Abstract
The brain constantly compares past and present experiences to predict the future, thereby enabling instantaneous and future behavioral adjustments. Integration of external information with the animal's current internal needs and behavioral state represents a key challenge of the nervous system. Recent advancements in dissecting the function of the Drosophila mushroom body (MB) at the single-cell level have uncovered its three-layered logic and parallel systems conveying positive and negative values during associative learning. This review explores a lesser-known role of the MB in detecting and integrating body states such as hunger, thirst, and sleep, ultimately modulating motivation and sensory-driven decisions based on the physiological state of the fly. State-dependent signals predominantly affect the activity of modulatory MB input neurons (dopaminergic, serotoninergic, and octopaminergic), but also induce plastic changes directly at the level of the MB intrinsic and output neurons. Thus, the MB emerges as a tightly regulated relay station in the insect brain, orchestrating neuroadaptations due to current internal and behavioral states leading to short- but also long-lasting changes in behavior. While these adaptations are crucial to ensure fitness and survival, recent findings also underscore how circuit motifs in the MB may reflect fundamental design principles that contribute to maladaptive behaviors such as addiction or depression-like symptoms.
To survive and thrive in challenging environments, the brain is constantly comparing the past and the present trying to predict the future. Doing so efficiently requires the nervous system to filter and integrate important information about the environment and the animal's physiological state on a moment-to-moment scale to adapt instantaneous and future behavior.
What happens in our brains when we are hungry, thirsty, or tired? And where and how is information about the external world integrated to modulate behavior according to our needs? Research on state-dependent plasticity in the insect brain has provided important insights to these questions. In addition to its well-known role in associative learning and memory, the mushroom body (MB) acts as a dynamic integrator of past and current (multi)sensory experience with physiological and behavioral states, and thereby dynamically influences a wide range of essential behaviors. In the past decade, the MB has been anatomically and functionally dissected to a single-cell level and our understanding of the circuit mechanisms underpinning behavioral adaptation has advanced immensely (Aso et al. 2014; Owald et al. 2015; Li et al. 2020a).
The general logic of the MB is composed of three layers. The intrinsic layer comprises the cholinergic Kenyon cells (KCs), which form five different lobes with their axons (γ, α/β, and α′/β′) and can be further subdivided into nine subtypes according to more detailed morphological and molecular analysis (see Figs. 1 and 2). The output layer includes about 20 MB output neurons (MBONs), which are GABAergic, glutamatergic, or cholinergic. The input layer predominantly contains dopaminergic neurons (DANs), but also different aminergic and peptidergic circuitries (Riemensperger et al. 2005; Mao and Davis 2009; Pech et al. 2013; Perisse et al. 2013; Aso et al. 2014; Yang et al. 2016; Li et al. 2020a; Scaplen et al. 2021). Here there are, at least at first approximation, two parallel systems in place: one conveying negative value (via PPL1 DANs) and the other conveying positive value (via PAM DANs) (Schwaerzel et al. 2003; Aso et al. 2012; Burke et al. 2012; Liu et al. 2012; Huetteroth et al. 2015; Yamagata et al. 2015). Associative memories or concurrent experience induce short- or long-term changes in synaptic weight at the output level, shifting the system either toward approach or avoidance behavior (Séjourné et al. 2011; Bräcker et al. 2013; Plaçais et al. 2013; Bouzaiane et al. 2015; Hige et al. 2015; Ichinose et al. 2015; Lewis et al. 2015; Owald et al. 2015; Perisse et al. 2016; Felsenberg et al. 2017, 2018; Berry et al. 2018; Handler et al. 2019; Zhang et al. 2019; Bilz et al. 2020; Li et al. 2020a; Ichinose et al. 2021; McCurdy et al. 2021; Villar et al. 2022).
Figure 1.
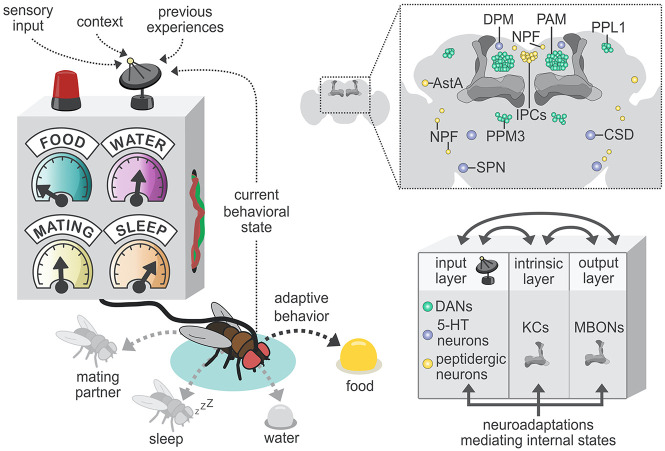
The MB as a panel control of body states. (Left) The MB integrates sensory information about the environment and the current context and behavioral state to regulate sensory valence according to the physiological needs of the fly in a moment-to-moment basis. As different internal states are being signaled in common or interconnected circuitries, this supports a tight regulation by body needs of the expression of the behavior that is most adaptive at each moment (e.g., eat and remember associated cues when starving). (Right, top and bottom) Information about the environment and behavioral state is sensed and signaled to the intrinsic and output layers of the MB (comprised of KCs and MB MBONs, respectively) through the input layer. The input layer contains a broad array of circuitries distributed all over the brain, including dopaminergic (DANs; green), serotonergic (5-HT; purple), and peptidergic (such as allatostatin A [AstA] and neuropeptide-F [NPF]; yellow) populations. The full name of these neural populations can be found in the text. (Bottom right) Short-lived and long-lasting plastic changes at the level of the three MB layers allow the fly to adapt its behavior instantaneously and in the future based on previous experience. Recurrent connectivity between the input, intrinsic, and output circuitries further potentiates plasticity across MB layers.
Figure 2.
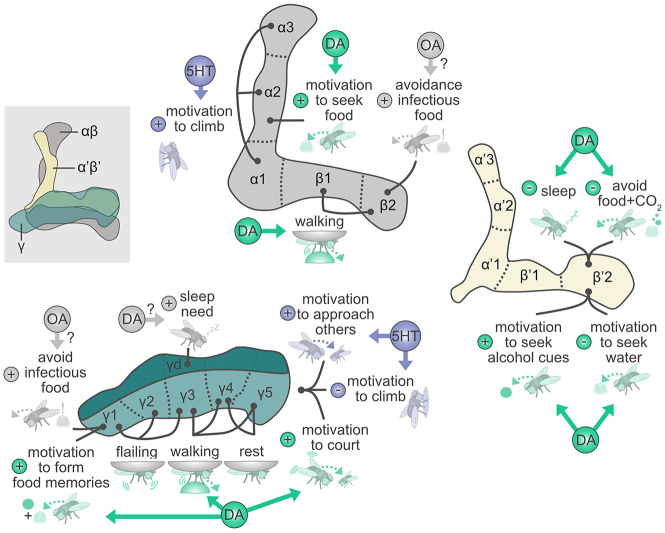
Complex integration of neuromodulatory signals by the MB mediates labile and enduring behavioral changes. Neuromodulators such as dopamine (DA), serotonin (5HT), and octopamine (OA) are signaled to specific MB lobes, allowing for representations of the behavioral state and behavioral adaptations. DAN and MBON innervations divide the MB lobes into 15 compartments and each type of DAN and MBON projecting to a specific compartment is named accordingly. The same compartment and circuits can integrate different signals to regulate distinct responses (e.g., γ5 mediating changes in climbing behavior and motivation to court in response to dopamine and serotonin, respectively). The modulation of different needs and behaviors by interconnected or common circuits might facilitate complex regulatory interactions (e.g., changes in activity in β′2 regulating arousal might affect the consolidation of ethanol memories). Arrows not linked to any compartment in the γ lobe denote not known compartment assignment. For simplicity, some KC subtypes are not shown. Based on data from Krashes et al. (2009), Keleman et al. (2012), Bräcker et al. (2013), Cohn et al. (2015), Musso et al. (2015), Sitaraman et al. (2015a,b), Perisse et al. (2016), Ries et al. (2017), Sayin et al. (2019), Senapati et al. (2019), Kobler et al. (2020), Scaplen et al. (2020), Sun et al. (2020), and Zolin et al. (2021). Please note that representative examples are shown due to space constraints. For a more detailed overview of behaviors, states, and circuits involved in different behavioral adaptations, see Supplemental Table S1.
Sophisticated behavioral assays, as well as advanced in vivo preparations for detailed multiphoton or fast whole-brain imaging, now allow for further unprecedented insights into behavioral and physiological adaptations in the behaving fly. In this review, we discuss recent important findings of the circuit mechanisms enabling the MB to detect and integrate important needs of the body such as eating, sleeping, and mating, thereby modulating sensory-driven decisions depending on the internal state or goals of the animal. While such state-dependent signals can act at all three layers, most of the state signals appear to affect DANs activity and thereby, change how positive and negative signals are conveyed—or not—to the MB (Krashes et al. 2009; Bräcker et al. 2013; Lin et al. 2014; Cohn et al. 2015; Lewis et al. 2015; Tsao et al. 2018; Siju et al. 2020; Zolin et al. 2021). However, other inputs to the MB can also be targeted by state-dependent modulation, such as serotoninergic (Keene et al. 2006; Haynes et al. 2015; Ries et al. 2017; Turrel et al. 2018; Scheunemann et al. 2019) and octopaminergic signals (Sayin et al. 2019; Kobler et al. 2020; Hermanns et al. 2022), as well as plasticity parameters directly at the KC or MBON level (Plaçais et al. 2017; Handler et al. 2019; Lee et al. 2021). Here, we review these and other state-dependent changes and the potential mechanisms by which they may affect behavior. We further discuss how these adaptations correlate with the current behavioral state of the animal and with long-lasting maladaptive states such as addiction and depression. By doing so, we highlight the role of the MB and connected circuits as a tightly regulated control hub of internal states in the fly brain (Fig. 1).
The social brain
The mushroom body modulates reproductive state-dependent choices and memories
Reproduction is a potent drive causing fundamental adaptations in animal behavior for finding suitable mating partners, executing successful copulation, and ensuring the fitness of the offspring. Naturally, this involves distinct adaptations in males and females. While hormones and neuromodulators act at several levels (e.g., olfactory and auditory pathways) (Auer and Benton 2016; Sayin et al. 2018), the convergence of external and internal signals at the MB circuit might allow for the optimization of goal-directed behavior in different contexts. At the level of the MB, changes in the mating state are integrated mainly at the dopamine input layer (Keleman et al. 2012; Azanchi et al. 2013; Zhao et al. 2018; Siju et al. 2020, 2021; Boehm et al. 2022), subsequently routing behavioral output by transiently or constantly changing plasticity at KC to MBON synapses.
Mating experience changes how males and females perceive their environment and members of their own species. One evident example of how mating status affects fly behavior is the transition of previously receptive females into a long-lasting state of rejection of copulation after the first mating encounter (Connolly and Cook 1973; Dickson 2008; Rezával et al. 2012). This, in turn, affects male fly courtship behavior by reducing courtship attempts toward mated females, presumably to avoid futile attempts and waste of resources. A key neuromodulator underlying this behavioral change is the sex pheromone 11-cis-Vaccenyl acetate (cVA) produced by males and transferred to females during mating (Butterworth 1969; Ejima and Griffith 2007; Datta et al. 2008; Griffith and Ejima 2009; Everaerts et al. 2010; Keleman et al. 2012; Montague and Baker 2016; Lim et al. 2018). In males, specific DANs (aSP13 and PAM cluster) lastingly change their response magnitude to future cVA encounters, which is integrated via the dopamine receptor 1 (Dop1R1) in downstream KCs and aversion-mediating MBONs (Keleman et al. 2012; Montague and Baker 2016). Such an experience-dependent change in response to pheromones could, in part, also induce female rejection behavior toward males after the first mating encounter. In line with this idea, mated females show a reduced response to cVA at the level of the antennal lobe (AL; DA1 glomerulus) (Lebreton et al. 2014). Although it is not understood how this could translate into persistent changes in cVA responses across MB layers, mating-dependent alterations in cVA responses in aversion-mediating MBONs might be a common feature in adapting male and female behavior according to mating state (Lebreton et al. 2014; Boehm et al. 2022).
Apart from inducing changes in mating behavior, cVA-dependent plasticity in DANs (β′1) might trigger an adaptation in odor-mediated food choices of females after mating. In virgins, avoidance-inducing PPL1 DANs (e.g., PPL1-α2α′2) reduce the olfactory preference for a highly nutritious food component, polyamine. Upon mating, females are attracted to polyamine odor through neuropeptidergic modulation of polyamine-sensitive olfactory sensory neurons and increased activity of PAM-β′1 DANs, which in turn reduces polyamine aversion through β′2 MBONs (Hussain et al. 2016a,b; Boehm et al. 2022). This behavioral adaptation also comprises the other higher-order olfactory center, the lateral horn (LH). In mated females, PPL1 DANs no longer counteract polyamine attraction, possibly through the modulation of MBON-α2sc and its direct connection to specific LH neurons.
In addition to dopamine, octopamine in males was shown to be important for female rejection-mediated courtship suppression (Zhou et al. 2012). Specific octopamine neurons (OANs) in the suboesophageal zone (SEZ, a region functionally similar to the mammalian brain stem) are both necessary and sufficient for cVA-dependent memory formation via octopamine receptors (OAMB) in KCs (Zhou et al. 2012). Interestingly, octopamine also mediates aggressive behavior toward potential male rivals (Zhou et al. 2008). Thus, the detection of cVA by OANs appears to suppress courtship in response to an encounter with a mated female eliciting aggressive behavior in the presence of another male (Wang and Anderson 2010). How these two behaviors are individually rooted and coordinated within the MB or other higher brain structures needs to be further investigated.
Ensuring the fitness and survival of the progeny also underlies highly adaptive processes. Interestingly, the MB seems to have a default inhibitory function on egg laying, since ablation of the MB increases egg laying even in virgin flies (Fleischmann et al. 2001). When a female Drosophila is in the course of egg laying, her sensory perception and decision-making undergo changes optimizing her reproductive success. Flies use fermenting fruit as a food source and as sites for oviposition, therefore, ethanol, acids, amines, and similar odors are predictors of high-quality egg laying grounds (Azanchi et al. 2013; Hussain et al. 2016a, b). On the other hand, geosmin (Stensmyr et al. 2012), an alarming smell indicating rotten food contaminated with certain bacteria, is highly aversive. Opposing DAN inputs to the MB (via PPL1/PAM and PPM3) reinforcing aversion and attraction behavior, respectively, are responsible for driving the behavioral choice of an optimal ethanol concentration (Azanchi et al. 2013). Such a mechanism could represent a universal design principle of the dopaminergic reinforcing system determining optimal conditions for reproductive behavior (e.g., for feeding, mating, and egg laying). Additionally, the LH may have a key role in selecting the optimal egg laying substrate, highlighting the multilayered integration of state-dependent behavioral adaptation (Chin et al. 2018).
Importantly, state-dependent plasticity in higher-order structures not only allows for adaptive behavioral choices, but also gates memory processes (Scheunemann et al. 2018). For example, virgin female Drosophila suppress consolidation of aversive olfactory long-term memory, via default inhibition of a pair of serotonergic neurons (SPNs) upstream of the avoidance-promoting DAN-MB memory circuit. After mating, phosphodiesterase activity in SPNs is reduced, which disinhibits their activity via increased cAMP signaling and promotes the consolidation of aversive memories (Scheunemann et al. 2018). This may be critical for fitness, as blocking long-term storage of aversive associations might engage flies in higher risk-taking behavior and promote foraging to find mating partners, while developing risk-averse behavior after mating could ensure the survival and safety of the progeny. Such a behavioral shift in response to reproductive state is commonly observed in mammals including humans (Pattwell et al. 2013, 2016); the neurobiological underpinning, however, is not well understood.
An intriguing aspect of MB modulation of behavior is its ability to integrate multiple sensory modalities; the detailed mechanisms are the subject of active investigation (Thiagarajan et al. 2022; Okray et al. 2023). For instance, the sensory stimuli driving DAN responses during egg laying decisions are multisensory, coming mainly from olfactory and gustatory but also undetermined sensory input (Chin et al. 2018). This cross-modal integration allows the MB to combine olfactory cues with other sensory inputs, such as gustatory and visual information. This integrated sensory processing could be crucial for making comprehensive reproductive decisions, as it enables the female to assess not only the suitability of potential mates but also the quality of egg laying sites and to form context-dependent memories for future behavior. Moreover, it will be interesting to decipher how multisensory input to important courtship-controlling neurons (e.g., P1/pC1) is integrated with MB processing (Clowney et al. 2015; Deutsch et al. 2020; Jung et al. 2020; Cheriyamkunnel et al. 2021; Shen et al. 2023).
The mushroom body brings flies together
Although flies mostly interact with each other to mate and compete for mating partners, recent evidence indicates that they are capable of simple types of sociability beyond these purposes. Indeed, Drosophila shows approach behavior to other individuals and a natural tendency to aggregate (Jiang et al. 2020; Sun et al. 2020). This moderate degree of sociability seems to be beneficial for flies, as it facilitates the spread of relevant information about their surroundings, learning, and avoidance of dangers within a group (Kacsoh et al. 2015; Ramdya et al. 2015; Muria et al. 2021; Wu et al. 2023).
Since group behavior and social interactions beyond mating could be critical for increasing the likelihood of survival and fitness, it is conceivable that the Drosophila brain contains defined circuits that regulate the drive to approach conspecifics according to previous social experience. This notion of a social drive is strengthened by studies showing the impact of lack of social experience on following social interactions and on the regulation of other internal drives. For instance, after being in isolation for 3–6 days, flies typically increase their activity levels, the frequency of social encounters, and the number of aggressive behaviors (Liu et al. 2018; Bentzur et al. 2021). This isolation-induced brain state appears to be long-lasting and characterized by a decreased need to sleep and overconsumption of food. These effects involve changes in NPF (homolog of NPY) signaling, a known input to MB-projecting DANs (PPL1-γ1-pedc) (Ganguly-Fitzgerald et al. 2006; Li et al. 2021).
Apart from regulating the need to mate, the MB has been shown to be required for social learning such as copying other flies’ mating preferences and avoidance of laying eggs in dangerous environments (Kacsoh et al. 2015; Nöbel et al. 2023). Recent evidence further indicates that this neuropil integrates visual and olfactory cues associated with social experience for generating the motivation to interact. Specifically, the activity of the γ lobe has been shown to be sensitive to such cues promoting social motivation and approach behavior (Fig. 2; Sun et al. 2020). Here, serotonin signaling from dorsal paired medial (DPM) neurons, which are involved in MB-dependent memory (Keene et al. 2006), is required for the expression of social drive (Sun et al. 2020). Interestingly, this serotonergic cluster also responds to putative social cues such as CO2 released by other individuals, thereby modulating the activity of α/β KCs to boost memory expression in a social context (Muria et al. 2021). These results stress the role of the MB and associated neurons as integration centers for social cues that regulate the drive of flies to be with others and other key functions of the brain according to the social environment.
Interestingly, previous research with flies indicates that a lack of social cues affecting future encounters and other internal drives can alter the sensitivity to pheromones and dopamine (Fernandez et al. 2017; Agrawal et al. 2019; Sethi et al. 2019). Bearing this in mind, it is conceivable that deprivation of social experience may also have long-lasting effects on the transcriptional landscape and activity levels of socially relevant subsets of MB and associated neurons. The extent to which such changes in neuron activity could impact information processing within the MB and its contribution in influencing or controlling relevant brain states more globally remain to be seen.
The maladaptive brain
Depression- and addiction-like states are influenced by the mushroom body
Brains contain a complex regulatory machinery that, when chronically dysregulated, may lead to maladaptive behavioral states that are typical of addiction and depression. Given the important role of the MB in balancing avoidance and approach based on state and experience, what is its role in the induction and maintenance of states of unhealthy motivation?
Similar to mammals, flies exposed to lasting and uncontrollable stress such as repeated vibrations, social defeats and deprivation (e.g., from food or sleep) fall into a persistent depression-like state characterized by a low motivation to seek natural rewards and avoid punishments (Yang et al. 2013; Ries et al. 2017; Araujo et al. 2018; Kim et al. 2018). This brain state also leads to cognitive deficits, pessimistic biases, increased aggression, and reduced drive to perform innate behaviors such as climbing and mating (Araujo et al. 2018; Deakin et al. 2018; Jia et al. 2021).
Not surprisingly, transitions in and out of this depression-like state are associated with different levels of activity within the MB and the fly's reward system. Supporting the notion that aminergic systems are key mediators of motivational states, enduring stress has been shown to reduce brain levels of dopamine and serotonin (Araujo et al. 2018). For example, chronic exposure to vibrations reduces serotonergic input from DPMs to KCs in the α lobe, which dampens the activity of these neurons and the motivation to climb (Ries et al. 2017). The depressive behavior can be alleviated by enhancing serotonergic signaling artificially or by exposing flies to natural rewards such as sweet sensation (Ries et al. 2017; Hu et al. 2020). Interestingly, this relief from sugar experience is mediated by rewarding PAM DANs, which respond to octopamine-mediated sweet signals and activate DPMs via α/β KCs, setting serotonin release to the α-lobes back to the levels that are required to promote climbing (Fig. 2; Hermanns et al. 2022). Although the activity of DANs such as PPL1-γ1-pedc gates the formation of aversive stressor associations (Kim et al. 2018), their output has also been shown to underlie the development of learning deficits after chronic stress, likely by leading to abnormal spontaneous activity in downstream MB neurons (Jia et al. 2021).
Contrary to depression, addiction leads to a long-lasting internal state characterized by a high motivation to seek the reward triggered by drugs and their cues even in the face of aversive consequences (Vanderschuren and Everitt 2004). The susceptibility to this state seems to be conserved from humans to flies, as Drosophila not only finds drugs of abuse and its cues rewarding, but also develops features of addiction such as voluntary self-administration, craving, withdrawal, and relapse (Devineni and Heberlein 2009; Kaun et al. 2011; Robinson et al. 2012; Ghezzi et al. 2014; Highfill et al. 2019; Kanno et al. 2021).
Critical for the maintenance of this state is the formation of appetitive memories of cues that are associated with drugs, as it facilitates seeking behavior and repeated exposure. Moreover, although still largely ununderstood, recent data suggest that the formation of associations related to opioids also influences the effects of the drug itself, leading to functional tolerance only when they are consumed in the context previously linked with drug intake (Hou et al. 2023). Just as preference to natural rewards such as sugar and water, the consolidation and expression of conditioned preference to drugs like alcohol requires the MB and dopaminergic input (Kaun et al. 2011; Scaplen et al. 2020; Kanno et al. 2021). A key difference is that alcohol experience does not rely on a specific subset of DANs but engages a broader population of PAM DANs, which could underlie the high motivational value of this substance. For instance, the encoding of positive alcohol associations requires the activity at the population level of PAM DANs, which increases with prolonged exposure (Scaplen et al. 2020). It has been proposed that this broad activation could lead to a highly stable memory and promote compulsive cue-seeking despite punishment by antagonizing the function of aversive DANs (Scaplen et al. 2020; Jovanoski et al. 2023). Once consolidated, the expression of positive alcohol memories is mediated by the activity of PPL1-α′2α2 and PAM-β′2a DANs projecting to the vertical and horizontal MB lobes, respectively (Scaplen et al. 2020).
Interestingly, the susceptibility of flies to display addiction-like behaviors seems to be influenced by past experiences or other motivational drives. For example, an increase of NPF levels that signal reward after mating interferes with the ability of flies to form rewarding alcohol associations, while NPF decreases after social rejection and promotes consumption (Shohat-Ophir et al. 2012). Importantly, the formation of positive alcohol associations and its progressive ingestion coincides with long-lasting alterations in gene expression and splicing in MB neurons. For example, the levels of Dop1R1 in β, β′, and γ lobes increase over days of self-administration, correlating with an escalation of alcohol intake and preference. Alcohol experience is also thought to induce functional changes in Dop1R2 in the MB via alterations in isoform expression (Petruccelli et al. 2018, 2020; Kanno et al. 2021). Suggesting common long-lasting effects of drug abuse, cocaine ingestion also alters the expression of genes associated with signal transduction at the KC level (Baker et al. 2021). Other neuroadaptations reported in mice after drug exposure, such as increases in mitochondrial trafficking in DANs, appear to be relevant in the dopaminergic system of Drosophila for forming positive alcohol associations (Knabbe et al. 2022). Although not yet investigated, similar long-lasting plastic changes across synapses between the MB and reward circuitry could be triggered by exposure to stressors and contribute to the development and maintenance of depressive-like states.
Future work with Drosophila will shed light on how MB plasticity, internal state, and context might facilitate entries and exits from these states of unbalanced motivation.
The hungry brain
Homeostasis and how it changes valence coding in the mushroom body
The processing of sensory information varies widely across behavioral states. Although olfaction is the most studied sensory modality for the MB circuit, also gustatory, temperature, mechanosensory (including proprioceptive), auditory, and visual inputs are integrated and may underlie state-dependent modulation (Hallem et al. 2006; Hong et al. 2008; Benton 2009; Gerber et al. 2009; Bang et al. 2011; Vogt et al. 2014, 2016; Kirkhart and Scott 2015; Sachse and Beshel 2016; Li et al. 2020b; Siju et al. 2020; Devineni et al. 2021). Metabolic states have been shown to strongly influence plasticity in the MB circuit mainly at the dopaminergic input level affecting both innate and learned behavior (Krashes et al. 2009; Bräcker et al. 2013; Lin et al. 2014; Tsao et al. 2018).
When flies interact with their environment and encounter specific sensory stimuli, the MB allows for the association between these stimuli and various outcomes or experiences. This is, for instance, crucial for recognizing food sources or avoiding dangers (Heisenberg 2003; Akalal et al. 2006; Owald and Waddell 2015; Cognigni et al. 2018; Mariano et al. 2020; Felsenberg 2021). Arguing against a strict functional division of memory processes and instinctive responses, work in the last decade demonstrated that the MB also regulates innate behaviors such as avoiding odors or cold temperatures according to the internal homeostatic needs (Hong et al. 2008; Bräcker et al. 2013; Siju et al. 2014; Lewis et al. 2015; Owald et al. 2015; Shih et al. 2015; Perisse et al. 2016; Sayin et al. 2019; Lin 2023). For example, hunger motivates flies to approach food odors, with DANs driving innate food odor-seeking through multiple MBON types (Tsao et al. 2018; Sayin et al. 2019). The MB also helps hungry flies modulate innate aversion, possibly as a gate for risk-taking behavior (Bräcker et al. 2013). For instance, while fed flies are strongly repelled by a mixture of the highly aversive odor CO2 and food odor vinegar, hungry flies show reduced aversion to it (Bräcker et al. 2013). This behavioral change is achieved by a vinegar-mediated activation of specific hunger-modulated PAM DANs (β2 and β′2), which suppress the output of avoidance-triggering MBONs (Bräcker et al. 2013; Lewis et al. 2015). Starvation also modulates odor-evoked responses by disinhibition of other MBONs, for instance at the MB heel (y1) (Fig. 2; Perisse et al. 2016). The metabolic state-dependent modulation of odor responses of MB-innervating DANs is mediated by several neuromodulators in response to satiety and hunger signals. To date, identified hunger signals include (short) neuropeptide F (NPF and sNPF) and serotonin while satiety signals are mainly conveyed by insulin and AstA (Krashes et al. 2009; Tsao et al. 2018). These studies and additional work suggest that DANs can redirect behavioral choices in food-deprived flies by modulating the synapse between KCs and MBONs (Bräcker et al. 2013; Plaçais and Preat 2013; Musso et al. 2015; Plaçais et al. 2017). Such a parallel mechanism, together with the LH, would ensure both hardwired and adaptive responses as needed, for instance, in hunger states.
In general, such state-dependent modulations can cause a change in the strength of the phasic DAN response to sensory input like odors (Lewis et al. 2015; Perisse et al. 2016; Jovanoski et al. 2023), but also changes tonic and spontaneous DAN activity (Ichinose et al. 2017). This is most visible in two MB-projecting DANs (PPL1-γ2′α1 and PPL1-γ1-pedc), which show spontaneous activity like slow oscillations in fed and water-sated flies. Their spontaneous activity is blocked by hunger (PPL1-γ1-pedc) and thirst (PPL1-γ2α′1) (Krashes et al. 2009; Plaçais et al. 2012; Plaçais and Preat 2013; Senapati et al. 2019), which is thought to increase motivation to seek food and water and to change the MB circuit to allow flies to be attracted to odors associated with these rewards during memory retrieval (Fig. 2; Krashes et al. 2009; Lin et al. 2014). Interestingly, studies training flies by pairing an odor with the optogenetic activation of subsets of DANs indicate that flies can form short-term sugar memories independent of the metabolic state with the input of specific PAM DANs (β′2 and γ4) to the MB (Huetteroth et al. 2015). Other PAM DANs (γ5) are sensitive to nutrient signals and form long-lasting food-seeking memories. Importantly, consolidation of such a sugar-rewarded memory trace is gated by increased ongoing activity of PPL1-γ1-pedc (Musso et al. 2015; Pavlowsky et al. 2018), the same DAN whose activity is blocked by nutrient deficiency. Thus, PPL1-γ1-pedc behaves like a state-dependent gatekeeper for inducing long-term changes to odor valence. In line with the idea of reward and punishment neurons, it seems that signals from food intake are conveyed by specific “rewarding” DANs (PAM DANs) while deficiency states for metabolites are integrated via changing spontaneous activity patterns in “punishment” DANs (PPL1 DANs). This could imply that the degree of inhibition from PPL1 gates a persistent behavioral change dependent on the perceived value of sugar-rewarded odors.
Likewise, water deprivation states are integrated by inhibition of specific “punishment” DANs (PPL1-γ2α′1), but also by inhibition of a subset of “rewarding” DANs (PAM-β′2a) via lateral horn leucokinin (LHLK) neurons and leucokinin signals (Senapati et al. 2019). The first is likely serving a deficiency detection mechanism similar as observed for food deprivation, while inhibition of PAM-β′2a might promote specificity for thirst rewards by blocking sugar reward processing in these neurons. Interestingly, leucokinin is a shared signal for both hunger and thirst and drives severely starved flies to approach food-associated odors through the activation of approach-promoting DANs. Activation of PAM-β′2p and PAM-β′2m and disinhibition of PPL1-γ2α′1 and PAM-β′2a DANs via additional neuromodulators override thirst-induced inhibition. Thus, fine-tuned modulation of the dopaminergic system allows flies to adapt behavior to the most relevant cues, while its malfunction can lead to compulsive behavior as described in the section above (Jovanoski et al. 2023).
Taken together, the DAN layer can bidirectionally modify behavior according to homeostatic needs through modulation of the synaptic weight of KC to MBON connections or directly by instructing the output layer. This function of DANs is mediated primarily by two dopamine receptors, inhibitory D2R and Dop1R2 (Sayin et al. 2018, 2019). Interestingly, the latter is linked to a second messenger pathway (PKCδ) that controls mitochondrial function and thereby energy fluxes that fuel memory processes (e.g., trigger protein synthesis–dependent long-term memory) (Comyn et al. 2023).
State-dependent plasticity occurs also directly at the level of MBONs (e.g., via direct input from octopamine and serotonin). Octopamine was shown to signal a successful food encounter directly at the MB output level to override and break starvation-dependent food-seeking behavior (Sayin et al. 2019). Serotonin was found to modulate specific output neurons in response to water deprivation, which supports the consolidation of water-reward learning (Lee et al. 2021). Interestingly, serotonin also modulates hunger state-dependent attraction and aversion toward odors at the first olfactory center, the AL (Vogt et al. 2021). A recent study showed that thirst-dependent modulation of gene expression is pronounced in glia. Interestingly, these cells modulate odor responses in a thirst-dependent fashion via tripartite synaptic connections with glutamatergic MBONs (γ5) and downstream neurons (Park et al. 2022). Together, while DAN activity appears to be the most prominent side of state-dependent modulation, KCs and MBONs are also direct targets of adaptive mechanisms, mediated fundamentally by glia-dependent signals.
Interestingly, protein synthesis–dependent aversive LTM is also sensitive to metabolic states and an increased frequency in the Ca2+ signals of “energy-signaling” PPL1-γ1-pedc DAN is indispensable for memory consolidation (Plaçais et al. 2012, 2017; Musso et al. 2015). Recent advances in the understanding of metabolic pathways in MB neurons demonstrated that the energy demands of neurons involved in memory formation are fueled by metabolites from glia cells surrounding the MB. In detail, it was shown that in the fed state, the formation of protein synthesis–dependent long-term memory relies on the regulation of both pyruvate (a glucose derivative) metabolism in MB neurons (Plaçais et al. 2017) and glucose metabolism in glial cells (de Tredern et al. 2021). When flies are starved, LTM formation is blocked, which is beneficial for surviving food restriction (Mery and Kawecki 2005; Plaçais and Preat 2013). This adaptive plasticity is specific to LTM, as starved flies maintain their ability to form consolidated—but protein synthesis–independent—memory fueled by ketone bodies from glia (Silva et al. 2022). Together, this demonstrates that (1) memory formation, in general, is sensitive to energy restriction, (2) energy transfer from the glia to the MB is regulated by metabolic state, and strikingly (3) increased energy flux in MB mitochondria can trigger the formation of stable protein synthesis–dependent LTM instead of a labile fast-decaying memory.
Finally, dietary adaptations like an increase in food consumption or preference for specific nutrients have been well described in female Drosophila in response to mating (Ribeiro and Dickson 2010; Vargas et al. 2010; Laturney et al. 2023). The MB might contribute significantly to such a crossover integration of different states (e.g., mating and nutrient homeostasis) (Hussain et al. 2016a; Boehm et al. 2022).
In summary, the MB is highly sensitive to metabolic deprivation states, ensuring the increased value of food-related sensory input is signaled only in contexts when physiological need is high and behavior has to be adapted to counteract the imbalance of metabolic homeostasis.
Movement and foraging: the mushroom body responds to behavioral states
Work over the past decades revealed that, apart from integrating external and homeostatic signals, the MB also responds to behavioral states of the body such as walking, resting, or flailing. Such moment-to-moment representation of locomotion and movement in DANs and MBONs might allow the brain to integrate the animal's ongoing behavior into decision-making, goal-directed behavior, and learning (Martin et al. 1998; Brembs 2009; Cohn et al. 2015; Aimon et al. 2019, 2023; Siju et al. 2020; Zolin et al. 2021).
Increased locomotion can be a consequence of state-dependent adaptations (e.g., in response to starvation) mediated by various neuromodulators (prominently dopamine) that, among other brain regions, are known to modulate MB plasticity (Lee et al. 2004; Eriksson et al. 2017; Lin et al. 2019). Although locomotion is vital for hungry animals to find food sources, it can also be a waste of already low-energy stores. Thus, the effort an animal invests into reaching a goal such as food must be tightly regulated. A deeper analysis of individual foraging sequences (like initiation and active search or engagement and disengagement of food sources) is providing a more detailed view of how DAN-driven motivational states control behavioral initiation and persistence and mediate the transition between tasks (Sayin et al. 2019; Zolin et al. 2021).
Ongoing dopamine release during locomotion might engage the same synaptic plasticity mechanisms as those driving memory formation, allowing both rewards and self-generated actions to modulate and reinforce behavior. For instance, dopamine versus octopamine feedback at the level of KCs and the MBON-γ1-pedc>αβ regulates persisting versus stopping food odor tracking, suggesting a tight regulation of the length and intensity of goal-directed behavior (Sayin et al. 2019). On its way to a goal, a fly might form new associations, potentially engaging similar DAN-dependent plasticity mechanisms to tune current as well as future behavior (Fisher et al. 2022). Interestingly, a similar, compartmentalized activity of DANs in the MB γ-lobe is triggered by electric shock or sugar ingestion and flailing or rest, respectively (see Fig. 2), suggesting that the animal's behavioral state could act as an additional reinforcement stimulus (Cohn et al. 2015). The combination of ongoing DAN activity during odor pursuit and rapid dopamine-dependent plasticity in the MB γ-lobe provides a potential mechanism for storing a permissive trace of the fly's recent actions. This mechanism may present evidence of history-dependent odor-tracking behavior or facilitate the pursuit of an odor plume (Sayin et al. 2019; Zolin et al. 2021; Fisher et al. 2022; Aso et al. 2023). Notably, other DANs innervating the other MB lobes also show walk-induced activity to different degrees (Siju et al. 2020; Aimon et al. 2023). Interestingly, DANs innervating certain compartments (e.g., γ3) increase activity before the animal starts to walk, consistent with a putative instructive role in behavior initiation (Aimon et al. 2023).
Work over recent years has revealed that locomotion and movement influence neural activity in many brain areas across many organisms (Busse et al. 2017; Kaplan and Zimmer 2020). Using whole-brain imaging with high temporal resolution, recent data describe global brain activity during free and forced walk in Drosophila (Aimon et al. 2019, 2023). As for other animals, Drosophila brain activity is widely correlated with locomotion representing a global change in brain state. These results challenge the assumption that most of the DAN activity is related to decision-making, top-down motor control, or prediction error detection from sensory feedback and instead provide evidence that walk itself and somatosensory bottom-up stimuli are largely responsible for the observed activity patterns (Aimon et al. 2023). Interestingly, while DAN activity increases with locomotion, specific serotonin signals are strongly inhibited when the fly engages in walking (Aimon et al. 2023), reminiscent of a functional discrimination between go and no-go signals by dopamine and serotonin in mammals (Desrochers et al. 2022).
Regarding the role of the MB in the brain-behavior loop, population and whole-brain imaging data in behaving animals, together with the whole-brain electron microscope (EM) connectomes for Drosophila, provide novel, highly complementary, resources for generating new hypotheses. This will help addressing the question of how goal-oriented, seemingly purposive, and spontaneous movements are governed by explicit and implicit reinforcing signals from the DAN system and account for statistical learning in a natural environment.
The brain under alert
Innate immune signaling triggers mushroom body–dependent behavioral adaptation
An animal's survival hinges not only on its ability to find sufficient food but to avoid harmful sources and remember that they caused sickness. Pathogens introduced through food navigate through the digestive system, inciting an immune response. Besides instigating direct antimicrobial measures like the production of antimicrobial peptides (AMPs), harmful microbes also prompt behavioral adjustments and memory formation. This is crucial for minimizing the impact of infection and preventing recurrent exposure to the same or similar contaminated food sources. These adaptive behaviors, which include conditioned taste aversion (CTA) of tastes associated with malaise, are widespread across the animal kingdom. Nevertheless, the mechanisms governing CTA or pathogen-modulated feeding behaviors remain uncertain, particularly concerning the necessary communication between the body and the brain.
Important work in honeybees and Caenorhabditis elegans suggests a role for serotonin in integrating negative postingestive signals during the formation of CTA (Zhang et al. 2005; Wright et al. 2010), while evidence from Drosophila points to the involvement of octopamine and immune signaling in the brain in the context of pathogen-modulated behavior (Babin et al. 2014; Kurz et al. 2017; Schretter et al. 2018; Masuzzo et al. 2019; Kobler et al. 2020). Importantly, Kobler et al. (2020) implicated the fly MB and proposed a model in which the intake of ecologically relevant pathogenic bacteria in flies induces CTA through innate immune signaling in OANs, KCs, and specific MBONs, directly linking pathogen ingestion, the immune system, and the behavioral adaptations regulated by the brain. Flies lacking the innate immune receptor PGRP-LC in the nervous system, specifically in OANs, lose their ability to differentiate between harmless and pathogenic bacteria postingestion while naive flies are strongly attracted to the odors of these pathogens. The additional requirement for the “synaptic plasticity” gene encoding the calcium-sensitive dependent adenylate cyclase rutabaga and synaptic output from β′2 and γ5 MBONs, indicates that the aversion to feeding on pathogenic bacteria involves an MB-dependent associative learning mechanism (Fig. 2). Moreover, flies lacking the important olfactory receptor Orco do not adapt their behavior and continue to consume pathogenic bacteria, suggesting an association between the bacterial odor and a negative postingestive experience.
PGRP-LC-mediated signaling in OANs, and possibly an involvement of other organs such as the fat body in conveying negative postingestion signals (Y Wang and IC Grunwald Kadow, unpubl.), indicate how complex neuromodulatory input from the body to the MB guides behavioral adaptations upon pathogen ingestion. Narrowing down the specific scenario that leads to the activation of PGRP-LC signaling in OANs is important. One hypothesis is that bacterial components or metabolites might traverse the gastrointestinal tract epithelium, activating PGRP-LC directly in the nervous system. Alternatively, bacterial fragments (e.g., peptidoglycans, PGN) could cross the epithelial barrier, reach the hemolymph, and activate immune responses in other organs that are detected by the brain through secondary signals.
A major output of PGRP signaling is the production of AMPs. Intriguingly, some AMPs may also play roles as modulators in the central nervous system (Barajas-Azpeleta et al. 2018; Kobler et al. 2020). Two specific AMPs were shown to be essential for the formation of two types of memories, appetitive associative olfactory memory and memories of an unsuccessful mating experience (Barajas-Azpeleta et al. 2018). AMPs, such as Diptericin B (DptB), were induced in the heads of adult flies following behavioral conditioning, and the activity of DptB and another AMP, Gram-negative bacteria binding protein like 3 (GNBP-like3), proved essential in modulating these forms of memory. These findings suggest that certain AMPs might have been repurposed in the nervous system, functioning as neuromodulators during evolution. However, the precise mechanism through which these immune peptides influence long-term memory remains unclear at this stage.
PGRP-LC signaling, and therefore possibly AMPs, could be directly involved in synaptic plasticity in the MB. A comprehensive forward genetic screen aimed at identifying genes involved in homeostatic plasticity, primarily using synaptic electrophysiology at the neuromuscular junction (NMJ) as the main assessment method, revealed mutations within the PGRP-LC locus that impede presynaptic homeostasis (Harris et al. 2015, 2018). Based on their findings, the authors hypothesized that PGRP-LC might serve as a receptor for a retrograde signal responsible for mediating homeostatic signaling from muscle to nerve. Whether it plays a similar role in the MB remains to be seen but is an exciting possibility.
Taken together, the MB's role in integrating sensory information with delayed postingestion signals from organs such as the gut or the fat body, forming memories of food quality, presents an intriguing avenue for future research.
The sleepy brain
The mushroom body regulates sleep and arousal
As other animals, insects transition between a wake and a sleep state according to the time of the day, level of tiredness, and the influence of other internal needs and environmental factors (Huber et al. 2004; Ganguly-Fitzgerald et al. 2006; Beckwith and French 2019; Shafer and Keene 2021). Sleep in flies can be defined as a period of quiescence marked by decreased responsiveness to the surroundings and characterized by changes in brain activity levels and electrical signatures relative to wakefulness (Hendricks et al. 2000; Shaw et al. 2000; van Alphen et al. 2013, 2021; Yap et al. 2017; Tainton-Heap et al. 2021). Although a unified theory of the function of sleep remains elusive, the last two decades of sleep research in Drosophila have provided further evidence of its key role in memory consolidation (Donlea et al. 2011; Dag et al. 2019; Lei et al. 2022; Marquand et al. 2023) and in recuperative processes of the brain such as synaptic homeostasis (Donlea et al. 2009; Gilestro et al. 2009; Bushey et al. 2011; Huang et al. 2020; Weiss and Donlea 2021). Since sleep and essential active behaviors such as foraging or mating are mutually exclusive, keeping the right balance between sleep and activity is critical.
To contribute to such balance, the MB comprises sleep- and wake-promoting KCs and MBONs and integrates inputs from different sleep-regulating circuits. These include signals from DANs activating glutamatergic wake-promoting MBONs (such as those innervating the γ, β, and β′ lobes) and GABAergic input from DPMs that promote sleep by inhibiting wake-promoting α′/β′-KCs (Fig. 2; Joiner et al. 2006; Pitman et al. 2006; Haynes et al. 2015; Sitaraman et al. 2015a,b; Driscoll et al. 2021; French et al. 2021). Once the fly has fallen asleep, the MB contributes to the state-dependent processing of salient olfactory information by promoting awakening in the presence of relevant stimuli (e.g., to food odors when hungry). This state-dependent sensory gate during sleep partly relies on an MBON in the β′2 and γ5 area that receives input from PAM DANs and signals to the sleep-regulatory fan-shaped body of the central complex (CC) (French et al. 2021).
An important hallmark of sleep that is conserved from flies to humans is its tight regulation by homeostatic drive that generates need to sleep according to the time spent awake and the complexity of visual inputs processed throughout the day (Borbely 1982; Ganguly-Fitzgerald et al. 2006; Donlea et al. 2009; Borbély et al. 2016; Donlea et al. 2017; Kirszenblat et al. 2019). When the need to sleep is high, awake flies are in a tired state in which important functions of the brain like attention and MB-dependent memory consolidation are compromised (Ganguly-Fitzgerald et al. 2006; Seugnet et al. 2008; Li et al. 2009). During this state, specific networks in the CC start displaying sleep-like state properties like slow wave activity, which promotes sensory decoupling and sleep need signaling (Raccuglia et al. 2019, 2022; Hasenhuetl et al. 2024). This finding indicates that tiredness is experienced at a network level, a notion supported by local sleep events during the wake in mammals that affect circuit function and lead to task-specific impairments (Vyazovskiy et al. 2011; Nir et al. 2017; Quercia et al. 2018; Suárez-Grimalt and Raccuglia 2021). Reminiscent of these local sleep events, KCs seem to go “offline” after a prolonged time awake, showing reduced spontaneous and evoked activity and being less likely to respond consistently to stimuli (Bushey et al. 2015). Although the signaling of the need to sleep has been mostly attributed to circuitries associated with the CC and to neurons in the ventral nerve cord (Donlea et al. 2014; Seidner et al. 2015; Liu et al. 2016; Blum et al. 2021; Ho et al. 2022; Jones et al. 2023), defined compartments of the MB are known to participate in the propagation of homeostatic signals. Specifically, sleep loss induces the activity of homeostatic recovery or rebound sleep by triggering the activity of a sleep-promoting KC–MBON circuit comprised of γd KCs and corresponding MBONs and reducing the activity of a wake-promoting one (Fig. 2; Sitaraman et al. 2015a).
Given the overlap between sleep-regulating and associative learning MB circuits (Keene et al. 2006; Claridge-Chang et al. 2009; Liu et al. 2012), learning-induced changes in these pathways might contribute to the build-up of tiredness or facilitate transitions to sleep. This might promote the regulation of sleep based on learning experience and the stabilization of specific memories. For example, the activity of the excitatory MBON-γ2α′1 has been recently shown to be sensitive to the duration of courtship learning and to trigger postlearning sleep via a CC circuit after an intense learning experience (Lei et al. 2022). This could not only be relevant for supporting the consolidation of these associations but also favor the downscaling of the synapses that were highly engaged in the task (Bushey et al. 2011; Diering 2023). Apart from facilitating memory storage by promoting sleep, the MB must also allow for the consolidation of memories during internal states in which falling asleep is not the best option (e.g., when starving). To support this, hunger signals after training mediated by NPF neurotransmission trigger the activity of a sleep-independent memory circuit composed of γ1 DANs, MBONs, and α′/β′m KCs. This pathway allows for memory consolidation with little postlearning sleep, unlike a different MB microcircuit comprised of γ2/α′1 DANs, MBON-γ2α′1, and α′/β′ap KCs that is activated when the fly is fed and that promotes sleep (Chouhan et al. 2021).
Despite the progress in the identification of the pathways transmitting homeostatic information, it is still not understood how all these signals are integrated to regulate sleep and how many circuits undergoing plastic changes upon sleep loss participate in signaling tiredness. Indeed, the increasing need to sleep as time awake passes is well reflected by brain-wide increases in the potentiation of synapses, which is evidenced by rising levels of the presynaptic protein Bruchpilot. Changing levels of this synaptic correlate of tiredness are required in a sleep need-encoding circuitry in the CC to regulate sleep according to time spent awake (Gilestro et al. 2009; Liu et al. 2016; Huang et al. 2020; Ho et al. 2022). At the level of the MB, KCs strongly contribute to the increase of Bruchpilot across different lobes, while presynaptic neurons such as DANs appear to be less sensitive to this form of presynaptic plasticity upon sleep loss (Weiss and Donlea 2021). Interestingly, KC to MBON synapses that are known to encode sleep-independent memories appear to display less changes in the number of synaptic connections as a result of sleep loss than those encoding sleep-dependent memories (Weiss and Donlea 2021). This opens the striking possibility that tiredness may not equally affect the function of all circuits and could perhaps spare the encoding function of some of them. Interestingly, recent evidence indicates that MBONs and DANs of distinct compartments involved in aversive and appetitive learning support memory at different times of the day (Dissel et al. 2024). This could potentially represent an additional strategy for preventing circuit damage, ensuring optimal circuit function and learning.
Future research on the mechanisms of sleep regulation in the MB will not only shed light on the underpinnings of tiredness signaling but also contribute to our understanding of the MB's role on memory formation during sleep–wake transitions.
Conclusion and outlook
In this review, we have featured the MB as a sophisticated control unit orchestrating the influence of a panel of diverse internal and behavioral states on goal-directed behavior, decision-making, and learning and memory by changing the valence of incoming sensory cues (for a list of selected publications covering each section, see Fig. 3; a brief summary of their main findings is in Supplemental Table S1). The functional parallels observed between the MB and mammalian brain regions such as the cerebellum and striatum underscore its intricate role in modulating instantaneous and future actions through the interactions of body needs and states and dopaminergic signals (Strausfeld et al. 1998; Farris 2011; Valjent and Gangarossa 2021; Perisse et al. 2023).
Figure 3.
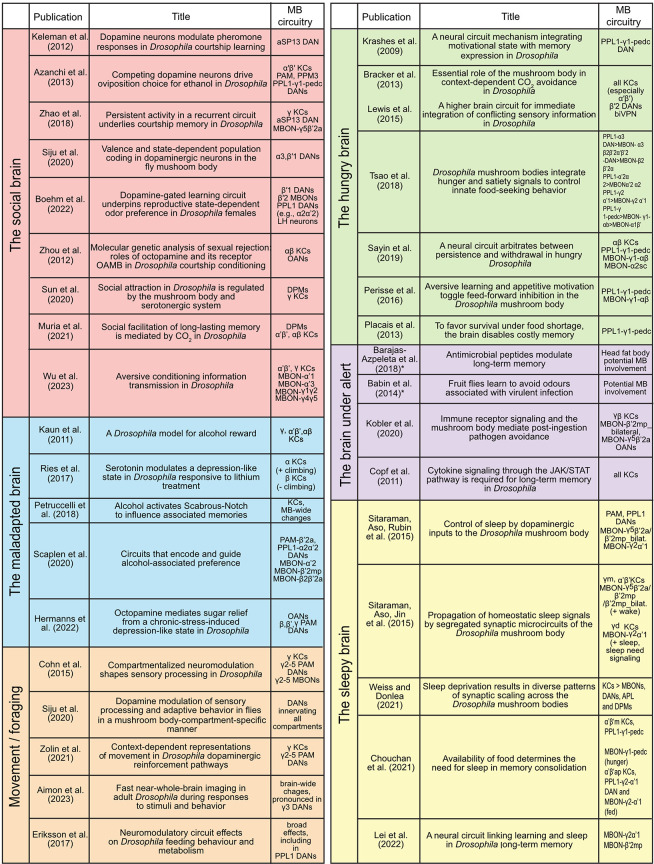
Selected publications for state-dependent modulations in the MB. Each physiological or behavioral state reviewed in this article for its role in modulating plasticity in the MB and changing behavioral output is represented by four to nine publications. Please note that these publications are selected to give a broad overview covering most of the diverse lines of research. The studies marked with an asterisk indicate that the involvement of the MB was not tested and remains to assessed. For a brief summary of the key findings of these studies, see Supplemental Table S1. (MB) Mushroom body, (KCs) Kenyon cells, (MBONs) mushroom body output neurons, (DANs) dopaminergic neurons, (PAM DANs) protocerebral anterior medial dopaminergic neurons, (PPL1 DANs) protocerebral posterior lateral dopaminergic neurons, (OANs) octopaminergic neurons, (APL) anterior paired lateral neurons (GABAergic), (DPM) dorsal paired medial neurons (GABAergic and serotonergic), (NPF) neuropeptide-F neurons, (AL) antennal lobe, (LH) lateral horn.
The common integration of different body needs within MB circuits may be key for facilitating complex regulatory interactions and the expression of the most adaptive behavioral output at every given moment (Lebreton et al. 2017; Beckwith and French 2019). This output is constantly updated by past and present contextual cues that leave state-dependent plasticity traces within sensing circuitries and MB neurons (Fig. 1). While substantial progress has been made in understanding state-dependent changes in activity within the MB network, the underlying mechanisms (e.g., varying input, pre- or postsynaptic adaptations) remain largely elusive. While we explored some adaptations such as alterations in presynaptic protein levels and cAMP pathway regulation (Tomchik and Davis 2009; Gervasi et al. 2010; Lee 2015; Scheunemann et al. 2018, 2019), other pre- and postsynaptic adaptations may contribute to state-dependent changes in activity (Zhang et al. 2019; Pribbenow et al. 2022; Davidson et al. 2023; Yamada et al. 2023). Additional fascinating avenues for future investigation include potential adaptations in the firing mode of neurons of the input layer in response to external or physiological signals (similarly to neurons encoding tiredness in the CC; Liu et al. 2016), and differential responses of downstream layers due to state-dependent changes in receptor expression and trafficking (Yamamoto and Seto 2014; Pribbenow et al. 2022). Bearing in mind that dopaminergic and other neuron types can release multiple neurotransmitters and are involved in promoting several behaviors (e.g., serotonergic and GABAergic DPMs promoting sleep and social approach) (Haynes et al. 2015; Aso et al. 2019; Sun et al. 2020; Yamazaki et al. 2023), it would be also interesting to explore potential context- and state-dependent alterations in cotransmission and their implications on behavior (Chen et al. 2023).
Despite tremendous progress in the understanding of MB function, it is still striking how MB neurons integrate such a complex and diverse array of information from changes in multisensory incoming signals. It is possible that different sensory inputs trigger plastic alterations in neurons of the input layer of distinct persistence and nature (e.g., changes in levels of activity, firing rates, cotransmitter release, or temporal coherence between dopaminergic, serotonergic, and other inputs), leading to specific plastic changes in downstream MB neurons. The coincidence of several signals that form a specific context boundary could further modify these factors, providing additional complexity to the array of the generated plastic changes and to the integration of inputs within the MB (Rodgers et al. 2011; Zeng et al. 2023).
Importantly, other brain areas receiving multisensory information described here in less detail, such as CC, AL, and LH, are known to contribute to the integration of and adaptation to body states and to behavioral organization (Pfeiffer and Homberg 2014; Sachse and Beshel 2016; Suárez-Grimalt and Raccuglia 2021; Vogt et al. 2021; Boehm et al. 2022; Sizemore et al. 2023). Considering the importance of generating robust responses to strong and salient stimuli, it is not surprising that parallel and partially redundant circuits may have evolved in the brain for the regulation of these outputs according to internal needs. In such a scenario, a potential key role of the MB might be to allow enduring state-dependent behavioral changes by undergoing long-lasting plastic synaptic reorganizations and supporting memory consolidation. Similar to the important role of synchronous activity between circuits and brain areas in memory consolidation (Plaçais et al. 2012; Miyamoto et al. 2016; Geva-Sagiv et al. 2023), the timing between the activity of MB neurons and that of other brain regions could also be a relevant factor for maintaining a unified representation of the fly's current state and induce a short- or long-term change in behavior. Future work will shed light on the intricate interplay of different brain circuits in contributing to the detection and generation of whole-brain states and their impact on the MB.
In conclusion, the Drosophila MB emerges as a multifaceted neuronal hub capable of decoding and integrating complex signals from internal and external environments. Unraveling the nuances of state-dependent plasticity at both the circuit and biochemical levels promises to deepen our understanding of adaptive behavior and cognition in this model organism and beyond. Future research should delve into additional plastic mechanisms and communication between MB and the body that allow efficient orchestration of behavioral responses by the MB.
Competing interest statement
The authors declare no competing interests.
Supplementary Material
Acknowledgments
I.C.G.K. was supported by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation FOR 2705 [TP3]) and by the iBehave Network (sponsored by the Ministry of Culture and Science of the State of North Rhine-Westphalia). L.S. received support from the DFG under Germany's Excellence Strategy—EXC-2049–390688087 and the DFG Emmy Noether Programme 1138 (495407463).
Author contributions: All the authors have accepted responsibility for the entire content of this manuscript and approved submission.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053918.124.
Freely available online through the Learning & Memory Open Access option.
References
- Agrawal P, Chung P, Heberlein U, Kent C. 2019. Enabling cell-type-specific behavioral epigenetics in Drosophila: a modified high-yield INTACT method reveals the impact of social environment on the epigenetic landscape in dopaminergic neurons. BMC Biol 17: 30. 10.1186/s12915-019-0646-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimon S, Katsuki T, Jia T, Grosenick L, Broxton M, Deisseroth K, Sejnowski TJ, Greenspan RJ. 2019. Fast near-whole-brain imaging in adult Drosophila during responses to stimuli and behavior. PLoS Biol 17: e2006732. 10.1371/journal.pbio.2006732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimon S, Cheng KY, Gjorgjieva J, Grunwald Kadow IC. 2023. Global change in brain state during spontaneous and forced walk in Drosophila is composed of combined activity patterns of different neuron classes. Elife 12: e85202. 10.7554/eLife.85202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akalal DB, Wilson CF, Zong L, Tanaka NK, Ito K, Davis RL. 2006. Roles for Drosophila mushroom body neurons in olfactory learning and memory. Learn Mem 13: 659–668. 10.1101/lm.221206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo SM, Poetini MR, Bortolotto VC, de Freitas Couto S, Pinheiro FC, Meichtry LB, de Almeida FP, Santos Musachio EA, de Paula MT, Prigol M. 2018. Chronic unpredictable mild stress-induced depressive-like behavior and dysregulation of brain levels of biogenic amines in Drosophila melanogaster. Behav Brain Res 351: 104–113. 10.1016/j.bbr.2018.05.016 [DOI] [PubMed] [Google Scholar]
- Aso Y, Herb A, Ogueta M, Siwanowicz I, Templier T, Friedrich AB, Ito K, Scholz H, Tanimoto H. 2012. Three dopamine pathways induce aversive odor memories with different stability. PLoS Genet 8: e1002768. 10.1371/journal.pgen.1002768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo TT, Dionne H, Abbott LF, Axel R, Tanimoto H, et al. 2014. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 3: e04577. 10.7554/eLife.04577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Ray RP, Long X, Bushey D, Cichewicz K, Ngo TT, Sharp B, Christoforou C, Hu A, Lemire AL, et al. 2019. Nitric oxide acts as a cotransmitter in a subset of dopaminergic neurons to diversify memory dynamics. Elife 8: e49257. 10.7554/eLife.49257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Yamada D, Bushey D, Hibbard KL, Sammons M, Otsuna H, Shuai Y, Hige T. 2023. Neural circuit mechanisms for transforming learned olfactory valences into wind-oriented movement. Elife 12: e85756. 10.7554/eLife.85756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer TO, Benton R. 2016. Sexual circuitry in Drosophila. Curr Opin Neurobiol 38: 18–26. 10.1016/j.conb.2016.01.004 [DOI] [PubMed] [Google Scholar]
- Azanchi R, Kaun KR, Heberlein U. 2013. Competing dopamine neurons drive oviposition choice for ethanol in Drosophila. Proc Natl Acad Sci 110: 21153–21158. 10.1073/pnas.1320208110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babin A, Kolly S, Schneider F, Dolivo V, Zini M, Kawecki TJ. 2014. Fruit flies learn to avoid odours associated with virulent infection. Biol Lett 10: 20140048. 10.1098/rsbl.2014.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BM, Mokashi SS, Shankar V, Hatfield JS, Hannah RC, Mackay TFC, Anholt RRH. 2021. The Drosophila brain on cocaine at single-cell resolution. Genome Res 31: 1927–1937. 10.1101/gr.268037.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Hyun S, Hong ST, Kang J, Jeong K, Park JJ, Choe J, Chung J. 2011. Dopamine signalling in mushroom bodies regulates temperature-preference behaviour in Drosophila. PLoS Genet 7: e1001346. 10.1371/journal.pgen.1001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barajas-Azpeleta R, Wu J, Gill J, Welte R, Seidel C, McKinney S, Dissel S, Si K. 2018. Antimicrobial peptides modulate long-term memory. PLoS Genet 14: e1007440. 10.1371/journal.pgen.1007440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith EJ, French AS. 2019. Sleep in Drosophila and its context. Front Physiol 10: 1167. 10.3389/fphys.2019.01167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R. 2009. Molecular basis of odor detection in insects. Ann NY Acad Sci 1170: 478–481. 10.1111/j.1749-6632.2009.03880.x [DOI] [PubMed] [Google Scholar]
- Bentzur A, Ben-Shaanan S, Benichou JIC, Costi E, Levi M, Ilany A, Shohat-Ophir G. 2021. Early life experience shapes male behavior and social networks in Drosophila. Curr Biol 31: 670. 10.1016/j.cub.2020.11.036 [DOI] [PubMed] [Google Scholar]
- Berry JA, Phan A, Davis RL. 2018. Dopamine neurons mediate learning and forgetting through bidirectional modulation of a memory trace. Cell Rep 25: 651–662.e5. 10.1016/j.celrep.2018.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilz F, Geurten BRH, Hancock CE, Widmann A, Fiala A. 2020. Visualization of a distributed synaptic memory code in the Drosophila brain. Neuron 106: 963–976.e4. 10.1016/j.neuron.2020.03.010 [DOI] [PubMed] [Google Scholar]
- Blum ID, Keleş MF, Baz ES, Han E, Park K, Luu S, Issa H, Brown M, Ho MCW, Tabuchi M, et al. 2021. Astroglial calcium signaling encodes sleep need in Drosophila. Curr Biol 31: 150–162.e7. 10.1016/j.cub.2020.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm AC, Friedrich AB, Hunt S, Bandow P, Siju KP, De Backer JF, Claussen J, Link MH, Hofmann TF, Dawid C, et al. 2022. A dopamine-gated learning circuit underpins reproductive state-dependent odor preference in Drosophila females. Elife 11: e77643. 10.7554/eLife.77643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA. 1982. A two process model of sleep regulation. Hum Neurobiol 1: 195–204. [PubMed] [Google Scholar]
- Borbély AA, Daan S, Wirz-Justice A, Deboer T. 2016. The two-process model of sleep regulation: a reappraisal. J Sleep Res 25: 131–143. 10.1111/jsr.12371 [DOI] [PubMed] [Google Scholar]
- Bouzaiane E, Trannoy S, Scheunemann L, Plaçais PY, Preat T. 2015. Two independent mushroom body output circuits retrieve the six discrete components of Drosophila aversive memory. Cell Rep 11: 1280–1292. 10.1016/j.celrep.2015.04.044 [DOI] [PubMed] [Google Scholar]
- Bräcker LB, Siju KP, Varela N, Aso Y, Zhang M, Hein I, Vasconcelos ML, Grunwald Kadow IC. 2013. Essential role of the mushroom body in context-dependent CO2 avoidance in Drosophila. Curr Biol 23: 1228–1234. 10.1016/j.cub.2013.05.029 [DOI] [PubMed] [Google Scholar]
- Brembs B. 2009. Mushroom bodies regulate habit formation in Drosophila. Curr Biol 19: 1351–1355. 10.1016/j.cub.2009.06.014 [DOI] [PubMed] [Google Scholar]
- Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. 2012. Layered reward signalling through octopamine and dopamine in Drosophila. Nature 492: 433–437. 10.1038/nature11614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Tononi G, Cirelli C. 2011. Sleep and synaptic homeostasis: structural evidence in Drosophila. Science 332: 1576–1581. 10.1126/science.1202839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Tononi G, Cirelli C. 2015. Sleep- and wake-dependent changes in neuronal activity and reactivity demonstrated in fly neurons using in vivo calcium imaging. Proc Natl Acad Sci 112: 4785–4790. 10.1073/pnas.1419603112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse L, Cardin JA, Chiappe ME, Halassa MM, McGinley MJ, Yamashita T, Saleem AB. 2017. Sensation during active behaviors. J Neurosci 37: 10826–10834. 10.1523/JNEUROSCI.1828-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth FM. 1969. Lipids of Drosophila: a newly detected lipid in the male. Science 163: 1356–1357. 10.1126/science.163.3873.1356 [DOI] [PubMed] [Google Scholar]
- Chen N, Zhang Y, Rivera-Rodriguez EJ, Yu AD, Hobin M, Rosbash M, Griffith LC. 2023. Widespread posttranscriptional regulation of cotransmission. Sci Adv 9: eadg9836. 10.1126/sciadv.adg9836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyamkunnel SJ, Rose S, Jacob PF, Blackburn LA, Glasgow S, Moorse J, Winstanley M, Moynihan PJ, Waddell S, Rezaval C. 2021. A neuronal mechanism controlling the choice between feeding and sexual behaviors in Drosophila. Curr Biol 31: 4231–4245.e4. 10.1016/j.cub.2021.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin SG, Maguire SE, Huoviala P, Jefferis G, Potter CJ. 2018. Olfactory neurons and brain centers directing oviposition decisions in Drosophila. Cell Rep 24: 1667–1678. 10.1016/j.celrep.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouhan NS, Griffith LC, Haynes P, Sehgal A. 2021. Availability of food determines the need for sleep in memory consolidation. Nature 589: 582–585. 10.1038/s41586-020-2997-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenböck G. 2009. Writing memories with light-addressable reinforcement circuitry. Cell 139: 405–415. 10.1016/j.cell.2009.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V. 2015. Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron 87: 1036–1049. 10.1016/j.neuron.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni P, Felsenberg J, Waddell S. 2018. Do the right thing: neural network mechanisms of memory formation, expression and update in Drosophila. Curr Opin Neurobiol 49: 51–58. 10.1016/j.conb.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R, Morantte I, Ruta V. 2015. Coordinated and compartmentalized neuromodulation shapes sensory processing in Drosophila. Cell 163: 1742–1755. 10.1016/j.cell.2015.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comyn T, Preat T, Pavlowsky A, Plaçais P-Y. 2023. PKCδ is an activator of neuronal mitochondrial metabolism that mediates the spacing effect on memory consolidation. bioRxiv 10.1101/2023.10.06.561186 [DOI] [Google Scholar]
- Connolly K, Cook R. 1973. Rejection responses by female Drosophila melanogaster: their ontogeny, causality and effects upon the behaviour of the courting male. Behaviour 44: 142–165. 10.1163/156853973X00364 [DOI] [Google Scholar]
- Dag U, Lei Z, Le JQ, Wong A, Bushey D, Keleman K. 2019. Neuronal reactivation during post-learning sleep consolidates long-term memory in Drosophila. Elife 8: e42786. 10.7554/eLife.42786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. 2008. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature 452: 473–477. 10.1038/nature06808 [DOI] [PubMed] [Google Scholar]
- Davidson AM, Kaushik S, Hige T. 2023. Dopamine-dependent plasticity is heterogeneously expressed by presynaptic calcium activity across individual boutons of the Drosophila mushroom body. eNeuro 10: ENEURO.0275-23.2023. 10.1523/ENEURO.0275-23.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin A, Mendl M, Browne WJ, Paul ES, Hodge JJL. 2018. State-dependent judgement bias in Drosophila: evidence for evolutionarily primitive affective processes. Biol Lett 14: 20170779. 10.1098/rsbl.2017.0779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrochers SS, Spring MG, Nautiyal KM. 2022. A role for serotonin in modulating opposing drive and brake circuits of impulsivity. Front Behav Neurosci 16: 791749. 10.3389/fnbeh.2022.791749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Tredern E, Rabah Y, Pasquer L, Minatchy J, Plaçais PY, Preat T. 2021. Glial glucose fuels the neuronal pentose phosphate pathway for long-term memory. Cell Rep 36: 109620. 10.1016/j.celrep.2021.109620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch D, Pacheco D, Encarnacion-Rivera L, Pereira T, Fathy R, Clemens J, Girardin C, Calhoun A, Ireland E, Burke A, et al. 2020. The neural basis for a persistent internal state in Drosophila females. Elife 9: e59502. 10.7554/eLife.59502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. 2009. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol 19: 2126–2132. 10.1016/j.cub.2009.10.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Deere JU, Sun B, Axel R. 2021. Individual bitter-sensing neurons in Drosophila exhibit both ON and OFF responses that influence synaptic plasticity. Curr Biol 31: 5533–5546.e7. 10.1016/j.cub.2021.10.020 [DOI] [PubMed] [Google Scholar]
- Dickson BJ. 2008. Wired for sex: the neurobiology of Drosophila mating decisions. Science 322: 904–909. 10.1126/science.1159276 [DOI] [PubMed] [Google Scholar]
- Diering GH. 2023. Remembering and forgetting in sleep: selective synaptic plasticity during sleep driven by scaling factors Homer1a and Arc. Neurobiol Stress 22: 100512. 10.1016/j.ynstr.2022.100512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissel S, Morgan E, Cao L, Wakefield ZP, Shetty S, Chan D, Duong V, Donlea J, Farah H, Loutrianakis V, et al. 2024. Breaking free from the clock's tyranny restores memory to brain damaged flies. bioRxiv 10.1101/2024.01.25.577231 [DOI] [Google Scholar]
- Donlea JM, Ramanan N, Shaw PJ. 2009. Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science 324: 105–108. 10.1126/science.1166657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. 2011. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science 332: 1571–1576. 10.1126/science.1202249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea JM, Pimentel D, Miesenböck G. 2014. Neuronal machinery of sleep homeostasis in Drosophila. Neuron 81: 1442. 10.1016/j.neuron.2014.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea JM, Alam MN, Szymusiak R. 2017. Neuronal substrates of sleep homeostasis; lessons from flies, rats and mice. Curr Opin Neurobiol 44: 228–235. 10.1016/j.conb.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Driscoll M, Buchert SN, Coleman V, McLaughlin M, Nguyen A, Sitaraman D. 2021. Compartment specific regulation of sleep by mushroom body requires GABA and dopaminergic signaling. Sci Rep 11: 20067. 10.1038/s41598-021-99531-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima A, Griffith LC. 2007. Measurement of courtship behavior in Drosophila melanogaster. CSH Protoc 2007: pdb.prot4847. 10.1101/pdb.prot4847 [DOI] [PubMed] [Google Scholar]
- Eriksson A, Raczkowska M, Navawongse R, Choudhury D, Stewart JC, Tang YL, Wang Z, Claridge-Chang A. 2017. Neuromodulatory circuit effects on Drosophila feeding behaviour and metabolism. Sci Rep 7: 8839. 10.1038/s41598-017-08466-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts C, Farine JP, Cobb M, Ferveur JF. 2010. Drosophila cuticular hydrocarbons revisited: mating status alters cuticular profiles. PLoS ONE 5: e9607. 10.1371/journal.pone.0009607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SM. 2011. Are mushroom bodies cerebellum-like structures? Arthropod Struct Dev 40: 368–379. 10.1016/j.asd.2011.02.004 [DOI] [PubMed] [Google Scholar]
- Felsenberg J. 2021. Changing memories on the fly: the neural circuits of memory re-evaluation in Drosophila melanogaster. Curr Opin Neurobiol 67: 190–198. 10.1016/j.conb.2020.12.003 [DOI] [PubMed] [Google Scholar]
- Felsenberg J, Barnstedt O, Cognigni P, Lin S, Waddell S. 2017. Re-evaluation of learned information in Drosophila. Nature 544: 240–244. 10.1038/nature21716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenberg J, Jacob PF, Walker T, Barnstedt O, Edmondson-Stait AJ, Pleijzier MW, Otto N, Schlegel P, Sharifi N, Perisse E, et al. 2018. Integration of parallel opposing memories underlies memory extinction. Cell 175: 709–722.e15. 10.1016/j.cell.2018.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez RW, Akinleye AA, Nurilov M, Feliciano O, Lollar M, Aijuri RR, O'Donnell JM, Simon AF. 2017. Modulation of social space by dopamine in Drosophila melanogaster, but no effect on the avoidance of the Drosophila stress odorant. Biol Lett 13: 20170369. 10.1098/rsbl.2017.0369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher YE, Marquis M, D'Alessandro I, Wilson RI. 2022. Dopamine promotes head direction plasticity during orienting movements. Nature 612: 316–322. 10.1038/s41586-022-05485-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann I, Cotton B, Choffat Y, Spengler M, Kubli E. 2001. Mushroom bodies and post-mating behaviors of Drosophila melanogaster females. J Neurogenet 15: 117–144. 10.3109/01677060109066198 [DOI] [PubMed] [Google Scholar]
- French AS, Geissmann Q, Beckwith EJ, Gilestro GF. 2021. Sensory processing during sleep in Drosophila melanogaster. Nature 598: 479–482. 10.1038/s41586-021-03954-w [DOI] [PubMed] [Google Scholar]
- Ganguly-Fitzgerald I, Donlea J, Shaw PJ. 2006. Waking experience affects sleep need in Drosophila. Science 313: 1775–1781. 10.1126/science.1130408 [DOI] [PubMed] [Google Scholar]
- Gerber B, Stocker RF, Tanimura T, Thum AS. 2009. Smelling, tasting, learning: Drosophila as a study case. Results Probl Cell Differ 47: 139–185. 10.1007/400_2008_9 [DOI] [PubMed] [Google Scholar]
- Gervasi N, Tchénio P, Preat T. 2010. PKA dynamics in a Drosophila learning center: coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. Neuron 65: 516–529. 10.1016/j.neuron.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Geva-Sagiv M, Mankin EA, Eliashiv D, Epstein S, Cherry N, Kalender G, Tchemodanov N, Nir Y, Fried I. 2023. Augmenting hippocampal–prefrontal neuronal synchrony during sleep enhances memory consolidation in humans. Nat Neurosci 26: 1100–1110. 10.1038/s41593-023-01324-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi A, Krishnan HR, Atkinson NS. 2014. Susceptibility to ethanol withdrawal seizures is produced by BK channel gene expression. Addict Biol 19: 332–337. 10.1111/j.1369-1600.2012.00465.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C. 2009. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science 324: 109–112. 10.1126/science.1166673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LC, Ejima A. 2009. Courtship learning in Drosophila melanogaster: diverse plasticity of a reproductive behavior. Learn Mem 16: 743–750. 10.1101/lm.956309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Dahanukar A, Carlson JR. 2006. Insect odor and taste receptors. Annu Rev Entomol 51: 113–135. 10.1146/annurev.ento.51.051705.113646 [DOI] [PubMed] [Google Scholar]
- Handler A, Graham TGW, Cohn R, Morantte I, Siliciano AF, Zeng J, Li Y, Ruta V. 2019. Distinct dopamine receptor pathways underlie the temporal sensitivity of associative learning. Cell 178: 60–75.e19. 10.1016/j.cell.2019.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N, Braiser DJ, Dickman DK, Fetter RD, Tong A, Davis GW. 2015. The innate immune receptor PGRP-LC controls presynaptic homeostatic plasticity. Neuron 88: 1157–1164. 10.1016/j.neuron.2015.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N, Fetter RD, Brasier DJ, Tong A, Davis GW. 2018. Molecular interface of neuronal innate immunity, synaptic vesicle stabilization, and presynaptic homeostatic plasticity. Neuron 100: 1163–1179.e4. 10.1016/j.neuron.2018.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenhuetl PS, Sarnataro R, Vrontou E, Rorsman HO, Talbot CB, Brain R, Miesenböck G. 2024. A half-centre oscillator encodes sleep pressure. bioRxiv 10.1101/2024.02.23.581780 [DOI] [Google Scholar]
- Haynes PR, Christmann BL, Griffith LC. 2015. A single pair of neurons links sleep to memory consolidation in Drosophila melanogaster. Elife 4: e03868. 10.7554/eLife.03868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg M. 2003. Mushroom body memoir: from maps to models. Nat Rev Neurosci 4: 266–275. 10.1038/nrn1074 [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. 2000. Rest in Drosophila is a sleep-like state. Neuron 25: 129–138. 10.1016/s0896-6273(00)80877-6 [DOI] [PubMed] [Google Scholar]
- Hermanns T, Graf-Boxhorn S, Poeck B, Strauss R. 2022. Octopamine mediates sugar relief from a chronic-stress-induced depression-like state in Drosophila. Curr Biol 32: 4048–4056.e3. 10.1016/j.cub.2022.07.016 [DOI] [PubMed] [Google Scholar]
- Hige T, Aso Y, Modi MN, Rubin GM, Turner GC. 2015. Heterosynaptic plasticity underlies aversive olfactory learning in Drosophila. Neuron 88: 985–998. 10.1016/j.neuron.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfill CA, Baker BM, Stevens SD, Anholt RRH, Mackay TFC. 2019. Genetics of cocaine and methamphetamine consumption and preference in Drosophila melanogaster. PLoS Genet 15: e1007834. 10.1371/journal.pgen.1007834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MCW, Tabuchi M, Xie X, Brown MP, Luu S, Wang S, Kolodkin AL, Liu S, Wu MN. 2022. Sleep need-dependent changes in functional connectivity facilitate transmission of homeostatic sleep drive. Curr Biol 32: 4957–4966.e5. 10.1016/j.cub.2022.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong ST, Bang S, Hyun S, Kang J, Jeong K, Paik D, Chung J, Kim J. 2008. cAMP signalling in mushroom bodies modulates temperature preference behaviour in Drosophila. Nature 454: 771–775. 10.1038/nature07090 [DOI] [PubMed] [Google Scholar]
- Hou Y, Zou G, Wang X, Guo H, Ma X, Cheng X, Xie Z, Zuo X, Xia J, Mao H, et al. 2023. Coordinated activity of a central pathway drives associative opioid analgesic tolerance. Sci Adv 9: eabo5627. 10.1126/sciadv.abo5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SW, Yang YT, Sun Y, Zhan YP, Zhu Y. 2020. Serotonin signals overcome loser mentality in Drosophila. iScience 23: 101651. 10.1016/j.isci.2020.101651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Piao C, Beuschel CB, Götz T, Sigrist SJ. 2020. Presynaptic active zone plasticity encodes sleep need in Drosophila. Curr Biol 30: 1077–1091.e5. 10.1016/j.cub.2020.01.019 [DOI] [PubMed] [Google Scholar]
- Huber R, Hill SL, Holladay C, Biesiadecki M, Tononi G, Cirelli C. 2004. Sleep homeostasis in Drosophila melanogaster. Sleep 27: 628–639. 10.1093/sleep/27.4.628 [DOI] [PubMed] [Google Scholar]
- Huetteroth W, Perisse E, Lin S, Klappenbach M, Burke C, Waddell S. 2015. Sweet taste and nutrient value subdivide rewarding dopaminergic neurons in Drosophila. Curr Biol 25: 751–758. 10.1016/j.cub.2015.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Üçpunar HK, Zhang M, Loschek LF, Grunwald Kadow IC. 2016a. Neuropeptides modulate female chemosensory processing upon mating in Drosophila. PLoS Biol 14: e1002455. 10.1371/journal.pbio.1002455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Zhang M, Üçpunar HK, Svensson T, Quillery E, Gompel N, Ignell R, Grunwald Kadow IC. 2016b. Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLoS Biol 14: e1002454. 10.1371/journal.pbio.1002454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Aso Y, Yamagata N, Abe A, Rubin GM, Tanimoto H. 2015. Reward signal in a recurrent circuit drives appetitive long-term memory formation. Elife 4: e10719. 10.7554/eLife.10719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Tanimoto H, Yamagata N. 2017. Behavioral modulation by spontaneous activity of dopamine neurons. Front Syst Neurosci 11: 88. 10.3389/fnsys.2017.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Kanno M, Wu H, Yamagata N, Sun H, Abe A, Tanimoto H. 2021. Mushroom body output differentiates memory processes and distinct memory-guided behaviors. Curr Biol 31: 1294–1302.e4. 10.1016/j.cub.2020.12.032 [DOI] [PubMed] [Google Scholar]
- Jia J, He L, Yang J, Shuai Y, Yang J, Wu Y, Liu X, Chen T, Wang G, Wang X, et al. 2021. A pair of dopamine neurons mediate chronic stress signals to induce learning deficit in Drosophila melanogaster. Proc Natl Acad Sci 118: e2023674118. 10.1073/pnas.2023674118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Cheng Y, Gao S, Zhong Y, Ma C, Wang T, Zhu Y. 2020. Emergence of social cluster by collective pairwise encounters in Drosophila. Elife 9: e51921. 10.7554/eLife.51921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Crocker A, White BH, Sehgal A. 2006. Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441: 757–760. 10.1038/nature04811 [DOI] [PubMed] [Google Scholar]
- Jones JD, Holder BL, Eiken KR, Vogt A, Velarde AI, Elder AJ, McEllin JA, Dissel S. 2023. Regulation of sleep by cholinergic neurons located outside the central brain in Drosophila. PLoS Biol 21: e3002012. 10.1371/journal.pbio.3002012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanoski KD, Duquenoy L, Mitchell J, Kapoor I, Treiber CD, Croset V, Dempsey G, Parepalli S, Cognigni P, Otto N, et al. 2023. Dopaminergic systems create reward seeking despite adverse consequences. Nature 623: 356–365. 10.1038/s41586-023-06671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y, Kennedy A, Chiu H, Mohammad F, Claridge-Chang A, Anderson DJ. 2020. Neurons that function within an integrator to promote a persistent behavioral state in Drosophila. Neuron 105: 322–333.e5. 10.1016/j.neuron.2019.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacsoh BZ, Bozler J, Ramaswami M, Bosco G. 2015. Social communication of predator-induced changes in Drosophila behavior and germ line physiology. Elife 4: e07423. 10.7554/eLife.07423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno M, Hiramatsu S, Kondo S, Tanimoto H, Ichinose T. 2021. Voluntary intake of psychoactive substances is regulated by the dopamine receptor Dop1R1 in Drosophila. Sci Rep 11: 3432. 10.1038/s41598-021-82813-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan HS, Zimmer M. 2020. Brain-wide representations of ongoing behavior: a universal principle? Curr Opin Neurobiol 64: 60–69. 10.1016/j.conb.2020.02.008 [DOI] [PubMed] [Google Scholar]
- Kaun KR, Azanchi R, Maung Z, Hirsh J, Heberlein U. 2011. A Drosophila model for alcohol reward. Nat Neurosci 14: 612–619. 10.1038/nn.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S. 2006. Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr Biol 16: 1524–1530. 10.1016/j.cub.2006.06.022 [DOI] [PubMed] [Google Scholar]
- Keleman K, Vrontou E, Krüttner S, Yu JY, Kurtovic-Kozaric A, Dickson BJ. 2012. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature 489: 145–149. 10.1038/nature11345 [DOI] [PubMed] [Google Scholar]
- Kim YK, Saver M, Simon J, Kent CF, Shao L, Eddison M, Agrawal P, Texada M, Truman JW, Heberlein U. 2018. Repetitive aggressive encounters generate a long-lasting internal state in Drosophila melanogaster males. Proc Natl Acad Sci 115: 1099–1104. 10.1073/pnas.1716612115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkhart C, Scott K. 2015. Gustatory learning and processing in the Drosophila mushroom bodies. J Neurosci 35: 5950–5958. 10.1523/JNEUROSCI.3930-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirszenblat L, Yaun R, van Swinderen B. 2019. Visual experience drives sleep need in Drosophila. Sleep 42: zsz102. 10.1093/sleep/zsz102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabbe J, Protzmann J, Schneider N, Berger M, Dannehl D, Wei S, Strahle C, Tegtmeier M, Jaiswal A, Zheng H, et al. 2022. Single-dose ethanol intoxication causes acute and lasting neuronal changes in the brain. Proc Natl Acad Sci 119: e2122477119. 10.1073/pnas.2122477119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobler JM, Rodriguez Jimenez FJ, Petcu I, Grunwald Kadow IC. 2020. Immune receptor signaling and the mushroom body mediate post-ingestion pathogen avoidance. Curr Biol 30: 4693–4709.e3. 10.1016/j.cub.2020.09.022 [DOI] [PubMed] [Google Scholar]
- Krashes MJ, DasGupta S, Vreede A, White B, Armstrong JD, Waddell S. 2009. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell 139: 416–427. 10.1016/j.cell.2009.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz CL, Charroux B, Chaduli D, Viallat-Lieutaud A, Royet J. 2017. Peptidoglycan sensing by octopaminergic neurons modulates Drosophila oviposition. Elife 6: e21937. 10.7554/eLife.21937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laturney M, Sterne GR, Scott K. 2023. Mating activates neuroendocrine pathways signaling hunger in Drosophila females. Elife 12: e85117. 10.7554/eLife.85117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton S, Grabe V, Omondi AB, Ignell R, Becher PG, Hansson BS, Sachse S, Witzgall P. 2014. Love makes smell blind: mating suppresses pheromone attraction in Drosophila females via Or65a olfactory neurons. Sci Rep 4: 7119. 10.1038/srep07119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton S, Carlsson MA, Witzgall P. 2017. Insulin signaling in the peripheral and central nervous system regulates female sexual receptivity during starvation in Drosophila. Front Physiol 8: 685. 10.3389/fphys.2017.00685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. 2015. Global and local missions of cAMP signaling in neural plasticity, learning, and memory. Front Pharmacol 6: 161. 10.3389/fphar.2015.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, You KH, Choo JK, Han YM, Yu K. 2004. Drosophila short neuropeptide F regulates food intake and body size. J Biol Chem 279: 50781–50789. 10.1074/jbc.M407842200 [DOI] [PubMed] [Google Scholar]
- Lee WP, Chiang MH, Chang LY, Shyu WH, Chiu TH, Fu TF, Wu T, Wu CL. 2021. Serotonin signals modulate mushroom body output neurons for sustaining water-reward long-term memory in Drosophila. Front Cell Dev Biol 9: 755574. 10.3389/fcell.2021.755574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z, Henderson K, Keleman K. 2022. A neural circuit linking learning and sleep in Drosophila long-term memory. Nat Commun 13: 609. 10.1038/s41467-022-28256-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LP, Siju KP, Aso Y, Friedrich AB, Bulteel AJ, Rubin GM, Grunwald Kadow IC. 2015. A higher brain circuit for immediate integration of conflicting sensory information in Drosophila. Curr Biol 25: 2203–2214. 10.1016/j.cub.2015.07.015 [DOI] [PubMed] [Google Scholar]
- Li X, Yu F, Guo A. 2009. Sleep deprivation specifically impairs short-term olfactory memory in Drosophila. Sleep 32: 1417–1424. 10.1093/sleep/32.11.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lindsey JW, Marin EC, Otto N, Dreher M, Dempsey G, Stark I, Bates AS, Pleijzier MW, Schlegel P, et al. 2020a. The connectome of the adult Drosophila mushroom body provides insights into function. Elife 9: e62576. 10.7554/eLife.62576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Mahoney BD, Jacob MS, Caron SJC. 2020b. Visual input into the Drosophila melanogaster mushroom body. Cell Rep 32: 108138. 10.1016/j.celrep.2020.108138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang Z, Syed S, Lyu C, Lincoln S, O'Neil J, Nguyen AD, Feng I, Young MW. 2021. Chronic social isolation signals starvation and reduces sleep in Drosophila. Nature 597: 239–244. 10.1038/s41586-021-03837-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Fernandez AI, Hinojos SJ, Aranda GP, James J, Seong CS, Han KA. 2018. The mushroom body D1 dopamine receptor controls innate courtship drive. Genes Brain Behav 17: 158–167. 10.1111/gbb.12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. 2023. Internal-state-dependent modulation of olfactory responses: a tale of dopamine neurons in the adult Drosophila mushroom body. Curr Opin Insect Sci 59: 101104. 10.1016/j.cois.2023.101104 [DOI] [PubMed] [Google Scholar]
- Lin S, Owald D, Chandra V, Talbot C, Huetteroth W, Waddell S. 2014. Neural correlates of water reward in thirsty Drosophila. Nat Neurosci 17: 1536–1542. 10.1038/nn.3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Senapati B, Tsao CH. 2019. Neural basis of hunger-driven behaviour in Drosophila. Open Biol 9: 180259. 10.1098/rsob.180259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Plaçais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. 2012. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature 488: 512–516. 10.1038/nature11304 [DOI] [PubMed] [Google Scholar]
- Liu S, Liu Q, Tabuchi M, Wu MN. 2016. Sleep drive is encoded by neural plastic changes in a dedicated circuit. Cell 165: 1347–1360. 10.1016/j.cell.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Nath T, Linneweber GA, Claeys A, Guo Z, Li J, Bengochea M, De Backer S, Weyn B, Sneyders M, et al. 2018. A simple computer vision pipeline reveals the effects of isolation on social interaction dynamics in Drosophila. PLoS Comput Biol 14: e1006410. 10.1371/journal.pcbi.1006410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Davis RL. 2009. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits 3: 5. 10.3389/neuro.04.005.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano V, Achsel T, Bagni C, Kanellopoulos AK. 2020. Modelling learning and memory in Drosophila to understand intellectual disabilities. Neuroscience 445: 12–30. 10.1016/j.neuroscience.2020.07.034 [DOI] [PubMed] [Google Scholar]
- Marquand K, Roselli C, Cervantes-Sandoval I, Boto T. 2023. Sleep benefits different stages of memory in Drosophila. Front Physiol 14: 1087025. 10.3389/fphys.2023.1087025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JR, Ernst R, Heisenberg M. 1998. Mushroom bodies suppress locomotor activity in Drosophila melanogaster. Learn Mem 5: 179–191. [PMC free article] [PubMed] [Google Scholar]
- Masuzzo A, Manière G, Viallat-Lieutaud A, Avazeri E, Zugasti O, Grosjean Y, Kurz CL, Royet J. 2019. Peptidoglycan-dependent NF-κB activation in a small subset of brain octopaminergic neurons controls female oviposition. Elife 8: e50559. 10.7554/eLife.50559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy LY, Sareen P, Davoudian PA, Nitabach MN. 2021. Dopaminergic mechanism underlying reward-encoding of punishment omission during reversal learning in Drosophila. Nat Commun 12: 1115. 10.1038/s41467-021-21388-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mery F, Kawecki TJ. 2005. A cost of long-term memory in Drosophila. Science 308: 1148. 10.1126/science.1111331 [DOI] [PubMed] [Google Scholar]
- Miyamoto D, Hirai D, Fung CC, Inutsuka A, Odagawa M, Suzuki T, Boehringer R, Adaikkan C, Matsubara C, Matsuki N, et al. 2016. Top-down cortical input during NREM sleep consolidates perceptual memory. Science 352: 1315–1318. 10.1126/science.aaf0902 [DOI] [PubMed] [Google Scholar]
- Montague SA, Baker BS. 2016. Memory elicited by courtship conditioning requires mushroom body neuronal subsets similar to those utilized in appetitive memory. PLoS ONE 11: e0164516. 10.1371/journal.pone.0164516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muria A, Musso PY, Durrieu M, Portugal FR, Ronsin B, Gordon MD, Jeanson R, Isabel G. 2021. Social facilitation of long-lasting memory is mediated by CO2 in Drosophila. Curr Biol 31: 2065–2074.e5. 10.1016/j.cub.2021.02.044 [DOI] [PubMed] [Google Scholar]
- Musso PY, Tchenio P, Preat T. 2015. Delayed dopamine signaling of energy level builds appetitive long-term memory in Drosophila. Cell Rep 10: 1023–1031. 10.1016/j.celrep.2015.01.036 [DOI] [PubMed] [Google Scholar]
- Nir Y, Andrillon T, Marmelshtein A, Suthana N, Cirelli C, Tononi G, Fried I. 2017. Selective neuronal lapses precede human cognitive lapses following sleep deprivation. Nat Med 23: 1474–1480. 10.1038/nm.4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöbel S, Danchin E, Isabel G. 2023. Mate copying requires the coincidence detector Rutabaga in the mushroom bodies of Drosophila melanogaster. iScience 26: 107682. 10.1016/j.isci.2023.107682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okray Z, Jacob PF, Stern C, Desmond K, Otto N, Talbot CB, Vargas-Gutierrez P, Waddell S. 2023. Multisensory learning binds neurons into a cross-modal memory engram. Nature 617: 777–784. 10.1038/s41586-023-06013-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Waddell S. 2015. Olfactory learning skews mushroom body output pathways to steer behavioral choice in Drosophila. Curr Opin Neurobiol 35: 178–184. 10.1016/j.conb.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Felsenberg J, Talbot CB, Das G, Perisse E, Huetteroth W, Waddell S. 2015. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron 86: 417–427. 10.1016/j.neuron.2015.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, Croset V, Otto N, Agarwal D, Treiber CD, Meschi E, Sims D, Waddell S. 2022. Gliotransmission of D-serine promotes thirst-directed behaviors in Drosophila. Curr Biol 32: 3952–3970.e8. 10.1016/j.cub.2022.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Casey BJ, Lee FS. 2013. The teenage brain: altered fear in humans and mice. Curr Dir Psychol Sci 22: 146–151. 10.1177/0963721412471323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Liston C, Jing D, Ninan I, Yang RR, Witztum J, Murdock MH, Dincheva I, Bath KG, Casey BJ, et al. 2016. Dynamic changes in neural circuitry during adolescence are associated with persistent attenuation of fear memories. Nat Commun 7: 11475. 10.1038/ncomms11475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlowsky A, Schor J, Plaçais PY, Preat T. 2018. A GABAergic feedback shapes dopaminergic input on the Drosophila mushroom body to promote appetitive long-term memory. Curr Biol 28: 1783–1793.e4. 10.1016/j.cub.2018.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech U, Pooryasin A, Birman S, Fiala A. 2013. Localization of the contacts between Kenyon cells and aminergic neurons in the Drosophila melanogaster brain using SplitGFP reconstitution. J Comp Neurol 521: 3992–4026. 10.1002/cne.23388 [DOI] [PubMed] [Google Scholar]
- Perisse E, Yin Y, Lin AC, Lin S, Huetteroth W, Waddell S. 2013. Different Kenyon cell populations drive learned approach and avoidance in Drosophila. Neuron 79: 945–956. 10.1016/j.neuron.2013.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisse E, Owald D, Barnstedt O, Talbot CB, Huetteroth W, Waddell S. 2016. Aversive learning and appetitive motivation toggle feed-forward inhibition in the Drosophila mushroom body. Neuron 90: 1086–1099. 10.1016/j.neuron.2016.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisse E, Miranda M, Trouche S. 2023. Modulation of aversive value coding in the vertebrate and invertebrate brain. Curr Opin Neurobiol 79: 102696. 10.1016/j.conb.2023.102696 [DOI] [PubMed] [Google Scholar]
- Petruccelli E, Feyder M, Ledru N, Jaques Y, Anderson E, Kaun KR. 2018. Alcohol activates scabrous-notch to influence associated memories. Neuron 100: 1209–1223.e4. 10.1016/j.neuron.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruccelli E, Brown T, Waterman A, Ledru N, Kaun KR. 2020. Alcohol causes lasting differential transcription in Drosophila mushroom body neurons. Genetics 215: 103–116. 10.1534/genetics.120.303101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer K, Homberg U. 2014. Organization and functional roles of the central complex in the insect brain. Annu Rev Entomol 59: 165–184. 10.1146/annurev-ento-011613-162031 [DOI] [PubMed] [Google Scholar]
- Pitman JL, McGill JJ, Keegan KP, Allada R. 2006. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 441: 753–756. 10.1038/nature04739 [DOI] [PubMed] [Google Scholar]
- Plaçais PY, Preat T. 2013. To favor survival under food shortage, the brain disables costly memory. Science 339: 440–442. 10.1126/science.1226018 [DOI] [PubMed] [Google Scholar]
- Plaçais PY, Trannoy S, Isabel G, Aso Y, Siwanowicz I, Belliart-Guerin G, Vernier P, Birman S, Tanimoto H, Preat T. 2012. Slow oscillations in two pairs of dopaminergic neurons gate long-term memory formation in Drosophila. Nat Neurosci 15: 592–599. 10.1038/nn.3055 [DOI] [PubMed] [Google Scholar]
- Plaçais PY, Trannoy S, Friedrich AB, Tanimoto H, Preat T. 2013. Two pairs of mushroom body efferent neurons are required for appetitive long-term memory retrieval in Drosophila. Cell Rep 5: 769–780. 10.1016/j.celrep.2013.09.032 [DOI] [PubMed] [Google Scholar]
- Plaçais PY, de Tredern E, Scheunemann L, Trannoy S, Goguel V, Han KA, Isabel G, Preat T. 2017. Upregulated energy metabolism in the Drosophila mushroom body is the trigger for long-term memory. Nat Commun 8: 15510. 10.1038/ncomms15510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribbenow C, Chen YC, Heim MM, Laber D, Reubold S, Reynolds E, Balles I, Fernández-d V Alquicira T, Suarez-Grimalt R, Scheunemann L, et al. 2022. Postsynaptic plasticity of cholinergic synapses underlies the induction and expression of appetitive and familiarity memories in Drosophila. Elife 11: e80445. 10.7554/eLife.80445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quercia A, Zappasodi F, Committeri G, Ferrara M. 2018. Local use-dependent sleep in wakefulness links performance errors to learning. Front Hum Neurosci 12: 122. 10.3389/fnhum.2018.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raccuglia D, Huang S, Ender A, Heim MM, Laber D, Suárez-Grimalt R, Liotta A, Sigrist SJ, Geiger JRP, Owald D. 2019. Network-specific synchronization of electrical slow-wave oscillations regulates sleep drive in Drosophila. Curr Biol 29: 3611–3621.e3. 10.1016/j.cub.2019.08.070 [DOI] [PubMed] [Google Scholar]
- Raccuglia D, Suárez-Grimalt R, Krumm L, Brodersen CB, Ender A, Jagannathan SR, Winter Y, Yvon-Durocher G, Kempter R, Geiger JR, et al. 2022. Coherent multi-level network oscillations create neural filters to favor quiescence over navigation in Drosophila. bioRxiv 10.1101/2022.03.11.483976 [DOI] [Google Scholar]
- Ramdya P, Lichocki P, Cruchet S, Frisch L, Tse W, Floreano D, Benton R. 2015. Mechanosensory interactions drive collective behaviour in Drosophila. Nature 519: 233–236. 10.1038/nature14024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezával C, Pavlou HJ, Dornan AJ, Chan YB, Kravitz EA, Goodwin SF. 2012. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr Biol 22: 1155–1165. 10.1016/j.cub.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C, Dickson BJ. 2010. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol 20: 1000–1005. 10.1016/j.cub.2010.03.061 [DOI] [PubMed] [Google Scholar]
- Riemensperger T, Völler T, Stock P, Buchner E, Fiala A. 2005. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol 15: 1953–1960. 10.1016/j.cub.2005.09.042 [DOI] [PubMed] [Google Scholar]
- Ries AS, Hermanns T, Poeck B, Strauss R. 2017. Serotonin modulates a depression-like state in Drosophila responsive to lithium treatment. Nat Commun 8: 15738. 10.1038/ncomms15738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BG, Khurana S, Kuperman A, Atkinson NS. 2012. Neural adaptation leads to cognitive ethanol dependence. Curr Biol 22: 2338–2341. 10.1016/j.cub.2012.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers EW, Krenz WD, Baro DJ. 2011. Tonic dopamine induces persistent changes in the transient potassium current through translational regulation. J Neurosci 31: 13046–13056. 10.1523/JNEUROSCI.2194-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse S, Beshel J. 2016. The good, the bad, and the hungry: how the central brain codes odor valence to facilitate food approach in Drosophila. Curr Opin Neurobiol 40: 53–58. 10.1016/j.conb.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin S, Boehm AC, Kobler JM, De Backer JF, Grunwald Kadow IC. 2018. Internal state dependent odor processing and perception-the role of neuromodulation in the fly olfactory system. Front Cell Neurosci 12: 11. 10.3389/fncel.2018.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayin S, De Backer JF, Siju KP, Wosniack ME, Lewis LP, Frisch LM, Gansen B, Schlegel P, Edmondson-Stait A, Sharifi N, et al. 2019. A neural circuit arbitrates between persistence and withdrawal in hungry Drosophila. Neuron 104: 544–558.e6. 10.1016/j.neuron.2019.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaplen KM, Talay M, Nunez KM, Salamon S, Waterman AG, Gang S, Song SL, Barnea G, Kaun KR. 2020. Circuits that encode and guide alcohol-associated preference. Elife 9: e48730. 10.7554/eLife.48730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaplen KM, Talay M, Fisher JD, Cohn R, Sorkaç A, Aso Y, Barnea G, Kaun KR. 2021. Transsynaptic mapping of Drosophila mushroom body output neurons. Elife 10: e63379. 10.7554/eLife.63379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheunemann L, Plaçais PY, Dromard Y, Schwarzel M, Preat T. 2018. Dunce phosphodiesterase acts as a checkpoint for Drosophila long-term memory in a pair of serotonergic neurons. Neuron 98: 350–365.e5. 10.1016/j.neuron.2018.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheunemann L, Lampin-Saint-Amaux A, Schor J, Preat T. 2019. A sperm peptide enhances long-term memory in female Drosophila. Sci Adv 5: eaax3432. 10.1126/sciadv.aax3432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretter CE, Vielmetter J, Bartos I, Marka Z, Marka S, Argade S, Mazmanian SK. 2018. A gut microbial factor modulates locomotor behaviour in Drosophila. Nature 563: 402–406. 10.1038/s41586-018-0634-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. 2003. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci 23: 10495–10502. 10.1523/JNEUROSCI.23-33-10495.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidner G, Robinson JE, Wu M, Worden K, Masek P, Roberts SW, Keene AC, Joiner WJ. 2015. Identification of neurons with a privileged role in sleep homeostasis in Drosophila melanogaster. Curr Biol 25: 2928–2938. 10.1016/j.cub.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séjourné J, Plaçais PY, Aso Y, Siwanowicz I, Trannoy S, Thoma V, Tedjakumala SR, Rubin GM, Tchénio P, Ito K, et al. 2011. Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat Neurosci 14: 903–910. 10.1038/nn.2846 [DOI] [PubMed] [Google Scholar]
- Senapati B, Tsao CH, Juan YA, Chiu TH, Wu CL, Waddell S, Lin S. 2019. A neural mechanism for deprivation state-specific expression of relevant memories in Drosophila. Nat Neurosci 22: 2029–2039. 10.1038/s41593-019-0515-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Lin HH, Shepherd AK, Volkan PC, Su CY, Wang JW. 2019. Social context enhances hormonal modulation of pheromone detection in Drosophila. Curr Biol 29: 3887–3898.e4. 10.1016/j.cub.2019.09.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. 2008. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol 18: 1110–1117. 10.1016/j.cub.2008.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Keene AC. 2021. The regulation of Drosophila sleep. Curr Biol 31: R38–R49. 10.1016/j.cub.2020.10.082 [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. 2000. Correlates of sleep and waking in Drosophila melanogaster. Science 287: 1834–1837. 10.1126/science.287.5459.1834 [DOI] [PubMed] [Google Scholar]
- Shen P, Wan X, Wu F, Shi K, Li J, Gao H, Zhao L, Zhou C. 2023. Neural circuit mechanisms linking courtship and reward in Drosophila males. Curr Biol 33: 2034–2050.e8. 10.1016/j.cub.2023.04.041 [DOI] [PubMed] [Google Scholar]
- Shih HW, Wu CL, Chang SW, Liu TH, Lai JS, Fu TF, Fu CC, Chiang AS. 2015. Parallel circuits control temperature preference in Drosophila during ageing. Nat Commun 6: 7775. 10.1038/ncomms8775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. 2012. Sexual deprivation increases ethanol intake in Drosophila. Science 335: 1351–1355. 10.1126/science.1215932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siju KP, Bräcker LB, Grunwald Kadow IC. 2014. Neural mechanisms of context-dependent processing of CO2 avoidance behavior in fruit flies. Fly 8: 68–74. 10.4161/fly.28000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siju KP, Štih V, Aimon S, Gjorgjieva J, Portugues R, Grunwald Kadow IC. 2020. Valence and state-dependent population coding in dopaminergic neurons in the fly mushroom body. Curr Biol 30: 2104–2115.e4. 10.1016/j.cub.2020.04.037 [DOI] [PubMed] [Google Scholar]
- Siju KP, De Backer JF, Grunwald Kadow IC. 2021. Dopamine modulation of sensory processing and adaptive behavior in flies. Cell Tissue Res 383: 207–225. 10.1007/s00441-020-03371-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva B, Mantha OL, Schor J, Pascual A, Plaçais PY, Pavlowsky A, Preat T. 2022. Glia fuel neurons with locally synthesized ketone bodies to sustain memory under starvation. Nat Metab 4: 213–224. 10.1038/s42255-022-00528-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D, Aso Y, Jin X, Chen N, Felix M, Rubin GM, Nitabach MN. 2015a. Propagation of homeostatic sleep signals by segregated synaptic microcircuits of the Drosophila mushroom body. Curr Biol 25: 2915–2927. 10.1016/j.cub.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D, Aso Y, Rubin GM, Nitabach MN. 2015b. Control of sleep by dopaminergic inputs to the Drosophila mushroom body. Front Neural Circuits 9: 73. 10.3389/fncir.2015.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore TR, Jonaitis J, Dacks AM. 2023. Heterogeneous receptor expression underlies non-uniform peptidergic modulation of olfaction in Drosophila. Nat Commun 14: 5280. 10.1038/s41467-023-41012-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensmyr MC, Dweck HK, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. 2012. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151: 1345–1357. 10.1016/j.cell.2012.09.046 [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Hansen L, Li Y, Gomez RS, Ito K. 1998. Evolution, discovery, and interpretations of arthropod mushroom bodies. Learn Mem 5: 11–37. [PMC free article] [PubMed] [Google Scholar]
- Suárez-Grimalt R, Raccuglia D. 2021. The neural architecture of sleep regulation – insights from Drosophila. Neuroforum 27: 189–199. 10.1515/nf-2021-0018 [DOI] [Google Scholar]
- Sun Y, Qiu R, Li X, Cheng Y, Gao S, Kong F, Liu L, Zhu Y. 2020. Social attraction in Drosophila is regulated by the mushroom body and serotonergic system. Nat Commun 11: 5350. 10.1038/s41467-020-19102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainton-Heap LAL, Kirszenblat LC, Notaras ET, Grabowska MJ, Jeans R, Feng K, Shaw PJ, van Swinderen B. 2021. A paradoxical kind of sleep in Drosophila melanogaster. Curr Biol 31: 578–590.e6. 10.1016/j.cub.2020.10.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan D, Eberl F, Veit D, Hansson BS, Knaden M, Sachse S. 2022. Aversive bimodal associations differently impact visual and olfactory memory performance in Drosophila. iScience 25: 105485. 10.1016/j.isci.2022.105485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik SM, Davis RL. 2009. Dynamics of learning-related cAMP signaling and stimulus integration in the Drosophila olfactory pathway. Neuron 64: 510–521. 10.1016/j.neuron.2009.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CH, Chen CC, Lin CH, Yang HY, Lin S. 2018. Drosophila mushroom bodies integrate hunger and satiety signals to control innate food-seeking behavior. Elife 7: e35264. 10.7554/eLife.35264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrel O, Goguel V, Preat T. 2018. Amnesiac is required in the adult mushroom body for memory formation. J Neurosci 38: 9202–9214. 10.1523/JNEUROSCI.0876-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Gangarossa G. 2021. The tail of the striatum: from anatomy to connectivity and function. Trends Neurosci 44: 203–214. 10.1016/j.tins.2020.10.016 [DOI] [PubMed] [Google Scholar]
- van Alphen B, Yap MH, Kirszenblat L, Kottler B, van Swinderen B. 2013. A dynamic deep sleep stage in Drosophila. J Neurosci 33: 6917–6927. 10.1523/JNEUROSCI.0061-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen B, Semenza ER, Yap M, van Swinderen B, Allada R. 2021. A deep sleep stage in Drosophila with a functional role in waste clearance. Sci Adv 7: eabc2999. 10.1126/sciadv.abc2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. 2004. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305: 1017–1019. 10.1126/science.1098975 [DOI] [PubMed] [Google Scholar]
- Vargas MA, Luo N, Yamaguchi A, Kapahi P. 2010. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr Biol 20: 1006–1011. 10.1016/j.cub.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar ME, Pavão-Delgado M, Amigo M, Jacob PF, Merabet N, Pinot A, Perry SA, Waddell S, Perisse E. 2022. Differential coding of absolute and relative aversive value in the Drosophila brain. Curr Biol 32: 4576–4592.e5. 10.1016/j.cub.2022.08.058 [DOI] [PubMed] [Google Scholar]
- Vogt K, Schnaitmann C, Dylla KV, Knapek S, Aso Y, Rubin GM, Tanimoto H. 2014. Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. Elife 3: e02395. 10.7554/eLife.02395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt K, Aso Y, Hige T, Knapek S, Ichinose T, Friedrich AB, Turner GC, Rubin GM, Tanimoto H. 2016. Direct neural pathways convey distinct visual information to Drosophila mushroom bodies. Elife 5: e14009. 10.7554/eLife.14009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt K, Zimmerman DM, Schlichting M, Hernandez-Nunez L, Qin S, Malacon K, Rosbash M, Pehlevan C, Cardona A, Samuel ADT. 2021. Internal state configures olfactory behavior and early sensory processing in Drosophila larvae. Sci Adv 7: eabd6900. 10.1126/sciadv.abd6900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. 2011. Local sleep in awake rats. Nature 472: 443–447. 10.1038/nature10009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Anderson DJ. 2010. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature 463: 227–231. 10.1038/nature08678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JT, Donlea JM. 2021. Sleep deprivation results in diverse patterns of synaptic scaling across the Drosophila mushroom bodies. Curr Biol 31: 3248–3261.e3. 10.1016/j.cub.2021.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright GA, Mustard JA, Simcock NK, Ross-Taylor AA, McNicholas LD, Popescu A, Marion-Poll F. 2010. Parallel reinforcement pathways for conditioned food aversions in the honeybee. Curr Biol 20: 2234–2240. 10.1016/j.cub.2010.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MS, Liao TW, Wu CY, Hsieh TH, Kuo PC, Li YC, Cheng KC, Chiang HC. 2023. Aversive conditioning information transmission in Drosophila. Cell Rep 42: 113207. 10.1016/j.celrep.2023.113207 [DOI] [PubMed] [Google Scholar]
- Yamada D, Davidson AM, Hige T. 2023. Cyclic nucleotide-induced bidirectional long-term synaptic plasticity in Drosophila mushroom body. bioRxiv 10.1101/2023.09.28.560058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata N, Ichinose T, Aso Y, Plaçais PY, Friedrich AB, Sima RJ, Preat T, Rubin GM, Tanimoto H. 2015. Distinct dopamine neurons mediate reward signals for short- and long-term memories. Proc Natl Acad Sci 112: 578–583. 10.1073/pnas.1421930112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Seto ES. 2014. Dopamine dynamics and signaling in Drosophila: an overview of genes, drugs and behavioral paradigms. Exp Anim 63: 107–119. 10.1538/expanim.63.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D, Maeyama Y, Tabata T. 2023. Combinatory actions of co-transmitters in dopaminergic systems modulate Drosophila olfactory memories. J Neurosci 43: 8294–8305. 10.1523/JNEUROSCI.2152-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Bertolucci F, Wolf R, Heisenberg M. 2013. Flies cope with uncontrollable stress by learned helplessness. Curr Biol 23: 799–803. 10.1016/j.cub.2013.03.054 [DOI] [PubMed] [Google Scholar]
- Yang CH, Shih MF, Chang CC, Chiang MH, Shih HW, Tsai YL, Chiang AS, Fu TF, Wu CL. 2016. Additive expression of consolidated memory through Drosophila mushroom body subsets. PLoS Genet 12: e1006061. 10.1371/journal.pgen.1006061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MHW, Grabowska MJ, Rohrscheib C, Jeans R, Troup M, Paulk AC, van Alphen B, Shaw PJ, van Swinderen B. 2017. Oscillatory brain activity in spontaneous and induced sleep stages in flies. Nat Commun 8: 1815. 10.1038/s41467-017-02024-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Li X, Zhang R, Lv M, Wang Y, Tan K, Xia X, Wan J, Jing M, Zhang X, et al. 2023. Local 5-HT signaling bi-directionally regulates the coincidence time window for associative learning. Neuron 111: 1118–1135.e5. 10.1016/j.neuron.2022.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI. 2005. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438: 179–184. 10.1038/nature04216 [DOI] [PubMed] [Google Scholar]
- Zhang X, Noyes NC, Zeng J, Li Y, Davis RL. 2019. Aversive training induces both presynaptic and postsynaptic suppression in Drosophila. J Neurosci 39: 9164–9172. 10.1523/JNEUROSCI.1420-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Lenek D, Dag U, Dickson BJ, Keleman K. 2018. Persistent activity in a recurrent circuit underlies courtship memory in Drosophila. Elife 7: e31425. 10.7554/eLife.31425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Rao Y, Rao Y. 2008. A subset of octopaminergic neurons are important for Drosophila aggression. Nat Neurosci 11: 1059–1067. 10.1038/nn.2164 [DOI] [PubMed] [Google Scholar]
- Zhou C, Huang H, Kim SM, Lin H, Meng X, Han KA, Chiang AS, Wang JW, Jiao R, Rao Y. 2012. Molecular genetic analysis of sexual rejection: roles of octopamine and its receptor OAMB in Drosophila courtship conditioning. J Neurosci 32: 14281–14287. 10.1523/JNEUROSCI.0517-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolin A, Cohn R, Pang R, Siliciano AF, Fairhall AL, Ruta V. 2021. Context-dependent representations of movement in Drosophila dopaminergic reinforcement pathways. Nat Neurosci 24: 1555–1566. 10.1038/s41593-021-00929-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.