Abstract
Background
Aflatoxin B1 (AFB1) food contamination is a global health hazard that has detrimental effects on both human and animal health. The objective of the current study is to assess the protective impact of carnosic acid against AFB1-induced toxicities in the liver, kidneys, and heart.
Methods
Forty male Wistar Albino rats (weighting 180 ~ 200 g) were allocated into 5 groups (8 rats each); the 1st group received saline as served as a control, the 2nd group received carnosic acid (CA100) at a dose of 100 mg/kg bw/day by gavage for 14 days, the 3rd group received AFB1 at a dose of 2.5 mg/kg bw, orally twice on days 12 and 14, the 4th group (AFB1-CA50) received AFB1 as in the 3rd group and CA at a dose of 50 mg/kg bw/day, and the 5th group (AFB1-CA100) received AFB1 as in the 3rd group and CA as in the 2nd group.
Results
CA significantly decreased the liver enzymes (ALT, AST. ALP), renal function products (LDH, BUN, creatinine), and cardiac enzymes (CK and CK-MB) to control levels after the high increment by AFB1 exposure. Moreover, CA significantly decreased the oxidative stress (MDA, NO, 8-OHdG) and increased the antioxidant enzyme activities (CAT, GSH, GSH-Px, and SOD) after severe disruption of oxidant/antioxidant balance by AFB1 exposure. Interestingly, CA significantly decreased the proinflammatory mediators (IL-6, IL-1β, and TNF-α) to the control levels after severe inflammation induced by AFB1 exposure.
Conclusions
Conclusively, CA had antioxidant, anti-inflammatory, and anti-DNA damage effects against hepatic, renal, and cardiac AFB1-induced toxicities.
Keywords: AFB1, carnosic acid, oxidative stress, inflammation, DNA damage, 8-OHdG
Introduction
Aflatoxins (AF) are produced by Aspergillus flavus, and Aspergillus parasiticus.1 Four compounds belonging to the AF class, which are aflatoxin B1 (AFB1), AFB2, AFG1, and AFG2.2 A major concern regarding aflatoxin food contamination worldwide, particularly in human-consumed food sources is increased as aflatoxins significantly contaminate animals’ food supply including grain, grain by-products, feed, or other products.2 Levels of aflatoxin contamination in feeds range between 20 and 300 ppb according to the Food and Drug Administration (FDA). The human gets AFB1 via the respiratory tract by inhalation of rotten misted bedding and via mouth by consumption of feeds with fungal action, leading to the weakening of the body’s antioxidant capacity,3 immunity,4 and internal organs damage, particularly the liver. AFB1 induced liver damage through an imbalance between the body’s antioxidant defense system and reactive oxygen species (ROS), leading to the liver’s hydropic degeneration, fatty vacuolar degeneration, and bile duct proliferation.5
The kidneys are also involved in the accumulation of toxin AFB1 since metabolites are selectively absorbed and concentrated by renal tubular cells before being excreted in the urine. The oxidative stress is a critical risk factor for AFB1 toxicity and exposure to AFB1 raises contents of reactive oxygen species (ROS), which can impair cellular redox homeostasis, leading to oxidative stress-induced kidney injury.6 AFB1 and AFM1 caused kidney toxicity by activating oxidative stress by altering the expression of the proline dehydrogenase (PRODH) gene and L-proline level, which then induced downstream apoptosis.7 Cardiac damage in AFB1 toxicity is due to mitochondrial dysfunction, ROS generation, and apoptosis.8 The precise mechanism for AFB1-induced cardiotoxicity is not clearly understood, although cardiomyocytes are particularly susceptible to attack by free radicals correlated to the lower antioxidant activity in cardiomyocytes. The significant reduction in the activities of the antioxidant system as well as increased MDA levels in AF-treated rats are responsible for induction of kidney and heart damage.9
Carnosic acid (CA) is one of the major phenolic compounds extracted from the leaf of rosemary (Rosmarinus officinalis L (Lamiaceae)). CA had various potential therapeutic effects, including antioxidant, anti-steatosis, anti-inflammation, and antitumor activity.10 CA induces apoptosis in most types of cancer cells and exerts its protective effect against organ injury by inhibiting apoptosis. For example, CA decreased the myocardial lipid peroxidation and cardiomyocyte apoptosis induced by isoproterenol toxicity.11 Moreover, CA has a protective effect on cisplatin-induced experimental nephrotoxicity by its antioxidant and antiapoptotic properties.12 Also, CA inhibited hepatic apoptosis induced by liver ischemia/reperfusion injury.13 CA has been reported to be a potential chemoprotective agent against aflatoxin B1 in vitro on cell lines cytotoxicity.14 The present study aimed to investigate the effect of CA against AFB1-induced hepatic, renal, and cardiac toxicity in rats and elucidate the antioxidant, anti-inflammatory, and anti-DNA damage activities.
Materials and methods
Chemicals
Pure AFB1 and CA were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). kits for serum biochemistry, oxidative indicators, and antioxidant activities were supplied by Biodiagnostics Co. (Cairo, Egypt). The lactate dehydrogenase (LDH) kit was supplied by Randox Laboratories (Ltd, Crumlin, UK).
Animals and experimental design
The experiment was ethically approved by Institutional Review Board (IRB), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia (Approval no. 22–1,036). A total of 40 mature male Wistar Albino rats (weighing 180 ~ 200 g, 10 weeks) were kept in the laboratory animal house. A 12-hour cycle of darkness and light and plenty of standard food and water were provided. The humidity and temperature in the animal house were kept at 50% and 25 degrees Celsius, respectively. After two weeks of acclimation, the rats were randomly allocated into five groups (Eight in each group); (1) received saline as control, (2) received carnosic acid (CA100) (100 mg/kg bw/day) by gavage for 14 days,15 (3) received AFB1 (2.5 mg/kg bw, orally) twice on days 12 and 14,16 (4) received AFB1 (2.5 mg/kg, orally) twice on days 12 and 14, and carnosic acid (CA50) (50 mg/kg bw/day) by gavage for 14 days, and (5) received AFB1 (2.5 mg/kg, orally) twice on days 12 and 14, and carnosic acid (CA100) (100 mg/kg bw/day) by gavage for 14 days.15
Blood collection and serum and tissue preparation
On day 15, retro-orbital venous plexus blood was drawn under the effect of isoflurane inhalation then all rats were sacrificed for further hepatic, renal, and cardiac tissue collections. After centrifuging blood at 3,000 × g for 15 min, sera were kept at −20 ° C for biochemical analysis of hepatic, renal, and cardiac function enzymes. Tissue oxidative stress biomarkers were evaluated in the liver, kidneys, and heart, so they were dissected and washed thoroughly with saline to remove blood clots and RBCs. Tissue homogenization was done in 5–10 mL of ice-cold buffer per gram tissue and then centrifugation at 5,000 rpm for 30 min. The supernatant was stored at −80 °C for spectrophotometric analysis.
Serum biochemistry
Serum hepatic function tests i.e. alkaline phosphatase [ALP] (Ref. 1001260), aspartate transaminase [AST] (Ref. 1001160), and alanine transaminase [ALT] (Ref. 1001170) were measured according to the manufacture’ kit. Serum concentrations of creatine kinase (CK) (Ref: 1002260), CK-MB (Ref: 1002260), and lactate dehydrogenase (LDH) (Ref: 1001260) for cardiac functions were determined using suitable manufacturing kits. Blood urea nitrogen (BUN) (Ref. 1001323) and creatinine (Ref: MD1001111) levels were also evaluated as renal function tests. The biomarkers were analyzed using commercially available standard kits (SPINREACT, Barcelona, Spain) following the manufacturer’s protocol.
Tissue antioxidant status and oxidative stress markers
The levels of malondialdehyde (MDA) (cat. No. MD-25-29)17 and nitric oxide (NO) (cat. No. 2533),18 markers for lipid peroxidation, were measured spectrophotometrically. Glutathione (GSH) (cat. No. GR-25-11),19 GSH peroxidase (GSH-Px) (Cat. No. E-BC-K096-S),20 catalase (CAT) (cat. No. E-BC-K031-S),21 and superoxide dismutase (SOD) (cat. No. SD-25-21)22 were purchased from Biodiagnostic and Elabscience Companies and determined based on the methods described by the manufacture’s kits.
DNA damage biomarker and proinflammatory cytokines.
Sera were kept at 20 °C for biochemical analysis as well as well as proinflammatory cytokines (IL-6, IL-1β, and TNF-α) analysis, in addition to DNA damage biomarker (8-OHdG) product. Quantitative analysis of TNF-α (cat. No. RTA00), IL-1β (cat. No. RLB00), or IL-6 (cat. No. R6000B) by sandwich enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Inc., MN, USA) according to the manufacturer’s instructions.23 The levels of 8-OHdG in serum samples were determined according to the protocol specified in the ELISA kit (Elabscience, E-EL-0028) using a Microplate Reader (Biotek ELx800, Biotek Intruments, Inc., Winooski, VT, USA).
Statistical analysis
One-way analysis of variance (ANOVA) was performed using SPSS 26.0 to figure out whether the results were statistically significant, and Tukey’s multiple range test was utilized to compare individuals. Data were presented as mean with standard error (SE), and statistical significance was determined by a p-value of less than 0.05.
Results
Effect of carnosic acid on the biochemical parameters in AFB1-treated rats
The serum liver enzyme activities of AST, ALT, ALP, and LDH were significantly increased in group 3 when compared to groups 1 and 2. No significant changes in liver enzymes in group 2 in comparison to control group 1. In groups 4 and 5, the serum liver enzymatic activity was significantly and dose-dependently decreased in comparison to group 3 and sometimes returned to normal control levels in Group 5 (Table 1).
Table 1.
Effect of carnosic on biochemical parameters in aflatoxin B1-treated rats.
| Parameters/Groups | Control | CA100 | AFB1 | AFB1-CA50 | AFB1-CA100 |
|---|---|---|---|---|---|
| AST (U/L) | 62.397 ± 1.416a | 61.840 ± 1.762a | 127.068 ± 2.103b | 90.981 ± 1.983c | 69.767 ± 1.342d |
| ALT (U/L) | 33.196 ± 0.893a | 32.387 ± 1.365a | 58.870 ± 3.059b | 42.341 ± 0.808c | 33.570 ± 1.125a |
| ALP (U/L) | 66.365 ± 0.800a | 64.531 ± 1.186a | 124.195 ± 3.637b | 89.836 ± 2.492c | 73.100 ± 1.447a |
| LDH (U/L) | 200.845 ± 3.926ab | 197.927 ± 3.777a | 482.282 ± 14.713d | 346.705 ± 15.594c | 242.541 ± 6.323b |
| BUN (mg/dL) | 30.287 ± 1.060a | 29.836 ± 0.815a | 63.756 ± 3.318b | 48.373 ± 1.455c | 33.430 ± 1.298a |
| Creatinine (mg/dl) | 0.376 ± 0.013a | 0.372 ± 0.010a | 5.375 ± 0.279b | 2.643 ± 0.173c | 0.707 ± 0.052a |
| CK (U/L) | 84.486 ± 1.584ab | 79.880 ± 1.270a | 195.967 ± 6.175c | 137.463 ± 1.424d | 95.651 ± 1.439b |
| CK-MB (U/L) | 38.838 ± 0.851a | 37.546 ± 0.801a | 102.552 ± 2.777b | 62.756 ± 0.791c | 42.046 ± 0.755a |
CA100; carnosic acid 100 mg/kg B. Wt., CA50; carnosic acid 50 mg/kg B. Wt., AFB1; aflatoxin B1, AST; aspartate transaminase, ALT; alanine transaminase, ALP; alkaline phosphatase, LDH; lactate dehydrogenase, BUN; Blood Urea Nitrogen, CK; creatine kinase, CK-MB; creatine kinase-MB. Data are expressed as mean ± standard error (n = 8). Values with different alphabetic superscripts (a, b, c and d) within the same row differ significantly (P < 0.05).
The cardiac enzymatic activity of CK, and CK-MB and the level of serum BUN and creatinine as renal function indicators were significantly increased in group 3 when compared to groups 1 and 2. No significant changes in cardiac enzymes and BUN and creatinine levels in group 2 in comparison to control group 1. In groups 4 and 5, the serum cardiac enzymatic activity and BUN and creatinine levels were significantly and dose-dependently decreased in comparison to group 3 and sometimes returned to normal control levels in Group 5 (Table 1).
Effect of carnosic acid on hepatic, renal, and cardiac oxidative stress, and antioxidant status in AFB1-treated rats
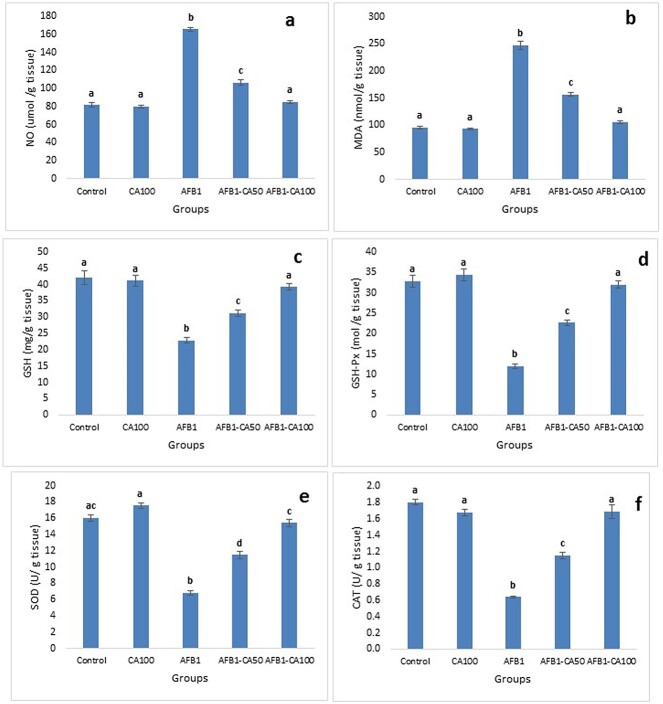
The results of the oxidative stress and antioxidant indicators were the same in hepatic (Fig. 1), renal (Fig. 2), and cardiac (Fig. 3) tissues.
Fig. 1.
Protective effects of carnosic acid on hepatic oxidative stress and antioxidant status in AFB1-treated rats. a) MDA, malondialdehyde concentration; b) NO, nitric oxide concentration; c) GSH, reduced glutathione concentration; d) GSH-Px, glutathione peroxidase activity; e) SOD, superoxide dismutase activity; and f) CAT, catalase activity. Data are presented as mean ± SE (number of rats in each group = 8). Columns labeled with different letters differ significantly (P < 0.05).
Fig. 2.
Protective effects of carnosic acid on renal oxidative stress and antioxidant status in AFB1-treated rats. a) MDA, malondialdehyde concentration; b) NO, nitric oxide concentration; c) GSH, reduced glutathione concentration; d) GSH-Px, glutathione peroxidase activity; e) SOD, superoxide dismutase activity; and f) CAT, catalase activity. Data are presented as mean ± SE (number of rats in each group = 8). Columns labeled with different letters differ significantly (P < 0.05).
Fig. 3.
Protective effects of carnosic acid on cardiac oxidative stress and antioxidant status in AFB1-treated rats. a) MDA, malondialdehyde concentration; b) NO, nitric oxide concentration; c) GSH, reduced glutathione concentration; d) GSH-Px, glutathione peroxidase activity; e) SOD, superoxide dismutase activity; and f) CAT, catalase activity. Data are presented as mean ± SE (number of rats in each group = 8). Columns labeled with different letters differ significantly (P < 0.05).
The levels of MDA and NO were significantly higher in group 3 than those in groups 1 and 2. No significant changes in MDA and NO levels in group 2 in comparison to control group 1. In groups 4 and 5, the MDA and NO levels were significantly and dose-dependently decreased in comparison to group 3 and returned to normal control levels in Group 5 (Fig. 1a and b, Fig. 2a and b, Fig. 3a and b).
The activities of the antioxidant enzymes including GSH, GSH-Px, SOD, and CAT were significantly decreased in group 3 in comparison with those in groups 1 and 2. No significant changes in the activities of antioxidant enzymes in group 2 in comparison to control group 1. In groups 4 and 5, the activities of antioxidant enzymes were significantly and dose-dependently increased in comparison to group 3 and returned to normal control levels in Group 5 (Fig. 1c–f; Fig. 2c–f’; Fig. 3c–f).
The level of MDA in hepatic tissue in Group 5 was significantly lower than those of Group 3 but still significantly higher than control groups 1 and 2 (Fig. 1a). In contrast, the level of MDA in renal (Fig. 2a) and cardiac (Fig. 3a) tissues in group 5 decreased to the control level. Also, the activity of GSH in the hepatic tissues of rats in group 5 was significantly increased in comparison to those of group 3 but still significantly lower than the control group (Fig. 1c).
Effect of carnosic acid on serum DNA damage and proinflammatory cytokines levels in AFB1-intoxicated rats
Regarding oxidative DNA damage markers, the level of 8-OHdG was significantly increased in group 3 in comparison to control groups 1 and 2. No significant changes in the level of 8-OHdG in group 2 correlated to group 1. In contrast, the levels of 8-OHdG were significantly decreased in groups 4 and 5 in comparison to group 3. The level of 8-OHdG in group 5 is comparable to those in control group 1 (Fig. 4a).
Fig. 4.

Protective effects of carnosic acid on DNA damage marker and proinflammatory cytokine levels in AFB1-treated rats. a) 8-OHdG, 8-hydroxy-2′-deoxyguanosine; b) IL-1β, interleukin-1β; c) IL-6, interleukin 6; and d) TNF-a, tumor necrosis factor-alpha. Data are presented as mean ± SE (number of rats in each group = 8). Columns labeled with different letters differ significantly (P < 0.05).
The proinflammatory cytokines i.e. IL-1β (Fig. 4b), IL-6 (Fig. 4c), and TNF-a (Fig. 4d) were evaluated, and their levels were significantly increased in group 3 in comparison to control groups 1 and 2. No significant changes in levels of the proinflammatory cytokines in group 2 correlated to group 1. In contrast, the levels of the proinflammatory cytokines were significantly decreased in groups 4 and 5 in comparison to group 3. The levels of the proinflammatory cytokines in group 5 are comparable to those in control group 1.
Discussion
The present study evaluates the potential effect of carnosic acid against acute AFB1-induced hepatic, renal, and cardiac toxicities in rats. In the present study, carnosic acid at a dose of 100 mg/kg B. Wt. considerably alleviated the inflammatory response and oxidative damage and reduced antioxidant activities induced by AFB1-acute toxicity. The protective effect of carnosic acid was doe-dependent and a dose of 100 mg/kg B. Wt. gives better protection than those of 50 mg/kg B. Wt.
Reactive oxygen species (ROS) is a normal cellular product, but overproduction causes oxidative damage.24 DNA damage and amino acid oxidation are possible results of this oxidative stress.25 AFB1 significantly elevated the DNA oxidation marker (8-OHdG) and proinflammatory cytokines. In contrast, carnosic acid 100 mg/kg B. Wt. subsided proinflammatory cytokines and DNA oxidation marker (8-OHdG) to the control levels this confirmed that carnosic acid has anti-inflammatory activity. CA has recently been reported to attenuate inflammatory damage in Indomethacin-induced gastric ulceration in rats by suppressing IL-1β, IL-6, and TNFα production and increasing Nrf2/HO-1 expressions.15 Moreover, carnosic acid inhibited the lipopolysaccharide (LPS)-induced cytokine production including hepatic mRNA levels of TNF-α, IL-6, IL-1β, and monocyte chemoattractant protein-1 (MCP-1) as well as phosphorylated forms of nuclear factor-κB (NF-κB) p65 and signal transducer and activator of transcription 3 (STAT3) and numbers of Ly6B.2-positively stained neutrophils in LPS-induced acute kidney injury in mice model.26 In the current study, CA similarly alleviated the proinflammatory cytokines TNF-α, IL-6, and IL-1β to the control levels. Unfortunately, the histopathology of the organs in the present study is not examined. R. officinalis and active principals carnosic acid and rosmarinic acid were evaluated for anti-inflammatory activity and exhibited anti-inflammatory activity before and after induction of treatments. The most evaluated proinflammatory biomarkers were tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-10, and peroxidative biomarkers were myeloperoxidase (MPO), catalase (CAT), glutathione (GSH), glutathione peroxidase (GPx), malondialdehyde (MDA), and superoxide dismutase (SOD). The best results were obtained via IP of carnosic acid (at a dose of 60 mg/kg,), via gavage of R. officinalis (at a dose of 400 mg/kg), and IP of rosmarinic acid (a dose of 10 mg/kg).27,28
In nuclear and mitochondrial DNA, 8-hydroxydeoxyguanosine (8-OHdG), an oxidized nucleoside of DNA, is the most frequently detected and studied DNA lesion. DNA oxidation marker (8-OHdG) is measured both in sera and urine to evaluate the public health of the individual. it is indicative of a generalized, cellular oxidative stress as well as a risk factor for cancer, atherosclerosis, and diabetes.29 Arsenic treatment significantly enhanced the ROS-mediated oxidative stress in the hepatic cells both in in vitro and in vivo systems through significant activation of MAPK, NF-κB, p53, and intrinsic and extrinsic apoptotic signaling and increment of cytosolic ATP level, DNA fragmentation, and oxidation. Carnosic acid could significantly counteract redox stress and ROS-mediated signaling and thereby attenuate arsenic-mediated hepatotoxicity.30 Similarly, in the present study, AFB1 significantly increased the level of DNA damage marker (8-OHdG), and treatment of carnosic acid interfered with DNA peroxidation and significantly decreased the biomarker and recovered to the control levels. At the cellular level, CA led to a clear, dose-dependent protective effect on cell toxicity, reducing cell death, levels of ROS, and concentration of 8-OH-deoxyguanine of HepG2 cells induced by exposure to AFB1.14 The literature concerning with role of 8-OHdG in toxicities, cancer, and diseases is still secant and further studies should be done to explore the mechanism of the biomarker in different pathologies.
AFB1 markedly reduced the levels of antioxidant enzymes GSH-Px, SOD, and CAT, and also increased the level of antioxidant enzymes MDA, while carnosic acid effectively alleviates the imbalance between oxidase and antioxidant enzymes, indicating that CA can effectively inhibit AFB1-caused oxidative stress in hepatic, renal and cardiac tissues. Carnosic acid was more effective in renal and cardiac tissues than hepatic tissues, this might be because the liver is the organ of detoxification and the hyperactivity of hepatocytes in oxidative enzymes.31 CA significantly decreased the MDA and NO and increased antioxidant enzyme activities of CAT, GSH, GSH-Px, and SOD. After a single dose of cisplatin (7.5 mg/kg), marked renal damage, characterized by a significant increase in serum creatinine, BUN, and relative weight of kidney with higher kidney MDA, total ROS, caspase 3, GSH levels and lowered tissue nitrite, SOD, CAT, GSH-Px, GR (glutathione reductase) and GST (glutathione S-transferase) levels was detected in rats. Carnosic acid treatment significantly (P < 0.05) attenuated the increase in lipid peroxidation, caspase-3, and ROS generation and enhanced the levels of reduced glutathione, tissue nitrite level, and activities of SOD, CAT, GSH-Px, GR, and GST compared to cisplatin-treated rats.12 similarly, in the present study, AFB1 significantly increased the serum creatinine BUN, and LDH as well as oxidative stress factors, and treatment with CA is dose-dependent demolished the adverse effect of AFB1 on the control levels. Similar results of anti-oxidative activity and restoration of biochemical parameters were obtained by ethanolic extract of rosemary against lead-induced hepato- and nephrotoxicity in rabbits.32 Moreover, CA significantly alleviated doxorubicin-induced cardiac apoptosis, inflammation, and fibrosis by suppressing oxidative stress and interfering with pathological signaling events in both isolated murine cardiomyocytes and rat hearts. CA treatment significantly countered doxorubicin-mediated pathological changes in blood parameters in rats.33 conclusively, CA ameliorated the changes in biochemical parameters and oxidative stress in hepatic, renal, and cardiac toxicities induced by AFB1 exposure.
Conclusion
AFB1 is a major contaminant of human and animal foods and is of high concern worldwide. High levels of AFB1 pollution increased the liver, kidney, and cardiac degenerative enzymes as well as proinflammatory mediators and oxidative stress. CA decreased the levels of hepatic, renal, and cardiac enzymes to control levels after the high increment by AFB1 exposure. Moreover, CA significantly increased the antioxidant enzyme activities and decreased the oxidative stress and the proinflammatory mediators. Conclusively, CA had regenerative, antioxidant, anti-inflammatory, and anti-DNA damage effects against hepatic, renal, and cardiac AFB1-induced toxicities. CA is promising as prophylactic in animal and poultry farming where feeds are always subjected to fungal bioactivity and contamination with AFB1 (Fig. 5).
Fig. 5.

Effect of carnosic acid on aflatoxin B1-induced oxidative stress, inflammation, and DNA damage. CA; carnosic acid, AFB1; aflatoxin B1, AST; aspartate transaminase, ALT; alanine transaminase, ALP; alkaline phosphatase, LDH; lactate dehydrogenase, BUN; blood urea nitrogen, CK; creatine kinase, CK-MB; creatine kinase-MB, ROS; reactive oxygen species, 8-OHdG; 8-hydroxy-2′-deoxyguanosine, IL-1β; interleukin-1β, IL-6; interleukin 6, and TNF-a; tumor necrosis factor-alpha,
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number RI-44-0836.
Contributor Information
Ghadeer M Albadrani, Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, PO Box 84428, Riyadh 11671, Saudi Arabia.
Ahmed E Altyar, Department of Pharmacy Practice, Faculty of Pharmacy, King Abdulaziz University, P.O. Box 80260, Jeddah 21589, Saudi Arabia; Pharmacy Program, Batterjee Medical College, P.O. Box 6231, Jeddah 21442, Saudi Arabia.
Osama A Kensara, Department of Clinical Nutrition, Faculty of Applied Medical Sciences, Umm Al-Qura University, P.O. Box 7067, Makkah 21955, Saudi Arabia.
Mohie A M Haridy, Department of Pathology and Laboratory Diagnosis, College of Veterinary Medicine, Qassim University, PO Box 6622, Buraydah 51452, Saudi Arabia; Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, South Valley University, Qena 83523, Egypt.
Mohamed Sayed Zaazouee, Faculty of Medicine, Al-Azhar University, P.O Box 71524, Assiut 71511, Egypt.
Alaa Ahmed Elshanbary, Faculty of Medicine, Alexandria University, P.O Box 21526, Alexandria 21563, Egypt.
Amany A Sayed, Zoology Department, Faculty of Science, Cairo University, Giza 12613, Egypt.
Mohamed M Abdel-Daim, Department of Pharmaceutical Sciences, Pharmacy Program, Batterjee Medical College, P.O. Box 6231, Jeddah 21442, Saudi Arabia; Pharmacology Department, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt.
Author contributions
Conceptualization: G.M.A, A.E.A, O.A.K., M.M.A-D. Data curation: G.M.A, A.E.A, O.A.K., M.A.M.H, A.A.S., M.S.Z, A.A.E., A.A.S., M.A.M.H, M.M.A-D. Formal Analysis: G.M.A, A.E.A, O.A.K., M.A.M.H, M.M.A-D. Funding acquisition: G.M.A, A.E.A, O.A.K., M.M.A-D. Investigation: G.M.A, A.E.A, O.A.K., M.M.A-D. Methodology: G.M.A, A.E.A, O.A.K., M.M.A-D. Project administration: G.M.A, A.E.A, O.A.K., M.M.A-D. Resources: G.M.A, A.E.A, O.A.K., M.M.A-D. Software: G.M.A, A.E.A, O.A.K., M.A.M.H, A.A.S., M.S.Z, A.A.E., A.A.S., M.A.M.H, M.M.A-D. Supervision: G.M.A, A.E.A, O.A.K., M.M.A-D. Validation: G.M.A, A.E.A, O.A.K., M.A.M.H, M.M.A-D. Visualization: G.M.A, A.E.A, O.A.K., M.A.M.H, M.M.A-D. Writing – original draft: G.M.A, A.E.A, O.A.K., M.A.M.H, A.A.S., M.S.Z, A.A.E., M.M.A-D. Writing – review & editing: G.M.A, A.E.A, O.A.K., M.A.M.H, A.A.S., M.S.Z, A.A.E., M.M.A-D.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through project number RI-44-0836.
Conflict of interest statement
All authors declare that there is no conflict of interest.
References
- 1. Rawal S, Kim JE, CoulombeR Jr. Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res Vet Sci. 2010:89(3):325–331. [DOI] [PubMed] [Google Scholar]
- 2. Rushing BR, Selim MI. Aflatoxin B1: a review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem Toxicol. 2019:124:81–100. [DOI] [PubMed] [Google Scholar]
- 3. Liu L, Chen F, Qin S, Ma J, Li L, Jin T, Zhao R. Effects of selenium-enriched yeast improved aflatoxin B1-induced changes in growth performance, antioxidation capacity, IL-2 and IFN-γ contents, and gene expression in mice. Biol Trace Elem Res. 2019:191(1):183–188. [DOI] [PubMed] [Google Scholar]
- 4. Reda FM, Ismail IE, el-Mekkawy MM, Farag MR, Mahmoud HK, Alagawany M. Dietary supplementation of potassium sorbate, hydrated sodium calcium almuniosilicate and methionine enhances growth, antioxidant status and immunity in growing rabbits exposed to aflatoxin B1 in the diet. J Anim Physiol Anim Nutr. 2020:104(1):196–203. [DOI] [PubMed] [Google Scholar]
- 5. Li S, Muhammad I, Yu H, Sun X, Zhang X. Detection of aflatoxin adducts as potential markers and the role of curcumin in alleviating AFB1-induced liver damage in chickens. Ecotoxicol Environ Saf. 2019:176:137–145. [DOI] [PubMed] [Google Scholar]
- 6. Rajput SA, Shaukat A, Wu K, Rajput IR, Baloch DM, Akhtar RW, Raza MA, Najda A, Rafał P, Albrakati A, et al. Luteolin alleviates aflatoxinB1-induced apoptosis and oxidative stress in the liver of mice through activation of Nrf2 signaling pathway. Antioxidants (Basel). 2021:10(8):1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li H, Xing L, Zhang M, Wang J, Zheng N. The toxic effects of aflatoxin B1 and aflatoxin M1 on kidney through regulating L-proline and downstream apoptosis. Biomed Res Int. 2018:2018:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ge J, Yu H, Li J, Lian Z, Zhang H, Fang H, Qian L. Assessment of aflatoxin B1 myocardial toxicity in rats: mitochondrial damage and cellular apoptosis in cardiomyocytes induced by aflatoxin B1. J Int Med Res. 2017:45(3):1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yilmaz S, Kaya E, Karaca A, Karatas O. Aflatoxin B1 induced renal and cardiac damage in rats: protective effect of lycopene. Res Vet Sci. 2018:119:268–275. [DOI] [PubMed] [Google Scholar]
- 10. Bahri S, Jameleddine S, Shlyonsky V. Relevance of carnosic acid to the treatment of several health disorders: molecular targets and mechanisms. Biomed Pharmacother. 2016:84:569–582. [DOI] [PubMed] [Google Scholar]
- 11. Sahu BD, Putcha UK, Kuncha M, Rachamalla SS, Sistla R. Carnosic acid promotes myocardial antioxidant response and prevents isoproterenol-induced myocardial oxidative stress and apoptosis in mice. Mol Cell Biochem. 2014:394(1–2):163–176. [DOI] [PubMed] [Google Scholar]
- 12. Sahu BD, Rentam KKR, Putcha UK, Kuncha M, Vegi GMN, Sistla R. Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food Chem Toxicol. 2011:49(12):3090–3097. [DOI] [PubMed] [Google Scholar]
- 13. Yan H, Jihong Y, Feng Z, Xiaomei X, Xiaohan Z, Guangzhi W, Zhenhai M, Dongyan G, Xiaochi M, Qing F, et al. Sirtuin 1-mediated inhibition of p66shc expression alleviates liver ischemia/reperfusion injury. Crit Care Med. 2014:42(5):e373–e381. [DOI] [PubMed] [Google Scholar]
- 14. Costa S, Utan A, Speroni E, Cervellati R, Piva G, Prandini A, Guerra MC. Carnosic acid from rosemary extracts: a potential chemoprotective agent against aflatoxin B1. An in vitro study. J Appl Toxicol. 2007:27(2):152–159. [DOI] [PubMed] [Google Scholar]
- 15. Danisman B, Cicek B, Yildirim S, Bolat I, Kantar D, Golokhvast KS, Nikitovic D, Tsatsakis A, Taghizadehghalehjoughi A. Carnosic acid ameliorates indomethacin-induced gastric ulceration in rats by alleviating oxidative stress and inflammation. Biomedicines. 2023:11(3):829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akinrinde AS, Ogunbunmi T, Akinrinmade FJ. Acute aflatoxin B1-induced gastro-duodenal and hepatic oxidative damage is preceded by time-dependent hyperlactatemia in rats. Mycotoxin Res. 2020:36(4):443–452. [DOI] [PubMed] [Google Scholar]
- 17. Uchiyama M, Mihara M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978:86(1):271–278. [DOI] [PubMed] [Google Scholar]
- 18. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982:126(1):131–138. [DOI] [PubMed] [Google Scholar]
- 19. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963:61:882–888. [PubMed] [Google Scholar]
- 20. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967:70(1):158–169. [PubMed] [Google Scholar]
- 21. Aebi H. [13] catalase in vitro. Methods enzymol. 1984:105:121–126. [DOI] [PubMed] [Google Scholar]
- 22. Nishikimi M, Appaji Rao N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972:46(2):849–854. [DOI] [PubMed] [Google Scholar]
- 23. Fu M-H, Chen IC, Lee CH, Wu CW, Lee YC, Kung YC, Hung CY, Wu KLH. Anti-neuroinflammation ameliorates systemic inflammation-induced mitochondrial DNA impairment in the nucleus of the solitary tract and cardiovascular reflex dysfunction. J Neuroinflammation. 2019:16(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Babizhayev MA. Generation of reactive oxygen species in the anterior eye segment. Synergistic codrugs of N-acetylcarnosine lubricant eye drops and mitochondria-targeted antioxidant act as a powerful therapeutic platform for the treatment of cataracts and primary open-angle glaucoma. BBA Clin. 2016:6:49–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Med Cell Longev. 2016:2016:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim J-Y, Hong HL, Kim GM, Leem J, Kwon HH. Protective effects of carnosic acid on lipopolysaccharide-induced acute kidney injury in mice. Molecules. 2021:26(24):7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gonçalves C, Fernandes D, Silva I, Mateus V. Potential anti-inflammatory effect of Rosmarinus officinalis in preclinical in vivo models of inflammation. Molecules. 2022:27(3):609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Habtemariam S. Anti-inflammatory therapeutic mechanisms of natural products: insight from rosemary Diterpenes, carnosic acid and carnosol. Biomedicines. 2023:11(2):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004:339(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 30. Das S, Joardar S, Manna P, Dua TK, Bhattacharjee N, Khanra R, Bhowmick S, Kalita J, Saha A, Ray S, et al. Carnosic acid, a natural diterpene, attenuates arsenic-induced hepatotoxicity via reducing oxidative stress, MAPK activation, and apoptotic cell death pathway. Oxidative Med Cell Longev. 2018:2018:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol: WJG. 2014:20(25):8082–8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mohamed WA, Abd-Elhakim YM, Farouk SM. Protective effects of ethanolic extract of rosemary against lead-induced hepato-renal damage in rabbits. Exp Toxicol Pathol. 2016:68(8):451–461. [DOI] [PubMed] [Google Scholar]
- 33. Manna P, Dewanjee S, Joardar S, Chakraborty P, Bhattacharya H, Bhanja S, Bhattacharyya C, Bhowmik M, Bhowmick S, Saha A, et al. Carnosic acid attenuates doxorubicin-induced cardiotoxicity by decreasing oxidative stress and its concomitant pathological consequences. Food Chem Toxicol. 2022:166:113205. [DOI] [PubMed] [Google Scholar]