pawar RS, Srigley C, Fong Sam J, Talavera C, Parker CH, & Noonan GO (2021). A survey of cannabinoids and toxic elements in hemp-derived products from the United States marketplace. Journal of Food Composition and Analysis, 97. 10.1016/j.jfca.2020.103800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, … Steardo L (2011). Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One, 6(12), e28668. 10.1371/journal.pone.0028668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova EV, Wong CF, Ataiants J, Iverson E, Conn BM, & Lankenau SE (2021). Cannabidiol (CBD) and other drug use among young adults who use cannabis in Los Angeles. Drug and Alcohol Dependence, 221, 108648. 10.1016/j.drugalcdep.2021.108648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florensa-Zanuy E, Garro-Martínez E, Adell A, Castro E, Díaz Á, Pazos Á, … Pilar-Cuéllar F (2021). Cannabidiol antidepressant-like effect in the lipopolysaccharide model in mice: Modulation of inflammatory pathways. Biochemical Pharmacology, 185, 114433. 10.1016/j.bcp.2021.114433 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. (2021). FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd
- Food and Drug Administration (2022). Warning Letters and Test Results for Cannabidiol-Related Products. https://www-fda-gov.proxy.cc.uic.edu/news-events/public-health-focus/warning-letters-and-test-results-cannabidiol-related-products
- Goodman S, Wadsworth E, Schauer G, & Hammond D (2022). Use and Perceptions of Cannabidiol Products in Canada and in the United States. Cannabis Cannabinoid Res, 7(3), 355–364. 10.1089/can.2020.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Weinberger AH, Kim JH, Wu M, & Galea S (2020). Trends in anxiety among adults in the United States, 2008-2018: Rapid increases among young adults. J Psychiatr Res, 130, 441–446. 10.1016/j.jpsychires.2020.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group., O. L. o. E. W. (2011). The Oxford Levels of Evidence 2. Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence [Google Scholar]
- Hammond D, Goodman S, Wadsworth E, Rynard V, Boudreau C, & Hall W (2020). Evaluating the impacts of cannabis legalization: The International Cannabis Policy Study. Int J Drug Policy, 77, 102698. 10.1016/j.drugpo.2020.102698 [DOI] [PubMed] [Google Scholar]
- Hegazy O, & Platnick H (2019). Cannabidiol (CBD) for Treatment of Neurofibromatosis-related Pain and Concomitant Mood Disorder: A Case Report. Cureus, 11(12), e6312. 10.7759/cureus.6312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, … Pertwee RG (2002). International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev, 54(2), 161–202. 10.1124/pr.54.2.161 [DOI] [PubMed] [Google Scholar]
- Izzo AA, & Sharkey KA (2010). Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther, 126(1), 21–38. 10.1016/j.pharmthera.2009.12.005 [DOI] [PubMed] [Google Scholar]
- Jurkus R, Day HL, Guimarães FS, Lee JL, Bertoglio LJ, & Stevenson CW (2016). Cannabidiol Regulation of Learned Fear: Implications for Treating Anxiety-Related Disorders. Front Pharmacol, 7, 454. 10.3389/fphar.2016.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JS, Wagner JK, Reid K, McGuinness F, Arvila S, Brooks M, … Predovich B (2021). Cannabidiol Exposure During the Mouse Adolescent Period Is Without Harmful Behavioral Effects on Locomotor Activity, Anxiety, and Spatial Memory. Front Behav Neurosci, 15, 711639. 10.3389/fnbeh.2021.711639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten CR, Zhang Y, & Boehm SL 2nd. (2019). Acute Cannabinoids Produce Robust Anxiety-Like and Locomotor Effects in Mice, but Long-Term Consequences Are Age- and Sex-Dependent. Front Behav Neurosci, 13, 32. 10.3389/fnbeh.2019.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedzior KK, & Laeber LT (2014). A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population--a meta-analysis of 31 studies. BMC Psychiatry, 14, 136. 10.1186/1471-244x-14-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, & Wittchen HU (2012). Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research, 21(3), 169–184. 10.1002/mpr.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Naveed S, Mian N, Fida A, Raafey MA, & Aedma KK (2020). The therapeutic role of Cannabidiol in mental health: a systematic review. J Cannabis Res, 2(1), 2. 10.1186/s42238-019-0012-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klier CM, de Gier C, Felnhofer A, Laczkovics C, & Amminger PG (2020). A Case Report of Cannabidiol Treatment of a Crohn’s Disease Patient With Anxiety Disorder. J Clin Psychopharmacol, 40(1), 90–92. 10.1097/jcp.0000000000001152 [DOI] [PubMed] [Google Scholar]
- Laczkovics C, Kothgassner OD, Felnhofer A, & Klier CM (2021). Cannabidiol treatment in an adolescent with multiple substance abuse, social anxiety and depression. Neuropsychiatr, 35(1), 31–34. 10.1007/s40211-020-00334-0 (Cannabidiol-Therapie eines Jugendlichen mit multiplem Substanzabusus, Sozialphobie und Depression.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, & Denovan-Wright EM (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol, 172(20), 4790–4805. 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma-Corvi S, Hernández-Hernández E, & García-Fuster MJ (2022). Exploring pharmacological options for adolescent depression: a preclinical evaluation with a sex perspective. Transl Psychiatry, 12(1), 220. 10.1038/s41398-022-01994-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci A, & Pinna G (2019). Stimulation of Peroxisome Proliferator-Activated Receptor-α by N-Palmitoylethanolamine Engages Allopregnanolone Biosynthesis to Modulate Emotional Behavior. Biological Psychiatry, 85(12), 1036–1045. 10.1016/j.biopsych.2019.02.006 [DOI] [PubMed] [Google Scholar]
- Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, … Kossowsky J (2017). Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo for Common Psychiatric Disorders Among Children and Adolescents: A Systematic Review and Meta-analysis. JAMA Psychiatry, 74(10), 1011–1020. 10.1001/jamapsychiatry.2017.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K (2005). Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol(168), 299–325. 10.1007/3-540-26573-2_10 [DOI] [PubMed] [Google Scholar]
- Mansell H, Quinn D, Kelly LE, & Alcorn J (2022). Cannabis for the Treatment of Attention Deficit Hyperactivity Disorder: A Report of 3 Cases. Med Cannabis Cannabinoids, 5(1), 1–6. 10.1159/000521370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masataka N (2019). Anxiolytic Effects of Repeated Cannabidiol Treatment in Teenagers With Social Anxiety Disorders. Frontiers in Psychology, 10, 2466. 10.3389/fpsyg.2019.02466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, & Pinna G (2021). PPAR-α Hypermethylation in the Hippocampus of Mice Exposed to Social Isolation Stress Is Associated with Enhanced Neuroinflammation and Aggressive Behavior. Int J Mol Sci, 22(19). 10.3390/ijms221910678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, & Bonner TI (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature, 346(6284), 561–564. 10.1038/346561a0 [DOI] [PubMed] [Google Scholar]
- McPartland JM, Glass M, & Pertwee RG (2007). Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol, 152(5), 583–593. 10.1038/sj.bjp.0707399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead A (2017). The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law. Epilepsy Behav, 70(Pt B), 288–291. 10.1016/j.yebeh.2016.11.021 [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, & Tapert SF (2007). Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry, 48(6), 592–600. 10.1111/j.1469-7610.2007.01728.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner H, & Cascella M (2022). Cannabidiol (CBD). StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK556048/ [PubMed]
- Melas PA, Scherma M, Fratta W, Cifani C, & Fadda P (2021). Cannabidiol as a Potential Treatment for Anxiety and Mood Disorders: Molecular Targets and Epigenetic Insights from Preclinical Research. Int J Mol Sci, 22(4). 10.3390/ijms22041863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HC, Lee FS, & Gee DG (2018). The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain Development. Neuropsychopharmacology, 43(1), 21–33. 10.1038/npp.2017.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SA, Stone NL, Yates AS, & O’Sullivan SE (2018). A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front Pharmacol, 9, 1365. 10.3389/fphar.2018.01365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Chadwick B, Dickstein DL, Purushothaman I, Egervari G, Rahman T, … Hurd YL (2019). Adolescent exposure to Δ(9)-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Mol Psychiatry, 24(4), 588–600. 10.1038/s41380-018-0243-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller OS, Elder EJ Jr., Jones KJ, & Gidal BE (2022). Analysis of cannabidiol (CBD) and THC in nonprescription consumer products: Implications for patients and practitioners. Epilepsy Behav, 127, 108514. 10.1016/j.yebeh.2021.108514 [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, & Abu-Shaar M (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature, 365(6441), 61–65. 10.1038/365061a0 [DOI] [PubMed] [Google Scholar]
- Murphy M, Mills S, Winstone J, Leishman E, Wager-Miller J, Bradshaw H, & Mackie K (2017). Chronic Adolescent Δ(9)-Tetrahydrocannabinol Treatment of Male Mice Leads to Long-Term Cognitive and Behavioral Dysfunction, Which Are Prevented by Concurrent Cannabidiol Treatment. Cannabis Cannabinoid Res, 2(1), 235–246. 10.1089/can.2017.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, E., and Medicine. (2017). The health effects of cannabis and cannabinoids: The current state of evidence and recommendations for research. The National Academies Press. https://www.ncbi.nlm.nih.gov/books/NBK423845/ [PubMed] [Google Scholar]
- O’Sullivan SE (2016). An update on PPAR activation by cannabinoids. Br J Pharmacol, 173(12), 1899–1910. 10.1111/bph.13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberbarnscheidt T, & Miller NS (2020). The Impact of Cannabidiol on Psychiatric and Medical Conditions. J Clin Med Res, 12(7), 393–403. 10.14740/jocmr4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, … Moher D (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, & Russo EB (2016). The Cannabis sativa Versus Cannabis indica Debate: An Interview with Ethan Russo, MD. Cannabis Cannabinoid Res, 1(1), 44–46. 10.1089/can.2015.29003.ebr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton JA, Smyth K, Soumbasis E, Llanos SA, Lewis M, Meerholz WA, & Tanguay RL (2020). A pediatric patient with autism spectrum disorder and epilepsy using cannabinoid extracts as complementary therapy: a case report. J Med Case Rep, 14(1), 162. 10.1186/s13256-020-02478-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, & Parolaro D (2016). The Impact of Exposure to Cannabinoids in Adolescence: Insights From Animal Models. Biol Psychiatry, 79(7), 578–585. 10.1016/j.biopsych.2015.07.024 [DOI] [PubMed] [Google Scholar]
- Sarris J, Sinclair J, Karamacoska D, Davidson M, & Firth J (2020). Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review. BMC Psychiatry, 20(1), 24. 10.1186/s12888-019-2409-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeler T, Theobald D, Pingault JB, Farrington DP, Coid JW, & Bhattacharyya S (2018). Developmental sensitivity to cannabis use patterns and risk for major depressive disorder in mid-life: findings from 40 years of follow-up. Psychol Med, 48(13), 2169–2176. 10.1017/s0033291717003658 [DOI] [PubMed] [Google Scholar]
- Scuderi C, Steardo L, & Esposito G (2014). Cannabidiol Promotes Amyloid Precursor Protein Ubiquitination and Reduction of Beta Amyloid Expression in SHSY5YAPP+ Cells Through PPARγ Involvement. Phytotherapy Research, 28(7), 1007–1013. 10.1002/ptr.5095 [DOI] [PubMed] [Google Scholar]
- Siegel RS, & Dickstein DP (2012). Anxiety in adolescents: Update on its diagnosis and treatment for primary care providers. Adolesc Health Med Ther, 3, 1–16. 10.2147/ahmt.S7597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silote GP, Sartim A, Sales A, Eskelund A, Guimarães FS, Wegener G, & Joca S (2019). Emerging evidence for the antidepressant effect of cannabidiol and the underlying molecular mechanisms. J Chem Neuroanat, 98, 104–116. 10.1016/j.jchemneu.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Simone JJ, Green MR, & McCormick CM (2022). Endocannabinoid system contributions to sex-specific adolescent neurodevelopment. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 113, 110438. 10.1016/j.pnpbp.2021.110438 [DOI] [PubMed] [Google Scholar]
- Stanciu CN, Brunette MF, Teja N, & Budney AJ (2021). Evidence for Use of Cannabinoids in Mood Disorders, Anxiety Disorders, and PTSD: A Systematic Review. Psychiatric Services, 72(4), 429–436. 10.1176/appi.ps.202000189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2014). The TEDS Report: Age of Substance Use Initiation among Treatment Admissions Aged 18 to 30. [PubMed]
- Substance Abuse and Mental Health Services Administration. (2019). Substance Misuse Prevention for Young Adults. Publication No. PEP19-PL-Guide-1, Rockville, MD. [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, & Pertwee RG (2007). Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol, 150(5), 613–623. 10.1038/sj.bjp.0707133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida A, Laurent A, Crivello F, Petit L, Pepe A, Beguedou N, … Mazoyer B (2021). Age-Related Variations in Regional White Matter Volumetry and Microstructure During the Post-adolescence Period: A Cross-Sectional Study of a Cohort of 1,713 University Students. Front Syst Neurosci, 15, 692152. 10.3389/fnsys.2021.692152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, S. A. a. M. H. S. A., Center for Behavioral Health Statistics and Quality. (2020). National Survey of Drug Use and Health. https://datafiles.samhsa.gov/
- Vaughn SE, Strawn JR, Poweleit EA, Sarangdhar M, & Ramsey LB (2021). The Impact of Marijuana on Antidepressant Treatment in Adolescents: Clinical and Pharmacologic Considerations. J Pers Med, 11(7). 10.3390/jpm11070615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Llorente R, Suarez J, Llorente-Berzal A, López-Gallardo M, & de Fonseca FR (2012). The endocannabinoid system in critical neurodevelopmental periods: sex differences and neuropsychiatric implications. J Psychopharmacol, 26(1), 164–176. 10.1177/0269881111408956 [DOI] [PubMed] [Google Scholar]
- Wheeler M, Merten JW, Gordon BT, & Hamadi H (2020). CBD (Cannabidiol) Product Attitudes, Knowledge, and Use Among Young Adults. Subst Use Misuse, 55(7), 1138–1145. 10.1080/10826084.2020.1729201 [DOI] [PubMed] [Google Scholar]


