Abstract
Intravascular routes of administration can provide a means to target gene- and virus-based therapies to multiple tumor foci located within an organ, such as the brain. However, we demonstrate here that rodent plasma inhibits cell transduction by replication-conditional (oncolytic) herpes simplex viruses (HSV), replication-defective HSV, and adenovirus vectors. In vitro depletion of complement with mild heat treatment or in vivo depletion by treatment of athymic rats with cobra venom factor (CVF) partially reverses this effect. Without CVF, inhibition of cell infection by HSV is observed at plasma dilution as high as 1:32, while plasma from CVF-treated animals displays anti-HSV activity at lower dilutions (1:8). When applied to the therapy of intracerebral brain tumors, in vivo complement depletion facilitates the initial infection (assayed at the 2-day time point) by an intra-arterial replication-conditional HSV of tumor cells, located within three separate and distinct human glioma masses. However, at the 4-day time point, no propagation of HSV from initially infected tumor cells could be observed. Previously, we have shown that the immunosuppressive agent, cyclophosphamide (CPA), facilitates the in vivo propagation of an oncolytic HSV, delivered intravascularly, within infected multiple intracerebral masses, by inhibition of both innate and elicited anti-HSV neutralizing antibody response (K. Ikeda et al., Nat. Med. 5:881–889, 1999). In this study, we thus show that the addition of CPA to the CVF treatment results in a significant increase in viral propagation within infected tumors, measured at the 4-day time period. The concerted action of CVF and CPA significantly increases the life span of athymic rodents harboring three separate and large glioma xenografts after treatment with intravascular, oncolytic HSV. Southern analysis of viral genomes analyzed by PCR reveals the presence of the oncolytic virus in the brains, livers, spleens, kidneys, and intestine of treated animals, although none of these tissues displays evidence of HSV-mediated gene expression. In light of clinical trials of oncolytic HSV for malignant brain tumors, these findings suggest that antitumor efficacy may be limited by the host innate and elicited humoral responses.
The identification of complementing interactions between viral genes and cellular pathways involved in tumorigenesis is providing a biological justification for the use of replication-conditional (oncolytic, replication-restricted) lytic viruses as anticancer agents (9, 10, 16, 19, 30, 47, 48, 52). Even before we have a full understanding of such interactions, clinical experimental safety trials of viral mutants based on replicating herpes simplex virus or adenovirus are being conducted for refractory head and neck, ovarian, and malignant glioblastoma (38; R. L. Martuza, personal communication). These tumors (and other malignant tumors) are commonly refractory to treatment by chemo- or radiotherapy and herald the rapid demise of the afflicted individual. Further complicating treatment is the finding that several malignant tumors manifest as multiple, discrete masses within an organ. This can be seen commonly with metastatic cancers to the liver, brain, and lung. Furthermore, the most malignant form of primary brain tumor (glioblastoma multiforme) can manifest as multicentrically distinct masses within the brain, a universally fatal occurrence (5, 63, 72).
Clinical trials of adenoviruses or herpes simplex viruses (HSV) primarily employ direct inoculation into the neoplastic mass through free-hand, stereotactic, or catheter-based techniques (8, 60, 61; J. B. Alavi, K. Judy, A. Alavi, D. Hackney, P. Phillips, J. Smith, A. Recio, J. Wilson, and S. Eck, Abstr. 1st Annu. Meet. Am. Soc. Gene Therapy, p. 444, 1998; T. W. Trask, E. Aguilar-Cordova, J. C. Goodman, R. Guevara, P. Wyde, H. D. Shine, and R. G. Grossman, Abstr. 1st Annu. Meet. Am. Soc. Gene Therapy, p. 445, 1998). This route of vector administration, while likely to produce focal necrosis within the injected tumor mass, is unlikely to directly generate viral lytic effects against other tumors located within the same organ. Immune-based “cross-priming” reactions may aid in this respect (71), but clearly the efficacy of any anticancer action would be greatly improved if some of the injected viruses were able to infect and replicate selectively within each of the tumor masses located in an organ. Therefore, the advantage of injecting oncolytic viruses within the circulation is related to the potential delivery into different tumor masses, commonly supplied by exuberant neovascular growth, with the possibility for lytic action against each tumor focus. However, several potential disadvantages of this approach may be envisioned. (i) It is likely that very few virus particles would be distributed into the neoplasms and infect its cells from the vasculature. (ii) Toxic side effects may occur in normal cells within organs affected by the neoplasm and/or to other organs. (iii) Injected viral particles may be inactivated by blood components. (iv) When trying to deliver molecules or viruses from the circulation into the brain, an additional limitation is presented by the blood-brain barrier (BBB) and the blood-brain tumor barrier (BTB) (7, 24, 25, 43, 56).
In an attempt to circumvent such limitations, regional delivery of virus vectors through the arterial blood supply may improve the chances of tumor infection by eliminating the “first-pass” effects by the liver. In fact, in experimental models of metastatic liver cancer, delivery through the portal supply to the liver can lead to tumor infection by replication-conditional HSV (74) or by replication-defective adenovirus vectors (2). However, when applied to the brain through intracarotid inoculation, no infection of tumors was observed (59). In an effort to improve this result, the addition of disruptors of the BBB, such as mannitol (56), or of disruptors of the BTB, such as bradykinin (59) or its agonist RMP7 (6), was shown to facilitate the infection of brain tumors by intra-arterial HSV or adenovirus. In spite of this result, the efficiency of tumor infection in the brain, as measured by the number of animals with positively infected tumors and by the anatomic extent of viral infection and/or propagation within a tumor, remained relatively poor (35). Concern about this perceived inefficiency thus has led us to consider other physiologic and/or molecular mechanisms that may contribute to this apparent inefficiency.
The inactivation of virus by blood components provides a likely mechanism that may limit the efficacy of intracerebral tumor infection. Serum lipoproteins (33, 67, 68), fatty acids (3, 70), immunoglobulin (20, 36), and complement (23, 50, 64) have been reported to bind to HSV and inactivate its ability to infect cells. Immunoglobulin has also been reported to bind to infected cells and inhibit further HSV infection by immune-mediated lysis through antibody-dependent cellular cytoxicity (40), opsonization of viral particles and/or cells by macrophages (39), and activation of classical complement pathways (39). To escape such innate antiviral responses, HSV has been shown to employ several mechanisms. First, the viral glycoprotein gC can bind to and inactivate the C3 component of complement (49, 50). Second, the viral glycoproteins gE and gI have been shown to bind to the Fc portion of IgG and thus inhibit its function, possibly by antibody bipolar bridging (22). Further evasion of immune responses derives from the function of the virus immediate-early gene transcript, ICP47, that inhibits TAP activity and major histocompatibility complex class I presentation in infected cells (29). The interaction between the innate immune response against virus and the viral evading mechanisms must represent an important variable in governing the efficacy and toxicity of lytic-virus-mediated destruction of tumors, particularly on exposure of the virus vector to host blood components.
To further investigate such aspects, we have recently showed that preimmune plasma harvested from athymic and immunocompetent rodents as well as from humans can inactivate the in vitro transducing ability of the replication-conditional HSV mutant, hrR3 (35). This innate activity is present at dilutions as high as 1:32 for athymic and 1:16 for immunocompetent rodents; it is calcium dependent; it is partially suppressed by in vivo pretreatment of rodents with agents that deplete complement, such as cobra venom factor (CVF), and it is partially lost upon mild heat inactivation, indicating that one of its components is complement. In athymic rats, additional insights into the characterization of this activity were provided by antibody neutralization studies against immunoglobulin M (IgM). In vivo pretreatment of rodents with cyclophosphamide (CPA), a generalized inhibitor of immunoglobulin production by B cells, could also partially suppress this innate antiviral activity. CPA pretreatment of rodents was found to decrease by more than half the IgM blood concentration within 48 h. In fact, when a single systemic dose of CPA was administered to rodents, significant increases both in the number of animals with positively infected intracerebral tumors and in the propagation of viral infection throughout the brain neoplasm were observed after intra-arterial administration with hrR3. These studies thus suggested that transient inhibition of the innate antiviral response, which involved IgM and its likely interaction with complement, resulted in an augmented anticancer effect in vivo. This model thus would predict that in vivo pretreatment of rodents with agents that deplete complement, such as CVF, should also result in an augmented anticancer effect. Since one of CPA's actions is to deplete plasma IgM, while CVF depletes complement, the model also would predict that the combination of CPA and CVF would result in further augmentation of the anticancer effect of hrR3, delivered intra-arterially. Herein, we show that CVF does reverse the antiviral action of plasma in vitro against three different HSV mutants derived from three different HSV strains and against a replication-defective adenovirus vector. In vivo, CVF pretreatment increases the number of positively infected tumor cells within a neoplastic intracerebral mass after intra-arterial administration of the oncolytic virus, hrR3. Addition of CPA appears to increase the propagation of virus within tumors and the combination of the two agents proves superior than other treatments in its anticancer effects. These results thus support a model of intravascular virus infection and propagation within tumors that is initially modulated by the host innate antiviral response, which can be pharmacologically modified to further augment viral anticancer effects.
MATERIALS AND METHODS
Vector stocks.
hrR3 is the genetically engineered HSV mutant, derived from HSV-1 KOS, which has an intact TK gene and a disruption of the UL39 gene through insertion of the Escherichia coli lacZ gene under the control of the ICP6 promoter (31). Viral stocks were generated in African green monkey kidney cell culture (Vero), and titers were determined by plaque assays. As a control, a mock-infection extract was prepared from mock-infected cells using the same procedures. MGH1 is a second-generation replication-conditional HSV-1 vector defective for both ribonucleotide reductase and the neurovirulence factor γ34.5 (42). The helper-free HSV amplicon (pHSVlac) has been described (28); it consists of a plasmid bearing the HSV-1 origin of DNA replication, the “Pac” sequence to support packaging, and an IE4/5 promoter driving lacZ. This amplicon is packaged in Vero cells by cotransfection with a set of five cosmids representing the entire HSV1 genome but lacking the Pac sequences needed for DNA cleavage and packaging (21). This allows for packaging of the amplicon plasmids without recombination and packaging of wild-type HSV or helper virus. The adenovirus vector was obtained originally from Alan Smith and Bruce Roberts (Genzyme), and it possesses a deletion in the E1A-E1B region, an intact E3 region, and a modified E4 region in which the entire E4 locus has been deleted and the E4 open reading frame and protein IX have been reinserted. A cytomegalovirus promoter-lacZ gene cassette has also been inserted into the E1 locus.
Cell culture.
African green monkey kidney (Vero) cells were purchased through the American Type Culture Collection. Human U87dEGFR glioma cells were a generous gift of H.-J. Su Huang (University of California at San Diego). This cell line was established by retroviral transfer of a mutant epidermal growth factor receptor (de 2-7 EGFR) into the U87 human glioblastoma cell line, enhancing its tumorigenic capacity in the brain of nude mice (55). U87dEGFR cells were propagated at 37°C in an atmosphere containing 5% carbon dioxide in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum containing 100 U of penicillin, 100 μg of streptomycin, and 500 μg of G418 (Sigma) per ml. RMP7 (Cereport) was a generous gift from R. T. Bartus (Alkermes, Inc., Cambridge, Mass.).
Assays for plasma antiviral activity.
Rat plasma was serially diluted with phosphate-buffered saline (PBS). For most experiments, 100 μl of plasma was incubated with 2 × 104 PFU/2 μl of viral vectors for 1.5 h at 37°C and then applied onto 4 × 104 Vero cells in 24-well plates. In some experiments (see Fig. 3), 40 μl of plasma was incubated with 8 × 103 PFU/2 μl of virus for 1.5 h at 37°C before addition onto 2 × 104 Vero cells in 48-well plates. Sixteen hours later, the cells were fixed and stained for β-galactosidase activity. Four random high-power fields were selected for counting lacZ-positive plaques or cells. There are 28 high-power fields in the well of a 48-well plate, allowing calculations of absolute numbers of infectious units. In pilot experiments, lacZ transduction of cells was essentially identical to the number of lacZ-expressing plaques measured 5 to 7 days later. For some experiments, blood samples were obtained from rats that had been injected intraperitoneally with CVF (Quidel, San Diego, California) at doses of 60 and 20 U/kg on the day before and the day of plasma preparation. For studies employing purified rat complement (Accurate Chemical and Scientific Co., Westbury, N.Y.) virus was preincubated with Hanks balanced salt solution (HBSS), complement (1 mg/ml) in HBSS, heat-inactivated plasma prepared from athymic rats (diluted 1:4), and complement re-added to heat-inactivated plasma to a concentration of 1 mg/ml.
FIG. 3.
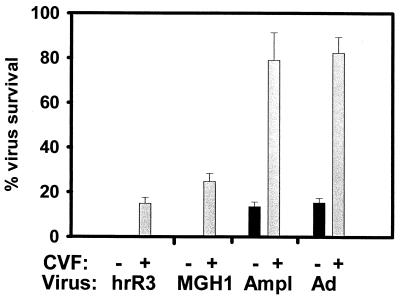
Effects of CVF-treated and control plasma on three HSV vectors and adenovirus. Plasma was prepared from athymic rats 2 days after treatment with CVF or carrier. It was then diluted 1:8 before being mixed with hrR3, an ICP6-defective HSV derived from KOS strain; MGH1, an ICP6- and ICP34.5-defective HSV derived from F strain; Amplicon (Ampl), a replication-defective HSV amplicon derived from strain 17; or an adenoviral (Ad) vector. The percent virus survival denotes the percentage of lacZ-expressing Vero cells, as enumerated 16 h after infection, compared to control dishes. Controls consisted of virus preincubated with HBSS for the same times before addition onto plates. The bars represent the average from triplicate dishes, and error bars represent the standard error of the mean.
Animal studies.
Adult female nude rats (rnu/rnu) or female adult immunocompetent Fisher 344 rats were anesthetized with an intraperitoneal injection of 0.5 ml of 0.9% NaCl containing 12.5 mg of ketamine and 2.5 mg of xylazine. After the rats were immobilized in a stereotactic apparatus and a linear skin incision was placed over the bregma, burr holes (1 mm in diameter) were drilled in the skull approximately 1 mm anterior to and 2 mm lateral to the bregma on both sides and 3 mm posterior and 2 mm lateral to the bregma on the right side. A total of 200,000 U87dEGFR cells (in a 2-μl volume) were injected at a depth of 3.5 mm from the dura by using a 5-μl Hamilton syringe.
Six days later, CVF (60 U/kg) was injected intraperitoneally. The next day, CVF (20 U/kg) administration was repeated, followed by intra-arterial catheterization with hrR3 and/or RMP7 (Alkermes, Inc., Cambridge, Mass.). In some animals, a single intraperitoneal dose of CPA (100 mg/kg) was also administered, 2 days after the last dose of CVF and the intra-arterial delivery of hrR3 and RMP7. The catheterization technique used was essentially identical to the one previously described (59). Briefly, RMP7 at a dose of 1.5 μg/kg or vehicle was infused over 10 min. Midway through the infusion, a 200-μl bolus of virus (2 × 109 PFU) or vehicle was given. For tumor transduction assays, animals were sacrificed at different days after catheterization and then perfused by intracardiac infusion of a solution containing 4% neutral paraformaldehyde in 0.9% sodium chloride and 10 mM sodium phosphate (pH 7), i.e., PBS. After harvesting, the brains were transferred to 30% sucrose in PBS for 2 days, frozen over liquid nitrogen, and stored at −80°C. For survival studies, rats were observed twice daily until they exhibited neurologic impairment (inability to feed, drink, or move), at which time they were euthanatized. Survival analysis was performed by employing the statistical software Microsoft Excel using Kaplan-Meier survival estimation and the Wilcoxon test for significance.
Histochemistry for virus distribution assay.
Brains and their tumors were analyzed by sectioning (20 μm thick) on a cryostat, and then the samples were air dried at room temperature. Sections were stained by histochemistry using the X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) substrate, as previously described (10), and were counterstained with neutral red.
Quantitative analysis of lacZ cDNA expression after virus injection.
Three randomly selected sections of brain tumors were selected from each animal and were analyzed using an Olympus BX60 microscope. The sections were scanned by Sony 3-chip Color Video Camera at ×20 magnification, and the entire tumor area and β-galactosidase-positive area were measured using Image Pro Plus Imaging Software. Selection of sections and scanning was performed by an observer (S.J.) blinded to the identity of the sections.
Analysis of viral genomes.
Two days after oncolytic virus administration, animals were sacrificed and genomic DNA was prepared from the brain tumor, brain tissue separate from the tumor, lung, liver, spleen, and kidney. PCR analysis was performed using primers specific for hrR3. The 5′ primer hybridizes to the 5′ region of the HSV ICP6 genome, and it is composed of sequence 5′-GAG GAC GAC TTT GGG CTT CT-3′. The 3′ primer hybridizes to the 5′ region of the lacZ cDNA, inserted in the ICP6 region of hrR3 (31), and its sequence is 5′-TCC CAC GCC ATC CCG CAT CT-3′. The resulting amplified product measures approximately 1,000 bp in length. After agarose gel electrophoresis, ethidium bromide staining, and transfer to nitrocellulose filters, Southern analysis of the PCR products was performed using a lacZ cDNA probe that hybridizes to the amplified PCR fragment. An enhanced chemiluminescence system (Amersham) was employed for nonradioactive probe labeling.
RESULTS
Effects of rat plasma on viral vector infectivity.
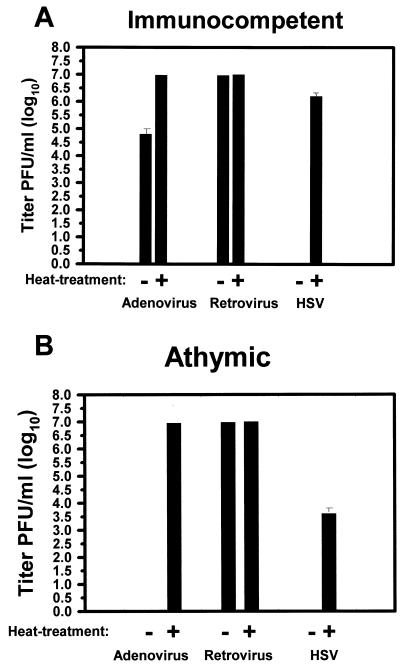
We sought to characterize the in vitro effect of undiluted plasma, harvested from immunocompetent and athymic rats on the infectivity of retrovirus, adenovirus, and HSV vectors expressing the lacZ reporter transgene. The replication-defective adenovirus-lacZ and murine retrovirus-lacZ cDNA were included in the assay as controls for specificity to HSV. Figure 1 shows that, for adenovirus incubated with immunocompetent rat plasma, the mean absolute reduction in titers was from 1 × 107 to 8 × 104 PFU/ml (a reduction of 99.2%, 125-fold, or 2.2 log10). For retrovirus there was no inhibition of infectivity. However, for the HSV mutant hrR3, infectivity was completely abrogated by preincubation with undiluted, untreated rodent plasma. There was no abrogation of infectivity in a parallel dish infected by hrR3, preincubated with HBSS (data not shown). As an additional control, preincubation with bovine serum albumin (10 mg/ml) did not inhibit virus infectivity, thus showing that the observed effect was not a nonspecific effect of serum proteins (data not shown).
FIG. 1.
The in vitro effect of control and heat-treated (complement-depleted) rodent plasma on virus vector infectivity of cells. Plasma was prepared from immunocompetent (A) and athymic (B) rats as described in Materials and Methods and either exposed to a temperature of 56°C for 30 min or not before incubation with an adenovirus, retrovirus, or HSV vector expressing the lacZ cDNA for 1.5 h. The viruses were then added onto Vero cells and, 16 h later, the number of lacZ cDNA-expressing cells was recorded. Values represent the mean titer of virus from triplicate dishes. In pilot experiments, virus preincubation with HBSS or with HBSS-bovine serum albumin (10 mg/ml) did not result in significant reductions of virus titers (data not shown). Error bars represent the standard error of the mean. If not shown, then the error bars were too small to graph.
One plasma candidate for this antiviral effect is complement. In fact, mild heat treatment, shown to inactivate complement (Table 1), completely reversed the antiadenovirus activity and partially reversed the anti-HSV activity of rodent plasma (Fig. 1). For HSV incubated with heat-inactivated immunocompetent rat plasma, the mean absolute reduction in titers was from 1 × 107 to 2.12 × 106 PFU/ml (an approximate reduction of 79.8%, fivefold, or 0.8 log10). For HSV incubated with heat-inactivated athymic rat plasma, the mean absolute reduction in titers was from 1 × 107 to 6 × 103 PFU/ml (a reduction of 99.94%, 1,667-fold, or 3.4 log10). Therefore, less HSV was inactivated after incubation with heat-treated plasma than after incubation with untreated plasma. Taken in conjunction, these experiments suggested that rodent complement inactivated HSV and adenovirus vectors, while, as expected, it did not affect retrovirus vectors (73). However, for HSV, because heat treatment only partially reversed plasma inactivation of the virus, complement-independent inactivation was also present, more so in athymic rats than in immunocompetent rats.
TABLE 1.
Complement function in athymic and immunocompetent rats
| Group | Complement function (U/ml) in ratsa
|
|
|---|---|---|
| Athymic | Immunocompetent | |
| Controlb | 393 | 396.5 |
| Heat treatmentc | 1 | 1 |
| CVFd | 35 | ND |
Values represent the average from two animals, as assayed by units per milliliter of 50% complement hemolytic activity. ND, not determined.
Control plasma was prepared from rodents as described in Materials and Methods.
Plasma from control rodents was exposed to 56°C for 30 min.
Athymic rats were injected with CVF at 60 and 20 U/Kg on two successive days. Plasma was prepared as described in Materials and Methods.
CVF, an in vivo depletor of complement, partially reverses the inactivation of HSV oncolytic viruses.
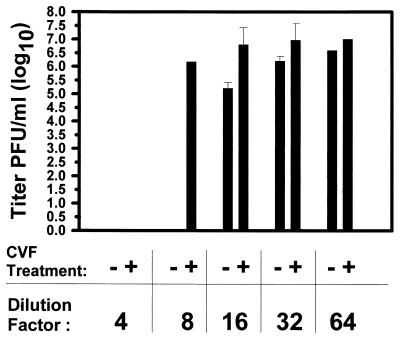
We then sought to characterize the highest dilution of plasma, prepared from naive athymic rats, which would inactivate the HSV viral vector, hrR3. Figure 2 shows that plasma neutralized approximately 80% of the virus at dilutions as high as 1:32 (from 1 × 107 to 2.1 × 106 PFU/ml), 98% of the virus at a dilution of 1:16 (from 1 × 107 to 2 × 105 PFU/ml), and 100% of the virus at lower dilutions. These results indicated that the antiviral action of plasma was present in relatively high concentrations.
FIG. 2.
Plasma anti-HSV activity and partial reversal by CVF. Plasma was prepared from athymic rats 48 h after intraperitoneal administration of CVF (or carrier) as detailed in Materials and Methods. After serial dilutions, it was mixed with oncolytic HSV before it was added onto Vero cells. Values represent the mean titer of virus from triplicate dishes. Controls, consisting of virus preincubated with HBSS for the same times before addition onto plates, did not show a reduction in mean titer (data not shown). Error bars represent the standard error of the mean. If not shown, then the error bars were too small to graph.
To provide further experimental evidence for the in vivo contribution of complement to this activity, athymic rats were treated with CVF whose C3 convertase action depletes complement levels in blood (4, 18, 32). Plasma harvested from these animals was not as effective in neutralizing hrR3, and the reversal of antiviral activity was evident even at a dilution of 1:8, where 83% of the virus was neutralized (from 1 × 107 to 1.7 × 106 PFU/ml) (Fig. 2). Taken in conjunction with the results described in Fig. 1, an in vivo role for complement inactivation of hrR3 appeared even more likely within the context of intravascular administration.
Strain differences in the inactivation of HSV by plasma and its reversal by CVF.
Since HSV is known to escape complement's antiviral action through glycoprotein C binding of C3 (50), we sought to determine if hrR3 was unusually sensitive to complement. We tested the inactivation of two other different HSV virus vectors that express the lacZ reporter transgene: MGH1 and an HSV amplicon. While hrR3 was derived from KOS strain by another laboratory (31), MGH1 was derived from F strain (42) and the HSV amplicon was packaged from strain 17+ by the authors of those studies (21, 62). For these experiments, results were graphed as the percentage of virus surviving after preincubation with plasma compared to a control in which virus was preincubated with buffer. Figure 3 shows that inactivation of all three virus vector strains was observed with diluted (1:8) athymic rat plasma and that this inactivation was partially reversed if plasma from animals treated with CVF was employed for the assay. The finding that three different HSV vectors derived from three distinct HSV strains in two different laboratories displayed relatively similar characteristics indicated that complement was relatively more effective against these three laboratory strains than was reported in previous publications, where complement effects were measured against a clinical isolate of HSV (27). Figure 3 also showed that there were differences among these three viral strains because the amplicon vector derived from strain 17+ was more resistant to plasma than the mutants derived from strain KOS and strain F.
Purified rat complement inhibits HSV vector infection of cells.
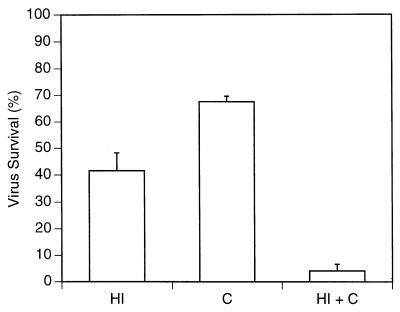
As further proof of complement's ability to inactivate hrR3, the virus was incubated with purified rat complement in serum-free medium and then added into cells in culture. Figure 4 shows that rat complement in serum-free medium partially inactivated the virus, from 2.25 × 106 to 1.52 × 106 PFU/ml (a reduction in titer of 33%, 1.5-fold, or 0.17 log10). Heat-inactivated plasma from athymic rats (diluted 1:4) also partially inactivated the virus, from 2.25 × 106 to 9.3 × 105 PFU/ml (a reduction in titer of 54%, 2.2-fold, or 0.35 log10). Re-addition of complement to the heat-inactivated plasma reduced virus titers from 2.25 × 106 to 9.1 × 104 PFU/ml (a reduction in titer of 96%, 25-fold, or 1.4 log10). These findings thus indicated that supplementation of heat-inactivated athymic rat plasma with purified rat complement could lead to a fairly effective barrier to hrR3 infection of cells.
FIG. 4.
Rat complement inactivates oncolytic HSV. The hrR3 mutant virus was preincubated with HBSS, purified rat complement (C; 1 mg/ml in HBSS), heat-inactivated rat plasma (HI; diluted 1:4), or rat complement re-added to heat-inactivated plasma (C + HI) for 1.5 h before adding it onto Vero cells in culture. The percent virus survival denotes the percentage of lacZ-expressing Vero cells (as enumerated 16 h after infection) compared to control dishes. Controls consisted of virus preincubated with HBSS for the same times before addition onto plates. In pilot experiments, virus preincubation with HBSS or with HBSS-bovine serum albumin (10 mg/ml) did not result in significant differences (data not shown). The bars represent the average from duplicate dishes, and the error bars represent the standard error of the mean.
CVF facilitates the transduction of multiple intracerebral tumors by the intravascular virus.
To show that CVF pretreatment depleted complement activity in rodents, blood was collected from animals, injected with 60 and 20 U of CVF per kg on two successive days. These dosages, this schedule, and the time of plasma harvesting were identical to the ones employed for the previous experiments and for subsequent experiments and did not result in clinical evidence of toxic side effects. Complement's hemolytic activity in these animals was significantly reduced compared to controls (Table 1). These findings thus indicated that CVF effectively eliminated complement function in animals.
In previous studies, we and others had shown that the combination of intra-arterial hrR3 and disruptors of the BBB or BTB, such as mannitol, bradykin, or RMP7, would lead to infection of a single syngeneic or xenogeneic tumor established in rodent brains (6, 56, 59). However, the number of positively transduced tumors was relatively low. The results described so far in this study indicated that animal pretreatment with CVF might increase the survival of hrR3 in rodent plasma and thus facilitate the transduction of an intracerebral tumor. In order to show the therapeutic power of this approach, we employed an animal model in which three separate and distinct tumor masses were established in the animal's brain. Athymic rats harboring three large and separate glioma tumors were thus pretreated with CVF (or saline) the day before and the day of intra-arterial injection with hrR3 (or mock injection) plus RMP7 (or vehicle). The anatomic extent of lacZ gene delivery within the three separate neoplasms was then measured 2 days later. Table 2 shows that complement depletion by CVF treatment of animals facilitated the transduction of three separate intracerebral tumors by intra-arterial hrR3. These findings thus confirmed that CVF pretreatment of rodents led to an increase in the number of positively transduced intracerebral tumors, probably through its depletion of complement.
TABLE 2.
Summary of lacZ cDNA transduction in each of three distinct intracerebral tumors at 2 days after the intravascular administration of hrR3 in the presence (+) or absence (−) of CVF and RMP7
| CVF | RMP7 | No. of positive tumors/ total no. of tumorsa | Avg % area of transduction in positive tumorsb |
|---|---|---|---|
| + | + | 10/12 | 3.2c |
| + | − | 5/9 | 3.5 |
| − | + | 1/9 | 2.3 |
| − | − | 0/9 | 0 |
Three tumors (right frontal and thalamic and left frontal) were established in each animal.
Percentages were calculated by computer-assisted analysis of lacZ-expressing tumor area, as determined 2 days after the intravascular administration of hrR3, divided by the total tumor area.
Values represent the mean transduced tumor area from five randomly selected sections analyzed in a blinded fashion by S.J.
CPA enhances the action of CVF.
The previous results showed that there was a significant increase in the number of transduced tumors 2 days after treatment with CVF and hrR3 (Table 2). However, when tumors were assayed for lacZ cDNA expression 4 days later, no further increases in the number of tumors nor increases in the area of intraneoplastic plaques (a marker of viral propagation) were observed (Table 3 and Fig. 5). We had previously shown that neutralizing (elicited) humoral responses could inhibit the propagation of hrR3 within transduced brain neoplasms; CPA pretreatment of rats partially suppressed these responses, permitting efficient and reliable oncolytic effects against multiple tumors by an intra-arterially administered virus (35). We thus sought to demonstrate if CVF and CPA would act in concert to anatomically increase the volume of tumor infected by the virus. For this experiment, CPA had to be administered 2 days after injection of hrR3 and CVF because same-day administration produced significant animal mortality. This time of CPA administration was different than that previously reported (36), when CPA was administered the same day as virus and not 2 days later, as in the present study. This regimen did result in a significant increase in the percent infection of the three intracerebral tumors by hrR3 in animals exposed to CVF followed 2 days later by CPA (Table 3). These results suggested a mechanism wherein the primary effect of CVF was to initially deplete complement, enhancing the initial infection of tumor cells by intravascular virus, while one of the actions of CPA was to inhibit both innate and elicited neutralizing humoral antiviral responses. This may have allowed for further viral propagation into the neoplasm.
TABLE 3.
Summary of lacZ cDNA transduction in each of three distinct intracerebral tumors at 4 days after the intravascular administration of hrR3 in the presence (+) or absence (−) of CVF and CPAa
| CVF | CPA | No. of positive tumors/ total no. of tumorsb | Avg % area of transduction in positive tumorsc |
|---|---|---|---|
| + | + | 8/9 | 64 |
| + | − | 5/9 | 1.2 |
| − | + | 1/9 | 45 |
| − | − | 0/9 | 0 |
All animals were treated with RMP7. CPA was administered 2 days after the intravascular injection of hrR3 and the second injection of CVF. The timing of CPA administration differs from the one previously reported (35), where CPA was administered the same day as virus, thus accounting for the difference in results.
Three tumors (right frontal and thalamic and left frontal) were established in each animal.
Percentages were calculated by computer-assisted analysis of lacZ-expressing tumor area, as determined 2 days after the intravascular administration of hrR3, divided by the total tumor area. Values represent the mean transduced tumor area from five randomly selected sections analyzed in a blinded fashion by S.J.
FIG. 5.
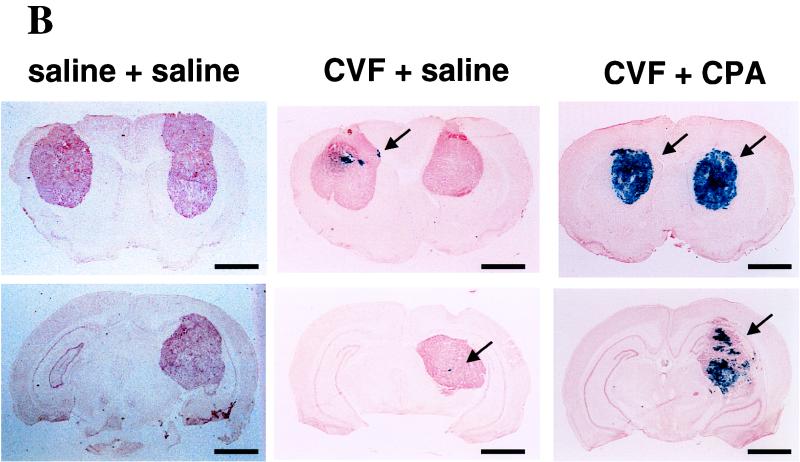
Histologic sections of brains with three neoplasms at 2 (A), 4 (B), and 8 (C) days after intravascular treatment with hrR3 in the presence or absence of CVF and CPA. Human U87dEGFR glioma cells were implanted into three separate intracerebral locations (right and left frontal lobes and right thalamus). Animals were treated with CVF (60 U/kg) or saline 6 days later and with another dose of CVF (20 U/kg) or saline 7 days later. At this time point, animals were treated with intravascular hrR3 and RMP7. Some animals also received an intraperitoneal injection of CPA (100 mg/kg) 2 days after intravascular treatment with hrR3. Animals were sacrificed 2, 4, and 8 days after virus administration, and their brains were harvested, sectioned, and stained for lacZ cDNA expression. The anatomic extent of tumor transduction was measured for each of the three neoplasms and tabulated in Table 3. Photomicrographs of sections showing brains with tumors stained for lacZ cDNA expression reveal transgene expression in a “plaque”-like configuration within tumors from CVF treated-animals at 2 days after administration of hrR3 (arrow in panel A). Four days later, the anatomic area of the lacZ-expressing plaques has not increased in the CVF-treated animals. However, the addition of CPA results in a significant augmentation of lacZ-expressing cells within the tumors (arrows in panel B). By 8 days, the oncolytic action of the virus has resulted in tumor involution (arrows in panel C). Bars, 4 mm.
Histologic analyses of brains harvested from treated animals provided some evidence for this mechanism. Figure 5 shows that CVF-treated animals harbored small lacZ-positive “plaques” within tumors 2 and 4 days after intra-arterial administration of hrR3, while control animals did not. Addition of CPA to the CVF treatment caused a significant increase in the size of lacZ-positive plaques that now appear to occupy the majority of each tumor. In fact, by day 8, involution and disappearance of tumors has occurred. These results thus show the facilitating action of CVF in providing for initial transduction, while CPA seemed to allow for subsequent propagation of the oncolytic virus within a neoplastic mass.
Survival analysis.
We then sought to characterize the anticancer effect of intra-arterial hrR3 after depletion of complement by CVF and inhibition of elicited and innate humoral responses by CPA. Figure 6 shows that the combination of intra-arterial hrR3, CVF, and CPA significantly increased the survival of athymic rats harboring three separate intracerebral human glioma xenografts compared to other treatments. Addition of RMP7 to this regimen increased this effect further, but not in a statistically significant fashion. These results thus suggested that strategies aimed at partially suppressing innate (complement) and elicited (neutralizing immunoglobulin) antiviral responses can significantly increase the oncolytic efficacy of an intravascular viral vector against multiple brain tumors.
FIG. 6.
Kaplan-Meier survival analyses of athymic rats harboring three separate human glioma xenografts. (A) Survival of rats injected with hrR3. (B) Survival of rats mock injected. Ten animals were treated in each group. Arrows indicate the time point of catheterization. In the hrR3-treated group, the differences in survival between animals treated with CVF+CPA versus CVF alone or CPA alone were both statistically significant (P < 0.001, Wilcoxon signed rank test). The addition of RMP7 to the hrR3+CVF+CPA treatment produced a slight increase in survival but was not statistically significant (P = 0.2). All treatments with hrR3 were significant compared to treatments without virus (P < 0.001).
Toxicity results.
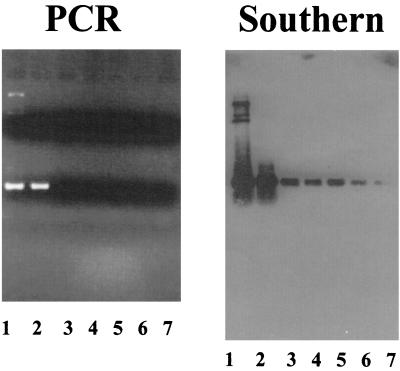
Although intra-arterial delivery can provide a route to target multiple tumor masses within an organ and inactivation of complement and humoral responses provided evidence of efficacy, it was important to determine if systemic infection with a replicating virus occurred in animals. There was no evidence of reporter transgene expression or viral antigen expression in the livers, lungs, spleens, kidneys, or brains of treated animals 4 days after intra-arterial administration of hrR3 (data not shown). PCR analysis revealed hrR3 viral genomes only in the brain tumor but not in contralateral brain, lung, liver, spleen, or kidney. However, when these PCR products were analyzed by Southern blotting, the presence of hrR3 genomes was evident in all organs (Fig. 7). This result thus showed that hrR3 genomes had established within the cells of these organs, although there was no evidence of active viral gene expression and replication within them.
FIG. 7.
Analysis of viral genomes in athymic rat tissues after intra-arterial administration of hrR3 in the presence of CVF and CPA. Genomic DNA was prepared from tissues of brain tumor (lane 2), brain surrounding the tumor (lane 3), lungs (lane 4), livers (lane 5), spleens (lane 6), and kidneys (lane 7) from athymic rats and was then analyzed by PCR using primers specific for hrR3. Lane 1 represents a positive control consisting of plasmid DNA containing the expected ICP6::lacZ cDNA sequence. The 5′ primer hybridizes to the 5′ region of the HSV ICP6 genome, and the 3′ primer hybridizes to the 5′ region of the inserted lacZ cDNA. After agarose gel electrophoresis and ethidium bromide staining (shown in the panel on the left), Southern analysis of the PCR products was performed using a lacZ cDNA probe that should hybridize to the amplified PCR fragments. The size of the PCR product is approximately 1,000 bp.
DISCUSSION
The primary objective of this report was to study the interaction of complement with virus vectors exposed to plasma. Delivery of such vectors through the vasculature may potentially target multiple and distinct tumor foci within an organ such as the brain. Because current gene- and virus-based therapies are commonly administered as intratumoral injections, they will necessarily remain limited to a local antitumor effect unless they elicit systemic anticancer immunity. Findings presented here demonstrate that (i) factors present in rat plasma are powerful inhibitors of viral vector infection of cells, (ii) one of the components in plasma responsible for this activity is complement, (iii) in vivo depletion of complement facilitates infection by an intra-arterial HSV of tumor cells located within three separate intracerebral neoplasms, and (iv) this depletion is not sufficient to allow for increased propagation of virus within tumors and additional treatment with CPA is needed to achieve anatomically extensive infection and propagation of oncolytic virus within tumors. These findings are relevant to our understanding of the interaction between oncolytic virus and the innate and elicited humoral immune response and how this interaction governs the process of viral infection of tumors and the subsequent propagation of progeny virions within the neoplastic mass.
In rats, pharmacologic or physical depletion of complement almost completely reversed plasma's inactivation of adenovirus and partially reversed the inactivation of HSV. However, complement is not the only innate anti-HSV factor in rat plasma. In fact, antibody-mediated depletion or neutralization of preimmune plasma IgM from athymic rats also led to a partial reversal of antiviral activity (35). The relevance of these findings in humans is under investigation, and additional studies in other species (such as mice) are needed to determine if the effects of complement on HSV vectors limit tumor transduction. However, even if the identity of innate anti-HSV factors was found to be different in humans, understanding the principles governing viral infection of and propagation within brain tumors in the animal model (athymic rat) would provide a significant benefit to the use of this method in the human setting.
We were initially surprised to find that undiluted rat plasma was able to completely inactivate hrR3. Previous reports have shown that the titer of a clinical isolate of HSV-1 (strain NS) was inhibited by normal human serum by only 0.3 log10 (twofold or 50%) (27). The infectivity of another virus strain (MP) was inhibited by normal human serum, diluted 1:3, by 35% (53). As stated by Friedman and colleagues: “Strain differences may be attributable to the ability of virus to activate complement or to the effectiveness of gC in modifying complement activation once it occurs” (27). These strain variations may thus explain the difference between complement effects against NS compared to the more pronounced effects against the three different laboratory strains (hrR3 [KOS], MGH1 [F], and amplicon [17+]) used in our work. Furthermore, there appear to be also species-specific differences, and HSV may not be able to evade rodent's complement as well as human's complement. Our results also indicate that very large differences may exist in HSV's ability to evade complement of immunocompetent versus athymic rodents.
Quantitatively, assuming that a rat's blood volume is approximately 20 ml and that we have saturated antiviral activity, then our assay says that at a dilution of plasma which abrogates antiviral activity (1:8), approximately 2 × 104 PFU of hrR3 are eliminated by 100 μl of plasma. Therefore, 20 ml of undiluted plasma might be expected to eliminate approximately 3.2 × 107 PFU of hrR3 if given as a single bolus. The observed inability of an intra-arterial bolus of 109 PFU of hrR3 to efficiently infect a brain neoplasm (35) still makes quantitative sense, taking into account that (i) over a 24-h time period circulating and/or infecting virus will be exposed to much higher volumes of plasma; (ii) physical barriers (such as the BBB and BTB and the splenic and hepatic trapping of circulating substances) will limit viral penetration into the tissue and organ; and (iii) innate antiviral responses, mediated by NK cells, neutrophils, and macrophages will also inactivate the virus. A threshold or “innate” barrier seems to exist to oncolytic virus infection of tumors, and one would predict that circumvention of this barrier requires either depleting one or more of its components or increasing the dose of injected virus. In fact, published and currently presented experimental evidence agree with this model. Evidence given here and previously (35) shows that the innate barrier can be lowered. Conversely, when higher doses of hrR3 (1010 instead of 109 PFU) were delivered intravascularly, an increase in anticancer efficacy was also observed.
In our studies, CVF increased the number of initially infected tumors and tumor cells 2 days after intravascular administration of oncolytic virus. However, 4 days later, there was no increase (and, in fact, by comparing the results in Table 2 versus those in Table 3, there was even a decrease) in the area of tumor transduction and in the size of the viral “plaques” within the tumors (compare the CVF-treated brains of Fig. 5A with those of Fig. 5B). This suggests that depletion of complement was sufficient to allow for the initial infection of tumor cells but was not sufficient to allow for subsequent rounds of viral propagation. In contrast, CPA does appear to possess two modes of action in this context: (i) although it does not deplete complement levels, it does inhibit complement antiviral function through the classical activation cascade; and (ii) at later time points (4 days), it inhibits the rise in neutralizing antibody titers (35). Based on these findings, we thus propose a model in which initial infection of brain tumors by intravascular virus limited by complement (and other blood components) can be circumvented by either depleting it with CVF or by inhibiting its function (with CPA). However, further propagation of virus from initially infected tumor cells becomes limited by the formation of neutralizing antibodies, a process that is abrogated by CPA (35). It should be noted that in the previous study (35) CPA was administered the same day as the virus. However, we could not administer CPA and CVF on the same day for the experiments described here because of animal toxicity. While CPA administration 2 days after CVF was not toxic, the effects of CPA alone at this later time point were not as significant in terms of tumor infection as those reported previously (35).
While the approach described here provide evidence of efficacy, as shown by the significant prolongation in the survival of animals harboring three intracerebral neoplasms, questions related to its safety do arise. In athymic rodents, we did not find evidence of oncolytic HSV replication in tissues other than the glioma, as evidenced by the lack of reporter transgene or HSV antigen expression. However, we did find viral genomes in cells from these organs, showing that infection of these tissues did occur. Since we are employing rodents for these studies, whose cells are notoriously impervious to HSV infection and replication, the definition of toxicity in rodents is not very reliable. Intravascular administration of a suitable oncolytic HSV in primate models of HSV toxicity will provide a more reliable measure of safety (34). Another issue relates to the safety of CVF, which specifically depletes the C3 component of complement (18). CVF is commonly and successfully used in animal models of xenotransplantation in order to avert the hyperacute rejection reaction caused by natural antibodies and complement (46, 69). In this regard, pharmacologic and monoclonal humoral anticomplement compounds are being developed and tested in clinical trials in humans to circumvent hyperacute immune rejection of xeno- and allotransplants (13, 14, 26, 41, 44, 54, 65, 66). The results given in present study tend to argue that intravascular, oncolytic HSV treatment of tumors will also be limited by a similar hyperacute rejection of the virus vector and of the initially infected tumor cells. Further clinical development of this approach may require the use of pharmacologic or monoclonal humoral methods to avert the innate immune response after appropriate safety testing in primate models.
The lytic ability of HSV is being harnessed as a novel cancer therapy. The primary issues that will affect the use of this virus as a clinically relevant anticancer agent are safety and efficacy. (i) Safety directly depends on the replication selectivity of the mutant virus. Several strategies exist to render HSV replication selective for tumor cells. Viral genes, needed for viral replication in postmitotic cells, can be deleted or mutated. Such genes encode enzymes that regulate nucleic acid metabolism in infected cells to allow for viral DNA synthesis (12, 52, 57). Another approach consists of deleting viral genes responsible for regulating viral progeny production, usually by modulation of the infected cell's apoptotic response (15, 37, 51). A third approach consists of using tumor-specific promoters to regulate the expression of essential viral genes (17). A fourth approach consists of altering the receptor specificity of HSV glycoproteins toward tumor rather than normal tissue (45). It is likely that a combination of these approaches may ultimately generate a series of oncolytic HSV that are extremely selective in their targeting of tumor cells. (ii) Efficacy depends on the ability of the virus to efficiently infect tumor cells and propagate within infected neoplastic masses. The results presented here and in an earlier publication (35) suggest that the mechanism of CPA action is not only as a direct antitumor agent but also as a facilitator of virus survival and propagation within infected tumors. The efficacy of an oncolytic HSV can also be augmented through its ability to deliver additional anticancer functions in infected cells. For instance, gene that encodes prodrug-activating enzymes can be engineered into the viral genome to combine a viral oncolytic and a chemotherapy-sensitizing effect (11, 12). We have recently shown that an oncolytic HSV can be used to deliver the CPA-susceptibility transgene, CYP2B1, thus producing an enhanced anticancer effect (16). These anticancer effects can be further enhanced by the addition of prodrug-activating genes that will pharmacologically synergize with CPA-CYP2B1 gene therapy (1). The ability to infect, transduce, and lyse multiple tumors within an organ such as the brain provides a first step towards rendering gene- or virus-based therapies useful applications for illnesses that are not currently treatable. Further elucidation of the mechanisms of viral passage from the vascular spaces into the tumor and of the effects of the early immune responses against the virus and virus-infected cells can provide refinement in this therapeutic strategy. If limited and transient manipulation of these innate and early humoral responses without alterations of more prolonged cellular responses provides an effective anticancer effect, it is possible that toxicity from a prolonged viral infection in tumors may be avoided.
ACKNOWLEDGMENTS
This work was supported by an NIH-NCI research grant (CA 69246). We also acknowledge the Berkowitz-Knott Fund for Brain Tumor Research at the Massachusetts General Hospital.
We thank R. Bartus (Alkermes, Inc.) for providing RMP7 (Cereport).
REFERENCES
- 1.Aghi M, Chou T C, Suling K, Breakefield X O, Chiocca E A. Multimodal cancer treatment mediated by a replicating oncolytic virus that delivers the oxazaphosphorine/rat cytochrome P450 2B1 and ganciclovir/herpes simplex virus thymidine kinase gene therapies. Cancer Res. 1999;59:3861–3865. [PubMed] [Google Scholar]
- 2.Anderson S C, Johnson D E, Harris M P, Engler H, Hancock W, Huang W M, Wills K N, Gregory R J, Sutjipto S, Wen S F, Lofgren S, Shepard H M, Maneval D C. p53 gene therapy in a rat model of hepatocellular carcinoma: intra-arterial delivery of a recombinant adenovirus. Clin Cancer Res. 1998;4:1649–1659. [PubMed] [Google Scholar]
- 3.Ash R J. Butyrate-induced reversal of herpes simplex virus restriction in neuroblastoma cells. Virology. 1986;155:584–592. doi: 10.1016/0042-6822(86)90218-7. [DOI] [PubMed] [Google Scholar]
- 4.Ballow M, Cochrane C G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969;103:944–952. [PubMed] [Google Scholar]
- 5.Barnard R O, Geddes J F. The incidence of multifocal cerebral gliomas. A histologic study of large hemisphere sections. Cancer. 1987;60:1519–1531. doi: 10.1002/1097-0142(19871001)60:7<1519::aid-cncr2820600719>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 6.Barnett F H, Rainov N G, Ikeda K, Schuback D E, Elliott P, Kramm C M, Chase M, Qureshi N H, Harsh G T, Chiocca E A, Breakefield X O. Selective delivery of herpes virus vectors to experimental brain tumors using RMP-7. Cancer Gene Ther. 1999;6:14–20. doi: 10.1038/sj.cgt.7700003. [DOI] [PubMed] [Google Scholar]
- 7.Bartus R T, Elliott P J, Dean R L, Hayward N J, Nagle T L, Huff M R, Snodgrass P A, Blunt D G. Controlled modulation of BBB permeability using the bradykinin agonist, RMP-7. Exp Neurol. 1996;142:14–28. doi: 10.1006/exnr.1996.0175. [DOI] [PubMed] [Google Scholar]
- 8.Berger M S, Prados M, VanGilder J C, et al. Gene therapy for the treatment of recurrent glioblastoma multiforme with in vivo transduction using the herpes simplex-thymidine kinase gene/ganciclovir system. J Neurosurg. 1997;1997:378A. [Google Scholar]
- 9.Bischoff J R, Kirn D H, Williams A, Heise C, Horn S, Muna M, Ng L, Nye J A, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 10.Boviatsis E J, Chase M, Wei M X, Tamiya T, Hurford R K, Jr, Kowall N W, Tepper R I, Breakefield X O, Chiocca E A. Gene transfer into experimental brain tumors mediated by adenovirus, herpes simplex virus, and retrovirus vectors. Hum Gene Ther. 1994;5:183–191. doi: 10.1089/hum.1994.5.2-183. [DOI] [PubMed] [Google Scholar]
- 11.Boviatsis E J, Park J S, Sena-Esteves M, Kramm C M, Chase M, Efird J T, Wei M X, Breakefield X O, Chiocca E A. Long-term survival of rats harboring brain neoplasms treated with ganciclovir and a herpes simplex virus vector that retains an intact thymidine kinase gene. Cancer Res. 1994;54:5745–5751. [PubMed] [Google Scholar]
- 12.Boviatsis E J, Scharf J M, Chase M, Harrington K, Kowall N W, Breakefield X O, Chiocca E A. Antitumor activity and reporter gene transfer into rat brain neoplasms inoculated with herpes simplex virus vectors defective in thymidine kinase or ribonucleotide reductase. Gene Ther. 1994;1:323–331. [PubMed] [Google Scholar]
- 13.Candinas D, Lesnikoski B A, Hancock W W, Otsu I, Koyamada N, Dalmasso A P, Robson S C, Bach F H. Inhibition of platelet integrin GPIIbIIIa prolongs survival of discordant cardiac xenografts. Transplantation. 1996;62:1–5. doi: 10.1097/00007890-199607150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Candinas D, Lesnikoski B A, Robson S C, Miyatake T, Scesney S M, Marsh H C, Jr, Ryan U S, Dalmasso A P, Hancock W W, Bach F H. Effect of repetitive high-dose treatment with soluble complement receptor type 1 and cobra venom factor on discordant xenograft survival. Transplantation. 1996;62:336–342. doi: 10.1097/00007890-199608150-00006. [DOI] [PubMed] [Google Scholar]
- 15.Chambers R, Gillespie G Y, Soroceanu L, Andreansky S, Chatterjee S, Chou J, Roizman B, Whitley R J. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci USA. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chase M, Chung R Y, Chiocca E A. An oncolytic viral mutant that delivers the CYP2B1 transgene and augments cyclophosphamide chemotherapy. Nat Biotechnol. 1998;16:444–448. doi: 10.1038/nbt0598-444. [DOI] [PubMed] [Google Scholar]
- 17.Chung R Y, Saeki Y, Chiocca E A. B-myb promoter retargeting of herpes simplex virus gamma34.5 gene-mediated virulence toward tumor and cycling cells. J Virol. 1999;73:7556–7564. doi: 10.1128/jvi.73.9.7556-7564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cochrane C G, Muller-Eberhard H J, Aikin B S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970;105:55–69. [PubMed] [Google Scholar]
- 19.Coffey M C, Strong J E, Forsyth P A, Lee P W. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–1334. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 20.Costa J, Rabson A S, Yee C, Tralka T S. Immunoglobulin binding to herpes virus-induced Fc receptors inhibits virus growth. Nature. 1977;269:251–252. doi: 10.1038/269251a0. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham C, Davison A J. A cosmid-based system for constructing mutants of herpes simplex virus type 1. Virology. 1993;197:116–124. doi: 10.1006/viro.1993.1572. [DOI] [PubMed] [Google Scholar]
- 22.Dubin G, Basu S, Mallory D L, Basu M, Tal-Singer R, Friedman H M. Characterization of domains of herpes simplex virus type 1 glycoprotein E involved in Fc binding activity for immunoglobulin G aggregates. J Virol. 1994;68:2478–2485. doi: 10.1128/jvi.68.4.2478-2485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubin G, Fishman N O, Eisenberg R J, Cohen G H, Friedman H M. The role of herpes simplex virus glycoproteins in immune evasion. Curr Top Microbiol Immunol. 1992;179:111–120. doi: 10.1007/978-3-642-77247-4_7. [DOI] [PubMed] [Google Scholar]
- 24.Elliott P J, Hayward N J, Huff M R, Nagle T L, Black K L, Bartus R T. Unlocking the blood-brain barrier: a role for RMP-7 in brain tumor therapy. Exp Neurol. 1996;141:214–224. doi: 10.1006/exnr.1996.0156. [DOI] [PubMed] [Google Scholar]
- 25.Emerich D F, Snodgrass P, Dean R, Agostino M, Hasler B, Pink M, Xiong H, Kim B S, Bartus R T. Enhanced delivery of carboplatin into brain tumours with intravenous Cereport (RMP-7): dramatic differences and insight gained from dosing parameters. Br J Cancer. 1999;80:964–970. doi: 10.1038/sj.bjc.6690450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferran C, Stroka D M, Badrichani A Z, Cooper J T, Wrighton C J, Soares M, Grey S T, Bach F H. A20 inhibits NF-kappaB activation in endothelial cells without sensitizing to tumor necrosis factor-mediated apoptosis. Blood. 1998;91:2249–2258. [PubMed] [Google Scholar]
- 27.Friedman H M, Wang L, Fishman N O, Lambris J D, Eisenberg R J, Cohen G H, Lubinski J. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J Virol. 1996;70:4253–4260. doi: 10.1128/jvi.70.7.4253-4260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geller A I, Freese A. Infection of cultured central nervous system neurons with a defective herpes simplex virus 1 vector results in stable expression of Escherichia coli beta-galactosidase. Proc Natl Acad Sci USA. 1990;87:1149–1153. doi: 10.1073/pnas.87.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldsmith K, Chen W, Johnson D C, Hendricks R L. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T-cell response. J Exp Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein D J, Weller S K. Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology. 1988;166:41–51. doi: 10.1016/0042-6822(88)90144-4. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein D J, Weller S K. An ICP6::lacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J Virol. 1988;62:2970–2977. doi: 10.1128/jvi.62.8.2970-2977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofmann C, Strauss M. Baculovirus-mediated gene transfer in the presence of human serum or blood facilitated by inhibition of the complement system. Gene Ther. 1998;5:531–536. doi: 10.1038/sj.gt.3300607. [DOI] [PubMed] [Google Scholar]
- 33.Huemer H P, Menzel H J, Potratz D, Brake B, Falke D, Utermann G, Dierich M P. Herpes simplex virus binds to human serum lipoprotein. Intervirology. 1988;29:68–76. doi: 10.1159/000150031. [DOI] [PubMed] [Google Scholar]
- 34.Hunter W D, Martuza R L, Feigenbaum F, Todo T, Mineta T, Yazaki T, Toda M, Newsome J T, Platenberg R C, Manz H J, Rabkin S D. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol. 1999;73:6319–6326. doi: 10.1128/jvi.73.8.6319-6326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda K, Ichikawa T, Wakimoto H, Silver J, Deisboeck T, Finkelstein D, Harsh G I, Louis D, Bartus R, Hochberg F, Chiocca E. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–889. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 36.Johansson P J, Kjellen L. Inhibition of herpes simplex virus growth caused by preparations of animal immunoglobulins is not dependent on Fc-Fc receptor interactions. Intervirology. 1988;29:334–338. doi: 10.1159/000150064. [DOI] [PubMed] [Google Scholar]
- 37.Kesari S, Randazzo B P, Valyi-Nagy T, Huang Q S, Brown S M, MacLean A R, Lee V M, Trojanowski J Q, Fraser N W. Therapy of experimental human brain tumors using a neuroattenuated herpes simplex virus mutant. Lab Investig. 1995;73:636–648. [PubMed] [Google Scholar]
- 38.Kirn D, Hermiston T, McCormick F. ONYX-015: clinical data are encouraging [letter; comment] Nat Med. 1998;4:1341–2. doi: 10.1038/3902. [DOI] [PubMed] [Google Scholar]
- 39.Kodukula P, Liu T, Rooijen N V, Jager M J, Hendricks R L. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol. 1999;162:2895–2905. [PubMed] [Google Scholar]
- 40.Kohl S. Role of antibody-dependent cellular cytotoxicity in defense against herpes simplex virus infections. Rev Infect Dis. 1991;13:108–114. doi: 10.1093/clinids/13.1.108. [DOI] [PubMed] [Google Scholar]
- 41.Koyamada N, Miyatake T, Candinas D, Hechenleitner P, Siegel J, Hancock W W, Bach F H, Robson S C. Apyrase administration prolongs discordant xenograft survival. Transplantation. 1996;62:1739–1743. doi: 10.1097/00007890-199612270-00008. [DOI] [PubMed] [Google Scholar]
- 42.Kramm C M, Chase M, Herrlinger U, Jacobs A, Pechan P A, Rainov N G, Sena-Esteves M, Aghi M, Barnett F H, Chiocca E A, Breakefield X O. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum Gene Ther. 1997;8:2057–2068. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- 43.Kroll R A, Neuwelt E A. Outwitting the blood-brain barrier for therapeutic purposes: osmotic opening and other means. Neurosurgery. 1998;42:1083–1100. doi: 10.1097/00006123-199805000-00082. [DOI] [PubMed] [Google Scholar]
- 44.Kroshus T J, Rollins S A, Dalmasso A P, Elliott E A, Matis L A, Squinto S P, Bolman R M., III Complement inhibition with an anti-C5 monoclonal antibody prevents acute cardiac tissue injury in an ex vivo model of pig-to-human xenotransplantation. Transplantation. 1995;60:1194–1202. [PubMed] [Google Scholar]
- 45.Laquerre S, Anderson D B, Stolz D B, Glorioso J C. Recombinant herpes simplex virus type 1 engineered for targeted binding to erythropoietin receptor-bearing cells. J Virol. 1998;72:9683–9697. doi: 10.1128/jvi.72.12.9683-9697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leventhal J R, Dalmasso A P, Cromwell J W, Platt J L, Manivel C J, Bolman R M D, Matas A J. Prolongation of cardiac xenograft survival by depletion of complement. Transplantation. 1993;55:857–866. doi: 10.1097/00007890-199304000-00033. [DOI] [PubMed] [Google Scholar]
- 47.Lorence R M, Katubig B B, Reichard K W, Reyes H M, Phuangsab A, Sassetti M D, Walter R J, Peeples M E. Complete regression of human fibrosarcoma xenografts after local Newcastle disease virus therapy. Cancer Res. 1994;54:6017–6021. [PubMed] [Google Scholar]
- 48.Lorence R M, Reichard K W, Katubig B B, Reyes H M, Phuangsab A, Mitchell B R, Cascino C J, Walter R J, Peeples M E. Complete regression of human neuroblastoma xenografts in athymic mice after local Newcastle disease virus therapy. J Natl Cancer Inst. 1994;86:1228–1233. doi: 10.1093/jnci/86.16.1228. [DOI] [PubMed] [Google Scholar]
- 49.Lubinski J, Nagashunmugam T, Friedman H M. Viral interference with antibody and complement. Semin Cell Dev Biol. 1998;9:329–337. doi: 10.1006/scdb.1998.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lubinski J M, Wang L, Soulika A M, Burger R, Wetsel R A, Colten H, Cohen G H, Eisenberg R J, Lambris J D, Friedman H M. Herpes simplex virus type 1 glycoprotein gC mediates immune evasion in vivo. J Virol. 1998;72:8257–8263. doi: 10.1128/jvi.72.10.8257-8263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markert J M, Malick A, Coen D M, Martuza R L. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Martuza R L, Malick A, Markert J M, Ruffner K L, Coen D M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 53.McNearney T A, Odell C, Holers V M, Spear P G, Atkinson J P. Herpes simplex virus glycoproteins gC-1 and gC-2 bind to the third component of complement and provide protection against complement-mediated neutralization of viral infectivity. J Exp Med. 1987;166:1525–1535. doi: 10.1084/jem.166.5.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyatake T, Koyamada N, Hancock W W, Soares M P, Bach F H. Survival of accommodated cardiac xenografts upon retransplantation into cyclosporine-treated recipients. Transplantation. 1998;65:1563–1569. doi: 10.1097/00007890-199806270-00005. [DOI] [PubMed] [Google Scholar]
- 55.Nagane M, Coufal F, Lin H, Bogler O, Cavenee W K, Huang H J. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 56.Nilaver G, Muldoon L L, Kroll R A, Pagel M A, Breakefield X O, Davidson B L, Neuwelt E A. Delivery of herpesvirus and adenovirus to nude rat intracerebral tumors after osmotic blood-brain barrier disruption. Proc Natl Acad Sci USA. 1995;92:9829–9833. doi: 10.1073/pnas.92.21.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pyles R B, Warnick R E, Chalk C L, Szanti B E, Parysek L M. A novel multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum Gene Ther. 1997;8:533–544. doi: 10.1089/hum.1997.8.5-533. [DOI] [PubMed] [Google Scholar]
- 58.Rainov N G, Dobberstein K U, Heidecke V, Dorant U, Chase M, Kramm C M, Chiocca E A, Breakefield X O. Long-term survival in a rodent brain tumor model by bradykinin-enhanced intra-arterial delivery of a therapeutic herpes simplex virus vector. Cancer Gene Ther. 1998;5:158–162. [PubMed] [Google Scholar]
- 59.Rainov N G, Zimmer C, Chase M, Kramm C M, Chiocca E A, Weissleder R, Breakefield X O. Selective uptake of viral and monocrystalline particles delivered intra-arterially to experimental brain neoplasms. Hum Gene Ther. 1995;6:1543–1552. doi: 10.1089/hum.1995.6.12-1543. [DOI] [PubMed] [Google Scholar]
- 60.Ram Z, Culver K W, Oshiro E M, Viola J J, DeVroom H L, Otto E, Long Z, Chiang Y, McGarrity G J, Muul L M, Katz D, Blaese R M, Oldfield E H. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 61.Roth J A, Swisher S G, Merritt J A, Lawrence D D, Kemp B L, Carrasco C H, El-Naggar A K, Fossella F V, Glisson B S, Hong W K, Khurl F R, Kurie J M, Nesbitt J C, Pisters K, Putnam J B, Schrump D S, Shin D M, Walsh G L. Gene therapy for non-small cell lung cancer: a preliminary report of a phase I trial of adenoviral p53 gene replacement. Semin Oncol. 1998;25:33–37. [PubMed] [Google Scholar]
- 62.Saeki Y, Ichikawa T, Saeki A, Chiocca E A, Tobler K, Ackermann M, Breakefield X O, Fraefel C. Herpes simplex virus type 1 DNA amplified as bacterial artificial chromosome in Escherichia coli: rescue of replication-competent virus progeny and packaging of amplicon vectors. Hum Gene Ther. 1998;9:2787–2794. doi: 10.1089/hum.1998.9.18-2787. [DOI] [PubMed] [Google Scholar]
- 63.Silbergeld D L, Rostomily R C, Alvord E C., Jr The cause of death in patients with glioblastoma is multifactorial: clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults. J Neurooncol. 1991;10:179–185. doi: 10.1007/BF00146880. [DOI] [PubMed] [Google Scholar]
- 64.Smiley M L, Friedman H M. Binding of complement component C3b to glycoprotein C is modulated by sialic acid on herpes simplex virus type 1-infected cells. J Virol. 1985;55:857–861. doi: 10.1128/jvi.55.3.857-861.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soares M P, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey S T, Colvin R B, Choi A M, Poss K D, Bach F H. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 66.Soares M P, Muniappan A, Kaczmarek E, Koziak K, Wrighton C J, Steinhauslin F, Ferran C, Winkler H, Bach F H, Anrather J. Adenovirus-mediated expression of a dominant negative mutant of p65/RelA inhibits proinflammatory gene expression in endothelial cells without sensitizing to apoptosis. J Immunol. 1998;161:4572–4582. [PubMed] [Google Scholar]
- 67.Srinivas R V, Birkedal B, Owens R J, Anantharamaiah G M, Segrest J P, Compans R W. Antiviral effects of apolipoprotein A-1 and its synthetic amphipathic peptide analogs. Virology. 1990;176:48–57. doi: 10.1016/0042-6822(90)90229-k. [DOI] [PubMed] [Google Scholar]
- 68.Srinivas R V, Venkatachalapathi Y V, Rui Z, Owens R J, Gupta K B, Srinivas S K, Anantharamaiah G M, Segrest J P, Compans R W. Inhibition of virus-induced cell fusion by apolipoprotein A-I and its amphipathic peptide analogs. J Cell Biochem. 1991;45:224–237. doi: 10.1002/jcb.240450214. [DOI] [PubMed] [Google Scholar]
- 69.Suh C H, Oaks M K, Dong N N, Pellegrini J G, Kress D C, Tector A J. Preoperative depletion of C3 improves the survival of guinea pig-to-rat cardiac xenograft recipients. J Investig Surg. 1997;10:37–40. doi: 10.3109/08941939709032123. [DOI] [PubMed] [Google Scholar]
- 70.Thormar H, Isaacs C E, Brown H R, Barshatzky M R, Pessolano T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1987;31:27–31. doi: 10.1128/aac.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toda M, Rabkin S D, Kojima H, Martuza R L. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–393. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 72.Wallner K E, Galicich J H, Krol G, Arbit E, Malkin M G. Patterns of failure following treatment for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1989;16:1405–1409. doi: 10.1016/0360-3016(89)90941-3. [DOI] [PubMed] [Google Scholar]
- 73.Welsh R M, O'Donnell C L, Reed D J, Rother R P. Evaluation of the Galα1-3Gal epitope as a host modification factor eliciting natural humoral immunity to enveloped viruses. J Virol. 1998;72:4650–4656. doi: 10.1128/jvi.72.6.4650-4656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon S, Chiocca E A, Tanabe K. Treatment of multiple liver metastases by intravascular delivery of an oncolytic HSV. FASEB J. 2000;14:301–311. [PubMed] [Google Scholar]