Abstract
Current in vitro biofilm modelling of the opportunistic pathogen, Pseudomonas aeruginosa (PA) in people with cystic fibrosis (PwCF) is limited in its ability to mimic the complexities of the cystic fibrosis (CF) lung environment. Recent adaptations of the Microbial Identification after Passive CLARITY Technique (MiPACT) in CF research have allowed for the direct imaging of PA biofilm spatial organization and structure in expectorated sputum. Here, we performed a comparative analysis of in vitro and within patient (ex vivo) measures of PA biofilms using sputa from new onset infected children with CF. MiPACT-fluorescent in situ hybridization (FISH) and fluorescent anti-Psl monoclonal antibody (mAb) staining was performed to directly visualize PA and Psl (exopolysaccharide in PA biofilm matrix) in 11 CF sputum specimens. Corresponding PA isolates, recovered from the same sputum samples, were grown as biofilms in a glass slide chamber model, then visualized by fluorescent live-cell and anti-Psl mAb staining. We observed that PA biovolume, aggregation and Psl antibody binding (normalized per PA biovolume) in CF sputum did not correlate with the in vitro model, although a trend towards significance in the biovolume relationship was observed with the addition of sputum supernatant to the in vitro model.
There is a clear disconnect between the results of in vitro testing of antimicrobials against bacterial pathogens and clinical outcomes in people with cystic fibrosis (pwCF), in part due to the challenge of replicating the complexities of cystic fibrosis (CF) airways in vitro [1,2]. The opportunistic pathogen, Pseudomonas aeruginosa (PA) is among the most studied bacterium in CF pulmonary infection and is often used in the design and testing of in vitro biofilm systems. Decades of research have focused on developing models of PA biofilm-associated lung infection with systems ranging from simple microtiter and minimum biofilm eradication concentration (MBEC) assays to complex flow cell and slide chamber models that generate three dimensional-biofilm structures of PA [3,4]. To mimic the conditions of the CF lung environment, adaptations of standardized in vitro models have also emerged, in which PA biofilms are cultivated on top of CF epithelial cell lines [5] in various formulations of synthetic [6] and pooled [7,8] CF sputa. Further advancements in biofilm modelling, including the use of bacteria-embedded gels [[9], [10], [11]] and encapsulating beads [12,13], have shown comparable imitation of PA biofilm structures to that described within pwCF (i.e., visualized in animal replicas, sputum smears and explanted lungs). These non-surface attached biofilms are substantially smaller compared to in vitro biofilm imitations and do not exhibit the classical mushroom-like structure [3,14].
Although in vitro assays have progressively contributed to the current knowledge of biofilm physiology, antimicrobial resistance, and drug development, very few investigations have examined the accuracy of these models, at an individual patient level, compared to direct in-patient observations of PA biofilm architecture and structure. The advent of novel methods for directly visualizing communities of bacteria within sputum, using a technique known as Microbial Identification after Passive CLARITY Technique (MiPACT), allows for such comparisons [15]. MiPACT is a tissue-clearing procedure, in which the specimen is first fixed in a bis-acrylamide gel matrix to maintain structural stability, then incubated in a sodium dodecyl sulfate (SDS) detergent to remove lipids—a major source of light diffraction in fluorescent microscopy [16]. Subsequently, fluorescence in situ hybridization (FISH) with species-specific rRNA probes can be performed to identify bacteria, as well as immunofluorescence staining to label other structural components within the sample [17]. The goal of our study was to utilize MiPACT-FISH and fluorescent monoclonal antibody (mAb) staining to determine the degree of correlation between ex vivo measures of PA biofilms in sputum from children with CF at the time of initial infection, compared to PA biofilms measured in vitro in a glass slide chamber when the same PA isolates were grown in the presence of sputum supernatant. Making direct comparisons between in vitro and ex vivo observations at an individual patient level is important given the known genotypic and phenotypic diversity of PA strains among pwCF.
1. Methods
This was a secondary analysis of a prospective, observational study of children with CF followed at SickKids Hospital (Toronto, Canada) who had a new onset PA infection; defined as a sputum culture positive for PA with at least 3 preceding negative cultures in the prior 12 months [7].
Expectorated sputum specimens (n = 11) were collected before the patients started antibiotic treatment with inhaled tobramycin. One half of each paraformaldehyde fixed sputum was polymerized in a hydrogel mixture, then cleared using MiPACT as previously described [7,15,17]. In brief, sputum plugs (approximately 0.2 g) were fixed in 4 % paraformaldehyde (PFA), then added to individual wells of a Nunc Lab-Tek eight-chamber cover glass slide (Thermo Fisher Scientific, Mississauga, ON) containing 250 μl of hydrogel mixture. The sputum hydrogel mixtures (within the chamber wells) were sealed in a BD GasPak EZ container (VWR, Mississauga, ON) with an anaerobic pack, then allowed to polymerize for 3 h at 37 °C. Following polymerization, the sputum hydrogels were cleared in 8 % SDS solution for 3–5 days at 37 °C, then stored in 0.01 % (wt/vol) sodium azide solution at 4 °C until imaging.
Ex vivo visualization of PA and Psl (exopolysaccharide in PA biofilm matrix) in the sputum hydrogels was performed using a fluorescent in situ hybridization (FISH) and antigen-antibody binding technique [7]. FISH was first performed on 1 mm diameter gel slices using the 16S rRNA, PseaerA probe (GGT AAC CGT CCC CCT TGC) specific to PA [18] and conjugated to Alexa488 (green). Psl antibody staining was then performed using the anti-Psl mAb, Psl0096 conjugated to Alexa594 (magenta). A total of 3 biological and 18 technical replicates of z-stack images were captured per sputum sample via confocal laser scanning microscopy (CLSM) with a 20× objective lens. Representative z-stack images in extended focus view were captured using a 100× oil objective lens.
PA was recovered from the remaining portion of sputum, and the isolates were grown in vitro as 48 h biofilms in Lysogeny Broth (LB) alone or LB with pooled 5 % sputum supernatant using our glass slide chamber model, previously described [4, 19]. The 5 % sputum supernatant was pooled from 10 randomly selected, pediatric sputa (comprised of excreted cellular products but filtered to remove host or other bacterial cells) [7]. The formed biofilms were then visualized by CLSM using the anti-Psl mAb, Psl0096 (magenta) and SYTO9 live-cell fluorescent (green) stain. A total of 3 biological and 18 technical replicates of z-stack images were captured per well with a 20× objective lens.
All z-stack images were analyzed using Volocity 6.3 to quantify PA biovolume (μm [3]) and anti-Psl mAb binding (total and per 100,000 μm3 of PA biovolume) [7, 20]. Comstat2 as a plugin for ImageJ was used to quantify surface-to-biovolume ratio (μm2/μm3), which is the ratio between surface area and PA volume, with lower values indicating greater PA aggregation [20,21]; also described as densely clustered bacterial cells [22,23]. All statistical comparisons were done using the GraphPad Prism software with Spearman correlation test; p-value <0.05 considered significant.
2. Results
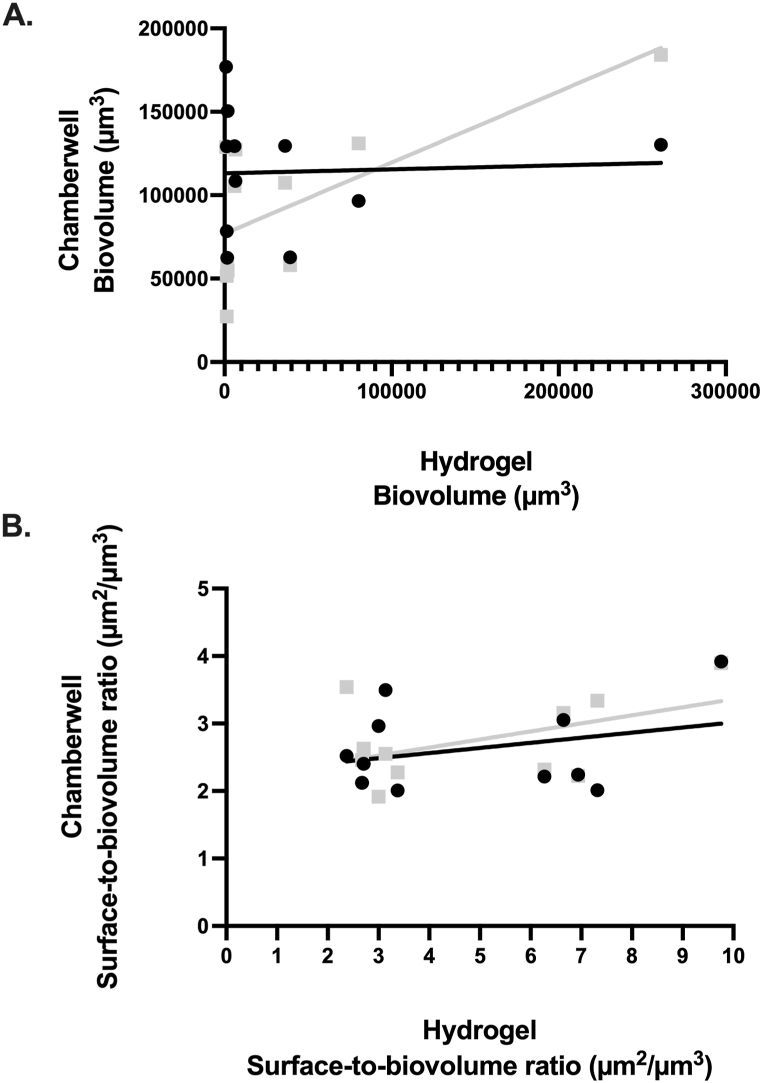
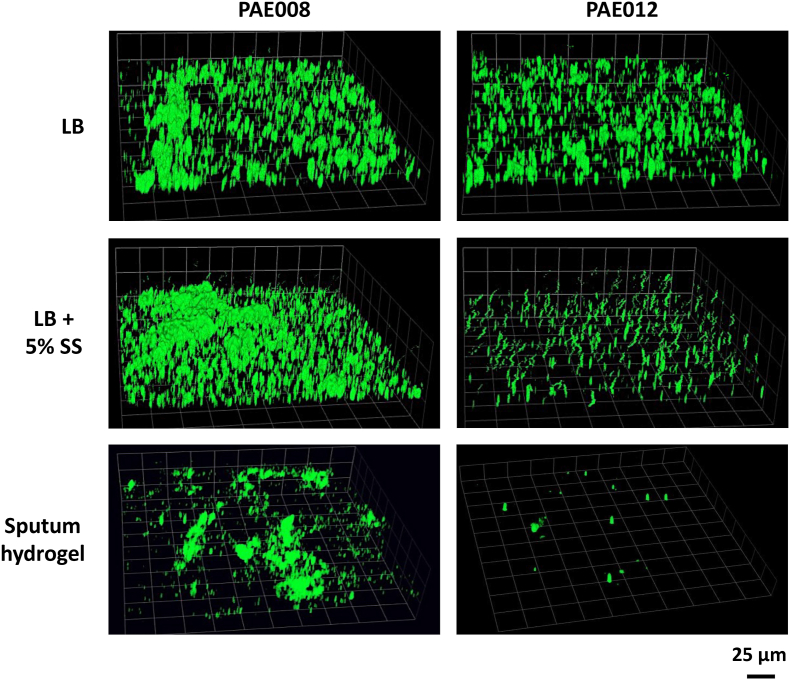
Comparisons of PA biovolume and aggregation (represented by surface-to-biovolume ratio) between the two assays are shown in Fig. 1. PA biovolume measured ex vivo in the sputum hydrogels compared to the in vitro, slide chamber model with recovered isolates grown in LB, showed no significant correlation (p = 0.75; r = −0.11). However, when PA was grown in the chamber wells in LB with 5 % sputum supernatant, the in vitro model showed a trend toward statistical significance with the ex vivo biovolume measures (p = 0.051; r = 0.6) (Fig. 1A). CLSM representative images of two sputum hydrogels (PAE008 and PAE012) and corresponding PA isolates are provided in Fig. 2, showing improved biovolume imitation between the bioassays with the addition of 5 % sputum supernatant to the in vitro model compared to LB alone. Measures of PA aggregation (surface-to-biovolume ratio) showed no significant correlation ex vivo compared to in vitro in LB alone or in LB with 5 % sputum supernatant (Fig. 1B).
Fig. 1.
Comparative analysis of ex vivo hydrogel model and in vitro chamber well model in LB alone (black circles) and in LB with 5 % sputum supernatant (grey squares). Individual circles/squares represent comparison of the mean value attained from 3 biological and 18 technical replicates from each bioassay. No significant correlation shown between measures of PA (A) biovolume in LB alone (r = −0.11, p = 0.75) or in LB with 5 % sputum supernatant (r = 0.6, p = 0.051), (B) surface-to-biovolume ratio in LB alone (r = −0.08, p = 0.82) or in LB with 5 % sputum supernatant (r = 0.16, p = 0.63). Surface-to-biovolume ratio is a measure of aggregation with lower values indicating greater number of aggregates [[20], [21], [22], [23]].
Fig. 2.
CLSM representative images of PA visualized in sputum hydrogels (PAE008 and PAE012) and corresponding PA isolates, recovered from the same sputum, then grown in a slide chamber well in LB alone and LB with 5 % sputum supernatant. The FISH PseaerA (green) probe and the SYTO9 (green) fluorescent nucleic acid stain were used to visualize PA in the sputum hydrogels and the chamber wells, respectively. Sputum hydrogel and chamber well images were captured with 20× objective.
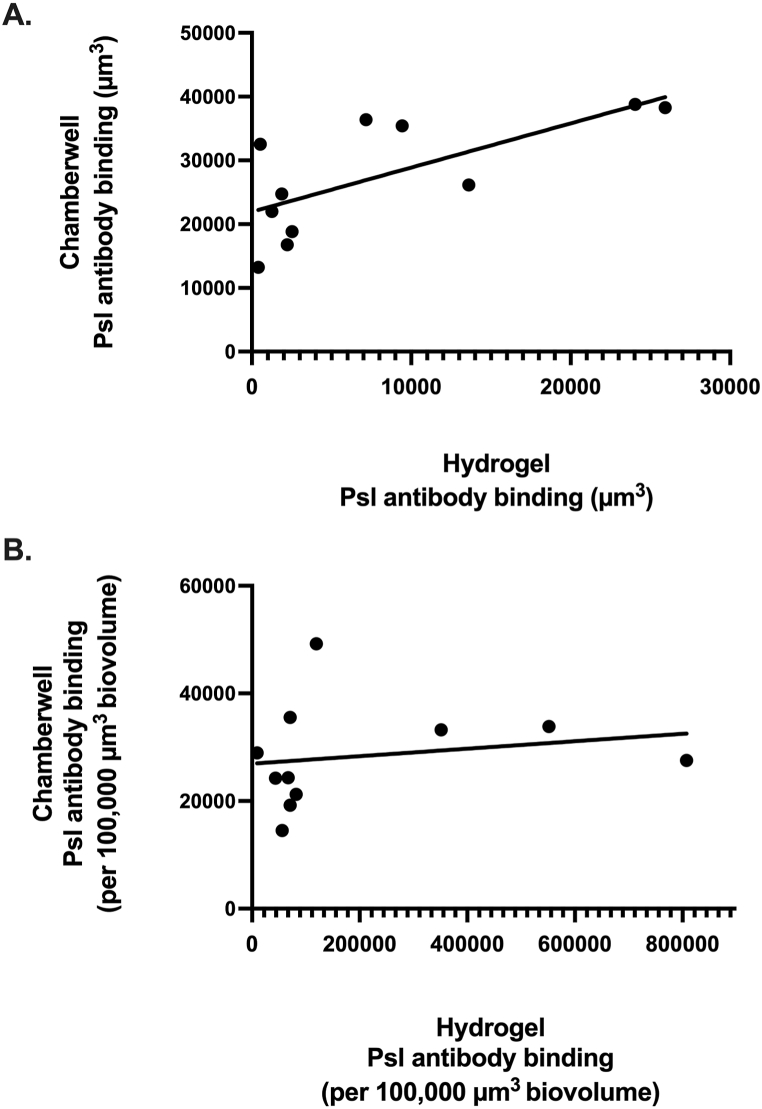
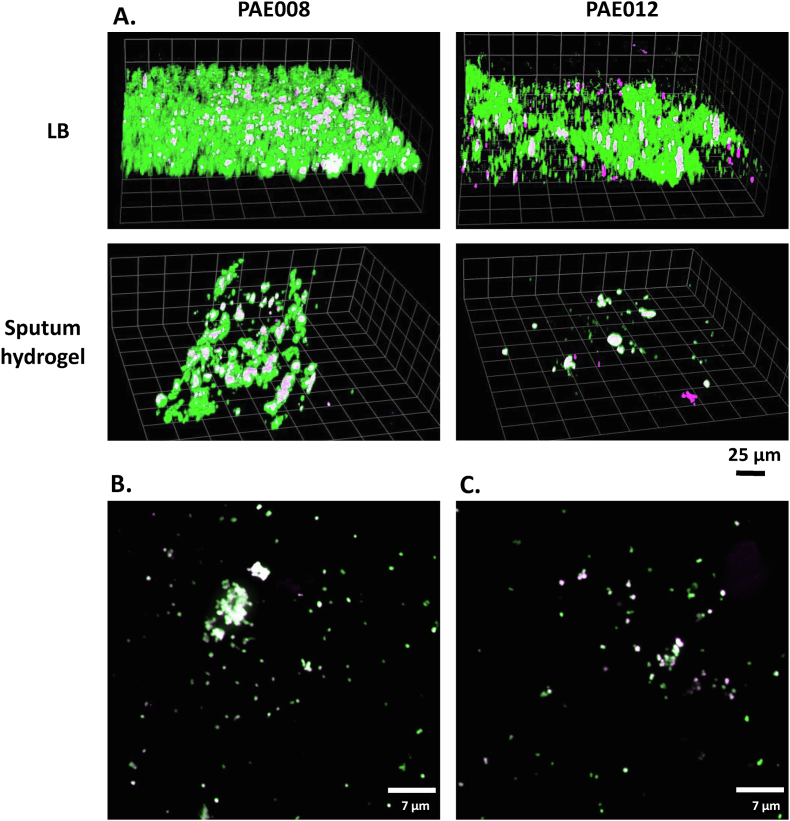
Comparison of Psl antibody binding between the two assays in LB alone is shown in Fig. 3. Although Psl antibody binding was significantly correlated with a strong linear relationship (p = 0.01; r = 0.73) between the ex vivo and in vitro models (Fig. 3A), there was no significant correlation when it was normalized per 100,000 μm3 of PA biovolume (Fig. 3B). CLSM images of the two representative sputum hydrogels and corresponding PA isolates are provided in Fig. 4A, showing a positive relationship, in both assays, between Psl antibody binding and the amount of PA biovolume present. Extended focus views of sputum hydrogels, PAE008 and PAE012, show large and numerous PA aggregates with high Psl antibody binding (Fig. 4B) and fewer PA aggregates with low biovolume and Psl antibody binding (Fig. 4C), respectively. CLSM images of all 11 sputum hydrogels and PA isolates under the varied conditions are provided in Fig. S1 and Fig. S2 (in Supplementary Material).
Fig. 3.
Psl antibody binding in the ex vivo sputum hydrogels and in vitro chamber well model. Individual circles represent comparison of the mean value attained from 3 biological and 18 technical replicates from each bioassay. (A) A significant correlation observed in Psl antibody binding between the hydrogel and chamber well models (r = 0.73, p = 0.01), (B) no significance correlation between the models when normalized per 100,000 μm3 of biovolume (r = 0.45, p = 0.17). Images captured with 20× objective.
Fig. 4.
CLSM representative images of PA and Psl visualized in (A) sputum hydrogels (PAE008 and PAE012) and corresponding PA isolates, recovered from the same sputum, then grown in a slide chamber well in LB alone. Images captured with 20× objective. Extended focus view of sputum hydrogels: (B) PAE008 showing large PA aggregates with high PA biovolume and high Psl antibody binding and (C) PAE012 showing fewer PA aggregates with low biovolume and low Psl antibody binding. Images captured with 100× oil objective. The FISH PseaerA (green) probe and the SYTO9 (green) fluorescent nucleic acid stain were used to visualize PA in the sputum hydrogels and chamber wells, respectively. The fluorescently labelled anti-Psl mAb, Psl0096 (magenta) was used to visualize Psl in both bioassays. White indicating areas of PA-Psl colocalization.
3. Discussion
Correlation between in vitro and in-patient model systems of biofilm-related infections is imperative for elucidating mechanisms of CF lung disease and ensuring efficacy of new (and existing) antimicrobials. In the present study, we directly visualized PA ex vivo in CF sputum measuring biovolume, aggregation and Psl antibody binding, and found little correlation to those of recovered isolates grown, in vitro, as biofilms.
There was no significant correlation between the in vitro and ex vivo models in terms of biovolume and aggregation. With the addition of sputum supernatant to the in vitro model, however, there was a trend towards statistical significance in the biovolume relationship, suggesting that mimicking CF lung growth conditions in vitro results in a better correlation, albeit moderate (r = 0.6), between the models. There are likely isolates that have growth advantages (or disadvantages) in a sputum milieu [9]. The lack of statistical significance may also have been influenced by the small sample size. With respect to aggregation (measured by surface-to-biovolume ratio), the addition of sputum supernatant generated minimal improvements to the correlation between the models. This may be due to the fact that the chamber well model, containing borosilicate cover glass, promotes surface-attached PA biofilm growth in the form of uniform layers whereas within CF sputa, PA appears planktonic or as non-surface adherent aggregates [7,[24], [25], [26]]. It is well documented that PA does not attach to epithelia in CF lungs [3,14]; thus, the stark difference in aggregation between the surface-attached biofilms and the suspended PA aggregates within sputum is as expected.
A significant correlation in total Psl antibody binding was determined between the models. Psl polymers visualized in surface-attached PA biofilms have shown to resemble non-attached PA aggregates when grown in vitro [27]. In the present study, once adjusted for biovolume, the Psl antibody binding correlation was no longer significant, primarily due to greater biovolumes (and thus lower Psl antibody binding/100,000 μm3 biovolume) in the glass slide chamber compared to within sputum aggregates (with higher Psl antibody binding/biovolume).
There were several limitations to this study. Only a single morphotype of PA recovered from CF sputum, was used for biofilm growth in the chamber wells. This approach does not truly reflect the intra-clonal genotypic and phenotypic diversity of biofilms visualized in CF sputum [28,29]. Additionally, PwCF may have a higher bacterial load either due to greater inoculum at the time of acquisition, more rapid bacterial growth in specific CF lung environments, or longer duration of growth prior to time of detection. None of these factors could be accounted for in our in vitro model but are important given the known inoculum effect.
In conclusion, in our secondary analysis of new onset PA infection in children with CF, we highlight the challenges of replicating aspects of in-patient PA biofilm communities in vitro. Given the weak correlation between model systems, translating in-patient observations to in vitro assays rather than vice versa, may lead to more clinically relevant research. The MiPACT methodology allows for the direct visualization of bacteria and host cells ex vivo and quantification of a wide variety of biogeographical parameters over large spatial scales. The resultant data can provide a foundation for infection-relevant in vitro model system design and can ultimately further our understanding of CF airway infections and response to antimicrobial treatment.
Ethical declaration statement
This study was approved by the SickKids Research Ethics Board (REB#1000079038); written informed consent was obtained from all participants/guardians for research and the publication of any images, clinical data and other data included in the manuscript, and the study was conducted in accordance with all relevant guidelines and regulations.
Data availability statement
All images that support the findings of this study are available in Supplementary Materials as Fig. S1 and Fig. S2.
CRediT authorship contribution statement
Amanda J. Morris: Writing – review & editing, Writing – original draft, Software, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Yvonne CW. Yau: Writing – review & editing, Supervision, Methodology, Investigation, Data curation, Conceptualization. William H. DePas: Writing – review & editing, Resources, Methodology. Valerie J. Waters: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Valerie Waters reports financial support was provided by Cystic Fibrosis Foundation. Valerie Waters reports financial support was provided by Cystic Fibrosis Canada. Amanda Morris reports financial support was provided by Cystic Fibrosis Canada. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was funded by the Cystic Fibrosis Foundation (CFF WATERS19AO-1) and Glyconet (ID-03). A.J.M. was funded by a CF Canada Fellowship. We thank Shafinaz Eisha, Research Technologist for assistance with compiling the Supplementary Material.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32424.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Waters V.J., Kidd T.J., Canton R., Ekkelenkamp M.B., Johansen H.K., LiPuma J.J., et al. Reconciling antimicrobial susceptibility testing and clinical response in antimicrobial treatment of chronic cystic fibrosis lung infections. Clin. Infect. Dis. 2019;69:1812–1816. doi: 10.1093/cid/ciz364. [DOI] [PubMed] [Google Scholar]
- 2.Somayaji R., Parkins M.D., Shah A., Martiniano S.L., Tunney M.M., Kahle J.S., et al. Antimicrobial susceptibility testing (AST) and associated clinical outcomes in individuals with cystic fibrosis: a systematic review. J. Cyst. Fibros. 2019;18:236–243. doi: 10.1016/j.jcf.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Bjarnsholt T., Alhede M., Eickhardt-Sørensen S.R., Moser C., Kühl M., Jensen P.Ø., et al. The in vivo biofilm. Trends Microbiol. 2013;21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Beaudoin T., Yau Y.C.W., Stapleton P.J., Gong Y., Wang P.W., Guttman D.S., et al. Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms Microbiomes. 2017;3:25. doi: 10.1038/s41522-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juntke J., Murgia X., Gunday Türeli N., Türeli A.E., Thorn C.R., Schneider M., et al. Testing of aerosolized ciprofloxacin nanocarriers on cystic fibrosis airway cells infected with P. aeruginosa biofilms. Drug Deliv Transl Res. 2021;11:1752–1765. doi: 10.1007/s13346-021-01002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer K.L., Aye L.M., Whiteley M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 2007;189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris A.J., Yau Y.C.W., Park S., Eisha S., McDonald N., Parsek M.R., et al. Pseudomonas aeruginosa aggregation and Psl expression in sputum is associated with antibiotic eradication failure in children with cystic fibrosis. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-25889-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben Hur D., Kapach G., Wani N.A., Kiper E., Ashkenazi M., Smollan G., et al. Antimicrobial peptides against multidrug-resistant Pseudomonas aeruginosa biofilm from cystic fibrosis patients. J. Med. Chem. 2022;65:9050–9062. doi: 10.1021/acs.jmedchem.2c00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staudinger B.J., Muller J.F., Halldórsson S., Boles B., Angermeyer A., Nguyen D., et al. Conditions associated with the cystic fibrosis defect promote chronic Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2014;189:812–824. doi: 10.1164/rccm.201312-2142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsui H., Wagner V.E., Hill D.B., Schwab U.E., Rogers T.D., Button B., et al. A physical linkage between cystic fibrosis airway surface dehydration and Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA. 2006;103:18131–18136. doi: 10.1073/pnas.0606428103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boboltz A., Yang S., Duncan G.A. Engineering in vitro models of cystic fibrosis lung disease using neutrophil extracellular trap inspired biomaterials. J. Mater. Chem. B. 2023;11:9419–9430. doi: 10.1039/d3tb01489d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sønderholm M., Kragh K.N., Koren K., Jakobsen T.H., Darch S.E., Alhede M., et al. Pseudomonas aeruginosa aggregate formation in an alginate bead model system exhibits in vivo-like characteristics. Appl. Environ. Microbiol. 2017;83(17) doi: 10.1128/AEM.00113-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres B.G.S., Awad R., Marchand S., Couet W., Tewes F. In vitro evaluation of Pseudomonas aeruginosa chronic lung infection models: are agar and calcium-alginate beads interchangeable? Eur. J. Pharm. Biopharm. 2019;143:35–43. doi: 10.1016/j.ejpb.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Kragh K.N., Tolker-Nielsen T., Lichtenberg M. The non-attached biofilm aggregate. Commun. Biol. 2023;6:898. doi: 10.1038/s42003-023-05281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DePas W.H., Starwalt-Lee R., Van Sambeek L., Ravindra Kumar S., Gradinaru V., Newman D.K. Exposing the three-dimensional biogeography and metabolic states of pathogens in cystic fibrosis sputum via hydrogel embedding, clearing, and rRNA labeling. mBio. 2016;7(16) doi: 10.1128/mBio.00796-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang B., Treweek J.B., Kulkarni R.P., Deverman B.E., Chen C.K., Lubeck E., et al. Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell. 2014;158:945–958. doi: 10.1016/j.cell.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson L., DePas W., Morris A.J., Guttman K., Yau Y.C.W., Waters V. Visualization of Pseudomonas aeruginosa within the sputum of cystic fibrosis patients. J. Vis. Exp. 2020 doi: 10.3791/61631. [DOI] [PubMed] [Google Scholar]
- 18.Hogardt M., Trebesius K., Geiger A.M., Hornef M., Rosenecker J., Heesemann J., et al. Rapid detection by fluorescent in situ hybridization of bacteria in clinical samples obtained from cystic fibrosis patients. J. Clin. Microbiol. 2000;38:818–825. doi: 10.1128/jcm.38.2.818-825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaudoin T., Stone T.A., Glibowicka M., Adams C., Yau Y., Ahmadi S.…Deber C.M. Activity of a novel antimicrobial peptide against Pseudomonas aeruginosa biofilms. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-33016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris A.J., Jackson L., Yau Y.C.W., Reichhardt C., Beaudoin T., Uwumarenogie S., et al. The role of Psl in the failure to eradicate Pseudomonas aeruginosa biofilms in children with cystic fibrosis. NPJ Biofilms Microbiomes. 2021;7:63. doi: 10.1038/s41522-021-00234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris A.J., Li A., Jackson L., Yau Y.C.W., Waters V. Quantifying the effects of antimicrobials on in vitro biofilm architecture using COMSTAT software. J. Vis. Exp. 2020 doi: 10.3791/61759. [DOI] [PubMed] [Google Scholar]
- 22.Ramos I., Dietrich L.E., Price-Whelan A., Newman D.K. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res. Microbiol. 2010;161:187–191. doi: 10.1016/j.resmic.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinandan C.S., Jadav V., Cecilia D., Nerurkar A.S. Nutrients determine the spatial architecture of Paracoccus sp. biofilm. Biofouling. 2010;26:449–459. doi: 10.1080/08927011003739760. [DOI] [PubMed] [Google Scholar]
- 24.Bjarnsholt T., Jensen P.O., Fiandaca M.J., Pedersen J., Hansen C.R., Andersen C.B., et al. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 2009;44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 25.Worlitzsch D., Tarran R., Ulrich M., Schwab U., Cekici A., Meyer K.C., et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrd M.S., Pang B., Hong W., Waligora E.A., Juneau R.A., Armbruster C.E., et al. Direct evaluation of Pseudomonas aeruginosa biofilm mediators in a chronic infection model. Infect. Immun. 2011;79:3087–3095. doi: 10.1128/IAI.00057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alhede M., Kragh K.N., Qvortrup K., Allesen-Holm M., van Gennip M., Christensen L.D., et al. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mowat E., Paterson S., Fothergill J.L., Wright E.A., Ledson M.J., Walshaw M.J., et al. Pseudomonas aeruginosa population diversity and turnover in cystic fibrosis chronic infections. Am. J. Respir. Crit. Care Med. 2011;183:1674–1679. doi: 10.1164/rccm.201009-1430OC. [DOI] [PubMed] [Google Scholar]
- 29.Clark S.T., Diaz Caballero J., Cheang M., Coburn B., Wang P.W., Donaldson S.L., et al. Phenotypic diversity within a Pseudomonas aeruginosa population infecting an adult with cystic fibrosis. Sci. Rep. 2015;5 doi: 10.1038/srep10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All images that support the findings of this study are available in Supplementary Materials as Fig. S1 and Fig. S2.