This cohort study compares lung donation rates and transplant survival outcomes in independent vs hospital-based donor care units (DCUs).
Key Points
Question
Does the survival of recipients with lungs recovered and transplanted from deceased donors after brain death differ between independent and hospital-based donor care units?
Findings
In this cohort study of 10 856 donors and 1657 recipients, although lung donation rates were higher among donors cared for in independent donor care units, graft survival was longer among donor lungs recovered from hospital-based units.
Meaning
These findings suggest that differences in lung donation and transplantation outcomes exist and depend on the type of donor care unit used for deceased organ donor management and organ recovery.
Abstract
Importance
Centralizing deceased organ donor management and organ recovery into donor care units (DCUs) may mitigate the critical organ shortage by positively impacting donation and recipient outcomes.
Objective
To compare donation and lung transplant outcomes between 2 common DCU models: independent (outside of acute-care hospitals) and hospital-based.
Design, Setting, and Participants
This is a retrospective cohort study of Organ Procurement and Transplantation Network deceased donor registry and lung transplant recipient files from 21 US donor service areas with an operating DCU. Characteristics and lung donation rates among deceased donors cared for in independent vs hospital-based DCUs were compared. Eligible participants included deceased organ donors (aged 16 years and older) after brain death, who underwent organ recovery procedures between April 26, 2017, and June 30, 2022, and patients who received lung transplants from those donors. Data analysis was conducted from May 2023 to March 2024.
Exposure
Organ recovery in an independent DCU (vs hospital-based DCU).
Main Outcome and Measures
The primary outcome was duration of transplanted lung survival (through December 31, 2023) among recipients of lung(s) transplanted from cohort donors. A Cox proportional hazards model stratified by transplant year and program, adjusting for donor and recipient characteristics was used to compare graft survival.
Results
Of 10 856 donors in the starting sample (mean [SD] age, 42.8 [15.2] years; 6625 male [61.0%] and 4231 female [39.0%]), 5149 (primary comparison group) underwent recovery procedures in DCUs including 1466 (28.4%) in 11 hospital-based DCUs and 3683 (71.5%) in 10 independent DCUs. Unadjusted lung donation rates were higher in DCUs than local hospitals, but lower in hospital-based vs independent DCUs (418 donors [28.5%] vs 1233 donors [33.5%]; P < .001). Among 1657 transplant recipients, 1250 (74.5%) received lung(s) from independent DCUs. Median (range) duration of follow-up after transplant was 734 (0-2292) days. Grafts recovered from independent DCUs had shorter restricted mean (SE) survival times than grafts from hospital-based DCUs (1548 [27] days vs 1665 [50] days; P = .04). After adjustment, graft failure remained higher among lungs recovered from independent DCUs than hospital-based DCUs (hazard ratio, 1.85; 95% CI, 1.28-2.65).
Conclusions and Relevance
In this retrospective analysis of national donor and transplant recipient data, although lung donation rates were higher from deceased organ donors after brain death cared for in independent DCUs, lungs recovered from donors in hospital-based DCUs survived longer. These findings suggest that further work is necessary to understand which factors (eg, donor transfer, management, or lung evaluation and acceptance practices) differ between DCU models and may contribute to these differences.
Introduction
Centralization of deceased organ donors after brain death into donor care units (DCU) addresses practical challenges associated with hospital-based management1 and is proposed to address the critical organ shortage in the US.2 DCUs are operated by organ procurement organizations (OPOs)—federal contractors responsible for organ donor identification, authorization, and organ allocation in discrete regions. Nearly one-half of OPOs operate a DCU in 1 of 2 commonly used models: (1) hospital-based, sharing some or all resources (including staff and beds) with hospitalized patients (under contract with the hospital), and (2) independent, which resemble intensive care units and operating rooms but lack hospital affiliations or licensed beds.3
While individual OPOs operate DCUs according to available local hospital resources, operational needs, and finances, each DCU model offers unique advantages, which include lower donor management costs among donors in independent DCUs,4 and access to consultant physicians or additional clinical resources (eg, cardiac catheterization laboratories) in hospital-based DCUs.3 Differences between models may be particularly impactful for lung donation because some acute complications of brain death (eg, atelectasis) are mitigated with lung donor–specific protocols5 or interventions (eg, lung-protective ventilation6 or prone positioning7), which may be delivered more consistently in independent DCUs than hospitals.8
Higher rates of lung donation are reported among donors cared for in independent DCUs,8,9 but recipient survival—a patient-centered measure and primary metric of lung transplant program performance10—has not been examined. Therefore, this study aims to compare graft survival duration between lungs recovered from deceased organ donors after brain death in each DCU model. Because many factors impact survival after transplant, we hypothesized that graft survival duration would not differ between lungs recovered from donors in hospital-based vs independent DCUs.
Methods
Study Design
We conducted a retrospective cohort study using preexisting data. The study protocol was determined exempt from human participants research and the requirement for informed consent by the Penn Medicine institutional review board and follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.11 This study used data from the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donors, wait-listed candidates, and transplant recipients in the US, submitted by members of the OPTN. The Health Resources and Services Administration of the US Department of Health and Human Services provides oversight to the activities of the OPTN contractor. Analyses reflect data captured in study datasets as of February 4, 2024.
Study Sample
The study included all deceased donors captured in the study dataset who underwent recovery procedures in the US between April 26, 2017 (the first day recovery location data were collected), and June 30, 2022 (the latest data available at study initiation), and recipients of transplanted lungs from those donors. We excluded donors cared for by OPOs without an operating DCU or with a newly opened DCU (contributing fewer than 6 months of data). We excluded donors unlikely to be transferred to a DCU, including those younger than 16 years and donors after circulatory death. We also excluded donors with recovery procedures before local DCU opening and missing or ambiguous recovery procedure locations.
Primary Exposure
The primary exposure was the type of DCU where the organ recovery procedure occurred, categorized as hospital-based or independent. Organ donors were classified using recovery procedure location and name variables in the study dataset as previously described,9 then categorized according to published literature.3 Recipients of lungs from cohort donors were also compared according to the site of lung recovery.
Most donor and recipient characteristics, including sex, race and ethnicity, donation outcomes, recipient severity of illness measures (including lung allocation score at the time of transplant12), transplant characteristics (eg, waitlist duration or single vs double transplant), and transplantation outcomes were defined by OPTN. Race and ethnicity were recorded by organ transplant coordinators and transplant program staff at the time of organ donor assessment and patient waitlist registration, respectively.13 Race and ethnicity categories included American Indian or Alaska Native, Asian, Black, Hispanic, Native Hawaiian or Other Pacific Islander, White, and multiracial. The study assessed these characteristics because of known differences in rates of deceased organ donation14 and lung transplant access and outcomes between groups.15,16 Recipient diagnoses were classified according to Valapour et al.17 We determined some transplant factors using combinations of individual donor and recipient characteristics, including extended criteria for lung donation18 and height mismatch.19
Study Outcomes
The primary outcome was the duration of transplanted lung survival (as of December 31, 2023, with graft failure defined as patient death or retransplant). Donation process outcomes included donor management time (from brain death diagnosis to aortic cross-clamp application) and total ischemic times. Organ donation outcomes included the donation of at least 1 transplanted lung and the number of lungs and other organs transplanted from each donor. We also examined secondary recipient outcomes 72 hours after transplant (including receipt of mechanical ventilation), at hospital discharge (including length of stay after transplant), and the incidence of graft survival at 1 year.
Statistical Analysis
We performed unadjusted comparisons of the demographic and clinical characteristics of cohort donors cared for in hospital-based vs independent DCUs. Then, we repeated these comparisons in the subgroup of donors who donated at least 1 lung for transplant. Next, we compared the characteristics and outcomes of patients who received lung transplants from cohort donors who underwent organ recovery in each DCU type. Other than organ recovery location data (excluded during cohort selection), missing data were minimal. Variables missing more than 10% of recorded values were not considered in analyses.
To examine the duration of graft survival (the primary outcome), we excluded recipients of simultaneous multiorgan transplants. We created Kaplan-Meier curves and compared restricted mean survival times between lungs recovered from donors in each DCU type over the first 5 years of follow-up.20 To account for differences in donor and transplant recipient characteristics likely to affect graft survival duration, we created a Cox proportional hazards model stratified by transplant year and transplant program (to account for changes in allocation policy that may have changed lung utilization practices and differences in transplant center characteristics associated with recipient outcomes). We adjusted for clinically relevant donor and recipient factors associated with primary graft dysfunction and short- and long-term recipient survival (see the eFigure in Supplement 1 for a proposed causal diagram).12,17,18,19,21,22,23 From the Cox model, we also estimated the average adjusted 1-year graft survival in each group. We tested the proportionality assumption in the adjusted model as described in the eMethods in Supplement 1.
Analyses were conducted using SAS 9.4 (SAS Institute). We considered a 2-sided P < .05 as the threshold of statistical significance. Data analysis occurred from May 2023 to March 2024.
Results
The study dataset captured 63 248 deceased donors after brain death, of which 10 856 (mean [SD] age, 42.8 [15.2] years; 6625 male [61.0%] and 4231 female [39.0%]) met inclusion criteria and were cared for by OPOs with an operating DCU. Of these, 5149 donors (47.4%) underwent recovery procedures in DCUs (Figure 1). A majority of these donors underwent recovery in 1 of 10 independent DCUs (3683 donors [71.5%]); the remainder (1466 donors [28.4%]) underwent recovery in 1 of 11 hospital-based DCUs (eTable 1 in Supplement 1). Among these 5149 donors, 1651 (32.1%) donated at least 1 lung for transplant; rates of lung donation (and the number of lungs transplanted) were higher among donors cared for in independent vs hospital-based DCUs (1233 donors [33.5%] vs 418 donors [28.5%]; P < .001), but higher in each DCU type than local hospitals (eTable 2 in Supplement 1).
Figure 1. Cohort Selection Diagram.
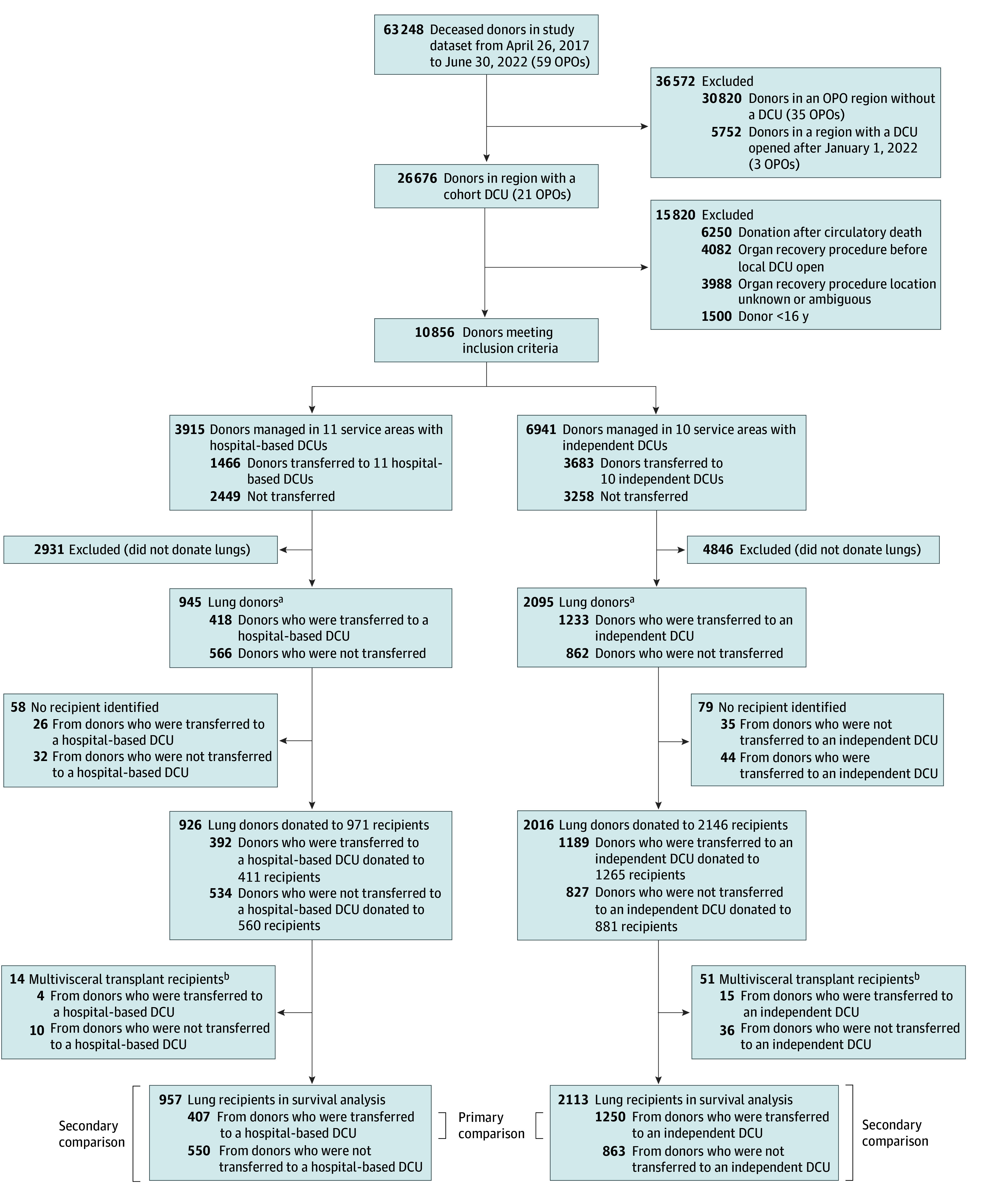
Secondary analyses compared donors in hospitals in donor service areas with operating DCUs but not transferred to a DCU (stratified by DCU type) and are presented in eTables 1-3 in Supplement 1. DCU indicates donor care unit; OPO, organ procurement organization.
aDonated at least 1 lung for transplant.
bRecipient received at least 1 donated lung from a cohort donor combined with 1 or more additional solid organs.
Compared with lung donors in hospital-based DCUs, those in independent DCUs were less likely to be Hispanic (162 of 1233 donors [13.1%] vs 93 of 418 donors [22.2%]) and more likely to be White (804 of 1233 donors [65.2%] vs 216 of 418 donors [51.7%]) (Table 1). Intracranial hemorrhage or stroke was the most common cause of death in both groups. Lung donors who underwent recovery in independent DCUs were significantly less likely to have documented pulmonary infection during donor management (838 of 1233 donors [68.0%] vs 325 of 418 donors [77.8%]; P < .001). Median terminal values for the ratio of partial pressure of oxygen in arterial blood to the fraction of inspiratory oxygen concentration (Pao2:Fio2) were similar between groups.
Table 1. Characteristics of Cohort Donors That Donated at Least 1 Lung for Transplant.
| Characteristic | Donors, No. (%) (N = 1651)a | P value | |
|---|---|---|---|
| Hospital-based DCU (n =418) | Independent DCU (n = 1233) | ||
| DCUs, No./Total No. | 11/21 (52.4) | 10/21 (47.6) | NA |
| Donation year, No./Total No. (%) | |||
| April to December 2017 | 28/102 (27.4) | 74/102 (72.5) | .02 |
| 2018 | 39/204 (19.1) | 165/204 (80.9) | |
| 2019 | 47/248 (19.0) | 201/248 (81.0) | |
| 2020 | 92/340 (27.1) | 248/340 (72.9) | |
| 2021 | 125/462 (27.1) | 337/462 (72.9) | |
| January to June 2022 | 87/295 (29.5) | 208/295 (70.5) | |
| Age, mean (SD), y | 36.1 (13.1) | 36.3 (12.8) | .80 |
| Sexb | |||
| Female | 163 (39.0) | 490 (39.7) | .79 |
| Male | 255 (61.0) | 743 (60.3) | |
| Race and ethnicityb | |||
| American Indian or Alaska Native | 2 (0.5) | 5 (0.4) | <.001 |
| Asian | 23 (5.5) | 11 (0.9) | |
| Black | 78 (18.7) | 245 (19.9) | |
| Hispanic | 93 (22.2) | 162 (13.1) | |
| Multiracial | 5 (1.2) | 5 (0.4) | |
| Native Hawaiian or Other Pacific Islander | 1 (0.2) | 1 (0.1) | |
| White | 216 (51.7) | 804 (65.2) | |
| History of tobacco use | 29 (6.9) | 118 (9.6) | .26 |
| History of cocaine use | 69 (16.5) | 258 (20.9) | .03 |
| History of other drug use | 225 (53.8) | 697 (56.5) | .62 |
| Clinical characteristics | |||
| Donor height, median (IQR), cm | 172 (163-179) | 173 (165-180) | .15 |
| Body mass index, median (IQR)c | 25.8 (22.5-29.6) | 26.3 (22.9-30.3) | .003 |
| Mechanism of death | |||
| Asphyxiation | 32 (7.7) | 53 (4.3) | .03 |
| Blunt injury | 84 (20.1) | 233 (18.9) | |
| Cardiovascular | 38 (9.1) | 125 (10.1) | |
| Death from natural causes | 8 (1.9) | 28 (2.3) | |
| Drowning | 2 (0.5) | 6 (0.5) | |
| Drug intoxication | 59 (14.1) | 255 (20.7) | |
| Electrical | 0 | 2 (0.2) | |
| Gunshot wound | 85 (20.3) | 204 (16.5) | |
| Intracranial hemorrhage or stroke | 105 (25.1) | 295 (23.9) | |
| Other | 1 (0.2) | 9 (0.7) | |
| Seizure | 4 (1.0) | 21 (1.7) | |
| Stab | 0 | 2 (0.2) | |
| Terminal Pao2:Fio2 ratio, mm Hg, median (IQR) | 439 (360-507) | 447 (361-502) | .53 |
| Pulmonary infection | 325 (77.8) | 838 (68.0) | <.001 |
Abbreviations: DCU, donor care unit; NA, not applicable; Pao2:Fio2, ratio of partial pressure of oxygen in arterial blood to the fraction of inspiratory oxygen concentration.
Not all cohort donors donated lungs to a lung transplant recipient included in survival analysis (see cohort selection diagram).
As classified by the Organ Procurement and Transplantation Network.13
Body mass index was calculated as weight in kilograms divided by height in meters squared.
Of 1657 recipients of lungs recovered from donors in DCUs (mean [SD] age, 58.6 [11.9] years; 1068 male [64.4%] and 589 female [35.6%]), 1250 (75.4%) received lungs recovered from independent DCUs and 407 (24.6%) received lungs from hospital-based DCUs (Table 2). Compared with recipients of lungs from hospital-based DCUs, recipients from independent DCUs were similar in age and sex but were less likely to be Asian or Hispanic. Recipients of lungs recovered from independent DCUs had several characteristics associated with better survival after transplant, including higher rates of chronic obstructive pulmonary disease (339 of 1250 recipients in independent DCUs [27.1%] vs 88 of 407 recipients in hospital-based DCUs [21.6%]) and lower rates of restrictive lung disease (779 of 1250 recipients in independent DCUs [62.3%] vs 289 of 407 in hospital-based DCUs [71.0%]). Compared with recipients of lungs from hospital-based DCUs, recipients of lungs from independent DCUs also had significantly longer median (IQR) 6-minute walk distances before transplant (767 [400-1050] ft vs 668 [295-1017] ft; P = .04) and lower median (IQR) lung allocation scores at transplant match (37.9 [34.0-45.7] vs 39.0 [34.7-46.8]; P = .02). Most transplant characteristics (including double lung transplant and total ischemic times) were similar between recipients of lungs from each DCU type. Cytomegalovirus mismatch rates were also lower among lung recipients from independent DCUs.
Table 2. Cohort Transplant Recipient Characteristics by Lung Recovery Location.
| Characteristic | Transplant recipients, No. (%) (N = 1657)a | P value | |
|---|---|---|---|
| Hospital-based DCU (n = 407) | Independent DCU (n = 1250) | ||
| Transplant programs, No./Total No.b | 56/71 (78.9) | 66/71 (92.3) | NA |
| Demographic characteristics | |||
| Transplant year, No./Total No. (%) | |||
| April to December 2017 | 26/99 (26.3) | 73/99 (73.7) | .001 |
| 2018 | 39/212 (18.4) | 173/212 (81.6) | |
| 2019 | 43/247 (17.4) | 204/247 (82.5) | |
| 2020 | 87/347 (25.1) | 260/347 (74.9) | |
| 2021 | 128/463 (27.6) | 335/463 (72.4) | |
| January to June 2022 | 84/289 (29.1) | 205/289 (70.9) | |
| Age, mean (SD), y | 59.5 (11.1) | 58.3 (12.1) | .07 |
| Sexc | |||
| Female | 138 (33.9) | 451 (36.1) | .43 |
| Male | 269 (66.1) | 799 (63.9) | |
| Race and ethnicityc | |||
| American Indian or Alaska Native | 6 (1.5) | 4 (0.3) | .003 |
| Asian | 23 (5.7) | 33 (2.6) | |
| Black | 37 (9.1) | 109 (8.7) | |
| Hispanic | 62 (15.2) | 147 (11.8) | |
| Multiracial | 4 (1.0) | 4 (0.3) | |
| Native Hawaiian or Other Pacific Islander | 2 (0.5) | 2 (0.2) | |
| White | 273 (67.1) | 951 (76.1) | |
| Clinical characteristics | |||
| Height, median (IQR), cm | 170 (163-178) | 170 (163-178) | .30 |
| Body mass index, median (IQR)d | 26.4 (23.5-29.0) | 26.2 (22.7-29.3) | .46 |
| Diagnosis groupe | |||
| A. Obstructive | 88 (21.6) | 339 (27.1) | .01 |
| B. Pulmonary vascular | 19 (4.7) | 72 (5.8) | |
| C. Cystic fibrosis and immunodeficiency | 11 (2.7) | 60 (4.8) | |
| D. Restrictive | 289 (71.0) | 779 (62.3) | |
| Hospitalized before transplant | |||
| Hospitalized (ICU) | 76 (18.7) | 180 (14.4) | .06 |
| Hospitalized (non–ICU) | 49 (12.0) | 131 (10.5) | |
| Not hospitalized | 282 (69.3) | 939 (75.1) | |
| Systolic pulmonary artery pressure, mm Hg, median (IQR) | 38 (30-49) | 38 (32-48) | .69 |
| Serum creatinine, median (IQR), mg/dL | 0.8 (0.7-1.0) | 0.8 (0.7-1.0) | .07 |
| Serum bilirubin, median (IQR), mg/dL | 0.5 (0.3-0.7) | 0.5 (0.3-0.6) | .16 |
| 6-min Walk distance, median (IQR), ft | 688 (295-1017) | 767 (400-1050) | .04 |
| Unable to ambulate | 31 (7.6) | 123 (9.8) | .18 |
| Panel reactive antibodies at time of transplant, median (IQR), No. | 0 (0-7) | 0 (0-3) | .31 |
| Mechanical ventilation at time of transplant | 29 (7.1) | 81 (6.5) | .65 |
| Extracorporeal membrane oxygenation at time of transplant | 38 (9.3) | 99 (7.9) | .37 |
| Time on waitlist, median (range), d | 41 (15-122) | 37 (13-97) | .38 |
| Lung allocation score at transplant, median (IQR) | 39.0 (34.7-46.8) | 37.9 (34.0-45.7) | .02 |
| Transplant characteristics | |||
| Type of transplant | |||
| Single | 81 (19.9) | 283 (22.6) | .25 |
| Double | 326 (80.1) | 967 (77.4) | |
| Retransplantf | 9 (2.2) | 10 (0.8) | .02 |
| Total ischemic time, median (IQR), h | 5.5 (4.6-6.9) | 5.4 (4.4-6.6) | .16 |
| Extended criteria lung donorg | 174 (42.8) | 548 (43.8) | .70 |
| Height mismatch (donor height vs recipient height) | |||
| >15 cm Taller | 29 (7.1) | 109 (8.7) | .53 |
| 10-15 cm Taller | 41 (10.1) | 141 (11.3) | |
| 5-10 cm Taller | 64 (15.7) | 224 (17.9) | |
| Within 5 cm | 164 (40.3) | 445 (35.6) | |
| 5-10 cm Shorter | 57 (14.0) | 189 (15.1) | |
| 10-15 cm Shorter | 30 (7.4) | 88 (7.0) | |
| >15 cm Shorter | 22 (5.4) | 54 (4.3) | |
| Cytomegalovirus mismatch | |||
| Donor positive and recipient negative | 110 (27.0) | 318 (25.4) | .002 |
| Donor and recipient positive | 183 (45.0) | 485 (38.8) | |
| Donor negative | 111 (27.3) | 446 (35.7) | |
Abbreviations: DCU, donor care unit; ICU, intensive care unit; NA, not applicable.
SI conversion factors: To convert serum bilirubin to micromoles per liter, multiply by 17.104; serum creatinine to micromoles per liter, multiply by 88.4.
Included in graft survival analyses.
Some recipients’ transplant programs accepted donor lungs from both DCU types.
As classified by the Organ Procurement and Transplantation Network.13
Calculated as weight in kilograms divided by height in meters squared.
Classified according to Valapour et al.17
Recipient with a history of at least 1 lung transplant before the study period.
As defined by Christie et al.18
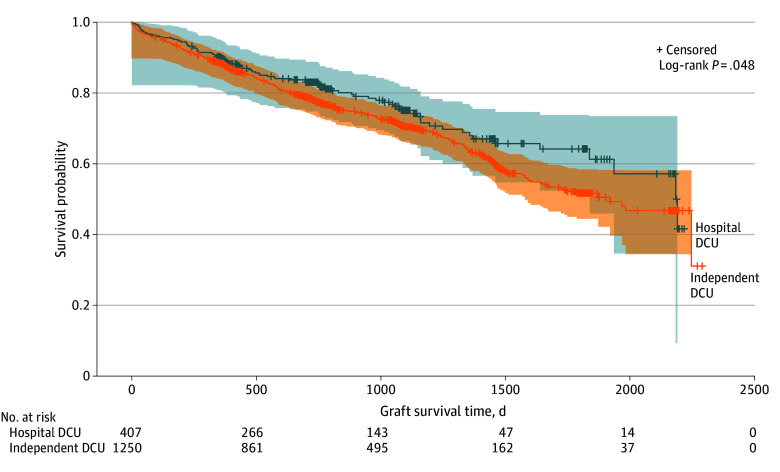
In survival analyses of recipients of transplanted lungs recovered from DCUs, the median (range) duration of follow-up was 734 (0-2292) days among all grafts and 762 (96-2292) days among those with censored outcomes. Among cohort recipients of lungs from DCUs, there were 451 patient deaths and 19 retransplants; 6 recipients were lost to follow-up during the study period (all among recipients of lungs from independent DCUs). The overall incidence of graft loss (death or retransplant) was higher among recipients of lungs recovered in independent DCUs than hospital-based DCUs (377 recipients [30.2%] vs 93 recipients [22.9%]; P = .005).
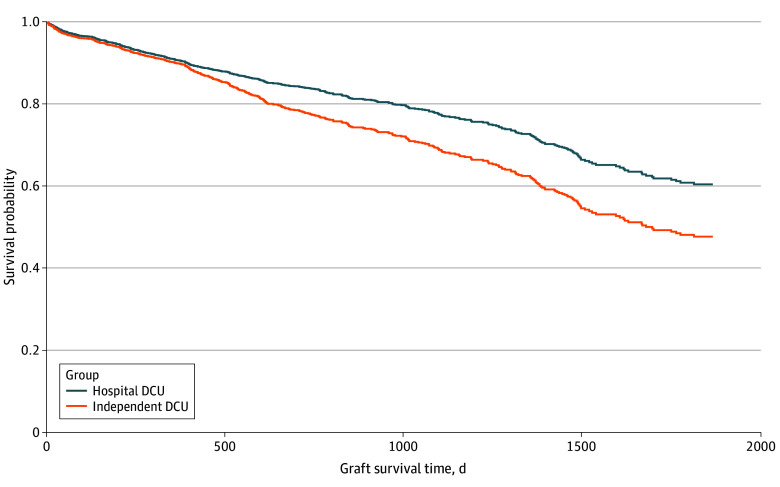
Unadjusted restricted mean (SE) graft survival time at 5 years was shorter among patients who received lungs from donors in independent DCUs than hospital-based DCUs (1548 [27] days vs 1665 [50] days; difference, 117 days; P = .04) (Figure 2). Hazards of graft failure were also higher among lungs recovered from independent DCUs (hazard ratio [HR], 1.26; 95% CI, 1.00-1.58). Additional analyses revealed no evidence against the proportionality assumption with respect to the effect of DCU type (see eResults in Supplement 1). After stratification by transplant center and year and adjustment for extended criteria lung donor status, recipient age, sex, race and ethnicity, lung allocation score at transplant, transplant type (double vs single), difference between donor and recipient height, and cytomegalovirus status, hazards of graft failure remained significantly higher among lungs recovered from independent DCUs than hospital-based DCUs (adjusted HR, 1.85; 95% CI, 1.28–2.65) (Figure 3).
Figure 2. Unadjusted Graft Survival Between Donors in Hospital-Based vs Independent Donor Care Units (DCUs).
The figure shows lung transplant recipients with lung(s) recovered in hospital-based DCUs and independent DCUs. Shading represents 95% CIs for the estimates. Crosses indicate censoring.
Figure 3. Adjusted Probability of Graft Survival From Hospital-Based vs Independent Donor Care Units (DCUs).
The figure shows estimated adjusted graft survival probability among recipients of lungs recovered from hospital-based DCUs and independent DCUs over time. Curves were adjusted for transplant year, transplant program, extended criteria lung donor status, recipient age, sex, race and ethnicity, lung allocation score at the time of transplant, transplant type (double or single lung), differences between donor and recipient height, and cytomegalovirus status.
Secondary Outcomes
Most donation process outcomes were similar between donors in independent and hospital-based DCUs. However, median (IQR) donor management time was significantly shorter among donors in independent DCUs than hospital-based DCUs (49 [8-153] hours vs 61 [10-153] hours; P < .001). Most secondary outcomes were similar between recipients of lungs from hospital-based vs independent DCUs, including receipt of mechanical ventilation at 72 hours and unadjusted and adjusted 1-year graft survival (eTable 3 in Supplement 1).
Discussion
In this retrospective cohort study of national organ donor and lung transplant recipient data, overall graft survival was nearly 4 months longer among lungs recovered from donors in hospital-based DCUs. This finding, which was unexpected and refuted the study hypothesis, was consistent across analyses and robust in analyses accounting for transplant program, transplant year, and donor and recipient factors associated with graft and recipient survival.
While understanding DCU utilization and outcomes is a priority for the US Centers for Medicare & Medicaid Services24 and the Health Resources and Services Administration,25 most studies of DCUs have focused on short-term donation outcomes (including the number and type of organs recovered from donors)8,9,26,27,28 and donation process measures.4,26,27,29 This study is consistent with past work suggesting that lungs are more frequently recovered and transplanted from donors after brain death cared for in independent DCUs than acute-care hospitals,8,9 but is unique in its examination of longer-term lung transplant recipient outcomes and direct comparisons between independent and hospital-based DCUs.
Three potential differences between DCU types may underlie study findings: (1) donor selection, (2) donor management, and (3) relationships with transplant programs. First, observational studies of DCU processes and outcomes are inherently limited by donor selection bias; even when DCUs are available, not all deceased donors are transferred. As in past studies,3,9,30 individual DCUs varied in the proportion of donors transferred from area hospitals. This may reflect systematic or individual differences in available local hospital or transportation resources or donor characteristics (including clinical stability and family authorization for transfer) between areas. In this study, detectable differences between donors in each DCU type were inconsistently associated with better donation outcomes, suggesting that differences in donor acceptance practices may not be systematic. Alternatively, donor selection processes may be unique and potentially modifiable features of DCU models (eg, through expansion of clinical services to accommodate more clinically heterogeneous donors) that may be used to optimize local donation system performance.
Second, critical care nurses, respiratory therapists, and physician consultants available in hospital-based DCUs may improve graft survival over lungs recovered from independent DCUs. In this study, the only detectable management difference between recovery locations was donor management times, which were shortest in independent DCUs and longest in hospital-based DCUs. While consistent with other work focused on independent DCUs,26,31 interpretation of these times in isolation is difficult; shorter times may indicate better efficiency but may also miss opportunities to rehabilitate injured lungs (or other organs) before allocation and recovery or indicate a greater willingness in some DCUs to delay organ recovery until additional organs can be allocated. Further work is needed to identify modifiable differences in clinical team composition or lung management practices that may be leveraged systematically to improve lung graft quality and associated recipient survival.
Last, it is possible that transplant programs may be more willing to consider, accept, and recover lungs from donors in independent DCUs than hospital-based DCUs, either due to expectations of higher quality donor management, fewer logistical barriers (eg, predictable operating room schedules), or lower organ acquisition costs.4,26,27,32 While the survival model stratified by transplant program (accounting for differences in individual program practices) and year (to address changing allocation rules33 and the COVID-19 pandemic), the present analyses cannot account for the myriad clinical, logistical, and programmatic factors that underlie individual organ acceptance decisions.34 Instead, differences in donor and recipient characteristics between groups in this study likely reflect complex relationships between waitlisted patients, transplant programs, and organ availability.
While secondary comparisons of survival duration did not identify differences between independent DCUs and area hospitals, higher rates of lung acceptance from DCUs have potential benefits beyond individual recipient survival. This study further illustrates the need to distinguish between factors associated with lung acceptance and donation,35 primary graft dysfunction,36 and recipient survival.37 For example, in a recent study by Marklin and colleagues,7 a demonstrable increase in potential donors’ Pao2:Fio2 ratio after prone positioning was associated with higher lung acceptance and donation rates but no difference in recipient survival at 3 months.
In this study, long-term graft survival was unexpectedly longer among recipients of lungs recovered from hospital-based DCUs than independent DCUs or hospitals. Given the very short period of donor management relative to recipient survival, the observed relationship requires further examination. Fundamentally, understanding DCU management and outcomes needs prospective study to address selection bias inherent in decisions to transfer donors to DCUs, to characterize expected but unmeasured differences in operations and resources between DCU models, and to define measures of donor management quality and DCU-specific performance in a rapidly changing system. At present, comprehensive understanding is limited by the quality and granularity of systematically collected data on deceased organ donor management38 and DCU operations.
To that end, we hope that this study spurs specific research to better understand how donors are cared for in DCUs vs hospitals, how the presence of DCUs impacts organ allocation and acceptance, and how DCUs are best utilized to facilitate local and national donation system improvements. In the meantime, before transplant programs incorporate study findings into assessments of graft quality, additional work is needed to replicate results and, if observed differences in graft survival are present, to leverage those differences to maximize transplant access and recipient survival.
Limitations
Study limitations include those inherent to the use of preexisting data. We assumed all donors were equally eligible for DCU transfer and could not account for all factors impacting decisions to transfer donors to a DCU or for donated lungs to be accepted and transplanted for individual recipients. In a US system characterized by heterogeneity of practices, resources, and outcomes among diverse hospitals,39 OPOs,40 and transplant programs,41,42 the impact of DCUs on individual stakeholders is also likely to vary. Furthermore, results may not be generalizable to newly opened DCUs and, given that donor management is rarely shared or transferred between organ procurement organizations, do not apply to the majority of US donor service areas without operating DCUs.3,30 As the study dataset lacks relevant information about the clinical conditions and management of deceased donors, we could not compare quality or intensity of donor management between groups.5,6,43 Similarly, we could not account for recipient care delivery variables (eg, ventilator settings) associated with short- and long-term outcomes but not captured in the study dataset.6,37 Additionally, some model factors, such as recipient race and ethnicity, are imprecise indicators of complex differences in social determinants of health that impact both transplant eligibility and recipient survival.15,16,44,45
Conclusions
In this cohort study, among deceased donors after brain death cared for in hospital-based donor care units, transplanted lungs survived 4 months longer, on average, than lungs recovered from independent DCUs. Overall, lung donation rates were highest among donors in independent DCUs but higher among deceased organ donors after brain death cared for in DCUs vs traditional hospital settings. Further work is needed to understand and validate these differences and to identify potential interventions to increase lung transplant recipient survival and availability of transplanted lungs.
eFigure. Proposed Causal Diagram Describing Potential Impact of Donor Care Units on Lung Transplantation and Recipient Survival
eMethods.
eTable 1. Organ Donor Characteristics by Organ Recovery Site
eTable 2. Organ Donation Processes and Outcomes by Organ Recovery Site
eResults.
eTable 3. Lung Transplant Recipient Outcomes by Organ Recovery Site
eReferences.
Data Sharing Statement
References
- 1.Razdan M, Degenholtz HB, Kahn JM, Driessen J. Breakdown in the organ donation process and its effect on organ availability. J Transplant. 2015;2015:831501. doi: 10.1155/2015/831501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kizere KW, English RA, Hackmann M, eds. National Research Council Realizing the Promise of Equity in the Organ Transplantation System. The National Academies Press; 2022. doi: 10.17226/26364 [DOI] [PubMed] [Google Scholar]
- 3.Marklin GF, Brockmeier D, Spector K. The 20-year paradigm shift toward organ recovery centers: 2500 donors at Mid-America Transplant and broader adoption across the United States. Am J Transplant. 2023;23(7):891-903. doi: 10.1016/j.ajt.2023.01.010 [DOI] [PubMed] [Google Scholar]
- 4.Gauthier JM, Doyle MBM, Chapman WC, et al. Economic evaluation of the specialized donor care facility for thoracic organ donor management. J Thorac Dis. 2020;12(10):5709-5717. doi: 10.21037/jtd-20-1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angel LF, Levine DJ, Restrepo MI, et al. Impact of a lung transplantation donor-management protocol on lung donation and recipient outcomes. Am J Respir Crit Care Med. 2006;174(6):710-716. doi: 10.1164/rccm.200603-432OC [DOI] [PubMed] [Google Scholar]
- 6.Mascia L, Pasero D, Slutsky AS, et al. Effect of a lung protective strategy for organ donors on eligibility and availability of lungs for transplantation: a randomized controlled trial. JAMA. 2010;304(23):2620-2627. doi: 10.1001/jama.2010.1796 [DOI] [PubMed] [Google Scholar]
- 7.Marklin GF, O’Sullivan C, Dhar R. Ventilation in the prone position improves oxygenation and results in more lungs being transplanted from organ donors with hypoxemia and atelectasis. J Heart Lung Transplant. 2021;40(2):120-127. doi: 10.1016/j.healun.2020.11.014 [DOI] [PubMed] [Google Scholar]
- 8.Chang SH, Kreisel D, Marklin GF, et al. Lung focused resuscitation at a specialized donor care facility improves lung procurement rates. Ann Thorac Surg. 2018;105(5):1531-1536. doi: 10.1016/j.athoracsur.2017.12.009 [DOI] [PubMed] [Google Scholar]
- 9.Vail EA, Schaubel DE, Abt PL, Martin ND, Reese PP, Neuman MD. Organ transplantation outcomes of deceased organ donors in organ procurement organization-based recovery facilities versus acute-care hospitals. Prog Transplant. 2023;33(2):110-120. doi: 10.1177/15269248231164176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasiske BL, McBride MA, Cornell DL, et al. Report of a consensus conference on transplant program quality and surveillance. Am J Transplant. 2012;12(8):1988-1996. doi: 10.1111/j.1600-6143.2012.04130.x [DOI] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 12.United Network for Organ Sharing . A guide to calculating the lung allocation score. Published July 14, 2020. Accessed March 1, 2023. https://unos.org/wp-content/uploads/unos/lung-allocation-score.pdf
- 13.United Network for Organ Sharing . How UNOS collects data. Published August 1, 2023. Accessed March 5, 2024. https://unos.org/data/data-collection/
- 14.Kernodle AB, Zhang W, Motter JD, et al. Examination of racial and ethnic differences in deceased organ donation ratio over time in the US. JAMA Surg. 2021;156(4):e207083. doi: 10.1001/jamasurg.2020.7083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mooney JJ, Hedlin H, Mohabir P, Bhattacharya J, Dhillon GS. Racial and ethnic disparities in lung transplant listing and waitlist outcomes. J Heart Lung Transplant. 2018;37(3):394-400. doi: 10.1016/j.healun.2017.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonner SN, Thumma JR, Valbuena VSM, et al. The intersection of race and ethnicity, gender, and primary diagnosis on lung transplantation outcomes. J Heart Lung Transplant. 2023;42(7):985-992. doi: 10.1016/j.healun.2023.02.1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valapour M, Lehr CJ, Wey A, Skeans MA, Miller J, Lease ED. Expected effect of the lung Composite Allocation Score system on US lung transplantation. Am J Transplant. 2022;22(12):2971-2980. doi: 10.1111/ajt.17160 [DOI] [PubMed] [Google Scholar]
- 18.Christie IG, Chan EG, Ryan JP, et al. National trends in extended criteria donor utilization and outcomes for lung transplantation. Ann Thorac Surg. 2021;111(2):421-426. doi: 10.1016/j.athoracsur.2020.05.087 [DOI] [PubMed] [Google Scholar]
- 19.Chambers DC, Cherikh WS, Harhay MO, et al. ; International Society for Heart and Lung Transplantation . The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant. 2019;38(10):1042-1055. doi: 10.1016/j.healun.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L, Tian L, Uno H, et al. Utilizing the integrated difference of two survival functions to quantify the treatment contrast for designing, monitoring, and analyzing a comparative clinical study. Clin Trials. 2012;9(5):570-577. doi: 10.1177/1740774512455464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lederer DJ, Kawut SM, Wickersham N, et al. ; Lung Transplant Outcomes Group . Obesity and primary graft dysfunction after lung transplantation: the Lung Transplant Outcomes Group Obesity Study. Am J Respir Crit Care Med. 2011;184(9):1055-1061. doi: 10.1164/rccm.201104-0728OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamond JM, Lee JC, Kawut SM, et al. ; Lung Transplant Outcomes Group . Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013;187(5):527-534. doi: 10.1164/rccm.201210-1865OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harhay MO, Porcher R, Thabut G, et al. Donor lung sequence number and survival after lung transplantation in the United States. Ann Am Thorac Soc. 2019;16(3):313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Medicare and Medicaid Services . Request for information: health and safety requirements for transplant programs, organ procurement organizations, and end-stage renal disease facilities. Federal Register. Published December 3, 2021. Accessed May 15, 2024. https://www.federalregister.gov/d/2021-26146
- 25.Organ Procurement and Transplantation Network . Organ Procurement and Transplantation Network (OPTN) RFI: HSB115C1031. US General Services Administration. Published April 8, 2022. Accessed July 10, 2023. https://sam.gov/opp/df25032b76b1467eabae79a2ba222ead/view
- 26.Jendrisak MD, Hruska K, Wagner J, Chandler D, Kappel D. Cadaveric-donor organ recovery at a hospital-independent facility. Transplantation. 2002;74(7):978-982. doi: 10.1097/00007890-200210150-00014 [DOI] [PubMed] [Google Scholar]
- 27.Doyle M, Subramanian V, Vachharajani N, et al. Organ donor recovery performed at an organ procurement organization-based facility is an effective way to minimize organ recovery costs and increase organ yield. J Am Coll Surg. 2016;222(4):591-600. doi: 10.1016/j.jamcollsurg.2015.12.032 [DOI] [PubMed] [Google Scholar]
- 28.Frye CC, Gauthier JM, Bery A, et al. Donor management using a specialized donor care facility is associated with higher organ utilization from drug overdose donors. Clin Transplant. 2021;35(3):e14178. doi: 10.1111/ctr.14178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vail EA, Schaubel DE, Potluri VS, et al. Deceased organ donor management and organ distribution from organ procurement organization-based recovery facilities versus acute-care hospitals. Prog Transplant. 2023;33(4):283-292. doi: 10.1177/15269248231212918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vail EA, Tam VW, Sonnenberg EM, et al. Characterizing proximity and transfers of deceased organ donors to donor care units in the United States. Am J Transplant. 2024;24(6):983-992. doi: 10.1016/j.ajt.2024.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moazami N, Javadi OH, Kappel DF, Wagner J, Jendrisak MD. The feasibility of organ procurement at a hospital-independent facility: a working model of efficiency. J Thorac Cardiovasc Surg. 2007;133(5):1389-1390. doi: 10.1016/j.jtcvs.2006.12.038 [DOI] [PubMed] [Google Scholar]
- 32.Lindemann J, Dageforde LA, Brockmeier D, et al. Organ procurement center allows for daytime liver transplantation with less resource utilization: may address burnout, pipeline, and safety for field of transplantation. Am J Transplant. 2019;19(5):1296-1304. doi: 10.1111/ajt.15129 [DOI] [PubMed] [Google Scholar]
- 33.Organ Procurement and Transplantation Network Lung Transplantation Committee . Briefing to the OPTN Board of Directors: establish continuous distribution of lungs. Health Services and Resources Administration. Published December 6, 2021. Accessed July 14, 2023. https://optn.transplant.hrsa.gov/media/esjb4ztn/20211206-bp-lung-establish-cont-dist-lungs.pdf
- 34.Schnellinger EM, Cantu E, Kimmel SE, Szymczak JE. A conceptual model for sources of differential selection in lung transplant allocation. Ann Am Thorac Soc. 2023;20(2):226-235. doi: 10.1513/AnnalsATS.202202-105OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heiden BT, Yang Z, Bai YZ, et al. Development and validation of the lung donor (LUNDON) acceptability score for pulmonary transplantation. Am J Transplant. 2023;23(4):540-548. doi: 10.1016/j.ajt.2022.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantu E, Diamond J, Ganjoo N, et al. Scoring donor lungs for graft failure risk: The Lung Donor Risk Index (LDRI). Am J Transplant. 2024;24(5):839-849. doi: 10.1016/j.ajt.2024.01.022 [DOI] [PubMed] [Google Scholar]
- 37.Mal H, Santin G, Cantrelle C, et al. Effect of lung-protective ventilation in organ donors on lung procurement and recipient survival. Am J Respir Crit Care Med. 2020;202(2):250-258. doi: 10.1164/rccm.201910-2067OC [DOI] [PubMed] [Google Scholar]
- 38.Doby BL, Boyarsky BJ, Gentry S, Segev DL. Improving OPO performance through national data availability. Am J Transplant. 2019;19(10):2675-2677. doi: 10.1111/ajt.15508 [DOI] [PubMed] [Google Scholar]
- 39.Johnson W, Kraft K, Chotai P, et al. Variability in organ procurement organization performance by individual hospital in the United States. JAMA Surg. 2023;158(4):404-409. doi: 10.1001/jamasurg.2022.7853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeRoos LJ, Zhou Y, Marrero WJ, et al. Assessment of national organ donation rates and organ procurement organization metrics. JAMA Surg. 2021;156(2):173-180. doi: 10.1001/jamasurg.2020.5395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thabut G, Christie JD, Kremers WK, Fournier M, Halpern SD. Survival differences following lung transplantation among US transplant centers. JAMA. 2010;304(1):53-60. doi: 10.1001/jama.2010.885 [DOI] [PubMed] [Google Scholar]
- 42.Wakeam E, Thumma JR, Bonner SN, et al. One-year mortality is not a reliable indicator of lung transplant center performance. Ann Thorac Surg. 2022;114(1):225-232. doi: 10.1016/j.athoracsur.2022.02.028 [DOI] [PubMed] [Google Scholar]
- 43.Swanson EA, Patel MS, Hutchens MP, et al. Critical care and ventilatory management of deceased organ donors impact lung use and recipient graft survival. Am J Transplant. 2021;21(12):4003-4011. doi: 10.1111/ajt.16719 [DOI] [PubMed] [Google Scholar]
- 44.Malas J, Chen Q, Megna D, et al. Lung transplantation outcomes in patients from socioeconomically distressed communities. J Heart Lung Transplant. 2023;42(12):1690-1699. doi: 10.1016/j.healun.2023.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lehr CJ, Valapour M, Gunsalus PR, et al. Association of socioeconomic position with racial and ethnic disparities in survival after lung transplant. JAMA Netw Open. 2023;6(4):e238306. doi: 10.1001/jamanetworkopen.2023.8306 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Proposed Causal Diagram Describing Potential Impact of Donor Care Units on Lung Transplantation and Recipient Survival
eMethods.
eTable 1. Organ Donor Characteristics by Organ Recovery Site
eTable 2. Organ Donation Processes and Outcomes by Organ Recovery Site
eResults.
eTable 3. Lung Transplant Recipient Outcomes by Organ Recovery Site
eReferences.
Data Sharing Statement