It is generally accepted that obesity is a health hazard because of its association with numerous metabolic complications such as dyslipidaemia, type 2 diabetes, and cardiovascular diseases.1 On that basis, health agencies2,3 have proposed that obesity should be defined on the basis of weight in kg expressed over height in m2, the so called body mass index,4 initially described by Quetelet in 1869 (table). Epidemiological studies have reported a progressive increase in the incidence of chronic diseases such as hypertension, diabetes, and coronary heart disease with increasing body mass index.1–3 However, despite this well documented evidence, physicians are, in their daily practice, perplexed by the remarkable heterogeneity found in their obese patients. For instance, some patients show a relatively “normal” profile of metabolic risk factors despite the presence of substantial excess body fat, whereas others who are only moderately overweight can nevertheless be characterised by a whole cluster of metabolic complications, increasing the risk of type 2 diabetes, coronary atherosclerosis, and cardiovascular disease.
Summary points
A simple measurement such as waist circumference can indicate accumulation of abdominal fat
Viscerally obese men are characterised by an atherogenic plasma lipoprotein profile
A triad of non-traditional markers for coronary heart disease found in viscerally obese middle aged men (hyperinsulinaemia, raised apolipoprotein B concentration, and small LDL particles) increases the risk of coronary heart disease 20-fold
Four out of five middle aged men with a waist measurement ⩾90 cm and triglyceride concentrations ⩾2 mmol/l are characterised by this triad
Even in the absence of hypercholesterolaemia, hyperglycaemia, or hypertension, obese patients could be at high risk of coronary heart disease if they have this “hypertriglyceridaemic waist” phenotype
In this regard, epidemiological and metabolic studies conducted over the past 15 years have re-emphasised a notion introduced in the mid-forties by a French physician, Dr Jean Vague, who reported that the complications commonly found in obese patients were more closely related to where the excess fat was rather than to excess weight per se.5 Since this early pioneering work, in which Vague described the high risk form of obesity by the term “android obesity” or male type (upper body) obesity, several studies have confirmed the notion that a high proportion of abdominal fat is a major risk factor for coronary heart disease, type 2 diabetes, and related mortality.6 Furthermore, recent studies have also shown that a preferential accumulation of body fat in the gluteofemoral region, commonly found in premenopausal women and initially described by Vague under the term “gynoid obesity,” is not a major threat to cardiovascular health.7,8
Therefore, there is currently overwhelming evidence that abdominal obesity is a major clinical and public health issue. In this review we discuss the clinical implications of this concept regarding the assessment and the management of risk in abdominally obese patients.
Visceral adipose tissue: the culprit
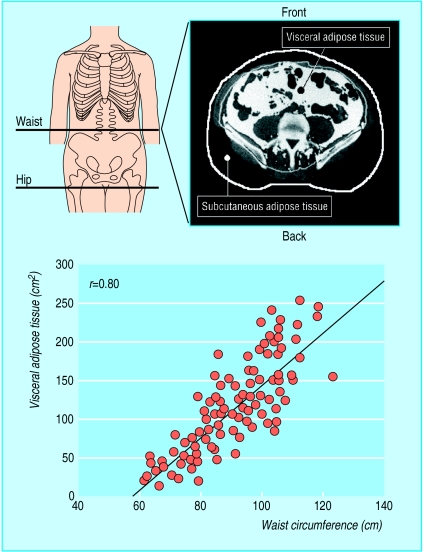
Epidemiological studies have mainly used anthropometric variables such as the ratio of waist to hip circumferences (waist:hip ratio) to estimate the proportion of abdominal adipose tissue (fig 1). Sophisticated imaging techniques such as magnetic resonance imaging and computed tomography, however, can distinguish, with a high level of precision, intra-abdominal or visceral fat depot from subcutaneous abdominal fat (fig 1).6,9 These techniques showed that a simple measurement such as waist circumference was the best anthropometric correlate of the amount of visceral adipose tissue (fig 1).9 Furthermore, a seven year longitudinal study conducted in women revealed that the change in waist measurement was a better correlate of the change in visceral adipose tissue observed over this period than the change in the waist:hip ratio10 for reasons that are illustrated in figure 2.
Figure 1.
Assessment of accumulation of abdominal fat by measurement of waist at mid-distance between bottom of rib cage and iliac crest. Amount of visceral adipose tissue that can be assessed by computed tomography can be estimated by waist measurement (adapted from Pouliot et al9)
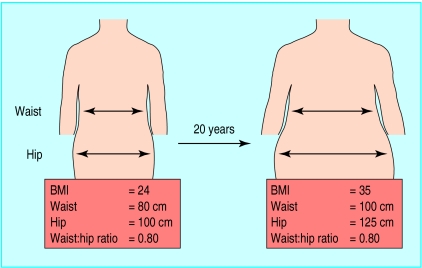
Figure 2.
Misleading information provided by follow up of changes in waist:hip ratio in woman followed over 20 years. Simultaneous increase in waist and hip measurements means ratio is stable over time despite considerable accumulation of visceral adipose tissue, which would have been predicted from 20 cm increase in waist observed over time. Thus, waist circumference provides crude index of absolute amount of abdominal adipose tissue whereas waist:hip ratio provides index of relative accumulation of abdominal fat
Metabolic complications associated with visceral obesity
Plasma glucose and insulin concentrations and risk of type 2 diabetes
Type 2 diabetes not only increases the risk of retinopathy, nephropathy, and neuropathy but is also a major risk factor for atherosclerotic macrovascular disease, as 75% of patients with type 2 diabetes will eventually die from this complication.11 Prospective studies have shown that abdominal obesity is a major risk factor for the development of type 2 diabetes.6 This increased risk can be largely attributed to the fact that a high accumulation of abdominal adipose tissue, especially of visceral adipose tissue (see fig 1),12 has been associated with glucose intolerance and with hyperinsulinaemia resulting from insulin resistance.6,13
To quantify the respective contributions of the two main abdominal adipose depots (subcutaneous versus visceral) to the development of glucose intolerance and hyperinsulinaemia in obesity, two groups of obese patients were carefully matched for the same amount of total body fat but with either low or high levels of visceral adipose tissue measured by computed tomography.13 Obese people with less visceral adipose tissue were found to have normal glucose tolerance when compared with lean controls.13 Obese people with a high accumulation of visceral adipose tissue, however, showed an increase in their glycaemic response to an oral glucose load which was measurably higher than that in obese people with less visceral adipose tissue or in non-obese controls.13 Major differences were also noted in the plasma insulin response to the oral glucose load.13 These comparisons show that viscerally obese people represent a subgroup of obese patients with the highest glycaemic and insulinaemic responses to an oral glucose challenge and that they are at the highest risk of developing type 2 diabetes.6,12,13
Atherogenic dyslipidaemia of visceral obesity: beyond low density lipoprotein cholesterol
Obese individuals with a high accumulation of visceral adipose tissue tend to have hypertriglyceridaemia and low concentrations of high density lipoprotein cholesterol.14 Furthermore, the reduction in plasma concentration of high density lipoprotein cholesterol in these viscerally obese people is the major factor responsible for the increase in their ratio of cholesterol:high density lipoprotein cholesterol,15 this ratio being a powerful predictor of risk of coronary heart disease.16
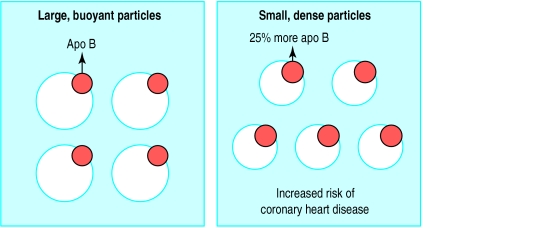
Despite the fact that viscerally obese patients often have plasma concentrations of low density lipoprotein cholesterol in the normal range, they have an increased proportion of atherogenic small, dense, low density lipoprotein particles and an increased concentration of apolipoprotein B (a marker of the concentration of atherogenic lipoproteins) (fig 3).17 Therefore viscerally obese patients have a very atherogenic plasma lipoprotein-lipid profile. Thus, physicians clearly need to go beyond the measurement and interpretation of cholesterolaemia and low density lipoprotein cholesterol concentrations for proper evaluation of risk of coronary heart disease in these patients. Simple lipid variables, generally obtained from a routine clinical biochemistry profile, can be used to identify high risk abdominally obese patients with high levels of visceral adipose tissue.
Figure 3.
Schematic example of how “normal” concentration of low density lipoprotein cholesterol could mislead assessment of risk of coronary heart disease in abdominally obese patients. Even when concentrations of low density lipoprotein cholesterol are the same, abdominally obese patients have increased proportions of smaller low density lipoprotein particles that are relatively depleted in cholesterol (esterified cholesterol). However, as there is one apolipoprotein B (apo B) molecule per low density lipoprotein particle, increased concentration of these atherogenic lipoproteins can be detected by measuring apolipoprotein B, which is about 25% higher among abdominally obese people. Presence of increased concentration of these small low density lipoprotein particles predicts substantially increased risk of coronary heart disease in middle aged men
Atherogenic metabolic profile of visceral obesity: beyond “classic” risk factors
Prospective studies have shown that the atherogenic metabolic profile of patients with visceral obesity contributes substantially to their increased risk of premature coronary heart disease.18 Results from a prospective study of middle aged men indicated that the cluster of metabolic abnormalities found in viscerally obese men was associated with a substantial increase in the risk of coronary heart disease, even in the absence of classic risk factors such as type 2 diabetes, hypercholesterolaemia, and hypertension.18 A triad of “new” atherogenic metabolic risk markers—fasting hyperinsulinaemia, increased apolipoprotein B concentration, and an increased proportion of small, dense, low density lipoprotein particles (abnormalities found together in viscerally obese men, even in the absence of type 2 diabetes)—was found to be associated with a 20-fold increase in the risk of developing coronary heart disease in initially asymptomatic middle aged men followed over a period of five years.18 Therefore, this atherogenic metabolic triad of risk markers observed in viscerally obese patients with insulin resistance is associated with a marked increase in the risk of coronary heart disease.
Atherothrombotic, pro-inflammatory abnormalities in viscerally obese patients
This cluster of complications substantially increases the risk of coronary heart disease in affected patients.
Insulin resistance
Hyperinsulinaemia
Glucose intolerance
Type 2 diabetes
Hypertriglyceridaemia
Hypoalphalipoproteinaemia
Increased apolipoprotein B
Small, dense, low and high density lipoprotein particles
Postprandial hyperlipidaemia
Hypertension
Impaired fibrinolysis and increased susceptibility to thrombosis (raised plasminogen activator inhibitor-1 and fibrinogen)
Low chronic inflammation state (raised interleukin 6, raised C reactive protein, etc)
Endothelial dysfunction
Screening tools to identify high risk abdominally obese individuals
The existence of this atherogenic metabolic triad indicates a need for a simple algorithm which could be used by general physicians and health professionals to screen rapidly for the risks associated with this cluster. A close relation between accumulation of visceral adipose tissue and plasma insulin and apolipoprotein B concentrations has been reported,10 but the direct measurement of accumulation of visceral adipose tissue by computed tomography is costly and involves irradiation of participants. Accumulation, however, can be estimated from anthropometric indices,19 and studies have shown that the waist circumference is a useful measurement not only to predict accumulation (see fig 1) but also to monitor its change over time.10,20 On the other hand, the variable which has been reported to display the closest association with the presence of atherogenic small, dense, low density lipoprotein particles is the plasma triglyceride concentration measured in the morning after a 12 hour fast.17,21
Simple screening variables, such as waist circumference and fasting triglyceride concentrations, have been tested for their ability to identify high risk, viscerally obese men who would be carriers of the atherogenic triad.15 Sensitivity and specificity analyses conducted in a sample of men aged between 30 and 65 years showed that a cut off point of a 90 cm waist measurement would provide the best discriminative ability to distinguish men with hyperinsulinaemia and increased apolipoprotein B concentration from those with normal concentrations for both variables.15 Furthermore, a fasting triglyceride concentration of 2 mmol/l provided the best cut off point to identify men with the small, dense, low density lipoprotein phenotype.15 By using these simple cut off values, more than 80% of men with a waist circumference ⩾90 cm and fasting triglyceride concentrations ⩾2 mmol/l were carriers of the atherogenic metabolic triad, whereas only 10% of men with a waist circumference <90 cm and fasting triglyceride <2 mmol/l were carriers.15 These results emphasise the importance of the measurement and interpretation of waist circumference and of fasting triglyceride concentration in the assessment of risk of coronary heart disease. Although this approach is promising, it is important to point out that there are sex and ethnic group differences in the relation of waist measurement to accumulation of visceral adipose tissue as well as to metabolic complications. Thus, it is likely that different cut off values defining the hypertriglyceridaemic waist phenotype may be found in women (before and after the menopause) as well as in other age and ethnic groups.
Hypertriglyceridaemic waist: a new clinical phenotype defining a high risk form of overweight/obesity
From the ability of the combined interpretation of waist measurement and fasting plasma triglyceride concentration to discriminate a large proportion of carriers from non-carriers of the features of the atherogenic metabolic triad, we believe that the hypertriglyceridaemic waist may help to refine our identification of high risk patients beyond the use of waist measurement alone.
For instance, the use of the waist measurement has been widely emphasised in the United Kingdom because of the pivotal studies of Lean et al.22–24 At the same time, our group had also documented a progressive increase in the prevalence of metabolic complications with increasing waist sizes, and the two cut off values that we had proposed (90 cm and 100 cm)19 are close to the 88 cm in women and 102 in men suggested by Lean and his group. Thus, if the clinician cannot obtain a 12 hour fasting blood sample to measure triglyceride concentrations, waist measurements of 100-102 cm in men and 88-90 cm in women provide useful reference values to identify obese patients who may be at high risk for chronic metabolic diseases. Furthermore, waist measurement also moves the focus from weight to the high risk form of obesity: abdominal obesity. Therefore, as waist circumference was found to be a useful and simple marker of abdominal fat accumulation, the World Health Organization3 had used the studies of Lean et al to propose cut off points of 102 cm for men and 88 cm for women. To define critical waist circumference values, however, a few additional issues need to be examined.
Firstly, with age there is a selective deposition of visceral adipose tissue.10,20 For example, a middle aged man with a waist measurement of 95 cm has, on average, more visceral adipose tissue than a young adult man with a similar waist circumference.19 Thus, different cut off measurements should be proposed on the basis of the patient's age, a notion not covered by the WHO recommendations. Secondly, and most importantly, although waist measurement improves our ability to identify high risk patients, there is still considerable variability in the metabolic risk profile within a group of patients with similar levels of abdominal fat. Adding fasting triglyceride concentration to the waist measurement improves physicians' ability to identify abdominally obese men likely to have the features of the insulin resistance syndrome. In their studies, Lean and colleagues rather focused on “traditional risk” factors such as cholesterol, high density lipoprotein cholesterol, and blood pressure,22–24 whereas we have assessed measurements of the metabolic syndrome, such as insulin, apolipoprotein B, and size of low density lipoprotein particles.16 Thirdly, although adding triglyceride concentration to waist measurement improves our ability to identify high risk abdominally obese men, we do not know whether this algorithm predicts risk of coronary heart disease equally well in women. This is because no prospective study with “hard” end points has documented the event rate for coronary heart disease associated with the atherogenic metabolic triad resulting from abdominal obesity in women. As the menopause is associated with a selective deposition of visceral adipose tissue as well as with an increased risk of coronary heart disease, such studies are urgently needed. Finally, there are major ethnic differences in the interrelations between waist circumference, accumulation of visceral adipose tissue, and the metabolic risk profile. Our hypertriglyceridaemic waist approach has been developed in white men and its validity should not be extrapolated to other ethnic groups. Additional studies are clearly warranted.
Management of obesity: focusing on high risk patients
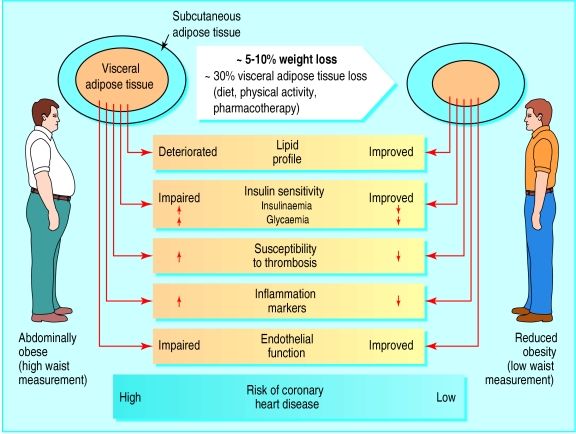
As obesity is often characterised by metabolic complications that harm health, it has been suggested that it should be considered as a disease.3 Unfortunately, if obesity is defined only on the basis of body mass index, some healthy men and women (at least from a metabolic point) with values of 30 and more will automatically be classified at high risk even if they have a fairly normal profile of metabolic risk factors. This may explain the rather disappointing results of weight loss trials that use pharmacotherapy, in which a large proportion of the participants were “metabolically healthy” women.25,26 However, on the basis of major metabolic improvements induced by moderate (5-10%) weight loss (fig 4), the relevance of an aggressive management of high risk abdominally obese people identified not on the basis of body weight but also by waist and fasting triglyceride measurements is emphasised. In this context, the benefits of pharmacotherapy are likely to outweigh the potential risks of drug treatment. Obesity as a health problem has to be defined beyond of weight and cosmetic considerations.
Figure 4.
Potential benefits of moderate (5-10%) weight loss in high risk patients with cluster of atherothrombotic, pro-inflammatory metabolic abnormalities associated with hypertriglyceridaemic waist. Weight loss in abdominally obese patients is associated with selective mobilisation of diabetogenic and atherogenic visceral adipose tissue, even 5-10% weight loss is associated with preferential mobilisation of visceral adipose tissue, leading to simultaneous improvement in all metabolic markers of coronary heart disease risk. Thus simultaneous metabolic improvements associated with mobilisation of visceral adipose tissue may contribute substantially to reduced risk of acute coronary event in high risk patients
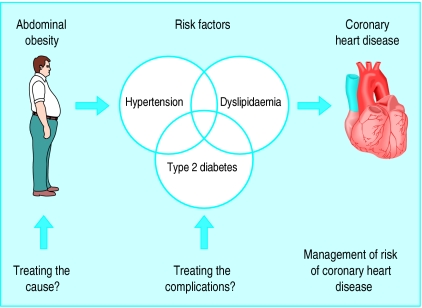
Physicians currently have a better understanding of the aetiology of chronic complications such as coronary heart disease and have access to a tremendous range of drugs to treat them. Therefore, there has been a legitimate emphasis on the treatment of complications that are powerful risk factors for coronary heart disease such as hypertension, dyslipidaemia, and diabetes (fig 5). However, for a long time obesity was not well defined as a risk factor for coronary heart disease because of its remarkable heterogeneity. By better identification of a high risk form of obesity, it is hoped that physicians will be encouraged to treat the cause of the metabolic complications by focusing on waist circumference as another therapeutic target. Waist girth should be considered as a “vital sign” and recorded in the medical chart of every patient. Obviously, as our sedentary lifestyle combined with our diet rich in saturated fats and trans fatty acids and in refined sugars is “toxic” to our metabolism, any approach aimed at the management of risk of coronary heart disease in abdominally obese patients will require a multifaceted approach (balanced nutrition with more vegetables and fruit, fewer refined products rich in fat and sugar, more physical activity) directed at the critical factors involved in the aetiology of the patient's condition. In a broader context of a comprehensive evaluation and treatment of risk (and not of body weight), it is hoped that this approach will help physicians to identify obese patients that may require pharmacotherapy aimed at waist rather than weight management.
Figure 5.
Contribution of abdominal obesity (increased waist measurement) as therapeutic target for better management of risk of coronary heart disease
Table.
Classification of obesity based on body mass index (BMI)2 3
| Classification | BMI* |
|---|---|
| Underweight | <18.5 |
| Normal range | 18.5-24.9 |
| Overweight: | ⩾25.0 |
| Pre-obese | 25.0-29.9 |
| Obese class I | 30.0-34.9 |
| Obese class II | 35.0-39.9 |
| Obese class III | ⩾40.0 |
kg/m2.
Editorial by Little and Byrne
Footnotes
Competing interests: J-PD has received honoraria for consultancy and lectures and funding for his laboratory from Servier Canada, Parke-Davis/Warner-Lambert Canada, Merck-Frosst Canada, Fournier Pharma, Gatorade, DuPont-Merck, Knoll Pharma, Weight Watchers International, Roche Pharma, and Eli Lilly.
Funding: This work was supported by the Canadian Institutes of Health Research (MT-14014 and MGC-15187). J-PD is chair professor of human nutrition and lipidology, which is supported by Parke-Davis/Warner-Lambert, Provigo, and the Foundation of the Quebec Heart Institute. IL is recipient of a fellowship from the Heart and Stroke Foundation of Canada.
References
- 1.Bray GA, Bouchard C, James WPT, editors. Handbook of obesity. New York: Marcel Dekker; 1998. [Google Scholar]
- 2.National Heart, Lung, and Blood Institute/National Institutes of Diabetes and Digestive and Kidney Diseases. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults. The evidence report. Bethesda: National Institutes of Health; 1998. pp. 1–228. [Google Scholar]
- 3.WHO Consultation on Obesity. Preventing and managing the global epidemic. Geneva: World Health Organization; 1997. pp. 1–276. [PubMed] [Google Scholar]
- 4.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–343. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- 5.Vague J. La différenciation sexuelle, facteur déterminant des formes de l'obésité. Presse Med. 1947;30:339–340. [PubMed] [Google Scholar]
- 6.Kissebah AH, Freedman DS, Peiris AN. Health risks of obesity. Med Clin North Am. 1989;73:111–138. doi: 10.1016/s0025-7125(16)30695-2. [DOI] [PubMed] [Google Scholar]
- 7.Terry RB, Stefanick ML, Haskell WL, Wood PD. Contributions of regional adipose tissue depots to plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism. 1991;40:733–740. doi: 10.1016/0026-0495(91)90093-c. [DOI] [PubMed] [Google Scholar]
- 8.Pouliot MC, Després JP, Nadeau A, Tremblay A, Moorjani S, Lupien PJ, et al. Associations between regional body fat distribution, fasting plasma free fatty acid levels and glucose tolerance in premenopausal women. Int J Obes. 1990;14:293–302. [PubMed] [Google Scholar]
- 9.Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 10.Lemieux S, Prud'homme D, Tremblay A, Bouchard C, Després JP. Anthropometric correlates to changes in visceral adipose tissue over 7 years in women. Int J Obes Relat Metab Disord. 1996;20:618–624. [PubMed] [Google Scholar]
- 11.Pyorala K, Laakso M, Uusitupa M. Diabetes and atherosclerosis: an epidemiologic view. Diabetes Metab Rev. 1987;3:463–524. doi: 10.1002/dmr.5610030206. [DOI] [PubMed] [Google Scholar]
- 12.Bergstrom RW, Newell-Morris LL, Leonetti DL, Shuman WP, Wahl PW, Fujimoto WY. Association of elevated fasting C-peptide level and increased intra-abdominal fat distribution with development of NIDDM in Japanese-American men. Diabetes. 1990;39:104–111. doi: 10.2337/diacare.39.1.104. [DOI] [PubMed] [Google Scholar]
- 13.Pouliot MC, Després JP, Nadeau A, Moorjani S, Prud'homme D, Lupien PJ, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 14.Després JP, Moorjani S, Lupien PJ, Tremblay A, Nadeau A, Bouchard C. Regional distribution of body fat, plasma lipoproteins, and cardiovascular disease. Arteriosclerosis. 1990;10:497–511. doi: 10.1161/01.atv.10.4.497. [DOI] [PubMed] [Google Scholar]
- 15.Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, et al. Hypertriglyceridemic waist. A marker of the atherogenic metabolic triad (hyperinsulinemia, hyperapolipoprotein B, small, dense LDL) in men? Circulation. 2000;102:179–184. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984;76:4–12. doi: 10.1016/0002-9343(84)90952-5. [DOI] [PubMed] [Google Scholar]
- 17.Tchernof A, Lamarche B, Prud'homme D, Nadeau A, Moorjani S, Labrie F, et al. The dense LDL phenotype. Association with plasma lipoprotein levels, visceral obesity, and hyperinsulinemia in men. Diabetes Care. 1996;19:629–637. doi: 10.2337/diacare.19.6.629. [DOI] [PubMed] [Google Scholar]
- 18.Lamarche B, Tchernof A, Mauriège P, Cantin B, Dagenais GR, Lupien PJ, et al. Fasting insulin and apolipoprotein B levels and low-density lipoprotein particle size as risk factors for ischemic heart disease. JAMA. 1998;279:1955–1961. doi: 10.1001/jama.279.24.1955. [DOI] [PubMed] [Google Scholar]
- 19.Lemieux S, Prud'homme D, Bouchard C, Tremblay A, Després JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64:685–693. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 20.Lemieux S, Prud'homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. Seven-year changes in body fat and visceral adipose tissue in women. Association with indexes of plasma glucose-insulin homeostasis. Diabetes Care. 1996;19:983–991. doi: 10.2337/diacare.19.9.983. [DOI] [PubMed] [Google Scholar]
- 21.McNamara JR, Jenner JL, Li Z, Wilson PW, Schaefer EJ. Change in LDL particle size is associated with change in plasma triglyceride concentration. Arterioscler Thromb. 1992;12:1284–1290. doi: 10.1161/01.atv.12.11.1284. [DOI] [PubMed] [Google Scholar]
- 22.Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–161. doi: 10.1136/bmj.311.6998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lean ME, Han TS, Seidell JC. Impairment of health and quality of life in people with large waist circumference. Lancet. 1998;351:853–856. doi: 10.1016/s0140-6736(97)10004-6. [DOI] [PubMed] [Google Scholar]
- 24.Han TS, van Leer EM, Seidell JC, Lean ME. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ. 1995;311:1401–1405. doi: 10.1136/bmj.311.7017.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson DF. Pharmacotherapy for obesity. JAMA. 1999;281:278–280. doi: 10.1001/jama.281.3.278. [DOI] [PubMed] [Google Scholar]
- 26.Sjostrom L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European multicentre orlistat study group. Lancet. 1998;352:167–172. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]