Summary
Background
Adjuvant abemaciclib plus endocrine therapy previously showed a significant improvement in invasive disease-free survival and distant relapse-free survival in hormone receptor-positive, human epidermal growth factor receptor 2 (HER2; also known as ERBB2)-negative, node-positive, high-risk, early breast cancer. Here, we report updated results from an interim analysis to assess overall survival as well as invasive disease-free survival and distant relapse-free survival with additional follow-up.
Methods
In monarchE, an open-label, randomised, phase 3 trial, adult patients (aged ≥18 years) who had hormone receptor-positive, HER2-negative, node-positive, early breast cancer at a high risk of recurrence with an Eastern Cooperative Oncology Group performance status of 0 or 1 were recruited from 603 sites including hospitals and academic and community centres in 38 countries. Patients were randomly assigned (1:1) by means of an interactive web-based response system (block size of 4), stratified by previous chemotherapy, menopausal status, and region, to receive standard-of-care endocrine therapy of physician’s choice for up to 10 years with or without abemaciclib 150 mg orally twice a day for 2 years (treatment period). All therapies were administered in an open-label manner without masking. High-risk disease was defined as either four or more positive axillary lymph nodes, or between one and three positive axillary lymph nodes and either grade 3 disease or tumour size of 5 cm or larger (cohort 1). A smaller group of patients were enrolled with between one and three positive axillary lymph nodes and Ki-67 of at least 20% as an additional risk feature (cohort 2). This was a prespecified overall survival interim analysis planned to occur 2 years after the primary outcome analysis for invasive disease-free survival. Efficacy was assessed in the intention-to-treat population. Safety was assessed in all treated patients. The study is registered with ClinicalTrials.gov, NCT03155997, and is ongoing.
Findings
Between July 17, 2017, and Aug 12, 2019, 5637 patients were randomly assigned (5601 [99∙4%] were women and 36 [0·6%] were men). 2808 were assigned to receive abemaciclib plus endocrine therapy and 2829 were assigned to receive endocrine therapy alone. At a median follow-up of 42 months (IQR 37–47), median invasive disease-free survival was not reached in either group and the invasive disease-free survival benefit previously reported was sustained: HR 0·664 (95% CI 0·578–0·762, nominal p<0∙0001). At 4 years, the absolute difference in invasive disease-free survival between the groups was 6·4% (85·8% [95% CI 84·2–87·3] in the abemaciclib plus endocrine therapy group vs 79·4% [77·5–81·1] in the endocrine therapy alone group). 157 (5·6%) of 2808 patients in the abemaciclib plus endocrine therapy group died compared with 173 (6·1%) of 2829 patients in the endocrine therapy alone group (HR 0·929, 95% CI 0·748–1·153; p=0·50). The most common grade 3–4 adverse events were neutropenia (in 548 [19·6%] of 2791 patients receiving abemaciclib plus endocrine therapy vs 24 [0·9%] of 2800 patients in the endocrine therapy alone group), leukopenia (318 [11·4%] vs 11 [0·4%]), and diarrhoea (218 [7·8%] vs six [0·2%]). Serious adverse events occurred in 433 (15·5%) of 2791 patients receiving abemaciclib plus endocrine therapy versus 256 (9·1%) of 2800 receiving endocrine therapy. There were two treatment-related deaths in the abemaciclib plus endocrine therapy group (diarrhoea and pneumonitis) and none in the endocrine therapy alone group.
Interpretation
Adjuvant abemaciclib reduces the risk of recurrence. The benefit is sustained beyond the completion of treatment with an absolute increase at 4 years, further supporting the use of abemaciclib in patients with high-risk hormone receptor-positive, HER2-negative early breast cancer. Further follow-up is needed to establish whether overall survival can be improved with abemaciclib plus endocrine therapy in these patients.
Funding
Eli Lilly.
Introduction
Despite historic advances in adjuvant treatment for early breast cancer, the cumulative risk of developing incurable distant metastatic disease for patients with high-risk hormone receptor-positive, human epidermal growth factor receptor 2 (HER2; also known as ERBB2)-negative early breast cancer at 20 years remains around 40%.1 monarchE is a global, randomised phase 3 trial investigating the addition of abemaciclib (a cyclin-dependent kinase 4 and 6 [CDK4 and 6] inhibitor) to current standard-of-care adjuvant endocrine therapy for patients with hormone receptor-positive, HER2-negative, node-positive early breast cancer at a high risk of recurrence on the basis of clinicopathological features.2,3 The study previously met its primary endpoint, showing that the addition of abemaciclib led to a significant and clinically meaningful improvement in invasive disease-free survival in the intention-to-treat (ITT) population compared with endocrine therapy alone.3,4 These data led to global regulatory approval of adjuvant abemaciclib in combination with endocrine therapy in selected patients with high-risk early breast cancer (US prescribing information 2021; EU summary of product characteristics 2022).5,6
Although these results led to the first drug approval for the adjuvant treatment of patients with hormone receptor-positive, HER2-negative early breast cancer since the introduction of aromatase inhibitors nearly two decades ago,7,8 important questions remain. Data from the last analysis at a median follow-up of 27 months indicated abemaciclib treatment benefit appeared to persist beyond the 2-year treatment period, but follow-up was relatively short for an adjuvant breast cancer study. Moreover, at the last analysis, immature overall survival results for the ITT population, with an overall survival hazard ratio (HR) point estimate greater than 1·0, raised concerns from some regulatory agencies about the benefit–risk of abemaciclib in the full monarchE study population.9 This resulted in approvals in some regions that were limited to a subset of the overall study (ie, patients with a high Ki-67 index), given that the overall survival HR in this subpopulation reflected a trend towards benefit.10 Here, we present updated efficacy outcomes, including the 4-year landmark analyses, from a preplanned overall survival interim analysis.
Methods
Study design and participants
monarchE is an open-label, randomised phase 3 trial, which was run at 603 sites including hospitals, and academic and community centres in 38 countries (appendix pp 32–63). Men and women aged 18 years or older who had hormone receptor-positive, HER2-negative, node-positive, early breast cancer at a high-risk of recurrence, and an Eastern Cooperative Oncology Group performance status of 0 or 1 and adequate organ function (absolute neutrophil count ≥1·5 × 109/L, platelets ≥100 × 109/L, total bilirubin ≤1·5 × upper limit of normality [ULN], aspartate aminotransferase [AST] and alanine aminotransferase [ALT] ≤3 × ULN) were eligible. Hormone receptor positivity, and HER2 negativity were assessed locally on primary tumours following international guidelines.11,12 Patients with metastatic disease, or node-negative breast cancer, and, after a protocol amendment, patients with inflammatory breast cancer, were excluded. Patients with a serious pre-existing medical condition that, in the judgement of the investigator, would preclude participation or patients with a history of thromboembolic events were not eligible. Patients must have completed definitive breast surgery, could have received up to 12 weeks of endocrine therapy after the last non-endocrine adjuvant therapy before randomisation, and must have been randomly assigned within 16 months of definitive breast cancer surgery.
Radiotherapy and neoadjuvant or adjuvant chemotherapy were allowed, but not required. All patients must have had bilateral breast imaging and abdominal–pelvic, chest, and bone imaging between diagnosis and randomisation as per standard clinical practice.
Eligible patients were assigned to one of two cohorts. Cohort 1 included patients with four or more positive pathological axillary lymph nodes or between one and three positive axillary lymph nodes and at least one of the following additional high-risk features: tumour size 5 cm or larger or histological grade 3 disease. Ki-67 was determined centrally in all patients in cohort 1 with a suitable pretreatment breast tumour tissue sample, but a Ki-67 index was not required for enrolment.4 Cohort 2 included patients with between one and three positive axillary lymph nodes, intermediate-risk clinicopathological features (tumour grade <3; tumour size <5 cm) and a centrally determined high Ki-67 index (≥20%) was required as an additional risk feature. Ki-67 index was centrally assessed in untreated breast tumour tissue sample by means of an investigational Ki-67 immunochemistry assay developed by Agilent Technologies (formerly Dako; Santa Clara, CA, USA).13
Patients provided written, informed consent for participation. This study, including all amendments, was approved by ethical and institutional review boards, and was done in accordance with consensus ethical principles derived from international ethical guidelines, including the Declaration of Helsinki, the Council for International Organizations of Medical Sciences International Ethical Guidelines, and the International Conference on Harmonization Good Clinical Practice Guidelines. The protocol is available online.
Randomisation and masking
Eligible patients were randomly assigned (1:1) by means of an interactive web-based response system, with a block size of 4, stratified by previous chemotherapy (neoadjuvant vs adjuvant vs no chemotherapy), menopausal status at the time of breast cancer diagnosis (premenopausal vs postmenopausal), and region (North America and Europe vs Asia vs other). Patients were randomly assigned to receive adjuvant endocrine therapy for up to 5–10 years with or without abemaciclib for 2 years (study treatment period; appendix p 2). All therapies were administered in an open-label manner without masking. Although this was an open-label study, the sponsor and all investigative sites remained masked to treatment group assignments for aggregate data until the study was confirmed as positive by an independent data monitoring committee.
Procedures
After randomisation, abemaciclib was administered orally at 150 mg twice daily for a maximum of 2 years. Endocrine therapy was administered per physician’s choice including antioestrogen agents (eg, tamoxifen) or aromatase inhibitors, with or without a gonadotropin-releasing hormone agonist per standard practice. Abemaciclib dose suspensions and dose reductions were required, as per protocol guidelines, to manage adverse events including haematological toxicities, ALT or AST increases, diarrhoea, and interstitial lung disease, with up to two dose reductions being allowed, to 100 mg and 50 mg. Patients requiring more than two dose reductions were required to discontinue abemaciclib. Dose adjustments or switch of endocrine therapy were to be established by the investigator per standard practice. In the abemaciclib plus endocrine therapy group, patients who discontinued abemaciclib and continued receiving the background endocrine therapy as part of the study treatment period until completion of 2 years were considered to have completed the on-study treatment period.
Visits occurred every 2 weeks for the first 2 months, monthly from months 3–6, and then every 3 months until the end of year 2. Thereafter, visits were every 6 months until year 5 and then annually from years 6–10. At each visit, patients were comprehensively and systematically assessed by a medically qualified individual for adverse events and any signs or symptoms of recurrence. If there was a suspicion of recurrence, a confirmation by imaging or by cytological or histopathological assessment was required. In addition, bilateral breast imaging was required at yearly intervals or as per local standards. Central chemistry and haematology laboratory tests were done, and performance status was assessed. Safety was graded according to the Common Terminology Criteria for Adverse Events version 4.0. All adverse events regardless of causality were reported up to 30 days following treatment discontinuation and all serious adverse events regardless of causality were collected from randomisation to the end of year 5. Venous thromboembolic events were closely monitored due to their clinical relevance and analysed as composite terms resulting from a comprehensive search of all adverse events under the appropriate Medical Dictionary for Regulatory Activities queries.14
Outcomes
The primary endpoint was invasive disease-free survival, defined as time from randomisation to the first occurrence of local or regional recurrence, contralateral recurrence, second primary non-breast invasive cancer, distant recurrence, or death attributable to any cause according to the STEEP criteria.15 Secondary endpoints were invasive disease-free survival in patients with high Ki-67 index (≥20%), distant relapse-free survival (defined as time from randomisation to the first occurrence of distant recurrence or death attributable to any cause), overall survival (defined as time from randomisation to the date of death due to any cause), patient-reported outcomes, and safety. Results for efficacy, safety, and patient-reported outcomes have been published previously.3,4,14
Statistical analysis
The statistical analysis plan was designed to characterise treatment effect in invasive disease-free survival, distant relapse-free survival, and overall survival for at least 5 years, which is considered appropriate for this disease setting. Patients were planned to be followed up according to the protocol and statistical analysis plan until the final overall survival analysis.
The study was powered at approximately 85% to detect the superiority of abemaciclib plus endocrine therapy versus endocrine therapy alone in invasive disease-free survival, with an assumed HR of 0·73 and two-sided alpha level of 0·05. This required approximately 390 invasive disease-free survival events in the ITT population (defined as all participants randomly assigned to a treatment group) at the time of the primary outcome analysis. The primary objective was achieved at an invasive disease-free survival interim analysis with a 2-sided p value of 0·01 (p value boundary of 0·026).3 At the primary outcome analysis, invasive disease-free survival was significantly improved in patients with high Ki-67 in the ITT and cohort 1 populations with 2-sided p values of 0·01 and 0·0042 (p value boundaries of 0·0424 and 0·0426), respectively.4 Because statistical significance was reached for invasive disease-free survival for each prespecified sequential test, invasive disease-free survival and distant relapse-free survival were assessed without alpha allocation at each subsequent analysis timepoint. Overall survival was an alpha-controlled secondary endpoint in the gatekeeping testing strategy to be assessed at each analysis timepoint. Overall type I error was controlled by means of the Lan-DeMets method with an O’Brien-Fleming type stopping boundary. This prespecified overall survival interim analysis was planned to occur 2 years following the primary outcome analysis and the data cutoff date was set as July 1, 2022.
Efficacy, including invasive disease-free survival, distant relapse-free survival, and overall survival, was assessed in the ITT population, and in cohort 1, cohort 2, and in prespecified subpopulations of cohort 1 Ki-67 high (≥20%) or cohort 1 Ki-67 low (<20%) populations. For each efficacy endpoint, the Kaplan-Meier method was used to estimate the efficacy outcomes in each treatment group, including the absolute difference in rates estimated by means of normal approximation at each year up to a timepoint when fewer than 200 patients were at risk. HRs were estimated by means of stratified Cox proportional hazard models. The proportional hazards assumption was tested by means of a time-varying Cox model and a regression of the weighted Schoenfeld residuals over time. A post-hoc exploratory analysis was done by assuming a Bayesian exponential hazard model within each year of the observation period to estimate piecewise yearly HR for invasive disease-free survival and distant relapse-free survival. Another post-hoc summary was done to present the number of patients who have died or developed metastatic disease by survival status at each analysis timepoint. For the assessment of effect size across prespecified subgroups defined by demographics and baseline disease characteristics, unstratified Cox proportional hazard models were fitted and presented in the forest plot, including treatment group, subgroup, and their interaction variable. A similar interaction model was also used to evaluate the consistency of the effect size across cohorts as a post-hoc analysis.
Updated safety was assessed in all participants who received at least one study treatment administration and summarised by means of descriptive statistics. All statistical analyses were done using SAS software (version 9.4) and RStudio (R version 4.0.3). This study is registered with ClinicalTrials.gov, NCT03155997.
Role of the funding source
The funder had a role in study design, data collection, data analysis, and data interpretation. The report was written in collaboration with all authors and was approved by the funder to be submitted for publication.
Results
Between July 17, 2017, and Aug 12, 2019, 5637 patients were randomly assigned to receive abemaciclib plus endocrine therapy (n=2808) or endocrine therapy alone (n=2829; figure 1). There were 5120 patients (91%) in cohort 1 and 517 patients (9%) in cohort 2. Baseline patient and disease characteristics and a summary of previous therapies for breast cancer are presented in table 1. 5601 (99∙4%) were women and 36 (0·6%) were men. Within cohort 1, 2003 patients had a Ki-67 index of at least 20% (cohort 1 Ki-67 high) and 1914 patients had a Ki-67 index <20% (cohort 1 Ki-67 low). At the data cutoff date for the overall survival interim analysis (July 1, 2022), median follow-up time for the overall ITT population was 42 months (IQR 37–47), and all treated patients were no longer on abemaciclib (figure 1). In the abemaciclib group, 2284 (81·3%) of 2808 patients completed the 2-year study treatment period (abemaciclib plus endocrine therapy or endocrine therapy alone), including 1938 (69·0%) of 2808 patients who received 2 years of abemaciclib plus endocrine therapy. In the control group, 2312 (81·7%) of 2829 patients completed the 2-year treatment period of endocrine therapy alone. Reasons for early discontinuation from the study treatment period are shown in figure 1.
Figure 1: Trial profile.
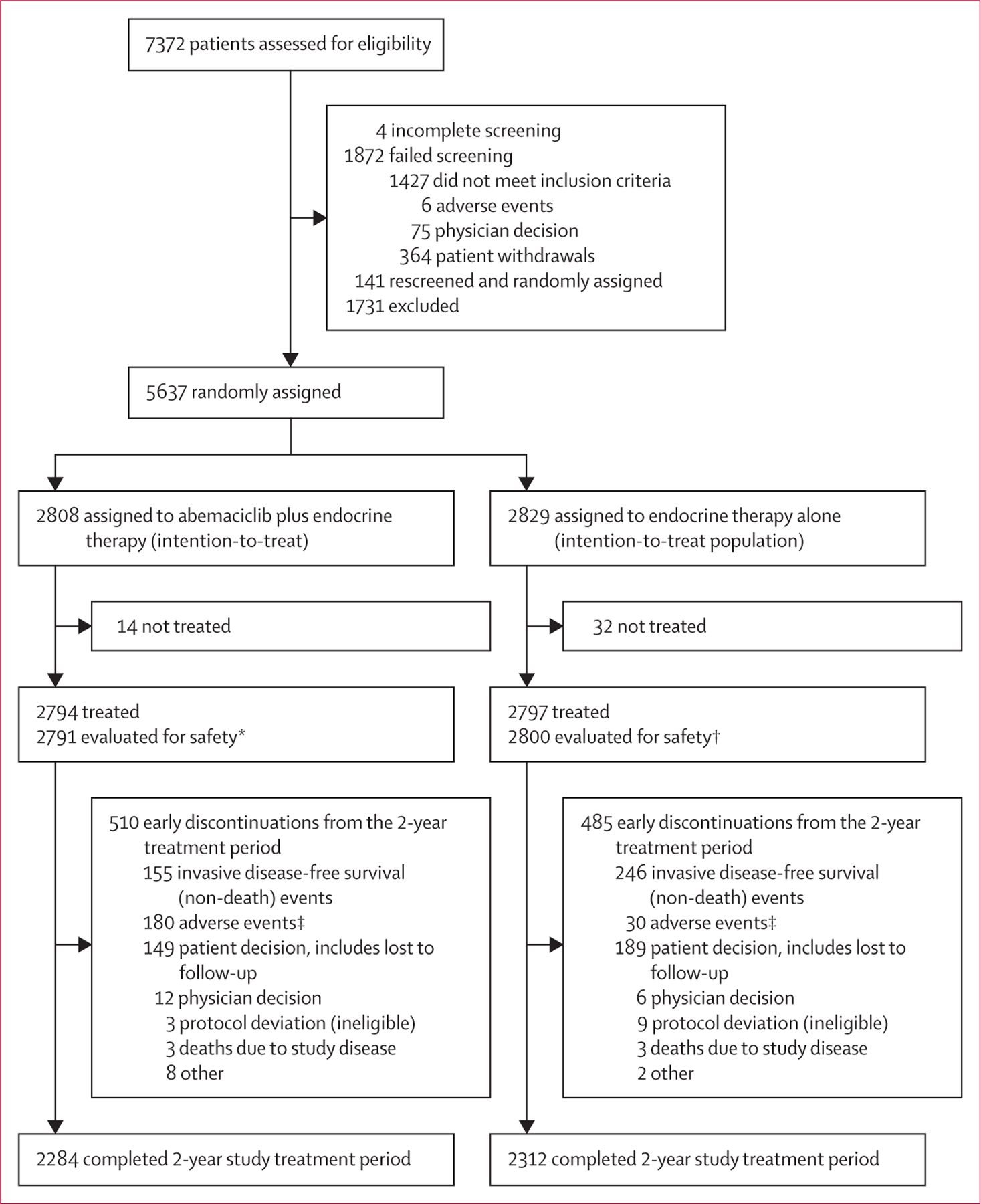
*Four patients randomly assigned to the abemaciclib group only received endocrine therapy and were evaluated for safety in the control group. †One patient randomly assigned to the control group received abemaciclib and was evaluated for safety in the abemaciclib group. ‡13 fatal adverse events in the abemaciclib plus endocrine therapy group and eight fatal adverse events in the endocrine therapy alone group.
Table 1:
Demographics, baseline characteristics, and previous systemic therapies in the intention-to-treat population
| Abemaciclib plus endocrine therapy group (n=2808)* | Endocrine therapy alone group (n=2829)* | |
|---|---|---|
| Age, median (IQR), years | 51 (44–60) | 51 (44–60) |
| <65 | 2371 (84·4%) | 2416 (85·4%) |
| ≥65 | 437 (15.6%) | 413 (14·6%) |
| Sex | ||
| Female | 2787 (99·3%) | 2814 (99·5%) |
| Male | 21 (0·7%) | 15 (0·5%) |
| Hormone receptor status | ||
| Oestrogen receptor positive | 2786 (99·2%) | 2810 (99·3%) |
| Oestrogen receptor negative | 16 (0·6%) | 17 (0·6%) |
| Progesterone receptor positive | 2426 (86·4%) | 2456 (86·8%) |
| Progesterone receptor negative | 298 (10·6%) | 295 (10·4%) |
| Menopausal status†‡ | ||
| Premenopausal | 1221 (43·5%) | 1232 (43·5%) |
| Postmenopausal | 1587 (56·5%) | 1597 (56·5%) |
| Region† | ||
| North American or Europe | 1470 (52·4%) | 1479 (52·3%) |
| Asia | 574 (20·4%) | 582 (20·6%) |
| Other | 764 (27·2%) | 768 (27·1%) |
| Race | ||
| American Indian or Alaska Native | 64 (2·3%) | 58 (2·1%) |
| Asian | 675 (24·0%) | 669 (23·6%) |
| Black or African American | 57 (2·0%) | 53 (1·9%) |
| Native Hawaiian or other Pacific Islander | 3 (0·1%) | 4 (0·1%) |
| White | 1947 (69·3%) | 1978 (69·9%) |
| Multiple | 22 (0·8%) | 25 (0·9%) |
| Ethnicity (reported in the USA only)§ | ||
| Hispanic or Latinx | 33 (8·3%) | 36 (8·7%) |
| Not Hispanic or Latinx | 364 (91·7%) | 377 (91·3%) |
| Positive axillary lymph nodes | ||
| 0 | 7 (0·2%) | 7 (0·2%) |
| 1–3 | 1118 (39·8%) | 1142 (40·4%) |
| ≥4 | 1682 (59·9%) | 1680 (59·4%) |
| Histopathological grade at diagnosis | ||
| Grade 1 | 209 (7·4%) | 216 (7·6%) |
| Grade 2 | 1377 (49·0%) | 1395 (49·3%) |
| Grade 3 | 1086 (38·7%) | 1064 (37·6%) |
| Not assessable | 126 (4·5%) | 141 (5·0%) |
| Pathological tumour size, cm | ||
| <2 | 781 (27·8%) | 767 (27·1%) |
| 2–5 | 1372 (48·9%) | 1419 (50·2%) |
| ≥5 | 607 (21·6%) | 610 (21·6%) |
| Ki-67 index | ||
| <20% | 953 (33·9%) | 974 (34·4%) |
| ≥20% | 1262 (44·9%) | 1233 (43·6%) |
| Tumour, node, metastasis stage¶ | ||
| IA | 2 (0·1%) | 1 (0·0%) |
| IIA | 324 (11·5%) | 353 (12·5%) |
| IIB | 392 (14·0%) | 387 (13·7%) |
| IIIA | 1029 (36·6%) | 1026 (36·3%) |
| IIIB | 99 (3·5%) | 88 (3·1%) |
| IIIC | 950 (33·8%) | 963 (34·0%) |
| Previous therapies for breast cancer | ||
| Previous chemotherapy | ||
| Neoadjuvant chemotherapy | 1026 (36·5%) | 1029 (36·4%) |
| Adjuvant chemotherapy | 1734 (61·8%) | 1731 (61·2%) |
| Previous endocrine therapy | ||
| Neoadjuvant endocrine therapy | 86 (3·1%) | 97 (3·4%) |
| Adjuvant endocrine therapy | 1764 (62·8%) | 1795 (63·4%) |
| Median time from start of endocrine therapy to randomisation, weeks | 7·6 (3·7–11·0) | 7·9 (3·9–11·3) |
| Previous radiotherapy | ||
| Neoadjuvant radiotherapy | 71 (2·5%) | 82 (2·9%) |
| Adjuvant radiotherapy | 2620 (93·3%) | 2628 (92·9%) |
| Surgical procedure with curative intent | 2804║ (99·9%) | 2829 (100·0%) |
| Median time from surgery to randomisation, months | 7·8 (4·7–9·6) | 7·9 (4·7–9·6) |
Data are n (%) or median (IQR).
Where values do not add up to 100%, remaining data are missing, unavailable, or could not be assessed.
Per interactive web response system.
Menopausal status is at the time of diagnosis and all male patients are considered postmenopausal.
397 patients in the abemaciclib plus endocrine therapy group and 413 in the endocrine therapy alone group.
Derived tumour, node, metastasis stage based on the pathological tumour size and number of positive lymph nodes following primary surgery.
Four patients who did not have definitive surgery in the breast were enrolled in the study without meeting eligibility criteria.
In the ITT population, invasive disease-free survival events were observed in 835 patients overall: 336 (12·0%) of 2808 patients in the abemaciclib plus endocrine therapy group and 499 (17·6%) of 2829 patients in the endocrine therapy alone group. Median invasive disease-free survival was not reached in either group, and the addition of abemaciclib to endocrine therapy reduced the risk of developing an invasive disease-free survival event (HR 0·664, 95% CI 0·578–0·762; nominal p<0∙0001; figure 2A). There is no evidence suggesting a violation of the proportional hazards assumption (appendix p 3). The estimated 4-year invasive disease-free survival rates were 85·8% (95% CI 84·2–87·3) in the abemaciclib plus endocrine therapy group versus 79·4% (77·5–81·1) in the endocrine therapy alone group, reflecting an absolute difference of 6·4%, compared with a 2·8% difference at 2 years (92·7% [91·6–93·6] vs 89·9% [88·7–91·0]) and a 4·8% difference at 3 years (89·2% [87·9–90·3] vs 84·4% [83·0–85·8]). The majority (589 [71%] of 835) of the invasive disease-free survival events were distant metastatic disease (appendix p 11).
Figure 2: Kaplan-Meier survival curves.
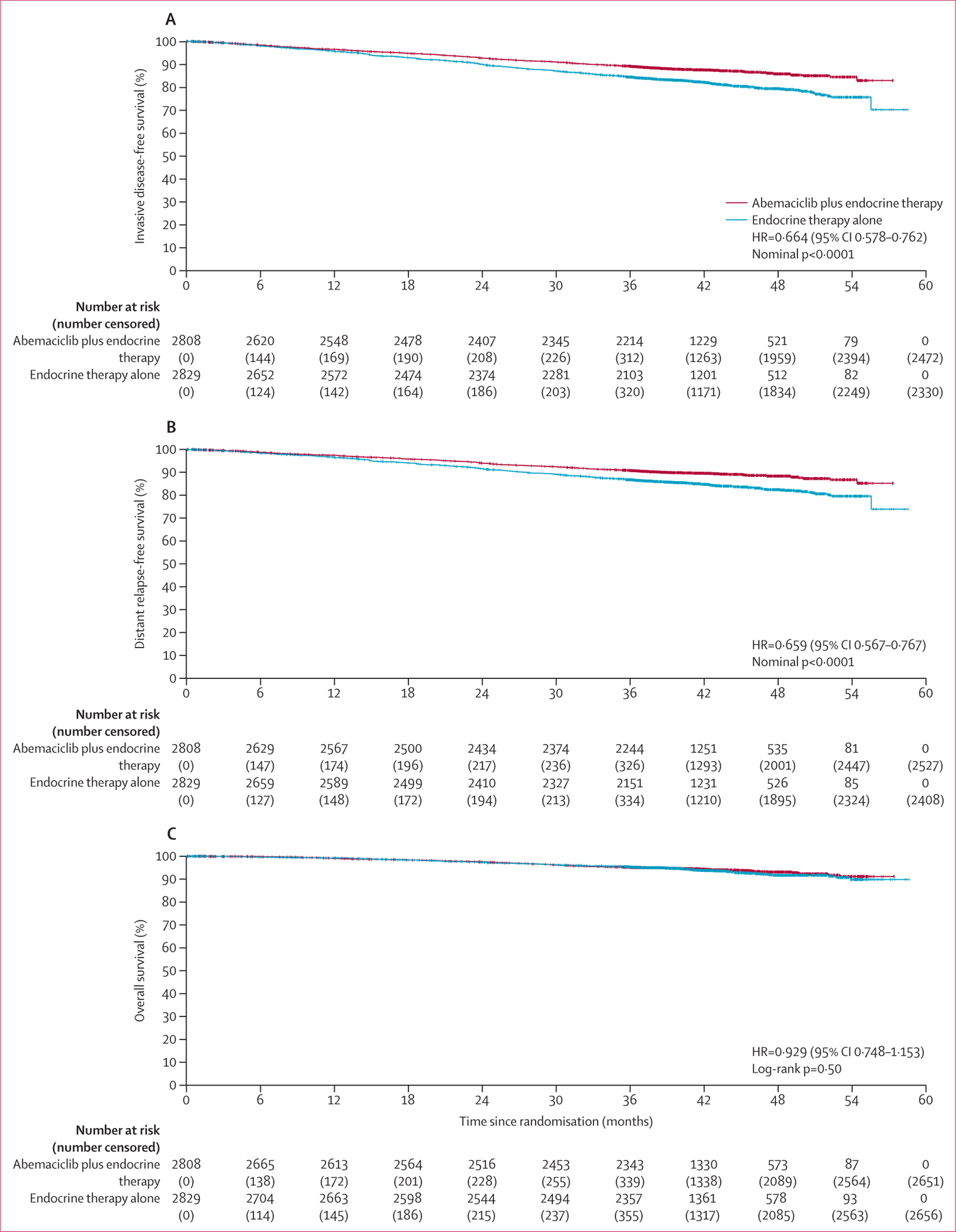
Invasive disease-free survival in the ITT population at the second overall survival interim analysis. (B) Distant relapse-free survival in the ITT population at the second overall survival interim analysis. (C) Overall survival in the ITT population at the second overall survival interim analysis. Vertical dashes denote censored patients. HR=hazard ratio. ITT=intention to treat.
Distant relapse-free survival events were observed in 281 (10·0%) of 2808 patients in the abemaciclib plus endocrine therapy group and 421 (14·9%) of 2829 patients in the endocrine therapy alone group. The addition of abemaciclib to endocrine therapy reduced the risk of developing a distant relapse-free survival event (HR 0·659, 95% CI 0·567–0·767; nominal p<0∙0001; figure 2B). The estimated 4-year distant relapse-free survival rates were 88·4% (95% CI 86·9–89·7) in the abemaciclib plus endocrine therapy group versus 82·5% (80·7–84·1) in the endocrine therapy alone group, reflecting an absolute difference of 5·9%, compared with a 2·5% difference at 2 years (94·0% [93·1–94·9] vs 91·6% [90·4%–92·5]) and a 4·1% difference at 3 years (90·8% [89·7–91·9] vs 86·8% [85·4–88·0]). Invasive disease-free survival and distant relapse-free survival in prespecified subgroups are shown in figure 3. In a post-hoc exploratory analysis, piecewise HR estimates for invasive disease-free survival and distant relapse-free survival suggest the magnitude of the effect size of abemaciclib increased over time, following completion of the 2-year study treatment period (appendix p 11).
Figure 3: Forest plots of subgroup analyses.
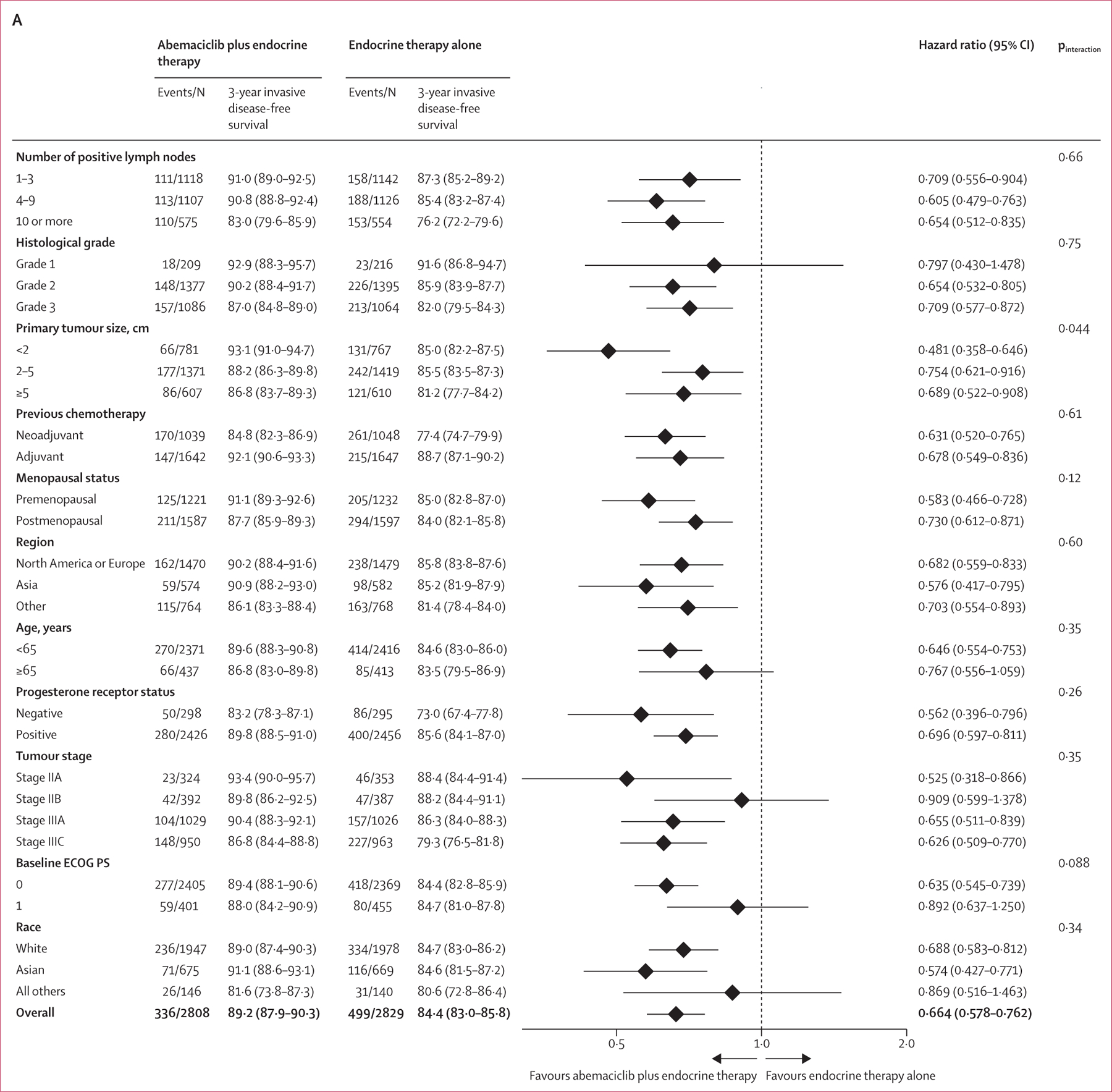
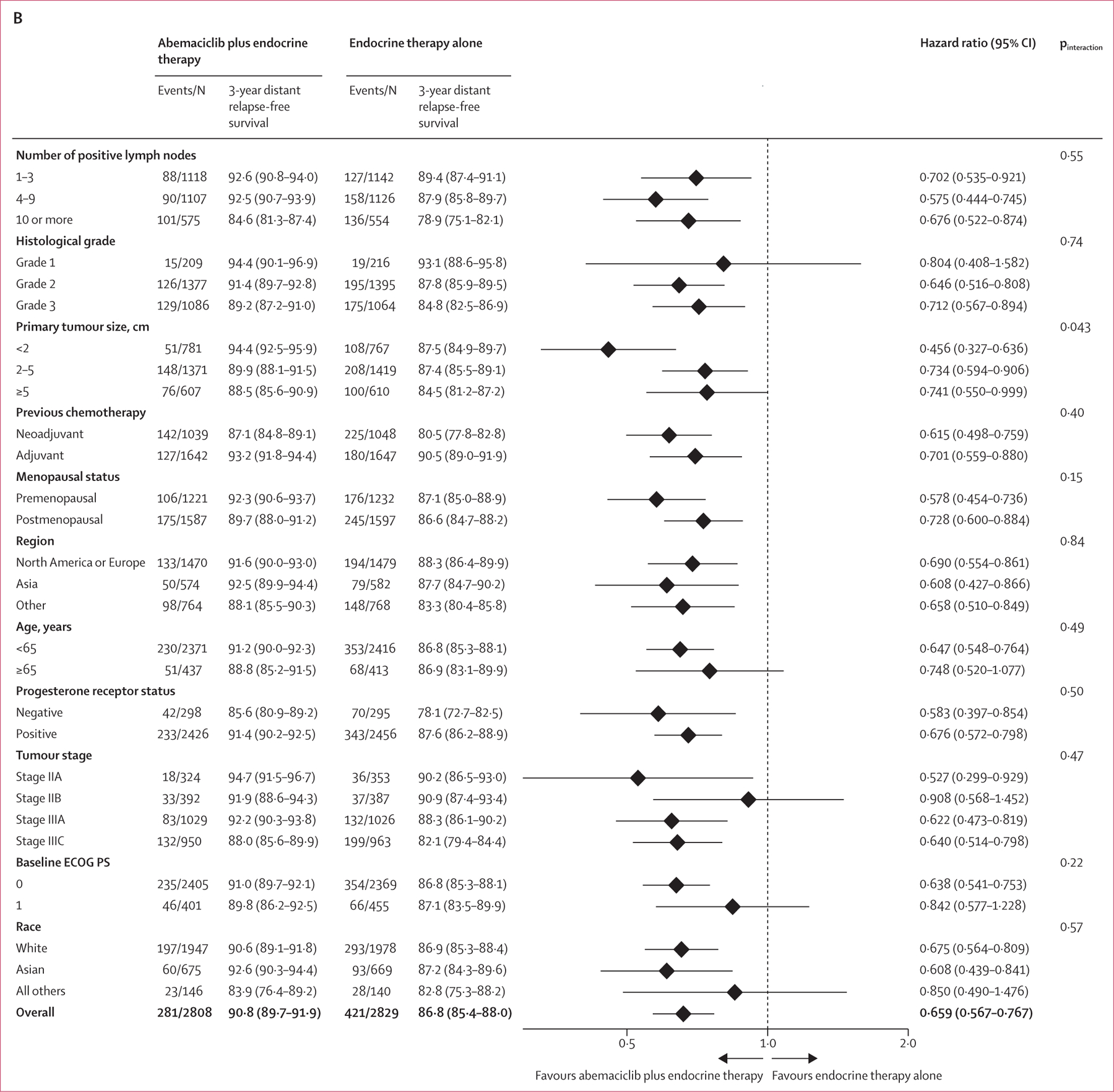
(A) Invasive disease-free survival in the ITT prespecified subgroups. (B) Distant relapse-free survival in the ITT prespecified subgroups. ECOG PS=Eastern Cooperative Oncology Group performance status. ITT=intention to treat.
At this prespecified overall survival analysis, overall survival data were immature and significance between the groups was not reached. 157 (5·6%) of 2808 patients in the abemaciclib plus endocrine therapy group died compared with 173 (6·1%) of 2829 patients in the endocrine therapy alone group (HR 0·929, 95% CI 0·748–1·153; log-rank p=0·50; figure 2C). There were 118 (4·2%) deaths due to breast cancer in the abemaciclib plus endocrine therapy group compared with 139 (4·9%) deaths due to breast cancer in the endocrine therapy alone group. To illustrate how the difference in distant relapse-free survival events affects the evolution of overall survival, the survival status of patients who have died or developed metastatic disease at each analysis timepoint was summarised as post-hoc analysis (appendix p 10). In addition to those who had already died from metastatic disease, 249 patients in the endocrine therapy alone group had developed and were living with metastatic disease compared with 125 patients in the abemaciclib plus endocrine therapy group.
Efficacy outcomes (invasive disease-free survival, distant relapse-free survival, and overall survival) in cohort 1, which accounts for 91% of enrolled patients, were generally concordant with results from the ITT population (appendix pp 4–5, 11). In cohort 1, the effect of abemaciclib in invasive disease-free survival was consistent between patients in cohort 1 with high and low Ki-67 index (appendix pp 6, 8, 11). Distant relapse-free survival results by Ki-67 index were consistent with invasive disease-free survival. Overall survival in the high and low Ki-67 subpopulations is also shown in the appendix (pp 7, 9, 12).
Cohort 2 is a small, exploratory subpopulation comprising 9% of the ITT population with accrual to this cohort beginning approximately 1 year after accrual initiated for cohort 1. At the overall survival interim analysis, the median duration of follow-up in cohort 2 was 39 months (IQR 36–43) in both treatment groups. The efficacy data in cohort 2 were immature at this longer follow-up. 19 (7·5%) of 253 patients had invasive disease-free survival events in the abemaciclib plus endocrine therapy group compared with 25 (9·5%) events in 264 patients in the endocrine therapy alone group (HR 0·773, 95% CI 0·420–1·420) and there were 14 (5·5%) distant relapse-free survival events in the abemaciclib plus endocrine therapy group compared with 19 (7·2%) events in the endocrine therapy alone group (HR 0·764, 95% CI 0·383–1·526). A post-hoc interaction analysis by cohort suggests that the effect size was consistent in both invasive disease-free survival (pinteraction=0·55) and distant relapse-free survival (pinteraction=0·66). Overall survival in cohort 2 was not formally evaluated given the immaturity of the data (15 [2·9%] patients died: ten in the abemaciclib plus endocrine therapy group and five in the endocrine therapy alone group).
All patients treated (2791 in the abemaciclib plus endocrine therapy and 2800 in the endocrine alone group) had discontinued study treatment at the data cutoff. Median duration of abemaciclib was 24 months (IQR 17–24). Median duration of endocrine therapy in the study treatment period was 24 months (IQR 23·5–24) in both treatment groups. The most frequent adverse events of any grade and any attribution were diarrhoea, neutropenia, and fatigue in the abemaciclib group, and arthralgia, hot flush, and fatigue in the control group (table 2). Venous thromboembolic events were more frequent with abemaciclib plus endocrine therapy (71 [2·5%] of 2791 patients) versus endocrine therapy alone (19 [0·7%] of 2800 patients).
Table 2:
Treatment-emergent adverse events in the safety population at the second overall survival interim analysis regardless of attribution
| Abemaciclib plus endocrine therapy (n=2791) |
Endocrine therapy alone (n=2800) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
| Any | 1353 (48·5%) | 1289 (46·2%) | 88 (3·2%) | 16 (0·6%) | 2016 (72·0%) | 439 (15·7%) | 22 (0·8%) | 11 (0·4%) |
| Diarrhoea | 2114 (75·7%) | 218 (7·8%) | 0 | 1 (<0·1%) | 238 (8·5%) | 6 (0·2%) | 0 | 0 |
| Fatigue | 1060 (38·0%) | 80 (2·9%) | 0 | 0 | 501 (17·9%) | 4 (0·1%) | 0 | 0 |
| Abdominal pain | 957 (34·3%) | 39 (1·4%) | 0 | 0 | 269 (9·6%) | 9 (0·3%) | 0 | 0 |
| Nausea | 811 (29·1%) | 14 (0·5%) | 0 | 0 | 251 (9·0%) | 2 (0·1%) | 0 | 0 |
| Leukopenia | 734 (26·3%) | 314 (11·3%) | 4 (0·1%) | 0 | 175 (6·3%) | 11 (0·4%) | 0 | 0 |
| Neutropenia | 733 (26·3%) | 529 (19·0%) | 19 (0·7%) | 0 | 134 (4·8%) | 20 (0·7%) | 4 (0·1%) | 0 |
| Arthralgia | 731 (26·2%) | 9 (0·3%) | 0 | 0 | 1031 (36·8%) | 29 (1·0%) | 0 | 0 |
| Anaemia | 626 (22·4%) | 57 (2·0%) | 1 (<0·1%) | 0 | 96 (3·4%) | 11 (0·4%) | 1 (<0·1%) | 0 |
| Headache | 545 (19·5%) | 8 (0·3%) | 0 | 0 | 420 (15·0%) | 5 (0·2%) | 0 | 0 |
| Vomiting | 476 (17·1%) | 15 (0·5%) | 0 | 0 | 127 (4·5%) | 4 (0·1%) | 0 | 0 |
| Hot flush | 427 (15·3%) | 4 (0·1%) | 0 | 0 | 634 (22·6%) | 10 (0·4%) | 0 | 0 |
| Cough | 390 (14·0%) | 1 (<0·1%) | 0 | 0 | 223 (8·0%) | 0 | 0 | 0 |
| Lymphoedema | 346 (12·4%) | 5 (0·2%) | 0 | 0 | 254 (9·1%) | 1 (<0·1%) | 0 | 0 |
| Thrombocytopenia | 337 (12·1%) | 28 (1·0%) | 8 (0·3%) | 0 | 48 (1·7%) | 2 (0·1%) | 2 (0·1%) | 0 |
| Constipation | 334 (12·0%) | 2 (0·1%) | 0 | 0 | 170 (6·1%) | 1 (<0·1%) | 0 | 0 |
| Urinary tract infection | 321 (11·5%) | 16 (0·6%) | 0 | 0 | 204 (7·3%) | 6 (0·2%) | 0 | 0 |
| Alopecia | 318 (11·4%) | 0 | 0 | 0 | 77 (2·8%) | 0 | 0 | 0 |
| Decreased appetite | 315 (11·3%) | 16 (0·6%) | 0 | 0 | 68 (2·4%) | 2 (0·1%) | 0 | 0 |
| Blood creatinine increased | 308 (11·0%) | 3 (0·1%) | 0 | 0 | 28 (1·0%) | 0 | 0 | 0 |
| Rash | 305 (10·9%) | 11 (0·4%) | 0 | 0 | 128 (4·6%) | 0 | 0 | 0 |
| Dizziness | 301 (10·8%) | 4 (0·1%) | 0 | 0 | 190 (6·8%) | 1 (<0·1%) | 0 | 0 |
| Upper respiratory tract infection | 296 (10·6%) | 6 (0·2%) | 0 | 0 | 238 (8·5%) | 0 | 0 | 0 |
| Pain in extremity | 284 (10·2%) | 3 (0·1%) | 0 | 0 | 323 (11·5%) | 4 (0·1%) | 0 | 0 |
| Aspartate aminotransferase increased | 283 (10·1%) | 50 (1·8%) | 3 (0·1%) | 0 | 125 (4·5%) | 15 (0·5%) | 0 | 0 |
| Pyrexia | 279 (10·0%) | 2 (0·1%) | 0 | 0 | 130 (4·6%) | 0 | 0 | 0 |
| Alanine aminotransferase increased | 274 (9·8%) | 72 (2·6%) | 5 (0·2%) | 0 | 138 (4·9%) | 19 (0·7) | 0 | 0 |
| Lymphopenia | 246 (8·8%) | 148 (5·3%) | 3 (0·1%) | 0 | 82 (2·9%) | 14 (0·5) | 0 | 0 |
| Hypertension | 106 (3·8%) | 30 (1·1%) | 0 | 0 | 122 (4·4%) | 49 (1·8%) | 0 | 0 |
| Hypokalaemia | 90 (3·2%) | 28 (1·0%) | 4 (0·1%) | 0 | 25 (0·9%) | 5 (0·2%) | 2 (0·1%) | 0 |
| Gamma-glutamyltransferase increased | 61 (2·2%) | 34 (1·2%) | 5 (0·2%) | 0 | 26 (0·9%) | 5 (0·2%) | 1 (<0·1%) | 0 |
| Other adverse events of interest, composite terms | ||||||||
| Venous thromboembolic event* | 33 (1·2%) | 32 (1·1%) | 6 (0·2%) | 0 | 10 (0.4%) | 8 (0·3%) | 0 | 1 (<0·1%) |
| Pulmonary embolism | 0 | 24 (0·9%) | 4 (0·1%) | 0 | 0 | 3 (0·1%) | 0 | 1 (<0·1%) |
Data are n (%). Data shown are grade 1–2 adverse events that occurred in ≥10% of patients and grade 3 or worse adverse events that occurred in ≥1% of patients.
Identified by selected terms in embolic and thrombotic events (Standardised Medical Dictionary for Regulatory Activities queries).
A higher frequency of grade 3 or worse adverse events and serious adverse events was observed with abemaciclib plus endocrine therapy versus endocrine therapy alone during treatment or within 30 days of study treatment discontinuation (1393 [49·9%] of 2791 patients vs 472 [16·9%] of 2800 patients and 433 [15·5%] of 2791 patients vs 256 [9·1%] of 2800 patients, respectively). The most common grade 3–4 adverse events were neutropenia (548 [19∙6%] of 2791 patients in the abemaciclib plus endocrine therapy group vs 24 [0∙9%] of 2800 patients in the endocrine therapy alone group), leukopenia (318 [11∙4%] vs 11 [0·4%]), and diarrhoea (218 [7·8%] vs six [0·2%]). The most frequently reported serious adverse events in both groups were infections (148 [5·3%] vs 81 [2·9%]) and gastrointestinal disorders (59 [2·1%] vs 17 [0·6%]; appendix p 25). 180 (6·4%) of 2791 patients discontinued both abemaciclib and endocrine therapy owing to adverse events, with the most frequent causes being diarrhoea (68 [2·4%]), and fatigue (28 [1·0%]); and 30 (1·1%) of 2800 patients in the control group discontinued endocrine therapy, with the most common adverse event leading to discontinuation being arthralgia (six [0·2%]). Abemaciclib treatment interruption due to adverse events occurred in 1721 (61·7%) of 2791 patients and dose reductions in 1216 (43·6%) of 2791 patients, generally related to diarrhoea, neutropenia, or fatigue. 15 (0·5%) of 2791 patients died due to adverse events on study treatment or within 30 days of study treatment discontinuation in the abemaciclib group and 11 (0·4%) of 2800 patients in the control group. Two of the 15 deaths in the abemaciclib group (diarrhoea and pneumonitis) were assessed by the investigators as possibly related to study treatment. No deaths in the control group were considered related to study treatment (appendix pp 26–27). Among patients who entered the long-term follow-up period, the incidence of serious adverse events in long-term follow-up regardless of causality was higher in the endocrine therapy alone group than in the abemaciclib plus endocrine therapy group (142 [5·4%] vs 116 [4·4%] appendix p 28), with no new safety concerns identified. Deaths due to adverse events in the long-term follow-up period are reported in the appendix (pp 26–27).
Discussion
At this prespecified overall survival interim analysis, with a median follow-up time of 42 months, the continued separation of the invasive disease-free survival and distant relapse-free survival Kaplan-Meier curves, together with the increased absolute improvement of 4-year invasive disease-free survival rates (6·4%) from the previous 2-year (2·8%) and 3-year rates (4·8%), suggests a sustained benefit beyond the treatment period. This is further shown by the post-hoc analysis assessing the evolution of the piecewise HR estimates within each year of the observation period. The numerical increase in treatment effect beyond the 2-year treatment period suggested a potential carryover effect as has been observed previously with adjuvant endocrine therapy.16
Disease recurrence, specifically distant recurrence, remains a common and life-threatening complication for patients with early breast cancer with high-risk clinicopathological features.1 In a systematic review and meta-analysis of the risk of recurrence, Salvo and colleagues17 concluded that approximately one in six patients with hormone receptor-positive, HER2-negative, lymph node-positive early breast cancer who received adjuvant endocrine therapy will have recurrence or death within 5 years. A real-world evidence study published in 2022 showed that patients with node-positive early breast cancer who met the monarchE high-risk eligibility criteria had up to a 30% risk of recurrence at 5 years.2 Notably in the endocrine therapy alone group of monarchE, 20·6% patients had already developed an invasive disease-free survival event and 17·5% had developed a distant relapse-free survival event at 4 years. These results confirm that patients included in monarchE were a selected, high-risk population, 95% of whom had received previous chemotherapy. As such, there remains an unmet need for improved adjuvant endocrine-based therapy for these patients with node-positive, high-risk early breast cancer.
Two other adjuvant studies with the CDK4 and 6 inhibitor palbociclib (PALLAS18 and Penelope-B19), did not meet their primary endpoint, and this had previously led some to question whether the benefits of abemaciclib would be maintained over time,20 especially given that previous monarchE data cutoffs had relatively short follow-up and included patients still receiving study treatment.4 The overall survival interim analysis from monarchE presented, including the 4-year estimated rates, address these concerns and show the persistent and deepening benefit of abemaciclib. These data highlight important differences between palbociclib and abemaciclib, as shown from a growing body of clinical and preclinical data.21,22 The increased selectivity of abemaciclib for CDK4 over CDK6 permits continuous dosing, compared with intermittent dosing of palbociclib. Continuous dosing leading to senescence and irreversible effects through apoptosis might be crucially important in the early breast cancer setting to eradicate microscopic residual hormone receptor-positive cells.4,23 Indeed, clinical data in the neoadjuvant setting have suggested that discontinuing CDK4 and 6 inhibition after 2 weeks of therapy led to proliferation rebound as measured by Ki-67 in the primary tumour after as little as 1 week’s cessation of drug.24–26 Additional ongoing clinical trials evaluating CDK4 and 6 inhibitors in the neoadjuvant or adjuvant setting will help to further establish the effect of these inhibitors in early breast cancer.27
Overall survival in monarchE remains immature at this interim analysis with 330 deaths (5·9% of the ITT population). The majority of overall survival events relate to deaths from metastatic disease in patients who had developed a recurrence of their breast cancer. Since the previous analysis,10 the higher number of deaths due to metastatic disease in the endocrine therapy alone group has shifted the overall survival difference between groups, resulting in numerically, but not significantly, fewer deaths in the abemaciclib group compared with endocrine therapy alone group at this analysis. The lower number of patients living with distant metastatic disease in the abemaciclib group at the overall survival interim analysis continues to be substantial (half the number in the abemaciclib group compared with the endocrine therapy alone group) and is expected to further affect differences in overall survival with additional follow-up.
The results of the subgroup analyses continue to illustrate the consistent treatment benefit associated with the addition of abemaciclib to standard endocrine therapy across prespecified subgroups for both invasive disease-free survival and distant relapse-free survival. Notably, the accumulation of recurrence events over time has allowed a more precise estimation of the magnitude of benefit, and all HR estimates now favour abemaciclib, even in subgroups with a better prognosis (ie, patients who are postmenopausal, patients with grade 1 or stage II tumours or a low Ki-67 index [<20%]). Within cohort 1, although patients with a Ki-67 index of at least 20% in the primary tumour have a worse prognosis, a similar relative effect size of abemaciclib treatment was observed in those with Ki-67 low (<20%) tumours, as well as those with Ki-67 high (≥20%) tumours, suggesting that abemaciclib benefit occurs regardless of Ki-67 index.
Cohort 2 enrolled patients with intermediate-risk clinicopathological features (ie, no more than three positive nodes, grade 1 or grade 2 and T1 or T2 tumours), with the additional risk factor of high cell proliferation defined as a Ki-67 of at least 20%. Enrolment into cohort 2 was started later in the trial, and with only 44 invasive disease-free survival events in 517 patients, the data remain immature. Notably, this small cohort was exploratory in nature and was not designed to provide definitive efficacy results on its own. Nonetheless, consistent with the ITT population, the number of invasive disease-free survival and distant relapse-free survival events within cohort 2 was lower in the abemaciclib plus endocrine therapy group than in the endocrine therapy alone group.
At the time of this analysis, all patients were no longer on study treatment, and safety continues to be consistent with the well-established safety profile of abemaciclib, which is considered manageable and acceptable for patients with high-risk early breast cancer. As expected, given the fact that the majority of abemaciclib toxicities occurred during the first few months of treatment, there were minimal changes in incidence of adverse events and no additional discontinuations due to adverse events compared with previous analyses. Of note, there were no new venous thromboembolic events since the previous interim analysis and no increased risk of venous thromboembolic events were observed with longer treatment duration of abemaciclib. Thus, the characterisation of toxicities, including time course, discontinuations due to adverse events, and dose adjustments reported by Rugo and colleagues remain valid.14 Given the risk of venous thromboembolic events, which might be affected by the choice of endocrine partner, and the early onset of toxicities such as diarrhoea, early intervention and risk monitoring are the most important measures to improve tolerability. The safety profile of abemaciclib in combination with endocrine therapy will continue to be monitored in the long-term follow-up.
The results of this trial are generalisable in clinical practice to patients with high risk of recurrence meeting the monarchE criteria, as the study enrolled patients on the basis of the commonly used clinicopathological features including Ki-67, a frequently used marker of cellular proliferation. One limitation of the monarchE study is the open-label design with no masking or placebo control because of the clinically detectable differences between safety profiles of abemaciclib and endocrine therapy. However, the potential bias in efficacy outcomes was effectively minimised through restricted access to unmasked aggregate data until the study was declared positive by the independent data monitoring committee. Another limitation of this analysis is the extent of follow-up time, especially since the overall survival data are immature and have not reached significance. Although it is considered sufficient to establish the benefit of abemaciclib in this high-risk population, longer follow-up remains important to fully characterise the treatment effect, including the survival effect, for at least 5 years in this disease setting.
In conclusion, with additional follow-up, monarchE has shown that the benefit of adjuvant abemaciclib added to endocrine therapy not only persisted, but deepened, resulting in an increase in absolute invasive disease-free survival and distant relapse-free survival benefit at 4 years. With a tolerable safety profile, these results further confirm the positive benefit–risk of adjuvant use of abemaciclib together with endocrine therapy in reducing the risk of recurrence for patients with high-risk, hormone receptor-positive, HER2-negative, node-positive, early breast cancer.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed on Oct 20, 2016, for clinical trials published between 2000 and 2016 in the English language using different combinations of the terms “breast cancer”, “early”, “adjuvant”, “HER2–”, “HR+”, and “CDK4 and 6 inhibitors”; we also retrieved American Society of Clinical Oncology, European Society for Medical Oncology, and National Comprehensive Cancer Network practice guidelines for the treatment of breast cancer. Our search found no published data describing outcomes for patients treated with CDK4 and 6 inhibitors in the adjuvant setting. Disease recurrence, specifically distant metastatic recurrence, remained a common and life-threatening complication for patients with high-risk hormone receptor-positive, human epidermal growth factor receptor 2 (HER2)-negative early breast cancer despite advances in neoadjuvant and adjuvant systemic therapies. Additionally, since the introduction of aromatase inhibitors in the early 2000s, there had been few new drug approvals to enhance the standard neoadjuvant and adjuvant therapies available. Previous clinical trials in patients with hormone receptor-positive, HER2-negative advanced or metastatic breast cancer have shown that the combination of a CDK4 and 6 inhibitor and endocrine therapy, both in the first-line and pretreated settings, improves progression-free survival. Given the benefits of CDK4 and 6 inhibitors in the metastatic setting, there was substantial interest in establishing whether these agents could improve outcomes for patients with hormone receptor-positive, HER2-negative early breast cancer in the adjuvant setting. In a phase 2 study in the neoadjuvant setting (neoMONARCH), the combination of the CDK4 and 6 inhibitor abemaciclib with anastrozole showed biological and clinical activity with a manageable safety profile, further supporting the concept of CDK4 and 6 inhibitors with endocrine therapy as treatment for early breast cancer. Four other randomised, phase 3 trials were set up to evaluate the combination of a CDK4 and 6 with endocrine therapy in the adjuvant setting: PALLAS and Penelope-B for palbociclib, monarchE for abemaciclib, and NATALEE for ribociclib.
Added value of this study
The positive outcome of monarchE represents a significant advancement in the adjuvant treatment of hormone receptor-positive, HER2-negative early breast cancer in a patient population with clinical and pathological features associated with a high likelihood of recurrence. On the basis of positive results from monarchE, abemaciclib became, to our knowledge, the first CDK4 and 6 inhibitor globally approved for use in the adjuvant setting.Notably, although the benefits of abemaciclib were seen early in the monarchE trial, the current analysis with longer follow-up is important to establish durable effect on disease recurrence and survival over time.
Implications of all the available evidence
monarchE results are practice changing and the updated analyses presented here, including the 4-year estimated rates, show the persistent and deepening benefit of adjuvant abemaciclib in a high-risk early breast cancer population. With a tolerable safety profile, these results further strengthen the positive benefit–risk for the adjuvant use of abemaciclib. Long-term follow-up to study the outcomes in these patients with high-risk early breast cancer is ongoing.
Acknowledgments
This study was funded by Eli Lilly. Additional support was provided by the National Institute for Health Research funding to the Royal Marsden and Institute of Cancer Research Biomedical Research Centre. Medical writing and editorial support were provided by Trish Huynh and Eglantine Julle-Daniere, who are both employees of Eli Lilly. All writing, editorial assistance, and statistical analysis were funded by Eli Lilly. All authors were not precluded from accessing the data in the study, and they accept responsibility for the decision to submit for publication. We thank the participants and their families or caregivers for participating in this trial. monarchE would not have been possible without the investigators and their support staff who participated in this work. Finally, the authors are grateful for the time and efforts of the monarchE Executive and Steering Committees.
Declaration of interests
SRDJ reports grants or contracts from Pfizer, Puma Biotechnology, Eli Lilly, AstraZeneca, Novartis, and Roche–Genentech for research funding to institute for laboratory studies and clinical trials; consulting fees from Eli Lilly, AstraZeneca, Puma Biotechnology, Pfizer, Novartis, and Sanofi Genzyme for consulting or an advisory role; and payment or honoraria from Pfizer, Eisai, AstraZeneca, and Roche–Genentech for speaker’s bureau. MT reports grants or contracts from Chugai, Takeda, Pfizer, Taiho, JBCRG, KBCRN, Eisai, Eli Lilly, Daiichi-Sankyo, AstraZeneca, Astellas, Shimadzu, Yakult, Nippon Kayaku, AFI technology, Luxonus, Shionogi, GL Science, and Sanwa Shurui for research grants to their department; payment or honoraria from Chugai, Takeda, Pfizer, Kyowa-Kirin, Taiho, Eisai, Daiichi-Sankyo, AstraZeneca, Eli Lilly, Merck Sharp and Dohme, Exact Science, Novartis, Shimadzu, Yakult, Nippon Kayaku, Devicore Medical Japan, and Sysmex for lecture honoraria or honoraria for lecture chair; participation on a data safety monitoring board or advisory board for Daiichi-Sankyo, Eli Lilly, Bristol Myers Squibb, Athenex Oncology, Bertis, Terumo, and Kansai Medical Net; and other financial or non-financial interest from British Journal of Cancer, Scientific Reports, Breast Cancer Research and Treatment, Cancer Science, Frontiers in Women’s Cancer, Asian Journal of Surgery, and Asian Journal of Breast Surgery for his role as Associate Editor. JO’S reports consulting fees and payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing or educational events from from AbbVie, Agendia, Amgen Biotechnology, Aptitude Health, AstraZeneca, Athenex, Bayer, Bristol Myers Squibb, Carrick Therapeutics, Celgene Corporation, Daiichi Sankyo, Eisai, G1 Therapeutics, Genentech, Genzyme, Gilead Sciences, GRAIL, Halozyme Therapeutics, Heron Therapeutics, Immunomedics, Ipsen Biopharmaceuticals, Eli Lilly, Merck, Myriad, Nektar Therapeutics, Novartis, Ontada, Pfizer, Pharmacyclics, Pierre Fabre Pharmaceuticals, Puma Biotechnology, Prime Oncology, Roche, Samsung Bioepsis, Sandoz, Sanofi, Seagen, Syndax Pharmaceuticals, Taiho Oncology, Takeda, Theralink, and Synthon. MC reports personal payment support from Eli Lilly for the present article, advisory board, and speaker’s bureau; payment or honoraria to institution from Novartis; support for attending meetings from Eli Lilly, Novartis, AstraZeneca, and Pfizer; and payment to institution from AstraZeneca, Novartis, Sanofi, Pfizer, Seagen, Gilead, Daiichi Sankyo for participation on a data safety monitoring board or advisory board. C-SH reports research grants to institution from Eli Lilly for the present article; research grants to institution or contracts from Daiichi Sankyo, AstraZeneca, EirGenix, Eli Lilly and Company, Merck Sharp and Dohme, OBI Pharma, Pfizer, Roche, and Novartis; payment or honoraria from Daiichi Sankyo, AstraZeneca, Pfizer, Novartis, Roche, and Eli Lilly for speaker’s bureau; support for attending meetings or travel from Astra Zeneca, Pfizer, Roche, and Novartis; and participation on advisory boards from Daiichi Sankyo, AstraZeneca, Eli Lilly, Pfizer, Novartis, and Roche. JH reports institutional grants or contracts from Celgene, Novartis, Hexal, and Eli Lilly; consulting fees paid to self from Eli Lilly, Novartis, Roche, Pfizer, Hexal, AstraZeneca, Merck Sharp and Dohme, Celgene, AbbVie, and Daiichi-Sankyo; honoraria or payments to self for lectures, presentation, speaker’s bureaus, manuscript writing, or educational events from Eli Lilly, Novartis, Roche, Pfizer, AstraZeneca, Merck Sharp and Dohme, Celgene, Eisai, Abbvie, Seagen, and Gilead; and support for attending meetings or travel paid to self from Roche, Pfizer, Novartis, Celgene, Daiichi-Sankyo, and Gilead. SMT reports institutional funding for study, consulting work to personal (honorariums), and manuscript preparation from Eli Lilly; institutional grants or contracts from AstraZeneca, Merck, Mektar, Novartis, Pfizer, Genentech–Roche, Gilead, Exelixis, Bristol Myers Squibb, Eisai, Nanostring, Cyclacel, Sanofi, and Seagen; and honoraria payments to self for participation in advisory boards or consulting from AstraZeneca, Eli Lilly, Merck, Novartis, Pfizer, Genentech–Roche, Gilead, Bristol Myers Squibb, Eisai, Sanofi, Seagen, Daiichi-Sankyo, Athenex, OncoPep, Kyowa Kirin Pharma, CytomX, Certara, Mersana Therapeutics, Ellipses Pharma, 4D Pharma, OncoSec, Infinity Therapeutics, BeyondSpring Pharma, OncXerna, Zymeworks, Zentalis, ARC Therapeutics, Reveal Genomics, Blueprint Medicines, Myovant, Umoja Biopharma, and Menarini–Stemline. MPG reports institutional grants or contracts from Pfizer, Eli Lilly, and Sermonix; consulting fees to institution from Eagle Pharmaceuticals, Eli Lilly, Biovica, Novartis, Sermonix, Pfizer, and ARC Therapeutics; honoraria or payment to self for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Research to Practice, Clinical Education Alliance, Medscape, Total Health Conferencing, Curio Science, and MJH Life Sciences; and support to institution from Eli Lilly, AstraZeneca, Novartis, Biotheranostics, Blueprint Medicines, Sanofi Genzyme, and ARC Therapeutics for participation on a data safety monitoring board or advisory board. HSR reports institutional research support from Pfizer, Novartis, Eli Lilly, Genentech–Roche, OBI, Merck, Gilead Sciences, Daiichi Sankyo, Seattle Genetics, Sermonix, AstraZeneca, and Astellas; travel support to academic meetings from Merck, AstraZeneca, and Gilead; and consulting or advisory support from Puma, NAPO, and Blueprint. ES reports support from Eli Lilly for the present article including investigator fees and medical writing; payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from AstraZeneca, Cancérodigest, Curio, Science, Egis, Eli Lilly and Company, Exact Sciences, Gilead, high5md, Merck Sharp and Dohme, Novartis, Pfizer, and Pierre Fabre; support for attending meetings or travel from Egis, Gilead, Novartis, Pfizer, and Roche; participation on a data safety monitoring board or advisory board from AstraZeneca, Egis, Eli Lilly, Exact Sciences, Merck Sharp and Dohme, Novartis, and Pfizer; a leadership or fiduciary role from Stowarzyszenie Różowy Motyl as Chair (unpaid); stock or stock options from AstraZeneca, Eli Lilly, and Pfizer; receipt of equipment, materials, drugs, medical writing, gifts or other services from Astellas, AstraZeneca, and Eli Lilly; and other financial or non-financial interests from Amgen, AstraZeneca, Eli Lilly, Novartis, OBI Pharma, Pfizer, Roche, and Samsung for contracted research. LT reports support from Eli Lilly as the trial sponsor for the study; grants or contracts from Novartis for clinical research; consulting fees from Merck Sharp and Dohme, Eli Lilly, and Novartis for advisory board; payment or honoraria from Merck Sharp and Dohme, Eli Lilly, Pfizer, Daiichi Sankyo, Novartis, and AstraZeneca for medical education; and support for attending meetings or travel from Pfizer and AstraZeneca. LDM reports an institutional research grant from Eli Lilly, Novartis, Roche, Daiichi Sankyo, and Seagen; consulting fees paid to self from Eli Lilly; honoraria or payment to self from Roche, Novartis, Pfizer, Eli Lilly, AstraZeneca, Merck Sharp and Dohme, Seagen, Gilead, Pierre Fabre, Eisa, Exact Sciences, and Ipsen for lectures, presentations, speaker’s bureaus, manuscript writing or educational events; support for attending meetings or travel from Roche, Pfizer, and Eisai; and fees paid to self for participation on a data safety monitoring board or advisory Board from Novartis, Roche, Eli Lilly, Pfizer, Daiichi-Sankyo, Exact Sciences, Gilead, Pierre Fabre, Eisai, AstraZeneca, and Agendia. CS reports research grants from Eli Lilly, and honoraria for lectures from Pfizer and Eli Lilly. RW, AS, MMu, BSA, VA are employees and stock shareholders of Eli Lilly. NH reports support for the present article (medical writing) from Eli Lilly; consulting fees from Gilead, Sandoz, and Seagen; payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Amgen, AsraZeneca, Daiichi-Sankyo, Gilead, Eli Lilly, Merck Sharp and Dohme, Novartis, Pierre-Fabre, Pfizer, Roche, and Seagen; participation on a data safety monitoring board or advisory board from Roche; and a leadership or fiduciary role with the West German Study Group and ESMO. MMa reports institutional grants from Roche, Novartis, and Puma; consulting fees paid to self from Roche, Eli Lilly, Pfizer, Daiichi-Sankyo, AstraZeneca, and Novartis; payment or honoraria to self for lectures, presentations, speaker’s bureaus, manuscript writing, or educational events from Roche, Eli Lilly, Astrazeneca, and Pfizer; support paid to self for attending meetings or travel from Daiichi-Sankyo and Roche; and support paid to self for participation on a data safety monitoring board or advisory board from Novartis. PR, PN, GGJ, and IC declare no competing interests.
Footnotes
Contributor Information
Stephen R D Johnston, The Royal Marsden NHS Foundation Trust, London, UK.
Masakazu Toi, Kyoto University, Kyoto, Japan.
Joyce O’Shaughnessy, Baylor University Medical Center, Texas Oncology, US Oncology, Dallas, TX, USA.
Priya Rastogi, University of Pittsburgh/UPMC, NSABP Foundation, Pittsburgh, PA, USA.
Mario Campone, Institute de Cancérologie de l’Ouest, Centre Rene Cauducheau, Saint-Herblain, Nantes, France.
Patrick Neven, Universitaire Ziekenhuizen Leuven, Campus Gasthuisberg, Leuven, Belgium.
Chiun-Sheng Huang, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Jens Huober, Cantonal Hospital St Gallen, Breast Centre St Gallen, Switzerland.
Georgina Garnica Jaliffe, Grupo Medico Camino SC, Mexico City, Mexico.
Irfan Cicin, Trakya University Faculty of Medicine, Edirne, Turkey.
Sara M Tolaney, Dana-Farber Cancer Institute, Boston, MA, USA.
Matthew P Goetz, Department of Oncology, Mayo Clinic, Rochester, MN, USA.
Hope S Rugo, University of California San Francisco Hellen Diller Family Comprehensive Cancer Center, San Francisco, CA, USA.
Elzbieta Senkus, Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk, Poland.
Laura Testa, Instituto D’Or de Pesquisa e Ensino (IDOR), Sao Paulo, Brazil.
Lucia Del Mastro, IRCSS Ospedale Policlinico San Martino, UO Breast Unit, Genoa, Italy, Università di Genova, Department of Internal Medicine and Medical Specialties (DIM), Genoa, Italy.
Chikako Shimizu, National Centre for Global Health and Medicine, Tokyo, Japan.
Ran Wei, Eli Lilly and Company, Indianapolis, IN, USA.
Ashwin Shahir, Loxo@Lilly, Indianapolis, IN, USA.
Maria Munoz, Loxo@Lilly, Indianapolis, IN, USA.
Belen San Antonio, Loxo@Lilly, Indianapolis, IN, USA.
Valérie André, Eli Lilly and Company, Indianapolis, IN, USA.
Nadia Harbeck, Breast Centre, Department of Gynaecology and Obstetrics, Comprehensive Cancer Centre München, LMU University Hospital, Munich, Germany.
Miguel Martin, Hospital General Universitario Gregorio Marañon, Universidad Complutense, CIBERONC, GEICAM, Madrid, Spain.
Data sharing
Eli Lilly will provide access to all individual participant data collected during the trial, after anonymisation, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU or after primary publication acceptance, whichever is later. No expiration date for data requests is set once the data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment.
References
- 1.Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017; 377: 1836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheffield KM, Peachey JR, Method M, et al. A real-world US study of recurrence risks using combined clinicopathological features in HR-positive, HER2-negative early breast cancer. Future Oncol 2022; 18: 2667–82. [DOI] [PubMed] [Google Scholar]
- 3.Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 2020; 38: 3987–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harbeck N, Rastogi P, Martin M, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol 2021; 32: 1571–81. [DOI] [PubMed] [Google Scholar]
- 5.Verzenio (abemaciclib) [package insert]. Indianapolis, IN: Eli Lilly, 2021. [Google Scholar]
- 6.Verzenios Summary of Product Characteristics. Netherlands: Eli Lilly Nederland, 2022. [Google Scholar]
- 7.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 2002; 359: 2131–39. [DOI] [PubMed] [Google Scholar]
- 8.Thürlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005; 353: 2747–57. [DOI] [PubMed] [Google Scholar]
- 9.Royce M, Osgood C, Mulkey F, et al. FDA approval summary: abemaciclib with endocrine therapy for high-risk early breast cancer. J Clin Oncol 2022; 40: 1155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harbeck N, Rastogi P, Shahir A, Johnston S, O’Shaughnessy J. Letter to the editor for ‘Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study’. Ann Oncol 2022; 33: 227–28. [DOI] [PubMed] [Google Scholar]
- 11.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 2010; 134: 907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol 2018; 36: 2105–22. [DOI] [PubMed] [Google Scholar]
- 13.Polewski MD, Nielsen GB, Gu Y, et al. A standardized investigational Ki-67 immunohistochemistry assay used to assess high-risk early breast cancer patients in the monarchE phase 3 clinical study identifies a population with greater risk of disease recurrence when treated with endocrine therapy alone. Appl Immunohistochem Mol Morphol 2022; 30: 237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rugo HS, O’Shaughnessy J, Boyle F, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: safety and patient-reported outcomes from the monarchE study. Ann Oncol 2022; 33: 616–27. [DOI] [PubMed] [Google Scholar]
- 15.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 2007; 25: 2127–32. [DOI] [PubMed] [Google Scholar]
- 16.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015; 386: 1341–52. [DOI] [PubMed] [Google Scholar]
- 17.Salvo EM, Ramirez AO, Cueto J, et al. Risk of recurrence among patients with HR-positive, HER2-negative, early breast cancer receiving adjuvant endocrine therapy: a systematic review and meta-analysis. Breast 2021; 57: 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer EL, Dueck AC, Martin M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2021; 22: 212–22. [DOI] [PubMed] [Google Scholar]
- 19.Loibl S, Marmé F, Martin M, et al. Palbociclib for residual high-risk invasive HR-positive and her2-negative early breast cancer—The Penelope-B Trial. J Clin Oncol 2021; 39: 1518–30. [DOI] [PubMed] [Google Scholar]
- 20.Spring L, Matikas A, Bardia A, Foukakis T. Adjuvant abemaciclib for high-risk breast cancer: the story continues. Ann Oncol 2021; 32: 1457–59. [DOI] [PubMed] [Google Scholar]
- 21.Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 2014; 32: 825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres-Guzmán R, Calsina B, Hermoso A, et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget 2017; 8: 69493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres-Guzmán R, Ganado MP, Mur C, et al. Continuous treatment with abemaciclib leads to sustained and efficient inhibition of breast cancer cell proliferation. Oncotarget 2022; 13: 864–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurvitz SA, Martin M, Press MF, et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase ii neoadjuvant study in HR+/HER2− breast cancer. Clin Cancer Res 2020; 26: 566–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma CX, Gao F, Luo J, et al. NeoPalAna: neoadjuvant palbociclib, a cyclin-dependent kinase 4/6 inhibitor, and anastrozole for clinical stage 2 or 3 estrogen receptor-positive breast cancer. Clin Cancer Res 2017; 23: 4055–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowsett M, Kilburn L, Rimawi MF, et al. Biomarkers of response and resistance to palbociclib plus letrozole in patients with ER+/HER2− breast cancer. Clin Cancer Res 2022; 28: 163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loibl S, Furlanetto J. Integrating CDK4/6 inhibitors in the treatment of patients with early breast cancer. Breast 2022; 62 (suppl 1): S70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Eli Lilly will provide access to all individual participant data collected during the trial, after anonymisation, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU or after primary publication acceptance, whichever is later. No expiration date for data requests is set once the data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment.


