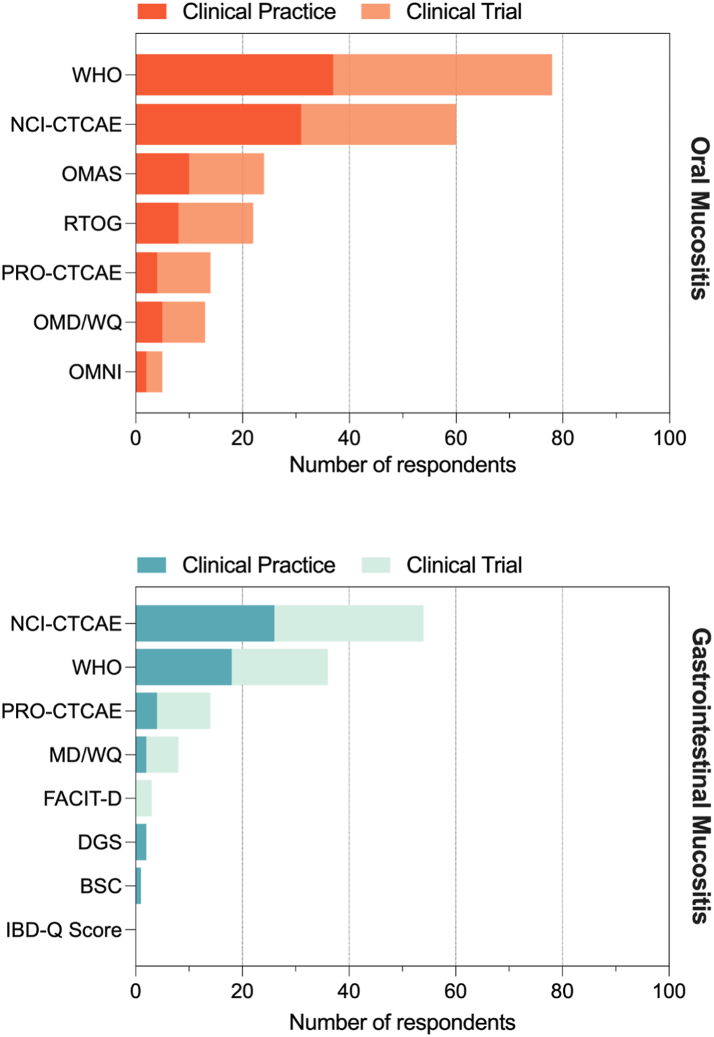
Fig. 2.
Assessment tools ranked by preference in sample of MASCC MSG members and aggregated based on their use in clinical trials vs clinical practice. The respondents were asked to mark their preferred assessment tool, in clinical practice and in clinical trials. Hence for each assessment tool, the same respondents answered two questions. BSC, Bristol stool scale; DGS, daily gut score; FACIT-D, Functional Assessment of Chronic Illness Therapy Diaerrhoea subscale; NCI-CTCAE, National Cancer Institute-Common Terminology Criteria for Adverse Events; IBD-Q Score, Inflammatory Bowel Disease Questionnaire; OMAS, Oral Mucositis Assessment Scale; OMDQ, Oral Mucositis Daily Questionnaire; OMNI, Oral Mucositis Nursing Instrument. OMWQ, Oral Mucositis Weekly Questionnaire; PRO-CTCAE, Patient-reported outcome-Common Terminology Criteria for Adverse Events; RTOG, Radiation Therapy Oncology Group; WHO, World Health Organization.