Abstract
Background
Human immunodeficiency virus (HIV) infection is highly prevalent and often coexists with other infectious diseases, especially Hepatitis B virus (HBV) and Hepatitis C virus (HCV). Men who have sex with men (MSM) represent a vulnerable population in terms of HIV infection. We aimed to determine the prevalence of HCV, HBV among HIV‐infected MSM.
Methods
This systematic review and meta‐analysis searched PubMed, Cochrane, Scopus, Web of Science, and ProQuest up‐to 2023/04/22. All studies reporting the prevalence of HBV or HCV infection in MSM PLHIV were included. Meta‐analysis used random effect model for synthesis and I 2 along with prediction interval for heterogeneity. Subgroup analysis based on continent and meta‐regression for study size, average age and year of publication were used to explore heterogeneity. Modified Newcastle‐Ottawa Scale was used to evaluate the quality of studies according to the protocol (PROSPERO: CRD42023428764).
Results
Fifty‐six of 5948 studies are included. In 53 studies with 3,07,589 participants, a pooled prevalence of 7% (95% confidence interval [CI]: 5–10) was found for HCV among MSM PLHIV, while a 9% (95% CI: 4–18) prevalence was found for HBV infection from five studies which included 5641 MSM PLHIV. Asia reported the lowest pooled prevalence at 5.84% (95% CI: 2.98–11.13) for HCV while Europe reported the highest pooled prevalence at 7.76% (95% CI: 4.35–13.45). Baujat plot and influence diagnostic identified contributors to influence and between‐study heterogeneity. Sensitivity analyses omitting these studies result in considerably more precise estimates. Another sensitivity analysis as leave‐one‐out meta‐analysis did not change any pooled estimate significantly.
Conclusion
There is a significant burden of HCV and HBV among MSM PLHIV worldwide, with varying prevalence rates. Future studies should focus on these multimorbidity clusters and investigate factors influencing disease burden, long‐term outcomes, optimal testing strategies, and tailored interventions.
Keywords: Hepatitis B virus, Hepatitis C virus, human immunodeficiency virus (HIV), infectious diseases, men having sex with men (MSM), people living with HIV (PLHIV), prevalence, sexually transmitted infections (STIs), systematic review and meta analysis
1. INTRODUCTION
The presence of three blood‐borne viruses, namely human immunodeficiency virus (HIV), hepatitis C virus (HCV), and hepatitis B virus (HBV), can have a substantial impact on morbidity and pose significant challenges to global health. Due to the similar blood‐borne transmission methods of these viruses, including sexual practices, vertical route, and use of shared needles, syringes, and other injection equipment, there is a substantial potential of concomitant infection in people. 1
WHO estimated that in 2022, approximately 242,000 people died from hepatitis C, mostly from cirrhosis and hepatocellular carcinoma. 2 There were 13,800 estimated new HBV infections and 67,400 estimated HCV infections in 2022. 3 In 2019, the worldwide mortality attributable to HCV infections exceeded 290 thousand, while HBV infections were responsible for over 820 thousand deaths. 2 However, the undetected rate of HBV or HCV viruses is close to 80% 4 and 96% of deaths from viral hepatitis are caused by HBV or HCV. 2 Globally, 37.7 million individuals were predicted to be HIV‐positive in 2020. In the year 2020, there were an estimated 1.5 million newly acquired HIV infections, and the global burden of HIV‐related mortality reached approximately 680,000 deaths. 2 Out of the estimated global population of 36.7 million people living with HIV (PLHIV), approximately 2.3 million individuals show serological evidence of previous or current HCV infection, indicated by positive results for HCV antibodies (anti‐HCV positive). Additionally, among the 36.7 million PLHIV, around 2.7 million individuals also have HBV infection. The worldwide prevalence of HBV infection in PLHIV is approximately 7.4%. 5 , 6 , 7
Compared to those who have only one of these diseases, those with or HIV‐HCV or HIV‐HBV coinfection are more likely to experience stigma and restricted access to healthcare facilities in some cases as well as rapid disease development, numerous liver‐related ailments, and non‐hepatic organ failure, all of which can lead to death. They might also be part of a population that is stigmatized as a result of using injectable drugs or engaging in certain sexual activities. 1 , 5 With shared health determinants, mechanisms of transmission, and high rates of coinfection, HIV and viral hepatitis together make up a cluster of interconnected communicable diseases. Within countries, certain demographic groups have different rates of HBV or HCV and HIV infection than others. 2 , 5 Men who have sex with men (MSM) are considered a high‐risk group for both HIV and hepatitis infections. They account for 23% of those who contract HIV. 2 Specifically, higher risk of HBV infection is associated with MSM. 8 Reinfection has also been reported even among HIV‐infected MSM who were successfully cured with treatment for hepatitis C. 9
Previous observational studies, and systematic reviews have looked the prevalence of HBV and HCV among people living with HIV/AIDS as well as prevalence of HIV, HCV, and HBV among high‐risk groups and at specific geographic regions. 10 , 11 , 12 , 13 , 14 , 15 , 16 Many recent studies are available which provide valuable insight 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 and are not included in the existing reviews. 12 , 26 This systematic review and meta‐analysis investigated the total pooled prevalence of HBV and HCV among the HIV‐infected MSM population including updated data with robust synthesis.
2. METHODS
The systematic review adhered to the PRISMA guidelines, as detailed in Table S1. The review protocol for this particular study was officially registered in PROSPERO, an international database for systematic review protocols. It was assigned the unique registration number CRD42023428764. Ethical approval and informed consent were nor applicable for this study since it is based on published data.
2.1. Inclusion and exclusion criteria
The aim of this systematic review was to assess the prevalence of HCV and HBV infections among MSM PLHIV. The review employed inclusive criteria, encompassing MSM PLHIV participants. All original studies reporting the prevalence of HBV or HCV infection in MSM PLHIV were included. The diagnostic criteria for HCV and HBV must be based on serum HCV‐RNA positivity or anti‐HCV IgG positivity. Narrative reviews, protocols, unpublished reports, editorials, clinical case reports, abstracts, and commentaries were excluded. Additionally, studies with a sample size of less than 100 were also excluded. This study included preprints and published articles that were available in English, without geographical or research environment restrictions. For further details on the eligibility criteria, please refer to Table S2.
2.2. Searching and screening
A comprehensive literature search was conducted on April 22, 2023, across several databases, including PubMed, Cochrane, Web of Science, Scopus, and ProQuest. The search strategy employed a combination of keywords, synonyms, and truncated words related to HIV, Hepatitis, HCV, HBV, and MSM. English‐language publications were specifically included, while there were no restrictions based on the publication year. The search strategy aimed to identify all relevant studies that mentioned on the number of HBV or HCV infections among MSM PLHIV. We have furnished a transparent reproducible search strategy across all databases in Table S3.
Upon obtaining the search results, duplicate entries were removed, and Mendeley was used for managing the references. The initial screening process was carried out by two researchers, A.Y. and M.S., utilizing Rayyan. They assessed the titles and abstracts of each study to exclude irrelevant articles. Subsequently, the full‐text readings of the remaining studies were carefully evaluated to confirm their eligibility for inclusion in the review. The screening process was conducted independently by the two researchers to ensure accuracy and consistency. In cases of disagreements or discrepancies, a third senior researcher, Q.S.Z., was consulted to provide a binding independent assessment of the study's eligibility.
2.3. Data extraction and quality assessment
Three investigators (A.Y., M.A., and M.N.K.) conducted data extraction from the included studies. The extracted data encompassed various aspects such as the first author's name, country of study, publication year, age, male percentage, type of population, total population of MSM PLHIV, and the number of HCV or HBV infections. To evaluate the quality of the included studies, a modified version of the Newcastle‐Ottawa Scale was employed, with a scoring range from 0 to 6. This scale assessed the methodological rigor and potential bias of each study based on four criteria: representativeness (scored 0‐2), sample size (scored 0‐1), definition of infections (scored 0‐2), and ascertainment of HCV or HBV infections (scored 0‐1). 27 , 28 A higher score indicated a higher level of methodological quality and a lower risk of bias in the respective study. 29
2.4. Statistical analysis
A random‐effect model was used to calculate the combined prevalence, taking into account the potential variation between studies. The prevalence from individual studies was logit‐transformed. These were synthesized using random intercept logistic regression model. Heterogeneity, or the degree of variability between study results, was assessed using prediction interval, I 2 and tau‐sqaured. I 2 values range from 0% to 100%, with higher values indicating greater heterogeneity. 30 95% prediction interval is a more intuitive and appropriate way of representing heterogeneity. 31 For future original studies answering the same research question, the individual study estimates are expected fall within this range 95% of the time. Tau‐squared is computed using maximum likelihood estimator. 32 A p ≤ 0.05 is considered to be significant. Then, subgroup analyses and meta‐regression were performed to investigate potential factors contributing to the observed heterogeneity. 33
For determining the risk of publication bias and small study effects, Doi plots with LFK index were utilized. Doi plots help assess publication bias in single‐group studies as they don't rely on the concept of significance. 34 They plot the individual study estimates against a folded normal quantile. Study symmetry is assessed visually, and quantified using LFK index. 35 The statistical analyses, including the calculation of pooled prevalence, assessment of heterogeneity, other analyses, and generation of plots, were conducted using R version 4.3. 36
3. RESULTS
3.1. Literature search
A total of 5948 records were obtained from multiple databases, out of which 1856 records were identified as duplicates. After removing these duplicates, the remaining 4092 records underwent primary screening based on reading title and abstract. A total of 114 records were deemed eligible for full‐text reading. Sixty‐seven studies were then excluded in which seven were with incorrect population, 11 were having wrong study design, 23 studies didn't mentioned prevalence among MSM, 25 were case reports, three were with duplicate data. Twelve records were identified from reference searching in which one study was excluded due to incorrect population, resulting in 11 studies. Overall, 56 studies met the eligibility criteria for inclusion in the systematic review and meta‐analysis (Figure 1).
Figure 1.
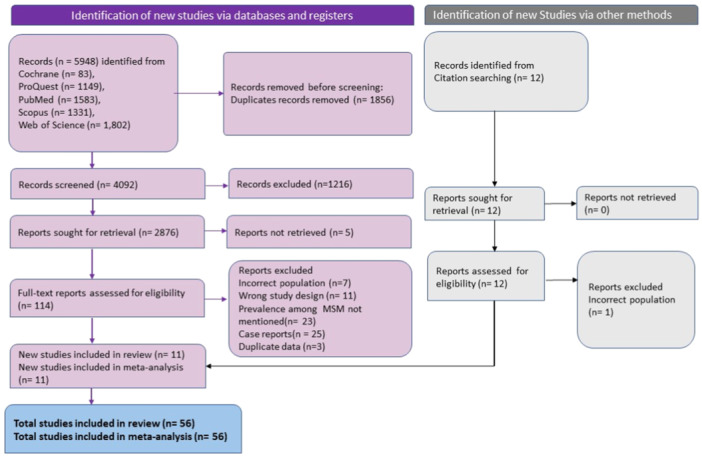
PRISMA flowchart depicting literature search and screening process.
3.2. Characteristics of included studies
Fifty‐six studies fulfilled the inclusion criteria, as shown in Table 1, comprising a total of 309203 MSM PLHIV. Switzerland contributed five studies, 18 , 20 , 37 , 38 , 39 United States had 11 studies, 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 Spain had four studies, 51 , 52 , 53 , 54 Netherlands had four studies, 55 , 56 , 57 , 58 three studies were from France, 17 , 59 , 60 six studies were from Taiwan, 22 , 61 , 62 , 63 , 64 , 65 eight studies were from United Kingdom, 19 , 21 , 23 , 25 , 66 , 67 , 68 , 69 two studies were from Europe, 70 , 71 two studies from Japan, 24 , 72 two studies from Australia. 73 , 74 One study each were from Belgium, 75 Denmark, 76 Canada, 77 Italy, 78 Sri Lanka, 79 Germany, 80 South Korea, 81 China, 82 and Thailand. 83 Two studies reported both HCV and HBV data, and eight studies were conducted across multiple centers. Among the study designs, eight were prospective cohort, 14 were observational, 13 were retrospective cohort, 10 were observational cohort, one was longitudinal, and nine were cross‐sectional. Additionally, one study used a nested case‐control design. Of the included studies, 53 reported HCV cases, while five reported HBV prevalence among MSM PLHIV. The studies included were of average quality, with seven studies scoring 6, 22 studies scoring 5, 17 studies scoring 4, six studies scoring 3, and four studies scoring 2 on the modified Newcastle‐Ottawa scale. Further Details regarding the quality assessment of the studies can be found in Table S4.
Table 1.
Characteristic of all included studies.
| Author | Year | Country/region | Study Design | Age (mean/median) | HCV infections | HBV infections | Total MSM PLHIV |
|---|---|---|---|---|---|---|---|
| Chen | 2019 | USA | Retrospective cohort | N/A | 371 | N/A | 1935 |
| Vanhommerig | 2017 | Europe | Observational cohort | 37.1 | 563 | N/A | 5038 |
| Gamage | 2011 | Sri Lanka | Retrospective cohort | 32 | 40 | N/A | 1445 |
| Castry | 2021 | France | Prospective cohort | 44 | 330 | N/A | 14,273 |
| Rooijen | 2016 | Netherlands | Longitudinal | 41 | 112 | N/A | 1742 |
| Pradat | 2018 | France | Observational cohort | 49 | 5557 | N/A | 40,714 |
| Apers | 2014 | Belgium | Retrospective cohort | 40.8 | 99 | N/A | 275 |
| Marcellin | 2015 | France | Cross‐sectional | N/A | 93 | N/A | 1037 |
| Dougan | 2007 | UK | Observational cohort | N/A | 11 | N/A | 242 |
| Barfod | 2011 | Denmark | Retrospective cohort | N/A | 41 | N/A | 1846 |
| Nwulia | 2015 | USA | Observational cohort | 32 | N/A | 277 | 2375 |
| Rauch | 2005 | Switzerland | Prospectivecohort | 35 | 2638 | N/A | 7899 |
| Huang | 2019 | Taiwan | Retrospective cohort | 33.7 | 120 | 26 | 219 |
| Nishijima | 2014 | Japan | Observational | 35 | 21 | N/A | 1182 |
| Helm | 2011 | Europe | Observational cohort | 31.6 | 124 | N/A | 4724 |
| Gabai | 2020 | USA | Observational | N/A | 2762 | N/A | 54,077 |
| Jongen | 2020 | Netherlands | Observational | N/A | 16 | N/A | 1372 |
| Cuomo | 2018 | Italy | Observational | 51 | 40 | N/A | 421 |
| Raymond | 2011 | USA | Cross‐sectional | N/A | 32 | N/A | 207 |
| Breskin | 2015 | USA | Observational | 34 | 2016 | N/A | 41,303 |
| Kouyos | 2014 | USA | Observational | 34 | 23 | N/A | 510 |
| Medland | 2017 | Australia | Retrospective cohort | 43.1 | 37 | N/A | 822 |
| Santen | 2021 | USA | Observational cohort | 35 | 361 | N/A | 9699 |
| Chen | 2016 | USA | Retrospective cohort | 38.4 | 42 | N/A | 1147 |
| Sun | 2014 | Taiwan | Observational | 28.5 | N/A | 37 | 767 |
| Newsum | 2019 | Netherland | Observational | 42.6 | 313 | N/A | 501 |
| Kusejko | 2022 | Switzerland | Prospective cohort | N/A | 28 | N/A | 4641 |
| Garg | 2013 | USA | Retrospective cohort | 38 | 64 | N/A | 1171 |
| Salazar‐Vizcaya | 2015 | Switzerland | Prospective cohort | N/A | 314 | N/A | 1455 |
| Turner | 2006 | UK | Cross sectional | 34 | 11 | N/A | 308 |
| Giraudon | 2007 | UK | Observational | N/A | 389 | N/A | 42,985 |
| Palacios | 2015 | Spain | Cross‐sectional, open observational | 42.9 | 61 | N/A | 725 |
| Jin | 2010 | Australia | Observational cohort | N/A | 23 | N/A | 245 |
| Taylor | 2011 | USA | Prospective cohort | N/A | 36 | N/A | 1830 |
| Sun | 2012 | Taiwan | Nested case‐control | 44 | 28 | N/A | 731 |
| Wandeler | 2012 | Switzerland | Prospective cohort | 38 | 147 | N/A | 4629 |
| Daskalopoulou | 2014 | UK | Cross‐sectional | N/A | 159 | N/A | 1195 |
| Lin | 2014 | China | Observational | 38 | 14 | N/A | 1311 |
| Sobrino‐Vegas | 2014 | Spain | Prospective cohort | N/A | 1227 | N/A | 7977 |
| Burchell | 2015 | Canada | Observational cohort | 41 | 51 | N/A | 1534 |
| Jansen | 2015 | Germany | Prospective cohort | 33 | 1784 | 468 | 1838 |
| Lee | 2016 | South Korea | Retrospective cohort | 44 | 41 | N/A | 790 |
| Ho | 2020 | Taiwan | Retrospective cohort | 28.7 | 277 | N/A | 3495 |
| Santen | 2017 | Netherlands | Observational | N/A | 703 | N/A | 10,061 |
| Willekens | 2021 | Spain | Cross‐sectional | N/A | 4 | N/A | 301 |
| Roche | 2022 | UK | Observational | N/A | 56 | N/A | 1497 |
| Serna | 2020 | Spain | Prospective cohort | 44.1 | 21 | N/A | 242 |
| Garvey | 2021 | UK | Retrospective cohort | 43 | 378 | N/A | 9278 |
| Braun | 2021 | Switzerland | Observational | N/A | 177 | N/A | 4640 |
| Huang | 2021 | Taiwan | Retrospective cohort | 34.5 | 428 | N/A | 5156 |
| Chen | 2020 | USA | Cross‐sectional | 40.9 | 472 | N/A | 1948 |
| Lee | 2021 | Taiwan | Cross‐sectional | 36.6 | 110 | N/A | 844 |
| Gouda | 2023 | UK | Cross‐sectional | 30 | N/A | 10 | 442 |
| Wansom | 2020 | Thailand | Observational cohort | 26 | 39 | N/A | 563 |
| Nishikawa | 2023 | Japan | Retrospective cohort | N/A | 45 | N/A | 1135 |
| Monin | 2022 | UK | Observational cohort | 41 | 55 | N/A | 464 |
Abbreviations; −HBV, Hepatitis B virus; HCV, Hepatitis C virus; HIV, Human immunodeficiency virus; MSM, men who have sex with men; N/A, not Available; PLHIV, People living with HIV.
3.3. Prevalence of HCV and HBV among MSM PLHIV
Overall combined prevalence estimates were obtained by pooling number HCV cases from the 53 included studies. Meta‐analysis of 3,07,589 participants revealed a global prevalence of HCV infection among MSM PLHIV to be 7% (95% confidence interval [CI]: 5–10) by using random effect model (Figure 2). A substantial degree of heterogeneity was observed among the included studies, as indicated by an I 2 value of 100%. The 95% prediction interval lies between 0% and 56%. Thus, an original study in the future is expected to give a prevalence between 0% and 56%.
Figure 2.
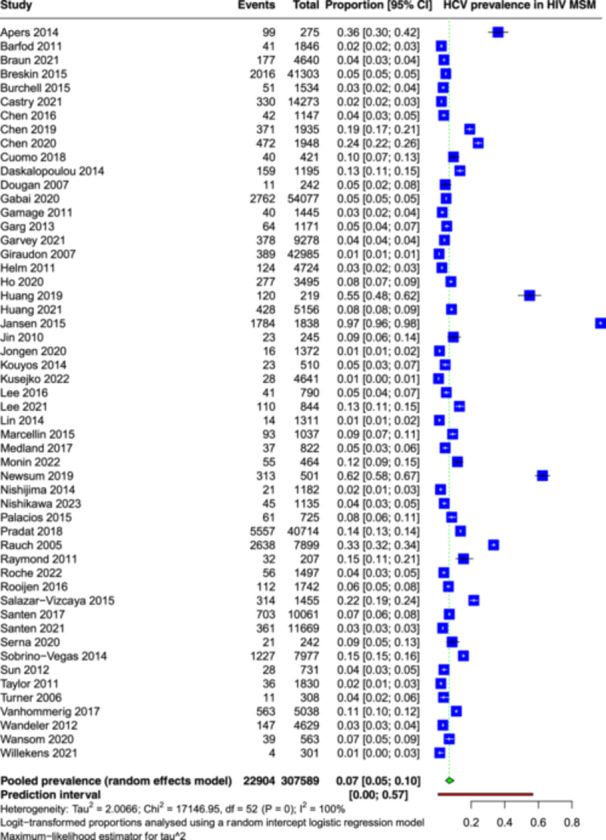
Forest plot showing the pooled prevalence of Hepatitis C virus among men having sex with men and people living with HIV.
Meta‐analysis of 5641 participants from five studies revealed a global prevalence of HBV infection among MSM PLHIV to be 9% (95% CI: 4–18) by using random effect model (Figure 3). A substantial degree of heterogeneity was observed among the included studies, as indicated by an I 2 value of 98%. The 95% prediction interval falls in the range of 0%–69%.
Figure 3.
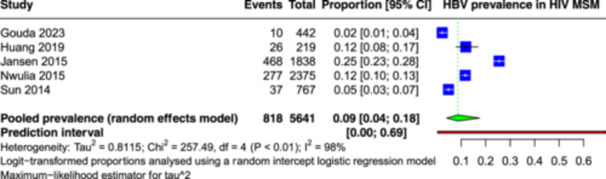
Forest plot showing the pooled prevalence of Hepatitis B virus among men having sex with men and people living with human immunodeficiency virus.
3.4. Heterogeneity and influence assessment
Both the pooled estimates for prevalence of HBV and HCV among MSM PLHIV are highly heterogenous with I 2 of 100% and 98% respectively. We further explored the heterogeneity graphically (Figure S1). The Baujat plot shows the contribution of individual studies to the heterogeneity and influence. The four studies in the upper or right portion contribute highly to both influence and heterogeneity (Figure S2). 37 , 59 , 67 , 80 Influence diagnostics have identified a study as an overly influential report (Figure S3). 61
3.5. Heterogeneity exploration
To explore the observed between‐study heterogeneity, we performed subgroup analysis based on continent for the prevalence of HCV in MSM PLHIV. It failed to reduce heterogeneity (p = 0.90). Asia reported the lowest pooled prevalence at 5.84% (95% CI: 2.98–11.13) while Europe reported the highest pooled prevalence at 7.76% (95% CI: 4.35–13.45) (Table 2). North America reported a prevalence of 6.09% (95% CI: 3.75–9.73) and Australia reported a prevalence of 6.28% (95% CI: 3.74–10.37) for HCV. Further, we performed meta‐regression based upon sample size (Figure S4), year of study (Figure S5), and average age of participants (Figure S6).
Table 2.
Subgroup analysis for prevalence of HCV among MSM PLHIV based on geography (continents).
| Subgrouping variable | Number of studies | Pooled estimate | 95% Confidence interval | tau‐squared | p Value |
|---|---|---|---|---|---|
| Continent | |||||
| Europe | 29 | 7.76 | 4.35–13.45 | 2.82 | p = 0.90 |
| North America | 11 | 6.09 | 3.75–9.73 | 0.73 | |
| Asia | 11 | 5.84 | 2.98–11.13 | 1.39 | |
| Australia | 2 | 6.28 | 3.74–10.37 | 0.11 | |
3.6. Sensitivity analysis
We performed several sensitivity analyses to assess the robustness of the pooled estimates. Omitting each study one‐by‐one in a leave‐one‐out meta‐analysis did not reveal any significant difference in the pooled estimate (Figure S7). Another sensitivity analysis excluded the overly influential study. The pooled estimate changed from 7% (95% CI: 5–10) to 7% (95% CI: 7–8), thus becoming much more precise (Figure S8). Excluding the four studies contributing highly to influence and heterogeneity also increased the precision of the pooled estimate without a significant change 6% (95% CI: 6–6) (Figure S9).
3.7. Publication bias
To determine publication bias in our meta‐analysis, we employed Doi plot with LFK index. The Doi plots for prevalence of HCV and HBV in MSM PLHIV show gross visual asymmetry. This is corroborated by the accompanying LFK indices of −2.26, and −3.21 respectively (Figure 4). This finding could also be by chance. And in the absence of a prespecified expectation of lower prevalence studies being more likely to be published, this does not prove publication bias.
Figure 4.
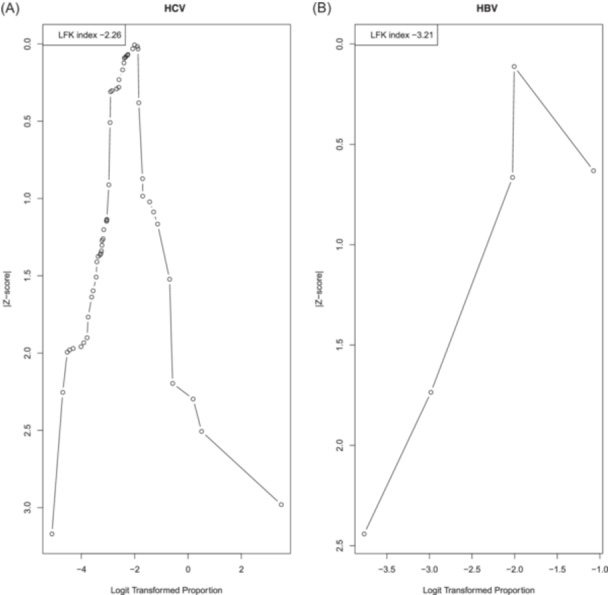
Doi plots and LFK indices for detecting publication bias. (A) Dot Plot of HCV. (B) Dot Plot of HBV.
4. DISCUSSION
The objective of this systematic review and meta‐analysis was to assess the prevalence of HCV and HBV infections among MSM PLHIV. Our analysis revealed an overall prevalence of 7% for HCV at the global level, indicating a substantial burden of HCV infection among this population. However, when considering the prevalence of HCV among MSM PLHIV at the continents, there was some variation. Asia reported the lowest prevalence and Europe reported the highest prevalence. There is no relation with year of study, age of participants, and size of study. However, omitting the highly influential and heterogeneity‐contributing studies made the pooled estimate considerably more precise. These studies collectively reported a pooled prevalence of 9% for HBV among MSM PLHIV. It is crucial to note the high heterogeneity observed among the included studies for both HCV and HBV, highlighting the need for cautious interpretation of the results. The sources of heterogeneity could not be identified despite conducting sensitivity analyses.
Comparing our results with a meta‐analysis by Jin et al., 12 which assessed pooled HCV prevalence in MSM, we found similar trends. Their study reported a global HCV prevalence of 3.4% among MSM, with higher rates in HIV‐positive people compared to HIV‐negative MSM. Our analysis, which included a larger number of studies specifically focusing on MSM PLHIV, obtained a higher prevalence of 9% for HCV. Similar trends of higher hepatitis incidence among MSM have also been reported in a meta‐analysis focusing on the US population, 84 as well as among HIV‐infected MSM and injectable drug users in another meta‐analysis. 85
The results of our meta‐analysis have important implications for clinical practice and society. Healthcare providers should prioritize routine screening for HCV and HBV among MSM PLHIV, ensuring early detection and timely interventions. Access to effective treatment options is crucial, and ongoing monitoring and care are needed to address complications. Prevention strategies should focus on safe sex practices, harm reduction programs, and education to increase awareness. Policies that protect the rights of MSM PLHIV and address social stigmas are necessary.
Future research perspectives in this field hold great promise for advancing our understanding of HCV and HBV among MSM PLHIV. First, additional research are required to explore the factors contributing to the observed variations in prevalence among different countries and regions. This could include investigating the impact of sociocultural factors, healthcare disparities, and differences in prevention and treatment strategies. Second, more longitudinal studies are needed to examine the long‐term outcomes of HBV and HCV infections among MSM PLHIV, including the progression of liver diseases and the effectiveness of interventions. Additionally, there is a need for research focused on the development and evaluation of tailored prevention and treatment interventions specifically targeting this population. This may involve exploring novel approaches such as community‐based interventions, peer support programs, and the integration of HCV and HBV screening and management within existing HIV care frameworks. Furthermore, collaborative efforts between researchers, healthcare providers, and community organizations can facilitate the implementation and evaluation of these interventions in real‐world settings. By addressing these research gaps, we can work towards improving the health outcomes and quality of life for MSM PLHIV affected by HCV and HBV infections.
Despite the valuable insights gained from our meta‐analysis, several limitations should be acknowledged. First, our inclusion criteria were limited to studies published in the English language, potentially introducing language bias. Secondly, significant heterogeneity was observed among the included studies, which could not be fully resolved through sensitivity analyses. This heterogeneity might be attributed to variations in study design, population characteristics, and other factors. Additionally, publication bias was detected, suggesting the possibility of selective publication of studies with significant results and potentially influencing the overall findings. Lastly, the generalizability of our results is limited due to the uneven distribution of studies, with a lack of representation from Africa, South America, the Middle East, and the Indian subcontinent. Only 5 studies on HBV in HIV‐positive MSM patients were available. The sample size was small, the clinical significance was also limited. In future studies, more studies should be available to summarize the prevalence of HBV in HIV‐positive patients with MSM.
This meta‐analysis highlights a substantial burden of HCV and HBV among PLHIV globally, with significant variation in prevalence rates. The high heterogeneity and wide prediction intervals emphasize the need for further research. Future studies should focus on understanding the factors contributing to variations in prevalence, exploring long‐term outcomes, and developing tailored interventions. By addressing these research gaps, we can work towards reducing the prevalence and impact of HCV and HBV among vulnerable populations like MSM PLHIV and improving their health outcomes.
AUTHOR CONTRIBUTIONS
Muhammed Shabil: Conceptualization; investigation; writing—original draft; methodology; data curation. Aarti Yadav: Conceptualization; investigation; data curation; methodology; writing—original draft. Muhammed A. Shamim: Validation; visualization; methodology; data curation; formal analysis; investigation. Mohammed Ahmed: Conceptualization; software; resources. Prakasini Satapathy: Conceptualization; investigation; writing—review and editing; project administration; resources; software. Ali A. Zaidan: Conceptualization; writing—review and editing; project administration; supervision. Mahalaqua N. Khatib: Supervision; project administration; validation; resources; writing—review and editing. Shilpa Gaidhane: Writing—review and editing; resources; data curation; conceptualization. Quazi S. Zahiruddin: Conceptualization; methodology; writing—review and editing; project administration; supervision. Ali A. Rabaan: Conceptualization; formal analysis; visualization; supervision; writing—review and editing. Nawal A. A. Kaabi: Validation; supervision; resources; project administration; software. Fadel A. M. Almosa: Software; supervision; visualization; writing—review and editing. Jehad AlSihati: Writing—review and editing; visualization; project administration; supervision; resources. Ranjit Sah: Project administration; writing—review and editing; investigation; data curation; conceptualization; methodology.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Quazi Syed Zahiruddin, Ranjit Sah affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors acknowledged the support provided by the members of Global Center for Evidence Synthesis (GCES), Chandigarh, India.
Shabil M, Yadav A, Shamim MA, et al. Prevalence of hepatitis B and C infections among HIV‐positive men who have sex with men: a systematic review and meta‐analysis. Health Sci Rep. 2024;7:e2206. 10.1002/hsr2.2206
Muhammed Shabil, Aarti Yadav, and Mahalaqua N. Khatib contributed equally to this work.
Contributor Information
Quazi S. Zahiruddin, Email: zahirquazi@gmail.com.
Ranjit Sah, Email: ranjitsah@iom.edu.np.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. All authors have read and approved the final version of the manuscript corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1. Shahriar S, Araf Y, Ahmad R, et al. Insights into the coinfections of human immunodeficiency virus‐hepatitis B virus, human immunodeficiency virus‐hepatitis C virus, and hepatitis B virus‐hepatitis C virus: prevalence, risk factors, pathogenesis, diagnosis, and treatment. Front Microbiol. 2022;12:4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections: Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact; 2021.
- 3.Centers for Disease Control and Prevention. 2024 Viral Hepatitis National Progress Report: Centers for Disease Control and Prevention; 2024. https://www.cdc.gov/hepatitis/policy/npr/2024/index.htm
- 4. World Health Organization . Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030; 2022.
- 5. World Health Organization . Global Hepatitis Report 2017. World Health Organization; 2017. [Google Scholar]
- 6. Shabil M, Kumar VU, Dhingra S, et al. Current scenario and strategies to tackle cardiovascular disease risk in HIV geriatrics. Curr Pharmacol Rep. 2023;9(6):523‐539. [Google Scholar]
- 7. Shabil M, Murti K, Kumar VU, et al. Older PLHIV are at higher cardiovascular risk with poor quality of life. Curr HIV Res. 2023;21(6):354‐360. [DOI] [PubMed] [Google Scholar]
- 8. Urbanus AT, Van Houdt R, van de Laar TJ, Coutinho RA. Viral hepatitis among men who have sex with men, epidemiology and public health consequences. Euro Surveill. 2009;14(47):19421. [DOI] [PubMed] [Google Scholar]
- 9. Ingiliz P, Martin TC, Rodger A, et al. HCV reinfection incidence and spontaneous clearance rates in HIV‐positive men who have sex with men in Western Europe. J Hepatol. 2017;66(2):282‐287. [DOI] [PubMed] [Google Scholar]
- 10. Vaux S, Chevaliez S, Saboni L, et al. Prevalence of hepatitis C infection, screening and associated factors among men who have sex with men attending gay venues: a cross‐sectional survey (PREVAGAY), France, 2015. BMC Infect Dis. 2019;19(1):315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davlidova S, Haley‐Johnson Z, Nyhan K, Farooq A, Vermund SH, Ali S. Prevalence of HIV, HCV and HBV in Central Asia and the Caucasus: a systematic review. Int J Infect Dis. 2021;104:510‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin F, Dore GJ, Matthews G, et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2021;6(1):39‐56. [DOI] [PubMed] [Google Scholar]
- 13. Yu S, Yu C, Li J, Liu S, Wang H, Deng M. Hepatitis B and hepatitis C prevalence among people living with HIV/AIDS in China: a systematic review and meta‐analysis. Virol J. 2020;17(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Remis RS, Liu J, Loutfy MR, et al. Prevalence of sexually transmitted viral and bacterial infections in HIV‐positive and HIV‐negative men who have sex with men in Toronto. PLoS One. 2016;11(7):e0158090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Falla AM, Hofstraat SHI, Duffell E, Hahné SJM, Tavoschi L, Veldhuijzen IK. Hepatitis B/C in the countries of the EU/EEA: a systematic review of the prevalence among at‐risk groups. BMC Infect Dis. 2018;18(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shabil M, Bushi G, Beig MA, Rais MA, Ahmed M, Padhi BK. Cardiovascular manifestation in tuberculosis cases: a systematic review and meta‐analysis. Curr Probl Cardiol. 2023;48:101666. [DOI] [PubMed] [Google Scholar]
- 17. Castry M, Cousien A, Bellet J, et al. Hepatitis C virus (HCV) incidence among men who have sex with men (MSM) living with HIV: results from the French Hospital Database on HIV (ANRS CO4‐FHDH) cohort study, 2014 to 2017. Euro Surveill. 2021;26(38):2001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kusejko K, Salazar‐Vizcaya L, Shah C, et al. Sustained effect on hepatitis C elimination among men who have sex with men in the Swiss HIV cohort study: a systematic re‐screening for hepatitis C RNA two years following a nation‐wide elimination program. Clin Infect Dis. 2022;75(10):1723‐1731. [DOI] [PubMed] [Google Scholar]
- 19. Roche R, Simmons R, Logan L, et al. Prevalence of hepatitis B immunity and infection in home self‐sampling HIV service users. Sex Transm Infect. 2022;98(4):286‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Braun DL, Hampel B, Ledergerber B, et al. A treatment‐as‐prevention trial to eliminate hepatitis C among men who have sex with men living with human immunodeficiency virus (HIV) in the Swiss HIV Cohort Study. Clin Infect Dis. 2021;73(7):e2194‐e2202. [DOI] [PubMed] [Google Scholar]
- 21. Garvey LJ, Cooke GS, Smith C, et al. Decline in hepatitis C virus (HCV) incidence in men who have sex with men living with human immunodeficiency virus: progress to HCV microelimination in the United Kingdom? Clin Infect Dis. 2021;72(2):233‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang M‐H, Sun H‐Y, Ho S‐Y, et al. Recently acquired hepatitis C virus infection among people living with human immunodeficiency virus at a university hospital in Taiwan. World J Gastroenterol. 2021;27(37):6277‐6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gouda M, Fitzpatrick C, Williams D, Richardson D. Prevalence of anti‐hepatitis B core in men who have sex with men attending a sexual health clinic in Brighton, UK, from 2012 to 2019. Sex Health. 2022;20(1):92‐95. [DOI] [PubMed] [Google Scholar]
- 24. Nishikawa K, Kimura M, Imamura J, Kimura K. Prevalence of hepatitis C virus infection among men who have sex with men with human immunodeficiency virus‐1 infection between 2010 and 2020 in Japan: a single‐center retrospective cohort study. J Infect Chemother. 2023;29(3):263‐268. [DOI] [PubMed] [Google Scholar]
- 25. Monin MB, Ingiliz P, Lutz T, et al. Low spontaneous clearance rates of recently acquired hepatitis C virus in human immunodeficiency Virus–Positive men who have sex with men (PROBE‐C Study). Clin Infect Dis. 2023;76(3):e607‐e612. [DOI] [PubMed] [Google Scholar]
- 26. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co‐infection in people living with HIV: a global systematic review and meta‐analysis. Lancet Infect Dis. 2016;16(7):797‐808. [DOI] [PubMed] [Google Scholar]
- 27. Bushi G, Padhi BK, Shabil M, et al. Cardiovascular disease outcomes associated with obstructive sleep apnea in diabetics: a systematic review and meta‐analysis. Diseases. 2023;11(3):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bushi G, Shabil M, Padhi BK, et al. Prevalence of acute kidney injury among dengue cases: a systematic review and meta‐analysis. Trans R Soc Trop Med Hyg. 2024;118(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 29. Shabil M, Bushi G, Beig MA, Rais MA, Ahmed M, Padhi BK. Cardiovascular manifestation in tuberculosis cases: a systematic review and meta‐analysis. Curr Probl Cardiol. 2023;48(7):101666. [DOI] [PubMed] [Google Scholar]
- 30. Alrahbeni T, Mahal A, Alkhouri A, et al. Surgical interventions for intractable migraine: a systematic review and meta‐analysis. Int J Surg. 2024:10.1097. https://pubmed.ncbi.nlm.nih.gov/38626410/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta‐analysis. BMJ Open. 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Langan D, Higgins JPT, Jackson D, et al. A comparison of heterogeneity variance estimators in simulated random‐effects meta‐analyses. Res Synth Methods. 2019;10(1):83‐98. [DOI] [PubMed] [Google Scholar]
- 33. Goel S, Shabil M, Kaur J, Chauhan A, Rinkoo AV. Safety, efficacy and health impact of electronic nicotine delivery systems (ENDS): an umbrella review protocol. BMJ Open. 2024;14(1):e080274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shamim MA, Dwivedi P, Padhi BK. Beyond the funnel plot: the advantages of Doi plots and prediction intervals in meta‐analyses. Asian J Psychiatry. 2023;84:103550. [DOI] [PubMed] [Google Scholar]
- 35. Furuya‐Kanamori L, Barendregt JJ, Doi S. A new improved graphical and quantitative method for detecting bias in meta‐analysis. Int J Evid Based Healthc. 2018;16(4):195‐203. [DOI] [PubMed] [Google Scholar]
- 36. Swarup SS, Padhi BK, Satapathy P, et al. Cardiovascular consequences of financial stress: a systematic review and meta‐analysis. Curr Probl Cardiol. 2023;49(2):102153. [DOI] [PubMed] [Google Scholar]
- 37. Rauch A, Martin M, Weber R, et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV‐infected men who have sex with men: the Swiss HIV cohort study. Clin Infect Dis. 2005;41(3):395‐402. [DOI] [PubMed] [Google Scholar]
- 38. Salazar‐Vizcaya L, Wandeler G, Fehr J, et al. editors Impact of direct‐acting antivirals on the burden of HCV infection among persons who inject drugs and men who have sex with men in the Swiss HIV Cohort Study. Open Forum Infect Dis. 2018; Oxford University Press US. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wandeler G, Gsponer T, Bregenzer A, et al. Hepatitis C virus infections in the Swiss HIV Cohort Study: a rapidly evolving epidemic. Clin Infect Dis. 2012;55(10):1408‐1416. [DOI] [PubMed] [Google Scholar]
- 40. Chen Y‐C, Thio CL, Cox AL, Ruhs S, Kamangar F, Wiberg KJ. Trends in hepatitis C treatment initiation among HIV/hepatitis C virus‐coinfected men engaged in primary care in a multisite community health centre in Maryland: a retrospective cohort study. BMJ Open. 2019;9(3):e027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Falade‐Nwulia O, Seaberg EC, Snider AE, et al. Incident Hepatitis B virus infection in HIV‐infected and HIV‐uninfected men who have sex with men from pre‐HAART to HAART periods. Ann Intern Med. 2015;163:673‐680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gabai CM, Moore MS, Penrose K, et al. Hepatitis C infection among men who have sex with men living with HIV in New York City, 2000–2015. Sex Transm Infect. 2020;96(6):445‐450. [DOI] [PubMed] [Google Scholar]
- 43. Raymond HF, Hughes A, O'Keefe K, Stall RD, McFarland W. Hepatitis C prevalence among HIV‐positive MSM in San Francisco: 2004 and 2008. Sex Transm Dis. 2011;38(3):219‐220. [DOI] [PubMed] [Google Scholar]
- 44. Breskin A, Drobnik A, Pathela P, et al. Factors associated with hepatitis C infection among HIV‐infected men who have sex with men with no reported injection drug use in New York City, 2000–2010. Sex Transm Dis. 2015;42(7):382‐386. [DOI] [PubMed] [Google Scholar]
- 45. Kouyos RD, Rauch A, Braun DL, et al. Higher risk of incident hepatitis C virus coinfection among men who have sex with men, in whom the HIV genetic bottleneck at transmission was wide. J Infect Dis. 2014;210(10):1555‐1561. [DOI] [PubMed] [Google Scholar]
- 46. Van Santen DK, Van Der Helm JJ, Touloumi G, et al. Effect of incident hepatitis C infection on CD4+ cell count and HIV RNA trajectories based on a multinational HIV seroconversion cohort. AIDS. 2019;33(2):327‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen YC, Thio CL, Kamangar F, Cox AL, Wiberg KJ. Evolving trends in the prevalence of hepatitis C virus antibody positivity among HIV‐infected men in a community‐based primary care setting. J Viral Hepatitis. 2020;27(11):1202‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen Y‐C, Wiberg KJ, Hsieh Y‐H, et al. Favorable socioeconomic status and recreational polydrug use are linked with sexual hepatitis C virus transmission among human immunodeficiency virus‐infected men who have sex with men. Open Forum Infect Dis. 2016;3:3 ofw137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Garg S, Taylor LE, Grasso C, Mayer KH. Prevalent and incident hepatitis C virus infection among HIV‐infected men who have sex with men engaged in primary care in a Boston community health center. Clin Infect Dis. 2013;56(10):1480‐1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taylor LE, Holubar M, Wu K, et al. Incident hepatitis C virus infection among US HIV‐infected men enrolled in clinical trials. Clin Infect Dis. 2011;52(6):812‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gonzalez‐Serna A, Macias J, Palacios R, et al. Incidence of recently acquired hepatitis C virus infection among HIV‐infected patients in Southern Spain. HIV Med. 2021;22(5):379‐386. [DOI] [PubMed] [Google Scholar]
- 52. Willekens R, Sánchez I, Miguel L, et al. Screening for asymptomatic STIs in HIV‐infected men who have sex with men. Sex Transm Infect. 2021;97(2):170‐171. [DOI] [PubMed] [Google Scholar]
- 53. Sobrino‐Vegas P, Monge Corella S, Serrano‐Villar S, et al. Incidence of hepatitis C virus (HCV) in a multicenter cohort of HIV‐positive patients in Spain 2004–2011: increasing rates of HCV diagnosis but not of HCV seroconversions. PLoS One. 2014;9(12):e116226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Palacios R, Mata R, Aguilar I, et al. High seroprevalence but low incidence of HCV infection in a cohort of patients with sexually transmitted HIV in Andalusia, Spain. J Int Assoc Physicians AIDS Care. 2009;8(2):100‐105. [DOI] [PubMed] [Google Scholar]
- 55. Newsum AM, Kooij KW, Boyd A, et al. Progression of liver fibrosis following acute hepatitis C virus infection in HIV‐positive MSM. AIDS. 2019;33(5):833‐844. [DOI] [PubMed] [Google Scholar]
- 56. Van Santen DK, Van Der Helm JJ, Del Amo J, et al. Lack of decline in hepatitis C virus incidence among HIV‐positive men who have sex with men during 1990–2014. J Hepatol. 2017;67(2):255‐262. [DOI] [PubMed] [Google Scholar]
- 57. Jongen VW, van Rooijen MS, Schim van der Loeff MF, et al. Evaluation of the hepatitis C testing strategy for human immunodeficiency virus–positive men who have sex with men at the sexually transmitted infections outpatient clinic of Amsterdam, the Netherlands. Sex Transm Dis. 2020;47(9):587‐595. [DOI] [PubMed] [Google Scholar]
- 58. van Rooijen M, Heijman T, de Vrieze N, et al. Earlier detection of hepatitis C virus infection through routine hepatitis C virus antibody screening of human immunodeficiency virus‐positive men who have sex with men attending a sexually transmitted infection outpatient clinic. Sex Transm Dis. 2016;43(9):560‐565. [DOI] [PubMed] [Google Scholar]
- 59. Pradat P, Huleux T, Raffi F, et al. Incidence of new hepatitis C virus infection is still increasing in French MSM living with HIV. AIDS. 2018;32(8):1077‐1082. [DOI] [PubMed] [Google Scholar]
- 60. Marcellin F, Lorente N, Demoulin B, et al. Comparison of risk factors in HIV‐infected men who have sex with men, coinfected or not with hepatitis C virus (ANRS VESPA2 French cross‐sectional national survey). Sex Transm Infect. 2015;91(1):21‐23. [DOI] [PubMed] [Google Scholar]
- 61. Huang MH, Chang SY, Liu CH, et al. HCV reinfections after viral clearance among HIV‐positive patients with recent HCV infection in Taiwan. Liver Int. 2019;39(10):1860‐1867. [DOI] [PubMed] [Google Scholar]
- 62. Sun H‐Y, Chang S‐Y, Yang Z‐Y, et al. Recent hepatitis C virus infections in HIV‐infected patients in Taiwan: incidence and risk factors. J Clin Microbiol. 2012;50(3):781‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sun H‐Y, Cheng C‐Y, Lee N‐Y, et al. Seroprevalence of hepatitis B virus among adults at high risk for HIV transmission two decades after implementation of nationwide hepatitis B virus vaccination program in Taiwan. PLoS One. 2014;9(2):e90194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ho S‐Y, Su L‐H, Sun H‐Y, et al. Trends of recent hepatitis C virus infection among HIV‐positive men who have sex with men in Taiwan, 2011–2018. EClinicalMedicine. 2020;24:100441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee C‐Y, Wu P‐H, Lu M‐W, Chen T‐C, Lu P‐L. High prevalence of unawareness of HCV infection status among both HCV‐seronegative and seropositive people living with human immunodeficiency virus in Taiwan. PLoS One. 2021;16(5):e0251158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Daskalopoulou M, Rodger A, Thornton A, et al. Sexual behaviour, recreational drug use and hepatitis C co‐infection in HIV‐diagnosed men who have sex with men in the United Kingdom: results from the ASTRA study. J Int AIDS Soc. 2014;17:19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Giraudon I, Ruf M, Maguire H, et al. Increase in diagnosed newly acquired hepatitis C in HIV‐positive men who have sex with men across London and Brighton, 2002–2006: is this an outbreak? Sex Transm Infect. 2008;84(2):111‐115. [DOI] [PubMed] [Google Scholar]
- 68. Turner JM. Behavioural predictors of subsequent hepatitis C diagnosis in a UK clinic sample of HIV positive men who have sex with men. Sex Transm Infect. 2006;82(4):298‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dougan S, Balogun MA, Elford J, et al. Can current national surveillance systems in England and Wales monitor sexual transmission of hepatitis C among HIV‐infected men who have sex with men? BMC Public Health. 2007;7(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. van der Helm JJ, Prins M, del Amo J, et al. The hepatitis C epidemic among HIV‐positive MSM: incidence estimates from 1990 to 2007. AIDS. 2011;25(8):1083‐1091. [DOI] [PubMed] [Google Scholar]
- 71. Vanhommerig JW, Bezemer D, Molenkamp R, et al. Limited overlap between phylogenetic HIV and hepatitis C virus clusters illustrates the dynamic sexual network structure of Dutch HIV‐infected MSM. AIDS. 2017;31(15):2147‐2158. [DOI] [PubMed] [Google Scholar]
- 72. Nishijima T, Shimbo T, Komatsu H, Hamada Y, Gatanaga H, Oka S. Incidence and risk factors for incident hepatitis C infection among men who have sex with men with HIV‐1 infection in a large urban HIV clinic in Tokyo. J Acquired Immune Deficiency Syndromes. 2014;65(2):213‐217. [DOI] [PubMed] [Google Scholar]
- 73. Jin F, Prestage GP, Matthews G, et al. Prevalence, incidence and risk factors for hepatitis C in homosexual men: data from two cohorts of HIV‐negative and HIV‐positive men in Sydney, Australia. Sex Transm Infect. 2010;86(1):25‐28. [DOI] [PubMed] [Google Scholar]
- 74. Medland NA, Chow EPF, Bradshaw CS, Read THR, Sasadeusz JJ, Fairley CK. Predictors and incidence of sexually transmitted hepatitis C virus infection in HIV positive men who have sex with men. BMC Infect Dis. 2017;17(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Apers L, Koole O, Bottieau E, et al. Incidence of HCV and sexually transmitted diseases among HIV positive MSM in Antwerp, Belgium, 2001‐2011. Acta Clin Belg. 2013;68(6):421‐426. [DOI] [PubMed] [Google Scholar]
- 76. Barfod TS, Omland LH, Katzenstein TL. Incidence and characteristics of sexually transmitted acute hepatitis C virus infection among HIV‐positive men who have sex with men in Copenhagen, Denmark during four years (2006–2009): a retrospective cohort study. Scand J Infect Dis. 2011;43(2):145‐148. [DOI] [PubMed] [Google Scholar]
- 77. Burchell AN, Gardner SL, Mazzulli T, et al. Hepatitis C virus seroconversion among HIV‐positive men who have sex with men with no history of injection drug use: results from a clinical HIV cohort. Can J Infect Dis Med Microbiol. 2015;26(1):17‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cuomo G, Digaetano M, Menozzi M, et al. Incidence of HCV infection amongst HIV‐positive men who had sex with men and prevalence data from patients followed at the infectious diseases clinic of Modena, Italy. Dig Liver Dis. 2018;50(12):1334‐1338. [DOI] [PubMed] [Google Scholar]
- 79. Gamage DG, Read TR, Bradshaw CS, et al. Incidence of hepatitis‐C among HIV infected men who have sex with men (MSM) attending a sexual health service: a cohort study. BMC Infect Dis. 2011;11(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jansen K, Thamm M, Bock C‐T, et al. High prevalence and high incidence of coinfection with hepatitis B, hepatitis C, and syphilis and low rate of effective vaccination against hepatitis B in HIV‐positive men who have sex with men with known date of HIV seroconversion in Germany. PLoS One. 2015;10(11):e0142515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lee S, Lee SH, Lee SJ, et al. Incidence and risk factors of hepatitis C virus infection among human immunodeficiency virus (HIV) patients in a large HIV clinic in South Korea. Korean J Intern Med. 2016;31(4):772‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wai Chi Lin A, Hing Wong K, Chan K. More safer sex intervention needed for HIV‐positive MSM with higher education level for prevention of sexually transmitted hepatitis C. J Int AIDS Soc. 2014;17:19663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wansom T, Pinyakorn S, Kolsteeg CJ, et al. Brief report: group sex and methamphetamine use fuel an explosive epidemic of hepatitis C among HIV‐infected men who have sex with men in Bangkok, Thailand. J Acquired Immune Deficiency Syndromes. 2020;84(4):331‐335. [DOI] [PubMed] [Google Scholar]
- 84. Gough E, Kempf MC, Graham L, et al. HIV and hepatitis B and C incidence rates in US correctional populations and high‐risk groups: a systematic review and meta‐analysis. BMC Public Health. 2010;10(1):777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jordan AE, Perlman DC, Neurer J, Smith DJ, Des Jarlais DC, Hagan H. Prevalence of hepatitis C virus infection among HIV+ men who have sex with men: a systematic review and meta‐analysis. Int J STD & AIDS. 2017;28(2):145‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. All authors have read and approved the final version of the manuscript corresponding author had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.


