Summary
Background
Mucositis is a common and highly impactful side effect of conventional and emerging cancer therapy and thus the subject of intense investigation. Although common practice, mucositis assessment is heterogeneously adopted and poorly guided, impacting evidence synthesis and translation. The Multinational Association of Supportive Care in Cancer (MASCC) Mucositis Study Group (MSG) therefore aimed to establish expert recommendations for how existing mucositis assessment tools should be used, in clinical care and trials contexts, to improve the consistency of mucositis assessment.
Methods
This study was conducted over two stages (January 2022–July 2023). The first phase involved a survey to MASCC-MSG members (January 2022–May 2022), capturing current practices, challenges and preferences. These then informed the second phase, in which a set of initial recommendations were prepared and refined using the Delphi method (February 2023–May 2023). Consensus was defined as agreement on a parameter by >80% of respondents.
Findings
Seventy-two MASCC-MSG members completed the first phase of the study (37 females, 34 males, mainly oral care specialists). High variability was noted in the use of mucositis assessment tools, with a high reliance on clinician assessment compared to patient reported outcome measures (PROMs, 47% vs 3%, 37% used a combination). The World Health Organization (WHO) and Common Terminology Criteria for Adverse Events (CTCAE) scales were most commonly used to assess mucositis across multiple settings. Initial recommendations were reviewed by experienced MSG members and following two rounds of Delphi survey consensus was achieved in 91 of 100 recommendations. For example, in patients receiving chemotherapy, the recommended tool for clinician assessment in clinical practice is WHO for oral mucositis (89.5% consensus), and WHO or CTCAE for gastrointestinal mucositis (85.7% consensus). The recommended PROM in clinical trials is OMD/WQ for oral mucositis (93.3% consensus), and PRO-CTCAE for gastrointestinal mucositis (83.3% consensus).
Interpretation
These new recommendations provide much needed guidance on mucositis assessment and may be applied in both clinical practice and research to streamline comparison and synthesis of global data sets, thus accelerating translation of new knowledge into clinical practice.
Funding
No funding was received.
Keywords: Oral mucositis, Gastrointestinal mucositis, Mucositis assessment tools, Patient-reported outcome measures
Research in context.
Evidence before this study
Based on an electronic PubMed search conducted from January 1st, 2000 to January 1st, 2022 on mucositis assessment tools, a number of high-quality, validated assessment tools exist. However, the assessment of mucositis is inconsistent with little to no guidance on which tools are appropriate in certain settings. The impact of this is heterogeneous evidence that cannot be synthesized to inform clinical practice.
Added value of this study
This study provides the first set of expert recommendations on how to assess mucositis in both clinical practice and trial settings. These recommendations were made on behalf of the highly esteemed international society Multinational Association of Supportive Care in Cancer (MASCC) and members of the Mucositis Study Group. To the best of our knowledge, these recommendations are the first of their kind and are well positioned to improve the consistency of mucositis in clinical practice and trial settings.
Implications of all the available evidence
Adherence to these recommendations could improve homogeneity in patient care and trial conduct. This might result in the next generation of mucositis research being more consistent and thus more readily synthesized to inform clinical practice. Our recommendations also highlight key areas where more evidence is required, including the development of assessment tools for mucositis-like lesions caused by novel anti-cancer treatments, such as immunotherapy and targeted therapies, best practice approaches in pediatrics and the development of novel biomarkers.
Introduction
Mucositis is a common and highly debilitating complication of almost all anti-cancer therapies, including chemotherapy (CT), radiotherapy (RT), hematopoietic cell transplantation (HCT), and targeted therapies.1 Mucositis is an acute side effect, characterized by breakdown of the alimentary mucosa, initiated by direct cytotoxic damage and presenting as ulcerative lesions in the mouth, upper and low gastrointestinal tract. Although mucositis is typically acute and self-limiting, it is frequently reported by patients as their most debilitating side effect of cancer therapy, associated with substantial pain and impaired functional capacity.2,3 In addition, mucositis is as a major catalyst for secondary complications including infection, diaerrhoea, malnutrition, cachexia, dehydration and renal failure, often requiring intensive and costly in-patient supportive care.4,5 As such, mucositis often interferes with the delivery of anti-cancer therapy, necessitating dose reductions and treatment cessation. Accordingly, mucositis has a profound impact on not only patient quality of life but also survival.5
Given the continued impact of mucositis on treatment adherence and quality of life, it is assessed as part of routine patient care and commonly the focus of many clinical trials. As a result, there are now a plethora of assessment tools used to assess mucositis, both with respect to observable pathology and functional implications.6,7 A number of clinical tools have been developed by societies and organizations such as the World Health Organization (WHO)8 and the National Cancer Institute (NCI),9 with other bespoke assessment tools developed such as the Oral Mucositis Assessment Scale (OMAS),10 the Radiation Therapy Oncology Group (RTOG) Scoring Tool,11 and the Oral Mucositis Index (OMI).12 These tools intend to be objective, aiming to inform treatment decision making based on the extent of ulceration, pain and functional deficits. More recently, patient reported outcome measures (PROMs) have been more readily adopted, reflecting the increasing need to understand the burden of mucositis symptoms and how they are perceived by the individual.
While there are a variety of options available for mucositis assessment, these tools vary considerably in their complexity and degree of validation,7,13 with no clear guidance on when and how they should be used. This heterogeneity has undoubtedly impacted the translation of clinical research into clinical practice, with varying adoption of mucositis assessment tools complicating evidence synthesis. We therefore aimed to establish guidance on mucositis assessment, tailored for a variety of clinical contexts, with the goal of informing the next generation of mucositis research to be more consistent and, by extension, better suited to impact clinical practice.
Methods
Study design and participants
The overarching goal of this study was to establish a set of recommendations for mucositis assessment. This was achieved by first engaging with members of the Multinational Association of Supportive Care in Cancer (MASCC) Mucositis Study Group (MSG) to understand their current approaches to mucositis assessment, and to identify barriers and enablers of assessment to inform our initial set of assessment recommendations. These recommendations were then iteratively optimized until consensus was achieved.
The MSG is the largest MASCC group, which consists of medical professionals, dentists, basic and translational scientists, nurses, pharmacists and bioinformaticians (Appendix 1). Collaborating closely with the International Society of Oral Oncology (ISOO), the group's major goal is to improve outcomes of patients experiencing oral and gastrointestinal mucositis.
Ethics
“The study was approved by the Chinese University of Hong Kong Survey and Behavioral Research Ethics (Reference number SBRE-21-0020A). All surveys were completed by respondents anonymously. The data were stored securely and confidentially, with only authorized research staff having access to it. The respondents were asked to only complete the sections that align with their expertise and experience and had the option to opt out the survey in any step. Considering this was a survey, completing it was deemed consent to participate in the study.
Procedures
Based on a literature review, the most frequently adopted and reported assessment tools were selected to be incorporated in the first survey. Using an electronic search of PubMed from January 1st, 2000 to January 1st, 2022, the following search strategy was used: “mucositis” AND/OR “assessment tools” AND “anticancer treatment” AND “chemotherapy” AND “targeted agents” AND “clinical trials”. The first survey (Appendix 2) was developed by the MSG leadership team (PB, HW), and disseminated to the MASCC MSG membership (N = 431) via email according to a distribution list. MSG members were invited to participate in the survey using the SurveyMonkey platform. Members were deemed eligible if they identified as age >18 years old; are healthcare providers (i.e., dentists, oral hygienists, oncologists, nurses, pharmacists among others) and/or are clinical researchers and were willing and able to complete the survey in English. The survey was distributed in April 2022. An email reminder was sent two weeks following the initial invitation to participate. The survey ended a month after the initial request.
To achieve consensus, the Delphi method was used to iteratively review and optimize a set of recommendations established (PB, DK, NB, PB) using insights gained from the consultation phase, whilst drawing on their expert opinion (Appendix 3). The study was conducted in accordance with the CREDES (Conducting and Reporting Delphi Studies) guidelines.
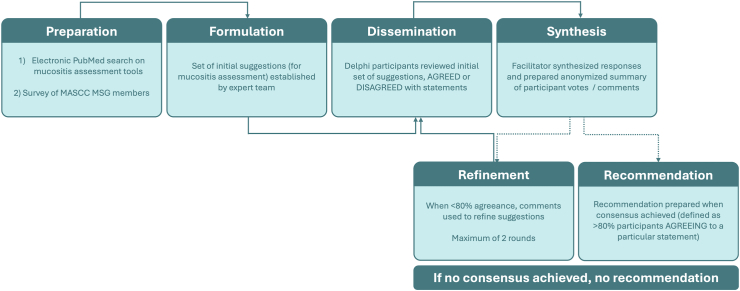
During February 2023, these initial suggestions were distributed to 31 experienced experts selected from the MSG, who were asked to review the suggestions and either agree or disagree and provide comments. These experts were chosen as having >10 years of experience in providing care for cancer patients, being MSG members and having a multidisciplinary view on the disease and on the adverse effects due to the treatments. A facilitator (PB) then provides an anonymized summary of the experts’ votes/comments. If ≥80% of the respondents agree with the initial suggestion, consensus was achieved. If <80% agree, the responses and comments were used to revise the suggestions, which were sent to the same 31 experts again for a second Delphi round. If no consensus is achieved, no recommendation was made. The respondents had 3 weeks to complete each round. Fig. 1 shows a flow chart demonstrating the stages of the Delphi process.
Fig. 1.
Flow chart demonstrating the stages of the Delphi process. MASCC, Multinational Association of Supportive Care in Cancer; MSG, Mucositis Study Group.
The recommendations were tailored to specific clinical contexts where mucositis presentation may differ (cyclic chemotherapy, head and neck radiotherapy, pelvic radiotherapy, hematopoietic stem cells transplant (HSCT), targeted therapy and immunotherapy), and separated based on whether they assessed oral or gastrointestinal mucositis, in a clinical context or clinical trial setting.
The names, affiliations, and description of the role of the members in the Delphi phase are outlined in Appendix 4.
Role of funding source
No funding was received for this study.
Results
First phase: consultation
Seventy-two respondents completed the survey (16.7% response rate, 72/431). Most respondents (77%, N = 55) were aged between 30 and 59 years, with a female-to-male ratio of 52%:48% (N = 37, 34). Most of the respondents identified themselves as clinicians (94%, N = 66), with the remainder being researchers that did not provide clinical care to patients. Clinicians were most commonly dental practitioners or oral care specialists (48%, N = 34), followed by medical oncologists (14%, N = 10) and radiation oncologists (11%, N = 8). Other professions included hematologists, gastroenterologists, supportive and palliative care physicians, nurse practitioners, dermatologists, internal medicine physician and pharmacist. Among clinicians, 82% (N = 57) reported they were also engaged in research. 77% (N = 51) of clinicians reported they had 10 to over 20 years of experience in providing care for people with cancer. Twenty-nine percent (N = 20), 19% (N = 13), 9% (N = 6) and 26% (N = 18) of the respondents reported they have led or participated in up to 5, 5–10, 10–15 and over 15 clinical trials, respectively. Most of the respondents were affiliated with a university hospital (49%, N = 35) or a community hospital (28%, N = 20), with others working in universities (13%, N = 9), research institutions (4%, N = 3), private practices (3%, N = 2) or community clinics (1.4%, N = 1). Most respondents were from Western Europe (31%, N = 22) followed by Asia–Pacific (24%, N = 17), North America (20%, N = 14), Latin America (17%, N = 12) and Eastern Europe (8%, N = 6).
Use of assessment tools
Clinical assessment tools (e.g. WHO/NCI) were most frequently used by healthcare professionals, with 47% of respondents reporting that they exclusively used clinical assessment tools and only 3% reporting they use PROMs or a combination of both (37%). Similarly, clinical researchers used clinical assessment tools more readily compared to PROMs, with WHO the most commonly reported tool (59%) by this sub-cohort.
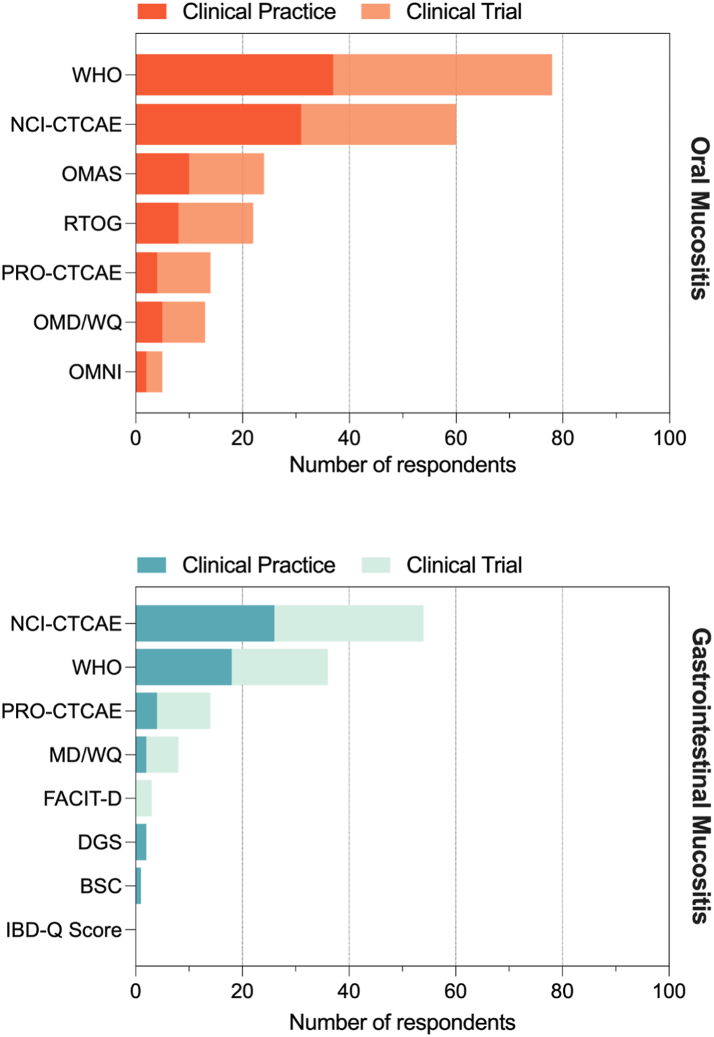
Healthcare professionals and clinical researchers reported similar preferences for the assessment of oral mucositis, with the WHO scale most commonly reported (52% vs 59%, respectively), followed by NCI-CTCAE scale (44% vs 41%), OMAS (14% vs 20%), RTOG (11% vs 20%), Oral Mucositis Daily Questionnaire (OMDQ, 7% vs 11%), Patient Reported Outcome- Common Terminology Criteria for Adverse Events (PRO-CTCAE, 6% vs 14%), Oral Mucositis Nursing Instrument (OMNI, 3% for both) and other scales.
Assessment of gastrointestinal mucositis by healthcare professionals and clinical researchers was also comparable, and included the NCI-CTCAE scale (38% vs 42%), followed by the WHO scale (27% for both), PRO-CTCAE (6% vs 15%), Mucositis Daily Questionnaire (3% vs 9%), Daily Gut Score (3% healthcare providers only), Bristol Stool Chart (1.5% healthcare providers only), the Functional Assessment of Chronic Illness Therapy (FACIT-D, 5% clinical researchers only) and other scales.
The various assessment tools in and preferences on their use in clinical trials and clinical practice are illustrated in Fig. 2.
Fig. 2.
Assessment tools ranked by preference in sample of MASCC MSG members and aggregated based on their use in clinical trials vs clinical practice. The respondents were asked to mark their preferred assessment tool, in clinical practice and in clinical trials. Hence for each assessment tool, the same respondents answered two questions. BSC, Bristol stool scale; DGS, daily gut score; FACIT-D, Functional Assessment of Chronic Illness Therapy Diaerrhoea subscale; NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events; IBD-Q Score, Inflammatory Bowel Disease Questionnaire; OMAS, Oral Mucositis Assessment Scale; OMDQ, Oral Mucositis Daily Questionnaire; OMNI, Oral Mucositis Nursing Instrument. OMWQ, Oral Mucositis Weekly Questionnaire; PRO-CTCAE, Patient-reported outcome-Common Terminology Criteria for Adverse Events; RTOG, Radiation Therapy Oncology Group; WHO, World Health Organization.
Confidence in clinical assessment tools, and reported strengths and weakness
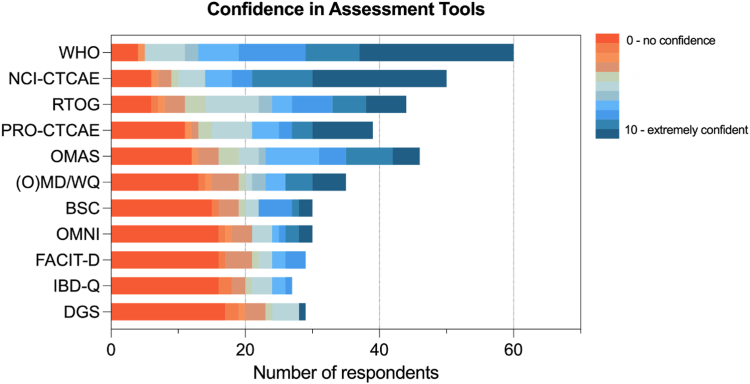
Respondents were asked to rate their degree of confidence in using each mucositis assessment tool, from 0 (not confident at all) to 10 (extremely confident in use) (Fig. 3). The WHO scale received the highest weighted average (8.8), followed by NCI-CTCAE (8.3), RTOG (6.8), PRO-CTCAE (6.23), OMAS (6.11), OMDQ (5) and other scales.
Fig. 3.
MASCC MSG member confidence in assessment tools with each tool ranked from 0 = no confident at all in using the tool to 10 = extremely confident in using the tool. BSC, Bristol stool scale; DGS, daily gut score; FACIT-D, Functional Assessment of Chronic Illness Therapy Diaerrhoea subscale; NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events; IBD-Q Score, Inflammatory Bowel Disease Questionnaire; OMAS, Oral Mucositis Assessment Scale; OMDQ, Oral Mucositis Daily Questionnaire; OMWQ, Oral Mucositis Weekly Questionnaire; PRO-CTCAE, Patient-reported outcome-Common Terminology Criteria for Adverse Events; RTOG, Radiation Therapy Oncology Group; WHO, World Health Organization.
Respondents were also asked to identify the key strengths and weaknesses of the various scales. For the WHO scale, the major strength was that it is easy to use (36% of respondents), followed by being universally accepted (31%), reproducible (9%), quick (6%) and patient friendly (2%). However, 18% of respondents reported that the WHO scale was not clear for patients (18%) and was not universally accepted (9%) or nor reproducible (9%). Respondents reported that the commonly used NCI-CTCAE scale was universally accepted (57%), easy to use (17%) and reproducible (7%). However, there were also responses that reported that it is not reproducible (26%), not clear to patients (17%) and too complicated for use (2%).
Use of digital tools to assess mucositis
In light of the increasing number of digital tools available to collect clinical and patient reported outcomes, we also asked respondents to report on how readily they adopted digital tools to facilitate mucositis assessment. Only 4.6% of respondents reported using digital tools for mucositis assessment. These tools included an application evaluating oral and gastrointestinal mucositis and PersonifyCare Digital Data Collection platform. The major barriers to digital data collection were the lack of universally accepted tools (39%), cost (23%), lack of appropriate infrastructure (22%), lack of literature (20%), patient hesitancy (15%) and time (12%). Additional reported barriers included unfamiliarity and lack of knowledge about the possibilities or resources available.
Biomarkers used in mucositis assessment
Despite a number of emerging biomarkers available for mucositis assessment, only 7.3% of respondents reported using biomarkers in routine mucositis assessment. Of respondents that did report on their use, biomarkers included radiation dose (presumably as a predictive factor), oral bacterial load/composition, doppler flowmetry and plasma citrulline.
Second phase: consensus
The first set of recommendations (Appendix 3) were reviewed by N = 19 respondents (of N = 31 identified experienced MSG members), 71/100 statements achieved consensus at the first Delphi round. Using the respondents' feedback, the statements were revised, and a second set of recommendations were sent to the same experienced respondents. This resulted in consensus being achieved in most (91/100) statements. Statements not reaching consensus were related to assessment tools in targeted therapy and immunotherapy, as well as the statements regarding frequency of assessment of oral mucositis (using the recommended clinical assessment tool) in people with head and neck cancer undergoing (chemo)radiotherapy in a clinical trials/research context.
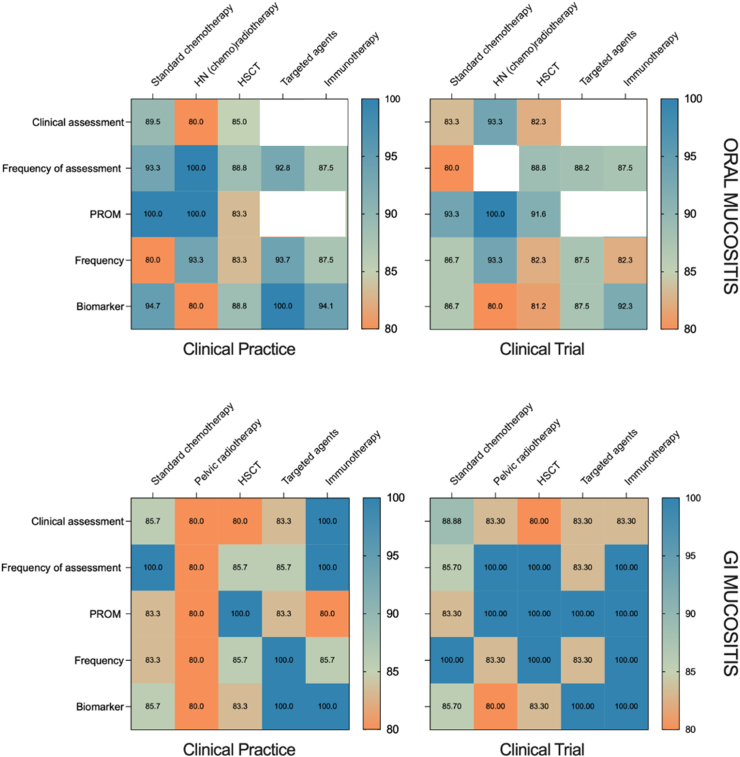
The final consensus recommendations are summarized in Table 1, Table 2, Table 3, Table 4. Rates of agreement for each recommendation is illustrated in Fig. 4.
Table 1.
Oral mucositis assessment in clinical practice.
| Clinical setting |
|||||
|---|---|---|---|---|---|
| Standard chemotherapywhere risk of OM is >20% | Head and neck (chemo)radiotherapy | HSCT | Targeted agents | Immunotherapy | |
| Recommended tool for clinician assessment | WHO | WHO | WHO | No consensus | No consensus |
| Frequency of assessment | Weekly and every time the symptoms manifest. Televisit/telehealth may complement this assessment | Weekly or more in high grade mucositis | Daily (if inpatient) Weekly (if feasible in outpatient setting) | As the trajectories vary, assessment should be performed at least at each cycle or clinic visit/telehealth appointment | At each cycle or clinic visit/telehealth appointment |
| Recommended PROM | OMWQ or PRO-CTCAE questions related to mucositis | OMWQ and OMDQ or PRO-CTCAE questions related to mucositis | OMWQ or OMDQ or PRO-CTCAE questions related to mucositis | No consensus | No consensus |
| Frequency of assessment | Weekly or whenever present with symptoms | Weekly or more frequently in severe mucositis | Weekly or whenever present with symptoms | At each cycle or clinic visit/telehealth appointment | At each cycle or clinic visit/telehealth appointment |
| Biomarkers to consider | No biomarkers recommended | No biomarkers recommended | No biomarkers recommended | No biomarkers recommended | No biomarkers recommended |
Table 2.
Oral mucositis assessment in clinical trials.
| Clinical trial |
|||||
|---|---|---|---|---|---|
| Standard chemotherapy | Head and neck (chemo)radiotherapy | HSCT | Targeted agents | Immunotherapy | |
| Recommended tool for clinician assessment | WHO | WHO accepted as standard by regulatory bodies. Another option is CTCAE | WHO | No consensus | No consensus |
| Frequency of assessment | Weekly and every time the symptoms manifest. Televisit/telehealth may complement this assessment | No consensus | Daily (if inpatient) Weekly (if feasible in outpatient setting) | At each cycle or clinic visit/telehealth appointment | At each cycle or clinic visit/telehealth appointment |
| Recommended PROM | OMWQ or OMDQ if a more frequent follow up schedule is needed OR PRO-CTCAE questions related to mucositis | OMWQ and OMDQ or PRO-CTCAE questions related to mucositis | OMWQ or OMDQ or PRO-CTCAE questions related to mucositis | No consensus | No consensus |
| Frequency of assessment | Weekly or more frequently in case of treatment at higher risk of mucositis | Weekly or more frequently in case of treatment at higher risk of mucositis | Daily if inpatient and using OMDQ. Weekly if feasible in outpatient setting | At each cycle or clinic visit/telehealth appointment | At each cycle or clinic visit/telehealth appointment |
| Biomarkers to consider | No biomarkers recommended | No biomarkers recommended | No biomarkers recommended | No biomarkers recommended | No biomarkers recommended |
Table 3.
Gastrointestinal mucositis assessment in clinical practice.
| Clinical setting |
|||||
|---|---|---|---|---|---|
| Standard chemotherapy | Pelvic radiotherapy | HSCT | Targeted agents | Immunotherapy | |
| Recommended tool for clinician assessment | WHO CTCAE |
WHO CTCAE |
Bristol stool chart | Bristol stool chart | Bristol stool chart |
| Frequency of assessment | First day of each cycle and at any clinic visit/telehealth appointment (especially when symptoms present) | 3-weekly | Daily (if in-patient) Weekly (if feasible in outpatient setting) |
Monthly (at each cycle or clinic visit/telehealth appointment) | Monthly (at each cycle or clinic visit/telehealth appointment) |
| Recommended PROM | PRO-CTCAE | PRO-CTCAE | PRO-CTCAE questions or OMDQ plus diarrhea questions may be suggested | No questionnaire specifically validated, suggestion for PRO-CTCAE use | No questionnaire specifically validated, suggestion for PRO-CTCAE use |
| Frequency of assessment | Weekly | Weekly | Daily if inpatient Weekly if outpatient |
At each cycle or clinic visit/telehealth appointment | At each cycle or clinic visit/telehealth appointment |
| Biomarkers to consider | No biomarkers recommended | Plasma citrulliine | No biomarkers recommended | No biomarkers recommended | No biomarkers recommended |
Table 4.
Gastrointestinal mucositis assessment in clinical trials.
| Clinical trial |
|||||
|---|---|---|---|---|---|
| Standard chemotherapy | Pelvic radiotherapy | HSCT | Targeted agents | Immunotherapy | |
| Recommended tool for clinician assessment | CTCAE | WHO CTCAE |
Bristol stool chart | Bristol stool chart | Bristol stool chart |
| Frequency of assessment | At beginning of each cycle and weekly if practicable | Every 1–3 weeks | Daily (if in-patient) Weekly (if feasible in outpatient setting) |
Monthly | Monthly |
| Recommended PROM | PRO-CTCAE | PRO-CTCAE OM(W/D)Q | PRO-CTCAE | No questionnaire specifically validated, suggestion for PRO-CTCAE use | No questionnaire specifically validated, suggestion for PRO-CTCAE use |
| Frequency of assessment | Weekly | Weekly for PRO-CTCAE/OMWQ or daily for OMDQ | Weekly | Monthly | At each cycle |
| Biomarkers to consider | Plasma citrulline and/or gut microbiome composition | Plasma citrulline and/or gut microbiome composition | Plasma citrulline and/or gut microbiome composition | No biomarkers recommended | No biomarkers recommended |
Fig. 4.
Rates of agreement of each recommendation following the second round of the Delphi survey. Recommendations that did not reach consensus are marked in white.
In summary, for the assessment of oral mucositis in clinical practice, the recommended tool for clinician assessment is WHO, used weekly or more as needed. The recommended PROM is Oral Mucositis Weekly/Daily Questionnaire (OMW/DQ) or the PRO-CTCAE, used weekly or more as needed. For biomarkers, a consensus was made not to recommend their routine use.
For oral mucositis assessment in clinical trials, the WHO is recommended, used in a variable frequency depending on the clinical setting. The recommended PROM is the OMD/WQ or PRO-CTCAE, used at least weekly. Consensus was made not to recommend the routine use of biomarkers.
For the assessment of gastrointestinal mucositis (GI) mucositis in clinical practice, the recommended tools for clinician assessment are the WHO, CTCAE, and the Bristol stool chart. The frequency of assessment vary depending on the setting. The recommended PROM is generally the PRO-CTCAE, with a varied frequency. For biomarkers, plasma citrulline is recommended for consideration in pelvic radiotherapy.
For the assessment of GI mucositis in clinical trials, the recommendations are similar to clinical practice, with an addition of a recommendation to use plasma citrulline or gut microbiome for consideration in chemotherapy, pelvic radiotherapy and HSCT.
Discussion
Mucositis remains a significant challenge in providing optimal care for people with cancer, and its assessment therefore represents the cornerstone of effective supportive cancer care both in clinical practice and research. Despite its importance, mucositis assessment lacks guidance and is typically informed by individual or institutional norms or preferences. This heterogeneity undoubtedly impacts our ability to synthesize data and translate new knowledge into clinical practice. The MASCC MSG is the peak professional group providing authoritative guidance on the prediction, prevention and management of mucositis. We therefore leveraged on this collective and global expertise to develop a set of expert opinion-guided recommendations on mucositis assessment, contextualized for various settings in clinical practice and research.
The heterogeneous approach to mucositis assessment we assumed from the available literature was underscored in the first phase of this study. Our data highlight that even in the MASCC MSG, a specialized group where the importance of appropriate mucositis assessment is highly valued, there is significant variability in preferences and practices. As such, it is highly likely that in a non-expert population of healthcare providers and researchers, the degree of variability and need for guidance is amplified.14, 15, 16
In addition to the significant heterogeneity in approaches used by respondents, a key finding in our survey was the limited use of PROMs for both oral and gastrointestinal mucositis. PROMs are a form of health status report, completed by the patient without interpretation or involvement of their healthcare team. A basic assumption of PROMs is that the meaning of an item to a respondent does not change over time, also called measurement invariance.17 However, when one experiences a change in health status, one may change one's internal standards, values and/or conceptualization of symptoms or other aspects of quality of life (i.e., recalibration, reprioritization, or reconceptualization). These underlying changes are termed response-shift effects, and may affect the meaning or interpretation of the measured change.18 Interpreting change over time is thus complicated by this fundamental human process of adaptation. Nevertheless, unlike clinical assessments, PROMs capture the more nuanced and subjective nature of a symptom, aiming to measure its burden irrespective of objective severity.19 Increasing advocacy has emerged for PROMs being integrated into clinical practice and research, especially in supportive cancer care as it is well documented that clinicians tend to under-report on the prevalence, emergence and severity of treatment side effects, including mucositis.13,19, 20, 21, 22, 23, 24, 25, 26 However, given the dynamic nature of mucositis and an individual's perceived symptom burden, especially during treatment and its recovery, PROMs should be collected frequently, which may result in inflated intra-individual variation or “response shifts'.17 Response shifts can be identified through patterns in longitudinal studies and incorporating measures of response shift can be valuable in assessing true change in these patients.17,18
The importance of PROMs in mucositis assessment are also amplified in contexts where mucositis affects regions of the alimentary tract that are difficult to access or visualize (e.g. throat or lower gastrointestinal tract). This therefore requires clinical assessments to be performed in person and requires the patient to describe the nature of their symptoms. Of course, this is the fundamental core of PROMs, however, the relative infrequency of in person clinic visits (which may not align with peak symptom burden) and doctor–patient interaction/power imbalance has the potential to influence the patient's ability or willingness to recall information and report on the true burden of their symptoms. Although PROMs empower patients to report on the burden of their symptoms more autonomously, effective implementation requires a high degree of logistical coordination with the appropriate infrastructure to support their collection. Further to this, while some PROMs are being translated and validated in languages other than English, this is not the case for all. As such, equitable access and use of PROMs on a global scale remains challenging, particularly when digital health tools are required. Although the regulatory bodies do not typically require PROMs as a tool for the process of approving new drugs, it is anticipated that their significance will grow in the future.27
Where PROMs or clinical assessments are challenging, biomarkers offer an objective method of assessment, although they have not yet maturely developed in the context of mucositis (especially oral). Our results indicated generally poor uptake or biomarkers in mucositis assessment and/or prediction, even for heavily studied/validated biomarkers such as plasma citrulline. This reiterates the need to establish clear guidance on mucositis assessment, and to work towards a multidisciplinary assessment approach combining a variety of tools, modalities and enablers that can accommodate settings with varying resources and infrastructure.20,28, 29, 30 This also reinforces the need to increase research on strong biomarkers that could be recognized and used by the health care community to predict the risk of mucositis and/or to confirm the severity of the process. The additive benefit of biomarkers use relies on the fact that they provide objective insights into the integrity of the mucosa.14,28 Their routine use is challenged by the need to collect biospecimens from patients often repeatedly throughout treatment. As such, biomarkers are typically reserved for clinical trials and in-patient populations and evidence-based research is awaited.
In working towards this vision, the insights gained from phase one were used to establish an initial set of mucositis assessment recommendations which were iteratively optimized through expert consultation. In line with some regulatory requirements and respondent confidence/preferences, WHO and NCI-CTCAE were highly prioritized and therefore part of the initial set of recommendations. Although a very common scale,6,30 the WHO has limitations as oral mucositis is not the only reason for food restrictions especially in patients with head and neck cancer undergoing RT.31 The NCI-CTCAE versions inquiring symptoms have limitations with the absence of information on how to assess symptoms in situations when analgesic medicines have been used, thus underestimating mucositis.31 Other limitations of clinician reported tools are inaccuracies in evaluation, underreporting of lower grade symptoms, and later reporting of symptoms compared to PROMs.13,19, 20, 21, 22, 23, 24, 25, 26 Ultimately, while our study provides guidance on improving the consistency of mucositis assessment, it is important to remain open-minded to other mucositis scales that may fit better to a certain patient population, special clinical-setting, specific research objectives or other unique circumstances.
Although it is important to note that across the board, respondents felt less confident in assessing gastrointestinal mucositis, compared to oral mucositis. This likely reflects the relative ease with which the oral cavity can be accessed and possibly bias in our respondents which contained more dental/oral care professionals compared to GI mucositis experts. Compared to the oral mucosa, direct visualization of the gastrointestinal mucosa is not feasible without the use of invasive techniques including endoscopy or colonoscopy. These are typically contraindicated in people with gastrointestinal mucositis due to the risk of perforation. As such, to confidently assess/grade gastrointestinal mucositis, healthcare providers and researchers must systematically discuss a range of bowel symptoms including diaerrhoea, bleeding, pain, fecal incontinence and flatulence. Navigating these symptoms requires the healthcare provider to delicately discuss problems that the patient may be hesitant to openly discuss, particularly in certain cultures and demographics. It also places an additional burden on the patient who must correctly understand or interpret the questions and recall symptoms that may have since resolved. In addition, gastrointestinal symptoms are not specific, and could arise from other complications such as infections or graft-vs-host disease, and may be impacted by other medications (e.g. opioids) which may affect stool frequency, but not necessarily consistency. As such, there is arguably a more pertinent need to optimize PROMs for gastrointestinal mucositis to capture burden, and use biomarkers more readily in its assessment.14 Of course, the selection of these PROMs is heavily influenced by the clinical context or research question, and attention must be placed on whether the goal is to capture mucosal damage, overall gut symptom burden, stool frequency or stool consistency. Hence, our recommendations differ based on the unique clinical context. For example, for assessing gastrointestinal mucositis in HSCT recipients, the panel recommends the Bristol Stool Chart as this will continue to capture diaerrhoea burden (based on consistency) even when concurrent medication, including anti-diaerrhoeal and opioids, are being used that will impact frequency. This selection also reflects the tendency for HSCT recipients to be treated in-patient, enabling more frequent assessments to be made. In contrast, where concurrent medication use is not as common and opportunities for clinical assessment are less frequent (e.g. standard chemotherapy), the NCI-CTCAE tool has been recommended. Unfortunately, there is no single tool that currently captures all of these parameters, forcing the use of multiple tools—an undertaking that should be approached with caution to avoid over-burdening the patient.
In working towards more sophisticated mucositis assessment methodologies, the opportunity to use digital tools and platforms must be considered. These tools reduce the burden of face-to-face assessments and/or hardcopy surveys/PROMs. Certainly, some tools offer unique capabilities in branching logic to eliminate unnecessary questions and optimize the experience for the user.32,33 However, our results show that only a minority use digital tools in assessing mucositis, citing the lack of universally accepted tools as the main barrier (39% of respondents), followed by cost (23%), lack of appropriate infrastructure (22%), lack of literature (20%), patient hesitancy (15%) and time (12%). This is not surprising, as not every medical community is prepared for advanced digital tools and parameters such as the patient's age/technical literacy should be carefully taken into consideration.32 However, patients that use digital health tools can become more independent, proactive and accepting of themselves.33 Further research is needed to explore the added benefit of digital tools in assessing mucositis, and societal leadership is needed to identify the desirable attributes of such platforms both in their design, development and implementation.
While the non-specific nature of gastrointestinal symptoms can make determining their origin challenging, it does simplify their assessment. In contrast, the highly nuanced nature of oral mucositis limits the applicability, utility and accuracy of certain assessment tools, particularly when assessing oral pathologies caused by novel anti-cancer treatments. As a result, there are an increasing number of assessment tools available for specific treatment modalities, e.g. the mTOR inhibitor associated stomatitis (mIAS) scale34 which attempt to overcome the limitations of applying “conventional” mucositis assessment tools to oral pathologies that clearly differ to oral mucositis caused by traditional cytotoxic therapies.35 Considering the heterogeneity of the clinical manifestations and the increasing reports of oral mucositis-like lesions caused by novel anti-cancer therapies, any new tool needs to be approached with caution. The wide use of immunotherapy in cancer patients has also opened the issue of oral complications induced by this treatment. The risk of immune checkpoint-derived mucositis is quite rare (about less than 2–3%36) but there are very heterogeneous patterns, including aphthous-like lesions, lichenoid lesions, xerostomia and taste alterations.35,37
Our recommendations have been purposely designed to match specific clinical contexts, defined by treatment modality and separated for oral mucositis and gastrointestinal mucositis, providing contextualized recommendations for both clinical assessment and relevant PROMs, as well as their frequency. Our guidelines have also been separated based on whether the assessment is in the clinical practice or clinical trial context, recognizing that each setting may have different objectives for mucositis assessment, e.g. clinical decision-making vs evaluating the efficacy of an anti-mucositis intervention. In everyday clinical setting, as well as in the clinical trial context where anti-mucositis interventions are being investigated, PROMs should be strongly prioritized given the goal of reducing symptom burden, not strictly mucosal damage, and the fact that objective tissue damage does not necessarily equate to perceived symptom burden.20, 21, 22,26 Also, it should be acknowledged that the use of the suggested scales to assess mucositis should not prevent physicians from evaluating other symptoms induced by mucositis with other scales. For instance, the evaluation of pain by means of the widely used visual analogue scale or the numeric rating scale should continue to be performed as an essential part of the clinical visit.
In implementing these recommendations, it is also critical that the longitudinal and often episodic nature of mucositis and its associated symptoms be acknowledged. Of course, this reiterates the need to perform frequent assessments where possible to capture the both the severity and duration of symptoms. This is ultimately what enables the true burden of mucositis to be defined, where 1–2 days of severe mucositis may indeed be equally burdensome as 7 days of mild mucositis. Calculating the area under the curve may be a simple way to synthesize both duration and severity, and thus easily compare burden across individual patients or study populations. However, in performing repeated assessments, the risk of the patient's perception of symptom burden may also shift as previously discussed. Including measures of response shift can be helpful in evaluating true change, and thus mucositis trajectories, in these patients.
These recommendations are intended to guide the community in mucositis assessment, aiming to improve the consistency and rigor of patient care and clinical trial conduct. Although this initiative is the first of its kind, the MASCC MSG is committed to the ongoing review and update of these recommendations. These will help address some of limitations of our approach, namely the low response rate in our initial consultation survey, the fact the medical and radiation oncologist comprised only 14% and 11% of respondents, the risk of facilitator bias in establishing the original recommendations and the fact that it was only distributed in English. The low response rate in the first phase does impact our results, as it may have resulted in a narrow set of initial suggestions which then informed the second phase. It is therefore critical to interpret these results with this in mind. Furthermore, we recognize that we have not engaged with patient representatives, consumers, patient partners or advocates in establishing these guidelines. MASCC is committed to strong engagement with lived experience experts, and the MSG will seek their perspectives on the current PROMs available for mucositis assessment and how they could be improved to better capture the breadth of symptoms associated with mucositis that are not readily captured in current PROMs (e.g. pain and xerostomia). Furthermore, these consensus statements apply to adult patients only. A similar project considering pediatric patients is planned. Finally, this study has highlighted the clear need to develop and validate new tools to accurately assess mucositis-like lesions/symptoms caused by novel anti-cancer agents, including immunotherapy and targeted therapies.
To conclude, patient care and the development of new mucositis interventions continues to be plagued by inconsistent mucositis assessment, limiting comparison and synthesis of disparate data. Here, we provide the first set of recommendations to guide mucositis assessment based on the expert-opinion of the MASCC MSG. It is anticipated that these recommendations provide much needed guidance in an area that has fallen victim to a plethora of choice. We hope that this guidance will ensure mucositis, its symptoms and broader patient experiences are accurately captured, and results are more easily synthesized across studies, accelerating translation of knowledge into clinical practice.
Contributors
Study concepts and design: RAA, HRW, PB.
Data acquisition and verification: RAA, PB, DK, NB, HRW, PB.
Data analysis: RAA, HRW, PB.
Data interpretation: RAA, PB, DK, NB, ERF, RM, JRD, JE, HRW, PB.
Drafting the manuscript or revising it critically for important intellectual content: RAA, PB, DK, NB, KC, YTC, ERF, RM, JRD, JE, YVS, EKF, AK, EMF, JF, VMC, YZ, GO, RAM, CMS, HRW, PB.
Final approval of version to be published: RAA, PB, DK, NB, KC, YTC, ERF, RM, JRD, JE, YVS, EKF, AK, EMF, JF, VMC, YZ, GO, RAM, CMS, HRW, PB.
Agreement to be accountable for all aspects of the work: RAA, PB, DK, NB, KC, YTC, ERF, RM, JRD, JE, YVS, EKF, AK, EMF, JF, VMC, YZ, GO, RAM, CMS, HRW, PB.
RAA, PB, DK, NB, KC, YTC, ERF, RM, JRD, JE, YVS, EKF, AK, EMF, JF, VMC, YZ, GO, RAM, CMS, HRW and PB read and approved the final version of the manuscript.
Data sharing statement
All data used for the study has been included in the manuscript and Supplementary materials.
Declaration of interests
The authors report no potential conflicts of interests to declare.
Acknowledgements
The authors wish to thank Steve Sonis DMD DMSc, Barbara Murphy MD and Sharon Elad DMD MSc for their continued and valuable input and advice. The authors are grateful for the members of the Mucositis Study Group of MASCC for participating in this Delphi survey. This publication was made possible by an unconditional grant from Angelini Pharma S.p.A.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102675.
Contributor Information
Paolo Bossi, Email: paolo.bossi@hunimed.eu.
MASCC Mucositis Study Group:
Alexa Laheij, Arghavan Tonkaboni, Jacqui Scott, Rania Abasaeed, Adel Kauzman, Adriana Do Socorro Lima Flato, Adwaita Gore, Anne-Marie Hardman, Agnes Horvath, Allan Hovan, Aisha Al-Jamaei, Aya koizumi, Alan Santos-Silva, Alessandra Majorana, Alexandre Giannini, Aléxia Teixeira, Muhammad Ali Shazib, Alison Melvin, Aluísio Miranda Filho, Amanda Gruza, Amber Brown-Dahl, Amit Harilall, Amr El Maghrabi, Ana Andabak Rogulj, Ana Raphaela Curvo, Ana Laura Soares, Andrea Stringer, Andréa Moreira, Andy Kurtzweil, Angelyn Salaberry, Anne Blazy, Anne Margrete Gussgard, Anne Marie Lynge Pedersen, Annette Freidank, Anura Ariyawardana, Adrian Ramseier, Jann Arends, Ariel Blanchard, Adriana Sesti Paz, Angela Thermann, Augusto Poropat, Azael Freites-Martinez, Abdul Rahman Al-Azri, Bente Brokstad Herlofson, Sitaraman BalajiSubramanian, Barbara Ballantyne, Kivanc Bektas-Kayhan, Bengt Hasséus, Benjamin Kaffenberger, Bernar Benites, Bernice Kwong, Beth Test, Fernando Chiantia, Bo Pettersson, Bomi Framroze, Pierluigi Bonomo, Božana Lončar Brzak, Brittany Dulmage, Sorin Buga, Caroline Spekssnijder, Carlton Brown, Antonio Carlos Moura de Melo, Ana Carolina Ribeiro, Caroline Silva, Caroline Fulop, Carryn Anderson, Catherine Flaitz, Cathy Massoud, Cesar Migliorati, Callie Gross, Chiara Gandini, Charles Loprinzi, Charlotte de Mooij, Catherine Hong, Ying Chu Choi, Maria Choy, Christine Boers-Doets, Leonard Schmeel, Cibele Nagano, Maria Coeli Franco, Courtney Subramaniam, Carolyn Patrick, Catherine Poh, Cristina Neuenschwander, Cesar Virgen, Dorothea Riesenbeck, Dale Weaver, Daniel Cohen Goldemberg, Daniel Sundaresan, Daniela Nunes, Danyel Perez, Daphine Travassos, David Yang, Daniela Ribeiro, Dean Kolbinson, Deborah Buick, Deborah Saunders, Deborah Buick, Juliane De Bortolli, Deepika Chugh, Denise Markstrom, Denise Travassos, Dianna Weikel, Dimitra Galiti, Dinusha Peiris, Fedja Djordjevic, Pankaj Singhai, Dorothy Keefe, Douglas Peterson, Douglas Fonseca, Doreen Pon, Iuliia Kovalenko, Aleksandra Polonskaia, Rogério Caldas, Kevin Saganski, Julia Néri, Dennis Abbott, Abhijna Vithal Yergolkar, Cristina Del Conte, Januaria Passos, Katia Uezu, Paula Silva, Steven Gilbert, Keng Yeoh, Kunal Jain, Madhup Rastogi, Satheeshkumar Poolakkad Sankaran, Deborah Manne, Evgeniya Shatokhina, Esther Adebayo-Olojo, Eszter Somogyi-Ganss, Eli Ehrenpreis, Wilber Bernaola-Paredes, Eduardo Fregnani, Elaci Cardoso, Elena Bardellini, Eleni Arvanitou, Elisa Kauark Fontes, Elise Bruning, Eloise Neumann, Elsa Madureira, Marcia Ramires, Erofili Papadopoulou, Etiene Munhoz, Fred Spijkervet, Fabiana Granzotto, Fabiana Martins, Fabio Alves, Farah Mougeot, Federica Aielli, Fernanda Pigatti, Fernanda Fonseca, Firoozeh Samim, Flavia Carvalho, Florence Cuadra Zelaya, Cesar Freytes, Gabriela da Silveira, Gabriela Torino, Gabriela Martins, Geisa Silva, Gemma Caro, Gemma Bryan, Georgette Radford, Ghanyah Al-Qadami, Giorgia Albini, Gisele Mainville, Georgios Gkardiakos, Gleidston Potter, Gulbin Hoeberechts, Gordon Howarth, Giulia Ottaviani, Grace Bradley, Gunjan Verma, Gustavo dos Santos, Margaret Randles-Guzzardi, Hanlie Engelbrecht, Hannah Wardill, Heidi Hansen, Iquebal Hasan, Hironobu Hata, Helena Ullgren, Heliton Spindola Antunes, Heloísa Laís dos Santos, Howard Weld, Helen McInnes, Hans Peter Jungbluth, Hsiaofen Weng, Ian Hewson, Ingrid Santos, Jorge Illarramendi, Ines Semendric, Rol Menge, Inger Von Bultzingslowen, Maria da Gloria de Melo, Iona Leong, Isabella Fonseca, Isadora Kalif, James Carroll, Janet Coller, Johann Beck-Mannagetta, Joanne Bowen, Jose Meurer, Ricky McCullough, Jennifer Powers, Jesus Gomez, Jimma Lenjisa, jaya Vangara, Jasna Leko, Jane Fall-Dickson, Jean-Luc Mougeot, Joan Fox, Joel Epstein, Jolien Robijns, Jonn Wu, Patricio Palma, Jaya Amaram-Davila, Jim Siderov, Judith Raber-Durlacher, Juliana Dantas, Juliana Jasper, Juliana Monteiro, Julia Bruno, julie pfeffer, Julija Jovanovic Ristivojevic, Juliana Brito, Jyothsna Kuriakose, Yuji Kabasawa, Kanan Dave, Karin Barczyszyn, Karol Sartori Lima, Kate Secombe, Kate White, Kate Cooper, Kouji Katsura, Karen Biggs, Katharine Ciarrocca, Kristopher Dennis, Ken Tomizuka, Kevin Hendler, Ikuko Komo, Kristina Skallsjö, Kristy Hodgins, Katia Rupel, Keiko Tanaka, Seema Kurup, Luiz Gueiros, Larissa Agatti, Laura Garzona-Navas, Letícia Guerra, Leila Portela, Leilani Iossi, Linda Elting, Lene Baad-Hansen, Leslie Reeder, Leticia Lang, Liciane Menezes, Liliana Braun, Liliane Grando, Mathew Lim, Lina Fernandez, Lucy McKeage, Luana Campos, Luciana Simonato, Luciana Muniz, Leah van Draanen, Mieko Mizutani, Tsai-Wei Huang, Mahfujul Riad, Mahnoor Nazar, Maíra Souza, Mariana Minamisako, Manoela Pereira, Carlos Mantelato, Márcio Diniz-Freitas, Marco Montezuma, Marco Andrade, Marcos Santos, Margherita Gobbo, Maria Caterina Fortuna, Mariana Vitor, Joana Marinho, Alina Markova, Marlyse Knuchel, Marta Carlesimo, Marta Neves, Andrew Mazar, Maria Cristina Gomez Amarilla, Mark Chambers, Melissa de Araujo, Alexandre Melo, Melody Cole, Mohamed Elsayed, Monica Fliedner, Martin Hauer-Jensen, Micaela Bouchacourt, Michael Brennan, Michael Thirlwell, Michio Nakamura, Midori Nakagaki, Camila Rossi, Robert Miller, Mireille Kaprilian, Michael Kase, Michael Dougan, Monique Stokman, Ragnhild Monsen, Alisha Morgan, Jocelyn Harding, Maryam Taleghani, Marie-Therese Genot, Mukund Seshadri, Brian Muzyka, Nancy Batista, Nancy Gadd, Naoko Tanda, Narmin Nasr, Natália Garcia, Nathan Lee, Natalia Palmier, Norman Brito-Dellan, Nancy Corbitt, Neli Pieralisi, Verônica Serrano, Nicola Alessandro Iacovelli, Nicole Blijlevens, Norma Lúcia Sampaio, Nour Karra, Niveditha Venkatesh, Noam Yarom, Renata Cristina Borin, Olivia Lemenchick, Ondina Mendes, Ourania Nicolatou-Galitis, Vasiliy Shchitka, Paolo Bossi, Paula Reis, Paulo Sérgio Santos, Paz Fernandez-Ortega, Ira Parker, Raquel García, Peter Fritz, Edmund Peters, Pamela Gardner, Pierre Saint Girons, Priya Tiwari, Pravin Chaturvedi, Tais de Moraes, Priscila Andrade, Raj Nair, Rachel Gibson, Rachita Gururaj, Ragda Abdalla-Aslan, Raghu Thota, Rajesh Lalla, Rui Amaral Mendes, Raquel Almeida Prado, Ravikiran Ongole, George Taybos, Regina Mackey, Renata Rego, Renata Camilotti, Renata Ferrari, Renato Junior, Rene-Jean Bensadoun, Richard Logan, Roberta Sales, Roberta Zanicotti, Roberta Tunes, Rodolfo Mauceri, Rosiene Feitoza, Kathryn Ruddy, Cynthia Rybczyk, Stephanie Trager, Sachiyo Mitsunaga, Sahani Gunathilake, Rajan Saini, Viola Salvestrini, Sandip Mukhopadhyay, Sandrina Angeloz, Pramod Sankar S, Luciana S Barbosa Barbosa, Elena Volkova, Sharon Elad, Sergio Cantoreggi, Sharon Gordon, Shelly Brown, Shu Yie Janine Tam, Sibelle Faleiro, Silmara da Silva, Silvia de Oliveira, Siri Beier Jensen, Ivana Skrinjar, Sophie Beaumont, Felipe Sperandio, Sandra Reese, Steven Roser, Sachiko Seo, Stephanie van Leeuwen, Stephen Sonis, Stephen Bernard, Stephen Rajan Samuel, Stuart Taylor, Suranjan Maitra, Susanne Skulski, Suzanne Carlisle, Sylvie Louise Avon, Tomoya Yokota, Takashi Yurikusa, Tabata Santos Polvora, Tabitha Kelock, Tauana Fernandes, Taylor Wain, Timothy Brown, Tetsuhito konishi, Thalyta Amanda Ferreira, Tomoko Kataoka, Thomas Kelly, Takehiko Mori, Tomoko Higuchi, Toshiaki Saeki, Nikolaos Tsoukalas, Typhaine Maupoint De Vandeul, Masatoshi Usubuchi, Vanessa Lacerda, Vanessa Tilly, Emmanuelle Vigarios, Alessandro Villa, Vinicius Torregrossa, Vinodh Kumar Selvaraj, Viviane Sarmento, Vivien Heng, Wagner Gomes-Silva, Abhishek Kandwal, Petter Wilberg, Wanessa Miranda e Silva, Wan Nor I'zzah Wan Mohamad Zain, Wonse Park, Wim Tissing, Yoshihiko Soga, Bella Van Sebille, Yuhei Matsuda, and Yehuda Zadik
Appendix A. Supplementary data
References
- 1.Elad S., Cheng K.K.F., Lalla R.V., et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2020;126:4423–4431. doi: 10.1002/cncr.33100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellm L.A., Epstein J.B., Rose-Ped A., et al. Patient reports of complications of bone marrow transplantation. Support Care Cancer. 2000;8:33–39. doi: 10.1007/s005209900095. [DOI] [PubMed] [Google Scholar]
- 3.Rose-Ped A.M., Bellm L.A., Epstein J.B., et al. Complications of radiation therapy for head and neck cancers. The patient's perspective. Cancer Nurs. 2002;25:461–467. doi: 10.1097/00002820-200212000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Sonis S.T., Oster G., Fuchs H., et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol. 2001;19:2201–2205. doi: 10.1200/JCO.2001.19.8.2201. [DOI] [PubMed] [Google Scholar]
- 5.Elting L.S., Cooksley C., Chambers M., et al. The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer. 2003;98:1531–1539. doi: 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- 6.Gibson F., Auld E.M., Bryan G., et al. A systematic review of oral assessment instruments: what can we recommend to practitioners in children's and young people's cancer care? Cancer Nurs. 2010;33:E1–E19. doi: 10.1097/NCC.0b013e3181cb40c0. [DOI] [PubMed] [Google Scholar]
- 7.Eilers J., Epstein J.B. Assessment and measurement of oral mucositis. Semin Oncol Nurs. 2004;20:22–29. doi: 10.1053/j.soncn.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . World Health Organization; 1979. WHO handbook for reporting results of cancer treatment. [Google Scholar]
- 9.National Cancer Institute (NCI) Common Terminology criteria for adverse event (CTCAE) 2018. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm Accessed August 2022.
- 10.Sonis S.T., Eilers J.P., Epstein J.B., et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis study group. Cancer. 1999;85:2103–2113. doi: 10.1002/(sici)1097-0142(19990515)85:10<2103::aid-cncr2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Cox J.D., Stetz J., Pajak T.F. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 12.Schubert M.M., Williams B.E., Lloid M.E., et al. Clinical assessment scale for the rating of oral mucosal changes associated with bone marrow transplantation. Development of an oral mucositis index. Cancer. 1992;69:2469–2477. doi: 10.1002/1097-0142(19920515)69:10<2469::aid-cncr2820691015>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez-Vargas R., Díaz-García M.L., Villasís-Keever M., et al. Instruments to measure the quality of life in patients with oral mucositis undergoing oncological treatment: a systematic review of the literature. Bol Med Hosp Infant Mex. 2016;73:457–466. doi: 10.1016/j.bmhimx.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Kuiken N.S.S., Rings E., Blijlevens N.M.A., et al. Biomarkers and non-invasive tests for gastrointestinal mucositis. Support Care Cancer. 2017;25:2933–2941. doi: 10.1007/s00520-017-3752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson D.E., Bensadoun R.J., Roila F. Management of oral and gastrointestinal mucositis: ESMO clinical practice guidelines. Ann Oncol. 2011;6:vi78–vi84. doi: 10.1093/annonc/mdr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuiken N.S., Rings E.H., Tissing W.J. Risk analysis, diagnosis and management of gastrointestinal mucositis in pediatric cancer patients. Crit Rev Oncol Hematol. 2015;94:87–97. doi: 10.1016/j.critrevonc.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Vanier A., Oort F.J., McClimans L., et al. Response shift in patient-reported outcomes: definition, theory, and a revised model. Qual Life Res. 2021;30:3309–3322. doi: 10.1007/s11136-021-02846-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz C.E., Finkelstein J.A. Understanding inconsistencies in patient-reported outcomes after spine treatment: response shift phenomena. Spine J. 2009;9:1039–1045. doi: 10.1016/j.spinee.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Franco P., Martini S., Di Muzio J., et al. Prospective assessment of oral mucositis and its impact on quality of life and patient-reported outcomes during radiotherapy for head and neck cancer. Med Oncol. 2017;34:81. doi: 10.1007/s12032-017-0950-1. [DOI] [PubMed] [Google Scholar]
- 20.Stiff P.J., Erder H., Bensinger W.I., et al. Reliability and validity of a patient self-administered daily questionnaire to assess impact of oral mucositis (OM) on pain and daily functioning in patients undergoing autologous hematopoietic stem cell transplantation (HSCT) Bone Marrow Transplant. 2006;37:393–401. doi: 10.1038/sj.bmt.1705250. [DOI] [PubMed] [Google Scholar]
- 21.Elting L.S., Keefe D.M., Sonis S.T., et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer. 2008;113:2704–2713. doi: 10.1002/cncr.23898. [DOI] [PubMed] [Google Scholar]
- 22.Epstein J.B., Beaumont J.L., Gwede C.K., et al. Longitudinal evaluation of the oral mucositis weekly questionnaire-head and neck cancer, a patient-reported outcomes questionnaire. Cancer. 2007;109:1914–1922. doi: 10.1002/cncr.22620. [DOI] [PubMed] [Google Scholar]
- 23.Kushner J.A., Lawrence H.P., Shoval I., et al. Development and validation of a patient-reported oral mucositis symptom (PROMS) scale. J Can Dent Assoc. 2008;74:59. [PubMed] [Google Scholar]
- 24.Webster K., Cella D., Yost K. The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:1477–7525. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagaki M., Gavin N.C., Clavarino A., et al. A real-world accuracy of oral mucositis grading in patients undergoing hematopoietic stem cell transplantation. Support Care Cancer. 2022;30:2705–2712. doi: 10.1007/s00520-021-06654-3. [DOI] [PubMed] [Google Scholar]
- 26.Basch E., Iasonos A., McDonough T., et al. Patient versus clinician symptom reporting using the national cancer Institute common Terminology criteria for adverse Events: results of a questionnaire-based study. Lancet Oncol. 2006;7:903–909. doi: 10.1016/S1470-2045(06)70910-X. [DOI] [PubMed] [Google Scholar]
- 27.Ge C., Guo K., Li Y., et al. Analysis of patient-reported outcomes in the approval of novel oncology drugs in the United States, 2017-2022. eClinicalMedicine. 2023;59:101953. doi: 10.1016/j.eclinm.2023.101953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Normando A.G.C., Rocha C.L., de Toledo I.P., et al. Biomarkers in the assessment of oral mucositis in head and neck cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2017;25:2969–2988. doi: 10.1007/s00520-017-3783-8. [DOI] [PubMed] [Google Scholar]
- 29.Villa A., Vollemans M., De Moraes A., et al. Concordance of the WHO, RTOG, and CTCAE v4.0 grading scales for the evaluation of oral mucositis associated with chemoradiation therapy for the treatment of oral and oropharyngeal cancers. Support Care Cancer. 2021;29:6061–6068. doi: 10.1007/s00520-021-06177-x. [DOI] [PubMed] [Google Scholar]
- 30.Quinn B., Potting C.M., Stone R., et al. Guidelines for the assessment of oral mucositis in adult chemotherapy, radiotherapy and haematopoietic stem cell transplant patients. Eur J Cancer. 2008;44:61–72. doi: 10.1016/j.ejca.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 31.de Freitas Neiva Lessa A., Meirelles D.P., Do Couto A.M., et al. Scales to graduate oral mucositis: what are the limitations? Oral Oncol. 2023;144 doi: 10.1016/j.oraloncology.2023.106489. [DOI] [PubMed] [Google Scholar]
- 32.Suchodolska G., Koelmer A., Puchowska M., et al. Are all societies ready for digital tools? Feasibility study on the use of mobile application in polish early breast cancer patients treated with perioperative chemotherapy. Healthcare. 2023;11:2114. doi: 10.3390/healthcare11142114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elkefi S., Trapani D., Ryan S. The role of digital health in supporting cancer patients' mental health and psychological well-being for a better quality of life: a systematic literature review. Int J Med Inform. 2023;176:9. doi: 10.1016/j.ijmedinf.2023.105065. [DOI] [PubMed] [Google Scholar]
- 34.Boers-Doets C.B., Lalla R. The mIAS scale: a scale to measure mTOR inhibitor-associated stomatitis. Support Care Cancer. 2013;21:S140. [Google Scholar]
- 35.Vigarios E., Epstein J.B., Sibaud V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint inhibitors. Support Care Cancer. 2017;25:1713–1739. doi: 10.1007/s00520-017-3629-4. [DOI] [PubMed] [Google Scholar]
- 36.Yura Y., Hamada M. Oral immune-related adverse Events caused by immune checkpoint inhibitors: salivary gland dysfunction and mucosal diseases. Cancers. 2022;14:792. doi: 10.3390/cancers14030792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacouture M., Sibaud V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am J Clin Dermatol. 2018;19:31–39. doi: 10.1007/s40257-018-0384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.