Abstract
Concerns regarding the safety of beta-2 agonists have led to revisions of the major asthma guidelines to better address these issues. Although these updates allow for a combination of previous and current strategies, they may confuse clinical practitioners. Beta-2 agonists are vital for alleviating asthma symptoms by relaxing smooth muscles; however, they also pose significant risks by inducing pro-inflammatory mediators both in vitro and in vivo. In addition to the risks of overuse and symptom masking, the use of beta-agonists alone at therapeutic doses can worsen airway inflammation and enhance virus-induced inflammation during asthma exacerbation. Inhaled corticosteroids (ICS) can effectively prevent these adverse effects. With new insights into the mechanisms of these adverse events, reserving short-acting beta-agonists for acute symptom relief during exacerbations and only for those who are already on ICS or oral steroids represents a careful approach to using beta-agonists with least adverse effects in patients with asthma. However, a major drawback of this approach is the potential non-compliance with ICS, leading to beta-agonist use without the necessary counteraction by ICS. An optimal strategy, both during and outside exacerbations, would integrate beta-agonists into an anti-inflammatory regimen that includes ICS, ideally combined with the same inhaler to ensure their concurrent use where finances allow. This would maintain the beneficial effects of beta-agonists, such as bronchodilation, while preventing the adverse effects from the induction of inflammatory mediators. This method is aligned with diverse clinical settings, maximizes the safe use of beta-agonists, and supports a comprehensive guideline-compliant management strategy.
Keywords: Asthma, adrenergic beta-2 receptor agonist, corticosteroids, inflammatory mediators, airway inflammation, adverse reactions, risk factors, safety, adherence
INTRODUCTION
Beta-adrenergic receptors are widely distributed throughout the body, with various subtypes known, from beta-1 to beta-3.1,2,3 Among these receptors, beta-2 receptors hold special significance because they are closely associated with the respiratory system. Beta-2 receptors are primarily distributed in smooth muscles throughout the body and their stimulation plays a crucial role in smooth muscle relaxation. Specifically, beta-2 receptors are found in the airway smooth muscle, intestine, bladder, vascular smooth muscle, liver, and pancreas.3 These receptors contribute to the fight-or-flight response and various physiological processes. The expression of beta-2 receptors in airway smooth muscle makes them essential for bronchodilation, a process that is vital for proper respiratory function. Owing to their role in bronchodilation, beta-agonists, together with anti-inflammatory inhaled corticosteroids (ICS), have long been central to the management of patients with asthma.1,3
For the last several decades, concerns regarding the safety of beta-agonists have persisted,4,5,6,7 leading to revisions of major asthma guidelines aimed at addressing these issues. Despite these updates, previous strategies are still permitted to be intermixed with the updated strategies, which may confuse clinical practitioners.8,9,10,11,12,13 In this review, we aim to explore background issues regarding the usage and side effects of beta-2 agonists by summarizing their indications in asthma management and presenting various adverse outcomes of using beta-agonists along with their possible mechanisms. Finally, we suggest practical strategies for the safer use of beta-2 agonists in real-world practice.
CURRENT RECOMMENDATIONS FOR USING BETA-2 AGONISTS IN VARIOUS ASTHMA MANAGEMENT GUIDELINES
The effectiveness of beta-2 agonists as bronchodilators is widely recognized in asthma guidelines, particularly in cases of acute exacerbation, owing to their rapid action and significant impact. The concurrent use of beta-2 agonists with ICS is advised to attain sustained asthma control and symptom relief. Moreover, the integrated approach of combining beta-2 agonists with ICS addresses both immediate symptoms and long-term aspects, offering a comprehensive and effective treatment strategy.
Global INitiative for Asthma (GINA) up to 2023
Within the GINA guidelines, beta-2 agonists, when used in conjunction with steroids, are recommended as pivotal medications for managing asthma symptoms and reducing risks in the long-term care of patients with asthma.8 However, the frequent recent revisions of these guidelines have resulted in an increasing demand for a clear understanding of the underlying principles governing the strategies for their utilization. In this context, Table provides a comprehensive overview of the use of beta-agonists in asthma management over the course of the past 6 years.8,14,15,16,17,18 To enhance the comprehension of reliever usage, Table also presents the controller (or maintenance medication) usage in patients with mild asthma, specifically focusing on steps 1 and 2 of maintenance strategies.
Table. Changes in the beta-agonist usage recommendations in the GINA guideline.
| Year | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|---|---|
| Comment | • ‘Preferred’ and ‘Options’ concept applied | • ‘Two track’ concept applied | • ‘Anti-inflammatory relievers’ concept applied | ||||
| • SABA only not permitted | • Emergence of the ICS-SABA combination | ||||||
| Preferred/track 1, Controller/maintenance, STEPS 1–2 | |||||||
| STEP 1 | (None) | • As-needed LD ICS-Form* | • As-needed LD ICS-Form | • As-needed LD ICS-Form | |||
| STEP 2 | LD ICS | • LD ICS or as-needed LD ICS-Form* | |||||
| Preferred/track 1, Reliever | |||||||
| STEPS 1–2 | As-needed SABA | • As-needed LD ICS-Form* | • As-needed LD ICS-form | • As-needed LD ICS-Form | |||
| STEPS 3–5 | As-needed SABA, or as-needed LD ICS-Form (MART) where applicable | • As-needed LD ICS-Form† | |||||
| (MART) where applicable | |||||||
| Alternative/track 2, Controller/Maintenance, STEPS 1–2 | |||||||
| STEP 1 | • LD ICS whenever SABA is taken | • Take ICS whenever SABA is taken | • Take ICS whenever SABA is taken | ||||
| STEP 2 | • Daily LTRA or LD ICS whenever SABA is taken | • LD ICS | • LD ICS | ||||
| Alternative/track 2, Reliever | |||||||
| STEPS 1–2 | • As-needed SABA | • As-needed SABA | • As-needed SABA, or as-neededICS-SABA | ||||
| STEPS 3–5 | |||||||
GINA, Global INitiative for Asthma; SABA, short-acting beta agonist; ICS, inhaled corticosteroid; LD, low-dose; Form, formoterol; MART, maintenance and reliever treatment; LTRA, leukotriene receptor antagonist.
*Budesonide-formoterol only. †Both budesonide-formoterol and beclomethasone-formoterol.
Until 2018, the approach to managing mild asthma primarily involved utilizing low-dose (LD) ICS as controllers and short-acting beta-agonists (SABA) as relievers on an as-needed basis.14 In this approach, if symptom control was achieved, it was possible to discontinue LD ICS as a controller, relying solely on SABA as needed. However, this transition sometimes leads to the overuse of SABA alone, eventually resulting in repeated exacerbations and even life-threatening episodes. Moreover, if asthma failed to be well controlled despite LD ICS maintenance inhalation, a combination of ICS and a long-acting beta-agonist (LABA) was recommended, with the option of choosing either salmeterol or formoterol as the LABA component. For individuals opting for rapid-acting formoterol as their preferred LABA in a combination inhaler, a maintenance and reliever therapy (MART) strategy was suggested, which allowed the use of maintenance medication as a reliever during exacerbations. Starting in 2019, SABA alone was no longer recommended for asthma management.15 The approach for managing mild asthma shifted towards employing a fixed ICS and LABA combination (ICS-LABA) of budesonide and formoterol, the only approved ICS-LABA preparation for as-needed use. The previous strategy was repositioned as an ‘alternative’ method of maintenance, advocating SABA usage alongside simultaneous ICS inhalation for individuals at Step 1, a stage in which controller drugs were not initially indicated. Furthermore, for the short-term prevention of exercise-induced bronchoconstriction (EIB) in patients with asthma, the conventional practice of SABA inhalation was replaced by the inhalation of LD ICS-formoterol. In 2021, a comprehensive two-track system was formally proposed, recommending both budesonide-formoterol and beclomethasone-formoterol as equally preferred options.17,18 Additionally, the approach to controlling medication became simpler. The use of combined ICS-LABA is recommended for all mild asthma cases, with adjustments to the corticosteroid content from the combination inhaler for more severe cases.
In the recent 2023 guideline, several terminology revisions have been made to minimize confusion.8 For instance, the term ‘maintenance medication’ has been suggested in place of the conventional ‘controller medication.’ Additionally, by introducing the term ‘anti-inflammatory reliever (AIR),’ the solitary use of SABA inhalers is challenged, and AIR or MART is recommended across all tracks of asthma management. This change also addresses the introduction of a fixed combination of budesonide and salbutamol in Track 2 management, in which the reliever medication differs from that in Track 1.
Furthermore, GINA recommends a sequential approach to manage asthma exacerbations, with beta-agonists playing an essential role. According to the 2018 report, for individuals experiencing acute asthma exacerbations, the approach is outlined through self-management, primary care, and in-hospital steps.14 During the self-management phase, it is highly recommended to increase the frequency of using relievers, primarily SABAs, alongside a recommendation to consider also increasing the ICS. For patients prescribed LD ICS-formoterol as a rescue medication, the inhalation frequency can be increased to a maximum of 12 times per day, followed by an earlier medical review in the subsequent primary care step.14 SABA is suggested to be administered with four to ten puffs every 20 minutes during the initial hour, with no recommendation regarding ICS use or simultaneous administration of systemic steroids for this first hour, although in our view, administering 40 puffs of SABA in the absence of ICS/oral corticosteroid is dangerous. Adjustments to the dosing interval should be made based on the patient response. If the patient responds well to initial treatment, additional inhalation is unnecessary; however, if the response is inadequate, SABA should be administered repeatedly during transfer to the hospital for acute exacerbations, ensuring the simultaneous administration of early systemic steroids and controlled-flow oxygen supplementation.14
In a hospital setting, SABA inhalation via a pressurized metered-dose inhaler frequently or via nebluizer continuously is initially suggested, followed by intermittent on-demand inhalation.14 Once symptoms improve and discharge with outpatient follow-up is considered appropriate, the SABA inhalation regimen should transition to an as-needed basis. Throughout this transition, the guidelines emphasize the importance of consistently administering controller medications. Thereafter, minor adjustments were subsequently made. In 2020, the self-management plan for patients prescribed beclomethasone-formoterol as their reliever recommended a maximum usage of eight times per day.16 In 2023, a limit of six times per day was advised for the budesonide-salbutamol combination inhaler, with immediate medical attention recommended for those exceeding this limit.8 Additionally, during the COVID-19 pandemic, the guidelines discouraged the use of nebulizers in emergency settings due to concerns about infection transmission.16
National Asthma Education and Prevention Program (NAEPP), UK, Korea, and Japanese guidelines
Tailored asthma management guidelines are being developed in various countries to align with medication availability and each nation’s unique medical practices.9,10,11,12,13,19 Variations in the use of beta-agonists are also evident across different countries. While the NAEPP guideline was updated in 2020, it has not yet endorsed the approach emphasized by GINA, which suggests ‘taking ICS whenever SABA is used.’20 Moreover, the NAEPP guideline only conditionally recommend that children aged 0–4 years with recurrent wheezing should start a short course of daily ICS with as-needed SABA at the onset of a respiratory tract infection for quick-relief.9 The NAEPP maintains the categorization of asthma into intermittent and persistent types, permitting the use of SABAs for monotherapy in intermittent asthma and as a pretreatment for EIB. For patients with persistent asthma, Step 2 recommends daily LD ICS use alongside as-needed SABA or concurrent on-demand ICS-LABA therapy. For higher stages, such as Step 3 and beyond, the NAEPP advocates the daily use of a combination of ICS-formoterol along with on-demand SABA inhalation as a reliever in conjunction with the existing medication regimen.
The British guidelines for asthma management, including the National Institute for Health and Care Excellence (NICE) guideline9 and the Scottish Intercollegiate Guidelines Network (SIGN),10 have not yet been fully aligned with GINA. The most recent 2021 NICE guideline (NG80) is closer to GINA’s Track 2, particularly in terms of beta-agonist usage.11 Symptom-based use of SABA is advised in the initial treatment of new asthma cases and those with infrequent wheezing and normal lung function. Similar to other guidelines, maintenance involves the proactive use of leukotriene receptor antagonists (LTRA), and like other guidelines, LABA is recommended alongside ICS. In uncontrolled asthma, options include escalating ICS dosing, increasing steroid doses within the MART approach, or raising fixed ICS dosing while using SABA as a reliever. The SIGN guideline (SIGN 158) also recommends short-acting bronchodilators, encompassing beta-agonists, theophylline, and inhaled ipratropium bromide, for managing infrequent and short-lived wheezing episodes.10 LABA is advised as an add-on therapy, co-administered with ICS. LABA is the first-choice treatment for adults and is equivalent to LTRA as an add-on treatment for children. Inhaled LABA is preferably administered using a single combination inhaler in a MART regimen. For acute exacerbations, SIGN 158 suggests immediate inhalation of beta-agonists as first-line agents, with SABA as the sole short-term reliever, while ICS co-administration is not explicitly recommended. Moreover, a pressurized metered-dose inhaler connected to a spacer is suggested for children. The use of intravenous (IV) beta-agonists is discouraged or reserved for severe cases, with IV use requiring lactate-level monitoring. Both SABA and LABAs are beneficial for EIB prophylaxis. However, SABA is recommended as the drug of choice for immediate administration before exercise.10
The asthma guidelines in South Korea and Japan exhibit distinct pathways. The updated 2022 asthma guideline by the Korean Academy of Tuberculosis and Respiratory Diseases predominantly incorporates the principles of the 2021 GINA guidelines.12 It delineates two distinct tracks: Track 1 advocates the use of low-dose ICS-formoterol as a reliever, designating it as the preferred approach, while Track 2 presents an alternative approach employing as-needed SABA as the reliever. Notably, Track 1 permits the omission of regular inhalation up to Step 2, whereas Track 2 recommends starting ICS at Step 1, even when using SABA as needed. Consistent with GINA 2021,17 the management of acute exacerbations entails the rapid and repeated inhalation of beta-2 agonists. Specifically, the recommendation entails inhaling 4 to 10 puffs of SABA every 20 minutes within the first hour to gauge the response. In contrast, the most recent revision of the Japanese asthma management guidelines adheres to a traditional Japan-specific approach.13 Asthma is stratified into four stages: mildly intermittent, mildly persistent, moderately persistent, and severely persistent. Throughout all stages, the guidelines advocate the use of SABA to relieve exacerbations. In Step 1, controllers are not recommended if asthma symptoms are rare.13 If symptoms worsen, LD ICS is introduced as a controller along with SABA. In Steps 2 and 3, the guidelines propose a MART regimen for controllers delivered through budesonide-formoterol, while recommending as-needed SABA for other cases. Intriguingly, certain medications developed in Japan, though lacking Food and Drug Administration (FDA) approval and international recognition, are utilized equivalently in long-term asthma management.13 The usage of MART for symptom relief during acute exacerbations is limited to a maximum of 12 inhalations per day, up to a duration of 3 days, with the necessity for prompt medical review if surpassing this limit.13
ADVERSE EFFECTS FROM USING BETA-AGONISTS
The use of beta-agonists can lead to multiple side effects due to the widespread distribution of their receptors in airway/lung cells beyond just airway smooth muscle and indeed throughout the entire body.1 Binding of beta-agonists to receptors in cells beyond and including airway smooth muscle may result in adverse effects, as well as their beneficial effects in relaxing airway smooth muscle. Initially, these adverse effects were found to be primarily associated with the inappropriate or excessive use of SABA in the absence of ICS. However, because all beta-agonists share the same mechanism of action, these adverse effects should theoretically occur with any beta-agonist.1,3 Indeed, similar adverse effects have been extensively reported with the use of LABAs in the absence of ICS.
Increased mortality
In the 1960s, 6 countries saw a surge in asthma-related deaths, which was suspected to be linked to the widespread use of a high-dose, non-selective beta agonist medication known as isoprenaline forte.21,22 Subsequently, another upsurge in asthma fatalities was observed in New Zealand, which coincided with the launch of a high-dose form of fenoterol.23,24 From the 1970s onwards, a gradual and consistent increase in asthma deaths was noticed in countries where fenoterol was frequently prescribed to patients with mild to moderate asthma.25 A review of asthma-related deaths in the UK showed that SABA overuse in the absence of ICS use is still a significant risk factor for asthma mortality.6,26 Even more concerning, the SABINA studies revealed a general overuse of SABA, which elevates the risk of asthma exacerbation and mortality.7 Even though no widespread increase in deaths has been reported, LABAs are reported to elevate the risk of asthma-related deaths, especially when salmeterol is prescribed regularly (and very likely overused at times of exacerbation) without accompanying ICS.27,28
Systemic adverse effects
Although beta-2 agonists exhibit receptor selectivity, this selectivity is not 100%. Given that most tissues express multiple subtypes of beta receptors, with one subtype usually being more predominant than the others, off-target effects are possible, especially with higher or more frequent dosing.29 Noteworthy side effects include palpitations, chest pain, tachycardia, and tremors, while headaches, nervousness, and muscle cramping are also commonly linked. These effects are most common with oral intake, followed by patches and inhaled agents,13 possibly intensified by excessive SABA inhaler use. Intravenous beta-agonist administration can induce serious outcomes such as hypokalemia, demanding vigilant monitoring, while high doses may lead to lactic acidosis.30,31 Therefore, caution is advised, especially in patients with conditions such as ischemic heart disease, hyperthyroidism, and diabetes, all of which can worsen by excessive beta-agonist use. Cardiac side effects, which have been previously implicated in increased asthma mortality,32 can be more dangerous under hypoxemic conditions.33 However, reductions in hospitalizations for asthma exacerbations (along with a reduction in asthma mortality) following the withdrawal of fenoterol in New Zealand indicated that the reduction in asthma mortality was not wholly due to a reduction in cardiac side effects, but at least partly due to a reduction in respiratory adverse effects. This is because if the reduction in mortality was only due to reduced cardiac side effects, hospitalization for asthma exacerbations should have remained unchanged.34
Poor asthma control and severe exacerbation
While beta-2 agonists aim to improve airway obstruction and alleviate asthma symptoms, the regular or excessive use of these agents is paradoxically associated with the exacerbation of airway hyperresponsiveness (AHR), loss of asthma control, prolonged asthma attacks, and declining lung function.35,36,37,38,39 These adverse effects, which were initially attributed to the overuse of SABAs in the absence of ICS, are observed across a broad spectrum of beta-agonists. Even in the routine utilization of LABAs in the absence of ICS, including salmeterol, formoterol, and the ultra-LABA indacaterol, there is a risk of asthma deterioration leading to hospitalization, intubation, and mortality.5,40,41,42 Conversely, employing SABAs/LABAs in conjunction with ICS in the same inhaler (so that the SABA/LABA cannot be used without ICS) has shown no additional risk of adverse events.43,44,45,46,47,48 For the as-needed and MART regimens of ICS-LABA, the risk of serious adverse events are not increased.49,50 In the case of salmeterol, although not explicitly approved as a MART regimen, the adverse effect profiles were comparable to those of formoterol when co-administered with ICS.51 Recent evidence indicates that even in patients who use beta-agonists judiciously, adverse events can arise if they are not accompanied by ICS.52 This underscores the fundamental rationale against recommending stand-alone beta-agonist use in patients with asthma and provides significant support for the evolving guidelines in this domain.
MECHANISMS UNDERLYING ADVERSE EVENTS FROM USING BETA-AGONISTS
Various hypotheses have been proposed to explain the mechanisms underlying the adverse effects associated with the use of beta-agonists. These include the issue of masking effects from temporary beta-stimulation effects,36 suboptimal utilization of anti-inflammatory agents,53 and problems such as induction of AHR, airway inflammation, and mucus secretion from beta-agonist usage in the absence of ICS.
Behavioral and psychosocial aspects
The increased frequency of severe asthma exacerbations requiring hospitalization and mortality is, in part, associated with patient behavior or psychosocial issues. As asthma symptoms can be almost immediately relieved by beta-2 agonists, patients may believe that using beta-2 agonists is sufficient to resolve their symptoms, and that ICS (which have no immediate symptom-relieving activity) are unnecessary and expensive.54,55 Moreover, some patients with asthma may regard ICS as being risky, although some of the long-term adverse effects of systemic steroids, including the exacerbation of diabetes mellitus, osteoporosis, and adrenal insufficiency, can be exaggerated.56,57 These issues can lead to the overuse of SABA and underuse of ICS, resulting in inadequate treatment of inflammation, which may cause a decline in lung function, worsened asthma control, an increased risk of exacerbation, and airway remodeling.53,58 Moreover, a previous experience of overcoming asthma exacerbation mainly with first-aid beta-agonists could make patients less sensitive in seeking medical review.59 As a result, patients with asthma, even mild asthma, frequently overuse SABA during asthma exacerbation, and unless appropriate personalized action plans including ICS use are given to them, their exacerbations become refractory to treatment when the patients come to an emergency room.6
Beta agonists induce inflammatory mediators and mucus secretion
The use of beta-agonists without ICS does not only result in the absence of the well known beneficial anti-inflammatory effects of ICS treatment. It also results in the loss of the protective benefits that ICS also have in directly protecting against the adverse effects of beta-agonists.
Evidence indicates that beta-2 agonists directly influence various airway cells, intensifying mucosal inflammation. Bronchial epithelial cells (BECs) are the cell type in the lower airways that are most directly exposed to inhalation therapy and they secrete interleukin (IL)-6 upon beta-2 agonist treatment, a phenomenon observed in both the human BEAS-2B cell line and primary normal human BECs.60 This induction of IL-6 in BECs can also be triggered by cyclic adenosine monophosphate (cAMP) or forskolin, providing evidence of the involvement of cAMP in the adverse effects of beta-2 agonists.60 Furthermore, during rhinovirus (RV) infection of BECs (simulating a virus-induced exacerbation), salmeterol was found to potently enhance IL-6 induction through the cAMP response element (CRE) in the IL-6 promoter, indicating a shared (cAMP-mediated) mechanism between the adverse effect in BECs of beta-2 agonist-induced IL-6 and cAMP-mediated relaxation effects in airway smooth muscle.60,61
Beta-2 agonists exert their influence on other mediators of asthma exacerbation. Two weeks of standard-dose salmeterol monotherapy increased AHR with elevated brain-derived neurotrophic factor (BDNF) in the serum and platelets of patients with asthma; the increased BDNF concentrations in serum and platelets correlated with the deterioration of AHR.62 Salmeterol has also been shown to increase BDNF release from mononuclear cells. As BDNF is known to enhance AHR63 and given that its regulation is mediated by the binding of CRE-binding protein (CREB) to the CRE in its gene promoter,64 similar to what is observed with IL-6, AHR aggravation could be attributed to the release of BDNF via the elevation of cAMP induced by salmeterol.61 In addition to inducing IL-6 and BDNF, salmeterol has been shown to significantly upregulate IL-11 and seven other genes that are less well-studied in asthma in BECs compared to control media. Among these genes, the addition of salmeterol to RV-infected cells resulted in further upregulation of all but one gene compared to cells infected with RV alone.65 Moreover, intravenous administration of SABA has been shown to elevate matrix metalloproteinase (MMP)-9 levels and activity in bronchoalveolar lavage fluid in patients.66 MMP-9 is also regulated by CREB binding to CRE in the MMP-9 gene promoter region in response to cAMP.67
Collectively, these findings suggest that the induction of beta-2 agonist-induced mediators through cAMP and CREs in humans likely mediates the adverse respiratory effects of beta-2 agonists. Factors such as IL-17, IL-33, cyclooxygenase (COX)-2, amphiregulin, thrombospondin (TSP)-1, vascular endothelial growth factor (VEGF)-A, MMP-1, MMP-2, MMP-10, mucin 2 (MUC2), MUC5AC, MUC5B, MUC7, and MUC8, all implicated in asthma exacerbation, may be induced by beta-2 agonists through cAMP induction, as they all have CREs in their promoter regions.68,69,70,71,72,73,74,75,76,77 However, evidence on their beta-2 agonist-induced expression in lung cells or patients with asthma remains limited. Theoretically, over 5,000 genes in humans can be regulated by CREs in their promoters, and 233 genes have been shown to be induced by cAMP-inducing agents in human cells,78 but relatively little information is available on respiratory cell types, such as airway macrophages and BECs. According to a recent study, beta-2 adrenoceptor signaling, mediated by beta-2 receptor expression on BECs, exacerbated IL-13-induced AHR, eosinophilic inflammation, and mucus-production.79
ICS protect against beta agonist induction of inflammatory mediators
Considering the impact of steroids on cell signaling and gene expression,80 the induction of CRE-regulated asthma-related mediators induced by increased cAMP due to beta-2 agonists might be suppressed by ICS. Studies have demonstrated the attenuation of salmeterol-induced IL-6 and the reduction in salmeterol augmentation of RV-induced IL-6 with the use of ICS in BECs in vitro.60 This was confirmed in a subsequent study, which also reported the attenuation of salmeterol-induced BDNF and the reduction in salmeterol augmentation of RV-induced BDNF with the use of ICS in BECs.65 Moreover, it has been confirmed that fluticasone inhibits the production of BDNF induced by salmeterol in serum and platelets in vivo, as well as its induction in mononuclear cells in vitro.62 Furthermore, evidence indicates that inhaled steroids and other corticosteroids suppress other exacerbating factors of asthma, including amphiregulin, TSP-1, VEGF A,73 IL-17,81 COX-2 transcription,82 the mRNA expression of MMP-2 and MMP-9,83,84 and the gene expression of MUC2 and MUC5AC.85 Clinical guidelines now advocate for the concurrent use of LABA and ICS, introducing ICS-containing AIRs in the 2023 GINA guidelines.8 Therefore, it becomes crucial to investigate whether beta-2 agonist-induced asthma exacerbation mediators, including IL-6, IL-11, IL-17, BDNF, COX-2, MMPs, mucins, and others, are effectively reduced by ICS treatment through in vitro and in vivo studies. The confirmation of these findings would provide compelling evidence for the use of AIRs.
Beta agonist induces a vicious cycle of worsening airway inflammation and refractory exacerbation
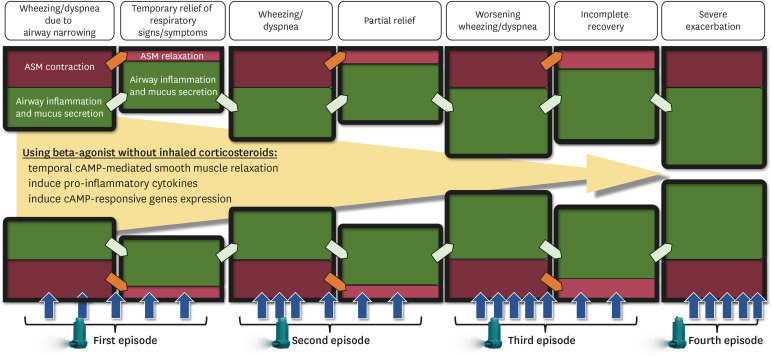
By combining the aforementioned factors, a possible mechanism underlying the exacerbation of asthma through unprotected (by ICS) beta-agonist use is illustrated in Figure. Airway obstruction is driven by increased inflammation, mucin secretion, and smooth muscle contraction. Administration of high-dose beta-agonists without ICS initially leads to temporary relief due to airway smooth muscle relaxation, but augments airway inflammation and mucus production through the induction of cAMP-regulated inflammatory mediators in BECs and other airway cells exposed to beta-agonists. Relief from smooth muscle relaxation wanes and requires escalated SABA doses owing to worsening airway inflammation and mucus secretion. Persistently escalated unprotected beta-agonist inhalation further elevates cAMP levels, inducing further airway inflammation and mucin secretion in BECs, lung macrophages, and other cell types. This cascade further exacerbates inflammation and mucin secretion, culminating in severe airway constriction and exacerbation that is refractory to, and indeed exacerbated by, further unprotected (by ICS) SABA use.
Figure. Putative mechanism of progressive luminal narrowing in the asthmatic airways due to beta-agonist inhalation unopposed by inhaled corticosteroids. Although patients inhale beta-agonists more frequently, which dampens the degree of ASM contraction, they worsen the degree of airway inflammation and mucus secretion.
ASM, airway smooth muscle; cAMP, cyclic adenosine monophosphate.
In contrast, high-dose beta-agonist use with ICS (preferably in the same inhaler so that the beta-agonist cannot be taken without the ICS) results in the adverse effects of the beta-agonist being blocked by the ICS, leaving only the beneficial beta-agonist effect of smooth muscle relaxation. Additionally, beyond the important property of blocking the adverse effects of beta-agonists, ICS also has the beneficial effect of dampening allergic (and likely virus-induced) airway inflammation.
PRACTICAL APPROACH WITH RELEVANCE TO THE REAL-WORLD SETTING
For the general, stable asthma patient with occasional aggravation
In clinical practice, the side effects that truly matter should be prioritized. Given that severe systemic adverse effects are uncommon in individuals with asthma, the primary concern of physicians should be minimizing asthma exacerbations. To achieve and maintain optimal asthma control, the recommended strategy involves combining ICS with beta-agonists, utilizing the most effective dosage with a maintenance and reliever plan.8,86 Indeed, using a budesonide-formoterol combination inhaler on an as-needed basis has shown comparable efficacy to twice-daily low-dose budesonide maintenance therapy combined with SABA used as-needed.87,88 The budesonide-formoterol combination inhaler effectively maintains asthma control while significantly reducing overall corticosteroid exposure compared to regular ICS use. Similarly, the beclomethasone-albuterol combination was successful in maintaining asthma control with minimal exposure to ICS.47 Moreover, the risk of severe asthma exacerbation has been shown to be effectively reduced by an as-needed use of the budesonide-albuterol combination rather than by the albuterol-only inhalation.48 Therefore, transitioning to the as-needed, fixed combination of ICS-beta-agonists is advisable when asthma management is successful and exacerbations are infrequent.8
Although guidelines correctly explicitly discourage the standalone use of LABA for patients with asthma,8,9,10,12 the stance on SABA monotherapy remains uncertain.86,89 Consequently, some experts advocate discontinuing standalone SABA inhalers from the market—a concept dubbed ‘banning blue inhalers’—to underscore this perspective.90 The recent introduction of the fixed-dose budesonide-albuterol combination inhaler, as a part of AIR medication, could facilitate this strategy.8,89 When prescribing as-needed SABA for intermittent or mild asthma, especially during exacerbations, it is crucial to consistently emphasize concurrent regular ICS use for control.88 Conversely, for those experiencing adverse effects from beta-agonist itself or in specific populations requiring caution, utilizing a fixed-dose steroid with a SABA reliever, in accordance with GINA Track 2, appears appropriate.8,9,10,11,12,13 Even though SABA needs to be utilized, it is crucial to inhale it in conjunction with ICS for control, rather than relying on beta-agonists alone.88
The most fundamental approach to avoid beta-agonist overusage is to reduce the risk of exacerbation, which involves the proficient management of non-pharmacological therapeutic factors. Reassessing triggers, evaluating adherence, assessing inhaler technique, and addressing untreated comorbidities, namely, rhinitis, polyps, gastroesophageal reflux disease, and obstructive sleep apnea, cannot be emphasized enough as being key factors to decrease unnecessary medication use.8 In cases where exacerbation occurs despite proper management, beyond combined ICS/SABA or ICS/LABA use, considering alternative strategies involving different bronchodilators other than beta-agonists could be beneficial. One of the first alternatives is muscarinic antagonists,10,91 which do not induce the same pro-inflammatory mediators as beta-agonists.92
Beta-agonist plus ICS in virus-induced asthma exacerbation
The chronic use of ICS is known to increase the risk of pneumonia in patients with obstructive respiratory diseases.93,94 ICS can also dampen innate immune responses to infectious agents, including RVs.95,96 However, in asthma, it is likely that ICS aid in restoring deficient innate antiviral defenses by mitigating type 2 cytokine responses, which are known to weaken innate antiviral immunity.97 ICS use is known to dramatically reduce the frequency of asthma exacerbation. Epidemiological data on hospital admissions for asthma exacerbations have indicated that non-users of ICS were more prevalent in the RV-positive group than in the RV-negative group.98 Thus, for patients with asthma, the benefits of using ICS outweigh the risks, as they help reduce hospitalizations due to virus-induced asthma exacerbations. This favorable outcome may be partially attributed to the role of ICS in reducing conditions that are prone to exacerbation,99 where inhaled beta-agonists enhance virus-induced inflammation.65
According to the existing literature, both in vitro and in vivo studies have shown that RV infection, the primary cause of the common cold, triggers the expression of a diverse array of cytokines (e.g., IL-1, IL-6, IL-11), growth factors (e.g., G-CSF, GM-CSF), and chemokines (e.g., CXCL8, CXCL5, CXCL10, and RANTES).100,101 This cascade leads to the activation and recruitment of inflammatory cells to the airways. In asthmatic individuals with RV infection, there is an increase in the epithelial alarmin IL-33, as well as IL-4, IL-5, and IL-13, along with airway eosinophils.70 Furthermore, the clinical severity of asthma exacerbation is closely correlated with the levels of IL-5 and IL-13. Another study involving human cell lines and primary human BECs demonstrated that RV reduced beta-agonist-induced cAMP levels, resulting in reduced beta-2 receptor agonist functionality.102 This reduction necessitates higher doses of beta-agonists, potentially leading to the induction of more inflammatory cytokines and chemokines if the beta-agonists are not countered by ICS.103
Considering that type 2 cytokines weaken the antiviral response in the respiratory epithelia97 and cannot be controlled (or may even be worsened) by beta-agonists alone,65,101 virus-induced asthma exacerbation can be effectively managed by an AIR strategy employing the combined use of beta-agonists and ICS, rather than relying solely on beta-agonist treatment. Consequently, the general approach of combining beta-agonists with ICS can also be applied to situations of asthma exacerbation triggered primarily by viral infections, particularly those caused by RV infections.104,105
Occasions where fixed combination preparation is unavailable
Although budesonide-formoterol is approved worldwide, such fixed combination treatments have not been approved for use in all countries for patients with asthma. The beclomethasone-formoterol combination has been sanctioned for use in the UK and Australia but is still pending approval from the US FDA, whereas the preparation of budesonide-salbutamol was approved in 2023. However, as of April 2024, this ICS-SABA combination has not yet garnered approval in other countries. In terms of age groups, for children aged ≥ 12 years, similar to adults, the ICS-LABA combination is authorized for maintenance, as-needed, or MART regimen use. However, for children between 6 and 11 years of age, recent approvals have been granted exclusively for maintenance and MART use, while as-needed inhalation of combination preparations still awaits FDA approval. Furthermore, LABA inhalers have not been approved for use in children under 6 years of age. Hence, it is reasonable to explore the optimal alternatives within the approved scope, considering factors such as country and age group, although this may seem complex at first glance.
Nevertheless, when we reviewed the recommendations of GINA 2023 and other guidelines, several common principles regarding the use of beta-agonists emerged. First, SABA should not be used as a controller in any asthma severity; rather, it should focus solely on short-term use for symptom relief, as use in the absence of smooth muscle contraction would result in no beneficial effects and only adverse effects. This should be applied regardless of the current treatment status, whether the patient is using an ICS as a controller or concurrently using LTRA or LAMA. Second, beta-agonists must be administered alongside corticosteroids.106 While employing fixed combinations in the same inhaler is preferable,8,86,89 SABA and ICS as separate inhalers are currently suggested as an alternative option. Furthermore, for patients employing the ICS-LABA combination as their maintenance, the MART regimen should be considered.49,107
Alternative, newly-emerging medications outside the US and Europe
In countries such as Japan, domestically developed medications play an active role in asthma management.13,19 These medications have not been introduced in the US or Europe, lack FDA approval, and are not endorsed by other guidelines. Representative forms include the tulobuterol transdermal patch, a dermal formulation of LABA designed to sustain bronchodilator effects for 24 hours.108 Owing to its unique crystal reservoir system, the therapeutic effect of the tulobuterol patch can be sustained for a prolonged period, positioning it as a LABA. According to the Japanese Pediatric Guideline for the treatment and management of asthma, tulobuterol is highly effective for asthma management when used alongside ICS.19 Beta-agonists in oral form are also recommended as valuable treatment options. Drugs such as procaterol, clenbuterol, and mabuterol function similarly to LABAs.13 Although the latest Japanese asthma treatment guidelines still advocate the use of SABA without concurrent ICS for asthma with rare symptoms, we advocate that all beta-agonists should only be used with concomitant ICS as stand alone beta-agonist use is clearly unsafe, resulting in completely preventable fatalities.
Time to move on: shifting from regular ICS plus as-needed SABA to MART/AIR strategies in asthma management
Some may still argue that while recent evidence supports the use of combination therapy, it would be premature to abandon the traditional approach of regular ICS plus SABA reliever to replace all SABA usage with ICS-LABA or ICS-SABA fixed combinations. However, we advocate transitioning to the AIR or MART strategy, as the traditional approach of regular ICS plus a SABA reliever still leaves poorly compliant individuals at risk of dying from SABA (and more rarely LABA) overuse in the absence of ICS.
For symptom relief outside of exacerbations and to prevent EIB, a fixed combination of formoterol and ICS could replace SABA. Additionally, a combination of budesonide and albuterol has recently been reported to be effective in the management of EIB. Furthermore, patients who consistently use ICS are likely to adhere well to MART. Recent studies indicate that the AIR strategy is quite effective compared to the traditional ICS plus SABA reliever, particularly under specific conditions. While SYGMA 1 and 2, as well as the BEST trial, showed similar rates of exacerbation for both treatments, regular ICS and SABA relievers required significantly higher doses of corticosteroids, up to four- to six-fold increases, to achieve better control.47,48,87,109 Moreover, AIR has shown clear benefits in reducing severe exacerbations at half the ICS dose in the Novel START and PRACTICAL studies, with fewer severe exacerbations and a longer duration before the first severe exacerbation than regular ICS and SABA relievers.110,111 These findings suggest that AIR is a superior option, offering effective asthma management with lower corticosteroid doses, especially in reducing severe symptoms.
To date, there is no evidence to suggest that as-needed ICS-formoterol is more effective than as-needed SABA for mild asthma in GINA Step 1, as research on as-needed ICS-formoterol has primarily focused on GINA Step 2.106 In contrast, 70% of participants in the BEST study were in GINA Step 1, which strongly supports the AIR regimen over SABA alone.47,48
Finally, a small subset of patients may exhibit minimal symptoms despite clear evidence of ongoing active allergic airway inflammation. In such cases, some clinicians may recommend regular ICS plus SABA over ‘as-needed’ AIR to prevent undertreatment of the active disease, which could lead to airway remodeling. For these individuals, adopting a MART strategy instead of regular ICS plus SABA as a reliever may mitigate this concern. Therefore, it is crucial to monitor how these considerations can be incorporated into upcoming asthma treatment guidelines.
CONCLUSION
Beta-agonists are essential medications for relieving asthma symptoms; however, they induce significant adverse events when used regularly or at high doses intermittently, both in the absence of ICS. This concern extends beyond overuse and the risk of symptom masking. Stand-alone administration of beta-agonists can exacerbate airway inflammation and increase mucus secretion, potentially rendering asthma more hazardous. As our understanding of the mechanisms behind adverse effects improves, a prudent strategy for beta-agonist use in patients with asthma involves maintaining ICS with as-needed SABA, where cost dictates, but preferably employing fixed-dose combinations of ICS with SABA/LABA in the same inhaler so that SABA/LABAs cannot be taken in the absence of ICS. In the context of the current understanding and various guidelines, a rational strategy involves the use of ICS-SABA or ICS-LABA as an AIR regimen or MART. This approach aligns with complex clinical scenarios and embraces a comprehensive guideline-compliance scheme.
ACKNOWLEDGMENTS
SLJ is a National Institute for Health Research (NIHR) Emeritus Senior Investigator and received support from the Asthma UK Clinical Chair (Grant CH11SJ), European Research Council Advanced Grants 233015 and 788575, Medical Research Council Centre Grant G1000758 and Asthma UK Centre Grant AUK-BC-2015-01.
Footnotes
Disclosure: SLJ has received consultancy fees from AstraZeneca, Bioforce, Enanta and GlaxoSmithKline and is an inventor on patents on the use of inhaled interferons for treatment of exacerbations of airway diseases and on rhinovirus vaccines. SLJ is Director and shareholder of Virtus Respiratory Research Ltd.
References
- 1.Johnson M. The beta-adrenoceptor. Am J Respir Crit Care Med. 1998;158:S146–S153. doi: 10.1164/ajrccm.158.supplement_2.13tac110. [DOI] [PubMed] [Google Scholar]
- 2.Billington CK, Penn RB, Hall IP. β2 agonists. Handb Exp Pharmacol. 2017;237:23–40. doi: 10.1007/164_2016_64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Velmurugan BK, Baskaran R, Huang CY. Detailed insight on β-adrenoceptors as therapeutic targets. Biomed Pharmacother. 2019;117:109039. doi: 10.1016/j.biopha.2019.109039. [DOI] [PubMed] [Google Scholar]
- 4.Crane J, Pearce N, Flatt A, Burgess C, Jackson R, Kwong T, et al. Prescribed fenoterol and death from asthma in New Zealand, 1981-83: case-control study. Lancet. 1989;1:917–922. doi: 10.1016/s0140-6736(89)92505-1. [DOI] [PubMed] [Google Scholar]
- 5.Chowdhury BA, Dal Pan G. The FDA and safe use of long-acting beta-agonists in the treatment of asthma. N Engl J Med. 2010;362:1169–1171. doi: 10.1056/NEJMp1002074. [DOI] [PubMed] [Google Scholar]
- 6.Levy ML. The national review of asthma deaths: what did we learn and what needs to change? Breathe (Sheff) 2015;11:14–24. doi: 10.1183/20734735.008914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55:1901872. doi: 10.1183/13993003.01872-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global INitiative for Asthma (GINA) Global strategy for asthma management and prevention [Internet] place unknown: GINA; 2023. [cited 2024 Apr 24]. Available from: https://ginasthma.org/2023-gina-main-report/ [Google Scholar]
- 9.Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC) Cloutier MM, Baptist AP, Blake KV, Brooks EG, Bryant-Stephens T, et al. 2020 Focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146:1217–1270. doi: 10.1016/j.jaci.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.British Thoracic Society and Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. London: Edinburgh; 2019. [Google Scholar]
- 11.National Institute for Health and Care Excellence. Asthma: diagnosis, monitoring and chronic asthma management NICE guideline (NG80) London: National Institute for Health and Care Excellence; 2017. [Google Scholar]
- 12.Rhee CK, Moon JY, Joo H, Jung JY, Lee JK, Min KH, et al. Summary of Korean asthma guideline. Tuberc Respir Dis (Seoul) 2023;86:158–165. doi: 10.4046/trd.2023.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niimi A, Fukunaga K, Taniguchi M, Nakamura Y, Tagaya E, Horiguchi T, et al. Executive summary: Japanese guidelines for adult asthma (JGL) 2021. Allergol Int. 2023;72:207–226. doi: 10.1016/j.alit.2023.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Global INitiative for Asthma (GINA) Global strategy for asthma management and prevention [Internet] place unknown: GINA; 2018. [cited 2024 Apr 24]. Available from: https://ginasthma.org/wp-content/uploads/2023/04/GINA-Main-Report-V1.3-2018-WMSA.pdf. [Google Scholar]
- 15.Global INitiative for Asthma (GINA) Global strategy for asthma management and prevention [Internet] place unknown: GINA; 2019. [cited 2024 Apr 24]. Available from: https://ginasthma.org/wp-content/uploads/2023/04/GINA-Main-Report-2019-WMSA.pdf. [Google Scholar]
- 16.Global INitiative for Asthma (GINA) Global strategy for asthma management and prevention [Internet] place unknown: GINA; 2020. [cited 2024 Aug 24]. Available from: https://ginasthma.org/wp-content/uploads/2023/04/GINA-2020-Main-Report-WMSA.pdf. [Google Scholar]
- 17.Global INitiative for Asthma (GINA) Global strategy for asthma management and prevention [Internet] place unknown: GINA; 2021. [cited 2024 Apr 24]. Available from: https://ginasthma.org/wp-content/uploads/2023/04/GINA-Main-Report-2021-V2-WMSA.pdf. [Google Scholar]
- 18.Global INitiative for Asthma (GINA) Global strategy for asthma management and prevention [Internet] place unknown: GINA; 2022. [cited 2024 Apr 24]. Available from: https://ginasthma.org/wp-content/uploads/2023/05/GINA-Main-Report-2022-WMSA.pdf. [Google Scholar]
- 19.Arakawa H, Adachi Y, Ebisawa M, Fujisawa T, et al. Committee for Japanese Pediatric Guideline for Childhood Asthma; Japanese Society of Pediatric Allergy and Clinical Immunology. Japanese guidelines for childhood asthma 2020. Allergol Int. 2020;69:314–330. doi: 10.1016/j.alit.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Larenas-Linnemann DE. Short-acting beta-2-agonist exposure and severe asthma exacerbations: analysis of the recently reported findings from the European and North American SABINA Datasets. J Allergy Clin Immunol Pract. 2022;10:2310–2311. doi: 10.1016/j.jaip.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Beasley R, Pearce N, Crane J, Windom H, Burgess C. Asthma mortality and inhaled beta agonist therapy. Aust N Z J Med. 1991;21:753–763. doi: 10.1111/j.1445-5994.1991.tb01385.x. [DOI] [PubMed] [Google Scholar]
- 22.Hills T, Beasley R. The history and future of short-acting beta2 -agonist therapy in asthma. Respirology. 2020;25:246–248. doi: 10.1111/resp.13727. [DOI] [PubMed] [Google Scholar]
- 23.Jackson RT, Beaglehole R, Rea HH, Sutherland DC. Mortality from asthma: a new epidemic in New Zealand. Br Med J (Clin Res Ed) 1982;285:771–774. doi: 10.1136/bmj.285.6344.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beasley R. A historical perspective of the New Zealand asthma mortality epidemics. J Allergy Clin Immunol. 2006;117:225–228. doi: 10.1016/j.jaci.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Crane J, Pearce N, Burgess C, Beasley R. Asthma and the beta agonist debate. Thorax. 1995;50(Suppl 1):S5–10. doi: 10.1136/thx.50.suppl_1.s5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sriprasart T, Waterer G, Garcia G, Rubin A, Andrade MA, Roguska A, et al. Safety of SABA monotherapy in asthma management: a systematic review and meta-analysis. Adv Ther. 2023;40:133–158. doi: 10.1007/s12325-022-02356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodrigo GJ, Castro-Rodríguez JA. Safety of long-acting β agonists for the treatment of asthma: clearing the air. Thorax. 2012;67:342–349. doi: 10.1136/thx.2010.155648. [DOI] [PubMed] [Google Scholar]
- 28.Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med. 2006;144:904–912. doi: 10.7326/0003-4819-144-12-200606200-00126. [DOI] [PubMed] [Google Scholar]
- 29.Williams DM, Rubin BK. Clinical pharmacology of bronchodilator medications. Respir Care. 2018;63:641–654. doi: 10.4187/respcare.06051. [DOI] [PubMed] [Google Scholar]
- 30.Gross TL, Sokol RJ. Severe hypokalemia and acidosis: a potential complication of beta-adrenergic treatment. Am J Obstet Gynecol. 1980;138:1225–1226. doi: 10.1016/s0002-9378(16)32800-9. [DOI] [PubMed] [Google Scholar]
- 31.Marazzini L, Pelucchi A, Bozzoni M, Mastropasqua B, Longhini E. The effect of intravenously administered salbutamol on serum potassium in asthmatic and nonasthmatic atopic subjects. J Clin Immunol. 1985;5:195–203. doi: 10.1007/BF00915511. [DOI] [PubMed] [Google Scholar]
- 32.Wong CS, Pavord ID, Williams J, Britton JR, Tattersfield AE. Bronchodilator, cardiovascular, and hypokalaemic effects of fenoterol, salbutamol, and terbutaline in asthma. Lancet. 1990;336:1396–1399. doi: 10.1016/0140-6736(90)93099-b. [DOI] [PubMed] [Google Scholar]
- 33.Burggraaf J, Westendorp RG, in’t Veen JC, Schoemaker RC, Sterk PJ, Cohen AF, et al. Cardiovascular side effects of inhaled salbutamol in hypoxic asthmatic patients. Thorax. 2001;56:567–569. doi: 10.1136/thorax.56.7.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sears MR. Adverse effects of beta-agonists. J Allergy Clin Immunol. 2002;110(Suppl):S322–S328. doi: 10.1067/mai.2002.129966. [DOI] [PubMed] [Google Scholar]
- 35.Aldridge RE, Hancox RJ, Robin Taylor D, Cowan JO, Winn MC, Frampton CM, et al. Effects of terbutaline and budesonide on sputum cells and bronchial hyperresponsiveness in asthma. Am J Respir Crit Care Med. 2000;161:1459–1464. doi: 10.1164/ajrccm.161.5.9906052. [DOI] [PubMed] [Google Scholar]
- 36.Beasley R, Pearce N, Crane J, Burgess C. β-agonists: what is the evidence that their use increases the risk of asthma morbidity and mortality? J Allergy Clin Immunol. 1999;104:S18–S30. doi: 10.1016/s0091-6749(99)70270-8. [DOI] [PubMed] [Google Scholar]
- 37.Taylor DR, Town GI, Herbison GP, Boothman-Burrell D, Flannery EM, Hancox B, et al. Asthma control during long-term treatment with regular inhaled salbutamol and salmeterol. Thorax. 1998;53:744–752. doi: 10.1136/thx.53.9.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor DR, Sears MR, Herbison GP, Flannery EM, Print CG, Lake DC, et al. Regular inhaled beta agonist in asthma: effects on exacerbations and lung function. Thorax. 1993;48:134–138. doi: 10.1136/thx.48.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drazen JM, Israel E, Boushey HA, Chinchilli VM, Fahy JV, Fish JE, et al. Comparison of regularly scheduled with as-needed use of albuterol in mild asthma. N Engl J Med. 1996;335:841–847. doi: 10.1056/NEJM199609193351202. [DOI] [PubMed] [Google Scholar]
- 40.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 41.Cates CJ, Cates MJ. Regular treatment with formoterol for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2012;4:CD006923. doi: 10.1002/14651858.CD006923.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chowdhury BA, Seymour SM, Michele TM, Durmowicz AG, Liu D, Rosebraugh CJ. The risks and benefits of indacaterol--the FDA’s review. N Engl J Med. 2011;365:2247–2249. doi: 10.1056/NEJMp1109621. [DOI] [PubMed] [Google Scholar]
- 43.Stempel DA, Raphiou IH, Kral KM, Yeakey AM, Emmett AH, Prazma CM, et al. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med. 2016;374:1822–1830. doi: 10.1056/NEJMoa1511049. [DOI] [PubMed] [Google Scholar]
- 44.Stempel DA, Szefler SJ, Pedersen S, Zeiger RS, Yeakey AM, Lee LA, et al. Safety of adding salmeterol to fluticasone propionate in children with asthma. N Engl J Med. 2016;375:840–849. doi: 10.1056/NEJMoa1606356. [DOI] [PubMed] [Google Scholar]
- 45.Peters SP, Bleecker ER, Canonica GW, Park YB, Ramirez R, Hollis S, et al. Serious asthma events with budesonide plus formoterol vs. budesonide alone. N Engl J Med. 2016;375:850–860. doi: 10.1056/NEJMoa1511190. [DOI] [PubMed] [Google Scholar]
- 46.Food and Drug Administration (FDA) FDA drug safety communication: FDA review finds no significant increase in risk of serious asthma outcomes with long-acting beta agonists (LABAs) used in combination with inhaled corticosteroids (ICS) [Internet] Silver Spring (MD): FDA; 2017. [cited 2024 Apr 24]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-review-finds-no-significant-increase-risk-serious-asthma-outcomes. [Google Scholar]
- 47.Papi A, Canonica GW, Maestrelli P, Paggiaro P, Olivieri D, Pozzi E, et al. Rescue use of beclomethasone and albuterol in a single inhaler for mild asthma. N Engl J Med. 2007;356:2040–2052. doi: 10.1056/NEJMoa063861. [DOI] [PubMed] [Google Scholar]
- 48.Papi A, Chipps BE, Beasley R, Panettieri RA, Jr, Israel E, Cooper M, et al. Albuterol-budesonide fixed-dose combination rescue inhaler for asthma. N Engl J Med. 2022;386:2071–2083. doi: 10.1056/NEJMoa2203163. [DOI] [PubMed] [Google Scholar]
- 49.Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA. 2018;319:1485–1496. doi: 10.1001/jama.2018.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogliani P, Ritondo BL, Ora J, Cazzola M, Calzetta L. SMART and as-needed therapies in mild-to-severe asthma: a network meta-analysis. Eur Respir J. 2020;56:2000625. doi: 10.1183/13993003.00625-2020. [DOI] [PubMed] [Google Scholar]
- 51.O’Shea O, Stovold E, Cates CJ. Regular treatment with formoterol and an inhaled corticosteroid versus regular treatment with salmeterol and an inhaled corticosteroid for chronic asthma: serious adverse events. Cochrane Database Syst Rev. 2021;4:CD007694. doi: 10.1002/14651858.CD007694.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Byrne PM, Jenkins C, Bateman ED. The paradoxes of asthma management: time for a new approach? Eur Respir J. 2017;50:1701103. doi: 10.1183/13993003.01103-2017. [DOI] [PubMed] [Google Scholar]
- 53.Amin S, Soliman M, McIvor A, Cave A, Cabrera C. Usage patterns of short-acting β2-agonists and inhaled corticosteroids in asthma: a targeted literature review. J Allergy Clin Immunol Pract. 2020;8:2556–2564.e8. doi: 10.1016/j.jaip.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 54.Cole S, Seale C, Griffiths C. ‘The blue one takes a battering’ why do young adults with asthma overuse bronchodilator inhalers? A qualitative study. BMJ Open. 2013;3:2247. doi: 10.1136/bmjopen-2012-002247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blakeston S, Harper G, Zabala Mancebo J. Identifying the drivers of patients’ reliance on short-acting β2-agonists in asthma. J Asthma. 2021;58:1094–1101. doi: 10.1080/02770903.2020.1761382. [DOI] [PubMed] [Google Scholar]
- 56.Boulet LP. Perception of the role and potential side effects of inhaled corticosteroids among asthmatic patients. Chest. 1998;113:587–592. doi: 10.1378/chest.113.3.587. [DOI] [PubMed] [Google Scholar]
- 57.Hui RW. Inhaled corticosteroid-phobia and childhood asthma: current understanding and management implications. Paediatr Respir Rev. 2020;33:62–66. doi: 10.1016/j.prrv.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 58.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161:1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 59.Pilcher J, Patel M, Pritchard A, Thayabaran D, Ebmeier S, Shaw D, et al. Beta-agonist overuse and delay in obtaining medical review in high risk asthma: a secondary analysis of data from a randomised controlled trial. NPJ Prim Care Respir Med. 2017;27:33. doi: 10.1038/s41533-017-0032-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edwards MR, Haas J, Panettieri RA, Jr, Johnson M, Johnston SL. Corticosteroids and beta2 agonists differentially regulate rhinovirus-induced interleukin-6 via distinct Cis-acting elements. J Biol Chem. 2007;282:15366–15375. doi: 10.1074/jbc.M701325200. [DOI] [PubMed] [Google Scholar]
- 61.Johnston SL, Edwards MR. Mechanisms of adverse effects of β-agonists in asthma. Thorax. 2009;64:739–741. doi: 10.1136/thx.2009.119230. [DOI] [PubMed] [Google Scholar]
- 62.Lommatzsch M, Lindner Y, Edner A, Bratke K, Kuepper M, Virchow JC. Adverse effects of salmeterol in asthma: a neuronal perspective. Thorax. 2009;64:763–769. doi: 10.1136/thx.2008.110916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bennedich Kahn L, Gustafsson LE, Olgart Höglund C. Brain-derived neurotrophic factor enhances histamine-induced airway responses and changes levels of exhaled nitric oxide in guinea pigs in vivo . Eur J Pharmacol. 2008;595:78–83. doi: 10.1016/j.ejphar.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 64.Tabuchi A, Sakaya H, Kisukeda T, Fushiki H, Tsuda M. Involvement of an upstream stimulatory factor as well as cAMP-responsive element-binding protein in the activation of brain-derived neurotrophic factor gene promoter I. J Biol Chem. 2002;277:35920–35931. doi: 10.1074/jbc.M204784200. [DOI] [PubMed] [Google Scholar]
- 65.Ritchie AI, Singanayagam A, Wiater E, Edwards MR, Montminy M, Johnston SL. β2-agonists enhance asthma-relevant inflammatory mediators in human airway epithelial cells. Am J Respir Cell Mol Biol. 2018;58:128–132. doi: 10.1165/rcmb.2017-0315LE. [DOI] [PubMed] [Google Scholar]
- 66.O’Kane CM, McKeown SW, Perkins GD, Bassford CR, Gao F, Thickett DR, et al. Salbutamol up-regulates matrix metalloproteinase-9 in the alveolar space in the acute respiratory distress syndrome. Crit Care Med. 2009;37:2242–2249. doi: 10.1097/CCM.0b013e3181a5506c. [DOI] [PubMed] [Google Scholar]
- 67.Park JK, Park SH, So K, Bae IH, Yoo YD, Um HD. ICAM-3 enhances the migratory and invasive potential of human non-small cell lung cancer cells by inducing MMP-2 and MMP-9 via Akt and CREB. Int J Oncol. 2010;36:181–192. [PubMed] [Google Scholar]
- 68.Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, et al. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yanagawa Y, Matsumoto M, Togashi H. Adrenoceptor-mediated enhancement of interleukin-33 production by dendritic cells. Brain Behav Immun. 2011;25:1427–1433. doi: 10.1016/j.bbi.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 70.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo . Am J Respir Crit Care Med. 2014;190:1373–1382. doi: 10.1164/rccm.201406-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu W, Silbajoris RA, Cao D, Bromberg PA, Zhang Q, Peden DB, et al. Regulation of cyclooxygenase-2 expression by cAMP response element and mRNA stability in a human airway epithelial cell line exposed to zinc. Toxicol Appl Pharmacol. 2008;231:260–266. doi: 10.1016/j.taap.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Du B, Altorki NK, Kopelovich L, Subbaramaiah K, Dannenberg AJ. Tobacco smoke stimulates the transcription of amphiregulin in human oral epithelial cells: evidence of a cyclic AMP-responsive element binding protein-dependent mechanism. Cancer Res. 2005;65:5982–5988. doi: 10.1158/0008-5472.CAN-05-0628. [DOI] [PubMed] [Google Scholar]
- 73.Nguyen HO, Salvi V, Tiberio L, Facchinetti F, Govoni M, Villetti G, et al. The PDE4 inhibitor tanimilast shows distinct immunomodulatory properties associated with a type 2 endotype and CD141 upregulation. J Transl Med. 2022;20:203. doi: 10.1186/s12967-022-03402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melnikova VO, Mourad-Zeidan AA, Lev DC, Bar-Eli M. Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J Biol Chem. 2006;281:2911–2922. doi: 10.1074/jbc.M508683200. [DOI] [PubMed] [Google Scholar]
- 75.Song KS, Seong JK, Chung KC, Lee WJ, Kim CH, Cho KN, et al. Induction of MUC8 gene expression by interleukin-1 beta is mediated by a sequential ERK MAPK/RSK1/CREB cascade pathway in human airway epithelial cells. J Biol Chem. 2003;278:34890–34896. doi: 10.1074/jbc.M303911200. [DOI] [PubMed] [Google Scholar]
- 76.Choi HJ, Chung YS, Kim HJ, Moon UY, Choi YH, Van Seuningen I, et al. Signal pathway of 17beta-estradiol-induced MUC5B expression in human airway epithelial cells. Am J Respir Cell Mol Biol. 2009;40:168–178. doi: 10.1165/rcmb.2007-0377OC. [DOI] [PubMed] [Google Scholar]
- 77.Song KS, Lee WJ, Chung KC, Koo JS, Yang EJ, Choi JY, et al. Interleukin-1 beta and tumor necrosis factor-alpha induce MUC5AC overexpression through a mechanism involving ERK/p38 mitogen-activated protein kinases-MSK1-CREB activation in human airway epithelial cells. J Biol Chem. 2003;278:23243–23250. doi: 10.1074/jbc.M300096200. [DOI] [PubMed] [Google Scholar]
- 78.Zhang X, Odom DT, Koo SH, Conkright MD, Canettieri G, Best J, et al. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen LP, Al-Sawalha NA, Parra S, Pokkunuri I, Omoluabi O, Okulate AA, et al. β2-Adrenoceptor signaling in airway epithelial cells promotes eosinophilic inflammation, mucous metaplasia, and airway contractility. Proc Natl Acad Sci U S A. 2017;114:E9163–E9171. doi: 10.1073/pnas.1710196114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barnes PJ. Corticosteroid effects on cell signalling. Eur Respir J. 2006;27:413–426. doi: 10.1183/09031936.06.00125404. [DOI] [PubMed] [Google Scholar]
- 81.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 82.Patil RH, Naveen Kumar M, Kiran Kumar KM, Nagesh R, Kavya K, Babu RL, et al. Dexamethasone inhibits inflammatory response via down regulation of AP-1 transcription factor in human lung epithelial cells. Gene. 2018;645:85–94. doi: 10.1016/j.gene.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 83.Namba M, Asano K, Kanai K, Kyo Y, Watanabe S, Hisamitsu T, et al. Suppression of matrix metalloproteinase production from nasal fibroblasts by fluticasone propionate in vitro . Acta Otolaryngol. 2004;124:964–969. doi: 10.1080/00016480310016947. [DOI] [PubMed] [Google Scholar]
- 84.Profita M, Gagliardo R, Di Giorgi R, Bruno A, Riccobono L, Bonanno A, et al. In vitro effects of flunisolide on MMP-9, TIMP-1, fibronectin, TGF-beta1 release and apoptosis in sputum cells freshly isolated from mild to moderate asthmatics. Allergy. 2004;59:927–932. doi: 10.1111/j.1398-9995.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 85.Kai H, Yoshitake K, Hisatsune A, Kido T, Isohama Y, Takahama K, et al. Dexamethasone suppresses mucus production and MUC-2 and MUC-5AC gene expression by NCI-H292 cells. Am J Physiol. 1996;271:L484–L488. doi: 10.1152/ajplung.1996.271.3.L484. [DOI] [PubMed] [Google Scholar]
- 86.Krings JG, Gerald JK, Blake KV, Krishnan JA, Reddel HK, Bacharier LB, et al. A call for the United States to accelerate the implementation of reliever combination inhaled corticosteroid-formoterol inhalers in asthma. Am J Respir Crit Care Med. 2023;207:390–405. doi: 10.1164/rccm.202209-1729PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bateman ED, Reddel HK, O’Byrne PM, Barnes PJ, Zhong N, Keen C, et al. As-needed budesonide–formoterol versus maintenance budesonide in mild asthma. N Engl J Med. 2018;378:1877–1887. doi: 10.1056/NEJMoa1715275. [DOI] [PubMed] [Google Scholar]
- 88.Cardet JC, Papi A, Reddel HK. “As-needed” inhaled corticosteroids for patients with asthma. J Allergy Clin Immunol Pract. 2023;11:726–734. doi: 10.1016/j.jaip.2023.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaplan A, Mitchell PD, Cave AJ, Gagnon R, Foran V, Ellis AK. Effective asthma management: is it time to let the AIR out of SABA? J Clin Med. 2020;9:921. doi: 10.3390/jcm9040921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Durham P. Another asthma expert calls blue inhaler a killer [Internet] Redfern: The Medical Republic; 2019. [cited 2024 Apr 24]. Available from: https://www.medicalrepublic.com.au/another-asthma-expert-calls-blue-inhaler-killer/23180. [Google Scholar]
- 91.Gosens R, Gross N. The mode of action of anticholinergics in asthma. Eur Respir J. 2018;52:1701247. doi: 10.1183/13993003.01247-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumar K, Kebadze T, Losa F, Mallia P, Singanayagam A, Edwards MR, et al. Clinically relevant β2-agonists induce and augment rhinovirus-induction of asthma-relevant pro-inflammatory mediators in human bronchial epithelial cells. Am J Respir Crit Care Med. 2020;201:A1184. [Google Scholar]
- 93.Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34:641–647. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- 94.Ekbom E, Quint J, Schöler L, Malinovschi A, Franklin K, Holm M, et al. Asthma and treatment with inhaled corticosteroids: associations with hospitalisations with pneumonia. BMC Pulm Med. 2019;19:254. doi: 10.1186/s12890-019-1025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singanayagam A, Glanville N, Girkin JL, Ching YM, Marcellini A, Porter JD, et al. Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations. Nat Commun. 2018;9:2229. doi: 10.1038/s41467-018-04574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomas BJ, Porritt RA, Hertzog PJ, Bardin PG, Tate MD. Glucocorticosteroids enhance replication of respiratory viruses: effect of adjuvant interferon. Sci Rep. 2014;4:7176. doi: 10.1038/srep07176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Contoli M, Ito K, Padovani A, Poletti D, Marku B, Edwards MR, et al. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy. 2015;70:910–920. doi: 10.1111/all.12627. [DOI] [PubMed] [Google Scholar]
- 98.Venarske DL, Busse WW, Griffin MR, Gebretsadik T, Shintani AK, Minton PA, et al. The relationship of rhinovirus-associated asthma hospitalizations with inhaled corticosteroids and smoking. J Infect Dis. 2006;193:1536–1543. doi: 10.1086/503809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsukura S, Kurokawa M, Homma T, Watanabe S, Suzuki S, Ieki K, et al. Basic research on virus-induced asthma exacerbation: inhibition of inflammatory chemokine expression by fluticasone propionate. Int Arch Allergy Immunol. 2013;161(Suppl 2):84–92. doi: 10.1159/000350455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamaya M, Kikuchi A, Sugawara M, Nishimura H. Anti-inflammatory effects of medications used for viral infection-induced respiratory diseases. Respir Investig. 2023;61:270–283. doi: 10.1016/j.resinv.2022.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hayashi Y, Sada M, Shirai T, Okayama K, Kimura R, Kondo M, et al. Rhinovirus infection and virus-induced asthma. Viruses. 2022;14:2616. doi: 10.3390/v14122616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van Ly D, Faiz A, Jenkins C, Crossett B, Black JL, McParland B, et al. Characterising the mechanism of airway smooth muscle β2 adrenoceptor desensitization by rhinovirus infected bronchial epithelial cells. PLoS One. 2013;8:e56058. doi: 10.1371/journal.pone.0056058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taylor DR, Hancox RJ. Interactions between corticosteroids and beta agonists. Thorax. 2000;55:595–602. doi: 10.1136/thorax.55.7.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Edwards MR, Johnson MW, Johnston SL. Combination therapy: synergistic suppression of virus-induced chemokines in airway epithelial cells. Am J Respir Cell Mol Biol. 2006;34:616–624. doi: 10.1165/rcmb.2005-0385OC. [DOI] [PubMed] [Google Scholar]
- 105.Tay H, Wark PA, Bartlett NW. Advances in the treatment of virus-induced asthma. Expert Rev Respir Med. 2016;10:629–641. doi: 10.1080/17476348.2016.1180249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Domingo C, Singh D. The changing asthma management landscape and need for appropriate SABA prescription. Adv Ther. 2023;40:1301–1316. doi: 10.1007/s12325-022-02410-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reddel HK, Bateman ED, Schatz M, Krishnan JA, Cloutier MM. A practical guide to implementing SMART in asthma management. J Allergy Clin Immunol Pract. 2022;10:S31–S38. doi: 10.1016/j.jaip.2021.10.011. [DOI] [PubMed] [Google Scholar]
- 108.Tamura G, Ichinose M, Fukuchi Y, Miyamoto T. Transdermal tulobuterol patch, a long-actingβ(2)-agonist. Allergol Int. 2012;61:219–229. doi: 10.2332/allergolint.11-RA-0358. [DOI] [PubMed] [Google Scholar]
- 109.O’Byrne PM, FitzGerald JM, Bateman ED, Barnes PJ, Zhong N, Keen C, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378:1865–1876. doi: 10.1056/NEJMoa1715274. [DOI] [PubMed] [Google Scholar]
- 110.Beasley R, Holliday M, Reddel HK, Braithwaite I, Ebmeier S, Hancox RJ, et al. Controlled trial of budesonide–formoterol as needed for mild asthma. N Engl J Med. 2019;380:2020–2030. doi: 10.1056/NEJMoa1901963. [DOI] [PubMed] [Google Scholar]
- 111.Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394:919–928. doi: 10.1016/S0140-6736(19)31948-8. [DOI] [PubMed] [Google Scholar]