Abstract
Rationale
Zuranolone, a newly FDA-approved synthetic neurosteroid, shows promise in treating depression.
Objectives
Our aim is to evaluate Zuranolone's efficacy and safety in treating depression.
Methods
Five databases were searched until September 2023 for relevant randomized clinical trials evaluating the efficacy and safety of zuranolone. The potential risk of bias in the included trials was evaluated by the Cochrane Risk of Bias II guideline Data were extracted and pooled using Review Manager Software (RevMan 5.3).
Results
An analysis of eight studies highlights Zuranolone's efficacy in treating depression compared to placebo across most of the outcomes. Notably, the 30mg and 50mg doses demonstrated significant improvements in reducing HAM-D scores by over 50% within a 15-day follow-up (RR) of 1.46 (95% CI [1.27, 1.68], p < 0.0001) and 1.14 (95% CI [1.01, 1.3], p = 0.04). Additionally, the HAM-D ≤ 7% score analysis revealed significant enhancements with the 30mg dose over both 15-day (RR = 1.82, 95% CI [1.44, 2.31], p < 0.0001) and 45-day (RR = 1.43, 95% CI [1.16, 1.77], p = 0.0008) durations. Adverse Events Drug Discontinuation demonstrated no overall significant difference (OR = 1.33, 95% CI: [0.79, 2.23], p = 0.282). Further, specific adverse events, such as headache, showed no significant overall difference between Zuranolone and placebo (OR = 1.11, 95% CI: [0.84, 1.47], p = 0.47), with dose-dependent analysis revealing less headache in the 30 mg group.
Conclusion
Zuranolone demonstrates favorable tolerability and safety, particularly at 30mg and 50mg doses after 15 days, suggesting its potential and effective treatment for depression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00213-024-06611-y.
Keywords: Zuranolone, Major depressive disorder, Postpartum depression, Synthetic neurosteroid
Introduction
Major Depressive Disorder (MDD) stands as a global mental health challenge, causing widespread disability (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators 2018). Its manifestations involve alterations in affect, as well as cognitive, social, and occupational functions (Otte et al. 2016). The global prevalence of depression has surged, with over 19 million individuals in the United States having experiencing multiple depressive episodes, more than half of whom struggle with severe functional impairment (2019 NSDUH Detailed Tables n.d; Kessler et al. 2003). Unfortunately, approximately 788,000 individuals, burdened by depression, commit suicide (World Health Organization [Internet] 2017). Postpartum Depression (PPD), a form of major depressive disorder that emerges within four weeks after childbirth, leads to a decline in overall well-being and mental functioning (Bauman et al. 2020; Da Costa et al. 2006). The profound impact of PPD is evident through the loss of maternal-infant bonding and an increased susceptibility to suicide (Kerstis et al. 2016).
Treatment typically involves Selective Serotonin Reuptake Inhibitors (SSRIs), Serotonin-Norepinephrine Reuptake Inhibitors (SNRIs), and Tricyclic Antidepressants (TCAs) (Hockenberry et al. 2019). However, the effectiveness of these interventions is not guaranteed, with remission rate of over a 30% and at least 50% resistance to treatment with combined antidepressants(Kulkarni & Dhir 2009). After initial treatment, a significant number of patients struggle to maintain remission, contending with persistent symptoms that affect their quality of life and increase the risk of relapse (Trivedi 2009; Trivedi et al. 2006). Factors such as discontinuation of treatment and poor compliance, often due to delayed improvement or intolerance to side effects like weight gain and sexual dysfunction, contribute to this challenge (Bull et al. 2002; Geddes et al. 2003; Kulkarni & Dhir 2009).
The pathophysiology of depression is multifaceted, involving genetic, epigenetic, and environmental risk factors (Batterham et al. 2009). Disruption in the excitatory-inhibitory balance, regulated by glutamatergic and GABAergic signaling, is posited to play a role in depression development (Lener et al. 2017). This hypothesis gains support by observed alterations in GABA levels in the plasma, cerebrospinal fluid (CSF), and brain tissue of depressed patients, along with downstream changes in the expression of GABA-synthesizing enzymes and mRNA of GABA type A in individuals who died by suicide (Cutler et al. 2023; Gerner & Hare 1981; Luscher et al. 2011; Luykx et al. 2012; Merali et al. 2004; Sanacora et al. 2004).
Preclinical studies have identified allopregnanolone levels as a risk factor influencing GABAergic signaling (Osborne et al. 2017). Allopregnanolone, a neuroactive steroid and positive allosteric modulator of GABAA receptors, shows promise as an antidepressant, as evidenced by its normalization in CSF following SSRI treatment for depression (Paul & Purdy 1992; Uzunova et al. 1998).
Recently, Zuranolone, a synthetic neurosteroid and positive allosteric modulator of GABAA receptors, has gained approval from the U.S. Food and Drug Administration (FDA) for use in postpartum depression, based on two phase 3 randomized controlled trials (FDA NEWS RELEASE 2023; Heo 2023). Our goal is to systematically assess the efficacy and safety of Zuranolone for major depressive disorder and postpartum depression based on available clinical trials.
Methodology
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (Page et al. 2021) and the Cochrane Handbook of Systematic Reviews and Meta-analysis (Higgins, et al. 2019). PRISMA checklist is illustrated in (See supplementary Tables S1 and S2, online resource).
Data sources & search strategy
We searched PubMed, Web of Science, SCOPUS, clinicaltrials.gov and Cochrane Central through September 2023, using the following keywords ((Zuranolone OR SAGE$217) AND (Depress* OR dysphoria OR Melancholia OR dysthymi* OR "adjustment disorder*" OR "mood disorder*" OR "affective disorder*" OR "affective symptoms")).
Eligibility criteria
We included clinical trials with the following PICO criteria: population (P): human patients with depression (e.g. MMD) or postpartum depression; intervention (I): Zuranolone; control (C): placebo. On the other hand, we excluded studies not fulfilling the previous criteria such as observational studies (cohort, case–control, cross-sectional, case series, and case reports), unpublished study protocols, letters to the editor, non-human studies, or those published in languages other than English.
Selection process
Two authors independently carried out a two-step selection process, screening titles and abstracts of retrieved records. Subsequently, full texts of potentially eligible records were retrieved and assessed for inclusion in the meta-analysis. Any conflicts were resolved through discussion to reach a consensus.
Data extraction
Four authors, using a pre-formed data extraction sheet, extracted the following data: study design characteristics (last authors' name, year of publication, NCT number, study design, country, inclusion criteria, exclusion criteria, intervention, control, outcomes, and Duration of treatment); Baseline sheet of the enrolled participants included study arms, number of participants in each arm, age in years, weight (kg), BMI (kg/m2), sex (%), ethnicity (%), race (%), Baseline antidepressant use No. (%) and baseline Hamilton Depression Rating Scale (HAMD) total score, mean (SD). All sheets were independently reviewed by the first author.
Risk of bias
Two authors independently evaluated the potential risk of bias in the included trials following the Cochrane Risk of Bias II guideline (Sterne et al. 2019). We accordingly considered the following: randomization process, deviation from intended intervention, bias in the measurement of outcomes, selection of reported results, missing outcome data, and overall bias. Conflicts were resolved by reaching a consensus through discussion.
Endpoints
Efficacy outcomes
HAMD-17 score improvement, Reduction of > 50% from baseline in HAM-D score, HAM-D ≤ 7% score, Clinical Global Impression Improvement (CGI-I) total score, Bech-6 total score, Montgomery-Åsberg Depression Rating Scale (MADRS) total score and Hamilton Rating Scale for Anxiety (HAM-A) total score.
Safety outcomes
Any treatment-emergent adverse event (TEAE), patients with a serious adverse event (any adverse event occurring while the patient was receiving the trial medication or placebo, that resulted in death, was immediately life-threatening, led to inpatient hospitalization or prolongation of hospitalization, caused persistent or clinically significant disability or incapacity, or resulted in a congenital abnormality or birth defect), patients with a severe adverse event (any event that was incapacitating or caused an inability to perform normal activities of daily living), adverse events drug discontinuation, and most common TEAEs.
Statistical analysis
This meta-analysis was conducted by using two programs; Review Manager Software (RevMan version 5.3 n.d; Cochrane Collaboration, Copenhagen, Denmark, 2014) and Open Meta Analyst (OMA n.d) (Computer program) (Version 5.4. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014). We presented all data as either (1) mean difference (MD) in pooling continuous outcomes, or (2) odds/risk ratio (OR) in pooling dichotomous outcomes with 95% confidence intervals (CIs). We tested the heterogeneity between pooled studies using chi-square and I-square tests. When the heterogeneity between studies at chi-square of I2 > 50% P-value < 0.05, we used a random-effect model for analysis. We performed subgroup analysis to test whether the effect estimate of zuranolone differs significantly according to the dose and duration.
Results
Literature search results
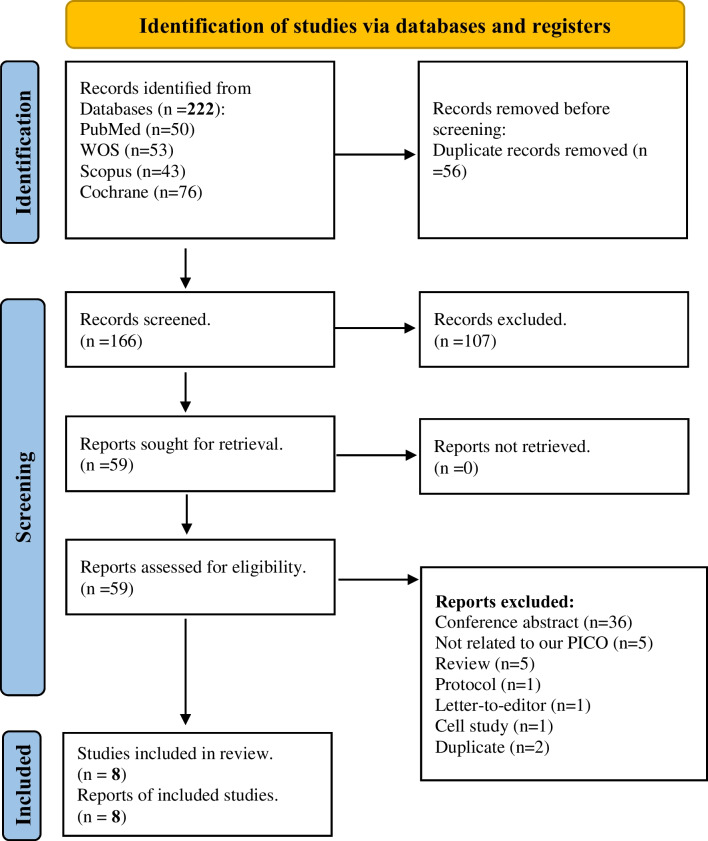
Our systematic search identified 222 potential studies; Among these, 56 were excluded as duplicates. Following title and abstract screening, an additional 107 studies were excluded. Subsequently, full-text screening led to the exclusion of 51 studies. Finally, eight eligible studies were included for quantitative and qualitative synthesis in this systematic review. An extra 107 studies were excluded after title/abstract screening and then 51 studies were excluded after the full-text screening. In the end, we got eight eligible studies to be included in the quantitative and qualitative synthesis of this systematic review (Fig. 1; PRISMA).
Fig. 1.
PRISMA flow diagram of the systematic review
Characteristics of included studies
All the included studies were RCTs with a total number of 2,176 patients. The eight included RCTs were controlled with a placebo, with two of them incorporating two intervention arms featuring different doses of Zuranolone. Additionally, one study compared Zuranolone in combination with antidepressant therapy (ADT) to placebo with ADT. The number of patients in these included studies ranged from 89 to 537 patients with mean age varying between 27.4 and 49.1 years, and The Hamilton Depression Rating Scale ranged between 24.5 to 28.8. Zuranolone was administered orally once daily, with doses ranging from 20 to 50 mg over 2 weeks. Seven trials were conducted in the United States, and one study was conducted in Japan (Tables 1 and 2).
Table 1.
Summary of included studies
| Study ID | Protocol registration (NCT) | Study Design | Setting (country) | Total number of patients | Intervention | Comparison | Duration of treatment | Outcomes | Participants (inclusion criteria) | Exclusion criteria |
|---|---|---|---|---|---|---|---|---|---|---|
| Deligiannidis et al. 2021 | NCT02978326 | RCT | USA | 150 | Zuranolone (30-mg/day) | Placebo | 14 days | Zuranolone was effective in improving symptoms of PPD indicated by HAMD-17 score, and generally well-tolerated | Female aged 18 to 45 years old, with PPD without psychosis (not more than 6 months postpartum) | Patients with known medical problems that prevent them from taking zuranolone |
| Gunduz-Bruce et al. 2019 | NCT03000530 | RCT | USA | 89 | SAGE 217 (30-mg/day) | Placebo | 14 days | SAGE-217 was effective in reducing depressive symptoms, but the safety and tolerability is still questionable | Males and females aged 18 to 65 years old with MDD | Patients with history of suicide, resistant to antidepressant, or known medical problems that prevent them from taking the intervention |
| Clayton et al. 2023 | NCT03672175 | RCT | USA | 482 | Zuranolone (20-mg/day or 30-mg/day) | Placebo | 14 days | Significant rapid improvements in depressive symptoms were observed with zuranolone 30 mg, and it was generally well-tolerated | Males and females aged 18 to 65 years old with MDD | Patients with history of suicide, resistant to antidepressant, or known medical problems that prevent them from taking the intervention |
| Deligiannidis et al. 2023 | NCT02978326 | RCT | USA | 150 | Zuranolone (30-mg/day) | Placebo | 14 days | Zuranolone was effective in improving depressive, anxiety, and associated insomnia symptoms | Female aged 18 to 45 years old, with PPD without psychosis (not more than 6 months postpartum) | Patients with known medical problems that prevent them from taking zuranolone |
| Suthoff et al. 2022 | NCT03000530 | RCT | USA | 89 | SAGE 217 (30-mg/day) | Placebo | 14 days | Zuranolone-treated patients reported rapid and significant improvements in HRQoL | Males and females aged 18 to 65 years old with MDD | Patients with history of suicide, resistant to antidepressant, or known medical problems that prevent them from taking the intervention |
| Kato et al. 2023a | JapicCTI-205276 | RCT | Japan | 249 | Zuranolone (20-mg/day or 30-mg/day) | Placebo | 14 days | Zuranolone was safe and demonstrated significant improvements in depressive symptoms | Males and females aged 18 to 75 years old with MDD | Patients with antidepressant resistance or known medical problems that prevent them from taking the intervention |
| Clayton et al. 2023 | NCT04442490 | RCT | USA | 537 | Zuranolone (50-mg/day) | Placebo | 14 days | Zuranolone demonstrated significant improvements in depressive symptoms | Males and females aged 18 to 64 years old with MDD | Patients with history of suicide, resistant to antidepressant, or known medical problems that prevent them from taking the intervention |
| Parikh et al. 2024 | NCT04476030 | RCT | USA | 430 | Zuranolone (50-mg/day) + ADT | Placebo + ADT | 14 days | Zuranolone demonstrated significant improvements in depressive symptoms | Males and females aged 18 to 64 years old with MDD | Patients with history of suicide, resistant to antidepressant, or known medical problems that prevent them from taking the intervention |
ADT Antidepressant Therapy, HAMD-17 17-item Hamilton Rating Scale for Depression, HRQoL Health-related Quality of Life, MMD Major Depressive Disorder, PPD Postpartum Depression, RCT Randomized Controlled Trial, USA United States of America
Table 2.
Baseline characteristics of the included studies
| Study ID | Study Arm | No. of participants | Age, mean (SD), y | Sex No. (%) | Weight, mean (SD), kg | BMI, mean (SD), kg/m2 | Ethnicity, No. (%) | Race, No. (%) | Baseline antidepressants use No. (%) | Baseline Hamilton Depression Rating Scale (HAMD) total score, mean (SD) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Hispanic or Latino | Not Hispanic or Latino | African American | White | Asian | Other | ||||||||
| Deligiannidis et al. 2021 | zuranolone 30 mg | 76 | 29.3 (5.4) | 0 | 100% | 85.1(19) | 31.1(6) | 16 (21) | 60 (79) | 31(41) | 44(58) | 1(1) | 16(21) | 28.4(2) | |
| placebo | 74 | 27.4(5.3) | 0 | 100% | 80.2(24) | 30.3(8) | 18(24) | 56(76) | 31(42) | 40(54) | 3(4) | 13(18) | 28.8(2) | ||
| Gunduz-Bruce et al. 2019 |
Zuranolone 30 mg |
45 | 49.1(13.6) | 20 (44) | 25(56) | _ | 30.0(6.3) | _ | _ | 36(80) | 7(16) | 1(2) | 1(2) | 12(27) | 25.2(2.6) |
| placebo | 44 | 38.3(12.2) | 14 (32) | 30 (68) | _ | 29.9(5.2) | _ | _ | 28(64) | 16(36) | 0 | 0 | 10(23) | 25.7(2.4) | |
| Clayton et al. 2023 | zuranolone 30 mg | 166 | 42.3 (11.8) | 45 (27.1) | 121 (72.9) | 89.7 (22.4) | _ | 27 (16.3) | 139(83.7) | 64 (38.6) | 94 (56.6) | 2 (1.2) | 6 (3.6) | 47 (28.3) | 25.9 (2.9) |
| zuranolone 20 mg | 159 | 41.9 (12.2) | 47 (29.6) | 112 (70.4) | 87.3 (20.2) | _ | 31 (19.5) | 128(80.5) | 56 (35.2) | 99 (62.3) | 3 (1.9) | 1 (0.6) | 46 (28.9) | 25.8 (2.8) | |
| placebo | 157 | 41.4 (12.2) | 51(32.5) | 106 (67.5) | 89.5 (22.9) | _ | 26 (16.6) | 131(83.4) | 54 (34.4) | 96 (61.1) | 3 (1.9) | 4 (2.5) | 49 (31.2) | 25.8 (3.1) | |
| Deligiannidis et al. 2023 | zuranolone 30 mg | 76 | 29.3 (5.4) | 0 | 100% | 85.1(19) | 31.1(6) | 16 (21) | 60 (79) | 31(41) | 44(58) | 1(1) | 16(21) | 28.4(2) | |
| placebo | 74 | 27.4(5.3) | 0 | 100% | 80.2(24) | 30.3(8) | 18(24) | 56(76) | 31(42) | 40(54) | 3(4) | 13(18) | 28.8(2) | ||
| Suthoff et al. 2022 | zuranolone 30 mg | 45 | 49.1(13.6) | 20 (44) | 25(56) | _ |
30.0 (6.3) |
_ | _ | 36(80) | 7(16) | 1(2) | 1(2) | 12(27) | 25.2(2.6) |
| placebo | 44 | 38.3(12.2) | 14 (32) | 30 (68) | _ | 29.9(5.2) | _ | _ | 28(64) | 16(36) | 0 | 0 | 10(23) | 25.7(2.4) | |
| Kato et al. 2023a | placebo | 82 | 40.8 (10.6) | 35 (42.7) | 47 (57.3) | 63.4 (16.5) | 23.6 (5.3) | 0 | 82 (100) | 0 | 0 | 82(100) | 0 | _ | 24.5 (2.1) |
| zuranolone 20 mg | 85 | 39.3 (12.6) | 36 (42.4) | 49 (57.6) | 64.6 (13.8) | 23.9 (4.4) | 0 | 85 (100) | 0 | 0 | 85(100) | 0 | _ | 24.8 (2.4) | |
| zuranolone 30 mg | 82 | 38.8 (12.0) | 35 (42.7) | 47 (57.3) | 61.0 (12.9) | 22.7 (4.0) | 0 | 82(100) | 0 | 0 | 82(100) | 0 | _ | 24.6 (2.2) | |
| Clayton et al. 2023 | zuranolone 50 mg | 268 | 39.4 (12.3) | 82(30.6) | 186 (69.4) | _ | 29.6 (6.3) | 58 (21.6) | 210 (78.4) | 75(28.0) | 169 (63.1) | 13 (4.9) | 11(4.1) | 79 (29.5) | 26.8 (2.6) |
| placebo | 269 | 40.1 (12.6) | 103(38.3) | 166 (61.7) | _ | 30.3 (6.2) | 54 (20.1) | 215 (79.9) | 46 (17.1) | 206 (76.6) | 4 (1.5) | 13(4.8) | 81 (30.1) | 26.9 (2.7) | |
| Parikh et al. 2024 | Zuranolone 50 mg + ADT | 212 | 38.6 (12.72) | 83 (39.2) | 129 (60.8) | _ | 29.1 (6.26) | 41 (19.3) | 171 (80.7) | 46 (21.7 | 153 (72.2) | 6 (2.8) | 3 (1.4) | 115 (54.2) |
26.8 (2.51) |
| Placebo + ADT | 218 | 37.7 (12.28) | 78 (35.8) | 140 (64.2) | _ |
29.9 (6.44) |
52 (23.9) | 166 (76.1) | 31 (14.2 | 168 (77.1) | 12 (5.5) | 3 (1.4) | 120 (55.0) | 26.6 (2.58) | |
Quality assessment
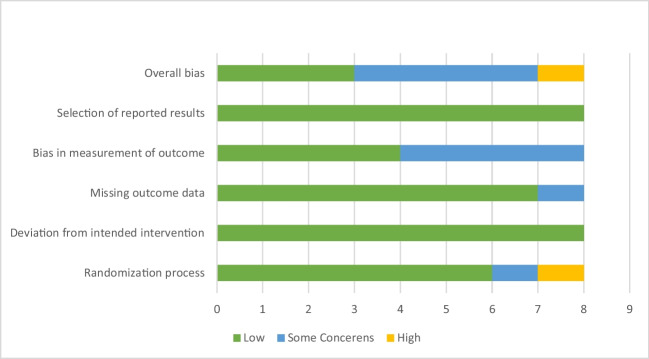
According to the Cochrane Risk of Bias Assessment Tool for Randomized Clinical Trials II (ROB-II), the quality of the included studies ranges from low to some concerned risk of bias. One study showed a potentially high risk of bias in the randomization process domain. While four studies showed some concerned risk of bias regarding the measurement of the outcomes domain, and one study in the missing data domain (Fig. 2 and Table 3).
Fig. 2.
Risk of bias assessment of the included studies
Table 3.
Risk of bias assessment of the included studies
| Domains | Risk of bias | Judgment of the authors |
|---|---|---|
| Deligiannidis et al. (2021) | ||
| Randomization Process | Low | The randomization scheme was performed using an interactive response technology system vendor using SAS statistical software version 9.4 (SAS Institute). There were no baseline differences between intervention groups |
| Deviation from the intended intervention | Low | Participants and personnel were blinded |
| Missing outcome data | Low | Intention to treat analysis |
| Bias in the measurement of outcome | Low | All other site personnel except site–designated pharmacy staff were blinded to treatment assignments during the study |
| Selection of reported results | Low | The protocol was available, and data were analyzed in accordance with pre-specified plan |
| Overall bias | Low | The study is judged to be at low risk of bias for all domains |
| Gunduz-Bruce et al. (2019) | ||
| Randomization Process | Low | Randomization was performed using interactive response technology created by 4G Clinical (Wellesley, MA). In a 1:1 ratio, patients were assigned to receive either SAGE-217 (30 mg) or placebo |
| Deviation from the intended intervention | Low | Participants and personnel were blinded, and analysis was appropriate |
| Missing outcome data | Low | Analyses were performed according to the intention-to-treat principle and included all patients who underwent randomization |
| Bias in the measurement of outcome | Some concerns | No information whether outcome assessors were aware of intervention or not and assessment can be influenced by knowledge of intervention |
| Selection of reported results | Low | The protocol was available, and data were analyzed in accordance with pre-specified plan |
| Overall bias | Some concerns | The study is judged to raise some concerns in one domain |
| Clayton et al. (2023) | ||
| Randomization Process | Some concern | No information about the allocation concealment |
| Deviation from the intended intervention | Low | Participants and personnel were blinded |
| Missing outcome data | Some concerns | There is large number of follow up loss, but proportions of missing outcome data were balanced between intervention groups |
| Bias in the measurement of outcome | Some concerns | No information whether outcome assessors were aware of intervention or not and assessment can be influenced by knowledge of intervention |
| Selection of reported results | Low | The protocol was available, and data were analyzed in accordance with pre-specified plan |
| Overall bias | Some concerns | The study is judged to raise some concerns in three domains |
| Deligiannidis et al. (2023) | ||
| Randomization Process | Low | The randomization scheme was performed using an interactive response technology system vendor using SAS statistical software version 9.4 (SAS Institute). There were no baseline differences between intervention groups |
| Deviation from the intended intervention | Low | Participants and personnel were blinded |
| Missing outcome data | Low | Intention to treat analysis |
| Bias in the measurement of outcome | Low | All other site personnel except site–designated pharmacy staff were blinded to treatment assignments during the study |
| Selection of reported results | Low | The protocol was available, and data were analyzed in accordance with pre-specified plan |
| Overall bias | Low | The study is judged to be at low risk of bias for all domains |
| Suthoff et al. 2022 | ||
| Randomization Process | Low | Randomization was performed using interactive response technology created by 4G Clinical (Wellesley, MA). In a 1:1 ratio, patients were assigned to receive either SAGE-217 (30 mg) or placebo |
| Deviation from the intended intervention | Low | Participants and personnel were blinded, and analysis was appropriate |
| Missing outcome data | Low | Analyses were performed according to the intention-to-treat principle and included all patients who underwent randomization |
| Bias in the measurement of outcome | Some concerns | No information whether outcome assessors were aware of intervention or not and assessment can be influenced by knowledge of intervention |
| Selection of reported results | Low | The protocol was available, and data were analyzed in accordance with pre-specified plan |
| Overall bias | Some concerns | The study is judged to raise some concerns in one domain |
| Kato et al. (2023a) | ||
| Randomization Process | Low | Patients were randomized (1:1:1) at baseline (visit 1), with stratification based on the 17-item Hamilton Depression Rating Scale (HAMD-17) total score at baseline (< 25 vs ≥ 25) and sex, to receive zuranolone 20 mg, zuranolone 30 mg, or matching placebo once daily for 14 days (double-blind treatment period) |
| Deviation from the intended intervention | Low | Study was double blinded, and analysis was appropriate |
| Missing outcome data | Low | "Full analysis set (FAS) comprised all patients randomly assigned to the study drug and administered at least one dose of the study drug”; Analysis was intention to treat |
| Bias in the measurement of outcome | Some concerns | No information whether outcome assessors were aware of intervention, however; the assessment tools were reliable |
| Selection of reported results | Low | The protocol was available, and data were analyzed in accordance with pre-specified plan |
| Overall bias | Some concerns | The study is judged to raise some concerns in one domain |
| Clayton et al. (2023) | ||
| Randomization Process | Low | Randomization, in a 1:1 ratio, was performed centrally via an interactive response technology system |
| Deviation from the intended intervention | Low | participants and personnel were blinded, and analysis was appropriate |
| Missing outcome data | Low | "543 were randomized, 537 were included in the safety set and 534 were included in the full analysis set", Nearly all randomized participants were involved in the analysis with missing outcome data is sufficiently small that their outcomes could have made no important difference to the estimated effect of intervention |
| Bias in the measurement of outcome | Low | Patients, clinicians, site personnel, the study sponsor, and the study team were blinded to treatment allocation. Blinding was maintained until database lock after all patients completed the study visit at day 42 |
| Selection of reported results | Low | The protocol was available, and data were analyzed in accordance with pre-specified plan |
| Overall bias | Low | The study is judged to be at low risk of bias for all domains |
| Parikh et al. (2024) | ||
| Randomization Process | High | Randomization was performed by stratification according to the co-initiated antidepressant and the administration of the antidepressants was open labelled |
| Deviation from the intended intervention | Low | Participants and personnel were blinded |
| Missing outcome data | Low | "Full analysis set (FAS) comprised all patients randomly assigned to the study drug and administered at least one dose of the study drug", Analysis was intention to treat |
| Bias in the measurement of outcome | Low | Outcome assessors were blinded |
| Selection of reported results | Low | The protocol was available, and data were analyzed in accordance with pre-specified plan |
| Overall bias | High | The study is judged to raise high risk of bias in one domain |
Outcomes
Efficacy outcomes
HAMD-17 score improvement
The overall effect estimates of 15-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (SMD = -0.3, 95% CI: [-0.43, -0.17], p < 0.00001). The pooled results were heterogeneous (p = 0.03, I2 = 53%) which could not be solved. After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in both Zuranolone 30-mg and Zuranolone 50-mg groups when compared to placebo group [(SMD = -0.44, 95% CI [-0.63, -0.24], p < 0.0001); p = 0.1, I2 = 49%] and [(SMD = -0.18, 95% CI [-0.31, -0.05], p = 0.008); p = 0.4, I2 = 0%], respectively. However, the effect estimate showed no significant difference in the Zuranolone group 20-mg when compared to the placebo group [SMD = -0.18, 95% CI [-0.45, 0.08], p = 0.18); p = 0.16, I2 = 49%] (Table 4). Also, see supplementary Fig. S1 (online resource).
Table 4.
Mean difference OR standardized mean difference of the efficacy outcomes
| Variables | Duration | Doses | Effect estimates | No. of participants | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|
| MD/SMD | 95% CI | P | Zurnaonlone | Placebo | I2 | P | |||
| HAMD-17 | 15 days | 20-mg | -0.18 | [-0.45, 0.08] | 0.18 | 233 | 223 | 49 | 0.16 |
| 30-mg | -0.44 | [-0.63, -0.24] | < 0.0001 | 424 | 414 | 49 | 0.1 | ||
| 50-mg | -0.18 | [-0.31, -0.05] | 0.008 | 437 | 466 | 0 | 0.4 | ||
| Total | -0.3 | [-0.43, -0.17] | < 0.00001 | 1098 | 1103 | 53 | 0.03 | ||
| 42–45 days | 20-mg | -0.12 | [-0.47, 0.022] | 0.48 | 215 | 209 | 66 | 0.08 | |
| 30-mg | -0.26 | [-0.45, -0.08] | 0.004 | 401 | 396 | 38 | 0.17 | ||
| 50-mg | -0.06 | [-0.19, 0.08] | 0.42 | 417 | 409 | 0 | 0.49 | ||
| Total | -0.16 | [-0.28, -0.04] | 0.008 | 1033 | 1014 | 42 | 0.09 | ||
| Bech-6 score | NA | NA | -7.75 | [-20.66, 5.15] | 0.24 | 125 | 126 | 84 | 0.01 |
| MADRS score | 15 days | 20-mg | -0.06 | [-0.29, 0.17] | 0.59 | 152 | 141 | NA | NA |
| 30-mg | -0.31 | [-0.46, -0.16] | < 0.0001 | 348 | 332 | 15 | 0.31 | ||
| 50-mg | -0.2 | [-0.38, -0.02] | 0.03 | 247 | 250 | NA | NA | ||
| Total | -0.22 | [-0.32, -0.12] | < 0.001 | 747 | 723 | 26 | 0.24 | ||
| 42–45 days | 20-mg | 0.05 | [-0.19, 0.28] | 0.69 | 140 | 135 | NA | NA | |
| 30-mg | -0.24 | [-0.4, -0.09] | 0.002 | 331 | 322 | 39 | 0.18 | ||
| Total | -0.16 | [-0.28, -0.03] | 0.02 | 471 | 457 | 56 | 0.06 | ||
| HAM-A score | 15 days | 20-mg | -1.03 | [-2.99, 0.93] | 0.3 | 232 | 223 | 28 | 0.24 |
| 30-mg | -2.34 | [-3.83, -0.85] | 0.002 | 354 | 341 | 40 | 0.17 | ||
| 50-mg | -1.29 | [-2.41, -0.17] | 0.02 | 247 | 250 | NA | NA | ||
| Total | -1.56 | [-2.37, -0.74] | 0.0002 | 833 | 814 | 25 | 0.24 | ||
| 42–45 days | 20-mg | -0.05 | [-1.44, 1.34] | 0.94 | 140 | 135 | NA | NA | |
| 30-mg | -2.42 | [-5.63, 0.8] | 0.14 | 258 | 253 | 76 | 0.01 | ||
| 50-mg | -0.81 | [-2.16, 0.54] | 0.24 | 240 | 232 | NA | NA | ||
| Total | -1.33 | [-2.83, 0.18] | 0.08 | 638 | 620 | 66 | 0.02 | ||
CI: Confidence interval; HAM-A: Hamilton Rating Scale for Anxiety; HAMD-17: Hamilton Rating Scale for Depression-17; MADRS: Montgomery–Åsberg Depression Rating Scale; MD: Mean difference; SMD: Standardized mean difference
The overall effect estimates of 42 to 45-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (SMD = -0.16, 95% CI: [-0.28, -0.04], p = 0.008). The pooled results were homogenous (p = 0.09, I2 = 42%). After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in Zuranolone 30-mg when compared to the placebo group [SMD = -0.26, 95% CI [-0.45, -0.08], p = 0.004); p = 0.17, I2 = 38%]. However, the effect estimates showed no significant difference in both Zuranolone 20-mg and Zuranolone 50-mg groups when compared to the placebo group [SMD = -0.12, 95% CI [-0.47, 0.22], p = 0.48); p = 0.08, I2 = 66%] and [SMD = -0.06, 95% CI [-0.19, 0.08], p = 0.42); p = 0.49, I2 = 0%], respectively (Table 4). Also, see supplementary Fig. S2 (online resource).
Reduction of > 50% from baseline in HAM-D score
The overall effect estimates of 15-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (RR = 1.25, 95% CI: [1.14, 1.36], p < 0.00001). The pooled results were homogenous (p = 0.05, I2 = 49%. After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in both Zuranolone 30-mg and Zuranolone 50-mg groups when compared to the placebo group [(RR = 1.46, 95% CI [1.27, 1.68], p < 0.0001); p = 0.2, I2 = 33%] and [(RR = 1.14, 95% CI [1.01, 1.3], p = 0.04); p = 0.48, I2 = 0%], respectively. However, the effect estimate showed no significant difference between Zuranolone 20-mg when compared to the placebo groups [RR = 1.07, 95% CI [0.84, 1.37], p = 0.58); p = 0.34, I2 = 0%] (Table 5). Also, see supplementary Fig. S3 (online resource).
Table 5.
Risk ratio of the efficacy outcomes
| Variables | Duration | Doses | Effect estimates | No. of participants | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|
| RR | 95% CI | P | Zurnaonlone | Placebo | I2 | P | |||
| Reduction of > 50% from baseline in HAM-D score | 15 days | 20-mg | 1.07 | [0.84, 1.37] | 0.58 | 233 | 223 | 0 | 0.34 |
| 30-mg | 1.46 | [1.27, 1.68] | < 0.00001 | 426 | 413 | 33 | 0.2 | ||
| 50-mg | 1.14 | [1.01, 1.3] | 0.04 | 437 | 448 | 0 | 0.48 | ||
| Total | 1.25 | [1.14, 1.36] | < 0.00001 | 1096 | 1084 | 49 | 0.05 | ||
| 42–45 days | 20-mg | 1.26 | [0.76, 2.1] | 0.36 | 215 | 209 | 77 | 0.04 | |
| 30-mg | 1.24 | [1.08, 1.43] | 0.003 | 398 | 391 | 9 | 0.35 | ||
| 50-mg | 1.02 | [0.82, 1.28] | 0.84 | 417 | 409 | 71 | 0.06 | ||
| Total | 1.16 | [1.02, 1.33] | 0.02 | 1030 | 1009 | 53 | 0.03 | ||
| HAM-D score ≤ 7 | 15 days | 20-mg | 1.15 | [0.78, 1.72] | 0.48 | 233 | 223 | 51 | 0.15 |
| 30-mg | 1.82 | [1.44, 2.31] | < 0.00001 | 426 | 413 | 6 | 0.37 | ||
| 50-mg | 1.19 | [0.96, 1.48] | 0.11 | 437 | 448 | 0 | 0.4 | ||
| Total | 1.41 | [1.21, 1.63] | < 0.00001 | 1096 | 1084 | 48 | 0.05 | ||
| 42–45 days | 20-mg | 1.11 | [0.79, 1.56] | 0.54 | 215 | 209 | 0 | 0.75 | |
| 30-mg | 1.43 | [1.16, 1.77] | 0.0008 | 398 | 391 | 53 | 0.07 | ||
| 50-mg | 1 | [0.83, 1.21] | 0.97 | 417 | 409 | 0 | 0.7 | ||
| Total | 1.17 | [1.03, 1.33] | 0.02 | 1030 | 1009 | 47 | 0.06 | ||
| CGI score of 1 or 2 | 15 days | 20-mg | 1.02 | [0.8, 1.3] | 0.16 | 151 | 141 | NA | NA |
| 30-mg | 1.34 | [1.15, 1.57] | 0.0002 | 273 | 259 | 38 | 0.2 | ||
| 50-mg | 1.59 | [1.16, 2.16] | 0.004 | 263 | 264 | NA | NA | ||
| Total | 1.3 | [1.15, 1.47] | < 0.0001 | 687 | 664 | 54 | 0.07 | ||
| 42–45 days | 20-mg | 1.06 | [0.83, 1.35] | 0.66 | 140 | 135 | NA | NA | |
| 30-mg | 1.22 | [1.05, 1.42] | 0.01 | 258 | 253 | 0 | 0.93 | ||
| Total | 1.17 | [1.03, 1.33] | 0.02 | 398 | 388 | 0 | 0.77 | ||
CI Confidence interval, CGI Clinical Global Impression, HAM-D Hamilton Rating Scale for Depression, RR Risk ratio
The overall effect estimates of 42 to 45-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (RR = 1.16, 95% CI: [1.02, 1.33], p = 0.02). The pooled results were heterogeneous (p = 0.03, I2 = 53%) which can’t be resolved. After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in Zuranolone 30-mg when compared to the placebo group [RR = 1.24, 95% CI [1.08, 1.43], p = 0.003); p = 0.35, I2 = 9%]. However, the effect estimates showed no significant difference in both Zuranolone 20-mg and Zuranolone 50-mg groups when compared to the placebo group [RR = 1.26, 95% CI [0.76, 2.1], p = 0.36); p = 0.04, I2 = 77%] and [RR = 1.02, 95% CI [0.82, 1.28], p = 0.84); p = 0.06, I2 = 71%], respectively (Table 5). Also, see supplementary Fig. S4 (online resource).
HAM-D ≤ 7% score
The overall effect estimates of 15-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (RR = 1.41, 95% CI: [1.21, 1.63], p < 0.00001). The pooled results were homogenous (p = 0.05, I2 = 48%. After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in the Zuranolone 30-mg group when compared to the placebo group [RR = 1.82, 95% CI [1.44, 2.31], p < 0.0001); p = 0.37, I2 = 6%]. However, the effect estimates showed no significant difference in both Zuranolone 20-mg and Zuranolone 50-mg groups when compared to the placebo group [RR = 1.15, 95% CI [0.78, 1.72], p = 0.48); p = 0.115, I2 = 51%] and [RR = 1.19, 95% CI [0.96, 1.48], p = 0.11); p = 0.4, I2 = 0%], respectively (Table 5). Also, see supplementary Fig. S5 (online resource).
The overall effect estimates of 42 to 45-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (RR = 1.17, 95% CI: [1.03, 1.33], p = 0.02). The pooled results were homogenous (p = 0.06, I2 = 47%). After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in Zuranolone 30-mg when compared to the placebo group [RR = 1.43, 95% CI [1.16, 1.77], p = 0.0008); p = 0.07, I2 = 53%]. However, the effect estimates showed no significant difference in both Zuranolone 20-mg and Zuranolone 50-mg groups when compared to the placebo group [RR = 1.11, 95% CI [0.79, 1.56], p = 0.54); p = 0.75, I2 = 0%] and [RR = 1, 95% CI [0.83, 1.21], p = 0.97); p = 0.7, I2 = 0%], respectively (Table 5). Also, see supplementary Fig. S6 (online resource).
CGI-I total score
The overall effect estimates of 15-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (RR = 1.3, 95% CI: [1.15, 1.47], p < 0.0001). The pooled results were homogenous (p = 0.07, I2 = 54%). After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in both Zuranolone 30-mg and 50-mg Zuranolone groups when compared to the placebo group [RR = 1.34, 95% CI [1.15, 1.57], p = 0.0002); p = 0.2, I2 = 38%] and [RR = 1.59, 95% CI [1.16, 2.16], p = 0.004)], respectively. However, the effect estimates showed no significant difference in the Zuranolone 20-mg group when compared to the placebo group [RR = 1.02, 95% CI [0.8, 1.3], p = 0.87)] (Table 5). Also, see supplementary Fig. S7 (online resource).
The overall effect estimates of 42 to 45-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (RR = 1.17, 95% CI: [1.03, 1.33], p = 0.02). The pooled results were homogenous (p = 0.77, I2 = 0%). After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in Zuranolone 30-mg when compared to the placebo group [RR = 1.22, 95% CI [1.05, 1.42], p = 0.01); p = 0.93, I2 = 0%]. However, the effect estimates showed no significant difference in the Zuranolone 20-mg group when compared to the placebo group [RR = 1.06, 95% CI [0.83, 1.35], p = 0.66] (Table 5). Also, see supplementary Fig. S8 (online resource).
Bech-6 total score
The overall effect estimates showed no significant difference between Zuranolone and placebo groups (MD = -7.75, 95% CI: [-20.66, 5.15], p = 0.24). The pooled results were heterogenous (p = 0.01, I2 = 84%) which can’t be resolved (Table 4). Also, see supplementary Fig. S9 (online resource)
MADRS total score
The overall effect estimates of 15-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (SMD = -0.22, 95% CI: [-0.32, -0.12], p < 0.0001). The pooled results were homogenous (p = 0.24, I2 = 26%). After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in both Zuranolone 30-mg and Zuranolone 50-mg groups when compared to placebo group [(SMD = -0.31, 95% CI [-0.46, -0.16], p < 0.0001); p = 0.31, I2 = 15%] and [(SMD = -0.2, 95% CI [-0.38, -0.02], p = 0.03)], respectively. However, the effect estimate showed no significant difference in the Zuranolone 20-mg group when compared to the placebo group [SMD = -0.06, 95% CI [-0.29, 0.17], p = 0.59] (Table 4). Also, see supplementary Fig. S10 (online resource).
The overall effect estimates of 42 to 45-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (SMD = -0.16, 95% CI: [-0.28, -0.03], p = 0.02). The pooled results were homogenous (p = 0.06, I2 = 56%). After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in Zuranolone 30-mg when compared to the placebo group [SMD = -0.24, 95% CI [-0.4, -0.09], p = 0.002); p = 0.18, I2 = 39%]. However, the effect estimates showed no significant difference in the Zuranolone 20-mg group when compared to the placebo group [SMD = 0.05, 95% CI [-0.19, 0.28], p = 0.69] (Table 4). Also, see supplementary Fig. S11 (online resource).
HAM-A total score
The overall effect estimates of 15-day follow-up duration showed a significant difference between Zuranolone and placebo groups favoring Zuranolone (MD = -1.56, 95% CI: [-2.37, -0.74], p = 0.0002). The pooled results were homogenous (p = 0.24, I2 = 25%). After introducing subgroup analysis based on Zuranolone dose, the effect estimates showed significant improvement in both Zuranolone 30-mg and Zuranolone 50-mg groups when compared to placebo group [(MD = -2.34, 95% CI [-3.83, -0.85], p = 0.002); p = 0.17, I2 = 40%] and [(MD = -1.29, 95% CI [-2.41, -0.17], p = 0.02], respectively. However, the effect estimate showed no significant difference in the Zuranolone group 20-mg when compared to the placebo group [SMD = -1.03, 95% CI [-2.99, 0.93], p = 0.3); p = 0.24, I2 = 28%] (Table 4). Also, see supplementary Fig. S12 (online resource).
The overall effect estimates of 42 to 45-day follow-up duration showed no significant difference between Zuranolone and placebo groups (MD = -1.33, 95% CI: [-2.83, 0.18], p = 0.08). The pooled results were heterogeneous (p = 0.02, I2 = 66%). After introducing subgroup analysis based on Zuranolone dose, the effect estimates also showed no significant improvement in Zuranolone 20-mg, Zuranolone 30-mg, and Zuranolone 50-mg groups when compared to the placebo group [MD = -0.05, 95% CI [-1.44, 1.34], p = 0.94], [MD = -2.42, 95% CI [-5.63, 0.8], p = 0.14); p = 0.01, I2 = 76%] and [MD = -0.81, 95% CI [-2.16, 0.54], p = 0.24], respectively (Table 4). Also, see supplementary Fig. S13 (online resource).
Safety outcomes
TEAEs
For this outcome the higher the odd ratio (OR) the worse the outcome. Meaning more events occurred with the intervention. Looking at zuranolone, regardless of the dose, the intervention showed more TEAEs compared with the placebo (OR = 1.46, 95% CI: [1.15, 1.84], p = 0.002).). The pooled results were homogenous (p = 0.06, I2 = 46%). After introducing subgroup analysis based on Zuranolone dose, the higher the dose the more TEAEs reported, as for 50 mg, 30 mg vs 20 mg (OR = 1.71, 95% CI: [1.31, 2.22], p > 0.0001), (OR = 1.49, 95% CI: [0.93, 2.38], p = 0.09).), (OR = 1.19, 95% CI: [0.85, 1.66], p = 0.32), respectively. A dose of 50 mg showed a significant difference compared to the placebo. However, doses 30 mg and 20 mg showed non-significant differences (Table 6). Also, see supplementary Fig. S14 (online resource).
Table 6.
Odds ratio of the safety outcomes
| Variables | Doses | Effect estimates | No. of participants | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | Zurnaonlone | Placebo | I2 | P | ||
| TEAEs | 20-mg | 1.19 | [0.85, 1.66] | 0.32 | 273 | 272 | 0 | 0.69 |
| 30-mg | 1.49 | [0.93, 2.38] | 0.09 | 275 | 462 | 64 | 0.02 | |
| 50-mg | 1.71 | [1.31, 2.22] | < 0.0001 | 480 | 487 | 0 | 0.42 | |
| Total | 1.46 | [1.15, 1.84] | 0.002 | 1228 | 1221 | 45 | 0.06 | |
| SAEs | 20-mg | 1.4 | [0.274, 7.23] | 0.682 | 273 | 272 | 0 | 0.836 |
| 30-mg | 1.46 | [0.42, 5.313] | 0.565 | 397 | 389 | 0 | 0.959 | |
| 50-mg | 1.63 | [0.31, 8.5] | 0.562 | 480 | 487 | 0 | 0.374 | |
| Total | 1.49 | [0.63, 3.54] | 0.366 | 1150 | 1148 | 0 | 0.992 | |
| Severe AEs | 30-mg | 0.94 | [0.21, 4.25] | 0.936 | 123 | 117 | 0 | 0.983 |
| 50-mg | 2.05 | [0.86, 4.87] | 0.104 | 480 | 487 | 0 | 0.584 | |
| Total | 1.69 | [0.8, 3.58] | 0.17 | 603 | 604 | 0 | 0.783 | |
| AEs- related drug discontinuation | 20-mg | 0.66 | [0.183, 2.38] | 0.526 | 273 | 272 | 0 | 0.322 |
| 30-mg | 0.94 | [0.36, 2.43] | 0.906 | 475 | 462 | 0 | 0.874 | |
| 50-mg | 2.01 | [0.983, 4.09] | 0.056 | 480 | 487 | 0 | 0.776 | |
| Total | 1.33 | [0.79, 2.23] | 0.282 | 1228 | 1221 | 0 | 0.735 | |
| Deaths | 20-mg | 1.93 | [0.16, 23.1] | 0.605 | 273 | 272 | 0 | 0.657 |
| 30-mg | 0.976 | [0.14, 6.97] | 0.98 | 397 | 389 | 0 | 1 | |
| Total | 1.27 | [0.27, 5.92] | 0.763 | 670 | 661 | 0 | 0.996 | |
| Headache | 20-mg | 1.66 | [0.88, 3.13] | 0.12 | 273 | 272 | 0 | 0.77 |
| 30-mg | 0.94 | [0.56, 1.57] | 0.81 | 379 | 389 | 0 | 0.77 | |
| 50-mg | 1.04 | [0.7, 1.56] | 0.85 | 480 | 487 | 54 | 0.14 | |
| Total | 1.11 | [0.84, 1.47] | 0.47 | 1150 | 1148 | 0 | 0.61 | |
| Dizziness | 20-mg | 2.18 | [1.04, 4.56] | 0.04 | 273 | 272 | 0 | 0.91 |
| 30-mg | 1.76 | [1, 3.11] | 0.05 | 475 | 462 | 0 | 0.86 | |
| 50-mg | 3.55 | [0.98, 12.78] | 0.05 | 480 | 487 | 82 | 0.02 | |
| Total | 2.33 | [1.62, 3.35] | < 0.00001 | 1228 | 1221 | 9 | 0.36 | |
| Nausea | 20-mg | 1.13 | [0.45, 2.85] | 0.8 | 188 | 190 | NA | NA |
| 30-mg | 0.93 | [0.3, 2.87] | 0.9 | 315 | 307 | 44 | 0.17 | |
| 50-mg | 0.32 | [0.18, 0.57] | < 0.0001 | 212 | 218 | NA | NA | |
| Total | 0.71 | [0.33, 1.52] | 0.38 | 715 | 715 | 62 | 0.03 | |
| Somnolence | 20-mg | 1.65 | [0.81, 3.38] | 0.17 | 273 | 272 | 0 | 0.62 |
| 30-mg | 1.97 | [1.24, 3.13] | 0.004 | 475 | 462 | 0 | 0.6 | |
| 50-mg | 3.58 | [2.25, 5.7] | < 0.00001 | 480 | 487 | 66 | 0.09 | |
| Total | 2.48 | [1.84, 3.33] | < 0.00001 | 1228 | 1221 | 21 | 0.26 | |
| Dry mouth | 30-mg | 2.16 | [0.47, 9.92] | 0.32 | 123 | 117 | 15 | 0.28 |
| 50-mg | 1.09 | [0.56, 2.11] | 0.8 | 212 | 218 | NA | NA | |
| Total | 1.23 | [0.67, 2.23] | 0.51 | 335 | 335 | 0 | 0.49 | |
| Sedation | 20-mg | 1.98 | [0.75, 5.22] | 0.166 | 273 | 272 | 0 | 0.803 |
| 30-mg | 1.83 | [0.8, 4.19] | 0.156 | 475 | 462 | 0 | 0.589 | |
| 50-mg | 5.7 | [0.6, 54.1] | 0.13 | 480 | 487 | 75.56 | 0.043 | |
| Total | 2.29 | [1.36, 3.84] | 0.002 | 1228 | 1221 | 0.44 | 0.43 | |
| Decreased appetite | 30-mg | 0.48 | [0.04, 5.46] | 0.55 | 45 | 44 | NA | NA |
| 50-mg | 1.81 | [0.7, 4.69] | 0.22 | 212 | 218 | NA | NA | |
| Total | 1.5 | [0.63, 3.56] | 0.36 | 257 | 262 | 0 | 0.32 | |
| Insomnia | 30-mg | 0.48 | [0.04, 5.46] | 0.55 | 45 | 44 | NA | NA |
| 50-mg | 1.3 | [0.67, 2.54] | 0.44 | 212 | 218 | NA | NA | |
| Total | 1.2 | [0.63, 2.29] | 0.57 | 257 | 262 | 0 | 0.44 | |
| Diarrhea | 20-mg | 1.12 | [0.46, 2.7] | 0.8 | 188 | 190 | NA | NA |
| 30-mg | 1.26 | [0.66, 2.39] | 0.48 | 393 | 380 | 12 | 0.33 | |
| 50-mg | 0.59 | [0.34, 1.03] | 0.07 | 480 | 487 | 0 | 0.88 | |
| Total | 0.86 | [0.59, 1.26] | 0.45 | 1061 | 1057 | 16 | 0.31 | |
| Upper respiratory tract infection | 20-mg | 1.48 | [0.4, 5.45] | 0.55 | 85 | 82 | NA | NA |
| 30-mg | 3.25 | [1.27, 8.34] | 0.01 | 238 | 228 | 0 | 0.49 | |
| Total | 2.54 | [1.19, 5.38] | 0.02 | 323 | 310 | 0 | 0.54 | |
| Fatigue | 20-mg | 0.6 | [0.14, 2.55] | 0.49 | 188 | 190 | NA | NA |
| 30-mg | 2.72 | [1.05, 7.07] | 0.04 | 270 | 263 | 0 | 0.96 | |
| 50-mg | 1.75 | [0.8, 3.79] | 0.04 | 212 | 218 | NA | NA | |
| Total | 1.74 | [1.01, 2.99] | 0.04 | 670 | 671 | 0 | 0.4 | |
AEs Adverse events, CI Confidence interval, OR Odds ratio, SAEs Serious adverse events, TEAEs Treatment emergent adverse events
Serious adverse events
The overall effect estimates showed a non-significant difference between Zuranolone and placebo groups (OR = 1.49, 95% CI: [0.62, 3.53], p = 0.366). After introducing subgroup analysis based on Zuranolone dose, the higher the dose the more serious adverse events reported, as for 50 mg, 30 mg vs 20 mg (OR = 1.62, 95% CI: [0.31, 8.5], p = 0.562), (OR = 1.46, 95% CI: [0.40, 5.313], p = 0.565), (OR = 1.40, 95% CI: [0.27, 7.23], p = 0.682), respectively. (Table 6). Also, see supplementary Fig. S15 (online resource).
Severe adverse events
Only two interventions (zuranolone 30, 50 mg) were included in this analysis. the overall effect estimates showed a non-significant difference between Zuranolone and placebo groups (OR = 1.69, 95% CI: [0.79, 3.58], p = 0.17). It is observed that the severe adverse events were more in dose 50 mg compared to dose 30mg (OR = 2.05, 95% CI: [0.86, 4.87], p = 0.104), (OR = 0.94, 95% CI: [0.20, 4.24], p = 0.936), respectively. (Table 6). Also, see supplementary Fig. S16 (online resource).
AEs-related drug discontinuation
The overall effect estimates showed a non-significant difference between Zuranolone and placebo groups (OR = 1.33, 95% CI: [0.79, 2.23], p = 0.282). After introducing subgroup analysis based on Zuranolone dose, the higher the dose the more adverse events drug discontinuation reported, as for 50 mg, 30 mg vs 20 mg (OR = 2.00, 95% CI: [0.98, 4.09], p = 0.056), (OR = 0.94, 95% CI: [0.36, 2.43], p = 0.906), (OR = 0.66, 95% CI: [0.18, 2.38], p = 0.526), respectively. (Table 6). Also, see supplementary Fig. S17 (online resource).
Most common TEAEs
Somnolence, dizziness, and headache were the most reported TEAEs in the zuranolone dose groups. However, the overall effect estimates showed a non-significant difference between Zuranolone and placebo groups (OR = 1.11, 95% CI: [0.84, 1.47], p = 0.47) for headache. In contrast, the zuranolone showed more dizziness compared with the placebo (OR = 2.33, 95% CI: [1.62, 3.35], p > 0.00001).) and Somnolence (OR = 2.48, 95% CI: [1.84, 3.33], p > 0.00001).). (Table 6). Also, see supplementary Fig. S18 (online resource).
Regardless of the dose, zuranolone showed more sedation (OR = 2.28, 95% CI: [1.36, 3.84], p = 0.002), fatigue (OR = 1.74, 95% CI: [1.01, 2.99], p = 0.04).), and upper respiratory tract infection (OR = 2.54, 95% CI: [1.19, 5.38], p = 0.02) compared to the placebo. In contrast, the overall effect estimate showed a non-significant difference between zuranolone and placebo in terms of insomnia, dry mouth, decreased appetite, nausea, diarrhea, and death [(OR = 1.20, 95% CI: [0.63, 2.29], p = 0.57), (OR = 1.23, 95% CI: [0.67, 2.23], p = 0.51), (OR = 1.50, 95% CI: [0.63, 3.56], p = 0.36), (OR = 0.71, 95% CI: [0.33, 1.52], p = 0.38), (OR = 0.86, 95% CI: [0.59, 1.26], p = 0.45), and (OR = 1.26, 95% CI: [0.27, 5.92], p = 0.763)], respectively. (Table 6). Also, see supplementary Fig. S18 (online resource).
Discussion
Depression, a pervasive mental health challenge affecting millions globally, prompts a continuous search for more effective and safer treatment options (Reddy 2010) Zuranolone, a novel selective neuroactive steroid GABAA receptor-positive allosteric modulator, exhibits promise in preclinical and early clinical trials for depression treatment (Althaus et al. 2020) This meta-analysis consolidates the latest data from 8 randomized controlled trials (RCTs) exploring zuranolone's efficacy and safety in major depressive disorder (MDD) and postpartum depression (PPD).
Our study evaluates zuranolone's impact compared to placebo across varied follow-up durations, employing common outcome measures in depression research (Rabin et al. 2022). The primary goal is to determine zuranolone's overall efficacy while scrutinizing its safety profile based on reported adverse events (AEs). Noteworthy findings reveal significant benefits favoring zuranolone in terms of efficacy outcomes. Specifically, both the 30-mg and 50-mg zuranolone groups exhibit substantial improvement at the 15-day follow-up, reflected in a reduction of over 50% from baseline in the Hamilton Depression Rating Scale (HAM-D) score. A similar trend is observed at the 42 to 45-day follow-up.
Efficacy outcomes
Unlike other common GABAA positive allosteric modulators like benzodiazepines, zuranolone can modulate both synaptic and extrasynaptic GABAA conductance due to binding to a non-benzodiazepine site on the receptor (Clayton et al. 2023; Stahl et al. 2023). Furthermore, it may restore the balance of GABAA receptor function that is disrupted by the rapid decline of allopregnanolone after childbirth (Stahl et al. 2023).
The overall effect estimates demonstrated a statistically significant improvement in HAMD-17 following 15 days after zuranolone administration and with a 42 to 45-day follow-up duration. Subgroup analysis based on zuranolone dose revealed that both the zuranolone 30-mg and 50-mg groups showed significant improvement in HAMD-17 scores compared to the placebo group. This is consistent with the study conducted on women with PPD which reported that rates of concurrent remission of depressive and anxiety symptoms were higher with zuranolone versus placebo (Deligiannidis et al. 2023) also a study conducted in the United States among adults with MDD reported significant improvement at days 3, 8, and 12 (Clayton et al. 2023).
On the other hand, the effect estimate for the zuranolone 20-mg group did not show a significant difference compared to the placebo group. However, a study conducted on Japanese patients aged between ≥ 18 years and ≤ 75 years with a diagnosis of MDD to test the efficacy and safety of zuranolone (Kato et al. 2023a) reported improvement in HAMD-17 scores and insomnia symptom score showed nominally significant differences in the zuranolone 20 mg groups when compared with the placebo group at Day 15. This suggests that the efficacy of zuranolone may vary depending on the specific dose used, and the 20-mg dose may not be as effective in improving HAMD-17 scores.
Regarding the Bech-6 scale (a shortened version of the HAMD-17 scale), our pooled analysis showed a great numerical improvement in zuranolone compared to placebo, but this numerical improvement didn’t reach statistical significance. Our finding was consistent with Kato et al. and Gunduz-Bruce et al. (Gunduz-Bruce et al. 2019; Kato et al. 2023a). However, Gunduz-Bruce et al. showed statistical significance. Unfortunately, the Bech-6 scale was reported by two studies, however; it’s more sensitive than the HAMD-17 scale in detecting treatment effects. The small sample size and high heterogeneity in our pooled analysis may mask the statistical significance of the treatment effects (Dunlop et al. 2019).
Regarding the reduction of > 50% from baseline in HAM-D score, this meta-analysis demonstrates that zuranolone, particularly at the 30-mg dose, is associated with a higher likelihood of achieving a reduction of > 50% from baseline in HAM-D score at both the 15-day and 42 to 45-day follow-up durations. However, there was no significant difference between Zuranolone 20-mg when compared to the placebo groups at 15 days and 42 to 45-day follow-ups as reported by a study conducted among Japanese adults (Kato et al. 2023a) and a study conducted in the United States among adults with MDD (Clayton et al. 2023). The negative results with these doses suggest the antidepressant effects of zuranolone may be threshold-dependent, requiring a minimum effective concentration to be reached. The 20 mg dose appears to be below this threshold level to consistently achieve clinically important reductions in depression severity (Walkery et al. 2021).
Additionally, zuranolone at 50 mg dosage showed no significant difference regarding the reduction of > 50% from baseline in HAM-D score between the treatment and placebo groups at 42 to 45-day follow-up duration. The lack of a significant difference seen with 50 mg dose versus placebo at 6 weeks also aligns with potential non-linear pharmacokinetics. Higher concentrations do not necessarily confer additional benefits and may come with an increased risk of adverse effects (Deligiannidis et al. 2021; Lin et al. 2023a, b). The lack of effect of 50 mg zuranolone compared to 30 mg may be attributed to a lack of compliance to 50 mg due to increased incidence of side effects (Lin et al. 2023a, b).
The ability to demonstrate a sustained response to antidepressant drugs was limited with the duration of follow-up of included trials. Nevertheless, it's important to evaluate the benefit of maintenance of medications over time to determine how long patients with MDD should take antidepressants. Kato et al. (Kato et al. 2021)recommended at least 6 months of maintenance therapy of antidepressants to prevent relapse and treatment failure as they found that the antidepressant maintenance group has a lower relapse rate than the antidepressant discontinuation group in both 6 months and 1 year maintenance periods. Another meta-analysis conducted by Kishi et al. (Kishi et al. 2023)recommended that maintenance treatment with antidepressants should be continued for at least 18 months or at least one year as the sustained response to antidepressants was more in 15 and 18 months than in 1 year or less. In contrast, the included studies, investigating the efficacy of zuranolone, only gave it for two weeks and the follow-up duration was relatively short; about 45 days. Furthermore, the overall effect achieved at 45 days of follow-ups was less than the effect achieved at 15 days of follow-ups in terms of HAMD-17 score, reduction of > 50% in HAM-D score, HAM-D score ≤ 7, CGI-I score of 1 or 2, MADRS score, and HAM-A score. Further long-term clinical trials are needed to evaluate the efficacy of a longer period of zuranolone maintenance in preventing relapse.
Safety outcomes
This analysis indicates a higher occurrence of treatment-emergent adverse events (TEAEs) in comparison to the placebo. Significantly, the relative risk of TEAEs rises with escalating zuranolone doses, particularly with the 50-mg dose posing the greatest risk compared to the placebo (Kato et al. 2023a) This dose-dependent safety profile aligns with zuranolone's pharmacokinetic properties, underscoring the need to consider dosage levels when assessing safety (Parikh et al. 2024). These findings are consistent with a study assessing the efficacy and safety of zuranolone among adults with major depressive disorder (MDD), reporting that 74.1% of patients who received zuranolone experienced at least one TEAE. While the 30-mg dose showed a nominally higher TEAE risk than the 20-mg dose, this difference did not reach statistical significance. This suggests that the safety risks associated with the 30-mg dose are comparable to those of the 20-mg dose, acknowledging the limitations of the included studies and sample sizes.
Concerning severe adverse events and adverse events related to drug discontinuation, the comparison between zuranolone and placebo groups yielded non-significant differences, indicating a similarity in the occurrence of severe adverse events between the two groups. This finding aligns with a study assessing the tolerability of zuranolone among U.S. patients (Hoffmann et al. 2020) This is supported by studies conducted by Sage Therapeutics and Biogen, where no notable difference was found in adverse events related to discontinuation of the drug between the zuranolone and placebo groups, and a separate study involving women with PPD (Deligiannidis et al. 2021; Sage Therapeutics and Biogen Announce Positive Pivotal, n.d.).
The most frequent adverse events include somnolence, dizziness, and fatigue, with no alarming safety concerns. This agrees with a study conducted among adults with MDD to assess the efficacy and safety of zuranolone (Parikh et al. 2024).
Strengths and limitations
This study was conducted based on PRISMA guidelines and pooled the results according to the available clinical trials to assess the efficacy and safety of zuranolone in depression. However, our review suffers from some limitations, including the limited number of randomized trials, and subsequently small sample size. Additionally, some of the measured outcomes were heterogeneous due to variations between the included studies. Our meta-analysis didn’t separate patients with postpartum depression and major depressive disorder. Although we acknowledge the importance of meticulously separate studies of zuranolone in postpartum patients from those involving MDD to better elucidate any nuanced effects and implications within each population, Lin et al. reported that the subgroup analysis didn’t show significant differences in outcomes between these two categories(Lin et al. 2023a, b). Also, most of the included studies were conducted in the US, making the generalizability of these results questionable.
Conclusion
In conclusion, these findings suggest zuranolone's potential as an effective treatment for depression, emphasizing the crucial need for cautious consideration regarding its safety. Notably, there is no significant difference in discontinuation rates between the zuranolone and placebo groups.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
we would like to express our gratitude to Mohamed Elsaid who guided us throughout this project. your useful advice and suggestions were really helpful to us during the project's completion. we would also like to thank Gehad S. Remeih who offered deep support to us.
Authors’ contributions
AMF: generating the research idea, writing the manuscript, data analysis, and solving any conflict; HAO: two-phase screening, quality assessment, and writing the manuscript; IAE: two-phase screening, and quality assessment; MATE: data extraction (summary and baseline); MMME: data extraction (outcomes); HKS: writing the manuscript, and data analysis.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding was received to this study.
Data availability
All data are available upon reasonable request from the corresponding author.
Declarations
Ethics approval
Not applicable.
Consent for publication
Not applicable.
Ethical considerations
Not applicable.
Conflict of interests
No conflict or competing interests between the manuscripts’ authors.
Footnotes
The original version of this article was revised: This article was originally published with a mispelled author name. It should be Elewidi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/22/2024
A Correction to this paper has been published: 10.1007/s00213-024-06642-5
References
- 2019 NSDUH Detailed Tables | CBHSQ Data. (n.d.). Retrieved May 18, 2024, from https://www.samhsa.gov/data/report/2019-nsduh-detailed-tables
- Althaus AL, Ackley MA, Belfort GM, Gee SM, Dai J, Nguyen DP, Kazdoba TM, Modgil A, Davies PA, Moss SJ, Salituro FG, Hoffmann E, Hammond RS, Robichaud AJ, Quirk MC, Doherty JJ (2020) Preclinical characterization of zuranolone (SAGE-217), a selective neuroactive steroid GABAA receptor positive allosteric modulator. Neuropharmacology 181. 10.1016/J.NEUROPHARM.2020.108333 [DOI] [PMC free article] [PubMed]
- Batterham PJ, Christensen H, Mackinnon AJ. Modifiable risk factors predicting major depressive disorder at four year follow-up: a decision tree approach. BMC Psychiatry. 2009;9:75. doi: 10.1186/1471-244X-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman BL, Ko JY, Cox S, D’Angelo MDV, Warner L, Folger S, Tevendale HD, Coy KC, Harrison L, Barfield WD. Vital Signs: Postpartum Depressive Symptoms and Provider Discussions About Perinatal Depression — United States, 2018. MMWR. Morb Mortal Wkly Rep. 2020;69(19):575–581. doi: 10.15585/mmwr.mm6919a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull SA, Hunkeler EM, Lee JY, Rowland CR, Williamson TE, Schwab JR, Hurt SW. Discontinuing or Switching Selective Serotonin-Reuptake Inhibitors. Ann Pharmacother. 2002;36(4):578–584. doi: 10.1345/aph.1A254. [DOI] [PubMed] [Google Scholar]
- Clayton AH, Lasser R, Nandy I, Sankoh AJ, Jonas J, Kanes SJ (2023) Zuranolone in Major Depressive Disorder: Results From MOUNTAIN—A Phase 3, Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial. J Clin Psychiatry 84(2). 10.4088/JCP.22M14445 [DOI] [PubMed]
- Cutler AJ, Mattingly GW, Maletic V (2023) Understanding the mechanism of action and clinical effects of neuroactive steroids and GABAergic compounds in major depressive disorder. Translational Psychiatry 13(1). 10.1038/S41398-023-02514-2 [DOI] [PMC free article] [PubMed]
- Da Costa D, Dritsa M, Rippen N, Lowensteyn I, Khalifé S. Health-related quality of life in postpartum depressed women. Arch Womens Ment Health. 2006;9(2):95–102. doi: 10.1007/s00737-005-0108-6. [DOI] [PubMed] [Google Scholar]
- Deligiannidis KM, Citrome L, Huang MY, Acaster S, Fridman M, Bonthapally V, Lasser R, Kanes SJ (2023) Effect of Zuranolone on Concurrent Anxiety and Insomnia Symptoms in Women With Postpartum Depression. J Clin Psychiatry 84(1). 10.4088/JCP.22M14475 [DOI] [PubMed]
- Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, Doherty J, Jonas J, Li S, Sankoh AJ, Silber C, Campbell AD, Werneburg B, Kanes SJ, Lasser R. Effect of Zuranolone vs Placebo in Postpartum Depression: A Randomized Clinical Trial. JAMA Psychiat. 2021;78(9):951–959. doi: 10.1001/JAMAPSYCHIATRY.2021.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Parikh SV, Rothschild AJ, Thase ME, DeBattista C, Conway CR, Forester BP, Mondimore FM, Shelton RC, Macaluso M, Logan J, Traxler P, Li J, Johnson H, Greden JF. Comparing sensitivity to change using the 6-item versus the 17-item Hamilton depression rating scale in the GUIDED randomized controlled trial. BMC Psychiat. 2019;19(1):420. doi: 10.1186/s12888-019-2410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA NEWS RELEASE (n.d) FDA Approves First Oral Treatment for Postpartum Depression | FDA. Retrieved May 18, 2024, from https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-treatment-postpartum-depression
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England), 392(10159), 1789–1858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed]
- Geddes JR, Carney SM, Davies C, Furukawa TA, Kupfer DJ, Frank E, Goodwin GM. Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet. 2003;361(9358):653–661. doi: 10.1016/S0140-6736(03)12599-8. [DOI] [PubMed] [Google Scholar]
- Gerner RH, Hare TA. CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. Am J Psychiatry. 1981;138(8):1098–1101. doi: 10.1176/ajp.138.8.1098. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Silber C, Kaul I, Rothschild AJ, Riesenberg R, Sankoh AJ, Li H, Lasser R, Zorumski CF, Rubinow DR, Paul SM, Jonas J, Doherty JJ, Kanes SJ. Trial of SAGE-217 in Patients with Major Depressive Disorder. N Engl J Med. 2019;381(10):903–911. doi: 10.1056/NEJMoa1815981. [DOI] [PubMed] [Google Scholar]
- Heo Y-A. Zuranolone: First Approval. Drugs. 2023;83(16):1559–1567. doi: 10.1007/s40265-023-01953-x. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (2019) Cochrane handbook for systematic reviews of interventions. Cochrane Handbook for Systematic Reviews of Interventions 1–694. 10.1002/9781119536604 [DOI] [PMC free article] [PubMed]
- Hockenberry JM, Joski P, Yarbrough C, Druss BG. Trends in Treatment and Spending for Patients Receiving Outpatient Treatment of Depression in the United States, 1998–2015. JAMA Psychiat. 2019;76(8):810–817. doi: 10.1001/jamapsychiatry.2019.0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E, Nomikos GG, Kaul I, Raines S, Wald J, Bullock A, Sankoh AJ, Doherty J, Kanes SJ, Colquhoun H. SAGE-217, A Novel GABAA Receptor Positive Allosteric Modulator: Clinical Pharmacology and Tolerability in Randomized Phase I Dose-Finding Studies. Clin Pharmacokinet. 2020;59(1):111–120. doi: 10.1007/S40262-019-00801-0/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Hori H, Inoue T, Iga J, Iwata M, Inagaki T, Shinohara K, Imai H, Murata A, Mishima K, Tajika A. Discontinuation of antidepressants after remission with antidepressant medication in major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2021;26(1):118–133. doi: 10.1038/s41380-020-0843-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Nakagome K, Baba T, Sonoyama T, Okutsu D, Yamanaka H, Shimizu R, Motomiya T, Inoue T. Efficacy and safety of zuranolone in Japanese adults with major depressive disorder: A double-blind, randomized, placebo-controlled, phase 2 clinical trial. Psychiatry Clin Neurosci. 2023;77(9):497–509. doi: 10.1111/pcn.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstis B, Aarts C, Tillman C, Persson H, Engström G, Edlund B, Öhrvik J, Sylvén S, Skalkidou A. Association between parental depressive symptoms and impaired bonding with the infant. Arch Womens Ment Health. 2016;19(1):87–94. doi: 10.1007/s00737-015-0522-3. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, Replication NCS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kishi T, Sakuma K, Hatano M, Okuya M, Matsuda Y, Kato M, Iwata N. Relapse and its modifiers in major depressive disorder after antidepressant discontinuation: meta-analysis and meta-regression. Mol Psychiatry. 2023;28(3):974–976. doi: 10.1038/s41380-022-01920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SK, Dhir A. Current investigational drugs for major depression. Expert Opin Investig Drugs. 2009;18(6):767–788. doi: 10.1517/13543780902880850. [DOI] [PubMed] [Google Scholar]
- Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, Zarate CA. Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biol Psychiat. 2017;81(10):886–897. doi: 10.1016/j.biopsych.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-W, Tu Y-K, Hung K-C, Liang C-S, Tseng P-T, Lin P-Y, Chia-Cheng Lai E, Hsu C-W. Efficacy and safety of zuranolone in major depressive disorder: a meta-analysis of factor effect and dose-response analyses. EClinicalMedicine. 2023;66:102308. doi: 10.1016/j.eclinm.2023.102308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-W, Tu Y-K, Hung K-C, Liang C-S, Tseng P-T, Lin P-Y, Lai EC-C, Hsu C-W. Efficacy and safety of zuranolone in major depressive disorder: a meta-analysis of factor effect and dose-response analyses. EClinicalMedicine. 2023;66:102308. doi: 10.1016/J.ECLINM.2023.102308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry. 2011;16(4):383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luykx JJ, Laban KG, Van Den Heuvel MP, Boks MP, Mandl RC, Kahn RS, Bakker SC. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of 1H-MRS findings. Neurosci Biobehav Rev. 2012;36(1):198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Merali Z, Du L, Hrdina P, Palkovits M, Faludi G, Poulter MO, Anisman H. Dysregulation in the suicide brain: mRNA expression of corticotropin-releasing hormone receptors and GABA(A) receptor subunits in frontal cortical brain region. J Neurosci : Official J Soc Neurosci. 2004;24(6):1478–1485. doi: 10.1523/JNEUROSCI.4734-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OpenMeta[Analyst] -- CEBM @ Brown (n.d.) Retrieved May 18, 2024, from http://www.cebm.brown.edu/openmeta/
- Osborne LM, Gispen F, Sanyal A, Yenokyan G, Meilman S, Payne JL. Lower allopregnanolone during pregnancy predicts postpartum depression: An exploratory study. Psychoneuroendocrinology. 2017;79:116–121. doi: 10.1016/j.psyneuen.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S … Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed]
- Parikh SV, Aaronson ST, Mathew SJ, Alva G, DeBattista C, Kanes S, Lasser R, Bullock A, Kotecha M, Jung J, Forrestal F, Jonas J, Vera T, Leclair B, Doherty J. Efficacy and safety of zuranolone co-initiated with an antidepressant in adults with major depressive disorder: results from the phase 3 CORAL study. Neuropsychopharmacol. 2024;49(2):467–475. doi: 10.1038/s41386-023-01751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J : Official Publ Fed Am Soc Exp Biol. 1992;6(6):2311–2322. doi: 10.1096/fasebj.6.6.1347506. [DOI] [PubMed] [Google Scholar]
- Rabin JS, Nyman AJ, Davidson B, Zakzanis KK, Giacobbe P, Hamani C, Nestor S, Lipsman N. Commonly used outcome measures in neurosurgical trials for major depressive disorder might not capture clinically meaningful treatment effects. J Neurol Neurosurg Psychiatry. 2022;93(4):455–456. doi: 10.1136/JNNP-2021-327688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MS. Depression: The Disorder and the Burden. Indian J Psychol Med. 2010;32(1):1. doi: 10.4103/0253-7176.70510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RevMan | Cochrane Training (n.d.) Retrieved May 18, 2024, from https://training.cochrane.org/onlinelearning/core-software/revman
- Sage Therapeutics and Biogen Announce Positive Pivotal (n.d.) Retrieved December 19, 2023, from https://www.globenewswire.com/news-release/2021/06/15/2247077/0/en/Sage-Therapeutics-and-Biogen-Announce-Positive-Pivotal-Phase-3-Results-for-Zuranolone-an-Investigational-Two-Week-Once-Daily-Therapeutic-Being-Evaluated-for-Major-Depressive-Disord.html
- Sanacora G, Gueorguieva R, Epperson CN, Wu Y-T, Appel M, Rothman DL, Krystal JH, Mason GF. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61(7):705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Hammond R, Garcia M, Kyaga S, Kotecha M, Pollack M, Doherty J. Zuranolone, a Positive Allosteric Modulator of the GABAA Receptor: Hypothesized Mechanism of Action in Major Depressive Disorder. CNS Spectr. 2023;28(2):260–261. doi: 10.1017/S1092852923002092. [DOI] [Google Scholar]
- Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Chen, H-Y, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T … Higgins JPT (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ l4898. 10.1136/bmj.l4898 [DOI] [PubMed]
- Suthoff E, Kosinski M, Arnaud A, Hodgkins P, Gunduz-Bruce H, Lasser R, Silber C, Sankoh AJ, Li H, Werneburg B, Jonas J, Doherty J, Kanes SJ, Bonthapally V. Patient-reported health-related quality of life from a randomized, placebo-controlled phase 2 trial of zuranolone in adults with major depressive disorder. J Affect Disord. 2022;308:19–26. doi: 10.1016/J.JAD.2022.03.068. [DOI] [PubMed] [Google Scholar]
- Trivedi MH. Treating Depression to Full Remission. J Clin Psychiatry. 2009;70(1):e01. doi: 10.4088/JCP.8017br6c.e01. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of Outcomes With Citalopram for Depression Using Measurement-Based Care in STAR*D: Implications for Clinical Practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95(6):3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkery A, Leader LD, Cooke E, Vandenberg A. Review of Allopregnanolone Agonist Therapy for the Treatment of Depressive Disorders. Drug Des Dev Ther. 2021;15:3017. doi: 10.2147/DDDT.S240856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization [Internet] (2017) Depression and Other Common Mental Disorders. https://www.who.int/Publications/i/Item/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon reasonable request from the corresponding author.