Abstract
Interleukin-10 (IL-10) is widely known as an immunosuppressive cytokine by virtue of its ability to inhibit macrophage-dependent antigen presentation, T-cell proliferation, and Th1 cytokine secretion. However, several studies have challenged the perception of IL-10 solely as an immunosuppressive cytokine. As part of an investigation on potentiation of the cytotoxic activity of human papillomavirus E7-specific CD8+ cytotoxic T lymphocytes (CTL) for adoptive transfusions to cervical cancer patients, we found that IL-10 in combination with IL-2, unlike several other combinations, including IL-2 with IL-12, gamma interferon (IFN-γ), tumor necrosis factor alpha, and transforming growth factor β, was able to consistently increase cytotoxicity. This augmentation in cytotoxic activity correlated with a significant increase in the cytoplasmic accumulation of perforin as detected by fluorescence-activated cell sorter. Surface expression of both the α and β chains of the CD8 heterodimeric coreceptor and CD56 molecules was increased by exposure of CTL to IL-10. More importantly, we found that administration of IL-10 in combination with IL-2 after antigen stimulation consistently increased the intracellular expression of Th1 cytokines (i.e., IFN-γ and IL-2) compared to results for control CD8+ T cells cultured in IL-2 alone. In kinetic studies, proliferation, intracellular perforin levels, cytotoxic activity, and IFN-γ expression were consistently elevated in CTL cultures containing IL-10 compared to control cultures, both at early and late time points following stimulation. In contrast, intracellular IL-2 expression was consistently increased only at early time points following stimulation with autologous tumor cells or solid-phase anti-CD3 antibody. Taken together, these data support the use of IL-10 in combination with IL-2 for the in vitro expansion and potentiation of tumor-specific CTL for clinical use in the therapy of cancer.
Interleukin-10 (IL-10) was originally described as a cytokine synthesis inhibitory factor by virtue of its ability to inhibit the production of several cytokines by Th1 clones (17). Since its original description, it has been shown to be secreted by multiple cell types, including T cells, monocytes, and B cells, after activation and to be endowed with pleiotropic and powerful immunosuppressive activity (for a review see reference 35). IL-10 has been reported to drastically reduce alloantigen-induced and antigen-specific T-cell proliferation, as well as cytotoxic T-cell responses (14, 15). These effects have been mainly related to an indirect inhibitory effect of IL-10 on antigen-presenting cells (APC), acting by inhibiting the production of various cytokines, including IL-12, and down-regulating the expression of surface costimulatory molecules (14, 15). However, a direct suppressive effect on T lymphocytes by IL-10 has also been demonstrated (16, 48). Overall, these biologic activities have strengthened the view of IL-10 as a potent negative regulator of immunoproliferative and inflammatory responses.
In the last few years, several in vitro and in vivo studies have challenged the view of IL-10 as an immunosuppressive cytokine. Indeed, IL-10, like IL-4, was thought to be exclusively secreted by Th2 T cells, but recently it has been reported that IL-12 may induce a stable phenotype in T-cell clones that coexpress large amounts of IL-10 and gamma interferon (IFN-γ) (18). Moreover, in a murine system, IL-10 has been shown to function as a cytotoxic T-cell differentiation factor, promoting a higher number of IL-2-activated cytotoxic T lymphocytes (CTL) to proliferate and differentiate into powerful cytotoxic effector cells (10). Murine tumor models genetically engineered to secrete large amounts of IL-10 are rejected by a combination of CD8+ T lymphocytes and NK cells (19, 55). Also, in a mouse tumor model genetically engineered to secrete multiple cytokines, including IL-2, IL-4, IL-6, IL-7, tumor necrosis factor alpha (TNF-α), granulocyte/macrophage colony-stimulating factor (GM-CSF), and IFN-γ, CTL activity and antibody responses induced by IL-10 stood out as the strongest (19). Finally, systemic administration of IL-10 may exacerbate allograft rejection (37) while an anti-IL10 antibody prolongs allograft survival in normal as well as presensitized recipients (28).
Recently, we have reported the successful generation by full-length E7-pulsed autologous dendritic cells (DC) of human papillomavirus (HPV)-specific, HLA class I-restricted CD8+ CTL in patients with invasive cervical cancer (40). These CTL recognize and kill autologous tumor cells from patients harboring HPV-infected adenocarcinomas and squamous cell carcinomas of the cervix and exhibit a striking dominance of type 1 intracellular cytokine expression by flow cytometric analysis (i.e., high IFN-γ, IL-2, and TNF-α expression and low IL-4 expression) (40, 41).
In an investigation of optimal culture conditions for potentiation of the cytotoxic activity of HPV E7-specific CTL for adoptive transfusions to cervical cancer patients, we found that IL-10 in combination with IL-2 consistently increased the cytotoxic potential of the CTL populations studied. Other combinations, notably IL-2 with IL-12, IFN-γ, TNF-α, or transforming growth factor β TGF-β, had no such effect. The ability of IL-10 to enhance proliferation, expression of immunologically important surface molecules, perforin content, cytotoxicity, and Th1 cytokine production by CD8+ CTL suggests that its use in combination with IL-2 may be a valuable adjunct for the in vitro expansion and potentiation of tumor-specific CTL in the therapy of cancer.
MATERIALS AND METHODS
Tumor cells.
The natural killer (NK)-sensitive target K562 (a human erythroleukemia cell line) was purchased from the American Type Culture Collection and was maintained at 37°C and 5% CO2 in RPMI 1640 (Gibco Life Technologies, Grand Island, N.Y.) and 10% fetal bovine serum (Gemini Bioproducts, Calabasas, Calif.). Fresh autologous tumor cells used in this study and derived from an HPV type 16 (HPV16)-positive cervical cancer patient have been previously described (40) and were cultured in serum-free keratinocyte medium (Gibco) supplemented with 5 ng of epidermal growth factor and 35 to 50 μg of bovine pituitary extract (Gibco)/ml at 37°C and 5% CO2. The Epstein-Barr virus (EBV)-transformed lymphoblastoid B-cell line (LCL) derived from the same cancer patient that provided the naturally HPV-infected primary tumor cell targets was established by coculture of peripheral blood mononuclear cells (PBMC) with EBV-containing supernatant from the B95.8 cell line in the presence of 1 μg of cyclosporin A (Sandoz, Camberley, United Kingdom)/ml and was maintained in RPMI 1640 supplemented with 10% human AB serum (Gemini Bioproducts).
DC cultures and generation of HPV E7-specific T cells.
The derivation of DC from the patients' PBMC and their subsequent use for generation of HPV E7-specific T cells were carried out essentially as described previously (40–42). E7-specific CD8+ T cells were derived from three cervical cancer patients, one with an HPV16+ squamous cell carcinoma and two with HPV18+ adenocarcinomas. Briefly, DC were generated from plastic-adherent PBMC by culture in AIM-V medium (Gibco) plus 800 U of GM-CSF (Immunex, Seattle, Wash.) and 1,000 U of IL-4 (R & D System, Minneapolis, Minn.)/ml. Cultures were fed by half changes of AIM-V plus GM-CSF and IL-4 every 2 days. After 6 to 7 days, DC were harvested and pulsed with 100 μg of recombinant HPV16 or HPV18 (as appropriate) incorporated in 125 μg of DOTAP cationic lipid (Boehringer Mannheim, Indianapolis, Ind.) in 2 to 5 ml of AIM-V for 3 h at 37°C with occasional agitation. The cells were then washed twice in phosphate-buffered saline (PBS) and resuspended in AIM-V. Fresh or cryopreserved responder PBMC were washed and resuspended in AIM-V at 107 to 2 × 107 cells/well in six-well culture plates with E7-pulsed DC at PBMC/DC ratios of 20:1 to 30:1. Cultures were initially supplemented with 10 U of IL-2 and 500 U of GM-CSF/ml. At day 21, CD8+ T cells were separated from the bulk cultures by positive selection with CD8 Dynabeads (Dynal Inc., Lake Success, N.Y.) and expanded by restimulation with 0.2 μg of anti-CD3 monoclonal antibodies (MAb)/ml and autologous or allogeneic irradiated (5,000 cGy) feeder PBMC (106 cells/ml). Demonstration of HLA class I restriction of tumor-specific CD8+ T-cell responses was achieved in standard cytotoxicity assays (40) in the presence of blocking MAb specific for a nonpolymorphic HLA class I determinant (W6/32) or isotype-matched control MAb (hybridomas were obtained from the American Type Culture Collection). All blocking MAb were used at 50 μg/ml. To evaluate the capacity of different human recombinant cytokines in combination with IL-2 to increase the cytotoxic activity of HPV-specific CTL, 106 CTL were cultured in AIM-V plus 5% human AB serum and 100 U of IL-2 (Aldesleukin, Chiron Therapeutics, Emeryville, Calif.)/ml (CM), in combination with IL-10 (range 1 to 20 ng/ml; specific activity, 2.9 × 104 U/μg), 50 U of human recombinant IL-12 (specific activity, 4.5 × 106 U/mg), 500 U of IFN-γ/ml (specific activity, 2.5 × 107 U/mg), 500 U of TNF-α/ml (specific activity, 1.0 × 107 U/mg), and 20 U of TGF-β/ml (specific activity, 3.2 × 104 U/μg) for 72 to 96 h before being tested in a standard 51Cr 6-h cytotoxicity assay against autologous tumor cells (CTL/tumor cell ratio, 5:1). All cytokines other than IL-2 were from R & D Systems. In kinetic experiments, CD8+ CTL (3 × 106 cells/well) rested for 4 to 6, 8 to 10, and 14 to 16 days from the last antigen stimulation with autologous irradiated tumor cells (5,000 cGy; tumor cell/effector cell ratio, 1:10) were resuspended in AIM-V medium with 5% human AB serum and 100 U of IL-2/ml and plated in the presence of 5 ng of IL-10/ml for 72 to 96 h before being tested for cytotoxicity against autologous tumor cells, LCL, or K562.
Proliferation assay.
CD8+ CTL (3 × 104 cells/well) rested 6 to 7 days from the last antigen stimulation with autologous irradiated tumor cells were resuspended in AIM-V medium with 5% human AB serum and 100 U of IL-2/ml and plated in the presence of different concentrations of IL-10 or in the absence of IL-10 in 96-well U-bottom plates in a total volume of 200 μl per well. In some cultures only IL-10 at different concentrations was added. Plates were then incubated at 37°C for 96 h. Cultures were pulsed with 1 μCi of [3H]thymidine/well for the last 16 h, and incorporated radioactivity was measured as described previously (40). All assays were carried out in triplicate wells. In kinetics experiments CD8+ CTL (3 × 104 cells/well) rested for 4 to 6, 8 to 10, and 14 to 16 days from the last antigen stimulation with autologous irradiated tumor cells were resuspended in AIM-V medium with 5% human AB serum and 100 U of IL-2/ml and plated in the presence of 5 ng of IL-10/ml in 96-well U-bottom plates in a total volume of 200 μl per well. Plates were then incubated at 37°C for 96 h. Cultures were pulsed with 1 μCi of [3H]thymidine/well for the last 16 h.
Phenotypic analysis of T cells.
Cultures of CD8+ T cells rested 6 to 7 days from the last antigen stimulation with autologous irradiated tumor cells (as described above) were cultured in CM in the presence or absence of 5 ng of IL-10/ml for 72 to 96 h and evaluated by flow cytometry for the expression of the following human leukocyte antigens: CD8 β chain (MCA1722) (Serotec, Oxford, United Kingdom), CD8 α chain, CD56, TcRα/β, CD2, CD3, and LFA-1 (all MAb were from Becton Dickinson, San Jose, Calif.). Control cells not exposed to IL-10 were always evaluated at the same time. For evaluation of intracellular perforin, cells rested for different times after antigen stimulation were harvested, washed, and fixed with 2% paraformaldehyde in PBS for 20 min at room temperature. Cells were then washed and permeabilized by incubation in PBS plus 1% bovine serum albumin (BSA) and 0.5% saponin (S-7900; Sigma, St. Louis, Mo.) for 10 min at room temperature. Experimental and control cells were stained with fluorescein isothiocyanate (FITC)-antiperforin MAb (Delta G9) (PharMingen, San Diego, Calif.) and isotype-matched control FITC–anti-immunoglobulin G2a (IgG2a; PharMingen). After the staining, cells were washed twice with PBS plus 1% BSA and 0.5% saponin and once with PBS plus 0.5% BSA and fixed a second time with 2% paraformaldehyde in PBS. All analyses were conducted with a FACScan utilizing Cell Quest software (Becton Dickinson).
Flow cytometric analysis of intracellular cytokines.
CD8+ CTL (3 × 106 cells/well in six-well plates [Costar, Cambridge, Mass.]) were stimulated with autologous irradiated tumor cells (5,000 cGy) (ratio of tumor cells to effector cells, 1:10) and cultured in CM (control cells). At 2 to 4, 8 to 10, and 14 to 16 days after antigen stimulation, 5 ng of IL-10/ml was added in some wells in a total volume of 3 ml per well. After 72 to 96 h of exposure to IL-10, experimental cultures and matched control cultures grown in IL-2 alone were restimulated with solid-phase anti-CD3 MAb (Ortho Pharmaceutical Corp., Raritan, N.J.) overnight in the presence of 1 μg of brefeldin A/ml. In some experiments, cells rested for 6 to 7 days after antigen stimulation were cultured in 5 ng of IL-10/ml for up to 2 weeks and then analyzed for intracellular cytokine content as described below. Briefly, 10 μg of anti-CD3 MAb/ml diluted in PBS was incubated for 4 h at 37°C in 24-well plates. After the plates were washed three times, 106 CD8+ T cells in 1 ml of CM containing 1 μg of brefeldin A/ml were added. CTL were harvested after overnight incubation and washed and fixed with 2% paraformaldehyde in PBS for 20 min at room temperature, after which they were washed and stored overnight in PBS at 4°C. For intracellular staining, the cells were washed and permeabilized by incubation in PBS plus 1% BSA and 0.5% saponin for 10 min at room temperature. Activated and control cells were stained with FITC–anti-IFN-γ, FITC–anti-IL-2, and phycoerythrin (PE)–anti-IL-4 and isotype-matched controls (FITC–anti-IgG2a and PE–anti-IgG1; Becton-Dickinson). After being stained, cells were washed twice with PBS plus 1% BSA and 0.5% saponin and once with PBS plus 0.5% BSA and fixed a second time with 2% paraformaldehyde in PBS. Analysis was conducted with a FACScan, utilizing Cell Quest software (Becton Dickinson).
RESULTS
Derivation of HPV E7-specific CD8+ CTL lines.
Three HPV E7-specific CD8+ CTL lines were derived, one from a patient with HPV16-associated squamous cell carcinoma of the cervix and two from patients with HPV18-associated adenocarcinomas. The results given below illustrate the responses of CD8+ CTL specific for HPV16 E7. These results are representative of all three CTL lines, which displayed essentially the same characteristics with respect to their responses to IL-10 treatment.
IL-10 increases IL-2-induced proliferation of cultured CD8+ CTL.
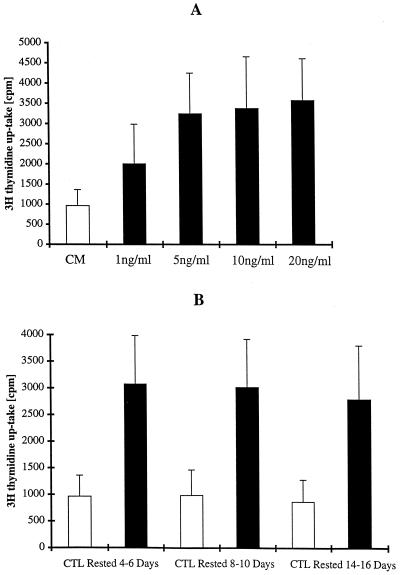
To determine whether IL-10 has a direct stimulatory activity on the proliferation of HPV E7-specific CD8+ T cells, pure populations of TcRα/β+ and CD8α/β+ T cells rested 6 to 7 days from the last antigen stimulation were cultured in 100 U of IL-2/ml alone, 1 to 20 ng of IL-10/ml alone, or 100 U of IL-2 plus 1 to 20 ng of IL-10/ml. IL-10 alone did not support a significant proliferation and survival of the CD8+ CTL population, as shown by [3H]thymidine uptake and vital dye exclusion (data not shown). In contrast, when we evaluated the proliferation of CD8+ T cells in response to various concentrations of IL-10 in the presence of 100 U of IL-2/ml, we found that IL-2-induced proliferation was significantly potentiated by the addition of IL-10 at concentrations of 1 ng/ml, with maximal proliferation at 5 ng/ml. At higher concentrations of IL-10 (i.e., 10 and 20 ng/ml), there was no further increment in proliferation above the level seen with 5 ng/ml (Fig. 1A). Having established 5 ng of IL-10/ml as an optimal dose for CTL proliferation in the presence of 100 U of IL-2/ml, we used this dose thereafter for kinetic analyses. To evaluate if IL-10-induced proliferation is dependent on a recent antigen stimulation, CTL rested for 4 to 6, 8 to 10, and 14 to 16 days from the last antigen stimulation were cultured with 5 ng of IL-10 plus 100 U of IL-2/ml or in 100 U of IL-2/ml alone for 72 to 96 h and evaluated by measuring [3H]thymidine uptake for the last 16 h. As shown in Fig. 1B, we found a significant and consistent increase in thymidine uptake in cultures containing IL-10 in combination with IL-2 compared to control cultures at all time points tested. These data, therefore, indicate that CD8+ CTL both at early and late stages after the last antigen stimulation can respond with increased proliferation when exposed to IL-10 in combination with IL-2.
FIG. 1.
(A) Effect of various concentrations of IL-10 on IL-2-induced proliferation of E7-specific CD8+ T cells at 6 to 7 days after antigen stimulation with autologous tumor cells. Pure populations of CD8+ T cells were cultured in CM (open bar) or in CM supplemented with increasing concentrations of IL-10 (solid bars). Cells were assayed for [3H]thymidine incorporation during the final 16 h of a 96-h culture. Results represent the means of triplicate wells ± standard deviations (SD). Thymidine incorporation in the presence of 1 to 20 ng of IL-10/ml plus IL-2, compared to that for control CTL cultured in IL-2 alone, was significant at P values <0.01 by Student's t test. No significant differences were noted when thymidine incorporation in the presence of 5 ng of IL-10/ml plus IL-2 was compared to levels in the presence of 10 and 20 ng of IL-10/ml plus IL-2. (B) Effect of 5 ng of IL-10/ml on IL-2-induced proliferation of E7-specific CD8+ T cells at 4 to 6, 8 to 10, and 14 to 16 days after antigen stimulation with autologous tumor cells. Pure populations of CD8+ T cells were cultured in CM (open bars) or in CM supplemented with 5 ng of IL-10/ml (solid bars). Cells were assayed for [3H]thymidine incorporation during the final 16 h of a 96-h culture. Results represent the means of triplicate wells ± SD. Thymidine incorporation in the presence of IL-10 plus IL-2, compared to that in control CTL cultured in IL-2 alone, was significant at P values <0.01 at all time points tested.
Effect of IL-10 on the expression of surface molecules in cultured CD8+ CTL compared to IL-2 alone.
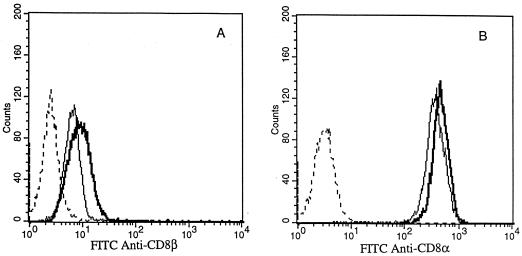
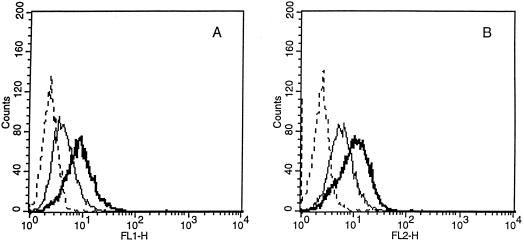
Flow cytometric analysis was used to determine the effect of 5 ng of IL-10/ml in combination with 100 U of IL-2/ml versus IL-2 alone on the expression of TcRα/β, CD2, CD3, CD8 α and β chains, CD56, and LFA-1 by CD8+ T cells 7 days after antigen stimulation. IL-10 did not significantly affect the surface expression of TcRα/β, CD3, CD2, and LFA-1 compared to that by the untreated counterparts (data not shown). In contrast, we found that the α and β chains of the CD8 heterodimeric molecules expressed by the CTL were consistently up-regulated by exposure to IL-10 (Fig. 2). CD8α and -β up-regulation by IL-10 was slight but reproducible and statistically significant (P < 0.05; Student's t test).
FIG. 2.
Effect of IL-10 on the expression of CD8β (A) and CD8α (B) by HPV-specific CTL as analyzed by flow cytometry. CD8+ T cells at 6 to 7 days after antigen stimulation with autologous tumor cells were stained with different MAb after incubation in CM alone (light lines) or in the presence of 5 ng of IL-10/ml for 72 to 96 h (heavy lines). Dashed lines, histograms from cells stained with control MAb.
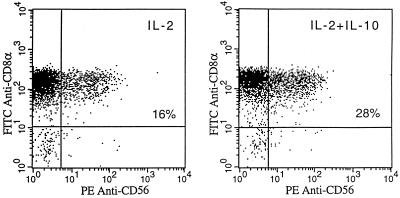
HPV-specific CD8α/β+ cytotoxic T cells cultured in vitro, as previously reported by us (40, 41) and others (23), may show significant CD56 expression, and such expression is strongly correlated with a high cytotoxic activity (23, 40, 41). Interestingly, when the expression of CD56 on T lymphocytes was analyzed by two-color immunofluorescence, we found that an increased percentage of CD8+ T lymphocytes (range, 15 to 33%) up-regulate and/or neoexpress the CD56 surface antigen during culture in the presence of IL-10 (Fig. 3). These data, therefore, suggest a direct effect of IL-10 on the expression of CD8 coreceptor and CD56 molecules by activated CD8+ T cells in the presence of IL-2.
FIG. 3.
Effect of IL-10 exposure on the percentage of CD56+ CD8+ T cells, as assessed by two-color flow cytometric analysis. The results from one experiment are shown and are representative of five separate experiments.
IL-10 increases the cytolytic activity of cultured CD8+ CTL compared to IL-2 alone.
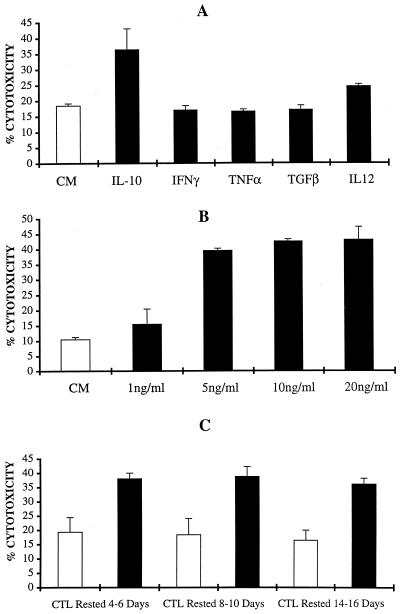
In preliminary experiments to evaluate if the cytotoxic activity of HPV-specific CTL against autologous tumor targets could be increased by the combination of IL-2 with other cytokines previously reported to be able to act as cytotoxic differentiation factors (7, 9, 11, 31, 51), we evaluated the effects of IL-12, IFN-γ, TNF-α, and TGF-β in combination with IL-2 on the cytotoxic activity of HPV-specific CTL and compared these effects to those induced by IL-10. We found that IL-10 at a dose of 5 ng/ml in combination with IL-2 stood out as the most effective cytokine in consistently increasing the cytotoxic potential of the HPV-specific CTL against autologous tumor cells in three repetitive experiments (Fig. 4A). Indeed, only IL-12 in our culture conditions was able to significantly increase CTL cytotoxicity above the control background, although at a lower level than IL-10 (Fig. 4A). In titration studies, we found that the optimal cytotoxicity induced by effector CTL against autologous HPV-infected tumor cells was induced by IL-10 at a dose of 5 ng/ml (Fig. 4B). This dose was congruent with the concentration required for the maximal enhancement of CD8+ T-cell proliferation. To evaluate if the IL-10-induced increase in the cytotoxic activity of CTL is related to the time following antigen stimulation of effector cells, we performed kinetic studies. As shown in Fig. 4C, for all times poststimulation tested (i.e., CTL rested for 4 to 6, 8 to 10, and 14 to 16 days from last antigen stimulation) increased cytotoxic activity against autologous tumor cells was consistently detected compared to that for control CTL cultures, but there were no significant differences between the three time points tested.
FIG. 4.
(A) Effect of different cytokines in combination with IL-2 on the cytotoxic activity of E7-specific CTL. CD8+ T cells at 6 to 7 days from the last antigen stimulation with autologous tumor cells were cultured in CM (open bar; control) or in CM with different cytokines (solid bars), as described in Materials and Methods for 72 to 96 h before being tested in cytotoxicity assays against autologous tumor cells. Percent lysis (± standard deviation) at a 5:1 effector/target cell ratio is shown. The increase in cytotoxic activity, compared to that for control CTL cultured in IL-2 alone, was significant at P values <0.01 in the presence of IL-10 plus IL-2 and at P values <0.05 in the presence of IL-12 plus IL-2 by Student's t test. (B) Effect of various concentrations of IL-10 in combination with IL-2 on the cytotoxic activity of E7-specific CTL against autologous tumor targets. CD8+ T cells were cultured in CM (open bar; control) or in CM with the addition of various doses of IL-10 (solid bars) for 72 to 96 h before being tested in cytotoxicity assays against autologous tumor cells. Percent lysis (± standard deviation) at a 5:1 effector/target cell ratio is shown. The increase in cytotoxic activity, compared to that for control CTL cultured in IL-2 alone, was significant at P values <0.01 in the presence of 5 to 20 ng of IL-10/ml (C) Effect of 5 ng of IL-10/ml on the cytotoxic activity of E7-specific CD8+ T cells at 4 to 6, 8 to 10, and 14 to 16 days after antigen stimulation with autologous tumor cells. Pure populations of CD8+ T cells were cultured in CM (open bars) or in CM supplemented with 5 ng of IL-10/ml (solid bars) for 72 to 96 h before being tested in cytotoxicity assays against autologous tumor cells. Percent lysis (± standard deviation) at a 5:1 effector/target cell ratio is shown. Increased cytotoxic activity by CTL cultured in the presence of IL-10 plus IL-2, compared to that for control CTL cultured in IL-2 alone, was significant at P values <0.01 at all time points tested.
IL-10-induced cytotoxic activity in cultured CD8+ CTL is HLA class I restricted.
The combination of IL-10 and IL-2 has been previously reported to have an additive effect on the cytotoxic activity of NK cells (6). To evaluate if the increased cytotoxic activity induced by IL-10 remained specific for HPV-infected autologous tumor cells or was also increased against other targets, CTL rested for 6 to 7 days after the last antigen stimulation were exposed to 5 ng of IL-10/ml in combination with IL-2 for 72 h and tested for their cytotoxic activity against autologous tumor cells in the presence or absence of blocking MAb against HLA class I molecules (W6/32), as well as against autologous EBV-transformed LCL and the NK-sensitive target K562. As can be seen in Fig. 5, while cytotoxic activity was consistently increased in the presence of IL-10 compared to that of control cultures, the percent reduction of autologous tumor killing by the anti-class I MAb remained at levels similar to that induced in control cultures (mean inhibition was 68% for IL-2 alone versus 64% for IL-2 plus IL-10). Importantly, killing of the autologous LCL and the NK-sensitive target remained negligible. These results suggest that IL-10 does not induce lymphokine-activated killer or NK activity in E7-specific HLA class I-restricted CD8+ T cells.
FIG. 5.
Effect of 5 ng of IL-10/ml in combination with IL-2 on the cytotoxic activity of E7-specific CTL measured in a 6-h 51Cr release assay against autologous tumor cells, autologous tumor cells plus anti-HLA class I blocking MAb (W6/32), autologous LCL, and K562. CD8+ T cells at 6 to 7 days after antigen stimulation with autologous tumor cells were cultured in CM (open bar; control) or in CM with the addition of 5 ng of IL-10/ml (solid bars) for 72 to 96 h before being tested in cytotoxicity assays. Percent lysis (± standard deviation) at a 5:1 effector/target cell ratio is shown. Inhibition of CTL-mediated killing by anti-HLA class I MAb (50 μg/ml) was significant at P values <0.01 for CD8+ T cells cultured in the presence of 5 ng of IL-10/ml plus IL-2 as well as control CTL cultured in IL-2 alone.
IL-10 increases intracellular accumulation of perforin in cultured CD8+ CTL.
Flow cytometric analysis was used to evaluate if the increased cytotoxic activity induced by IL-10 against HPV-infected autologous tumor cells correlated with an increase in intracellular perforin levels. CTL at an early or late stage after activation (i.e., 6 to 7 days or 14 to 16 days from the last antigen stimulation, respectively) were exposed to 5 ng of IL-10/ml in combination with IL-2 for 72 to 96 h and tested for intracellular perforin content. As shown in Fig. 6, CTL cultured in IL-2 alone demonstrated significant intracellular perforin levels when cultured in CM. However, when CTL were cultured in the presence of IL-10, a striking increase in perforin levels was consistently detected in CTL at both early and late stages after antigen stimulation.
FIG. 6.
Effect of 72 to 96 h of exposure to IL-10 on the expression of intracellular perforin by HPV-specific CTL, as analyzed by flow cytometry. CD8+ T cells at 6 to 7 days (A) and 14 to 16 days (B) after antigen stimulation with autologous tumor cells were cultured in CM alone (light lines) or in the presence of 5 ng of IL-10/ml (heavy lines) before being stained with FITC-conjugated MAb against perforin, as described in Materials and Methods. Dashed lines, histograms from cells stained with isotype control MAb.
IL-10 increases intracellular production of Th1 cytokines in cultured CD8+ CTL.
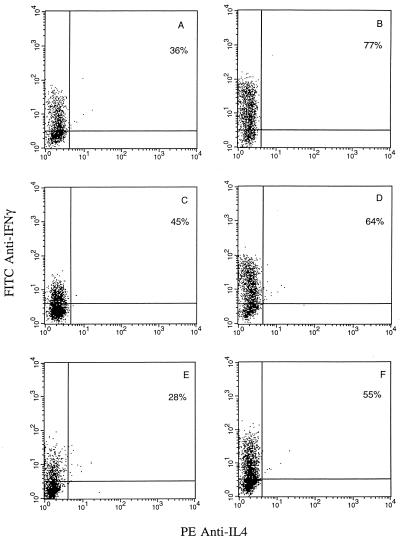
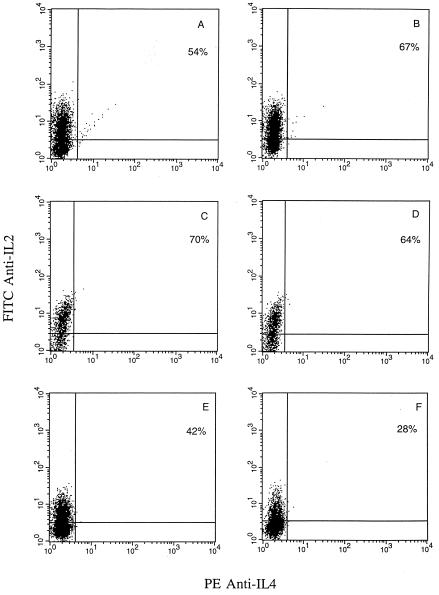
Flow cytometric analysis of intracellular IFN-γ, IL-2, and IL-4 expression by HPV-specific CTL cultured in IL-2 alone and restimulated every 7 to 10 days with irradiated autologous tumor cells indicated a type 1 cytokine phenotype, with negligible IL-4 expression (40, 41) (Fig. 7 and 8). Because of the previously reported powerfully inhibitory effect of IL-10 on cytokine synthesis in Th1 CD4+ T-cell clones (15, 17, 35) as well as the crucial importance of Th1 cytokine secretion by CTL for effective adoptive cancer immunotherapy (2, 49), we evaluated in kinetic studies the effects of IL-10 on the intracellular expression of IFN-γ and IL-2 by CTL. In addition, to evaluate if IL-10 exposure may induce a Th2 cytokine switch in these strongly Th1-polarized T cells, IL-4 expression was also analyzed. CTL rested for 2 to 4, 8 to 10, and 14 to 16 days from the last antigen stimulation were cultured with 5 ng of IL-10 plus 100 U of IL-2/ml or in 100 U of IL-2/ml alone (control cultures) for 72 to 96 h before being restimulated overnight with solid-phase anti-CD3 in the presence of brefeldin A. As shown in Fig. 7, at all time points tested we found a consistent increase in intracellular IFN-γ cytokine expression in CTL cultures treated with IL-10 in combination with IL-2 compared to that in control cultures treated with IL-2 alone. No induction of IL-4 expression was detected when CTL were exposed to IL-10 for 72-96 h at different stages of activation (Fig. 7) as well as when CTL were maintained continuously for up to 2 weeks in the presence of IL-10 (data not shown). In contrast, when IL-2 cytokine expression by CTL was evaluated, we found that augmentation of IL-2 cytokine expression by CTL was strictly dependent on a recent antigen stimulation (within 2 to 4 days) (Fig. 8). Indeed, when IL-2 expression was evaluated at later time points (i.e., 8 to 16 days), unmodified or reduced levels of IL-2 were noted. Taken together, these data demonstrate that exposure to IL-10 in association with IL-2 may increase the expression of IFN-γ and IL-2 by CD8+ T cells but that augmentation of IL-2 expression, unlike that of IFN-γ, is dependent on recent antigen stimulation. Finally, IL-10 treatments did not induce a switch in cytokine expression (i.e., from Th1 to Th2) in these strongly Th1-committed CTL.
FIG. 7.
Two-color flow cytometric analysis of intracellular IFN-γ and IL-4 expression by tumor-specific CD8+ T cells. CD8+ T cells at 2 to 4 days (A and B), 8 to 10 days (C and D), and 14 to 16 days (E and F) after antigen stimulation with autologous tumor cells were cultured in CM alone (A, C, and E) or in the presence of 5 ng of IL-10/ml for 72 to 96 h (B, D, and F) before being activated overnight with solid-phase anti-CD3 in the presence of brefeldin A, as described in Materials and Methods. A representative experiment is shown.
FIG. 8.
Two-color flow cytometric analysis of intracellular IL-2 and IL-4 expression by tumor specific CD8+ T cells. CD8+ T cells at 2 to 4 days (A and B), 8 to 10 days (C and D), and 14 to 16 days (E and F) after antigen stimulation with autologous tumor cells were cultured in CM alone (A, C, and E) or in the presence of 5 ng of IL-10/ml for 72 to 96 h (B, D, and F) before being activated overnight with solid-phase anti-CD3 in the presence of brefeldin A, as described in Materials and Methods. A representative experiment is shown.
DISCUSSION
Patients and animals harboring advanced tumor burdens have been shown to progressively develop impaired immune responses against autologous tumor cells that precede the development of a more generalized state of immunosuppression (3, 24, 34, 44, 46). In several of these studies, diminished T-cell function has been correlated with specific alterations in the T-cell signal transduction pathways (24, 27, 34, 50). Several mechanisms have been suggested to account for tumor-induced subversion of the immune system including soluble tumor-derived inhibitory factors (26, 43, 53), tolerogenic presentation of antigens by tumor cells (8), and activation of inhibitory regulatory elements of the immune system (1, 12). Importantly, however, poor immune responses by recently explanted T lymphocytes could be normalized upon in vitro culture in recombinant IL-2 (33, 50) or by T-cell stimulation with anti-CD3 and anti-CD28 (38). Adoptive transfusions of tumor-specific in vitro-activated T cells, which avoid the potential problems associated with inducing a CTL response in vivo, might therefore be a more effective approach for control of tumor growth in patients harboring advanced-stage cancer.
Recently, we have reported the successful in vitro generation by full-length E7-pulsed autologous DC of HPV-specific cytotoxic CD8+ T cells able to kill naturally HPV-infected autologous tumor cells in three consecutive patients harboring invasive cervical cancer (40). These HLA class I-restricted CTL populations demonstrated a striking dominance of type 1 intracellular cytokine expression (i.e., high IFN-γ, IL-2, and TNF-α expression and low IL-4 expression) (40) and the ability to persistently accumulate around metastatic disease in vivo (41). However, one of the most critical components of successful adoptive immunotherapy of cancer remains the identification, isolation, and in vitro expansion of large numbers of lymphocytes that are endowed with and retain potent and specific antitumor activity. In our search for improved culture conditions able to induce high cytotoxic activity in HPV-specific CTL, we found that IL-10 (in combination with IL-2) was the only cytokine able to consistently increase the cytotoxic potential of the CTL populations studied. In this regard, several other cytokines previously reported to act as cytotoxic T-cell differentiation factors for CD8+ T cells when used in combination with IL-2, including IL-12 (31, 51), IFN-γ (9), TNF-α (11), and TGF-β (7), were significantly less effective or not effective at all in our system. Indeed, only IL-12 in our culture conditions was able to increase tumor-specific CD8+ T-cell cytotoxicity above control levels, although to a significantly lesser extent than IL-10. On the basis of these findings, we have extensively characterized the functional consequences of IL-10 exposure in combination with IL-2 on HPV-specific CTL.
IL-10 has been regarded as a powerful regulatory cytokine endowed with immunosuppressive activity because of its capacity to (i) inhibit allogeneic and major histocompatibility complex (MHC)-specific proliferative and CTL responses (17, 35), (ii) suppress IL-2 and IFN-γ production by activated CD4 T cells (17, 35, 48), (iii) down-regulate the expression of MHC class II antigens and costimulatory molecules on APC (14, 35), and (iv) inhibit the synthesis of inflammatory cytokines, such as TNF-α, IL-1β, and IL-12 by activated macrophages (15). However, although a variety of in vivo studies have supported an immunosuppressive role for IL-10, others have challenged this view. Indeed, tumor cells genetically manipulated to secrete IL-10 have been shown to be rapidly rejected by CD8+ and NK effector cells in murine models (19, 42, 55). Furthermore, systemic administration of cellular IL-10 induces an effective, specific, and long-lived immune response against established tumors in mice (4). Administration of IL-10 has been associated with accelerated rejection of cardiac allografts as well as increased generation of donor-specific CTL and cytotoxic alloantibody responses (37). Production of IL-10 by islet cells accelerates the onset and increases the prevalence of diabetes in mice (54). Finally, systemic IL-10 administration has been associated with graft versus host disease and decreased survival in mouse recipients of bone marrow allografts (5), while anti-IL-10 antibodies prolonged allograft survival in normal as well as presensitized recipients (28).
While the indirect suppressive effects of IL-10 on T-cell activity (mediated by macrophages and DC) have been demonstrated by several investigators (13, 14, 17, 35), analysis of the direct in vitro effects of IL-10 on pure populations of human T lymphocytes has produced controversial results (16, 21, 22, 29, 48). In this regard, CD4+ and CD8+ T cells seem to significantly differ in their susceptibilities to IL-10. Indeed, IL-10 has been shown to be a specific chemotactic factor for CD8+ T cells but not CD4+ T cells (25). In addition, IL-10 inhibits the ability of CD4+ T cells, but not CD8+ T cells, to migrate in response to the T-cell chemotactic cytokine IL-8 (25). While several reports found IL-10 able to directly inhibit CD4+ T-cell functions, including proliferation and Th1 cytokine secretion (14, 16, 17, 21, 35), both inhibitory and stimulatory effects of IL-10 on human CD8+ T cells have recently been described (22).
In this study, we found that human IL-10 can directly augment IL-2-induced HPV-specific CD8+ T-cell proliferation and cytotoxic function. Our results support and extend the finding that a virally encoded homolog of IL-10 could enhance EBV-specific human CD8+ CTL responses (45). These data are also in agreement with previous reports highlighting the function of IL-10 as a powerful growth and cytotoxic differentiation factor for mouse CD8+ T cells (10, 29). In addition, we found that IL-10 induced a significant increase in intracellular perforin levels in HPV-specific CTL. These results strongly suggest that the increased cytotoxic activity shown by CTL exposed to IL-10 may be perforin mediated, although it must be noted that we have not examined the effect of IL-10 on FasL expression. Because differential effects of IL-10 on CD8+ T cells, depending on their state of activation, have recently been described (22), we evaluated the effects of IL-10 on CTL at different time points following antigen stimulation. We found that CD8+ T cells cultured in the presence of IL-10 consistently exhibited increased proliferation and cytotoxic function, not only when recently restimulated with tumor cells but also when relatively distant in time from the last antigen stimulation. We found that CD8+ T cells cultured in the presence of IL-10 consistently exhibited increased proliferation and cytotoxic function, not only when recently restimulated with tumor cells but also when relatively distant in time from the last antigen stimulation (i.e., 14 days). These data, therefore, suggest that IL-10 may act as a CTL growth and cytotoxic differentiation factor at both early and late stages after antigen stimulation for human CD8+ T cells, as well as mouse CD8+ T cells (10, 29).
No significant changes in the expression of TcRα/β, CD3, CD2, and LFA-1 were observed in CTL exposed to IL-10 compared to control cells cultured in IL-2 alone. However, we consistently detected an increase in the percentage of CD56+ CD8 T cells in the presence of IL-10. In this regard, in agreement with the findings of others (23), we have previously reported the concomitant expression of CD56 molecules on strongly activated and HLA class I-restricted CD8α/β+ CTL (40, 41). Although the function of CD56 expression is not presently known, in these previous studies (40, 41) we consistently found higher cytotoxic activity against cervical cancer cells when CD8+ T cells coexpressed CD56 compared to that by CD8+ CD56− CTL. These observations, together with the finding that increased expression of CD56 may also be induced by IL-10 exposure, support the hypothesis that CD56 expressed by HLA class I-restricted CD8+ CTL may be an activation antigen associated with cytotoxic function rather than a lineage-specific marker (23, 36, 40, 41).
Significant up-regulatory effects of IL-10 exposure on CD8 molecule expression have previously been reported in fetal as well as adult mouse thymocytes (29). CD8+ heterodimeric (i.e., CD8α/β) molecules expressed by thymus-derived CD8 T cells are known to be physically associated with the TcRα/β heterodimer, and down-regulation or inhibition of its function by anti-CD8 antibodies may inhibit specific target cell lysis (32). It is thus tempting to speculate that increased expression of the CD8 coreceptor on CTL due to IL-10 exposure might also contribute to the increased cytotoxic activity of CTL by augmenting their affinity for target cells. Paradoxically, this effect on CTL, although not formally tested in this study, might counteract the opposing effect on tumor cells, which can be rendered insensitive to CTL lysis by IL-10-induced down-regulation of HLA class I expression (a consequence of reduced expression and function of the transporter associated with antigen processing) (26, 30, 39).
Cytokine synthesis by Th1-committed and strongly cytotoxic CD8+ T cells may be a crucial quality for effective cancer immunotherapy. Indeed, the importance of this issue for the immunotherapeutic treatment of cancer by adoptive immunotherapy has been highlighted by studies showing that regressions in established murine tumor models correlated more with type 1 cytokine secretion by tumor-specific CD8 T cells than with the in vitro cytotoxic activity of the reinfused CTL (2, 20, 49). While IL-10 was originally described as a cytokine synthesis-inhibiting factor by virtue of its ability to down-regulate type 1 cytokine secretion in Th1 T cells (14, 17, 35), the evidence presented by Fiorentino et al. (17) suggested that IL-10 acts indirectly, via APC, to inhibit cytokine synthesis. Recently, however, it has been shown that IL-10, in the absence of APC, can directly suppress proliferation and IL-2 and TNF-α secretion by CD4+ T cells (16, 21, 48). However, to our knowledge, this study is the first to describe a direct effect of IL-10 on cytokine synthesis by CD8+ T cells.
We found that IL-10 combined with IL-2 consistently augmented intracellular type 1 cytokine expression by CD8+ CTL, compared to that by control cultures. Interestingly, although CTL exposure to IL-10 soon after antigen stimulation (i.e., between 2 to 4 days from stimulation) was able to increase the expression of both intracellular IL-2 and IFN-γ, delayed exposure to IL-10, late after the last antigen stimulation (i.e., between 8 and 14 days), was only able to increase the expression of IFN-γ, not that of IL-2. Indeed, IL-2 levels were either unmodified or significantly reduced at these late time points compared to those for control cells. Furthermore, prolonged exposure to IL-10 (i.e., up to 2 weeks) resulted in increased IFN-γ expression compared to that for matched control cells but had a negative effect on IL-2 (not shown), suggesting, in the long run, a down-regulatory effect of IL-10 on IL-2 secretion. These data extend previous observations by Groux et al. (22) showing that differential effects of IL-10 on CD8+ T cells may crucially depend on their state of activation. Moreover, while the reasons for differential cytokine responses by CD8+ T cells are not clear, these data clearly suggest that IFN-γ production by CTL directly exposed to IL-10, as previously shown for CD4+ T cells (15), can be regulated independently from production of IL-2. Collectively, our results demonstrate that exposure to IL-10 in association with IL-2 significantly increases the expression of IFN-γ and IL-2, but this effect on IL-2 is dependent on a recent antigen stimulation. Finally, we found that prolonged, continuous exposure to IL-10 did not induce a type 1-to-type 2 switch in these antigen-specific and strongly polarized CD8+ T cells.
In summary, these data show that the direct effects of IL-10 on CD8+ CTL differ from the reported inhibitory effects on CD4+ T cells (16, 21). As optimal T-cell immunotherapy would likely also incorporate CD4+ T cells, which are important for the maintenance of CD8+ T cells in vivo (52), we would thus favor independent generation of E7-specific CD4+ and CD8+ T cells, with IL-10 being used as a supplement only for the CD8+ T-cell cultures. A further question centers on the durability of the IL-10-induced functional enhancement following IL-10 withdrawal (for example, after infusion into a patient). We find that enhanced cytotoxic activity is maintained for at least 5 to 6 days following withdrawal or washout of IL-10 from in vitro culture (data not shown). However, additional experimentation will be needed to establish the long-term effects of IL-10, which are an important consideration for immunotherapeutic purposes.
The presence of well-defined tumor antigens (i.e., HPV E6 and E7) that are constantly expressed by cervical tumor cells and that serve as CTL target antigens and the readily available supply of recombinant E6 and E7 oncoproteins from the high-risk HPV genotypes render E6- and E7-pulsed DC stimulation of T cells for adoptive immunotherapy of advanced cervical cancer patients a feasible strategy. Taken together, the findings presented in this paper support the use of IL-10 in combination with IL-2 as a promising cytokine combination for the in vitro expansion and potentiation of tumor-specific CTL for clinical use in the therapy of cancer.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Camillo Golgi Foundation, Brescia, Italy, to A.D.S., the Lega Nazionale contro i Tumori Sezione di Brescia to S.P., NIH grant CA 63931 to M.J.C., and a grant from the Arkansas Science & Technology Authority to G.P.P.
We thank Donna Dunn, Cathy Buzbee, and Janet Linam for excellent technical support and assistance and Joshua Epstein for use of the flow cytometer in the Division of Hematology and Oncology at the University of Arkansas for Medical Sciences.
REFERENCES
- 1.Aoe T, Okamoto Y, Saito T. Activated macrophages induce structural abnormalities of the T cell receptor-CD3 complex. J Exp Med. 1995;181:1881–1886. doi: 10.1084/jem.181.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth R J J, Mule J J, Spiess P J, Rosenberg S A. Interferon gamma and tumor necrosis factor have a role in tumor regression mediated by murine CD8+ tumor infiltrating lymphocytes. J Exp Med. 1991;173:647–654. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxevanis C N, Papamichail M. Characterization of an anti-tumor immune response in human cancers and strategies for immunotherapy. Crit Rev Oncol Hematol. 1994;16:157–179. doi: 10.1016/1040-8428(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 4.Berman R M, Suzuki T, Tahara H, Robbins P D, Narula S, Lotze M T. Systemic administration of cellular interleukin-10 induces an effective, specific, and long lived immune response against established tumors in mice. J Immunol. 1996;157:231–237. [PubMed] [Google Scholar]
- 5.Blazar B R, Taylor P A, Smith S, Vallera D A. Interleukin-10 administration decreases survival in murine recipients of major histocompatibility complex disparate donor bone marrow grafts. Blood. 1995;85:842–850. [PubMed] [Google Scholar]
- 6.Carson W E, Lindemann M J, Baiocchi R, Linett M, Tan J C, Chou C-C, Narula S, Caligiuri M A. The functional characterization of interleukin-10 receptor expression on human natural killer cells. Blood. 1995;85:3577–3585. [PubMed] [Google Scholar]
- 7.Cerwenka A, Bevec D, Majdic O, Knapp W, Holter W. TGF-β1 is a potent inducer of human effector T cells. J Immunol. 1994;153:4367–4372. [PubMed] [Google Scholar]
- 8.Chen L, Linsley P S, Hellstrom K E. Costimulation of T cells for tumor immunity. Immunol Today. 1993;14:483–486. doi: 10.1016/0167-5699(93)90262-J. [DOI] [PubMed] [Google Scholar]
- 9.Chen L B, Tourvieille B, Burns G F, Bach F H, Mathieu-Mahul D, Sasportes M, Bensussan A. Interferon; a cytotoxic T lymphocyte differentiation factor. Eur J Immunol. 1986;16:767–773. doi: 10.1002/eji.1830160709. [DOI] [PubMed] [Google Scholar]
- 10.Chen W-F, Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991;147:528–533. [PubMed] [Google Scholar]
- 11.Chouaib S, Bertoglio J, Blay Y, Marchiol-Fournigault C, Fradelizi D. Generation of lymphokine activated killer cells: synergy between tumor necrosis factor and interleukin-2. Proc Natl Acad Sci USA. 1988;85:6875–6880. doi: 10.1073/pnas.85.18.6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobbold S, Waldmann H. Infectious tolerance. Curr Opin Immunol. 1998;10:518–524. doi: 10.1016/s0952-7915(98)80217-3. [DOI] [PubMed] [Google Scholar]
- 13.de Smedt T, van Mechelen M, de Becker G, Urbain J, Leo O, Moser M. Effects of interleukin-10 on dendritic cell maturation and function. Eur J Immunol. 1997;27:1229–1234. doi: 10.1002/eji.1830270526. [DOI] [PubMed] [Google Scholar]
- 14.de Waal Malefyt R, Haanem J, Spits H, Roncarolo M-G, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries J E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–921. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Waal Malefyt R, Abrams J, Bennet B, Figdor C, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1214. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Waal Malefyt R, Yssel H, de Vries J E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 17.Fiorentino D F, Bond M W, Mosmann T M. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerosa F, Paganin C, Peritt D, Paiola F, Scupoli M T, Aste-Amezaga M, Frank I, Trinchieri G. Interleukin-12 primes human CD4 and CD8 T cell clones for high production of both interferon-γ and interleukin-10. J Exp Med. 1996;183:2559–2565. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovarelli M, Musiani P, Modesti A, Dellabona P, Casorati G, Allione A, Consalvo M, Cavallo F, di Pierro F, de Giovanni C, Musso T, Forni G. Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol. 1995;155:3112–3123. [PubMed] [Google Scholar]
- 20.Goedegebuure P S, Zuber M, Leonard-Vidal D L, Burger U L, Cusack J C, Chang M P, Douville L M, Eberlein T J. Reactivation of murine tumor-infiltrating lymphocytes with solid phase anti-CD3 antibody: in vitro cytokine production is associated with in vivo efficacy. Surg Oncol. 1994;3:79–89. doi: 10.1016/0960-7404(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 21.Groux H, Bigler M, de Vries J E, Roncaralo M G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groux H, Bigler M, de Vries J E, Roncoralo M G. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–3193. [PubMed] [Google Scholar]
- 23.Hilders C G J M, Ras L, van Eendenburg J D H, Nooyen Y, Fleuren G J. Isolation and characterization of tumor-infiltrating lymphocytes from cervical carcinoma. Int J Cancer. 1994;57:805–812. doi: 10.1002/ijc.2910570608. [DOI] [PubMed] [Google Scholar]
- 24.Horiguchi S, Petersson M, Nakazaua T, Kanda M, Zea A H, Ochoa A C, Kiessling R. Primary chemically induced tumors induce profound immunosuppression concomitant with apoptosis and alteration in signal transduction in T cells and NK cells. Cancer Res. 1999;59:2950–2956. [PubMed] [Google Scholar]
- 25.Jinquan T, Gronhoj-Larsen C, Gesser B, Matsushima K, Thestrup-Pedersen K. Human IL-10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocyte migration. J Immunol. 1993;151:4545–4551. [PubMed] [Google Scholar]
- 26.Kim J, Modlin R L, Moy R L, Dubinett S M, McHugh T, Nickoloff B J, Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 27.Kono K, Ressing M E, Brandt R M P, Melief J M, Potkul R K, Andersson B, Petersson M, Kast W M, Kiessling R. Decreased expression of signal-transducing ζ chain in peripheral T cells and natural killer cells in patients with cervical cancer. Clin Cancer Res. 1996;2:1825–1828. [PubMed] [Google Scholar]
- 28.Li W, Fu F, Lu L, Narula S K, Fung J J, Thomson A W, Qian S. Systemic administration of anti-interleukin-10 antibody prolongs organ allograft survival in normal and presensitized recipients. Transplantation. 1998;66:1587–1592. doi: 10.1097/00007890-199812270-00004. [DOI] [PubMed] [Google Scholar]
- 29.MacNeil I A, Suda T, Moore K W, Mosmann T R, Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145:4167–4173. [PubMed] [Google Scholar]
- 30.Matsuda M, Salazar F, Petersson M, Masucci G, Hansson J, Pisa P, Zhang Q-J, Masucci M G, Kiessling R. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994;180:2371–2376. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehrotra P T, Wu D, Crim J A, Mostowski H S, Siegel J P. Effects of IL-12 on the generation of cytotoxic activity in human CD8+ T lymphocytes. J Immunol. 1993;151:2444–2450. [PubMed] [Google Scholar]
- 32.Mescher M F. Surface contact requirements for activation of cytotoxic T lymphocytes. J Immunol. 1992;149:2402–2405. [PubMed] [Google Scholar]
- 33.Mischer S, Whiteside T L, Carrel S, Fliedner V. Functional properties of tumor infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986;136:1899–1907. [PubMed] [Google Scholar]
- 34.Mizoguchi H, O'Shea J J, Longo D L, Loeffler C M, McVicar D W, Ochoa A C. Alteration in signal transduction molecules in T lymphocytes from tumor bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 35.Moore K W, O'Garra A, de Vaal Malefyt R, Vieira P, Mosmann T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 36.Pittet M J, Speiser D E, Valmori D, Cerottini J-C, Romero P. Cytolytic effector function in human circulating CD8+ T cells closely correlated with CD56 surface expression. J Immunol. 2000;164:1148–1152. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 37.Qian S, Li W, Li Y, Fu F, Lu L, Fung J J, Thomson A W. Systemic administration of cellular interleukin-10 can exacerbate cardiac allograft rejection in mice. Transplantation. 1996;62:1709–1713. doi: 10.1097/00007890-199612270-00002. [DOI] [PubMed] [Google Scholar]
- 38.Renner C, Ohnesorge S, Held G, Bauer S, Jung W, Pfitzenmeier J-P, Pfreundschuh M. T cells from patients with Hodgkin's disease have a defective T-cell receptor ζ chain expression that is reversible by T-cell stimulation with CD3 and CD28. Blood. 1996;88:236–241. [PubMed] [Google Scholar]
- 39.Salazar-Onfray F, Charo J, Freland S, Noffz G, Qin Z, Blankenstein T, Ljunggren H-G, Kiessling R. Down-regulation of the expression and function of the transporter associated with antigen processing in murine tumor cell lines expressing IL-10. J Immunol. 1997;159:3195–3202. [PubMed] [Google Scholar]
- 40.Santin A D, Hermonat P l, Ravaggi A, Chiriva-Internati M, Zhan D, Pecorelli S, Parham G P, Cannon M J. Induction of human papillomavirus-specific CD4+ and CD8+ lymphocytes by E7-pulsed autologous dendritic cells in patients with human papillomavirus type 16- and 18-positive cervical cancer. J Virol. 1999;73:5402–5410. doi: 10.1128/jvi.73.7.5402-5410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santin A D, Hermonat P l, Ravaggi A, Bellone S, Cowan C, Korourian S, Pecorelli S, Cannon M J, Parham G P. Development, characterization, and distribution of adoptively transferred peripheral blood lymphocytes primed by human papillomavirus 18 E7-pulsed autologous dendritic cells in a patient with metastatic adenocarcinoma of the uterine cervix. Eur J Gynaecol Oncol. 2000;21:17–23. [PubMed] [Google Scholar]
- 42.Santin A D, Hermonat P l, Ravaggi A, Chiriva-Internati M, Cannon M J, Hiserod J C, Pecorelli S, Parham G P. Expression of surface antigens during the differentiation of human dendritic cells vs macrophages from blood monocytes in vitro. Immunobiology. 1999;200:187–204. doi: 10.1016/s0171-2985(99)80069-2. [DOI] [PubMed] [Google Scholar]
- 43.Santin A D, Hermonat P L, Ravaggi A, Cannon M J, Pecorelli S, Parham G P. Secretion of vascular endothelial growth factor in adenocarcinoma and squamous cell carcinoma of uterine cervix. Obstet Gynecol. 1999;94:78–82. doi: 10.1016/s0029-7844(99)00282-3. [DOI] [PubMed] [Google Scholar]
- 44.Staveley-O'Carrol K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart J P, Rooney C M. The interleukin-10 homolog encoded by Epstein-Barr virus enhances the reactivation of virus-specific cytotoxic T cell and HLA-unrestricted killer cell responses. Virology. 1992;191:773–782. doi: 10.1016/0042-6822(92)90253-l. [DOI] [PubMed] [Google Scholar]
- 46.Stuntman O. Immunodepression and malignancy. Adv Cancer Res. 1975;22:261–272. doi: 10.1016/s0065-230x(08)60179-7. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki T, Tahara H, Narula S, Moore K W, Robbins P D, Lotze M T. Viral interleukin-10 (IL-10), the human herpes virus 4 cellular IL-10 homologue, induces local anergy to allogeneic and syngeneic tumors. J Exp Med. 1995;182:477–486. doi: 10.1084/jem.182.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964–2971. [PubMed] [Google Scholar]
- 49.Takashi N, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, Ohta A. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–624. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tartour E, Latour S, Mathiot C, Thiounn N, Mosseri V, Joyeux I, Dubois D'Enghien C, Lee R, Debre B, Fridman W H. Variable expression of CD3-ζ chain in tumor infiltrating lymphocytes (TIL) derived from renal-cell carcinoma: relationship with TIL phenotype and function. Int J Cancer. 1995;63:205–212. doi: 10.1002/ijc.2910630210. [DOI] [PubMed] [Google Scholar]
- 51.Trinchieri G. Interleukin-12 and its role in the generation of Th1 cells. Immunol Today. 1993;14:335–339. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 52.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 53.Whal S M. Transforming growth factor β: the good, the bad, and the ugly. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wogensen L, Lee M-S, Sarvetnick N. Production of interleukin-10 by islet cells accelerates immune-mediated destruction of β cells in nonobese diabetic mice. J Exp Med. 1994;179:1379–1384. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng L M, Ojcius D M, Graud F, Roth C, Maxwell E, Li Z, Rong H, Chen J, Wang X Y, Catino J J, King I. Interleukin-10 inhibits tumor metastasis through an NK cell-dependent mechanism. J Exp Med. 1996;184:579–584. doi: 10.1084/jem.184.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]