Trauma and orthopaedics have seen many changes over the past 20 years. The next 20 promise even greater advances, particularly in the areas of materials science, computer aided manufacturing technology, and molecular biology. In addition to technical advances there have been changes in the provision of care, such as the greater subspecialisation of surgeons and improvements in the organisation of trauma and oncology services. Perhaps the most important change is the increase in evidence based practice which uses studies of patients' outcomes.
Methods
It has been difficult to select specific advances to focus on within such a diverse specialty. We have tried to identify developments in common areas of practice, such as trauma, paediatrics, and joint replacement surgery. A computer based literature search and search of abstracts of presentations at conferences was used to identify the evidence that supports the changes made in current practice. Some of the advances, such as the use of flexible nailing in children's fractures, are already well established in some countries. Others, such as using gene therapy to enhance the repair of fractures, are still at the laboratory stage but have the potential to revolutionise practice. The era of the “orthopaedic molecular biologist” may soon be a reality.
Treating younger patients
Autologous chondrocyte transplantation
Articular cartilage is vulnerable to injury and has poor potential for repair so damage can lead to arthritis many years after injury.1,2 In elderly people, joint replacement surgery has revolutionised the treatment of arthritis, but the management of damage to articular cartilage in young patients remains a problem.
The ideal treatment would replace damaged articular cartilage, restore joint function, and prevent the development of arthritis. Fibrocartilage is unable to withstand the high mechanical loads within a joint and only hyaline cartilage, which has predominantly type II collagen, has the potential for good, long term results. Transplantation of hyaline cartilage has been used for a number of years, but there are few sites where donor articular cartilage can be harvested without damaging the joint. Thus, only small articular defects can be treated with this method. However, a new patented technique allows small amounts of hyaline cartilage to be harvested, the chondrocytes extracted, and the cell population increased in tissue culture. The number of cells increases by about 15 times over four weeks. These cells can then be reimplanted beneath a periosteal patch which is sutured over the articular defect.
Recent advances
Autologous chondrocyte transplantation is being used in some centres to provide a better quality of life for and to prevent arthritis in younger patients who have had damage to their articular cartilage
Internal fixation of long bone fractures in children reduces the time spent in hospital
Botulinum toxin is useful in treating muscle spasticity in children with cerebral palsy
Metal on metal hip replacement prostheses can give good results, and new designs may allow routine hip replacement in middle aged patients
In 1994 Brittberg reported on a series of 23 patients who had had full thickness articular surface defects treated using this technique. Two years later, 14 of 16 had good or excellent results, and biopsies showed the repair to be hyaline-like in appearance with predominantly type II collagen.3 Based largely on this success, the US Food and Drug Administration granted a licence to Genzyme Tissue Repair in 1997 for Carticel autologous cultured chondrocytes. Genzyme has patented the method of culturing the isolated cells. Their register now includes information on repairs to over 1000 knees (www.genzymebiosurgery.com/). The longest published follow up is nine years, and biopsies taken at that time showed no signs of ossification within the grafts.1 Independent centres are now undertaking similar programmes of cartilage cell culture, but it will be some time before the technique has had adequate follow up, possible side effects have been minimised, the best results have been consistently reproduced, and the procedure becomes widely available.4
This is an exciting approach to a difficult problem and could result in a great improvement in the quality of life of active young people. The same technology may one day be used to grow a patient's own cartilage cells on a collagen template to create new menisci that can be surgically implanted.5 In England and Wales, the National Institute for Clinical Excellence recently evaluated this new technology and recommended that autologous cartilage transplantation should continue to be evaluated by prospective research (www.nice.org.uk/cat.asp?c=13461). If further studies confirm its potential, then these advances could represent an important step towards ultimately preventing osteoarthritis and avoiding later joint replacement.
Flexible nailing of long bone fractures
Femoral fractures in children have traditionally been treated with traction and hospital stays of 4-12 weeks depending on the child's age. Earlier hospital discharge is possible if a plaster spica is used, but these are uncomfortable and difficult for children and parents to manage. Flexible nailing of the femur—first developed by specialist centres in continental Europe—allows early mobilisation. Flexible nails produced from new metal alloys are small enough to fit the intramedullary canal in children but are also able to maintain their shape after contouring and are strong enough to provide stable fixation. Two nails with opposing curves are inserted to provide maximum stability. They are inserted percutaneously through a 5 mm incision (fig 1).
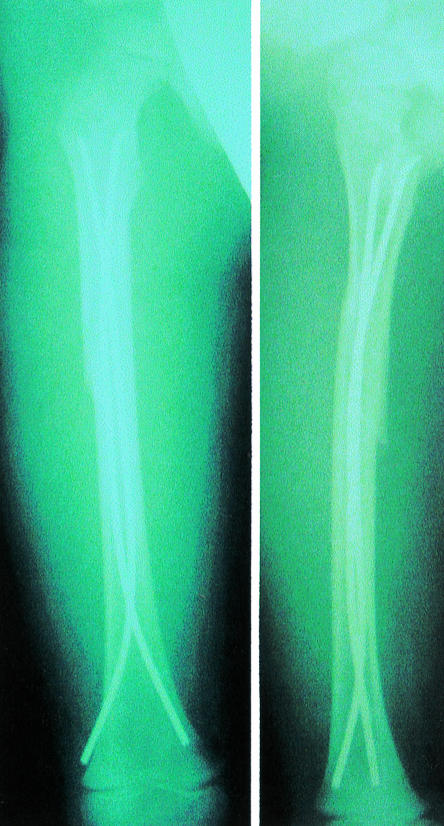
Figure 1.
x Ray picture of flexible nails used in a 6 year old with a fractured femur (left, anteroposterior; right, lateral)
The largest reported series of patients treated with flexible nails was published earlier this year and included 308 patients.6They had had no serious complications. The length of time spent in hospital was reduced: indeed children with femoral fractures, who would otherwise have spent several weeks in traction, were able to return to school within 15-20 days. Compared with external fixation, flexible nailing allows earlier weight bearing, movement, and return to school, in addition to fewer complications.7 The indications for flexible nailing have now been extended to include unstable fractures of the tibia and forearm. The results are equivalent to plating but with better cosmesis, ease of removal, and shorter time spent under anaesthesia.8–10 The nails are removed after union of the fracture and patients are seen as day cases. Most hospitals caring for children's fractures are likely to introduce this technique over the next few years. Indeed, it is already available in some district general hospitals in the United Kingdom where consultants have received training in the technique. The website of the Association for the Study of Internal Fixation, which is based in Switzerland, has information about devices used to treat different types of fractures (www.ao-asif.ch/).
Botulinum toxin for cerebral palsy
Cerebral palsy affects 2 to 3 children in every 1000 births. About 70-80% of these children have severe muscle spasticity, resulting in an imbalance across joints which causes deformity. When this involves the calf muscles it leads to talipes equinus and impairs the ability of these children to walk. Botulinum toxin has recently been licensed in the United Kingdom for the treatment of this condition and has proved to be a useful adjunct to physiotherapy, splints, and surgery.11 It is also licensed for use in Europe, the United States, and Australia.
Botulinum toxin type A is derived from Clostridium botulinum. It is a neurotoxin with high affinity and specificity for binding to the presynaptic membranes of cholinergic neurones, preventing the release of acetylcholine into the synaptic cleft. Injection of the toxin into muscle results in a dose dependent, chemical denervation of the muscle. New nerve terminals sprout, rendering the process reversible, but clinical effects last up to 12 weeks.
Treatment is most effective in patients with dynamic muscle shortening that is localised to a few muscle groups. Younger patients benefit more, perhaps because fibrous contractures have not had time to develop. Patients can be treated as outpatients. Side effects are rare but include pain at the injection site. The number of injections depends on the muscle groups affected and their response.11,12
The use of botulinum toxin type A reduces the stiffness of muscles making them more amenable to stretching. This allows better ankle movement, increases the strength of antagonist muscles, and results in a considerable improvement in walking.12,13 Botulinum toxin type A is as effective as serial casting and costs a similar amount but has fewer side effectsand the improvements last longer.14
Treating adults
Metal on metal prostheses for hip replacement
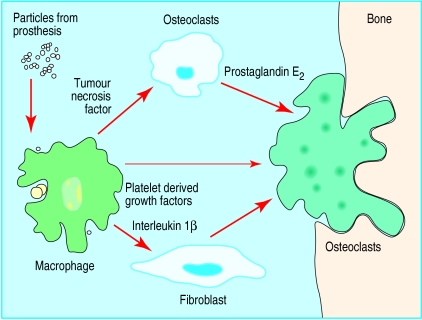
Total hip replacement is the treatment of choice for arthritis in elderly people. The most commonly used prostheses are metal femoral components with plastic acetabular cups. The greatest long term problem after hip replacement is loosening of the components which results in bone loss and pain. This often requires complex revision surgery. This is one of the most important factors that restricts the use of total hip replacement among middle aged patients. The problems are caused by small plastic particles produced by the wearing of the cup.10 Activated macrophages attempt to clear the particles, and this results in an inflammatory reaction that produces foreign body giant cells; chemical mediators then activate osteoclasts which resorb bone from around the prosthesis producing bone cysts and loosening (fig 2 and fig 3). An understanding of the mechanism behind this type of loosening has stimulated research into the potential use of other materials, such as ceramics and metal on metal hips.
Figure 2.
Loosening and pain after hip replacement are caused by the release of small plastic particles that stimulate macrophages. These activate osteoclasts which resorb bone around the prosthesis
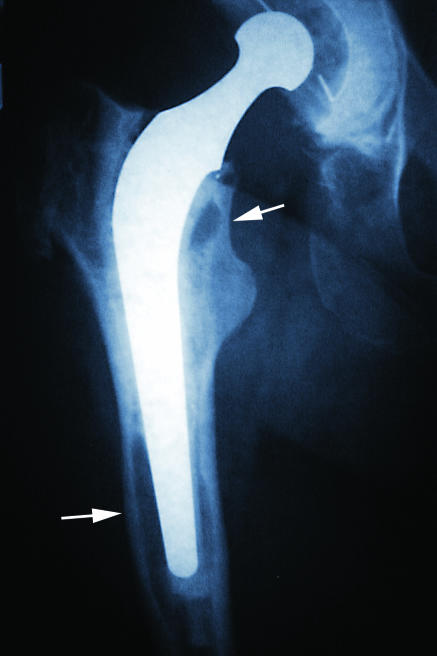
Figure 3.
x Ray picture of hip replacement. Arrows show resorption around the tip of the prosthesis and a cystic area just below the neck
Metal on metal hips were first used in the 1950s but the results were largely disappointing, and the metal on plastic hip superseded them. However, a small number of patients had remarkable results from their metal on metal hips: these devices survived for more than 40 years in pristine condition. It is now thought that by chance these patients had perfectly matched ball and socket joints and so wear particles were kept to a minimum.
Advances in the aviation industry allow precise surfaces to be manufactured for the turbines of jet engines. Computerised manufacturing techniques are now being used to produce a second generation of metal on metal replacement hips with precisely matched articulating surfaces. These prostheses have been used in Europe since 1988, and after extensive trials are now in use in the United States. Over 100 000 of these replacement hips have been implanted.
In a review of the volumetric wear of retrieved prostheses, metal on metal hips had up to 60 times less wear than metal on plastic prostheses. Most wear occurred in the first year, and this was reduced by a factor of five after three years.15 Researchers have suggested that the metal surfaces have a degree of “self polishing” and smooth out their own scratches over time. Artificial testing stations have confirmed that the wear of metal on metal produces 100 times less debris than metal on plastic.16The metal particles produced are also much smaller than plastic ones, and this results in less cellular reaction.15,17 Particles about 0.2-0.8 μm in size stimulate macrophage activation; the benefit of metal on metal may result from the smaller size of particles rather than the volume of wear.18The new generation of metal on metal prostheses represents an important advance in the treatment of osteoarthritis. However, their introduction must be closely controlled. In England and Wales guidelines from the National Institute for Clinical Excellence must be applied and properly instituted and controlled trials undertaken. (Further information about the history of metal on metal hips can be found at www.metasul.com, where there is an extensive list of relevant literature.)
Gene therapy in fracture healing
The fracture of a bone initiates a complex healing cascade similar to that in other tissues: haemostasis, inflammation, and infiltration of granulation tissue, followed by callus formation and remodelling. The process may be influenced by systemic factors, such as nutrition and calcium balance, and local factors, such as blood supply and the mechanical stability of the fracture. Delayed healing of fractures causes disability and has considerable socioeconomic effects resulting from the loss of work and the cost of treatment. Advances in molecular biology have improved our understanding of the complex chemical mediators responsible for the healing cascade. Manipulation of these mediators has the potential to enhance fracture healing and accelerate recovery.
Bone morphogenic proteins and transforming growth factor β enhance fracture healing. Other factors, such as insulin-like growth factor II and platelet derived growth factor, are also important. Bone morphogenic proteins regulate chemotaxis, mitosis, and differentiation, and are fundamental in initiating fracture repair.19 Transforming growth factor β is 100 times more concentrated in bone than in other tissues, and osteoblasts have a high concentration of receptors for it. The activity of transforming growth factor β is not fully understood but the net effect is an increase in bone matrix.20The use of these growth factors may stimulate fracture repair and minimise the rate of non-union.19,20They could be delivered to the fracture site by direct injection, and bone defects could be filled with a collagen or hydroxyapatite matrix containing these factors to stimulate new bone formation. Unfortunately these proteins have short biological half lives and must be maintained at therapeutic concentrations at the fracture site to be effective.
Additional educational resources
Selected review articles
Graham HK, Aoki KR, Autti-Ramo I, Boyd RN, Delgado MR, Gaebler-Spira DJ, et al. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait and Posture 2000;11:67-79
Minas T, Nehrer S. Current concepts in the treatment of articular cartilage defects. Orthopaedics 1997;20:525-38
Websites
Information for clinicians
American Academy of Orthopaedic Surgeons and American Association of Orthopaedic Surgeons—www.aaos.org
Provides information about devices used to treat different types of fractures; has many links to educational resources
Wheeless' Textbook of Orthopaedics—www.medmedia.com
Comprehensive trauma and orthopaedic textbook; easy to search
WorldMedicus/WorldOrtho—www.worldortho.com
Contains a database of lectures, a section on important articles, and information on emerging topics; also includes discussion forum and advice for doctors
Information for patients
Arthritis Foundation—www.arthritis.org
Information for patients and their families
Mayo Clinic—www.mayohealth.org
Information for patients and the general public
Developments in gene therapy may allow the gene encoding for a given growth factor to be isolated and transferred to a recipient cell using a viral or non-viral vector. The recipient cell might then produce the growth factor at the fracture site and concentrations may be able to be maintained for an extended time.21 In one animal study gene expression was detected in a fracture for up to six weeks, which is certainly sufficient time to accelerate repair.22 These techniques have been tested on both small and large animals and they have accelerated bone formation and fracture union.23
Similar techniques could also be used for tissue engineering. It may become possible to mould spare muscle with a vascular pedicle to the shape of a specific bone. Treatment with growth factors may then transform muscle to woven bone. The new vascularised bone might then be transplanted to fill a bone defect, and in the correct biomechanical environment it may have the potential to mature into cortical bone. These recent developments may herald a revolution in reconstructive surgery that will be based on molecular biology and tissue engineering.24
Footnotes
Competing interests: None declared.
References
- 1.Minas T, Nehrer S. Current concepts in the treatment of articular cartilage defects [Review] Orthopedics. 1997;20:525–538. doi: 10.3928/0147-7447-19970601-08. [DOI] [PubMed] [Google Scholar]
- 2.Messner K, Maletius W. The long-term prognosis for severe damage to weight bearing cartilage in the knee. Acta Orthop Scand. 1996;67:165–168. doi: 10.3109/17453679608994664. [DOI] [PubMed] [Google Scholar]
- 3.Brittberg M, Lindahl A, Nilsson A. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 4.Bentley G, Minas T. Treating joint damage in young people. BMJ. 2000;320:1585–1588. doi: 10.1136/bmj.320.7249.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vransky P, Bourdelat D, Al Faour A. Flexible intramedullary pinning technique in the treatment of paediatric fractures. J Pediatr Orthop. 2000;20(1):23–27. [PubMed] [Google Scholar]
- 6.Bar-On E, Sagiv S, Porat S. External fixation or flexible intramedullary nailing for femoral shaft fractures in children: a prospective, randomised study. J Bone Joint Surg Br. 1997;79:975–978. doi: 10.1302/0301-620x.79b6.7740. [DOI] [PubMed] [Google Scholar]
- 7.Van der Reis WL, Otsuka NY, Moroz P, Mah J. Intramedullary nailing versus plate fixation for unstable forearm fractures in children. J Pediatr Orthop. 1998;18:9–13. [PubMed] [Google Scholar]
- 8.Richter D, Ostermann AW, Ekkernkamp A, Muhr G, Hahn MP. Elastic intramedullary nailing: a minimally invasive concept in the treatment of unstable forearm fractures in children. J Pediatr Orthop. 1998;18:457–461. [PubMed] [Google Scholar]
- 9.Luhmann SJ, Gordon JE, Schoenecker PL. Intramedullary fixation of unstable both-bone forearm fractures in children. J Pediatr Orthop. 1998;18:451–456. [PubMed] [Google Scholar]
- 10.Willert HG, Bertram H, Buchhorn GH. Osteolysis in alloarthroplasty of the hip: the role of ultra-high molecular weight polyethylene wear particles. Clin Orthop. 1990;258:95–107. [PubMed] [Google Scholar]
- 11.Graham HK, Aoki KR, Autti-Ramo I, Boyd RN, Delgado MR, Gaebler-Spira DJ, et al. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy [Review] Gait and Posture. 2000;11:67–79. doi: 10.1016/s0966-6362(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 12.Koman LA, Mooney JF, Smith BP, Walker F, Leon JM. Botulinum toxin type A neuromuscular blockade in the treatment of lower extremity spasticity in cerebral palsy: a randomized, double-blind, placebo-controlled trial. J Pediatr Orthop. 2000;20:108–115. [PubMed] [Google Scholar]
- 13.Boyd RN, Pliatsios V, Starr R, Wolfe R, Graham HK. Biomechanical transformation of the gastroc-soleus muscle with botulinum toxin A in children with cerebral palsy. Dev Med Child Neurol. 2000;42:32–41. doi: 10.1017/s0012162200000074. [DOI] [PubMed] [Google Scholar]
- 14.Corry IS, Cosgrove AP, Duffy CM, McNeill S, Taylor TC, Graham HK. Botulinum toxin A compared with stretching casts in the treatment of spastic equinus: a randomised prospective trial. J Pediatr Orthop. 1998;18:304–311. [PubMed] [Google Scholar]
- 15.Seiber HP, Rieker CB, Kottig P. Analysis of 118 second-generation metal-on-metal retrieved hip implants. J Bone Joint Surg Br. 1999;81:46–50. doi: 10.1302/0301-620x.81b1.9047. [DOI] [PubMed] [Google Scholar]
- 16.Anissian HL, Stark A, Gustafson A, Good V, Clarke IC. Metal-on-metal bearing in hip prosthesis generates a 100-fold less wear debris than metal-on-polythene. Acta Orthop Scand. 1999;70:578–582. doi: 10.3109/17453679908997845. [DOI] [PubMed] [Google Scholar]
- 17.Goldsmith AA, Dowson D, Isaac GH, Lancaster JG. A comparative joint simulator study of the wear of metal-on-metal and alternative material combinations in hip replacements. Proc Inst Mech Eng [H] 2000;214:39–47. doi: 10.1243/0954411001535228. [DOI] [PubMed] [Google Scholar]
- 18.Ingham E, Fisher J. Biological reactions to wear debris in total joint replacement. Proc Inst Mech Eng [H] 2000;214:21–37. doi: 10.1243/0954411001535219. [DOI] [PubMed] [Google Scholar]
- 19.Reddi AH. Initiation of fracture repair by bone morphogenic proteins. Clin Orthop. 1998;355(suppl):S66–S72. doi: 10.1097/00003086-199810001-00008. [DOI] [PubMed] [Google Scholar]
- 20.Bostrom MPG, Asnis P. Transforming growth factor beta in fracture repair. Clin Orthop. 1998;355(suppl):S124–S131. doi: 10.1097/00003086-199810001-00014. [DOI] [PubMed] [Google Scholar]
- 21.Niyibizi C, Baltzer A, Lattermann C, Oyama M, Whalen JD, Robbins PD, et al. Potential role for gene therapy in the enhancement of fracture healing. Clin Orthop. 1998;355(suppl):S148–S153. doi: 10.1097/00003086-199810001-00016. [DOI] [PubMed] [Google Scholar]
- 22.Baltzer A, Lattermann C, Whalen JD, Braunstein S, Robbins PD, Evans CH. A gene therapy approach to accelerating bone healing. Evaluation of gene expression in a New Zealand white rabbit model. Knee Surg Sports Traumatol Arthrosc. 1999;7:197–202. doi: 10.1007/s001670050147. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein SA, Bonadio J. Potential role for direct gene transfer in the enhancement of fracture healing. Clin Orthop. 1998;355(suppl):S154–S162. doi: 10.1097/00003086-199810001-00017. [DOI] [PubMed] [Google Scholar]
- 24.Oreffo R, Triffitt JT. Future potentials for using osteogenic stem cells and biomaterials in orthopaedics. Bone. 1999;25(suppl):S5–S9. doi: 10.1016/s8756-3282(99)00124-6. [DOI] [PubMed] [Google Scholar]