Abstract
Studies in Western populations have shown that Black and Hispanic patients have an earlier age of Multiple Sclerosis (MS) onset and a more severe disease course characterised by faster disability accrual compared to Whites. It is yet unclear whether MS disease characteristics and clinical course differ amongst Asian racial groups. Singapore is uniquely poised to investigate this as its multi-racial population comprises three genetically diverse Asian racial groups—Chinese, Malay and South Asian. Herein, we sought to elucidate differences in the clinical phenotypes, disease-modifying therapy (DMT) usage, and disease course amongst these three Asian racial groups by performinga retrospective observational study on MS patients seen at the National Neuroscience Institute, Singapore. Data on demographics, disease characteristics, ancillary investigations, and DMT usage were collected. One hundred and eighty-eight patients were included (90 Chinese, 32 Malay, and 66 South Asian). Our findings showed that MS prevalence was the highest in South Asians followed by Malays and Chinese, while demographics, healthcare access, and longer-term disease course were identical across the racial groups. However, several differences and trends were elucidated: (1) South Asian patients had milder sentinel attacks (p = 0.006), (2) a higher proportion of Malay patients had enhancing lesions on their initial MRI (p = 0.057) and the lesion topography differed across the races (p = 0.034), and (3) more Malay patients switched out of their initial DMT (p = 0.051). In conclusion, MS disease characteristics were largely similar across these three Asian racial groups, and while there were some clinical and radiological differences at presentation, these did not influence longer-term outcomes.
Subject terms: Demyelinating diseases, Multiple sclerosis
Introduction
While a high Multiple Sclerosis (MS) disease prevalence is present within Western populations, this is not uniform across the different racial groups. A recent large study in the United States showed that MS prevalence was highest in Whites, followed by Blacks, other non-Hispanic racial groups, and lowest in Hispanic individuals1, while studies from the UK demonstrated higher prevalence in Whites compared to Blacks and South Asians2,3. Beyond prevalence differences, it has been reported that MS disease characteristics differ across racial groups in the West. Black and Hispanic patients appear to have an earlier age of disease onset and a more severe disease course characterised by faster disability accrual compared to Whites4–6. Ancillary measures such as optical coherence tomography and MRI also reveal greater accelerated retinal damage and brain tissue loss in Blacks compared to Whites7,8.
To date, MS demographic and disease-related information in Asia have been mostly derived from countries that are largely racially homogenous, thus it is difficult to ascertain if there are differences in prevalence and disease characteristics between various Asian racial groups, especially those residing within the same geographical region (which may suggest shared environmental exposures). While MS prevalence is low in South-East Asia (~ 8 to 9 per 100,000)9, epidemiological studies from Singapore and Malaysia have offered some insights into MS prevalence within these multi-racial populations—prevalence is highest in Indians (South Asian ancestry), followed by Malays (Austronesian/Austroasiatic ancestry), and lowest in Chinese (East Asian ancestry)10,11. However, it is still unclear if there are differences in MS disease characteristics amongst these geographically proximate, yet genetically diverse Asian racial groups12. Singapore is uniquely poised to investigate this given its multi-racial population comprises three genetically distinct (demonstrated on a large-scale whole genome sequencing study in Singapore) Asian racial groups—Chinese, Malay, and South Asian, residing in a small island city state12. In this study, we sought to compare Chinese, Malay, and South Asian MS patients in Singapore with regards to their disease characteristics; specifically to use race as an exploratory variable to: (1) explore differences in MS phenotypes during initial presentation, (2) identify disparities in healthcare access and disease-modifying therapy (DMT) usage, and (3) highlight distinctions in disease severity and longer-term disability between these three Asian racial groups.
Methods
Study population
This retrospective observational study was performed at the National Neuroscience Institute (Tan Tock Seng Hospital Campus), which sees the majority of MS patients in Singapore. Consented patients who fulfilled the 2017 McDonald criteria for MS and were on active follow-up (to ensure robust data capture) were included13. Race was determined by that indicated on each individual’s national registration identity card in Singapore which was self-identified; for this study, racial groups were defined as Chinese, Malays, and South Asians (individuals whose ancestry can be traced back to India, Bangladesh, Pakistan, and Sri Lanka). Patients were classified into these racial groups regardless of generation (e.g. first, second) or time since migration.
Data collection
Chart review was performed using electronic medical records up till the most recent clinical entry, with a defined data collection cut-off date of 1st December 2022. Data collected included: demographics, initial presentation of MS (severity, clinical phenotype, MS type, investigations), DMT usage (DMT efficacy, time on DMT, DMT changes), and longer-term disease course (total number of relapses, disability severity).
DMTs were categorised into high and low-to-moderate efficacy treatments; high efficacy DMTs being Fingolimod, Natalizumab, Cladribine, Alemtuzumab, anti-CD20 therapies, and autologous haematopoietic stem cell transplantation, while all other DMTs were categorised as low-to-moderate efficacy14. For the few patients who were started on DMTs prior to a formal diagnosis of MS or had DMTs initiated for other rheumatological conditions prior to the onset of MS, the time interval from diagnosis to DMT initiation was taken as 0. The percentage time spent on DMTs was calculated with the denominator being the total MS duration from first symptom onset.
Three disability indices were used during data collection: EDMUS Grading Scale (EGS), Expanded Disability Status Scale (EDSS) and The Multiple Sclerosis Severity Score (MSSS). The EGS was utilised to determine the severity of the first attack; it is a simplified version of the EDSS and has been shown to have excellent correlation with the equivalent score in the EDSS15,16. The advantage of using the EGS in this instance was the simplicity to ascribe a score from physical examination notes in the clinical records, some of which dated back many years ago. The EDSS is an established and validated MS disability rating scale; this was readily obtained for all patients using their recent clinical records to measure current and longer-term disability17. EDSS 6.0 was taken as a disability milestone and was confirmed on two clinical visits with an interval of ≥ 3 months. The MSSS is a score of a patient’s EDSS adjusted for the total duration of MS ranked against a large pool of patients18; this was derived for every patient based on their most recent EDSS (≥ 3 months away from last relapse, if applicable) prior to 1st December 2022.
Statistical analysis
All statistical analyses were performed using SAS software version 9.4 for Windows (Cary, NC: SAS Institute Inc) and GraphPad Prism (version 8, GraphPad Software, San Diego, California USA). To identify differences in MS characteristics, univariate analyses were done on continuous data using Kruskal–Wallis one-way analysis of variance followed by Dunn’s test for post-hoc analysis, while Fisher exact test was utilised for categorical data with post-hoc Bonferroni correction. Univariate Cox proportional-hazards regression was performed to analyse the time taken to reach EDSS 6.0 amongst the three racial groups. P-values were two-tailed and significance set at < 0.05.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. This study was performed with IRB approval (SingHealth CIRB 2007/813/A, 2022/2462). Informed consent was obtained from all participants.
Results
Demographics
A total of 219 patients were initially identified, comprising 90 Chinese, 32 Malays, 66 South Asians; 31 were classified as ‘Others’ (Eurasians/mixed race, Caucasians, Filipinos, Vietnamese, and Indonesians) and were excluded from analysis. The three main racial groups, i.e., Chinese, Malays, and South Asians (n = 188) were included for analysis.
Singapore’s population has an ethnic composition of 74.1% Chinese, 13.6% Malay, 9% Indian, and 3.3% ‘Others’19. We observed a higher number of South Asians compared to Malay patients in our cohort even though South Asians was the smallest racial group in Singapore. To compare the relative MS prevalence across the three racial groups in this cohort, we calculated pair-wise prevalence ratios adjusted for the current population statistics for each racial group19; Chinese/Malay was 0.52, Chinese/South Asian was 0.17, and Malay/South Asian was 0.32. This finding was in line with a recent local MS epidemiology study which observed that Indians had the highest prevalence of MS, followed by Malays and then Chinese10.
Consistent with the known demographic features of MS patients, the age of onset of MS in our cohort was in the late 20 s (median 28.0 years) and there was a strong female preponderance (82.4%) (Table 1). The majority of patients (83.1%) were non-smokers and concomitant autoimmune conditions were uncommon (8.5%). No differences in demographic variables were observed between the racial groups.
Table 1.
Demographic, clinical and investigational information of the study cohort, stratified by racial group.
| Chinese (n = 90) | Malay (n = 32) | South Asian (n = 66) | Total (n = 188) | p-value | |
|---|---|---|---|---|---|
| Age at onset in years, median (Q1, Q3) | 28.0 (23.0, 36.0) | 25.0 (22.5, 31.5) | 27.0 (25.0, 33.0) | 28.0 (24.0, 35.0) | 0.673 |
| Gender (%) | |||||
| Female | 77 (85.6) | 26 (81.3) | 52 (78.8) | 155 (82.4) | |
| Male | 13 (14.4) | 6 (18.8) | 14 (21.2) | 33 (17.6) | 0.725 |
| Smoking status at first visit (%) | |||||
| Smoker | 10 (14.7) | 6 (26.1) | 8 (15.7) | 24 (16.9) | |
| Non-smoker | 58 (85.3) | 17 (73.9) | 43 (84.3) | 118 (83.1) | 0.641 |
| Any other autoimmune conditions (%) | |||||
| Yes | 6 (6.7) | 1 (3.1) | 9 (13.6) | 16 (8.5) | |
| No | 84 (93.3) | 31 (96.9) | 57 (86.4) | 172 (91.5) | 0.336 |
| Initial MS type (%) | |||||
| RRMS | 82 (91.1) | 32 (100.0) | 63 (95.5) | 177 (94.1) | |
| PPMS | 8 (8.9) | 0 (0.0) | 3 (4.5) | 11 (5.9) | 0.195 |
| Phenotype of onset attack (%)a | |||||
| TM | 34 (41.5) | 11 (34.4) | 35 (56.5) | 80 (45.5) | |
| Infratentorial | 21 (25.6) | 11 (34.4) | 7 (11.3) | 39 (22.2) | |
| ON | 18 (22.0) | 5 (15.6) | 12 (19.4) | 35 (19.9) | |
| Supratentorial | 2 (2.4) | 0 (0.0) | 1 (1.6) | 3 (1.7) | |
| Multiple | 7 (8.5) | 5 (15.6) | 7 (11.3) | 19 (10.8) | 0.230 |
| Severity of first MS attack by EGS, median (Q1, Q3)a | 3.0 (2.0, 3.0)* | 3.0 (2.0, 3.0)+ | 2.0 (2.0, 3.0)*+ | 3.0 (2.0, 3.0) | 0.006 |
| Enhancing lesion on initial MRI (%) | |||||
| Yes | 42 (63.6) | 20 (87.0) | 37 (77.1) | 99 (82.8) | |
| No | 24 (36.4) | 3 (13.0) | 10 (22.9) | 37 (27.2) | 0.057 |
| Location of enhancing lesion on initial MRI (%) | |||||
| Brain | 24 (36.4) | 9 (39.1) | 21 (44.7) | 54 (39.7) | |
| Spine | 6 (9.1) | 9 (39.1) | 8 (17.0) | 23 (16.9) | |
| Both brain and spine | 12 (18.2) | 2 (8.7) | 8 (17.0) | 22 (16.2) | |
| No enhancing lesion | 24 (36.4) | 3 (13.0) | 10 (21.3) | 37 (27.2) | 0.034Ψ |
| First MRI brain T2 lesion load (%)b | |||||
| High | 19 (35.2) | 9 (52.9) | 13 (35.1) | 41 (38.0) | |
| Intermediate | 11 (20.4) | 3 (17.6) | 12 (32.4) | 26 (24.1) | |
| Low | 24 (44.4) | 5 (29.4) | 12 (32.4) | 41 (38.0) | 0.436 |
| CSF-restricted OCB (%) | |||||
| Present | 40 (69.0) | 22 (88.0) | 35 (81.4) | 97 (77.0) | |
| Absent | 18 (31.0) | 3 (12.0) | 8 (18.6) | 29 (23.0) | 0.139 |
For variables ‘Enhancing lesion on initial MRI’, ‘Location of enhancing lesion on initial MRI’, ‘First MRI brain T2 lesion load’, and ‘CSF-restricted OCB’, not all patients in the study population was included due to missing data either due from the procedure not being performed or unavailability of records. Percentages indicated in these variables were based on the denominator of those with available data.
CSF cerebrospinal fluid, EGS EDMUS grading scale, MRI magnetic resonance imaging, OCB oligoclonal bands, PPMS primary progressive multiple sclerosis, RRMS relapsing remitting multiple sclerosis, TM transverse myelitis.
aWithin RRMS patients only (Chinese n = 82, Malays n = 32, South Asian n = 63) as most PPMS patients do not present with relapses.
bThe number of lesions were manually counted using axial T2-weighted sequence and cross referenced with the neuroradiologist report. Low lesion load was defined as 1–5 lesions, intermediate as 6–10 lesions and high as > 10 lesions.
*Pairwise comparison of Chinese vs. South Asian, Dunn’s post-hoc p = 0.010.
+Pairwise comparison of Malays vs. South Asian, Dunn’s post-hoc p = 0.006.
ΨPairwise comparison of Chinese vs. Malays, Bonferroni correction p = 0.019.
Significant values are in italics.
Healthcare access
As a surrogate measure of health-seeking behaviour and healthcare access, the number of relapses between first symptom onset and first medical consult for MS was tabulated within patients subsequently diagnosed with relapsing remitting MS (RRMS) (Table 2). The median number of relapses between onset and consult were comparable among the three races at 1.0, indicating that most patients sought medical consult during or soon after their first attack. This observation suggests strong health-seeking behaviour across the racial groups which is likely to be facilitated by the ease of access to neurological care in Singapore. Next, we examined the number of relapses between the first medical consult for MS and diagnosis; the median was 0.0 within the cohort with no differences between the races. This suggests that despite the low prevalence of MS in Singapore, we were diagnosing MS in a timely manner (i.e., after the first attack and before subsequent attacks) and also highlights the fact the most patients already fulfilled the McDonald criteria for MS at their sentinel attack. Expectedly, the number of relapses from first onset to diagnosis was not statistically different amongst the racial groups.
Table 2.
Number of relapses between key timepoints at disease onset within RRMS patients.
| Chinese (n = 82) | Malay (n = 32) | South Asian (n = 63) | Total (n = 177) | p-value | |
|---|---|---|---|---|---|
| Number of relapses between onset and first consult, median (Q1, Q3) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 0.462 |
| Number of relapses between onset and diagnosis, median (Q1, Q3) | 1.0. (1.0, 2.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 2.0) | 1.5 (1.0, 2.0) | 0.442 |
| Number of relapses between first consult and diagnosis, median (Q1, Q3) | 0.0 (0.0, 1.0) | 0.5 (0.0, 1.25) | 0.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.569 |
RRMS relapsing remitting multiple sclerosis.
Initial MS presentation and workup
Table 1 details the various clinical and investigational parameters at MS onset. Within RRMS patients, we observed that the severity of the first attack was milder in South Asian patients compared to Chinese and Malay patients (median EGS; South Asian, 2.0 vs. Chinese, 3.0 vs. Malay, 3.0; p = 0.006) (Fig. 1A). An EGS of 2 represents minimal, not ambulation-related disability and able to run, whereas an EGS of 3 indicates significant, not ambulation-related disability or unlimited walking distance but unable to run15,16. There were no differences in the clinical phenotype of the first attack. Proportions of MS subtype (RRMS or primary progressive MS [PPMS]) were similar between the racial groups; the majority of the cohort had RRMS (94.1%) who presented predominantly with transverse myelitis (45.5%), followed by infratentorial syndromes (22.2%) and optic neuritis (19.9%).
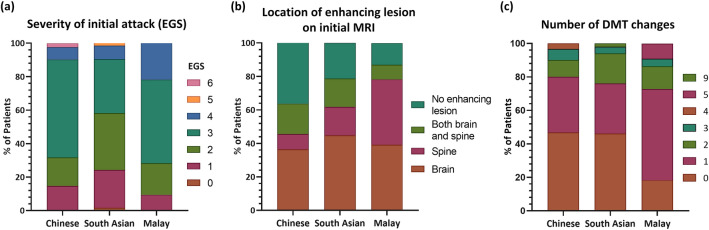
Figure 1.
Stacked bar charts illustrating the comparative proportions between the three racial groups with regards to: (a) severity of initial attack within RRMS patients, (b) location of enhancing lesion(s) on initial MRI, and (c) number of DMT changes during MS disease course. DMT disease-modifying therapy; EGS EDMUS grading scale, MRI magnetic resonance imaging, RRMS relapsing remitting multiple sclerosis.
We observed a trend in the proportions of patients who had gadolinium-enhancing lesions on initial MRI amongst the three racial groups, with Malays having the highest proportion (Malay, 87.0% vs. South Asian, 77.1% vs. Chinese, 63.6%; p = 0.057). Further stratification based on the topography of enhancing lesions revealed significant differences between the three groups (p = 0.034), particularly between Chinese and Malay patients (p = 0.019, Bonferroni correction), with a greater proportion of Malay patients having spinal cord restricted enhancing lesion(s) (39.1% vs. 9.1%) (Fig. 1B). There appeared to be more Malay patients (52.9%) with high (> 10) brain T2 lesion load on MRI compared to Chinese (35.2%) and South Asians (35.1%), while a lower proportion of Chinese patients had positive CSF-restricted oligoclonal bands (OCB) (69.0%) compared to Malays (88.0%) and South Asians (81.4%); however, these observations did not reach statistical significance.
DMT usage
DMT usage (i.e. ever started on DMT), time taken to initiate DMT from MS diagnosis, and time on DMT (over MS disease duration) were similar between the comparative groups (Table 3). We observed a trend that fewer Malay patients commenced treatment with high efficacy DMTs compared to Chinese and South Asian patients (Malay, 18.2% vs. Chinese, 38.3% vs. South Asian, 34.7%; p = 0.248). Malay patients also had more frequent DMT changes (Fig. 1C); more Malay patients changed their DMT at least once as compared to Chinese and South Asian patients (Malay, 81.8% vs. Chinese, 53.3% vs. South Asian, 55.1%; p = 0.051).
Table 3.
DMT uptake and usage pattern within RRMS patients.
| Chinese (n = 82) | Malay (n = 32) | South Asian (n = 63) | Total (n = 177) | p-value | |
|---|---|---|---|---|---|
| DMT usage (%) | |||||
| Yes | 60 (73.2) | 22 (68.8) | 49 (77.8) | 131 (74.0) | |
| No | 22 (26.8) | 10 (31.3) | 14 (22.2) | 46 (26.0) | 0.467 |
| Efficacy of first DMT (%) | |||||
| High | 23 (38.3) | 4 (18.2) | 17 (34.7) | 44 (33.6) | |
| Low-to-moderate | 37 (61.7) | 18 (81.8) | 32 (65.3) | 87 (66.4) | 0.248 |
| Type of first DMT (%) | |||||
| Interferons | 33 (55.5) | 17 (77.3) | 22 (44.9) | 72 (55.0) | |
| Glatiramer acetate | 1 (1.7) | – | 2 (4.1) | 3 (2.3) | |
| Teriflunomide | 1 (1.7) | – | 2 (4.1) | 3 (2.3) | |
| Dimethyl fumarate | – | 1 (4.5) | 3 (6.1) | 4 (3.1) | |
| Leflunomide | 1 (1.7) | – | 1 (2.0) | 2 (1.5) | |
| Azathioprine | 1 (1.7) | 1 (4.5) | 1 (2.0) | 3 (2.3) | |
| Fingolimod | 2 (3.3) | – | 4 (8.2) | 6 (4.6) | |
| Cladribine | 6 (10.0) | – | 5 (10.2) | 11 (8.4) | |
| Alemtuzumab | 1 (1.7) | 3 (13.6) | 1 (2.0) | 5 (3.8) | |
| Natalizumab | 5 (8.3) | – | 1 (2.0) | 6 (4.6) | |
| Rituximab | 7 (11.7) | – | 5 (10.2) | 12 (9.2) | |
| Ocrelizumab | 2 (3.3) | – | 1 (2.0) | 3 (2.3) | |
| Secukinumab | – | – | 1 (2.0) | 1 (0.8) | 0.458 |
| Time taken to initiate DMT from MS diagnosis in days, median (Q1, Q3) | 241 (30.5, 975) | 152 (28.5, 568) | 121 (28, 527) | 124 (29, 731) | 0.535 |
| Percentage time on DMT, median (Q1, Q3) | |||||
| High efficacy | 36.4 (6.5, 74.2) | 30.7 (0.0, 53.2) | 26.5 (0.0, 59.1) | 30.0 (0.3, 61.5) | 0.225 |
| Low-to-moderate efficacy | 9.9 (0.0, 41.4) | 19.9 (9.0, 58.3) | 19.6 (0.0, 52.7) | 16.6 (0.0, 48.1) | 0.188 |
| All DMTs | 69.8 (46.1, 94.1) | 58.0 (37.8, 86.5) | 68.7 (31.9, 95.3) | 65.8 (38.3, 93.1) | 0.661 |
| Number of DMT changes, median (Q1, Q3) | 1.0 (0.0, 1.0) | 1.0 (1.0, 2.0) | 1.0 (0.0, 1.5) | 1.0 (0.0, 1.0) | 0.126 |
| Switched out of initial DMT (%) | |||||
| Yes | 32 (53.3) | 18 (81.8) | 27 (55.1) | 70 (56.0) | |
| No | 28 (46.7) | 4 (18.2) | 22 (44.9) | 55 (44.0) | 0.051 |
Table includes data within RRMS patients only as most PPMS patients were historically not commenced on DMTs.
DMT disease-modifying therapy, PPMS primary progressive multiple sclerosis, RRMS relapsing remitting multiple sclerosis.
Longer-term disease course and disability
The median MS disease duration among the racial groups were identical at around 10 years (Table 4). There were no significant differences amongst the racial groups with regards to progression and longer-term disability, conversion from RRMS to secondary progressive MS (SPMS), total number of relapses, proportion reaching EDSS 6.0, and latest MSSS (Table 4). Figure 2A shows the Kaplan–Meier curve for the time taken to reach EDSS 6.0; no differences were observed amongst the groups (log rank test, p = 0.873). The median time taken to reach EDSS 6.0 of the entire study cohort was 23.0 years (95% confidence interval 19.2–29.0) (Fig. 2B).
Table 4.
Longer-term disability and disease severity of the study cohort.
| Chinese (n = 90) | Malay (n = 32) | South Asian (n = 66) | Total (n = 188) | p-value | |
|---|---|---|---|---|---|
| Conversion to SPMS (from RRMS) (%)a | |||||
| Yes | 17 (20.7) | 6 (18.8) | 10 (15.9) | 33 (18.6) | |
| No | 65 (79.3) | 26 (81.3) | 53 (84.1) | 144 (81.4) | 0.763 |
| Total duration of MS in years, median (Q1, Q3) | 10.1 (5.28, 15.7) | 10.4 (6.83, 13.2) | 9.30 (5.37, 13.0) | 10.0 (5.56, 15.6) | 0.641 |
| Total number of relapses, median (Q1, Q3)a | 3.0 (2.0, 5.0) | 4.0 (2.0, 8.0) | 3.0 (2.0, 5.0) | 3.0 (2.0, 5.8) | 0.407 |
| Reached EDSS 6.0 (%) | |||||
| Yes | 25 (27.8) | 8 (25.0) | 14 (21.2) | 47 (25.0) | |
| No | 65 (72.2) | 24 (75.0) | 52 (78.8) | 141 (75.0) | 0.646 |
| MSSS, median (Q1, Q3) | 2.3 (1.1, 6.6) | 1.8 (0.7, 3.6) | 2.4 (1.1, 4.8) | 2.2 (1.0, 5.4) | 0.495 |
DMT disease-modifying therapy, EDSS expanded disability status scale, MSSS multiple sclerosis severity score, RRMS relapsing remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis.
aAnalysed within RRMS only.
Figure 2.
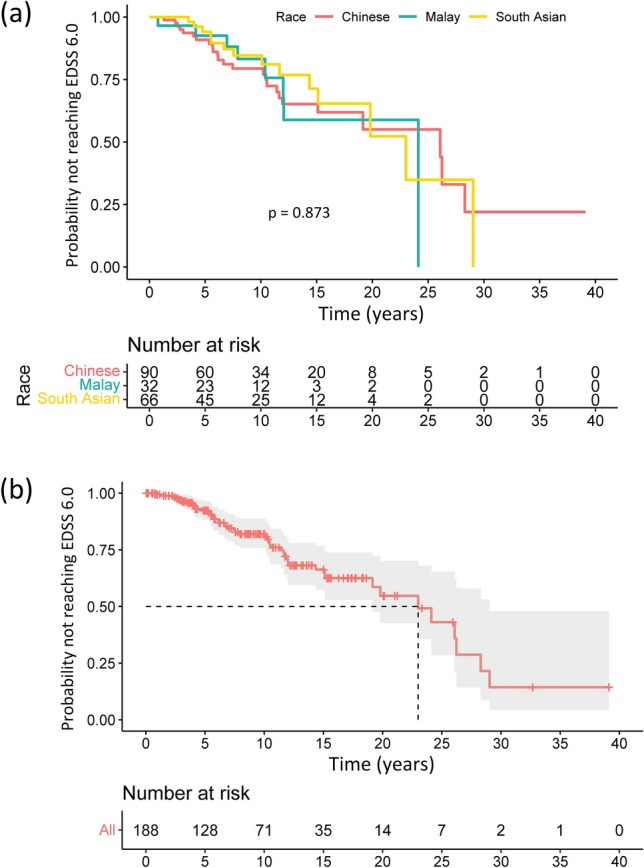
Kaplan–Meier curves showing time to reach EDSS 6.0 (a) stratified by race, and (b) of the entire study cohort. The median time to reach EDSS 6.0 was 23.0 years (dash line) for the entire cohort. EDSS expanded disability status scale.
Discussion
In this study, we compared Chinese, Malay, and South Asian MS patients in Singapore to identify potential differences in disease characteristics during initial presentation, healthcare access, DMT usage, and disease course. Consistent with our previous study, we found a clear difference in MS prevalence among the races (highest in South Asians, followed by Malays then Chinese). This is likely to be attributable to differential underlying genetic susceptibility given that these racial groups are genetically diverse (as demonstrated in a large whole-genome sequencing study of 4810 Singaporean Chinese, Malays, and Indians) and that environmental exposures are conceivably similar especially since Singapore is a densely populated, small island city state12. This difference in genetic susceptibility is suggested by human leucocyte antigen (HLA) haplotyping studies; a large study on bone marrow donors in Singapore showed that Indians have the highest frequency of the HLA-DRB1*15:01 allele (the HLA allele reported to have strongest effect on MS risk) while another study from India confirmed the association between MS with the HLA-DRB1*15:01 allele20,21.
Despite prevalence differences, the observed similarities in the other clinical features suggest that MS is by and large the same disease across these three Asian racial groups. We found that demographic characteristics and MS disease course were similar across the groups, save for a few minor differences at the initial presentation, namely, the milder severity of initial attacks in South Asian patients compared to Chinese and Malays, and to a certain extent, the proportions of patients with gadolinium-enhancing lesions (highest in Malays) and the location of these enhancing lesions on initial MRI. These differences, however, did not appear to influence longer-term disability between the racial groups. Of note, it is unlikely that the greater severity of the sentinel attack in Chinese and Malays was confounded by the higher rates of Neuromyelitis Optica Spectrum Disorder (NMOSD) in these racial groups with resultant diagnostic misclassification10. NMOSD is a well-recognised clinical entity in Singapore and cell-based assays for aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies were available in our institution for ~ 15 years and ~ 5 years respectively.
The findings from our study also enabled us to juxtapose the MS disease characteristics of our Asian cohort (all three racial groups combined) with those reported in other populations. We found that most of these characteristics were generally similar to those reported in Western populations, consistent with a study by Kim et al. demonstrating that MS disease characteristics in South Korean patients showed no significant differences when compared to Canadian patients22. However, we wish to highlight several notable observations which will be discussed below.
Prevailing Western literature reports that 85–90% of individuals with MS have CSF-restricted OCB at baseline23,24. Whilst the majority of Malay and South Asian patients in our study had CSF-restricted OCB (~ 85%) at initial workup, this was only present in 69% of Chinese patients (although this difference was not statistically significant). Approximately 83% (68/82) of our Chinese RRMS patients had clinically definite MS (CDMS) (i.e. more than one clinical attack), and when we restricted our analysis to these Chinese CDMS patients, the proportion of those having positive CSF-restricted OCB remained low at 60% (27/45 who had CSF examination). This observation is in line with several studies reporting low CSF-restricted OCB positivity rate in Chinese patients25,26. The reason for this finding in Singaporean Chinese patients is unclear although it is possible that early MS disease at CSF sampling, lower latitude, and differential genetic susceptibility could be contributory27–29.
Three quarters of RRMS patients in our study cohort were commenced on DMTs, comparable to the high rates of DMT usage in Australia and Scandinavian countries30. Within those who were treated, one third were initiated on high efficacy DMTs. These observations are likely attributable to the access to a wide range of DMTs in Singapore including Rituximab biosimilars (often used as an off-label treatment). Furthermore, our institution has implemented the early use of high efficacy DMTs as current evidence points towards the association of this treatment strategy with lower long-term disability and likelihood of progressive disease31,32. We observed that a lower proportion of Malay patients were initiated on high efficacy DMTs, and consequently, a higher proportion switched out of their initial DMT which is likely due to breakthrough disease activity on lower efficacy treatments. There may be possible socio-behavioural factors affecting the uptake of high efficacy treatments amongst the racial groups in Singapore which merit further investigation.
It has been reported that approximately 50% of untreated RRMS patients convert to SPMS after 10–15 years from disease onset33. A more recent study by Fambiatos et al. observed a median time to SPMS of 32.4 years, taking into account the effect of DMTs (median proportion of time on treatment was 10.9%)34. The rate of secondary conversion in our study cohort was 18.6% after a median disease duration of 10 years which is comparable to that reported in the study by Fambiatos et al. (~ 10% after 10 years disease duration). Comparing disease severity between Asian and Caucasian patient populations, a previous study highlighted that MS disease course appear to be milder in Japanese patients compared to UK patients, with median MSSS of 3.34 and 5.87 respectively35. However, disparities were present between the two cohorts, in particular, the higher DMT usage in Japanese patients (83.7% vs. 17%). The median MSSS in our cohort was 2.2, comparable to recent studies in both Asian (South Korea: MSSS 1.77, mean disease duration 8 years) and Western cohorts (United States: MSSS 2.2, mean disease duration 20.1 years)36,37. We also calculated that the median time to reach EDSS 6.0 (from disease onset) of our study cohort to be 23.0 years, which is comparable to other contemporaneous population-based studies worldwide (Sweden, 30.4 years; Norway, 29.8 years; Wales 22.1 years)38–40. Older studies have reported a shorter time to EDSS 6.0 (France, 18.0 years; Canada 18.9 years) and it has been shown that the time taken to reach EDSS 6.0 from EDSS 1.0 was 18.5 years amongst patients in the placebo arms of randomised controlled trials41–43. These differences are likely to be explained by wider DMT usage as well as the use of more efficacious DMTs in the modern era43. Future local studies will explore if the rates of SPMS conversion, time to reach EDSS 6.0, and MSSS improve with more patients being initiated high efficacy treatments earlier in their disease course.
The main limitation of this study is the limited sample size of patients owing to the low prevalence of MS in Singapore. While several trends were noted, they were not statistically significant. We also ascribed patients into the defined racial groups regardless of generation or time since migration. This may be important for first generation patients as it is plausible that certain environmental factors may have already been acquired and modulated disease characteristics, prior to residing in Singapore. Despite these limitations, our study represents a novel attempt to comprehensively detail MS disease characteristics in three genetically distinct racial groups in Asia, and with this, enable comparisons to other MS cohorts globally.
Conclusion
MS disease characteristics are largely similar across the main racial groups in Singapore and while there were some differences at initial presentation and in DMT use, these did not influence longer-term outcomes. Further studies encompassing more patients and the inclusion of sensitive disability indices would represent significant steps to deepening our understanding of MS among Asian races and with Western populations.
Author contributions
M.J.K.: Conceptualisation; Formal analysis; Investigation; Methodology; Visualisation; Writing—original draft. S.E.S.: Formal analysis; Visualisation; Writing—review & editing. J.S.N.T.: Data curation; Investigation; Writing—review & editing. A.Y.Y.A.: Investigation; Writing—review & editing. R.W.E.S.: Investigation; Writing—review & editing. X.P.: Investigation; Writing—review & editing. J.M.M.T.: Investigation; Resources; Writing—review & editing. K.T.: Investigation; Resources; Writing—review & editing. T.Y.: Conceptualisation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Visualisation; Writing—original draft.
Funding
T.Y. is funded by the Singapore Ministry of Health’s National Medical Research Council Transition Award (MOH-TA20nov-002).
Data availability
Anonymised data can be made available to qualified investigators on reasonable request to the corresponding author.
Competing interests
J.M.M.T. has received honoraria from Merck for speaker’s fees and research grants from the National Neuroscience Institute (Singapore). K.T. has received travel grants and compensation from Novartis, Merck, Sanofi, Eisai, Roche, and Terumo BCT for consulting services and education activities. T.Y. has received honoraria from ASNA, decodeMR, Edanz Pharma, Euroimmun AG, Merck, Novartis, Terumo BCT for consulting services and speaker’s fees, and research grants from the National Medical Research Council (NMRC Singapore) and Roche. He has also received travel grants and awards from PACTRIMS, ACTRIMS, ECTRIMS, Orebro University, UCB, and Merck. M.J.K., S.E.S., J.S.N.T., A.Y.Y.A., R.W.E.S., and X.P. report no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hittle M, et al. Population-based estimates for the prevalence of multiple sclerosis in the United States by race, ethnicity, age, sex, and geographic region. JAMA Neurol. 2023;80:693–701. doi: 10.1001/jamaneurol.2023.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albor C, et al. Ethnicity and prevalence of multiple sclerosis in east London. Mult. Scler. 2017;23:36–42. doi: 10.1177/1352458516638746. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs BM, et al. Modifiable risk factors for multiple sclerosis have consistent directions of effect across diverse ethnic backgrounds: A nested case-control study in an English population-based cohort. J. Neurol. 2023 doi: 10.1007/s00415-023-11971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amezcua L, McCauley JL. Race and ethnicity on MS presentation and disease course. Mult. Scler. 2020;26:561–567. doi: 10.1177/1352458519887328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langer-Gould A, Brara SM, Beaber BE, Zhang JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology. 2013;80:1734–1739. doi: 10.1212/WNL.0b013e3182918cc2. [DOI] [PubMed] [Google Scholar]
- 6.Ventura RE, Antezana AO, Bacon T, Kister I. Hispanic Americans and African Americans with multiple sclerosis have more severe disease course than Caucasian Americans. Mult. Scler. 2017;23:1554–1557. doi: 10.1177/1352458516679894. [DOI] [PubMed] [Google Scholar]
- 7.Caldito NG, et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: A longitudinal study. Brain. 2018;141:3115–3129. doi: 10.1093/brain/awy245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimbrough DJ, et al. Retinal damage and vision loss in African American multiple sclerosis patients. Ann. Neurol. 2015;77:228–236. doi: 10.1002/ana.24308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walton C, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020;26:1816–1821. doi: 10.1177/1352458520970841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan K, et al. Central nervous system inflammatory demyelinating diseases and neuroimmunology in Singapore—Epidemiology and evolution of an emerging subspecialty. Neurol. Clin. Neurosci. 2021;9:259–265. doi: 10.1111/ncn3.12479. [DOI] [Google Scholar]
- 11.Viswanathan S, Wah LM. A nationwide epidemiological study on the prevalence of multiple sclerosis and neuromyelitis optica spectrum disorder with important multi-ethnic differences in Malaysia. Mult. Scler. 2019;25:1452–1461. doi: 10.1177/1352458518792430. [DOI] [PubMed] [Google Scholar]
- 12.Wu D, et al. Large-scale whole-genome sequencing of three diverse Asian populations in Singapore. Cell. 2019;179:736–749. doi: 10.1016/j.cell.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 13.Thompson AJ, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 14.Samjoo IA, et al. Efficacy classification of modern therapies in multiple sclerosis. J. Comp. Eff. Res. 2021;10:495–507. doi: 10.2217/cer-2020-0267. [DOI] [PubMed] [Google Scholar]
- 15.Amato MP, et al. European validation of a standardized clinical description of multiple sclerosis. J. Neurol. 2004;251:1472–1480. doi: 10.1007/s00415-004-0567-0. [DOI] [PubMed] [Google Scholar]
- 16.Confavreux C, Compston DA, Hommes OR, McDonald WI, Thompson AJ. EDMUS, a European database for multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1992;55:671–676. doi: 10.1136/jnnp.55.8.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 18.Roxburgh RH, et al. Multiple sclerosis severity score: Using disability and disease duration to rate disease severity. Neurology. 2005;64:1144–1151. doi: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- 19.Singapore Department of Statistics. SingStat. https://www.singstat.gov.sg (Accessed 4 February 2023) (2023).
- 20.Yu-Jin AN, et al. Human leukocyte antigen allele and haplotype frequencies in Singapore bone marrow donors and cord blood units. Blood Cell Ther. 2022;5:99–106. doi: 10.31547/bct-2022-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandit L, et al. HLA associations in South Asian multiple sclerosis. Mult. Scler. 2016;22:19–24. doi: 10.1177/1352458515581439. [DOI] [PubMed] [Google Scholar]
- 22.Kim S-H, et al. Clinical characteristics and outcome of multiple sclerosis in Korea: Does multiple sclerosis in Korea really differ from that in the Caucasian populations? Mult. Scler. J. 2013;19:1493–1498. doi: 10.1177/1352458513477712. [DOI] [PubMed] [Google Scholar]
- 23.Kuhle J, et al. Conversion from clinically isolated syndrome to multiple sclerosis: A large multicentre study. Mult. Scler. 2015;21:1013–1024. doi: 10.1177/1352458514568827. [DOI] [PubMed] [Google Scholar]
- 24.Dobson R, Ramagopalan S, Davis A, Giovannoni G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: A meta-analysis of prevalence, prognosis and effect of latitude. J. Neurol. Neurosurg. Psychiatry. 2013;84:909–914. doi: 10.1136/jnnp-2012-304695. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, et al. Application of the 2017 McDonald criteria in a Chinese population with clinically isolated syndrome. Ther. Adv. Neurol. Disord. 2020;13:1756286419898083. doi: 10.1177/1756286419898083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu T, et al. Comparison of multiple sclerosis patients with and without oligoclonal IgG bands in South China. J. Clin. Neurosci. 2019;66:51–55. doi: 10.1016/j.jocn.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Zeman AZ, et al. A study of oligoclonal band negative multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1996;60:27–30. doi: 10.1136/jnnp.60.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lechner-Scott J, et al. The frequency of CSF oligoclonal banding in multiple sclerosis increases with latitude. Mult. Scler. 2012;18:974–982. doi: 10.1177/1352458511431729. [DOI] [PubMed] [Google Scholar]
- 29.Niino M, et al. Latitude and HLA-DRB1 alleles independently affect the emergence of cerebrospinal fluid IgG abnormality in multiple sclerosis. Mult. Scler. 2015;21:1112–1120. doi: 10.1177/1352458514560924. [DOI] [PubMed] [Google Scholar]
- 30.Giovannoni G, et al. Brain health: Time matters in multiple sclerosis. Mult. Scler. Relat. Disord. 2016;9(Suppl 1):S5–S48. doi: 10.1016/j.msard.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Brown JWL, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321:175–187. doi: 10.1001/jama.2018.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He A, et al. Timing of high-efficacy therapy for multiple sclerosis: A retrospective observational cohort study. Lancet Neurol. 2020;19:307–316. doi: 10.1016/S1474-4422(20)30067-3. [DOI] [PubMed] [Google Scholar]
- 33.Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2014;85:67–75. doi: 10.1136/jnnp-2012-304333. [DOI] [PubMed] [Google Scholar]
- 34.Fambiatos A, et al. Risk of secondary progressive multiple sclerosis: A longitudinal study. Mult. Scler. 2020;26:79–90. doi: 10.1177/1352458519868990. [DOI] [PubMed] [Google Scholar]
- 35.Piccolo L, et al. Multiple sclerosis in Japan appears to be a milder disease compared to the UK. J. Neurol. 2015;262:831–836. doi: 10.1007/s00415-015-7637-3. [DOI] [PubMed] [Google Scholar]
- 36.Kim SH, et al. Real-world effectiveness of disease-modifying therapies in Korean patients with relapsing multiple sclerosis. J. Clin. Neurol. 2019;15:20–26. doi: 10.3988/jcn.2019.15.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cree BAC, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann. Neurol. 2019;85:653–666. doi: 10.1002/ana.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harding KE, et al. Contemporary study of multiple sclerosis disability in South East Wales. J. Neurol. Neurosurg. Psychiatry. 2023;94:272–279. doi: 10.1136/jnnp-2022-330013. [DOI] [PubMed] [Google Scholar]
- 39.Simonsen CS, et al. The course of multiple sclerosis rewritten: A Norwegian population-based study on disease demographics and progression. J. Neurol. 2021;268:1330–1341. doi: 10.1007/s00415-020-10279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manouchehrinia A, Beiki O, Hillert J. Clinical course of multiple sclerosis: A nationwide cohort study. Mult. Scler. 2017;23:1488–1495. doi: 10.1177/1352458516681197. [DOI] [PubMed] [Google Scholar]
- 41.Leray E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain. 2010;133:1900–1913. doi: 10.1093/brain/awq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tremlett H, Yinshan Z, Devonshire V. Natural history of secondary-progressive multiple sclerosis. Mult. Scler. 2008;14:314–324. doi: 10.1177/1352458507084264. [DOI] [PubMed] [Google Scholar]
- 43.Lublin FD, et al. How patients with multiple sclerosis acquire disability. Brain. 2022;145:3147–3161. doi: 10.1093/brain/awac016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymised data can be made available to qualified investigators on reasonable request to the corresponding author.