Abstract
The aim of this study was to investigate the distribution of human T-cell leukemia virus type 1 (HTLV-1) in various organs of serially sacrificed squirrel monkeys (Saimiri sciureus) in order to localize the reservoir of the virus and to evaluate the relationship between viral expression and the humoral or cellular immune response during infection. Six squirrel monkeys infected with HTLV-1 were sacrificed 6, 12, and 35 days and 3, 6, and 26 months after inoculation, and 20 organs and tissues were collected from each animal. PCR and reverse transcription-PCR (RT-PCR) were performed with gag and tax primers. Proviral DNA was detected by PCR in peripheral blood mononuclear cells (PBMCs) of monkeys sacrificed 6 days after inoculation and in PBMCs, spleens, and lymph nodes of monkeys sacrificed 12 and 35 days and 3, 6, and 26 months after inoculation. Furthermore, tax/rex mRNA was detected by RT-PCR in the PBMCs of two monkeys 8 to 12 days after inoculation and in the spleens and lymph nodes of the monkey sacrificed on day 12. In this animal, scattered HTLV-1 tax/rex mRNA-positive lymphocytes were detected by in situ hybridization in frozen sections of the spleen, around the germinal centers and close to the arterial capillaries. Anti-HTLV-1 cell-mediated immunity was evaluated at various times after inoculation. Anti-p40Tax and anti-Env cytolytic T-cell responses were detected 2 months after infection and remained detectable thereafter. When Tax peptides were used, this response appeared to be directed against various Tax epitopes. Our results indicate that squirrel monkeys represent a promising animal model for studying the early events of HTLV-1 infection and for evaluating candidate vaccines against HTLV-1.
Human T-cell leukemia/lymphoma virus type 1 (HTLV-1), the causative agent of adult T-cell leukemia (36) and tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM) (10), has also been associated with pediatric infectious dermatitis (22), uveitis (24), and some cases of arthropathy (13) and polymyositis (25).
Owing to the inherent difficulty of obtaining human specimens in early HTLV-1 infection, a relevant animal model is essential for better understanding of the seeding of HTLV-1 in various organs. We showed recently that the squirrel monkey Saimiri sciureus, a South American nonhuman primate which is free of simian T-cell leukemia virus, is susceptible to experimental infection with syngeneic or allogeneic HTLV-1-immortalized cells (19). As in humans, such experimental inoculation leads to chronic infection, HTLV-1 provirus being detectable in peripheral blood mononuclear cells (PBMCs) by PCR up to 36 months after inoculation. Chronically infected monkeys, like HTLV-1-infected humans, develop high titers of antibodies against the structural proteins of the virus (19).
In infections with human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV), the lymph nodes are reservoirs that contain a large viral load during the asymptomatic stage (8, 29). A significant percentage of latently infected lymphocytes are found in the lymph nodes and constitute a target for later viral reactivation (7). Indeed, during clinical latency in HIV or SIV infection, productively infected cells are detected at higher frequency in lymph nodes than in peripheral blood, indicating the progressive involvement of the lymphoid organs in HIV infection (2, 21, 27). These findings show that HIV is widely disseminated in lymphoid tissue relatively early in infection. In contrast, little is known about the role of the lymphoid tissues in the early phases of HTLV-1 infection.
The aim of the present study was to investigate the early events in HTLV-1 infection and specifically the distribution of the HTLV-1 provirus in various organs of serially sacrificed squirrel monkeys, to localize the reservoir of the latent virus, and to evaluate the humoral and cellular immune responses to infection.
MATERIALS AND METHODS
Animals and HTLV-1 infection.
Three 6-year-old male, two 9-year-old male, and two 7-year-old female squirrel monkeys from the primate breeding center of the Pasteur Institute of French Guiana were used in this study. The experiments performed complied with French laws relative to animal experimentation. Five animals were inoculated intravenously with 5 × 107 EVO/798 monkey HTLV-1-transformed cells, and one monkey (1491) had been infected previously with 107 of these cells; the seventh animal (1657) had been infected previously by intravenous inoculation with 107 monkey HTLV-1-transformed EVO/1540 cells (19). The monkeys were bled at various times after inoculation, and their PBMCs were separated by Ficoll-Paque. The sera were diluted 1/100, and antibodies against HTLV-1 were measured by enzyme-linked immunosorbent assay (ELISA; Diagnostic Biotechnology, Singapore) at various times after infection and then confirmed by Western blotting (HTLV blot 2.3; Diagnostic Biotechnology).
Six monkeys were sacrificed at 6, 12, and 35 days and 3, 6, and 26 months after inoculation of the cells (Table 1). Before necropsy, the blood was removed by perfusion with 1 liter of physiological saline. Fragments of dissected organs were then snap-frozen for in situ hybridization or fixed in 4% paraformaldehyde for histological examination. Specimens from all organs, including lymphatic tissues and various regions of the brain and spinal cord, were examined histologically and tested by PCR for HTLV-1 provirus. The tissue sections were stained with hematoxylin-eosin and blood; bone marrow smears were stained with May-Grünwald-Giemsa.
TABLE 1.
Distribution of HTLV-1 provirus by PCR in PBMCs and various organs of serially sacrificed squirrel monkeys
| Samplea | Distributionb
|
|||||
|---|---|---|---|---|---|---|
| 90047 6 D | 92038 12 D | 90083 35 D | 92106 3 M | 92039 6 M | 1657 26 M | |
| PBMC | + | + | + | + | + | + |
| Spleen | − | + | + | + | + | + |
| Mesenteric LN | − | + | + | + | + | + |
| Submaxillary LN | − | + | + | + | + | + |
| Axillary LN | − | − | + | + | + | + |
| Inguinal LN | − | − | + | + | + | + |
| Bone marrow | + | − | + | + | + | + |
| Salivary gland | − | − | + | − | + | + |
| Thyroid gland | − | − | + | − | + | − |
| Lung | − | − | + | − | − | + |
| Liver | + | − | − | − | − | − |
| Pancreas | − | − | − | + | − | + |
| Intestine | − | − | − | + | − | + |
| Stomach | − | − | − | − | − | − |
| Heart | − | − | − | − | − | − |
| Muscle | − | − | + | − | − | − |
| Brain | − | − | − | − | − | − |
| TSC | − | − | + | − | − | − |
| CSC | − | − | − | − | − | + |
| LSC | − | − | + | − | − | + |
LN, lymph node; TSC, thoracic spinal cord; CSC, cervical spinal cord; LSC, lumbar spinal cord.
Monkey number, time of necropsy. D, day; M, month.
Detection of the HTLV-1 provirus by PCR and quantitative PCR.
Genomic DNA was extracted from PBMCs and various organs by the method described by Ibrahim et al. (11). PCR (35 cycles) was performed, as previously described, with primers Rmtax1 and Rmtax2 (antisense) for the tax region (202 nucleotides) (23) and with primers gag1 and gag2 for the gag region (17). One microgram of genomic DNA from the PBMCs or organs was used for each PCR amplification. The amplified products were submitted to electrophoresis on a 1.4% agarose gel, transferred to nylon membranes, and hybridized with 32P-labeled specific internal oligonucleotide probes for tax (5′ GGGGCCCTAATAATTCTACCCGAAGACT 3′) and gag (5′ GCAAAGGTACTGCAGGAGGT 3′). The membranes were washed and exposed to Hyperfilm MP (Amersham, Little Chalfont, United Kingdom) at −80°C for 24 h and 1 week. The sensitivity of the PCR was evaluated with HTLV-1 plasmid p4.39, cloned from HTLV-1 cell line 2060 (26). Plasmid p4.39 DNA was diluted at various concentrations in 1 μg (150,000 cell equivalents) of DNA from uninfected PBMCs and analyzed by PCR.
Copies of proviral HTLV-1 in PBMCs from infected monkeys were quantified by the method described by Cimarelli et al. (5). Briefly, competitive PCRs were performed with genomic DNA purified from PBMCs of infected monkeys obtained at various times after inoculation. The number of HTLV-1 copies was determined in 0.7 μg of DNA, corresponding to the amount of genomic DNA extracted from 105 PBMCs.
Detection of tax/rex mRNA by RT-PCR.
Total cellular RNA was extracted from PBMCs and tissues with Trizol reagent (Gibco BRL, Grand Island, N.Y.). The RNA was suspended in water and diluted to a final concentration of 0.1 μg/μl. It was then mixed with 15 pmol of the random hexamer primers in a final volume of 12.7 μl. Reverse transcription (RT) and cDNA synthesis were performed as described previously (30). Immediately after cDNA synthesis, a seminested PCR was carried out on the pX region, with RPX3 and RPX5 as the outer primers and RPX3 and RPX4 as the inner primers (20). The sequence of the RPX5 primer was 5′ AGGCGGGCCGAACATAGTCCC 3′ (antisense). To confirm the presence of amplifiable cDNA in the samples, primers 18SL (5′ CCATGGAGAAGGCTGGGG 3′; sense) and 20SL (5′ CAAAGTTGTCATGGATGACC 3′; antisense) were used to detect a portion of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Fate of inoculated cells.
To confirm that the expression of tax/rex mRNA of HTLV-1 and HTLV-1 provirus that was detected was not that of surviving cells from the inoculum, we evaluated the fate of the inoculum by inoculating two females with the HTLV-1-transformed cell line EVO/798, derived from a male, and sacrificing the two females 6 and 35 days after inoculation. By PCR with primers VIB and SRY Omega (35), a portion of the Saimiri Y chromosome gene SRY was amplified and identified by hybridization with the SRY probe (5′ TATACCTTCTCATACAGAGATAACTGTACAAAATCC 3′). This probe was defined after cloning and sequencing of the amplified Saimiri SRY product.
In situ hybridization.
In situ hybridization was performed on serial sections of various frozen tissues as described by Vazeux et al. (33) and Boche et al. (3). Briefly, 33P- or 35S-labeled antisense riboprobes corresponding to the complete tax mRNA sequence were used. These riboprobes were derived from the T3 promoter of a Bluescript vector. The sections were exposed for 10 to 20 days to 106 cpm/slide. The monkey HTLV-1-transformed cell line EVO/798 was used as a positive control, and negative controls were performed with PBMCs from an HTLV-1-seronegative monkey and frozen monkey tissues in which tax expression was not detected by RT-PCR.
Detection of anti-HTLV-1 cell-mediated immunity.
Autologous Epstein-Barr virus-transformed B-lymphoblastoid cell lines were obtained as described previously (4) and used as target cells in the assay for cytolytic activity after infection with vaccinia virus–HTLV-1 constructs or loading with exogenous HTLV-1 p40Tax peptides. The recombinant vaccinia viruses were kindly provided by H. Shida, Kyoto University, Kyoto, Japan. The overlapping p40Tax peptides described by Parker et al. (28) were kindly provided by C. Pique, Institut Cochin de Génétique Moléculaire, Paris, France.
Effector cells were prepared as follows. Frozen PBMCs or splenocytes were thawed and cultured for 3 days at 37°C in a CO2 incubator in RPMI 1640 culture medium containing 10% fetal calf serum, glutamine, penicillin-streptomycin, and sodium pyruvate. The cultured cells were depleted of CD4+ T lymphocytes by magnetic microbeads coupled with monoclonal antibody Leu-3a (miniMACS system; Miltenyi Biotec, Auburn, Calif.), which is a mouse anti-human CD4 chain that cross-reacts with the squirrel monkey CD4 chain. Autologous irradiated CD4+ T cells were used as feeder cells for expansion of the CD4+-depleted CD8+-enriched lymphocytes in vitro in the presence of phytohemagglutinin (5 μg/ml; Difco, Detroit, Mich.) and human recombinant interleukin 2 (40 U/ml; Boehringer Mannheim, Mannheim, Germany). The medium was changed every 3 to 4 days. After 2 weeks, the expanded T cells were used in a cytolytic chromium release assay. Briefly, 3 × 103 51Cr-loaded target cells were distributed at 100 μl per well in a round-bottom 96-well microtiter plate (Costar, OSI), and 100 μl of effector cells at various ratios of effector/target was added to each well in triplicate or duplicate. The plates were incubated for 4 h at 37°C, 100-μl aliquots of supernatant were collected, and the release of chromium was counted in a gamma counter (LKB, Les Ulis, France). Maximal release was counted in target cells lysed with aqueous sodium hypochlorite, and spontaneous release was counted in target cells incubated alone. The percentage of specific lysis was calculated as [(cpm experimental release − cpm spontaneous release)/(cpm maximal release − cpm spontaneous release)] × 100. When background lysis due to vaccinia virus or Epstein-Barr virus antigens was considerable, a 25- to 50-fold excess of cold autologous vaccinia virus-infected B-lymphoblastoid cell lines was added to reduce it.
Nucleotide sequence accession number.
The GenBank accession number of the Saimiri SRY sequence reported (359 nucleotides) is AF151695.
RESULTS
Detection of the HTLV-1 provirus and its distribution in organs of squirrel monkeys.
The distribution of the HTLV-1 provirus was evaluated by PCR with gag and tax primers in 20 organs from the six experimentally infected squirrel monkeys sacrificed at various times after infection (Table 1). This method has been shown to detect five copies of plasmid p4.39 diluted in 1 μg of carrier DNA.
In the animal sacrificed 6 days after inoculation, HTLV-1 provirus was detected only in PBMCs, liver, and bone marrow. In the animal sacrificed 12 days after inoculation, the provirus was detected in PBMCs, spleen, and mesenteric and submaxillary lymph nodes. In the four animals sacrificed at 35 days and 3, 6, and 26 months after inoculation of the cells, the most common sites of detection of HTLV-1 provirus were PBMCs, spleen, bone marrow, and all tested lymph nodes. In these four animals, positive signals were recorded sporadically in salivary gland, thyroid gland, lung, pancreas, intestine, and spinal cord (Table 1).
Fate of the inoculum.
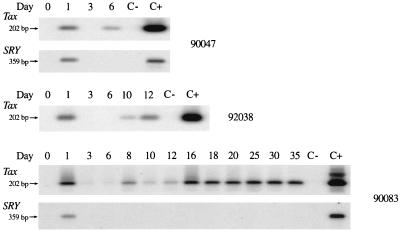
The fate of the inoculum was evaluated in two females (90047 and 90083) sacrificed 6 and 35 days after inoculation. As seen in Fig. 1, PCR with primers that detect part of the SRY sequence of the Y chromosome showed the presence of the inoculum 24 h later but not thereafter. The presence of the inoculum correlates with the detection of HTLV-1 provirus (202 bp of the tax region) by PCR and tax/rex mRNA by RT-PCR at 24 h in the PBMCs of these inoculated monkeys (Fig. 1 and 2A). No positive signal for the SRY was detected in PBMCs or in any organs at necropsy. The absence of a positive signal for the SRY in PBMCs and organs shows that the presence of HTLV-1 provirus was due to infection of the PBMCs and not to persistence of the inoculum.
FIG. 1.
Detection of HTLV-1 tax sequence (202 bp) (first line) and SRY sequence of Y chromosome (second line) by PCR in PBMCs of monkeys 90047 (female), 92038 (male), and 90083 (female) sacrificed 6, 12, and 35 days after inoculation. C− and C+ represent negative and positive controls, respectively.
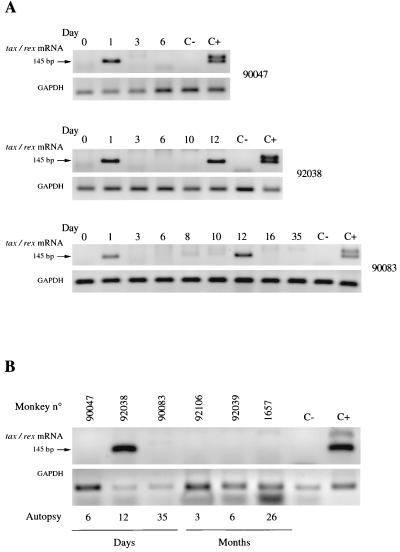
FIG. 2.
Detection of HTLV-1 provirus expression (tax/rex mRNA) by RT-PCR. (A) Detection of tax/rex mRNA (first line) and GAPDH sequence (second line) in PBMCs of monkeys 90047, 92038, and 90083 sacrificed 6, 12, and 35 days after inoculation. (B) Detection of tax/rex mRNA (first line) and GAPDH sequence (second line) in spleens of different monkeys sacrificed at various times after inoculation. C− and C+ represent negative and positive controls; respectively.
Dynamics of virus infection in PBMCs as detected by PCR.
As seen in Fig. 1, no or very low positive signals were detected by PCR in the PBMCs of three monkeys 3 days after inoculation, but the signals increased after 8 days and became stable after 16 days in the monkey sacrificed at 35 days (Fig. 1). Furthermore, in three other animals (92106, 92039, and 1657) sacrificed at 3, 6, and 26 months after inoculation, the HTLV-1 provirus could be detected by PCR at 3 weeks after inoculation and thereafter. Quantitative PCR was performed to determine the proviral load in 105 PBMCs from two monkeys, 90083 and 92039, sacrificed at 35 days and at 6 months. In the monkey sacrificed at 35 days, we detected 167 HTLV-1 proviral copies 8 days after infection and 557 copies at 35 days. In the animal sacrificed at 6 months, we detected 354 proviral copies 3 weeks after infection and 413 copies on the day of necropsy.
Detection of HTLV-1 provirus expression (tax/rex mRNA) and in situ hybridization in various organs of squirrel monkeys.
RT-PCR was used to examine the expression of tax/rex mRNA in fresh PBMCs and in organs obtained from the animals at necropsy. tax/rex mRNA was detected by seminested PCR only in the PBMCs, spleen, and mesenteric and submaxillary lymph nodes of the animal sacrificed 12 days after inoculation and in PBMCs collected at 12 days from the animal sacrificed at 35 days (Fig. 2). In other animals, tax/rex mRNA could not be detected in PBMCs at 3 weeks or in organs at necropsy. In the animal sacrificed at 12 days, in situ hybridization on several frozen sections of the spleen revealed scattered HTLV-1-positive lymphocytes around the germinal centers and next to arterial capillaries (Fig. 3). Cells with signals for tax/rex mRNA were observed reproducibly in serial sections hybridized with 33P- and 35S-labeled riboprobes. In contrast, we observed no positive cells in frozen or paraffin-embedded organs, including spleen, lymph nodes, and PBMCs, from monkeys infected longer.
FIG. 3.
In situ hybridization with HTLV-1 tax riboprobe on frozen spleen sections from an infected squirrel monkey sacrificed 12 days after inoculation. (A and B) Positive signals in the spleen observed with antisense 33P-labeled riboprobe; arrows indicate positive spleen cells after 10 days of in situ hybridization. (C) Negative control (PBMCs from squirrel monkey not infected with HTLV-1). (D) Positive control (EVO/798 HTLV-1 monkey-transformed cell line).
Thus, in circulating blood cells, spleen, and lymph nodes, the HTLV-1 provirus is transcribed only transiently in vivo, at a very low level.
Pathological and histological investigations.
Some lesions and lymphocyte infiltrations were observed in the salivary glands of two monkeys sacrificed at 12 days and 3 months, and follicular hyperplasia was observed in the spleen and lymph nodes of four of the six monkeys.
Humoral responses of inoculated monkeys.
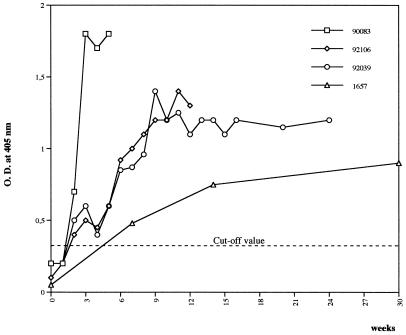
When the antibody response to HTLV-1 was evaluated by ELISA, no anti-HTLV-1 antibodies were detected in the two animals sacrificed at 6 and 12 days; however, as seen in Fig. 4, the monkey sacrificed at 35 days had high antibody responses from 16 days after infection, and the three monkeys sacrificed at 3, 6, and 26 months after inoculation had antibodies 3 to 6 weeks after infection. The positive sera reacted on Western blotting against Gag proteins p19 and p24 and Env gp46 peptide (amino acids 162 to 209) and recombinant gp21. This antibody response remained stable for 3 to 26 months.
FIG. 4.
Antibody responses of monkeys against HTLV-1, tested by ELISA after inoculation with homologous HTLV-1 monkey-transformed cell lines. Monkey 90083 was sacrificed at 35 days, monkey 92106 was sacrificed at 3 months, monkey 92039 was sacrificed at 6 months, and monkey 1657 was sacrificed at 26 months. O.D., optical density.
Anti-HTLV-1 cell-mediated immunity.
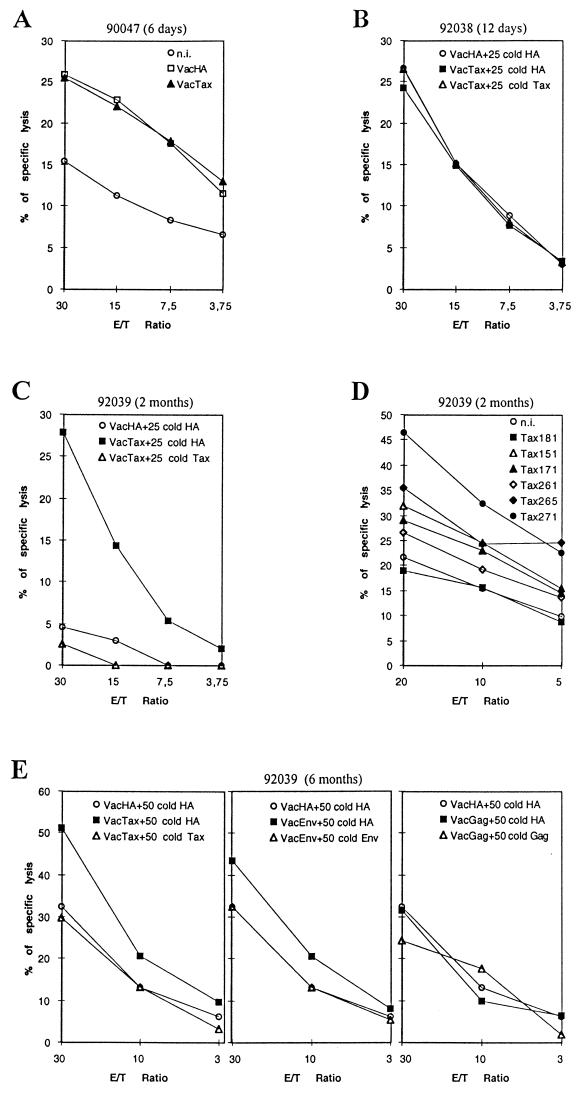
In the two monkeys sacrificed 6 and 12 days after inoculation, no significant cytolytic activity was detected in splenocytes against target cells infected with recombinant vaccinia virus expressing the whole p40Tax protein (VacTax; Fig. 5A and B) or loaded with p40Tax peptides. The monkey sacrificed 6 months after inoculation showed cytolytic activity in PBMCs against VacTax-infected target cells at 2 months (Fig. 5C) but not at 2 or 6 weeks (data not shown). This response against p40Tax seemed to be directed against various Tax epitopes, as we detected cell-mediated immune responses against Tax peptides 151-165, 171-185, 261-275, 265-279, and 271-285 (Fig. 5D). Furthermore, at necropsy at 6 months, we observed strong cytolytic activity in splenocytes against p40Tax but only a weak response against Env and no specific cytolytic activity against Gag (Fig. 5E).
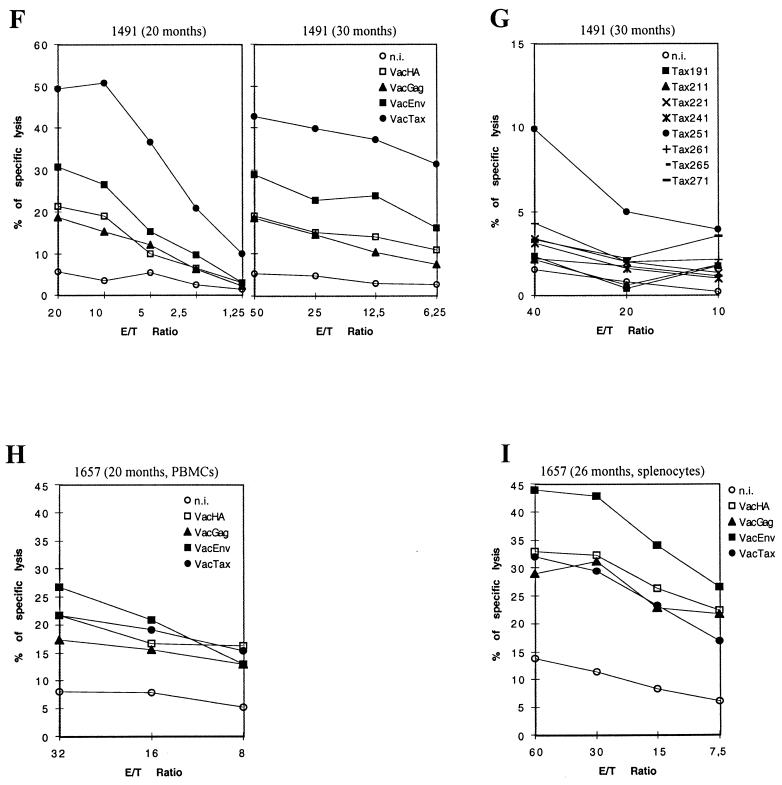
FIG. 5.
Cultured CD4+-depleted CD8+-enriched cells obtained from PBMCs or splenocytes, used as effectors in cytolytic chromium release assays. Target cells were autologous B-lymphoblastoid cell lines either uninfected (n.i.), vaccinia virus infected, or Tax peptide loaded. In some cases, a 25- or 50-fold excess of cold autologous vaccinia virus-infected B-lymphoblastoid target cells was added to reduce background. (A) Monkey 90047 sacrificed day 6 after inoculation; CD4-depleted splenocytes by day 12 of culture. (B) Monkey 92038 sacrificed day 12 after inoculation; CD4-depleted splenocytes by day 18 of culture. (C) Blood from monkey 92039 sampled at 2 months; CD4-depleted PBMCs by day 18 of culture. (D) Blood from monkey 92039 sampled at 2 months; CD4-depleted PBMCs by day 16 of culture. (E) Monkey 92039 sacrificed at 6 months; CD4-depleted splenocytes by day 24 of culture. (F) Blood from monkey 1491 sampled 20 and 30 months after inoculation; CD4-depleted PBMCs by days 23 and 15 of culture, respectively. (G) Blood from monkey 1491 sampled 20 and 30 months after inoculation; CD4-depleted PBMCs obtained from blood sample at 30 months after inoculation, by day 19 of culture. (H) Blood from monkey 1657 sampled 20 months after inoculation; CD4-depleted PBMCs by day 26 of culture. (I) Monkey 1657 sacrificed 26 months after inoculation; CD4-depleted splenocytes by day 21 of culture.
A cell-mediated immune response in PBMCs against HTLV-1 proteins was observed in one monkey with long-term infection at both 20 and 30 months (Fig. 5F) with a very strong response against p40Tax and a weaker response against the Env protein; no response was observed against the Gag product. In this animal, the anti-Tax cytolytic response seemed to be directed against a unique epitope contained in the Tax 251-265 peptide (Fig. 5G). In the second monkey, the cell-mediated immune response was weaker than in the first animal, and the only cytolytic response, detectable in PBMCs at 20 months and in splenocytes at 26 months, was directed against the Env protein (Fig. 5H and I).
DISCUSSION
We show here that during early HTLV-1 infection of a nonhuman primate (S. sciureus), PBMCs, the spleen, and lymph nodes serve as major reservoirs for HTLV-1.
In our previous studies (11, 18), in which adult rats were used as the experimental model to examine the distribution of the HTLV-1 provirus in serially sacrificed animals, the most frequent locations of the provirus 12 weeks after infection were PBMCs and the spinal cord; however, 22 weeks after inoculation, when the provirus could no longer be detected in these organs, it was present in sympathetic nodes. In a study reported by Ishiguro et al. (14) in which rats were infected as neonates, the HTLV-1 provirus was widely disseminated in the lymphoid system, central nervous system, heart, liver, lung, and kidney. The difference from our results could be linked to the age at which the animals were infected, as neonates rather than as adults, and to the fact that the rats and monkeys in our studies were thoroughly perfused before necropsy. PBMCs and mesenteric lymph nodes were also found to be major reservoirs in HTLV-1-infected rabbits (6). Ibuki et al. (12) showed, however, the presence of HTLV-1 provirus in PBMCs, spleens, and lymph nodes of cynomolgus monkeys 51 weeks after inoculation of an HTLV-1-producing T-cell line derived from a macaque monkey.
No data are available on the reservoirs of HTLV-1 in humans during the asymptomatic period. In HIV or SIV infection, it is now accepted that the major viral reservoir during the asymptomatic period is lymphoid tissue (2, 8, 21, 27). In the case of lentiretroviruses, the lymphoid tissue harbors numerous virus-producing cells and virions trapped as immune complexes in the follicular dendritic cell network of the germinal centers. These trapped virions could act as a source of infectious virus (2). Infected cells can migrate into and through lymphoid tissue, and naive CD4+ cells, in particular, can be infected within the germinal centers of the lymphoid tissue.
In the present study, HTLV-1 provirus could be detected by PCR 6 days after infection, and we showed by competitive PCR that the proviral load increases in PBMCs of infected animals shortly after infection and remains stable thereafter. RT-PCR and in situ hybridization indicated, however, that HTLV-1 tax/rex mRNA is transcribed transiently during the early stage of infection (12 days). We cannot conclude that tax/rex expression is not maintained in PBMCs, but it is too weak to be detected by our method. Nevertheless, it is likely that tax/rex mRNA expression does not last long and that the latency occurs very early after infection of squirrel monkeys with HTLV-1. HTLV-1 expression is rarely detected in asymptomatic human chronic carriers of HTLV-1, despite a high proviral load, suggesting that HTLV-1 replication in vivo results predominantly from mitosis of latently infected cells rather than from RT (34). The genetic stability of HTLV-1, its weak expression, and the high proviral load cited above favor such a hypothesis. Wattel et al. (34) further suggested that primary infection with HTLV-1 must involve infected allogeneic cells, followed by RT and viral replication. Once a few rounds of RT have taken place, clonal expansion of the infected cells should predominate. Our results in the squirrel monkey model seem to confirm this observation, as we found expression of tax/rex mRNA in PBMCs and lymphoid organs only during the early stage of infection and found only latent HTLV-1 proviral sequences at these sites thereafter. This could reflect clonal expansion of infected cells lacking detectable viral RNA. Since only a small proportion of HTLV-1 carriers develop malignant disease after 30 to 50 years of infection, such clonal expansion must be actively controlled by specific humoral or cell-mediated immunity.
As a first step to addressing the role of the cell-mediated immune response in controlling HTLV-1 infection in the squirrel monkey model, we analyzed its presence at various times after infection in serially sacrificed animals. No clear phenotyping of the cytolytic effectors or of major histocompatibility complex (MHC) restriction could be done, as there are no relevant monoclonal antibodies and MHC class I molecules in the squirrel monkey have not been well characterized. Work is in progress to better characterize this response. Nevertheless, two observations support the hypothesis that the cytolytic effectors responsible for the responses we observed are CD8 T lymphocytes. (i) PBMCs were CD4 depleted before addition of T mitogen, which would favor expansion of CD8 T lymphocytes in the culture medium. (ii) NK activity is unlikely, as the B cells used as targets express large amounts of class I molecules, which are known to inhibit NK activity; furthermore, NK activity against unloaded viral peptide or wild vaccinia virus-infected B cells was not observed. We therefore conclude that the cell-mediated immune responses observed in our study are due to cytotoxic T-lymphocyte (CTL) activity.
While there was no detectable CTL response during the early stage of infection (6 and 12 days and 2 or 6 weeks after inoculation), activity was detected after 2 months and was present during chronic infection (up to 20 months). The CTL activity was directed mainly against HTLV-1 p40Tax protein. In rats infected with HTLV-1, CTL responses can be detected against Gag, Env, and Tax, with a stronger reaction against Env protein (31). In asymptomatic human carriers of HTLV-1 and in patients with TSP/HAM, the CTL response is also directed mainly against p40Tax protein (15). Bangham et al. (1) suggested that this response could play a major role in controlling HTLV-1 replication, but others consider that it could be detrimental in some genetically susceptible individuals, leading them to develop TSP/HAM (15). In our squirrel monkey model, the CTL response seem to be comparable to that observed in asymptomatic human HTLV-1 carriers but with different epitopic targets, probably because of differences in antigen presentation by MHC class I molecules.
It was of interest that virus expression in squirrel monkeys was detected only in the absence of humoral and CTL responses. In SIV infection, both neutralizing antibodies and the CTL response play important roles in limiting viral replication and in clearance of the agent (9, 16). The CTL response in our nonhuman primate model, which is probably mediated by CD8+-enriched lymphocytes, could be of interest for evaluating the role of cytotoxic CD8+ T-cell responses during HTLV-1 infection; however, immunological tools such as monoclonal antibodies against squirrel monkey CD4 and CD8 molecules will be necessary. Although one monoclonal antibody cross-reactive between human and Saimiri CD4 molecules has been identified (Leu-3a), none of the anti-human CD8 monoclonal antibodies tested has similar cross-reactivity. In an attempt to derive anti-squirrel monkey CD8 monoclonal antibodies, we cloned and sequenced the cDNAs encoding squirrel monkey CD8 α and β chains (32). The expression of these chains in appropriate mouse cells should enable us to derive the monoclonal antibodies needed to evaluate the role of the antiviral CTL response in chronic HTLV-1 infection and in HTLV-1-induced neurological disease.
ACKNOWLEDGMENTS
We thank E. Wattel for helpful discussion, C. Nicot for the p4.39 plasmid, and J. F. Pouliquen and V. Trouplin for technical assistance. We acknowledge the financial support of the Association pour la Recherche contre la Cancer (ARC), Fondation pour la Recherche Médicale (FRM/SIDACTION) and Association Virus Cancer Prévention (VCP).
REFERENCES
- 1.Bangham C, Kermode A G, Hall S E, Daenke S. The cytotoxic T-lymphocyte response to HTLV-I: the main determinant of disease. Semin Virol. 1996;7:41–48. [Google Scholar]
- 2.Blacklaws B. Quantification of the reservoir of HIV-I. Trends Microbiol. 1997;5:215–216. doi: 10.1016/S0966-842X(97)01047-0. [DOI] [PubMed] [Google Scholar]
- 3.Boche D, Khatissian E, Gray F, Falanga P, Montagnier L, Hurtrel B. Viral load and neuropathology in the SIV model. J Neurovirol. 1999;5:232–240. doi: 10.3109/13550289909015809. [DOI] [PubMed] [Google Scholar]
- 4.Chizzolini C, Sulzer A J, Olsen-Rasmussen M A, Collins W E. Epstein-Barr virus transformation of Saimiri sciureus (squirrel monkey) B cells and generation of a Plasmodium brasilianum-specific monoclonal antibody in P. brasilianum-infected monkeys. Infect Immun. 1991;59:2285–2290. doi: 10.1128/iai.59.7.2285-2290.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cimarelli A, Duclos C A, Gessain A, Cattaneo E, Casoli C, Biglione M, Mauclere P, Bertazzoni U. Quantification of HTLV-II proviral copies by competitive polymerase chain reaction in peripheral blood mononuclear cells of Italian injecting drug users, central Africans, and Amerindians. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;10:198–204. doi: 10.1097/00042560-199510020-00014. [DOI] [PubMed] [Google Scholar]
- 6.Cockerell G L, Lairmore M, De B, Rovnak J, Hartley T M, Miyoshi I. Persistent infection of rabbits with HTLV-I: patterns of anti-viral antibody reactivity and detection of virus by gene amplification. Int J Cancer. 1990;45:127–130. doi: 10.1002/ijc.2910450123. [DOI] [PubMed] [Google Scholar]
- 7.Embretson J, Zupancic M, Ribas J L, Burke A, Racz P, Tenner-Racz K, Haase A T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 8.Fox C H, Cottler-Fox M. The pathobiology of HIV infection. Immunol Today. 1992;13:353–356. doi: 10.1016/0167-5699(92)90171-3. [DOI] [PubMed] [Google Scholar]
- 9.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 10.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim F, Fiette L, Gessain A, Buisson N, de Thé G, Bomford R. Infection of rats with human T-cell leukemia virus type-I: susceptibility of inbred strains, antibody response and provirus location. Int J Cancer. 1994;58:446–451. doi: 10.1002/ijc.2910580324. [DOI] [PubMed] [Google Scholar]
- 12.Ibuki K, Funahashi S I, Yamamoto H, Nakamura M, Igarashi T, Miura T, Ido E, Hayami M, Shida H. Long-term persistence of protective immunity in cynomolgus monkeys immunized with a recombinant vaccinia virus expressing the human T cell leukaemia virus type I envelope gene. J Gen Virol. 1997;78:147–152. doi: 10.1099/0022-1317-78-1-147. [DOI] [PubMed] [Google Scholar]
- 13.Ijichi S, Matsuda T, Maruyama I, Izumihara T, Kojima K, Niimura T, Maruyama Y, Sonoda S, Yoshida A, Osame M. Arthritis in a human T-lymphotropic virus type-I (HTLV-I) carrier. Ann Rheum Dis. 1990;49:718–721. doi: 10.1136/ard.49.9.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishiguro N, Abe M, Seto K, Sakurai H, Ikeda H, Wakisaka A, Togashi T, Tateno M, Yoshiki T. A rat model of human T lymphocyte virus type I (HTLV-I) infection. 1. Humoral antibody response, provirus integration, and HTLV-I-associated myelopathy/tropical spastic paraparesis-like myelopathy in seronegative HTLV-I carrier rats. J Exp Med. 1992;176:981–989. doi: 10.1084/jem.176.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson S, Shida H, McFarlin D E, Fauci A S, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 16.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawase K, Katamine S, Moriuchi R, Miyamoto T, Kubota K, Igarashi H, Doi H, Tsuji Y, Yamabe T, Hino S. Maternal transmission of HTLV-I other than through breast milk: discrepancy between the polymerase chain reaction positivity of cord blood samples for HTLV-I and the subsequent seropositivity of individuals. Jpn J Cancer Res. 1992;83:968–977. doi: 10.1111/j.1349-7006.1992.tb02009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazanji M, Ibrahim F, Fiette L, Bomford R, de Thé G. Role of the genetic background of rats in infection by HTLV-I and HTLV-II and in the development of associated diseases. Int J Cancer. 1997;73:131–136. doi: 10.1002/(sici)1097-0215(19970926)73:1<131::aid-ijc20>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Kazanji M, Moreau J P, Mahieux R, Bonnemains B, Bomford R, Gessain A, de Thé G. HTLV-I infection in squirrel monkey (Saïmiri sciureus) using autologous, homologous or heterologous HTLV-I-transformed cell lines. Virology. 1997;231:258–266. doi: 10.1006/viro.1997.8528. [DOI] [PubMed] [Google Scholar]
- 20.Kinoshita T, Shimoyama M, Tobinai K, Ito M, Ito S, Ikeda S, Tajima K, Shimotohno K, Sugimura T. Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc Natl Acad Sci USA. 1989;86:5620–5624. doi: 10.1073/pnas.86.14.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lafeuillade A, Poggi C, Tamalet C, Profizi N. Human immunodeficiency virus type 1 dynamics in different lymphoid tissue compartments. J Infect Dis. 1997;176:804–806. doi: 10.1086/517307. [DOI] [PubMed] [Google Scholar]
- 22.Lagrenade L, Hanchard B, Fletcher V, Cranston B, Blattner W. Infective dermatitis of Jamaican children—a marker for HTLV-I infection. Lancet. 1990;336:1345–1347. doi: 10.1016/0140-6736(90)92896-p. [DOI] [PubMed] [Google Scholar]
- 23.Mahieux R, de Thé G, Gessain A. The tax mutation at nucleotide 7959 of human T-cell leukemia virus type 1 (HTLV-1) is not associated with tropical spastic paraparesis/HTLV-1-associated myelopathy but is linked to the cosmopolitan molecular genotype. J Virol. 1995;69:5925–5927. doi: 10.1128/jvi.69.9.5925-5927.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mochizuki M, Yamaguchi K, Takatsuki K, Watanabe T, Mori S, Tajima K. HTLV-I and uveitis. Lancet. 1992;339:1110. doi: 10.1016/0140-6736(92)90699-4. [DOI] [PubMed] [Google Scholar]
- 25.Morgan O, Rodgers-Johnson P, Mora C, Char G. HTLV-I and polymyositis in Jamaica. Lancet. 1989;ii:1184–1186. doi: 10.1016/s0140-6736(89)91793-5. [DOI] [PubMed] [Google Scholar]
- 26.Nicot C, Astier-Gin T, Edouard E, Legrand E, Moynet D, Vital A, Londos-Gagliardi D, Moreau J P, Guillemain B. Establishment of HTLV-I-infected cell lines from French, Guianese and West Indian patients and isolation of a proviral clone producing viral particles. Virus Res. 1993;30:317–334. doi: 10.1016/0168-1702(93)90099-9. [DOI] [PubMed] [Google Scholar]
- 27.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 28.Parker C E, Nightingale S, Taylor G P, Weber J, Bangham C R. Circulating anti-Tax cytotoxic T lymphocytes from human T-cell leukemia virus type I-infected people, with and without tropical spastic paraparesis, recognize multiple epitopes simultaneously. J Virol. 1994;68:2860–2868. doi: 10.1128/jvi.68.5.2860-2868.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuurman H J, Krone W J, Broekhuizen R, Goudsmit J. Expression of RNA and antigens of human immunodeficiency virus type-1 (HIV-1) in lymph nodes from HIV-1 infected individuals. Am J Pathol. 1988;133:516–524. [PMC free article] [PubMed] [Google Scholar]
- 30.Talarmin A, Chandler L J, Kazanji M, de Thoisy B, Debon P, Lelarge J, Labeau B, Bourreau E, Vie J C, Shope R E, Sarthou J L. Mayaro virus fever in French Guiana: isolation, identification, and seroprevalence. Am J Trop Med Hyg. 1998;159:452–456. doi: 10.4269/ajtmh.1998.59.452. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka Y, Tozawa H, Koyanagi Y, Shida H. Recognition of human T cell leukemia virus type I (HTLV-I) gag and pX gene products by MHC-restricted cytotoxic T lymphocytes induced in rats against syngeneic HTLV-I-infected cells. J Immunol. 1990;144:4202–4211. [PubMed] [Google Scholar]
- 32.Ureta-Vidal A, Garcia Z, Lemonier F, Kazanji M. Molecular characterization of cDNAs encoding squirrel monkeys (Saïmiri sciureus) CD8 α and β chains. Immunogenetics. 1999;49:718–721. doi: 10.1007/s002510050672. [DOI] [PubMed] [Google Scholar]
- 33.Vazeux R, Brousse N, Jarry A, Henin D, Marche C, Vedrenne C, Mikol J, Wolff M, Michon C, Rozenbaum W, Bureau J F, Montagnier L, Brahic M. AIDS subacute encephalitis: identification of HIV infected cells. Am J Pathol. 1987;126:403–410. [PMC free article] [PubMed] [Google Scholar]
- 34.Wattel E, Vartanian J P, Pannetier C, Wain-Hobson S. Clonal expansion of human T-cell leukemia virus type I-infected cells in asymptomatic and symptomatic carriers without malignancy. J Virol. 1995;69:2863–2868. doi: 10.1128/jvi.69.5.2863-2868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitfield L S, Lovell-Badge R, Goodfellow P N. Rapid sequence evolution of the mammalian sex-determining gene SRY. Nature. 1993;364:713–715. doi: 10.1038/364713a0. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida M, Seiki M, Yamaguchi K, Takatsuki K. Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA. 1984;81:2534–2537. doi: 10.1073/pnas.81.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]