Abstract
To begin a successful infection, viruses must first cross the host cell plasma membrane, either by direct fusion with the membrane or by receptor-mediated endocytosis. After release into the cytoplasm those viruses that replicate in the nucleus must target their genome to that location. We examined the role of cytoplasmic transport of the canine parvovirus (CPV) capsid in productive infection by microinjecting two antibodies that recognize the intact CPV capsid into the cytoplasm of cells and also by using intracellular expression of variable domains of a neutralizing antibody fused to green fluorescence protein. The two antibodies tested and the expressed scFv all efficiently blocked virus infection, probably by binding to virus particles while they were in the cytoplasm and before entering the nucleus. The injected antibodies were able to block most infections even when injected 8 h after virus inoculation. In control studies, microinjected capsid antibodies did not interfere with CPV replication when they were coinjected with an infectious plasmid clone of CPV. Cytoplasmically injected full and empty capsids were able to move through the cytosol towards the nuclear membrane in a process that could be blocked by nocodazole treatment of the cells. Nuclear transport of the capsids was slow, with significant amounts being found in the nucleus only 3 to 6 h after injection.
The infection of cells by viruses is a multistep process that requires that the virus pass through a series of barriers so as to deliver the genome into the appropriate region of the cell for replication. The early steps of virus entry into cells involve attachment to the cell surface, followed by penetration of the virion or its components into the cytoplasm either by direct fusion with the plasma membrane or after uptake into the endocytic pathway. During uptake in endosomes the release of enveloped virus nucleocapsids to the cytoplasm is in many cases associated with structural changes of viral proteins triggered by exposure to an acidic pH or binding to cellular receptors. Fusion of the viral envelope releases the viral genome and other components into the cytoplasm (reviewed in reference 19, 30, and 32 to 34).
Less is known about the mechanisms by which nonenveloped viruses enter a host cell, although many of those viruses use interactions between the hydrophobic portions of the outer capsid proteins or of lipid-conjugated proteins to allow the particle to penetrate the cellular membranes (33, 34). Nonenveloped viruses may enter the cell through either pH-dependent pathways (e.g., picornaviruses, adenoviruses, reoviruses, and parvoviruses [6, 20, 36, 39, 40, 44, 45, 51]) or pH-independent pathways (papovaviruses or poliovirus [27, 47]), and some may also directly penetrate the plasma membrane (rotaviruses [25]).
Nuclear replicating viruses must pass through the cytoplasm to access the nuclear pore or the nucleus (28, 55). Although it has been assumed that this was a largely passive process, it is now clear that the intracytosolic movements of adenovirus and herpes simplex virus 1 capsids use the active transportation system of the cell, mediated by the microtubule cytoskeleton, to move through the cytoplasm to the vicinity of the nuclear pore (49, 50). Polyomavirus virions are endocytosed via small monopinocytotic vesicles derived from the plasma membrane, and then it is suggested that they enter the nuclei via direct fusion of the vesicles with the nuclear membrane (21). The slow caveola-mediated entry of simian virus 40 (SV40) particles into cells appears to be followed by targeting of virions to the endoplasmic reticulum, where the viruses induce the formation of interconnected tubular smooth membrane structures (2, 9, 27). However, mechanisms by which the virion or viral genome is translocated from the endoplasmic reticulum to the nucleus for replication are not known. It appears that the SV40 capsids enter the cytoplasm of cells during infection, as infection can be blocked by intracytoplasmic injection of antibodies against VP1 or VP3 (38). Microinjected SV40 particles in the cytoplasm of cells were imported into the nucleus within 1 h, and they infected the cell, indicating that trafficking through the cytoplasm is part of the infectious pathway (10).
Before the genome of the incoming virion can replicate, it must be released from its capsid, and uncoating may occur at one or more of several sites in the cell, ranging from the cell surface to the nuclear matrix (19, 20, 22, 29). For viruses that replicate in the nucleus, the genome and possibly some associated proteins must enter that compartment. Most viruses utilize the nuclear import system of the cell, including the nuclear pore complex, receptors, and import factors (33, 55). The nuclear pore has an effective diameter of about 26 nm when Xenopus oocytes are injected with coated gold beads (13). Larger viruses appear to localize to the nuclear pore and then to release their DNA for transport to the nucleus associated with specific viral proteins (18, 26, 49). SV40 particles within the nuclear pore appeared to be about 21 to 24 nm in diameter, while those seen within the nucleus had a diameter of 38.2 nm, suggesting that the capsid structure is distorted during nuclear import (56).
Autonomous parvoviruses have a nonenveloped 25-nm-diameter capsid that contains a single-stranded DNA genome. Canine parvovirus (CPV) is a member of the feline parvovirus subgroup, which includes several host range variants that infect carnivores. CPV enters cells by receptor-mediated endocytosis into clathrin-coated vesicles (42). CPV infection can be prevented by lysosomotropic bases, indicating that infection involves an acidic intracellular compartment (6), and temperature- and microtubule-dependent transport steps are also required (53). CPV capsids associated with cells appear not to be degraded (54). However, the mechanisms of penetration of CPV from the endosomes into the cytoplasm and nuclear targeting of the virus or its genome prior to replication are still poorly understood. In previous studies only a small proportion of CPV capsids injected into the cytoplasm of cells were seen to enter the nucleus within 1 or 2 h (53, 54). CPV capsids added to cells are detected only in endosomes for several hours (6, 42, 53, 54). Adeno-associated virus (AAV) capsids are taken into cells in a dynamin-dependent endocytic process (12), and labeled capsids added to cells are seen in the nucleus (4, 5). Up to 50% of the input single-stranded DNA-AAV capsids may be filled in after uptake into some cells (14, 15).
Here we examine the steps in CPV entry that occur between internalization via receptor-mediated endocytosis and nuclear replication of the virus. Both microinjection of monoclonal anti-capsid antibodies and intracellular expression of a single-chain antibody prevented CPV replication, showing that the capsid enters the cytosol during productive infection. Full or empty CPV capsids microinjected into the cytoplasm of cells accumulated in a perinuclear area and were mostly transported into the nucleus only after 3 to 6 h. Depolymerization of microtubules by nocodazole treatment of cells resulted in the capsids being found distributed throughout the cytoplasm, and this treatment also inhibited nuclear transport.
MATERIALS AND METHODS
Viruses and cells.
CPV type 2 (CPV-d) was grown in NLFK cells, and aliquots were stored frozen at −70°C (43). Empty and full CPV particles were purified and quantified as previously described and stored in 10 mM Tris-HCl (pH 7.5) at 4°C (1). NLFK cells were grown and maintained in a 1:1 mixture of McCoy's 5A medium and Leibovitz L15 medium with 5% fetal bovine serum (43). Virus-infected cells were detected by staining for the newly produced viral NS1 protein using a monoclonal antibody (MAb), obtained from Caroline Astell (57), conjugated to Texas red (TxR).
Antibodies and intracellular expression of single-chain antibody (scFv).
MAb 8 and MAb 14, which both recognize the intact CPV capsid, were purified from hybridoma culture supernatant by chromatography on protein G (54). Purified mouse immunoglobulin G (IgG) was obtained from Sigma (St. Louis, Mo.).
The variable domain sequences of MAb 8 light and heavy chain were cloned and expressed as a single-chain antibody (scFv) in bacteria as detailed by Yuan and Parrish (58), and that purified protein bound and efficiently neutralized CPV capsids. That scFv was recloned into the vector pEGFP-N (Clontech, Palo Alto, Calif.) so that it was fused at its C terminus with the enhanced green fluorescent protein (EGFP). Cells transfected with the plasmid expressing the scFv-EGFP fusion protein or with pEGFP-N alone were inoculated 24 h later with CPV and then incubated for a further 24 h. Cells were then incubated with 3.7% (wt/vol) paraformaldehyde in phosphate-buffered saline for 15 min, then with 0.1% Triton X-100 in phosphate-buffered saline and 1% (wt/vol) bovine serum albumin. The transfected cells were detected by EGFP expression, and the CPV-infected cells were detected by staining with anti-NS1-TxR antibody. After being washed, coverslips were mounted in Prolong medium (Molecular Probes, Eugene, Oreg.) and examined by fluorescence microscopy. The proportions of scFv-EGFP- or EGFP-expressing cells that became infected were compared to the infection of the nontransfected cells in the same culture.
Cytoplasmic microinjection of antibodies.
Purified MAb 8 and Mab 14 IgGs were dialyzed against 10 mM Tris-HCl–120 mM KCl (pH 7.4). Cells at 7 × 103 per cm2 on Microgrid coverslips (12-mm diameter, 175-μm grid size; Eppendorf, Hamburg, Germany) were injected with 0.1 to 0.5 pl of antibodies at 5 mg/ml using an Eppendorf 5246 Microinjector and Eppendorf 5171 Micromanipulator with Eppendorf capillaries. The cells were inoculated with CPV either before the time of microinjection or up to 12 h afterwards. The cells were then incubated for a total time after inoculation of 24 h before paraformaldehyde fixation. Fluorescein isothiocyante-labeled goat anti-mouse IgG was used to detect injected cells, while virus infection was detected using TxR-conjugated anti-NS1.
In control studies MAb 8 at 5 mg/ml was microinjected along with an infectious plasmid clone of CPV (43) at a concentration of 200 μg/ml, and the cells were analyzed by staining for IgG and viral NS1. The colocalization of mouse IgG with viral capsids was visualized using an FITC-labeled goat anti-mouse IgG and rabbit polyclonal anticapsid antibody, followed by TxR-conjugated anti-rabbit secondary antibody. Cells were examined with a confocal fluorescence microscope (Bio-Rad Laboratories, Hercules, Calif.). The proportion of cells injected with MAb 8 and plasmid that became infected was compared to the proportion of control mouse IgG-injected cells and also to the noninjected cells in the same culture.
Capsid microinjection and nocodazole treatment.
To further examine the distribution and transport of capsids within the cell, we injected full or empty CPV capsids at 2.5 mg/ml into the cytoplasm of cells and then incubated the cells at 37°C for 0, 1, 3, 6, 12, or 24 h. The cells were then fixed and permeabilized as described above and stained with MAb 8 (to detect the intact capsids) and with TxR-anti-NS1, and the cells were then examined by confocal microscopy. In some experiments the cells were incubated in medium containing 20 μM nocodazole for 1 h before microinjection; then, the drug was maintained thereafter for 1, 6, or 12 h before fixation in 3.7% paraformaldehyde. Some nocodazole-treated cells injected with capsids were first incubated with drug for 6 h and then washed and incubated in normal medium for an additional 6 h before fixation and staining were carried out as described above. Control cells were stained with antitubulin antibody (Amersham, Little Chalfont, Buckinghamshire, United Kingdom) to confirm the nocodazole affect on the microtubule structure.
RESULTS
Anticapsid antibodies in the cytoplasm and the nucleus of cells block CPV infection.
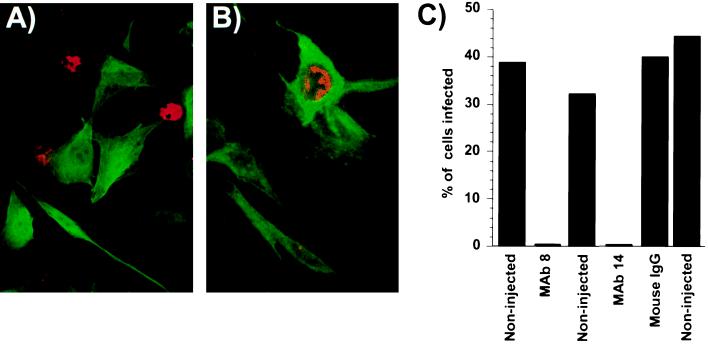
We microinjected two MAbs against assembled capsids into cells prior to CPV inoculation. The antibodies were seen throughout the cytoplasm and nucleus (Fig. 1A and B). In several independent experiments only between 0 and 0.35% of the MAb 8- or MAb 14-injected cells became infected and expressed NS1, while 32 to 45% of the noninjected cells in the same dishes became infected (Fig. 1C). The effect of the injected MAb 8 or MAb 14 was specific for the virus capsid and was not a nonspecific effect of microinjection on the cells, as control IgG injected into the cells did not affect virus infection (Fig. 1B and C).
FIG. 1.
Microinjection of cells with of MAb 8 or MAb 14, but not with control antibodies, resulted in the cells becoming resistant to infection. A) NLFK cells were injected with MAb 8 and then inoculated with CPV. The cells were dual stained for IgG (green) or for the viral NS1 protein (red). B) NLFK cells were injected with a control IgG and then inoculated with CPV. The cells were stained for IgG (green) or for the viral NS1 protein (red). C) The percentage of MAb 8- or MAb 14-injected cells infected in the various experiments compared to noninjected cells in the same dishes. Controls include NLFK cells injected with control mouse IgG.
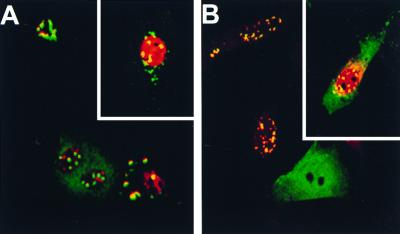
To determine whether the block to infection was due to a specific effect on the infecting capsid and not to a nonspecific effect on some later step in viral replication, we coinjected cells with MAb 8 and the infectious plasmid clone of CPV. In different experiments NS1 expression was seen in between 18 and 33% of the injected cells, not significantly different from values seen for plasmid microinjected alone in the same experiment (Fig. 2; results not shown). It is likely that the injected cells that did not show virus infection did not enter S phase during the time of incubation, preventing virus DNA replication. In MAb 8-injected and CPV-infected cells, the IgG appeared as small labeled aggregations which were either in the perinuclear cytoplasm or within the nucleus (Fig. 2A). Immunofluorescence staining using anti-mouse IgG to detect MAb 8 and polyclonal anticapsid antibodies confirmed that those spots represented aggregated viral capsids (Fig. 2B). In contrast, in noninfected cells diffuse immunofluorescence of the IgG was seen throughout the cytoplasm and the nucleus (Fig. 1 and 2).
FIG. 2.
CPV infection of cells microinjected simultaneously with MAb 8 (5 mg/ml) and the infectious plasmid clone of CPV (200 μg/ml). (A) MAb 8 detected using FITC-labeled goat anti-mouse IgG (green) and viral infection visualized with TxR-labeled anti-NS1 antibody (red). The insert shows another cell subjected to the same treatment. (B) Capsids were present within the aggregates seen in the cells. Injected MAb 8 was detected using FITC-labeled goat anti-mouse IgG (green), while capsids were detected using rabbit polyclonal anti-capsid IgG and TxR-conjugated anti-rabbit antibody (red). The insert shows another cell subjected to the same treatment.
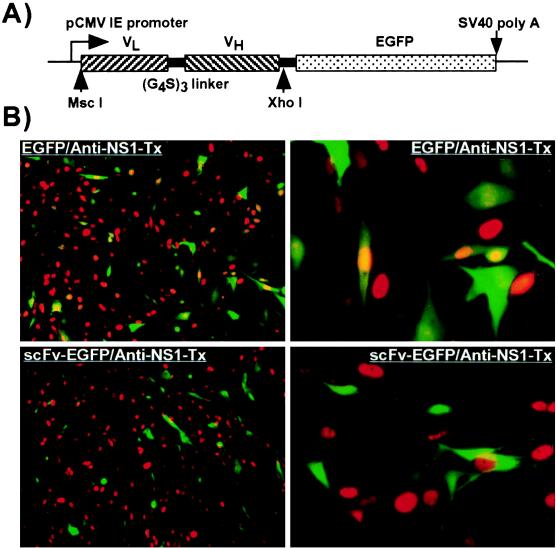
MAb 8 expressed as an scFv fused to EGFP accumulated in both the nucleus and the cytoplasm of the transfected NLFK cells. Cells which expressed the scFV-EGFP completely resisted infection (0% infection), while control cells expressing only EGFP showed normal levels of infection (20 to 30%) (Fig. 3).
FIG. 3.
(A) The insert in the plasmid expressing the variable domains of MAb 8 fused to EGFP. The light variable domain (VL) was linked to the heavy variable domain (VH) with three repeats of GlyGlyGlyGlySer sequence (G4S)3 and then fused in frame with EGFP. (B) Intracellular expression of the variable domain ScFv of MAb 8 and susceptibility to infection with CPV. Cells transfected with a plasmid expressing MAb 8 scFv fused to EGFP (scFv-EGFP) were inoculated with CPV. Control cells (EGFP) were transfected with a plasmid expressing EGFP alone. After a further 24 h of incubation, the cells were fixed, examined for GFP expression (green), and stained with a TxR-labeled anti-NS1 (red). Low- and high-magnification views of typical fields are shown in each case (to the left and right, respectively).
Antibody injected after virus inoculation blocks infection.
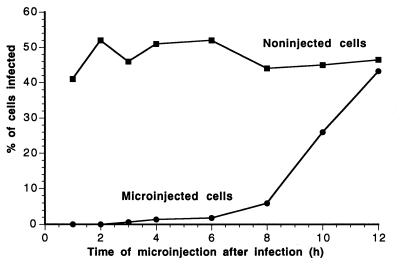
The kinetics of the blockade of CPV infection by antibody was examined by inoculating the cells with virus, injecting MAb 8 into the cytoplasm at various times afterwards, and then determining the proportions of infected cells (Fig. 4). A large increase in the proportion of virus-infected cells was observed only after 8 h, and by 12 h there was little effect on the rate of the infection (Fig. 4).
FIG. 4.
Effect of the timing of MAb 8 injection on infection by CPV. Cells incubated with CPV for 1 h at 0°C were warmed to 37°C and then injected with MAb 8 at various times between 1 and 12 h later. The percentage of cells that became infected after injection at the various times after warming was determined by staining for the injected MAb and for NS1 to indicate the virus infection. The percentage of infection of noninjected cells in the same cultures was used as a control.
Virus in the cytoplasm is transported into the nucleus after a period of delay.
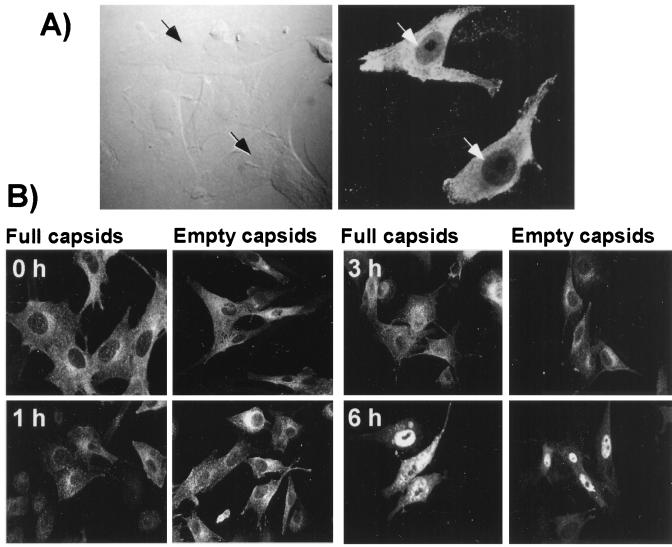
Both full and empty particles were distributed throughout the cytoplasm immediately after microinjection (Fig. 5). By 1 h of incubation at 37°C the capsids were more concentrated around the nucleus. Capsids appeared to be transported into the nucleus in two phases. Up to 3 h after injection, only small amounts of capsids were seen inside the nucleus, but by 6 h, 30 to 50% of the injected cells showed substantial nuclear localization of the capsids (Fig. 5). Twenty-four hours after injection of empty capsids, almost all of the capsids were seen inside the nucleus (results not shown), and those cells did not show viral infection detectable by staining for NS1. However, in cultures injected with full capsids many of the injected cells and cells surrounding them showed NS1 expression at 24 h but not at 1, 3, or 6 h (results not shown).
FIG. 5.
Localization of CPV capsids after microinjection into the cytoplasm of cells. (A) Differential interference contrast and fluorescence confocal image of cells 2 h after injection, showing the specificity of staining for the virus in the injected cells. (B) Full or empty CPV capsids injected into the cytoplasm of the cells that were either fixed immediately (0 h), or incubated for 1, 3, or 6 h at 37°C prior to fixation. The cells were stained for the capsid with MAb 8, followed by FITC-conjugated anti-mouse secondary antibody.
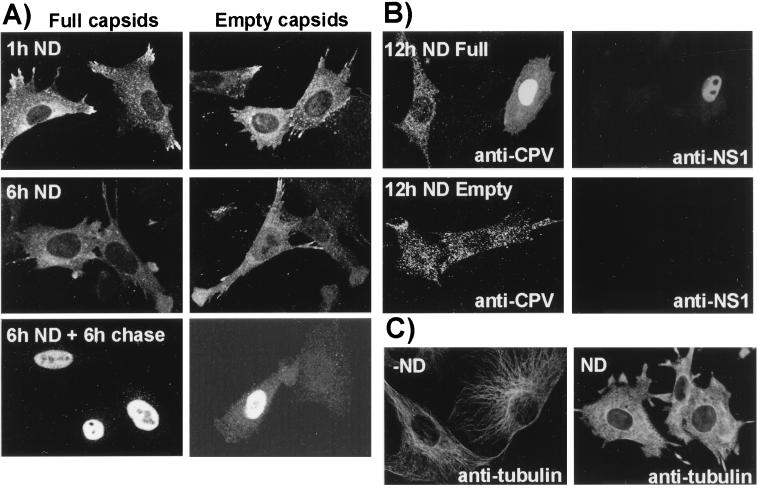
To determine whether capsid transport through the cytosol towards the nuclear area depended on an intact microtubule network, we monitored the localization of full and empty capsids between 1 and 6 h after cytoplasmic injection in cells pretreated for 1 h with nocodazole. Immunofluorescence staining of nocodazole-treated cells for tubulin showed extensive disruption of microtubules (Fig. 6C). At 1 and 6 h full and empty capsids were both distributed randomly throughout the cytoplasm with little perinuclear accumulation or nuclear localization (Fig. 6A), and none of the cells was infected as seen by anti-NS1 antibody staining (results not shown). In some cases the capsid-injected cells treated with nocodazole were washed and incubated in normal medium for a further 6 h, in which case nuclear accumulation of capsids was observed in between 10 and 15% of cells, indicating the reversible nature of inhibition (Fig. 6A). Empty or full capsids injected into cells and incubated in the presence of the drug for 12 h appeared to be aggregated, but they showed little nuclear localization (Fig. 6B). Some of the cells injected with full capsids and incubated for 12 h with nocodazole became infected and showed NS1 expression (Fig. 6B), but this was not seen for the empty capsid-injected cells.
FIG. 6.
(A) Intracellular localization of capsids injected into cells in the presence of nocodazole. Full or empty capsids were injected into cells in the presence of nocodazole and then incubated for 1 h (1h ND) or for 6 h (6h ND). Other cells were injected in the presence of nocodazole, incubated for 6 h, washed, and incubated for a further 6 h in normal medium (6h ND + 6h chase). (B) Cells injected with full or empty capsids in the presence of nocodazole and then incubated for 12 h with the drug (12h ND). The cells were then fixed and stained with MAb 8 or with TxR-labeled anti-NS1 to distinguish those that were infected. (C) Cells that were untreated (−ND) or incubated for 1 h with 20 μM nocodazole (ND) were fixed and then stained with an antibody against tubulin.
DISCUSSION
Intracellular antibodies show that capsids enter the cytoplasm during infection.
Here we show that the intact parvovirus capsid must enter the cytoplasm of the host cell during infectious entry, as infection could be almost completely blocked by intracytoplasmic injection of MAb 8 and 14, which only recognize assembled capsids, or by expression of the MAb 8 variable domain fused to EGFP. This effect was virus specific and not due to an indirect effect of the microinjection process, as injection of cells with nonspecific IgG did not affect infection.
That the blocking effect was due to antibody binding to the capsid was confirmed by injection of infectious plasmid DNA, where the infection was not blocked by either antiviral MAb 8 or 14. In the MAb-injected cells which became infected, the capsid-antibody complexes were frequently observed in the cytoplasm. Although capsids normally accumulate in the nuclei of parvovirus-infected cells, neither the site of assembly nor any process of assembled capsid transport into the nucleus or cytoplasm has been defined (16, 48). These data suggest that the CPV capsids can assemble in the cytoplasm, that they can be transported there after assembly in the nucleus, or that both processes can occur.
After CPV inoculation most cells were still protected from infection by injection with MAb 8 up to 8 h later, indicating that separation of the viral DNA from the capsid occurred only slowly after cellular entry. The reasons for this delayed process are not clear. Slow trafficking of the capsids through the endosomal pathway has also been suggested by the finding that bafilomycin A1 added to cells 90 min after virus inoculation reduces infection by about 70% (42). However, there may also be a slow transport of the capsid through the cytoplasm or into the nucleus, or DNA release from the capsid may require cellular factors associated with S-phase. Several attempts to inject antibodies into isoleucine-deprived and aphidicolin-treated cells resulted in few cells surviving (results not shown), and so we have not been able to test the last hypothesis. The slow kinetics of infection are similar to those seen during the uptake of SV40, where infection could be blocked by injection of antibodies against nucleoporin up to 8 but not 12 h after virus inoculation (56).
The mechanism(s) of intracellular neutralization is not known for certain, but the antibodies would likely bind directly the incoming virus in the cytoplasm, interfering with capsid functions by masking structures required for interactions with cellular factors or for nuclear transport, or they may prevent essential conformational changes of the capsid required for infection or DNA release. Where sufficient virus was present the antibodies clearly aggregated the capsids, as seen in cells infected with CPV plasmids (Fig. 3).
In previous studies we did not observe infection by the injected capsids (53), while that was sometimes seen in these studies 12 or 24 h after we injected full virus capsids. We believe that this difference was due to small variations in the procedures used. It is not clear that capsid injection would recapitulate normal virus transport during infection; very large amounts of capsids were injected into the cytoplasm, and it is also likely that capsids were deposited onto the plasma membrane during the injection process.
Capsids are transported within the cytoplasm and enter the nucleus slowly.
When we injected CPV full or empty capsids into normal cells, they rapidly became localized to a perinuclear region. This suggests that capsid transport was controlled by microtubules, perhaps in a manner similar to that reported for the transport of adenovirus and herpesvirus capsids or their proteins (23, 49, 50). The association between the microtubules and nuclear transport was also shown when the cells were preincubated with nocodazole to cause microtubule breakdown, where the capsids became distributed throughout the cytoplasm (Fig. 6). The cytoskeleton is closely associated with the nuclear pore complex (17). It is therefore likely that capsid transport with the microtubular system could facilitate nuclear transport and that capsids would not otherwise be efficiently transported through the cytoplasm due to the high viscosity and other steric obstacles present (31).
We showed in previous studies that during a 1- to 2-h period after injection, most CPV capsids are found within the cytoplasm, with only small amounts being transported to the nucleus (53, 54). Here we confirm those findings but also show that by 6 h after injection there was significant nuclear transport of capsids in between 30 and 50% of the cells. Nuclear transport required intact microtubules, as it was blocked for up to 12 h by incubation of the cells with nocodazole, while further incubation of the treated cells in normal medium restored nuclear transport (Fig. 6). The 26-nm diameter of the parvovirus particle is close to the maximum size that is reported to pass through the nuclear pore, and it is not clear whether any structural change in the capsids occurs during the process. However, MAb 8 used to detect the virus recognizes only intact particles, so the intranuclear particles seen were largely intact. As nuclear transport of the capsids appears to require virus transport to the vicinity of the nuclear pore, the small amounts of capsid found within the nucleus immediately after microinjection may be those that were deposited in the vicinity of the nucleus by the injection (Fig. 5A).
Other changes in either the capsids or the cells which may facilitate nuclear import of the capsids after 3 h include exposure of the apparent nuclear localization sequence in the VP1 N terminus (11, 52), removal of bound calcium ions from the capsid, modification of the capsid by cellular enzymes, or binding of the virions to cellular components. As no differences were seen between the transport of injected full or empty capsids, the transport events examined here do not appear to be affected by the exposed N-terminal sequences of VP2 or by the viral DNA in full capsids.
The transport of infecting viral capsids or nucleocapsids within the cytoplasm of cells has been found to be a tightly regulated process in most cases that have been examined (7, 28, 35, 37, 55). The processes often involve multiple cellular factors, changes in the virus components or in the cell, and a variety of specific interactions between viral and host cell components. AAV capsids have been reported to be transported into the nucleus when added to cells in vivo (4, 5), and some of the viral DNA is converted to a double-stranded form in receptive cells (14). The autonomous parvoviruses appear to differ in that most endocytosed capsids are retained within endosomes for long periods and neither the capsid nor the viral DNA appears to reach the nucleus at significant levels (42, 46, 53, 54). However, the infection process must be closely regulated and depend on specific cellular factors, as one or two amino acid sequence substitutions on the surface of autonomous parvovirus capsids can alter the infectivity for specific cells by over 105-fold, and the block to infection appears to occur after virus binding and endocytosis but before successful DNA replication in the nucleus (3, 8, 24, 41).
In our future studies we will examine the process of virus transport through the cytoplasm in detail and the role of the microtubule network, as well as the specific cellular and viral factors that control nuclear transport.
ACKNOWLEDGMENTS
Gail Sullivan and Wendy Weichert provided excellent technical assistance.
This work was supported by grants NR 64951 from the Academy of Finland and AI28385 from the National Institutes of Health (to C.R.P.).
REFERENCES
- 1.Agbandje M, McKenna R, Rossmann M G, Strassheim M L, Parrish C R. Structure determination of feline panleucopenia virus empty particles. Proteins. 1993;16:155–171. doi: 10.1002/prot.340160204. [DOI] [PubMed] [Google Scholar]
- 2.Anderson H A, Chen Y, Norkin L C. Bound simian virus 40 translocates to caveolin-enriched domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell. 1996;7:1825–1834. doi: 10.1091/mbc.7.11.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball-Goodrich L J, Tattersall P. Two amino acid substitutions within the capsid are coordinately required for acquisition of fibrotropism by the lymphotropic strain of minute virus of mice. J Virol. 1992;66:3415–3423. doi: 10.1128/jvi.66.6.3415-3423.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartlett J S, Samulski R J. Fluorescent viral vectors: a new technique for the pharmacological analysis of gene therapy. Nat Biotechnol. 1998;4:635–673. doi: 10.1038/nm0598-635. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett J S, Samulski R J, McCown T J. Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum Gene Ther. 1998;9:1181–1186. doi: 10.1089/hum.1998.9.8-1181. [DOI] [PubMed] [Google Scholar]
- 6.Basak S, Turner H. Infectious entry pathway for canine parvovirus. Virology. 1992;186:368–376. doi: 10.1016/0042-6822(92)90002-7. [DOI] [PubMed] [Google Scholar]
- 7.Bui M, Whittaker G, Helenius A. Effect of M1 protein and low pH on nuclear transport of influenza virus ribonucleoproteins. J Virol. 1996;70:8391–8401. doi: 10.1128/jvi.70.12.8391-8401.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang S-F, Sgro J-Y, Parrish C R. Multiple amino acids in the capsid structure of canine parvovirus coordinately determine the canine host range and specific antigenic and hemagglutination properties. J Virol. 1992;66:6858–6867. doi: 10.1128/jvi.66.12.6858-6867.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Norkin L C. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp Cell Res. 1999;246:83–90. doi: 10.1006/excr.1998.4301. [DOI] [PubMed] [Google Scholar]
- 10.Clever J, Yamada M, Kasamatsu H. Import of simian virus 40 virions through nuclear pore complexes. Proc Natl Acad Sci USA. 1991;88:7333–7337. doi: 10.1073/pnas.88.16.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotmore S F, D'Abramo A M, Ticknor C M, Tattersall P. Controlled conformational transitions in the MVM virion expose the VP1 N-terminus and viral genome without particle disassembly. Virology. 1999;254:169–181. doi: 10.1006/viro.1998.9520. [DOI] [PubMed] [Google Scholar]
- 12.Duan D, Li Q, Kao A W, Yue Y, Pessin J E, Engelhardt J F. Dynamin is required for recombinant adeno-associated virus type 2 infection. J Virol. 1999;73:10371–10376. doi: 10.1128/jvi.73.12.10371-10376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dworetzky S I, Landford R E, Feldherr C M. The effects of variations in the number and sequence of targeting signals on nuclear uptake. J Cell Biol. 1988;107:1279–1287. doi: 10.1083/jcb.107.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher K J, Gao G P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox E, Moen P T, Jr, Bodnar J W. Replication of minute virus of mice DNA in adenovirus-infected or adenovirus-transformed cells. Virology. 1990;176:403–412. doi: 10.1016/0042-6822(90)90010-o. [DOI] [PubMed] [Google Scholar]
- 17.Gorlich D, Mattaj I W. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 18.Greber U, Suomalainen M, Stidwill R P, Boucke K, Ebersold M W, Helenius A. The role of the nuclear pore complex in adenovirus DNA entry. EMBO J. 1997;16:5998–6007. doi: 10.1093/emboj/16.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greber U F, Singh I, Helenius A. Mechanisms of virus uncoating. Trends Microbiol. 1994;2:52–56. doi: 10.1016/0966-842x(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 20.Greber U F, Willets M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 21.Griffith G R, Marriot S J, Rintoul D A, Consigli R A. Early events in polyomavirus infection: fusion of monopinocytotic vesicles containing virions with mouse kidney cell nuclei. Virus Res. 1988;10:41–52. doi: 10.1016/0168-1702(88)90056-1. [DOI] [PubMed] [Google Scholar]
- 22.Helenius A. Unpacking the incoming influenza virus. Cell. 1992;69:577–578. doi: 10.1016/0092-8674(92)90219-3. [DOI] [PubMed] [Google Scholar]
- 23.Hong S S, Gay B, Karayan L, Dabauvalle M C, Boulanger P. Cellular uptake and nuclear delivery of recombinant adenovirus penton base. Virology. 1999;262:163–177. doi: 10.1006/viro.1999.9864. [DOI] [PubMed] [Google Scholar]
- 24.Horiuchi M, Goto H, Ishiguro N, Shinagawa M. Mapping of determinants of the host range for canine cells in the genome of canine parvovirus using canine parvovirus/mink enteritis virus chimeric viruses. J Gen Virol. 1994;75:1319–1328. doi: 10.1099/0022-1317-75-6-1319. [DOI] [PubMed] [Google Scholar]
- 25.Kaljot K T, Shaw R D, Rubin D H, Greenberg H B. Infectious rotavirus enters cells by direct cell membrane penetration, not by endocytosis. J Virol. 1988;62:1136–1144. doi: 10.1128/jvi.62.4.1136-1144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kann M, Sodeik B, Vlachou A, Gerlich W H, Helenius A. Phosphorylation-dependent binding of hepatitis B virus core particles to the nuclear pore complex. J Cell Biol. 1999;145:45–55. doi: 10.1083/jcb.145.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kartenbeck J, Stukenbrok H, Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989;109:2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kasamatsu H, Nakanishi A. How do animal DNA viruses get to the nucleus. Annu Rev Microbiol. 1998;52:627–286. doi: 10.1146/annurev.micro.52.1.627. [DOI] [PubMed] [Google Scholar]
- 29.Knipe D M. Virus-host cell interactions. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 273–299. [Google Scholar]
- 30.Lanzrein M, Schlegel A, Kempf C. Entry and uncoating of enveloped viruses. Biochem J. 1994;302:313–320. doi: 10.1042/bj3020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luby-Phelps K. Physical properties of cytoplasm. Curr Opin Cell Biol. 1994;6:3–9. doi: 10.1016/0955-0674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 32.Marsh M. The entry of enveloped viruses into cells by endocytosis. Biochem J. 1984;218:1–10. doi: 10.1042/bj2180001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsh M, Pelchen-Matthews A. Entry of animal viruses into cells. Rev Med Virol. 1993;3:173–185. [Google Scholar]
- 35.Martin K, Helenius A. Transport of incoming influenza virus nucleocapsids into the nucleus. J Virol. 1991;65:232–244. doi: 10.1128/jvi.65.1.232-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martínez C G, Guinea R, Benavente J, Carrasco L. The entry of reovirus into L cells is dependent on vacuolar proton-ATPase activity. J Virol. 1996;70:576–579. doi: 10.1128/jvi.70.1.576-579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyazawa N, Leopold P L, Hackett N R, Ferris B, Worgall S, Falck-Pedersen E, Crystal R G. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J Virol. 1999;73:6056–6065. doi: 10.1128/jvi.73.7.6056-6065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakanishi A, Clever J, Yamada M, Li P P, Kasamatsu H. Association with capsid proteins promotes nuclear targeting of simian virus 40 DNA. Proc Natl Acad Sci USA. 1996;93:96–100. doi: 10.1073/pnas.93.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neubauer C, Frasel L, Kuechler, Blaas D. Mechanism of entry of human rhinovirus 2 into Hela cells. Virology. 1987;158:255–258. doi: 10.1016/0042-6822(87)90264-9. [DOI] [PubMed] [Google Scholar]
- 40.Nibert M L, Schiff L A, Fields B N. Reoviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Vol. 2. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1557–1624. [Google Scholar]
- 41.Parker J S L, Parrish C R. Canine parvovirus host range is determined by the specific conformation of an additional region of the capsid. J Virol. 1997;71:9214–9222. doi: 10.1128/jvi.71.12.9214-9222.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker S L, Parrish C R. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J Virol. 1999;74:1919–1930. doi: 10.1128/jvi.74.4.1919-1930.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parrish C R. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology. 1991;183:195–205. doi: 10.1016/0042-6822(91)90132-u. [DOI] [PubMed] [Google Scholar]
- 44.Prchla E, Kuechler E, Blaas D, Fuchs R. Uncoating of human rhinovirus serotype 2 from late endosomes. J Virol. 1994;68:3713–3723. doi: 10.1128/jvi.68.6.3713-3723.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prchla E, Plank C, Wagner E, Blaas D, Fuchs R. Virus-mediated release of endosomal content in vitro: different behavior of adenovirus and rhinovirus serotype 2. J Cell Biol. 1995;131:111–123. doi: 10.1083/jcb.131.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Previsani N, Fontana S, Hirt B, Beard P. Growth of the parvovirus minute virus of mice MVMp3 in EL4 lymphocytes is restricted after cell entry and before viral DNA amplification: cell-specific differences in virus uncoating in vitro. J Virol. 1997;71:7769–7780. doi: 10.1128/jvi.71.10.7769-7780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimura H, Umeno Y, Kimura G. Effects of inhibitors of the cytoplasmic structures and functions on the early phase of infection of cultured cells with simian virus 40. Virology. 1987;158:34–43. doi: 10.1016/0042-6822(87)90235-2. [DOI] [PubMed] [Google Scholar]
- 48.Singer I I. Ultrastructural studies of HI-1 parvovirus replication. III. Intracellular localization of viral antigens with immunocytochrome C. Exp Cell Res. 1976;99:346–356. doi: 10.1016/0014-4827(76)90592-9. [DOI] [PubMed] [Google Scholar]
- 49.Sodeik B, Ebersold M W, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suomalainen M, Nakano M Y, Keller S, Boucke K, Stidwill R P, Greber U F. Microtubule-dependent plus- and minus end-directed motilities are competing processes for nuclear targeting of adenoviruses. J Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varga M J, Weibull C, Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991;65:6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vihinen-Ranta M, Kakkola L, Kalela A, Vilja P, Vuento M. Characterization of a nuclear localization signal of canine parvovirus capsid proteins. Eur J Biochem. 1997;250:389–394. doi: 10.1111/j.1432-1033.1997.0389a.x. [DOI] [PubMed] [Google Scholar]
- 53.Vihinen-Ranta M, Kalela A, Mäkinen P, Kakkola L, Marjomäki V, Vuento M. Intracellular route of canine parvovirus entry. J Virol. 1998;72:802–806. doi: 10.1128/jvi.72.1.802-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weichert W S, Parker J S, Wahid A T M, Chang S-F, Meier E, Parrish C R. Assaying for structural variation in the parvovirus capsid and its role in infection. Virology. 1998;250:106–117. doi: 10.1006/viro.1998.9352. [DOI] [PubMed] [Google Scholar]
- 55.Whittaker G R, Helenius A. Nuclear import and export of viruses and virus genomes. Virology. 1998;246:1–23. doi: 10.1006/viro.1998.9165. [DOI] [PubMed] [Google Scholar]
- 56.Yamada M, Kasamatsu H. Role of nuclear pore complex in simian virus 40 nuclear targeting. J Virol. 1993;67:119–130. doi: 10.1128/jvi.67.1.119-130.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeung D E, Brown G W, Tam P, Russnak R H, Wilson G, Clark-Lewis I, Astell C R. Monoclonal antibodies to major nonstructural nuclear protein of minute virus of mice. Virology. 1991;181:35–45. doi: 10.1016/0042-6822(91)90467-p. [DOI] [PubMed] [Google Scholar]
- 58.Yuan, W., and C. R. Parrish. Comparison of two single chain antibodies which neutralize canine parvovirus—analysis of antibody combining site and mechanisms of neutralization. Virology, in press. [DOI] [PubMed]