Abstract
In Argentina, circulation of hepatitis E virus (HEV) genotype 3 has been described, producing sporadic cases of acute and chronic hepatitis. Limited information is available regarding HEV infection in children, so we aimed to investigate this virus in a pediatric population from the country. Serum samples from Argentine children (0–18 years old) (n = 213) were studied for IgG anti-HEV, IgM anti-HEV and RNA-HEV: 202 samples belonged to individuals attending health-care centers for routine check-ups, and 11 samples from patients with acute hepatitis of unknown etiology. Seropositivity for IgG anti-HEV was 1.49 % (3/202). One sample from an 18-years-old female patient with acute hepatitis tested positive for IgM anti-HEV detection, negative for IgG anti-HEV and RNA-HEV, but also positive for IgM anti-EBV. The HEV prevalence was low and showed circulation among children in central Argentina.
Keywords: HEV, Argentina, Children, Infection, Coinfection
1. Introduction
The hepatitis E virus (HEV) (Hepeviridae family, Orthohepevirinae subfamily, Paslahepevirus genus, specie Paslahepevirus balayani) is an important pathogen with a worldwide distribution, causing enterically transmitted hepatitis, with a single-stranded positive sense RNA genome [[1], [2], [3]]. The virus is transmitted mainly by the fecal-oral route and as a zoonosis, although vertical transmission and blood transfusion have also been described [1,2]. Eight viral genotypes have been described, from which 1, 2, 3, 4, and 7 cause disease in humans. Historically, genotypes 1 (HEV-1) and 2 (HEV-2) have been described only in humans, causing epidemic outbreaks by fecal-oral transmission in Asia and Africa, and sporadic cases in America. However, a recent study demonstrated that HEV-1 efficiently infected Mongolian gerbils, expanding the possible host spectrum for this genotype [4]. Genotypes 3 (HEV-3) and 4 (HEV-4) are zoonotic viruses; HEV-3 has a worldwide distribution, while the HEV-4 circulates in Asia and Europe. Genotype 7 (HEV-7) was been detected in camels and humans only in the Middle East [2,5].
Usually, the course of the infection is benign and self-limiting (incubation period: 2–10 weeks). Infected people begin to shed the virus a few days before the onset of symptoms until about 3–4 weeks after. Generally, the course of the infection is asymptomatic, especially in pediatrics, or its symptoms are mild and indistinguishable from those of other hepatitis, which is why cases often remain without an etiological diagnosis [2]. Pregnant women infected with HEV-1 may present a higher mortality rate (up to 20 %) [2,5], and immunosuppressed individuals infected with HEV-3 or HEV-4 can progress to chronicity [1,2].
The first HEV serological in America date from the 1990s and early 2000s, reporting prevalence rates from 0.1 % to 29 % among different populations [5]. Although reports of HEV circulation in our region have increased significantly in recent years [1,5,6], research has focused on the adult general population, patients with underlying pathologies and immunosuppressed patients. Thus, there are few studies on HEV in the pediatric population, and the impact of infection in this group still remains unknown [3].
In Argentina, IgG anti-HEV prevalences between 0.15 % and 35 % have been described, depending on the population studied, the region and the serological kit used, with higher infection rates in immunosuppressed patients (HIV+, transplant patients, dialysis, individuals with cirrhosis) [5,7]. In addition, sporadic cases of acute and chronic hepatitis E have been reported [5], and HEV-3 RNA has been detected in environmental (sewage, dam and river) and animal (pigs and wild boards) samples [5]. Only two previous studies of HEV circulation in children have been performed in our country, reporting seroprevalences of 0.15 % and 1.7 % [8,9]. However, one of them was performed in the 1990s decade [8], and the other one (performed between 2016 and 2018) was localized to a rural area in the North of the country (Chaco province) [9]. Therefore, there is still no updated and representative information about the circulation and impact of HEV in the pediatric population in our country. With this background, we aimed to study HEV infection in a children population in Córdoba, an urban central area of Argentina, conducting a viral survey for HEV and studying cases of acute hepatitis without etiology.
2. Material and methods
2.1. Samples
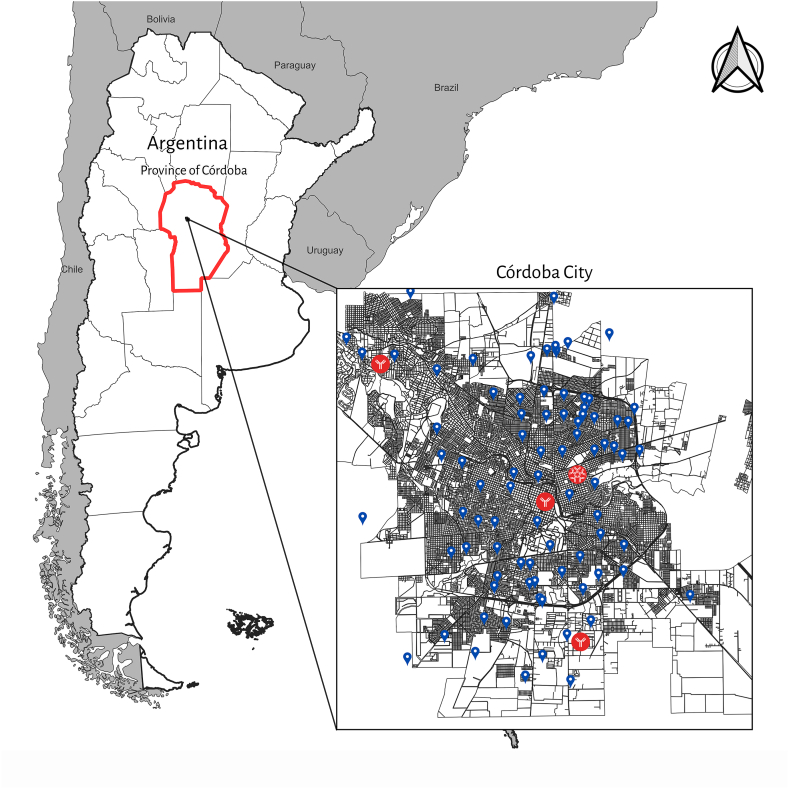
This is an anonymous, descriptive, retrospective, observational study, in which a total of 213 serum samples from children and adolescents (age range: 0–18 years old), collected during 2022 in two health centers from the city of Córdoba, central region of Argentina (Fig. 1), were analyzed for HEV detection. The samples were divided in 2 groups: a-202 samples which belonged to individuals who attended health-care centers for a routine checkup (school control, control for low weight, control for mild anemia, etc.) and agreed to participate in a HEV survey study, which were analyzed for IgG anti-HEV and RNA-HEV detection; b-11 samples from patients diagnosed with acute hepatitis derived to our laboratory for hepatitis E diagnosis, which were tested for IgG anti-HEV, IgM anti-HEV and RNA-HEV detection. This last group of patients had negative results for hepatitis A (HAV), B (HBV) and C (HCV) viruses, cytomegalovirus (CMV), autoimmune or toxic hepatitis.
Fig. 1.
Map of Córdoba city showing the neighborhoods from which samples were taken and studied, labeled in blue. Positive IgG and IgM anti-HEV samples are labeled in red.
This study was approved by the ethics committee of the Hospital Infantil Municipal (Córdoba), CIEIS Repis N°184.
Age, sex and neighborhood data were collected from all patients. For the analyses, age was stratified into 4 groups: 0–4 years, 5–9 years, 10–14 years and 15–18 years, and, according to the neighborhood, the presence of a sewer or a septic tank in the home could be known (Table 1). Other socioeconomical, clinical and epidemiological variables could be registered for some of the individuals (n = 76) (Supplementary Table 1).
Table 1.
Age, sex and excreta disposal data from children and adolescents from group a (202 individuals enrolled in a HEV survey) studied from Córdoba province, Argentina, and the IgG anti-HEV serological reactivity.
| Number of participants |
HEV IgG reactive participants |
HEV IgG non-reactive participants |
|||
|---|---|---|---|---|---|
| Age range | n | n | % | n | % |
| 0 - 4 | 51 | 1 | 1.96 | 50 | 98.04 |
| 5 - 9 | 46 | 0 | 0 | 46 | 100 |
| 10 - 14 | 50 | 0 | 0 | 50 | 100 |
| 15 -18 | 55 | 2 | 3.63 | 53 | 96.37 |
| TOTAL | 202 | 3 | 1.49 | 199 | 98.51 |
| Sex | |||||
| Female | 102 | 2 | 1.96 | 100 | 98.04 |
| Male | 100 | 1 | 1 | 99 | 99 |
| TOTAL | 202 | 3 | 1.49 | 199 | 98.51 |
| Excreta disposal data | |||||
| Sewage system | 72 | 1 | 1.38 | 71 | 98.62 |
| Septic tank | 48 | 2 | 4.16 | 46 | 95.84 |
| NDa | 82 | 0 | 0 | 82 | 100 |
| TOTAL | 202 | 3 | 1.49 | 199 | 98.51 |
No data.
2.1.1. Serological detection
IgG and IgM anti-HEV detections were performed by ELISA assays (Diapro, Italy; diagnostic specificity ≥98 %, diagnostic sensitivity ≥98 %, limit of detection: 0.5 WHO UI/mL), following the manufacturer's instructions. All samples were subjected to specific IgG detection, while only IgG positive and acute samples were subjected to IgM anti-HEV detection.
2.1.2. Molecular detection
Viral nucleic acids were extracted from 200 μL serum samples using High Pure Viral Nucleic Acid kit (Roche®), following the manufacturer's instructions. RNA-HEV amplification was performed by single-step real time RT-PCR, according to the protocol previously described by Jothikumar et al. (2006) (limit of detection: 4 copies of plasmid DNA) [10], using the TaqMan® Fast Applied Biosystems single-step RT-qPCR Kit (Carlsbad, CA, USA), in a StepOne™ Real-Time PCR equipment (Applied Biosystems).
2.1.3. Statistical analyzes
The Raosoft ® software (http://www.raosoft.com/samplesize.html) was used to calculate the sample size to be analyzed for IgG anti-HEV prevalence studies, with a 95 % confidence and 3 % error (minimum of 164 samples with a previously known HEV seroprevalence in adults of 4 %) [7]. Presence or absence of IgG anti-HEV was defined as the dependent variable.
Categorical variables were summarized as counts and percentages, and continuous variables as medians and inter-quartile ranges (IQR). Formal statistical analyses assessing the association between HEV IgG reactivity and demographic, epidemiological and socioeconomical variables were not performed due to only three IgG anti-HEV-reactive samples obtained.
Proportion for IgG and IgM anti-HEV with their 95 % confidence intervals (95 % CI) was performed using the R package “stats” (with Yates' continuity correction [11]. Moreover, and based on the method proposed by Lang and Reiczigel [12], these crude seroprevalences and their confidence intervals were adjusted for the sensitivity and specificity of the kits provided by the manufacturer, using the R function prevSeSp(.) in the package “asht” [13]. All statistical analyses were performed using RStudio version 2023.03.0.386 [14].
3. Results
Epidemiological data of the studied population from group a is shown in Table 1. The median age of the studied individuals was 10 years (interquartile range 4–15 years); 50 % participants were female and 50 % males. The overall seropositivity rate for IgG anti-HEV in the studied pediatric population (group a) was 1.49 % (3/202) (95 % CI: 0.38–4.63) and it remained the same when adjusting this crude estimate by the assay sensitivity and specificity, (95 % CI: 0.00–4.21 %).
Two positive samples belonged to 15-years-old individuals, while the other positive sample was collected from a 4-year-old child. When divided into categories according to the age, the group over 15 years old had the highest prevalence value (3.57 %, 95 % CI: 0.62–13.38 %, adjusted CI: 0.00–12.21 %) (Table 1). All positive samples belonged to subjects that had access to tap drinking water and only one of them had sewage system at their house. None of the immunosuppressed individuals, with a previous history of blood transfusion, or who had traveled abroad, presented IgG anti-HEV (Supplementary Table 1). Geographical location of the positive individuals among and around the city showed no specific distribution pattern, and no difference was observed in the HEV seroprevalence according to the neighborhood (Fig. 1). None of the 3 IgG anti-HEV positive samples were reactive for IgM anti-HEV. All samples resulted negative for RNA-HEV detection.
From the 11 samples belonging to patients with acute hepatitis (group b), one of them, from a female 18-years-old patient, resulted positive to IgM anti-HEV detection, with negative results for IgG anti-HEV and RNA-HEV. It is worth mentioning that this patient showed positive result for IgM anti-Epstein Barr virus (EBV). The remaining 10 samples (of group b) were negative for all HEV markers tested.
After performing the IgM analysis, the crude seroprevalence obtained for this marker was 7.14 % (1/14) (95 % CI: 0.37–35.83 %) and an adjusted seroprevalence of 5.35 % (95 % CI: 0.00–33.10 %).
4. Discussion
In this study we explored HEV infection in children aged 0–18 years old living in Córdoba city, an urban area of central Argentina, providing updated and novel data about HEV infection in this population group for our country. We found an IgG anti-HEV prevalence of 1.49 %, similar to that previously described in children from other regions of Argentina (0.15 % and 1.7 %), including a rural area [8,9], suggesting that the urban/rural environment would not be determinant in the circulation of the virus in our region. This low HEV seroprevalence found is also in line with the few studies performed in children in Latin America [3]. Some of them documented prevalences of 1.2 % in Chile [15], 1.7 % in Bolivia [16], 1.1 % in Colombia [3] and 1.6 % in Brazil [17], while others reported slightly higher seroprevalence rates of 4.5 % in Mato Grosso State in Brazil [18], or 3 % and 3.9 % in Mexico [19,20]. Although the different diagnostic kits used could be a factor of variability in the reported prevalences, in all studies low circulation values were recorded in the pediatric population.
In this study, the highest HEV seroprevalence was found in the 15–18 years old group (3.9 %), and the patient with IgM anti-HEV was 18 years old, showing that infections may occur at older ages, and not mainly in childhood, as occurs in the case of hepatitis A virus (HAV). This finding is consistent with previous studies conducted in different countries, which have demonstrated an age-related increase in HEV seroprevalence, even among children and adolescents. A study involving a pediatric population of Germany documented an increase in HEV prevalence from 0.4 % in infants younger than two years, to 1.5 % in adolescents of 15–17 years of age [21]. Another study from Mexico also showed this trend, revealing an increase in seroprevalence from 1.1 % among children aged 1–4 years to 9.6 % in the 15–19 years old age group [20]. This age-related increase in seroprevalence might be attributed to greater exposure to the virus as individuals grow older. However, this trend should be confirmed by analyzing a larger number of samples.
Several factors associated with HEV infection have been described worldwide, including Latin America, such as contact with pigs, consumption of pork meat, travel to endemic regions, living in rural areas or low levels of education [3,22]. Nevertheless, in this study, no other variable studied besides age (such as consumption of tap water, consumption of pork meat, contact with animals, presence of a cesspool or sewer at home, neighborhood, travel abroad, blood transfusion or immunosuppression) was found to be related to the presence of anti-HEV IgG, in agreement with previous reports in children [3]. However, the number of samples for which these epidemiological data is available is limited (n = 76), due to incomplete data collection by health personnel, which constitutes a limitation of our work.
From the group of patients with acute hepatitis, only one sample (18-years-old female patient) tested positive for IgM anti-HEV, although without detection of specific IgG or RNA-HEV. Unfortunately, subsequent samples of the patient, to detect the appearance of IgG anti-HEV, or stool samples, to increase the possibility of RNA-HEV detection, were unavailable. This case corresponded to an immunocompetent individual with acute hepatitis, with no prior history of liver disease, and a positive result for IgM anti-EBV as well. Two hypotheses can be considered for this case. Firstly, it could be a false positive result for IgM anti-HEV, since cross-reactivity has been previously observed between HEV, EBV and CMV IgM results. In such cases, the search of nucleic acids and IgG anti-HEV is recommended to achieve a conclusive diagnosis [23,24]. However, in our study, the IgM anti-HEV S/CO value was 9 (sample value was 9 times higher than the cut off), providing evidence that it might be a true positive. Taking this into account, the second hypothesis is coinfection with both viruses (HEV and EBV), which could have resulted in a symptomatic infection (than the usual asymptomatic HEV infection), as the patient presented elevated liver enzymes. In this sense, a previous case of HEV and HAV coinfection was registered in a 1-year-old boy from Salta province, in the north region of Argentina, who presented fulminant hepatic failure and died [25]. Hence, considering both hypotheses, it would be important to inform and alert the healthcare professional team about the silent circulation of HEV and the possibility of: a-symptomatic infections when they occur as coinfections with other agents or in patients with underlying pathologies, as previously described [26]; and b-cross-reactions with other agents (such as EBV and CMV) that can affect the diagnosis of hepatitis E. Therefore, the presence of more than one marker, in combination with clinical features and PCR analysis when appropriate, is strongly recommended for an accurate diagnosis [24,26].
Our work presents some limitations: the number of samples obtained was relatively small, mainly from cases of acute hepatitis without diagnosis. This is because sampling in the pediatric population is difficult to access, since children are considered a vulnerable population. Furthermore, the number of samples for which epidemiological data was available was even smaller, limiting robust conclusions. Another limitation was the time of sampling in the acute hepatitis group (these were not early samples), which could influence the negative results for RNA-HEV.
5. Conclusions
In conclusion, we found a low HEV prevalence among children from an urban area of Argentina. Although previous studies have shown that this virus circulates in the region [5,7], infection in children appears to be rare and generally asymptomatic. However, it is crucial to take this agent into consideration since it could cause symptomatic or more severe cases of acute hepatitis when it occurs as a coinfection with other infectious agents or in individuals with underlying pathologies. In this context, the search for HEV in cases of acute hepatitis or severe hepatitis would be recommended.
Data availability statement
Data associated with this study have not been deposited into a publicly available repository since all the information generated is included in the manuscript and supplementary material.
Ethics approval
The study was carried out in compliance with the regulations of the Declaration of Helsinki and Good Clinical Practices, respecting the confidentiality of the subjects, and was approved by the Ethics Committee of the Hospital Infantil Municipal, Repis N°184. Informed consent was obtained from all individual participants included in the study (and from their parents).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Lorena L. Cervella: Writing – original draft, Investigation, Formal analysis, Conceptualization. Cecilia García Oro: Investigation. Anabella C. Fantilli: Investigation, Formal analysis. Guadalupe Di Cola: Investigation, Formal analysis. Maribel G. Martínez Wassa: Methodology, Investigation. Gabriela Oropeza: Investigation. Paola Sicilia: Writing – review & editing, Investigation, Formal analysis, Conceptualization. Gonzalo M. Castro: Methodology, Investigation. Maria Belén Pisano: Writing – original draft, Supervision, Project administration, Conceptualization. Viviana E. Ré: Writing – review & editing, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We appreciate and thank the predisposition and collaboration of the staff of the Laboratory of Hospital Infantil Municipal de Córdoba for the collection of samples, and the staff of the Central Laboratory of the Province of Córdoba for their collaboration in the search for data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32284.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Pisano M.B., Giadans C.G., Flichman D.M., Ré V.E., Preciado M.V., Valva P. Viral hepatitis update: progress and perspectives. World J. Gastroenterol. 2021;27(26):4018–4044. doi: 10.3748/wjg.v27.i26.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Fact sheet hepatitis E. July 2023. https://www.who.int/news-room/fact-sheets/detail/hepatitis-e
- 3.Fernández Villalobos N.V., Kessel B., Torres Páez J.C., Strömpl J., Kerrinnes T., de la Hoz Restrepo F.P., Strengert M., Krause G. Seroprevalence of hepatitis E virus in children and adolescents living in urban Bogotá: an explorative cross-sectional study. Front. Public Health. 2023;11 doi: 10.3389/fpubh.2023.981172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lui T., He Q., Yang X., Li Y., Yuan D., Lu Q., Tang T., Guan G., Zheng L., Zhang H., Xia C., Yin X., Wei G., Chen X., Lu F., Wang L. An immunocompetent Mongolian gerbil model for hepatitis E virus genotype 1 infection. Gastroenterology. 2024 doi: 10.1053/j.gastro.2024.03.038. in press. [DOI] [PubMed] [Google Scholar]
- 5.Pisano M.B., Martinez-Wassaf M.G., Mirazo S., Fantilli A., Arbiza J., Debes J.D., Ré V.E. Hepatitis E virus in South America: the current scenario. Liver Int. 2018;38:1536–1546. doi: 10.1111/liv.13881. [DOI] [PubMed] [Google Scholar]
- 6.De Oliveira J.M., Dos Santos D.R.L., Pinto M.A. Hepatitis E virus research in Brazil: looking back and forwards. Viruses. 2023;15(2):548. doi: 10.3390/v15020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fantilli A.C., Trinks J., Marciano S., Zárate F., Balderramo D.C., Wassaf M.M., Haddad L., Gadano A., Debes J., Pisano M.B., Ré V.E. Unexpected high seroprevalence of hepatitis E virus in patients with alcogol-related cirrhosis. PLoS One. 2019;4(10) doi: 10.1371/journal.pone.0224404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rey J.A., Findov J.A., Daruich J.R., Velaxco C.C., Igavtua E.B., Schmee E., Kohan A. Prevalence of IgG anti-HEV in Buenos Aires, a nonendemic area for hepatitis E. J. Trav. Med. 1997;4(2):100–101. doi: 10.1111/j.1708-8305.1997.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 9.Martínez A.P., Pereson M.J., Perez P.S., Baecjk P.M., Saubidet I.L., Di Lello F.A. Prevalence of hepatitis E virus in children from Northeast of Argentina. J. Med. Virol. 2021;93:4015–4017. doi: 10.1002/jmv.26274. [DOI] [PubMed] [Google Scholar]
- 10.Jothikumar N., Cromeans T.L., Robertson B.H., Meng X.J., Hill V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol Methods. 2006;131:65–71. doi: 10.1016/j.jviromet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 11.R Core Team . R Foundation for Statistical Computing; Vienna, Austgria: 2023. R: A Language and Environment for Statistical Computing.https://www.r-project.org/ [Google Scholar]
- 12.Lang Z., Reiczigel J. Confidence limits for prevalence of disease adjusted for estimated sensitivity and specificity. Prev. Vet. Med. 2014;113:13–22. doi: 10.1016/j.prevetmed.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Fay M.P. Asht: applied statistical hypothesis tests. 2022. https://cran.r-project.org/package=asht Available online at:
- 14.Posit team . PBC; Boston, MA: 2023. RStudio: Integrated Development Environment for R. Posit Software.http://www.posit.co/ URL. [Google Scholar]
- 15.Ibarra H., Riedemann S., Toledo C. Hepatitis A and E virus antibodies in Chilean children of low socioeconomic status: a one-year follow-up study. Rev. Med. Chile. 2006;134(2):139–144. doi: 10.4067/s0034-98872006000200001. [DOI] [PubMed] [Google Scholar]
- 16.Gandolfo G.M., Ferri G.M., Conti L., Antenucci A., Marrone R., Frasca A.M., Vitelli G. Prevalence of infections by hepatitis A, B, C, and E viruses in two different socioeconomic groups of children from Santa Cruz, Bolivia. Med. Clin. 2003;120:725–727. doi: 10.1016/s0025-7753(03)73826-3. [DOI] [PubMed] [Google Scholar]
- 17.Silva G.R.D.C.E., Martins T.L.S., Silva C.A., Caetano K.A.A., Carneiro M.A.D.S., Silva B.V.D.E., Pacheco L.R., Villar L.M., Paula V.S., Martins R.M.B., Teles S.A. Hepatitis A and E among immigrants and refugees in Central Brazil. Rev. Saude Publica. 2022;56:29. doi: 10.11606/s1518-8787.2022056003839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assis S.B., Souto F.J.D., Fontes C.J.F., Gaspar A.M.C. Prevalence of hepatitis A and E virus infection in school children of an Amazonian municipality in Mato Grosso State. Rev. Soc. Bras. Med. Trop. 2002;35:155–158. doi: 10.1590/s0037-86822002000200005. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Santaella T., Álvarez y Muñoz T., Madeiros-Domingo M., Moreno-Espinosa S., Sanchez A.C., Muñoz-Hernández O., Sarmiento-Silvia R.E., Sotomayor-Gonzalez A., Trujillo-Ortega M.E., García-Hernández M.E., Taboada-Ramírez B.I., Arenas-Huertero F. Serological and molecular study of hepatitis E virus in pediatric patients in Mexico. Ann. Hepatol. 2020;19:295–301. doi: 10.1016/j.aohep.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Alvarez-Muñoz M.T., Torres J., Damasio L., Gómez A., Tapia-Conyer R., Muñoz O. Seroepidemiology of hepatitis E virus infection in Mexican subjects 1 to 29 years of age. Arch. Med. Res. 1999;30:251–254. doi: 10.1016/s0188-0128(99)00019-6. [DOI] [PubMed] [Google Scholar]
- 21.Krumbholz A., Neubert A., Joel S., Girschick H., Huppertz H.I., Kaiser P., Liese J., Streng A., Niehues T., Peters J., Sauerbrey A., Schroten H., Tenenbaum T., Wirth S., Zell R., Sauerbrei A. Prevalence of hepatitis E virus antibodies in children in Germany. Pediatr. Infect. Dis. J. 2014;33(3):258–262. doi: 10.1097/INF.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 22.Li P., Liu J., Li Y., Su J., Ma Z., Bramer W.M., Cao W., de Man R.A., Peppelenbosch M.P., Pan Q. The global epidemiology of hepatitis E virus infection: a systematic review and meta-analysis. Liver Int. 2020;40:1516–1528. doi: 10.1111/liv.14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fogeda M., de Ory F., Avellón A., Echevarría J.M. Differential diagnosis of hepatitis E virus, cytomegalovirus and Epstein-Barr virus infection in patients with suspected hepatitis E. J. Clin. Virol. 2009;45:259–261. doi: 10.1016/j.jcv.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Hyams C., Mabayoje D.A., Copping R., Maranao D., Patel M., Labbett W., Haque T., Webster D.P. Serological cross reactivity to CMV and EBV causes problems in the diagnosis of acute hepatitis E virus infection. J. Med. Virol. 2014;86:478–483. doi: 10.1002/jmv.23827. [DOI] [PubMed] [Google Scholar]
- 25.Munné M.S., Altabert N.R., Vladimirsky S.N., Moreiro R., Otegui Mares L.S., Soto S.S., Brajterman L.S., Castro R.E., Gonzalez J.E. Identifications of polyphyletic variants in acute hepatitis suggest an underdiagnosed circulation of hepatitis E virus in Argentina. J. Clin. Virol. 2011;52:138–141. doi: 10.1016/j.jcv.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 26.European Association for the Study of the Liver EASL clinical practice guidelines on hepatitis E virus infection. Elsevier B.V. J Hepatol. 2018;68j:1256–1271. doi: 10.1016/j.jhep.2018.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study have not been deposited into a publicly available repository since all the information generated is included in the manuscript and supplementary material.