Abstract
Background:
Diabetes mellitus (DM) is a long-term condition marked by high blood glucose levels caused by insulin resistance which will lead to complications of other diseases such as dyslipidemia, which also affects the health of the liver and kidneys. Butterfly pea flower (Clitorea ternatea L.) has phenolic and flavonoid compounds which have the potential as herbal medicines for antidiabetics.
Aim:
The purpose of this study is to examine the potential of butterfly pea flower extract (BPE) as an antidiabetic, anti-dyslipidemia, and renoprotection.
Methods:
In vivo test was performed on Sprague Dawley rats (Rattus norvegicus L.) induced by Streptozotocin-Nicotinamide and High Fat Diet-Propylthiouracil as models of DM and dyslipidemia, and BPE was administered orally (200, 400, and 800 mg/kg BW) for 28 days. glutathione peroxidase (GSH-Px), glutathione S-transferase (GST), tumor necrosis factor-α (TNF-α), nuclear factor-kappa beta (NF-kB), alkaline phosphatase (ALP), liver albumin levels, serum blood urea nitrogen (BUN), serum creatinine, and serum uric acid (UA), were measured by ELISA and colorimetry methods.
Results:
Treatment of BPE 800 mg/kg BW increased levels of GSH-Px, GST, albumin, and serum protein. BPE decreased TNF-α, NF-kB, and ALP. BPE also decreased BUN, serum CR, and serum UA.
Conclusion:
BPE has the potential to be used as a drug alternative for the treatment of DM and dyslipidemia as well as a hepatoprotective and renoprotective agent.
Keywords: Antidiabetic, Clitorea ternatea L., Diabetes mellitus, Dyslipidemia, Renoprotective
Introduction
Diabetes mellitus (DM) is a long-term condition identified by a deficient amount of insulin production or the body’s incapability to effectively use insulin, resulting in elevated blood glucose levels (Aamir et al., 2021). Insulin works by converting glucose into glycogen to be stored in the liver (Dimitriadis et al., 2021). DM is one of the most challenging global public health issues, with a prediction that DM cases will reach 578 million in 2030. DM disease is classified into three groups, namely type 1 DM, type 2 DM, and gestational DM (Mukhtar et al., 2020). Type 1 DM disease is marked by the autoimmune destruction of pancreatic β-cells so that the pancreas cannot produce insulin hormone (Paschou et al., 2018). The combination of genetic and environmental factors can influence causing type 1 DM (Persson et al., 2019). Type 2 DM is a metabolic disorder characterized by insulin resistance and pancreatic β-cell dysfunction. The majority of cases of diabetes worldwide (90%–95%) are of type 2 (Atlas et al., 2015), which has been linked to changes in lifestyle, lack of awareness of initial detection of DM disease, lack of physical activity, and poor diet (Mukhtar et al., 2020).
Insulin resistance in DM patients can also cause dyslipidemia, which is a condition characterized by blood lipid levels exceeding normal limits due to a lack of insulin to activate the lipoprotein lipase enzyme which hydrolyzes triglycerides (TG) in the blood (Tahereh and Saeed, 2014). DM conditions coupled with dyslipidemia will further increase oxidative stress, inflammation, and damage to tissues and organs as a result of the formation of cholesterol (CHOL) deposits in blood vessel walls which will undergo oxidation and increase excess reactive oxygen species (ROS) production (Rendra et al., 2019). The liver is an organ that is susceptible to hyperglycemia induced by oxidative stress which will lead to liver damage (Mohamed et al., 2016). Liver damage is characterized by increased enzymes in the liver, such as alkaline phosphatase (ALP), and reduced production of protein albumin for blood by the liver, which functions to carry hormones, vitamins, and enzymes throughout the body (El-Hadary and Ramadan, 2019). Numerous studies have also disclosed that tumor necrosis factor-α (TNF-α) and nuclear factor-kappa beta (NF-kB) play a big role in the onset of inflammation associated with liver damage (Park et al., 2014; Zhou et al., 2016). Apart from the liver, other organs that are affected by DM and dyslipidemia are the kidneys, which play a role in the excretory system. Kidney damage can be detected by measuring the levels of blood urea nitrogen (BUN), serum creatinine (SCr), and uric acid (UA), which are the end products of nitrogen and purines from protein metabolism that are toxic in the blood, so that they are excreted in the urine.
Antioxidant therapy can be an option for treating oxidative stress in patients with DM and dyslipidemia because the human body does not produce excess antioxidant reserves to reduce free radical activity (Djama'an et al., 2012; Tsalamandris et al., 2019). Catalase (CAT), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD) are the three main enzymes in the antioxidant defense system (Dworzański et al., 2020). Glutathione S-transferase (GST) enzymes also participate in overcoming antioxidants by fighting reactive electrophiles and fatty acid hydroperoxides that result from oxidative stress (Jaid et al., 2022). Many plants can be used as alternative natural antioxidants because they contain phenolic compounds, one of which has been widely studied is the butterfly pea flower (Clitoria ternatea L.). The butterfly pea flower is a perennial herbaceous plant that has piqued the interest of researchers due to its potential applications in both modern medicine and agriculture (Oguis et al., 2019). The butterfly pea flower exhibits promising pharmacological properties, including antidiabetic, antidyslipidemic, antioxidant, analgesic, anti-inflammatory, antihistamine, antibacterial, and anticancer (Tahereh and Saeed, 2014; Jamil et al., 2018; Jeyaraj et al., 2021; Widowati et al., 2023). Research by Talpate et al. (2013) showed that butterfly pea extract (BPE) has antihyperglycemic activity by decreasing fasting serum glucose and nitric oxide (NO), as well as increasing SOD and CAT in streptozotocin (STZ)-induced DM rats. Other research shows that BPE can reduce total serum CHOL, TG, and low-density lipoprotein (LDL) and normalize the ratio of high-density lipoprotein (HDL) to LDL in hyperlipidemia-induced mice (Al-Snafi, 2016). The potential of BPE as a hepatoprotection and renoprotection from the late effects of DM and dyslipidemia needs to be studied, as there has been no research conducted on this topic to date. Therefore, this study aims to assess the potential of BPE as an antioxidant, anti-inflammatory, hepatoprotection, and renoprotection effect in the DM and dyslipidemia rats model.
Material and Methods
Plant’s extraction
Butterfly pea flowers were obtained from Sukolilo Village, Pasuruan, Indonesia, and were determined at LIPI Bogor, Indonesia. A 70% ethanol maceration method was used to extract 500 g of dried simplicia. The resulting BPE was processed following good manufacturing practices. Further processing was done by PT. FAST (Depok, Indonesia) (CoA Batch 00103211072( (Widowati et al., 2023).
Animal experimental
This study used 32 male Sprague Dawley rats (average weight: 120–140 g, average age: 6 weeks) from IratCo Laboratory, Bogor. After a 7-day acclimatization period, they were given standard feed and aquades ad libitum (Widowati et al., 2022a), then randomly assigned to 8 groups. Group I: normal; II: positive control (induced nicotinamide/NA) (Sigma-Aldrich, N3376) 120 mg/kg BW+STZ (Sigma-Aldrich, SO130) 60 mg/kg BW and given a high-fat diet (HFD) 50 g/head/day+propylthiouracil (PTU) 0.01% as a model of DM and dyslipidemia; III: positive control+BPE 200 mg/kg BW/day; IV: positive control+BPE 400 mg/kg BW/day; V: positive control+BPE 800 mg/kg BW/day. Groups VI-VII are comparison control groups, using glibenclamide and simvastatin (Generic, GKL9520905004A2; GKL131670271A); VI: positive control+glibenclamide 0.45 mg/kg BW/day; VII: positive control+simvastatin 0.9 mg/kg BW/day; VIII: positive control+glibenclamide 0.45 mg/kg BW+simvastatin 0.9 mg/kg BW (Widowati et al., 2023). After inducing DM and dyslipidemia with STZ, NA, HFD, and PTU for 5 days, rats' blood sugar and total CHOL levels were checked following a 6-hour fast using Autocheck. Successful induction was indicated by blood sugar levels >200 mg/dl, and dyslipidemia was confirmed if serum CHOL levels were ≥200 mg/dl using the CHOL Kit (Elabscsi,.E-BC-K109-M). Rats with DM and dyslipidemia were then treated orally with BPE, glibenclamide, and simvastatin for 28 days according to their assigned groups (Yimdee et al., 2014).
Serum collection, rat termination, and liver collection
Rat blood samples collected on days 14 and 28 after a 12-hour fast and BPE treatment were refrigerated for 2 hours at 4°C, then put into centrifugation at 3,500 g for 10 minutes to obtain serum. On day 28, rats were euthanized with ketamine (Ikapharmindo Putramas) (100 mg/kg BW) and xylazine (Interchemie,.361453) (3 mg/kg BW), which were administered intracardially. Liver tissues were collected, weighed, and preserved at −80°C for protein level analysis (Widowati et al., 2022a).
Protein assay
Standard Bovine Serum Albumin solutions (Sigma-Aldrich,.A9576) (20 µl) and serum samples (20 µl) were added to designated wells, followed by the addition of Quick Start Dye Reagent 1X (Biorad,.5000205) (200 µl). After a five-minute incubation, the samples were read at 595 nm using a microplate reader (Widowati et al., 2019).
Measurement of GSH-Px, GST, TNF-α, NF-kB, ALP, and ALB levels
Parameter measurements were conducted using ELISA kits for Rat TNF-α and Rat NFkB-p65 (Elabsci, E-EL-R2856 and E-EL-R0674, respectively), as well as GSH-Px, ALP, ALB, and GST activity assays (Elabsci, E-BC-K096-S; E-BC-K091-M; E-BC-K057-S; and E-BC-K2785, respectively). Absorbance was measured at 405 and 450 nm using a microplate reader (Multiskan.Go.Thermo.Scientific), following the manufacturer's instructions (Widowati et al., 2019).
Measurement of BUN, SCr, and UA levels
Serum BUN, SCr, and UA levels were analyzed with dedicated kits: BUN Colorimetric Assay Kit (Urease Method), Creatinine Colorimetric Assay Kit (Sarcosine Oxidase Method), and UA Colorimetric Assay Kit. Absorbance was read at 405 and 450 nm using a microplate reader (Multiskan.Go.Thermo.Scientific) per the manufacturer's instructions (Widowati et al., 2022a).
Data analysis
Data analysis involved IBM SPSS Statistics 26.0, encompassing normality testing (Shapiro-Wilk), homogeneity testing (Levene), and one-way ANOVA (p < 0.05). Homogeneous data were assessed with the Tukey test, while heterogeneous data underwent Dunnett's T3 Post Hoc test. Abnormally distributed data were assessed using Kruskal-Wallis and Mann-Whitney Post Hoc tests (p ≤ 0.05) (Widowati et al., 2022b).
Ethical approval
This study received an ethical approval from the Research Ethics Commission of the Faculty of Medicine, Maranatha Christian University–Immanuel Hospital Bandung (099/KEP/VII/2022).
Results
GSH-Px hepatic activity of DM and dyslipidemia rat models
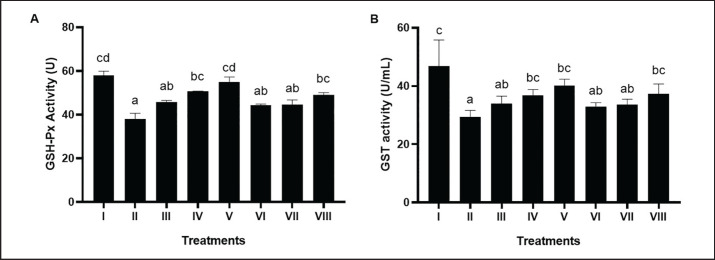
BPE significantly (p < 0.05) increases hepatic GSH-Px activity in DM and dyslipidemia rat models (Fig. 1A). The best BPE administration is BPE 800 mg/kg BW/day (V) with a value of 54.83 U.
Fig. 1. Effects of various treatments on GSH-Px activity and GST activity in DM and dyslipidemia rat models; (A) GSHPx activity, (B) GST activity. *Data are presented as means ± SD of four repetitions. The different superscript marks a, ab, bc, cd on GSH-Px activity (U) and a, ab, bc, c on GST activity (U/ml) showed a significant difference (p < 0.05). Group I: negative control (aquadest), Group II: positive control (HFD, PTU, STZ 60 mg/kg BW, NA 120 mg/kg BW), Group III: positive control + BPE 200 mg/kg BW/day, Group IV: positive control + BPE 400 mg/kg BW/day, Group V: positive control + BPE 800 mg/kg BW/day, Group VI: positive control + Simvastatin 0.9 mg/kg BW, Group VII: positive control + Glibenclamide 0.45 mg/kg BW, Group VIII: positive control + Glibenclamide 0.45 mg/kg BW + Simvastatin 0.9 mg/kg BW.
GST hepatic activity of DM and dyslipidemia rats model
Rats induced by STZ, NA, HFD, and PLU (II) had reduced GST activity compared to the negative controls (Fig. 1B). Higher doses of BPE increased GST levels, with the most efficient dose being BPE at 800 mg/kg BW (V) with GST activity at 40.21 U/ml.
TNF-α hepatic levels of DM and dyslipidemia rats model
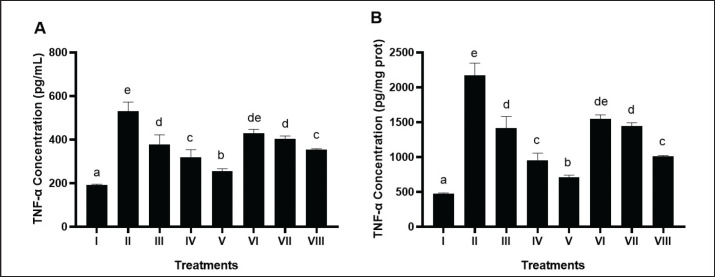
Mice induced by STZ and NA as well as HFD and PLU (II) demonstrated a significant increase in TNF-α levels compared to negative control (Fig. 2A and B). The results showed that BPE at a dose of 800 mg/kg BW (V) significantly reduced TNF-α levels (706.66 pg/mg protein) compared to the positive control (2,174.46 pg/mg protein).
Fig. 2. Effects of various treatments on TNF-α levels in DM and dyslipidemia rat models. *Data are presented as means ± SD of four repetitions. The different superscript marks a, b, c, d, de, e on TNF-α level (pg/ml, pg/mg prot) showed a significant difference among treatments (p < 0.05). Group I: negative control (aquadest), Group II: positive control (HFD, PTU, STZ 60 mg/kg BW, NA 120 mg/kg BW), Group III: positive control + BPE 200 mg/kg BW/day, Group IV: positive control + BPE 400 mg/kg BW/day, Group V: positive control + BPE 800 mg/kg BW/day, Group VI: positive control + Simvastatin 0.9 mg/kg BW, Group VII: positive control + Glibenclamide 0.45 mg/kg BW, Group VIII: positive control + Glibenclamide 0.45 mg/kg BW + Simvastatin 0.9 mg/kg BW.
NF-kB hepatic levels of DM and dyslipidemia rats model
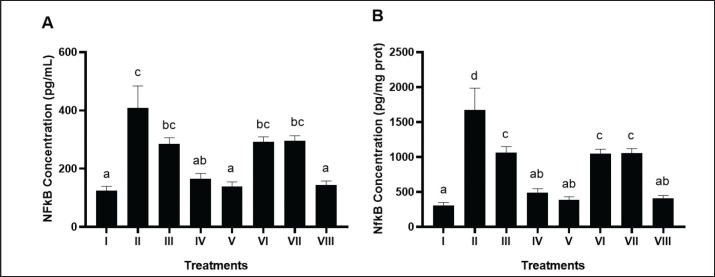
BPE notably (p < 0.05) reduced hepatic NF-kB levels in the DM and dyslipidemia rat model (Fig. 3A and B). The most significant reduction was observed with BPE at 800 mg/kg BW/day (V), measuring 384.45 pg/mg protein, surpassing the comparison control of simvastatin and glibenclamide (VIII) at 410.7 pg/mg protein.
Fig. 3. Effects of various treatments on NF-kB levels in DM and dyslipidemia rats model. *Data are presented as means ± SD of four repetitions. The different superscript marks a, ab, bc, c on NF-kB levels (pg/ml) and a, ab, c, d on NF-kB levels (pg/mg prot) showed a significant difference among treatment (p < 0.05). Group I: negative control (aquadest), Group II: positive control (HFD, PTU, STZ 60 mg/kg BW, NA 120 mg/kg BW), Group III: positive control + BPE 200 mg/kg BW/day, Group IV: positive control + BPE 400 mg/kg BW/day, Group V: positive control + BPE 800 mg/kg BW/day, Group VI: positive control + Simvastatin 0.9 mg/kg BW, Group VII: positive control + Glibenclamide 0.45 mg/kg BW, Group VIII: positive control + Glibenclamide 0.45 mg/kg BW + Simvastatin 0.9 mg/kg BW.
ALP hepatic levels of DM and dyslipidemia rats model
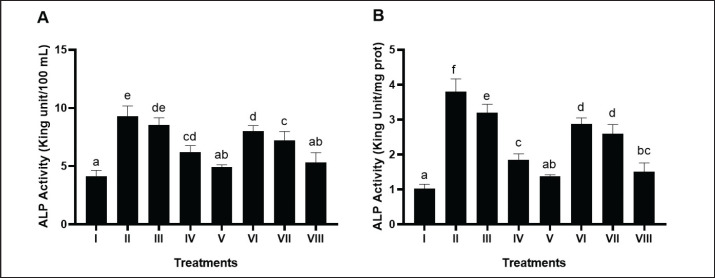
Rats induced with STZ, NA, HFD, and PLU (II) had significantly elevated ALP levels compared to the negative control (Fig. 4A and B). Higher doses of BPE, particularly BPE at 800 mg/kg BW (V), effectively reduced ALP levels to 1.37 King Unit/mg protein, outperforming the positive control.
Fig. 4. Effects of various treatments on ALP activity in DM and dyslipidemia rats model. *Data are presented as means ± SD of four repetitions. Different superscript marks a, ab, c, cd, d, de, e on ALP activity (King Unit/100 ml) and a, ab, bc, c, d, e, f on ALP activity (King Unit/mg protein) showed a significant difference among treatments (p < 0.05). Group I: negative control (aquadest), Group II: positive control (HFD, PTU, STZ 60 mg/kg BW, NA 120 mg/kg BW), Group III: positive control + BPE 200 mg/kg BW/day, Group IV: positive control + BPE 400 mg/kg BW/day, Group V: positive control + BPE 800 mg/kg BW/day, Group VI: positive control + Simvastatin 0.9 mg/kg BW, Group VII: positive control + Glibenclamide 0.45 mg/kg BW, Group VIII: positive control + Glibenclamide 0.45 mg/kg BW + Simvastatin 0.9 mg/kg BW.
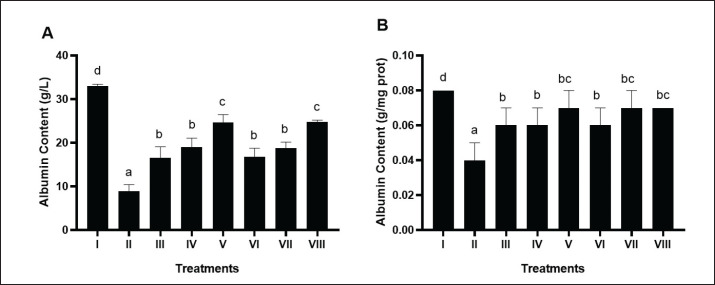
Albumin hepatic levels of DM and dyslipidemia rats model
BPE notably (p < 0.05) increased hepatic albumin levels in the DM and dyslipidemia rat model (Fig. 5A and B). The most significant change occurred by BPE at 800 mg/kg BW/day (V), measuring 0.07 g/mg protein, matching the comparison control of simvastatin and glibenclamide (VIII).
Fig. 5. Effects of various treatments on albumin levels in DM and dyslipidemia rat models. *Data are presented as means ± SD of four repetitions. The different superscript marks a, b, c, d for albumin content (g/l) and a, b, bc, d for albumin content (g/mg prot) showed significant differences among treatments (p < 0.05). Group I: negative control (aquadest), Group II: positive control (HFD, PTU, STZ 60 mg/kg BW, NA 120 mg/kg BW), Group III: positive control + BPE 200 mg/kg BW/day, Group IV: positive control + BPE 400 mg/kg BW/day, Group V: positive control + BPE 800 mg/kg BW/day, Group VI: positive control + Simvastatin 0.9 mg/kg BW, Group VII: positive control + Glibenclamide 0.45 mg/kg BW, Group VIII: positive control + Glibenclamide 0.45 mg/kg BW + Simvastatin 0.9 mg/kg BW.
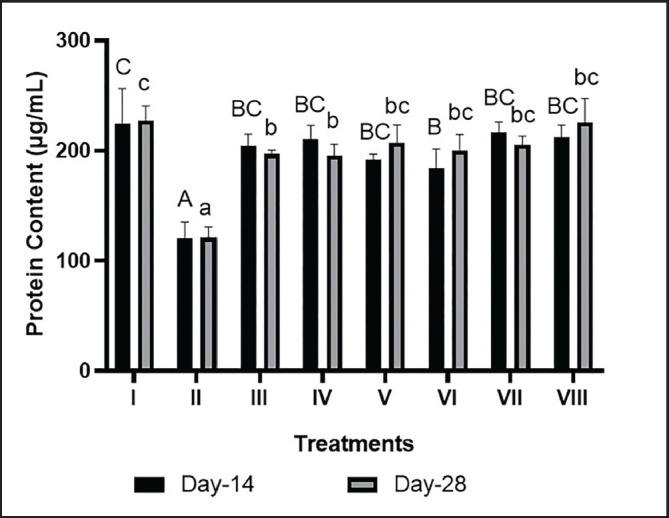
Protein serum levels in the liver of DM and dyslipidemia models rat
Serum protein levels with BPE treatment at 800 mg/kg BW/day (V) significantly increased on day 14 (191.64 μg/ml) and day 28 (206.98 μg/ml) compared to the positive control. These levels were comparable to the combination of simvastatin and glibenclamide (212.03 μg/ml on day 14 and 225.5 μg/l on day 28) (Fig. 6).
Fig. 6. Effects of BPE on protein content in DM and Dyslipidemia Rats. * The data are shown as mean ± standard deviation from 4 repetitions. Different superscript marks A, B, BC, and C on protein content day 14 (μmol/l) and a, b, bc, and c on protein content day 28 (mmol/l) showed significant differences (p < 0.05). Group I: negative control (aquadest), Group II: positive control (HFD, PTU, STZ 60 mg/kg BW, NA 120 mg/kg BW), Group III: Positive control + BPE 200 mg/kg BW/day, Group IV: Positive control + BPE 400 mg/kg BW/day, Group V: Positive control + BPE 800 mg/kg BW/day, Group VI: Positive control + Simvastatin 0.9 mg/kg BW, Group VII: Positive control + Glibenclamide 0.45 mg/kg BW, Group VIII: positive control + Glibenclamide 0.45 mg/kg BW + Simvastatin 0.9 mg/kg BW.
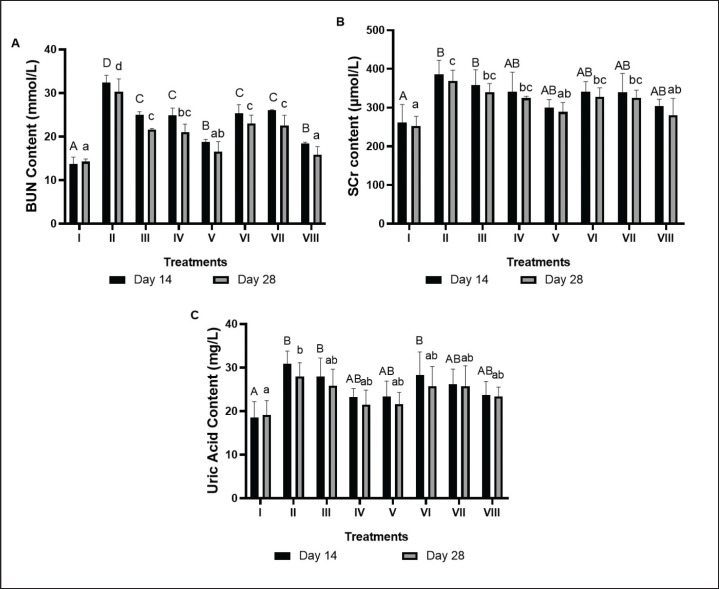
Serum BUN levels in the liver of DM and dyslipidemia rat models
Positive control (II), mice induced by HFD, PTU, NA, and STZ showed a notable increase in serum BUN contrasted to negative controls (I). Treatment with BPE decreased serum BUN levels in the DM rat model (Fig. 7A). A decrease in serum BUN by BPE was seen on day 14, namely 25.05 mmol/l (III), 24.91 mmol/l (IV), and 18.78 mmol/l (V), while on day 28 it was 21.64 mmol/l (III), 20.98 mmol/l (IV), and 16.59 mmol/l (V) when compared to the positive control. The most effective dose to reduce serum BUN levels is BPE 800 mg/kg BW/day (V).
Fig. 7. Effects of BPE on BUN, Creatinin, and UA content in DM and Dyslipidemia rats; (A) BUN, (B) Creatinin, (C) UA. * The data are shown as mean ± standard deviation from 4 repetitions. The different superscript marks A, AB, and B on BUN Content day 14 (μmol/l) and a, ab, and b on BUN content day 28 (mmol/l) showed significant differences (p < 0.05). Group I: negative control (aquadest), Group II: positive control (HFD, PTU, STZ 60 mg/kg BW, NA 120 mg/kg BW), Group III: Positive control + BPE 200 mg/kg BW/day, Group IV: Positive control + BPE 400 mg/kg BW/day, Group V: Positive control + BPE 800 mg/kg BW/day, Group VI: Positive control + Simvastatin 0.9 mg/kg BW, Group VII: Positive control + Glibenclamide 0.45 mg/kg BW, Group VIII: positive control + Glibenclamide 0.45 mg/kg BW + Simvastatin 0.9 mg/kg BW.
Creatinin serum levels in the liver of DM and dyslipidemia rat models
In the DM rat model, the positive control (II) induced by HFD, PTU, NA, and STZ exhibited a notable increase in SCr compared to the negative control group (I). Treatment with BPE reduced SCr levels (Fig. 7B). In the BPE treatment at 800 mg/kg BW/day (V), SCr decreased significantly on day 14 (300.06 μmol/l), and day 28 (289.03 μmol/l) compared to the positive control. This reduction was similar to the effect achieved by the combination of simvastatin and glibenclamide (280.34 μmol/l) on day 28.
UA serum levels in the liver of DM and dyslipidemia rat models
The positive control (II) demonstrated a notable increase in serum UA compared to the negative control (I). BPE treatment reduced serum UA levels in the DM rat model (Fig. 7C). Serum UA levels significantly decreased with BPE treatment at 400 mg/kg BW/day (IV) on day 14 (23.26 mg/l) and day 28 (21.51 mg/l) compared to the positive control. These levels approached the reduction effect produced by the combination of simvastatin and glibenclamide, which measured 23.75 mg/l on day 14 and 23.37 mg/l on day 28.
Discussion
In this study, DM model rats were made by STZ and NA induction, which increases blood glucose levels in rats due to insufficient insulin (Cruz et al., 2021). Giving STZ and NA can also cause partial insulin deficiency similar to type 2 DM (Del Giudice and Gangestad, 2018; Widowati et al., 2023). STZ activity is linked to the production of ROS, which causes oxidative stress and cell damage which is characterized by increased lipid peroxidase and depleted activity of endogenous antioxidant enzymes (Peiró et al., 2017). To create a dyslipidemia model in rats, HFD and PTU are administered, with PTU inhibiting thyroxine hormone synthesis, leading to reduced lipid metabolism and increased TG levels. In the dyslipidemia rat model, there was an elevation in CHOL, LDL, TG, and a decrease in HDL (Widi et al., 2015; Widowati et al., 2023).
In DM, mitochondria are the main source of potentially harmful and cell-damaging highly reactive molecules known as ROS. During mitochondrial oxidative metabolism, oxygen is reduced to water, while the remaining oxygen is altered into ROS like superoxide (O-), which can further convert into different forms, including peroxynitrite (ONOO-), hydroxide (OH-), and H2O2 (Widowati et al., 2023). Excessive ROS production disrupts the balance with natural antioxidants, causing oxidative damage that contributes to tissue injury via mechanisms including DNA damage, lipid peroxidation, enzyme oxidation, and the activation of proinflammatory cytokines. The results showed that after induction, the activity of endogenous antioxidant enzymes namely GSH-Px and GST decreased (Fig. 1A and B). BPE treatment can significantly increase the antioxidant activity of GSH-Px and GST compared to the positive control, even better than the comparison control. According to a different study, BPE evaluated in an STZ-induced mouse model showed strong antihyperglycemic and antioxidant effects by reducing TBARS levels and total NO content and raising antioxidant defense enzymes (CAT, GSH-Px, and SOD) (Talpate et al., 2013). This could be due to the antioxidant activity in BPE. BPE is known to contain many bioactive components that have antioxidant roles such as flavonoids, tannins, saponins, anthocyanins, gallic acid, and phenols (Singh and Jain, 2022). Anthocyanins display antioxidant activity against ROS through hydrogen atom transfer and single electron transfer mechanisms, stabilizing ROS and averting oxidative damage (Gollen et al., 2018).
Previous studies revealed links between diabetes, dyslipidemia, kidney dysfunction, and the presence of inflammatory cytokines, particularly TNF-α and NF-kB (Melasari et al., 2021; Li et al., 2023). Proinflammatory cytokines induce endoplasmic reticulum stress, increasing insulin metabolism. In DM, reduced insulin production and resistance are notable. NF-kB and TNF-α trigger low-grade inflammation in type 2 DM, contributing to complications like dyslipidemia, liver dysfunction, and atherosclerosis (Mehnati et al., 2020). The study's results indicated that positive controls, used as models for DM and dyslipidemia, exhibited significantly higher levels of NF-kB and TNF-α expression compared to the negative controls (Figs. 2 and 3). Treatment with BPE caused a significant decline in the expression of NF-kB and TNF-a compared to the positive control. This is supported by previous research that in vitro the leaves and flowers of butterfly peas showed anti-inflammatory activity (Gollen et al., 2018). Flavonoids typically have anti-inflammatory effects by targeting key mediators of inflammation through mechanisms such as inhibiting transcription factors and regulatory enzymes. Additionally, they hinder the activity of various kinases, including protein kinase C, phosphoinositide kinase, tyrosine kinase, phosphatidylinositol kinase, and cyclin-dependent kinase-4 (Yokoyama et al., 2015).
DM is linked to the health of various organs, including the liver, which stores glycogen. The ALP enzyme is a widely used marker for liver health, and research has shown a connection between ALP, glucose metabolism, insulin resistance, and metabolic syndrome. This association is attributed to ALP’s function as a hepatobiliary marker (Kim et al., 2020; Martuza et al., 2022). The results showed that the positive control found a fairly high level of the ALP enzyme. Therapy with BPE 800 mg/kg can reduce liver damage by significantly reducing ALP enzyme levels compared to negative controls (Fig. 4). In line with previous studies, CCl4-induced rats treated with BPE at a dose of 500 mg/kg could reduce ALP, SGOT, SGPT, and Serum Bilirubin levels (Balaji et al., 2015). This hepatoprotective capacity is influenced by the phenolic content such as flavonoids in BPE which are proven to be hepatoprotective agents by increasing liver cell regeneration (Wan and Jiang, 2018).
Albumins facilitate the transport of hormones and are involved in the exchange of nutrients and minerals, as well as water. Albumin can also function as an extracellular antioxidant agent (Sitar et al., 2013). Other studies have shown that serum albumin concentration is regarding metabolic disorders such as type 2 DM and metabolic syndrome (Jun et al., 2017). Reduced serum albumin elevates type 2 DM risk, as seen in this study where serum albumin and protein levels dropped significantly after STZ, NA, HFD, and PTU induction compared to negative controls. BPE treatment for 28 days, particularly at the highest dose (800 mg/kg), notably increased serum albumin and protein levels compared to positive controls (Figs. 5 and 6). This aligns with other studies demonstrating that extracts from C. ternatea, including gallic acid in BPE, can raise total protein and serum albumin levels, as well as enhance hepatoprotective effects through the regulation of SOD, CAT, GSH, protein, and serum albumin concentrations in in vivo tests (Nabavi et al., 2013).
DM and dyslipidemia are closely linked to kidney dysfunction, with oxidative stress potentially impairing glomerular filtration, leading to toxin retention in the blood due to reduced excretion through urine. Serum BUN, SCr and UA levels are markers of kidney damage that are commonly used through blood tests (Zhu et al., 2020). The results showed that blood levels of BUN, SCr, and UA increased significantly after STZ, NA, HFD, and PTU induction compared to negative controls (Fig. 7A–C). After BPE treatment with different dose variants for up to 28 days, the highest dose of BPE, 800 mg/kg, reduced these three parameters better than other doses. This is mediated by the content of flavonoids in BPE which act as a renoprotector. Many studies mention the effectiveness of flavonoids as renoprotectors by increasing the glomerular filtration rate (GFR) (Li et al., 2022). A rise in the glint in the kidneys will result in increased excretion of BUN, SCr, and UA, so that the levels of BUN, SCr, and UA in the blood decrease.
Based on the results, BPE is known to have antioxidant activity by increasing enzyme activity namely GSH-Px and GST, antiinflammatory activity by inhibiting NF-kB and TNF-a protein expression, hepatoprotection by inhibiting ALP activity, increasing ALB and Protein Total Serum, and renoprotection by decreasing BUN, SCr and UA Serum. The mechanism of BPE as anti-DM and antidyslipidemia can be seen in Figure 8. In conclusion, BPE 800 mg/kg BW treatment increases GSH-Px, GST Albumin, and protein serum, BPE also reduces TNF-α, NF-kB, ALP, BUN Serum, SCr, and UA Serum. BPE is potent enough to be used as a medication to treat DM and dyslipidemia, and also as a hepatoprotective and renoprotective.
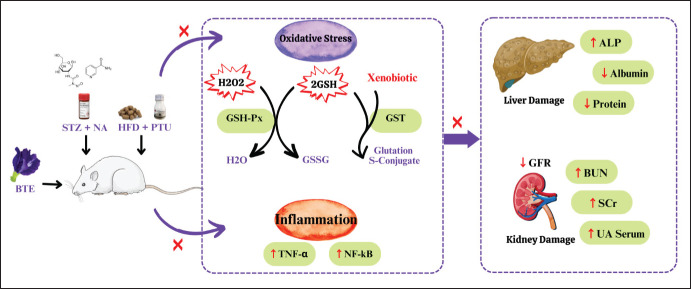
Fig. 8. Proposed mechanism of BPE as hepatoprotector and renoprotector in DM and Dyslipidemia rat model. In diabetic conditions, ROS in the body such as H2O2 are produced in excess. Normally this H2O2 can be overcome by the activity of the GPX enzyme to become H2O through the oxidation of GSH to (Glutathione Disulfide) GSSG. However, the large number of H2O2 molecules disrupts the activity of the GSH-Px enzyme. On the other hand, other antioxidant enzymes, namely GST, can also convert GSH and xenobiotics, namely foreign substances that are not beneficial to the body, into safer Glutathione S-Conjugates. The presence of free radicals can also activate NFkB which causes the production of TNF-α which has an impact on inflammation. The presence of oxidative stress and inflammation in cells over time will develop into damage to organs such as the liver and kidneys. Liver damage is characterized by increased ALP enzymes, as well as decreased protein and albumin. In the kidney, inflammation and oxidative stress have an impact on decreasing the GFR which causes BUN, SCr, and UA in the blood cannot be excreted. Treatment with BPE can neutralize H2O2 in cells by increasing GSH-Px and GST enzyme activity, also downregulating NF-kB and TNF-a, as well as reducing the risk of liver damage by reducing ALP enzyme expression and increasing total protein and albumin. BPE also increases GFR so that BUN, SCr, and UA can be excreted properly.
Acknowledgments
The authors appreciate the additional funding from Lembaga Penelitian dan Pengembangan Masyarakat (LPPM) of Maranatha Christian University. Aretha Medika Utama Biomolecular and Biomedical Research Center, Bandung, Indonesia, provided the methodology and laboratory resources for this research.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
None.
Authors’ contributions
WW, LD, HSU, and RR conceived and designed the study and also drafted the manuscript. MRN, NWW, AMGT, BHS, RO, and FHZ contributed to the design of the study, conducted the experiment, and collected the data. MRN, NWW, AMGT, BHS, RO, and FHZ provided the analysis and interpretation of data, drafted and revised the manuscript. All authors read and approved the final manuscript.
Data availability
The information backing the discoveries of this research is not openly accessible due to sensitivity concerns but can be obtained from the corresponding author upon reasonable inquiry.
References
- Aamir K.M., Sarfraz L., Ramzan M., Bilal M., Shafi J., Attique M. A fuzzy rule-based system for classification of diabetes. Sensors. 2021;21:8095–8112. doi: 10.3390/s21238095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Snafi A.E. Pharmacological importance of Clitoria ternatea–a review. IOSR. J. Pharm. 2016;6:68–83. [Google Scholar]
- Atlas D. International diabetes federation. IDF Diabetes Atlas, 7th edn. Brussels, Belgium: Int. Diabetes Federation. 2015;33:1–304. [Google Scholar]
- Balaji B., Bookya K., Bai G.V., Mangilal T. Hepatoprotective agent present in pods of Clitoria ternatea with evidence of histopathological analysis. Int. J. 2015;4:33–38. [Google Scholar]
- Cruz P.L., Moraes-Silva I.C., Ribeiro A.A., Machi J.F., de Melo M.D.T., Dos Santos F., Irigoyen M.C. Nicotinamide attenuates streptozotocin-induced diabetes complications and increases survival rate in rats: role of autonomic nervous system. BMC Endocr. Disord. 2021;21:1–10. doi: 10.1186/s12902-021-00795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice M., Gangestad S.W. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav. Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G.D., Maratou E., Kountouri A., Board M., Lambadiari V. Regulation of postabsorptive and postprandial glucose metabolism by insulin-dependent and insulin-independent mechanisms: an integrative approach. Nutrients. 2021;13:159–192. doi: 10.3390/nu13010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djama’an Q., Goenarwo E., Mashoedi I.D. Effect of Ginger Juice Water on Blood Glucose Levels and Histopathology of Pancreatic Beta Cells. Sains Med. J. Kedokt. dan Kesehat. 2012;4:165–173. [Google Scholar]
- Dworzański J., Strycharz-Dudziak M., Kliszczewska E., Kiełczykowska M., Dworzańska A., Drop B., Polz-Dacewicz M. Glutathione peroxidase (GPx) and superoxide dismutase (SOD) activity in patients with diabetes mellitus type 2 infected with Epstein-Barr virus. PLoS One. 2020;15:e0230374–e0230384. doi: 10.1371/journal.pone.0230374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hadary A.E., Ramadan M.F. Antioxidant traits and protective impact of Moringa oleifera leaf extract against diclofenac sodium-induced liver toxicity in rats. J. Food Biochem. 2019;43:e12704–e12712. doi: 10.1111/jfbc.12704. [DOI] [PubMed] [Google Scholar]
- Gollen B., Mehla J., Gupta P. Clitoria ternatea Linn: a herb with potential pharmacological activities: future prospects as therapeutic herbal medicine. J. Pharm. Rep. 2018;3:1–8. [Google Scholar]
- Jaid H.K., Khaleel F.M., Salman I.N., Abd B.A. Evaluation of insulin resistance and glutathione-s-transferase in Iraqi patients with type 2 diabetes mellitus and diabetic peripheral neuropathy. Ibn AL-Haitham J. Pure Appl. Sci. 2022;35:194–205. [Google Scholar]
- Jamil N., Zairi M.N.M., Nasim N.A.I.M., Pa'ee F. Influences of environmental conditions to phytoconstituents in Clitoria ternatea (butterfly pea flower)–a review. J. Sci. Technol. 2018;10:208–228. [Google Scholar]
- Jeyaraj E.J., Lim Y.Y., Choo W.S. Extraction methods of butterfly pea (Clitoria ternatea) flower and biological activities of its phytochemicals. J. Food Sci. Technol. 2021;58:2054–2067. doi: 10.1007/s13197-020-04745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun J.E., Lee S.E., Lee Y.B., Jee J.H., Bae J.C., Jin S.M., Hur K.Y., Lee M.K., Kim J.H. Increase in serum albumin concentration is associated with prediabetes development and progression to overt diabetes independently of metabolic syndrome. PloS One. 2017;12:1–13. doi: 10.1371/journal.pone.0176209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Lee H.S., Park H.M., Lee Y.J. Serum alkaline phosphatase level is positively associated with metabolic syndrome: a nationwide population-based study. Clin. Chim. Acta. 2020;500:189–194. doi: 10.1016/j.cca.2019.10.015. [DOI] [PubMed] [Google Scholar]
- Li Z., Deng H., Guo X., Yan S., Lu C., Zhao Z., Ma X. Effective dose/duration of natural flavonoid quercetin for treatment of diabetic nephropathy: a systematic review and meta-analysis of rodent data. Phytomedicine. 2022;105:154348. doi: 10.1016/j.phymed.2022.154348. [DOI] [PubMed] [Google Scholar]
- Li J., Liu H., Shang L. Tert-butylhydroquinone mitigates renal dysfunction in pregnant diabetic rats via attenuation of oxidative stress and modulation of the iNOs/NFkB/TNF alpha signalling pathway. Endocr. Metab. Immune Disord. Drug Targets. 2023;23:633–646. doi: 10.2174/1871530322666220908153118. [DOI] [PubMed] [Google Scholar]
- Martuza N., Haque R., Afrin S., Morshed A.J.M.T., Shahnaj A., Hossain M.T., Zaman M.S. High sensitive C reactive protein (hsCRP) and alkaline phosphatase (ALP) in type 2 diabetes patients. Sch. Int. J. Biochem. 2022;5:22–27. [Google Scholar]
- Mehnati P., Baradaran B., Vahidian F., Nadiriazam S. Functional response difference between diabetic/normal cancerous patients to inflammatory cytokines and oxidative stresses after radiotherapy. Rep. Pract. Oncol. Radiother. 2020;25:730–737. doi: 10.1016/j.rpor.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melasari W.P., Suharjono S., Samsulhadi W. Effect of atorvastatin in lipid profile changes and inflammation marker TNF-alpha on diabetes patient with dyslipidemia. Folia Med. Indones. 2021;57:6–10. [Google Scholar]
- Mohamed J., Nafizah A.N., Zariyantey A.H., Budin S. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016;16:e132–e141. doi: 10.18295/squmj.2016.16.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar Y., Galalain A., Yunusa U. A modern overview on diabetes mellitus: a chronic endocrine disorder. Europ. J. Biol. 2020;5:1–14. [Google Scholar]
- Nabavi S.F., Habtemariam S., Sureda A., Hajizadeh Moghaddam A., Daglia M., Nabavi S.M. In vivo protective effects of gallic acid isolated from Peltiphyllum peltatum against sodium fluoride-induced oxidative stress in rat erythrocytes. Arh. Hig. Rada. Toksikol. 2013;64:553–558. doi: 10.2478/10004-1254-64-2013-2353. [DOI] [PubMed] [Google Scholar]
- Oguis G.K., Gilding E.K., Jackson M.A., Craik D.J. Butterfly pea (Clitoria ternatea), a cyclotide-bearing plant with applications in agriculture and medicine. Front. Plant Sci. 2019;10:645–667. doi: 10.3389/fpls.2019.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Lee W.R., Kim H.S., Han S.M., Chang Y.C., Park K.K. Protective effects of melittin on tumor necrosis factor-α induced hepatic damage through suppression of apoptotic pathway and nuclear factor-kappa B activation. Exp. Biol. Med. 2014;239:1705–1714. doi: 10.1177/1535370214533880. [DOI] [PubMed] [Google Scholar]
- Paschou S.A., Papadopoulou-Marketou N., Chrousos G.P., Kanaka-Gantenbein C. On type 1 diabetes mellitus pathogenesis. Endocr. Connect. 2018;7:R38–R46. doi: 10.1530/EC-17-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiró C., Lorenzo Ó., Carraro R., Sánchez-Ferrer C.F. IL-1β inhibition in cardiovascular complications associated to diabetes mellitus. Front. Pharmacol. 2017;8:363–375. doi: 10.3389/fphar.2017.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson E., Persson S., Gerdtham U.G., Steen Carlsson K. Swedish Childhood Diabetes Study Group. Effect of type 1 diabetes on school performance in a dynamic world: new analysis exploring Swedish register data. Appl. Econ. 2019;51:2606–2622. [Google Scholar]
- Rendra E., Riabov V., Mossel D.M., Sevastyanova T., Harmsen M.C., Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiol. 2019;224:242–253. doi: 10.1016/j.imbio.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Singh A., Jain A.K. Qualitative and quantitative analysis of phytochemical constituents in Clitoria ternatea L. Ind. J. Agric.Biochem. 2022;35:51–57. [Google Scholar]
- Sitar M.E., Aydin S., Cakatay U.F.U.K. Human serum albumin and its relation with oxidative stress. Clin. Lab. 2013;59:945–952. [PubMed] [Google Scholar]
- Tahereh F., Saeed S. The effect of saffron (Crocus sativus L.) and its ingredients on the management of diabetes mellitus and dislipidemia. Afr. J. Pharm. Pharmacol. 2014;8:541–549. [Google Scholar]
- Talpate K.A., Bhosale U.A., Zambare M.R., Somani R. Antihyperglycemic and antioxidant activity of Clitorea ternatea Linn. on streptozotocin-induced diabetic rats. Ayu. 2013;34:433–439. doi: 10.4103/0974-8520.127730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalamandris S., Antonopoulos A.S., Oikonomou E., Papamikroulis G.A., Vogiatzi G., Papaioannou S., Deftereos S., Tousoulis D. The role of inflammation in diabetes: current concepts and future perspectives. Eur. Cardiol. Rev. 2019;14:50–59. doi: 10.15420/ecr.2018.33.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L., Jiang J.G. Protective effects of plant-derived flavonoids on hepatic injury. J. Funct. Foods. 2018;44:283–291. [Google Scholar]
- Widi N.A., Sitorus T.D., Rita C. Red ear fungi (Auricularia auricula) infusion reduce blood triglyceride level in dyslipidemic rats. Althea Med. J. 2015;2:143–146. [Google Scholar]
- Widowati W., Darsono L., Lucianus J., Setiabudi E., Obeng S.S., Stefani S., Rizal R. Butterfly pea flower (Clitoria ternatea L.) extract displayed antidiabetic effect through antioxidant, anti-inflammatory, lower hepatic GSK-3β, and pancreatic glycogen on diabetes mellitus and dyslipidemia rat. J. King Saud Univ. Sci. 2023;35:102579–102589. [Google Scholar]
- Widowati W., Prahastuti S., Ekayanti N.L.W., Munshy U.Z., Kusuma H.S.W., Wibowo S.H.B., Rizal R. Anti-inflammation assay of black soybean extract and its compounds on lipopolysaccharide-induced RAW 264.7 cell. J. Phys. Conf. Ser. 2019;1374:012052–012062. [Google Scholar]
- Widowati W., Prahastuti S., Hidayat M., Hasianna S.T., Wahyudianingsih R., Eltania T.F., Kusuma H.S.W. Detam 1 black soybean against cisplatin-induced acute ren failure on rat model via antioxidant, antiinflammatory and antiapoptosis potential. J. Tradit. Complement. Med. 2022a;12:426–435. doi: 10.1016/j.jtcme.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widowati W., Wargasetia T.L., Rahardja F., Gunanegara R.F., Priyandoko D., Gondokesumo M.E., Rizal R. Human Wharton’s jelly mesenchymal stem cells inhibit cytokine storm in acute respiratory distress syndrome in a rat model. Asian Pac. J. Trop. Biomed. 2022b;12:343–350. [Google Scholar]
- Yimdee J., Uabundit N., Chaisiwamonkol K., Iamsaard S., Hipkaeo W., Wattanathorn J. Evaluation of cognitive enhancing effects of Clitoria ternatea flowers water extract in normal male mice. Asian Pac. J. Sci. Technol. 2014;14:59–66. [Google Scholar]
- Yokoyama T., Kosaka Y., Mizuguchi M. Structural insight into the interactions between death-associated protein kinase 1 and natural flavonoids. J. Med. Chem. 2015;58:7400–7408. doi: 10.1021/acs.jmedchem.5b00893. [DOI] [PubMed] [Google Scholar]
- Zhou G.Y., Yi Y.X., Jin L.X., Lin W., Fang P.P., Lin X.Z., Pan C.W. The protective effect of juglanin on fructose-induced hepatitis by inhibiting inflammation and apoptosis through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed rats. Biomed. Pharmacother. 2016;81:318–328. doi: 10.1016/j.biopha.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Zhu H., Zhu X., Lin H., Liu D., Dai Y., Su X., Li L. Association of serum PSP/REG Iα with renal function in type 2 DM. J. Diabet. Res. 2020;2020:1–6. doi: 10.1155/2020/9787839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The information backing the discoveries of this research is not openly accessible due to sensitivity concerns but can be obtained from the corresponding author upon reasonable inquiry.