Abstract
Vertebrate testosterone, an androgen present in the testes, is essential for male fertility. Vertebrate-type steroid hormones have been identified in insects, but their function remains unknown. Insect vitellogenin (Vg) is usually a female-specific protein involved in reproductive processes. However, males of some species, such as the green lacewing Chrysopa pallens, have Vg. Here, we demonstrated that the knockdown of C. pallens male Vg by RNAi significantly shortened the lifespan of males, suppressed the reproduction of post-mating females, and strikingly reduced the abundance of several immune-related compounds, including testosterone. LC-MS/MS revealed that C. pallens male testosterone had the same structure and molecular mass as vertebrate testosterone. Topical testosterone application partially restored the lifespan of Vg-deficient males and the reproduction of post-mating females. These results suggest that vertebrate-type testosterone maintains male longevity and female reproduction under the control of the male Vg in C. pallens.
1. Introduction
Insect spermatogenesis is regulated by hormones, such as 20-fydroxyecdysone (20E) and juvenile hormone (JH) [1,2]. It has been demonstrated that high levels of 20E promote the mitotic division of spermatogonia. However, JH generally exerts an inhibitory effect on spermatogenesis. JH also controls the development and secretion of male accessory glands. The levels of certain secretory peptides in the glands are increased by JH and reduced by 20E [2,3]. Recently, more than a dozen vertebrate-type steroids were identified in insects using specific and sensitive analytical techniques such as gas chromatography-mass spectrometry and radioimmunoassay [4,5]. For example, eleven non-ecdysteroid steroids such as testosterone, 5α-dihydrotestosterone, 11β-hydroxytestosterone and 11-ketotestosterone were identified in the hemolymph of Sarcophaga bullata (Diptera: Sarcophagidae) larvae [6]. Both C21 and C19 steroids were identified and quantified in the hemolymph of Leptinotarsa decemlineata [7]. Two typical fragments of testosterone were identified at the proper retention time and with the right abundance ratios when the contents of testosterone, pregnenolone, and estradiol in ovaria and testes of the locust Locusta migratoria were measured using gas chromatography combined with mass spectrometry [4]. Taken together, the presence of these steroids in insects is remarkable as it implies that the ‘vertebrate-type’ steroid hormone system is very ancient and may be shared by all phyla of animal kingdom. However, up to now, no correlations are proved between steroid titer fluctuations and divergent physiological processes. The vertebral-like steroids present in insect tissue extracts have not been shown to have a physiological role. The primary reason is that application of the typical steroids of higher vertebrates did not cause significant effects on insect physiology [8,9].
Most vertebrate testosterone is produced in the testes from the substrates, cholesterol and acetate. At least four steroidogenic enzymes are required for testosterone synthesis in Leydig cells: cholesterol side chain cleavage enzyme (CYP11A1) [10], HSD3B [11], CYP17A1 [12], and HSD17B3 [13]. However, the major enzymatic steps relevant to synthesis and catabolism, as revealed by studies with tissues of vertebrates, are not, or rarely observed in insects. The current conclusion is that typical vertebrate-type steroids presumably do not serve as physiological active substances in insects [9]. In L. migratoria, metabolic enzymes such as 17β-hydroxy steroid dehydrogenase (HSD), 20 α-HSD and 20 β-HSD are found. However, important biosynthetic enzymes such as 17α-hydroxylase and aromatase are absent, demonstrating that the biosynthesis of vertebrate-type steroids in insects does not follow the commonly used pathways in vertebrates [14].
Previously, the metabolism of vertebrate-type steroids was usually analyzed in a straightforward manner. The steroids were directly administrated to insects or insect tissues and then the metabolites were identified. In most of these studies, radioactive labelled steroids were used as precursors for a high sensitivity. Therefore, conversions of a tiny amount of steroids can easily be detected. These amounts may exactly match the physiological concentrations of endogenous substrates. In most in vivo studies, vertebrate-type steroids were delivered to insects by injection or feeding [15,16]. When progesterone was injected into S. bullata larvae, its metabolism into 17α-hydroxy-progesterone and testosterone was detected by chromatographic methods [15]. However, detection of the steroid metabolites in these insects is not necessarily an indication of their physiological relevance.
In most insects, vitellogenin (Vg) synthesis and deposition in developing oocytes during oogenesis are crucial for successful female reproduction [17,18]. These processes are also modulated by endocrine hormones such as JH, ecdysteroids, and neuropeptides [[19], [20], [21], [22]]. The roles of JH and ecdysteroids in controlling reproduction vary among insect groups. JH is the major hormone that regulates vitellogenesis in hemimetabolous insect orders, including Orthoptera, Blattodea, and Hemiptera. However, reproduction in the insect orders Diptera, Hymenoptera, and Lepidoptera is governed by ecdysteroids [[23], [24], [25], [26]].
Vg is a fundamental protein that is indispensable for reproduction. Its primary function is to supply nutrients and functional substances such as amino acids, fats, carbohydrates, phosphorus, and sulfur to developing oocytes [27]. It has long been established that insect Vg is usually female-specific. However, a male Vg gene was recently identified in the green lacewing, Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). The C. pallens male Vg has conserved cysteine residues, GL/ICG, RXXR, and other specific domains, similar to Vgs in other insects. Its expression level was stable at different ages but was significantly higher in the abdomen than in the head and chest [28]. C. pallens is one of the most prolific predators of insect pests, including various aphids, caterpillars, and larvae and eggs of other insects in the world's most agriculturally important regions [29,30]. These insect pests damage leaves, flowers, and fruits of many economically important crops, such as rice, maize, wheat, cotton, soybean and vegetables, and transmit a variety of destructive plant viruses. C. pallens has been extensively studied for its biological control potential for development of integrated pest management strategies to reduce the use of chemical insecticides in agricultural areas of the world [[31], [32], [33], [34]].
In this study, we found that C. pallens male Vg regulated male life expectancy and post-mating reproduction by maintaining the synthesis of immune-related compounds, including vertebrate-type testosterone. Topical testosterone application significantly recovered the life expectancy of Vg-deficient males and reproduction of post-mating females, suggesting that vertebrate-type steroid maintains male longevity and female reproduction under the control of male vitellogenin in C. pallens.
2. Material and methods
2.1. Insects
C. pallens culture used in the study was collected from the Beijing suburbs (40 that vertebrate-typeand reared in a greenhouse for more than 20 generations after identification using an EZ4 dissecting microscope (Leica, Wetzlar, Germany). C. pallens was fed pea aphids, Acyrthosiphon pisum (Hemiptera: Aphididae) reared on broad beans, Vicia faba L. (Fabales: Leguminosae) and kept at 25 °C with a photoperiod of 16:8 h light/dark cycle and a relative humidity of 70 %.
2.2. RNA interference (RNAi)
Male C. pallens Vg was depleted using dsRNA-mediated RNAi, as previously described [30,35,36]. A unique target fragment of 469 bp showing very low homology with other insect Vgs was selected from the C-terminus of the male C. pallens Vg transcript. Total RNA was isolated from the whole body of C. pallens adult male using TRIzol™ reagent (ThermoFisher, Waltham, MA, USA) on day six after eclosion. First-strand cDNA was synthesized using TransCript First-Strand cDNA Synthesis SuperMix (Transgene, Beijing, China). T7 primers (T7 promoter plus exon-specific sequence) were designed to amplify the Vg and gfp fragments. In vitro dsRNA synthesis was performed using the MEGAscript® RNAi Kit (Ambion, Austin, TX, USA). Template DNA and ssRNA in the dsRNA solution were removed by DNase/RNase digestion. Primers used are listed in Supplementary Table S1.
C. pallens cocoons of wild type were maintained under standard rearing conditions. Once adults emerged, males and females were distinguished by observing the externalia and kept separated. The dsRNA (2 μg, 2 mg/ml) was injected into 2-day-old male adults using an Eppendorf InjectMan NI 2 and FemtoJet express microinjector system (Eppendorf, Hamburg, Germany). Each male was injected at the joint of the fourth and fifth abdominal segments and housed with a virgin female. Thirty-four to thirty-seven male adults were injected for each dsRNA. The male survival rates were recorded daily until all males died. When female adults began to oviposit, the eggs were counted, and lifetime fecundity was calculated.
2.3. Quantitative PCR (qRT-PCR)
Gene transcript levels were examined by qRT-PCR. Using a total reaction volume of 25 μL, the mixture contained 200 nM each of forward and reverse gene-specific primers, 11.25 μL Go Taq qPCR Master Mix (Promega, Madison, WI, USA), and cDNA produced from 2 μg total RNA. qRT-PCR was conducted on a LightCycler® 96 (Roche, Basel, Switzerland) using the following thermal program: initial incubation at 95 °C for 60 s, followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. The housekeeping gene actin from C. pallens was used as an internal control for normalization of gene expression. Four technical replicates were used for each treatment. Relative mRNA levels were quantified using the 2−ΔΔCt approach [37]. The specific primers for the tested genes are listed in Table S1.
2.4. Microscopy of female ovaries
On day six post-emergence, three females were randomly selected for each dsRNA treatment. The ovaries were dissected and observed at 20-fold magnification using a VHX-2000 digital microscope (KEYENCE, Neu-Isenburg, Germany) as previously described [30]. Three ovarioles were randomly selected from a single ovary and the length of primary follicles was measured.
2.5. Quasi-targeted metabolomics analysis
On day 5 post-eclosion, the dsRNA-treated males were dissected in PBS, and the terminal 3.5 abdominal segments containing the entire reproductive tract were cut from the body and ground in liquid nitrogen. The homogenate (100 mg) was suspended in pre-chilled 80 % methanol (500 μL) with vortexing and centrifuged at 15,000×g for 20 min at 4 °C. The supernatant was diluted with methanol to a final concentration of 53 %. The LC-MS/MS analysis was performed using an Exion LC system (SCIEX, Framingham, MA, USA) coupled to a QTRAP 6500 Plus spectrometer (SCIEX). An Xselect HSS T3 column (Waters, Framingham, MA, USA) was used to separate the extracts with a solvent gradient at a flow rate of 0.4 ml/min. The solvent gradient was as follows: 2 min, 98 % A; 15 min, 98-0% A; 17 min, 0 % A; 17.1 min, 0–98 % A; 20 min, 98 % A. The eluents used for the positive/negative polarity mode were eluent A (0.1 % formic acid in water) and eluent B (0.1 % formic acid in acetonitrile). Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were performed using the MetaX software (http://metax.genomics.cn/). The KEGG, HMDB, and LIPIDMaps databases were used to annotate the identified metabolites. Five to six replicates were analyzed for each dsRNA treatment.
2.6. Testosterone measurement
Testosterone samples were prepared using liquid-liquid extraction (LLE) [38]. The abdomens of ten male C. pallens were ground in liquid nitrogen on days 0, 10, and 15 post eclosion. The homogenate (100 mg) was suspended in 200 μl buffer A (0.5 mol/L ammonium acetate; pH, 5.5) and mixed at room temperature for 5 min. The LLE was performed twice using a mixture of ethyl acetate and n-hexane solution (3:2, v/v). The upper organic phase was transferred and evaporated to dryness using nitrogen blowing apparatus. The extract was dissolved in 200 μl of basic buffer (0.2 mol/L sodium carbonate; pH, 9.8). The solution was extracted twice using n-hexane (500 μl each time). The organic phase was dried at 40 °C and redissolved in 100 μl of methanol for mass spectrum analysis.
To confirm the presence of testosterone in C. pallens males, LC-MS/MS was performed by a Vanquish™ Flex UHPLC system with a Q Exactive™ GC Orbitrap™ mass spectrometer (Thermo Fisher Scientific™, Waltham, MA, USA) as previously described [39]. Testosterone (≥98.33 %) was purchased from Dr. Ehrenstorfer (Augsburg, Germany) and used as the calibration standard. Testosterone was separated on an Accucore™ Vanquish™ C18 + UHPLC column (1.5 μm, 100 × 2.1 mm, Thermo Fisher Scientific™) kept at 30 °C using a gradient program. Solvents A and B comprised 0.1 % formic acid in water and acetonitrile, respectively, with program 0–20 min, 5%–95 % B; 20–22 min, 100 % B, 22–25 min, 5 % B. The flow rate was maintained at 0.4 ml/min and the injection volume was 5 μl.
To quantify testosterone in C. pallens males at different developmental stages, UPLC-MS analysis was performed with an ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm) (Waters) using a gradient program. Three replicates were analyzed for each stage. Solvents A and B comprised 0.1 % formic acid in water and acetonitrile, respectively with a program of 0–5 min, 40%–65 % B; 5.25–7.25 min, 100 % B, 7.5–10 min, 40 % B. The flow rate was maintained at 0.3 ml/min. The injection volume was 5 μl and the samples were kept at 4 °C in the autosampler.
2.7. Testosterone treatment
To test the recovery of fecundity by testosterone, a testosterone reagent (Virtue-Clara, Beijing, China) was solubilized in acetone at 20 mg/ml. Two micrograms of dsVg were injected into males on day 2 post-emergence and then testosterone solution (1 μl) was dropped onto the joint of the fourth and fifth abdominal segments of males on days 3 and 4 post-emergence for easy absorption. Two control groups were established in this study. One comprised males treated with 2 μg of dsVg on day 2 post-emergence and treated with 1 μl of acetone on days 3 and 4 post-emergence. The other consisted of males treated with 2 μg of dsgfp on day 2 post-emergence and treated with 1 μl of acetone on days 3 and 4 post-emergence. After the second testosterone and acetone treatments, each male was kept with a virgin female (2-day-old) and reproductive parameters were recorded.
3. Results
3.1. Male Vg is crucial for male lifespan and female reproduction
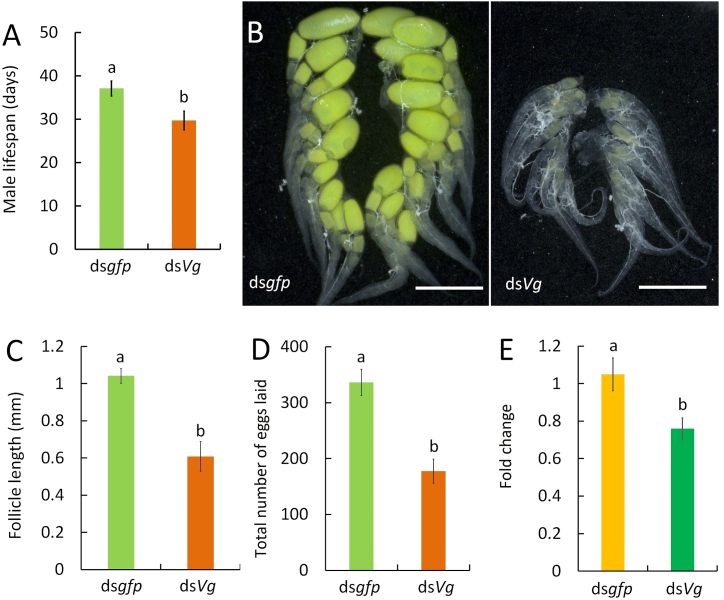
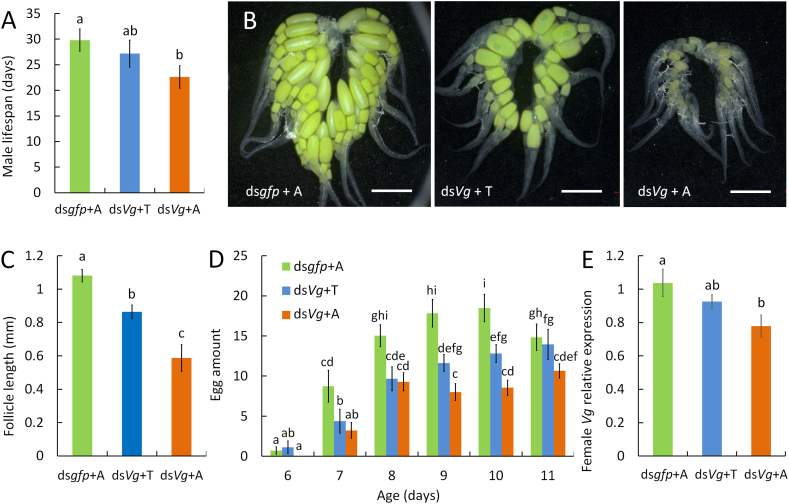
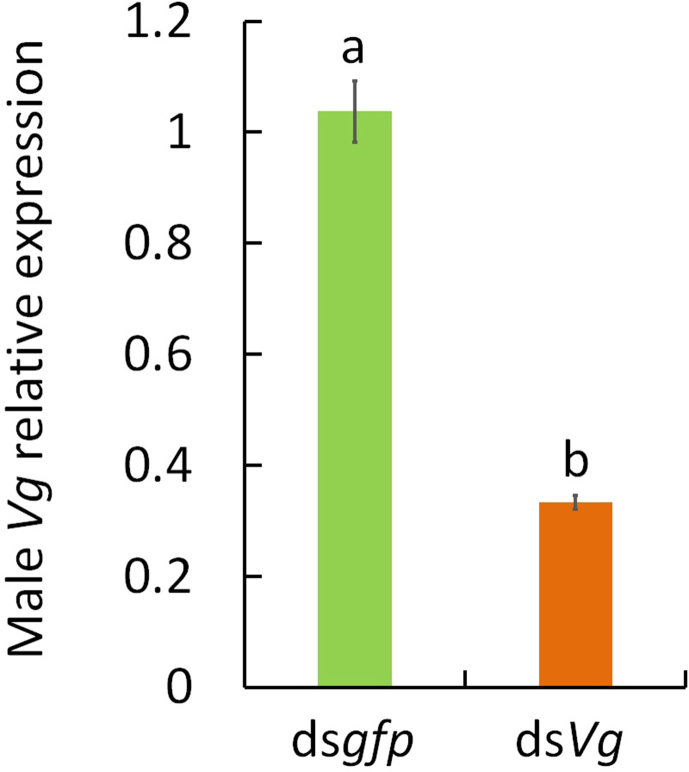
Male Vg transcripts were significantly reduced following dsVg injection (Fig. S1) (P < 0.01). The lifespan of dsVg-treated males was significantly shorter than that of dsgfp-treated males (Fig. 1A) (P < 0.01). Six days after emergence, females mated with dsgfp-treated males exhibited mature ovaries with vitellogenic primary follicles. However, ovarian development in females mated with dsVg-treated males was arrested (Fig. 1B). The primary follicles in females mated with dsVg-treated males were significantly smaller than those in females mated with dsgfp-treated males (Fig. 1C) (P < 0.01). Total fecundity (P < 0.01) (Fig. 1D) and Vg expression (Fig. 1E) (P = 0.02) in females were significantly impaired after mating with dsVg-treated males.
Fig. 1.
Knockdown of male Vg shorted male lifespan and impaired female reproduction. Male life expectancy was significantly shortened by Vg knockdown (A) (t-test, n = 34–37). Mating with dsVg-treated males suppressed ovarian development (B), reduced primary follicle size (C) (t-test, n = 9) and reproductive output (D) (t-test, n = 34–37), and significantly reduced female Vg expression (E) (t-test, n = 4). B, scale bar = 1 mm. Bars (mean ± SE) with different letters indicate significant differences.
3.2. Differentially accumulated metabolites after male Vg knockdown
Metabolome analysis yielded 425 and 361 compounds in positive and negative ion modes, respectively. The thresholds for significant differences in metabolite abundance were VIP >1, FC > 1.2, and P < 0.05. The principal component analysis (PCA) revealed that dsVg and dsgfp had different effects on metabolite accumulation in the reproductive tracts (Fig. 2A). Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis of differential metabolites showed that five pathways, including tryptophan metabolism and steroid hormone synthesis, were enriched in the dsVg-versus-dsgfp differential metabolites (Fig. 2B). The knockdown of male Vg led to the downregulation of 22 compounds. Among them, the level of the steroid hormone testosterone was reduced by 41.1 % following the knockdown of male Vg. The top ten downregulated metabolites are shown in Table 1.
Fig. 2.
PCA plot and KEGG enrichment of differentially accumulated metabolites post male Vg knockdown.
PCA plot of metabolite features in males treated with dsVg and dsgfp (A). KEGG enrichment map of differentially accumulated metabolites post male Vg knockdown (B). In B, the vertical axis represents pathway entries and the horizontal axis represents the ratio of the number of differentially accumulated metabolites in the pathway to the total number of metabolites in the pathway. Circle size corresponds to the number of enriched metabolites.
Table 1.
Differential metabolites post male Vg knockdown.
| Compound ID | Annotation | Classification | Log2FC |
|---|---|---|---|
| Com_519_Pos | Dl-Threitol | Sugar Alcohols | −0.92843 |
| Com_343_Neg | Phenylpyruvic Acid | Organic Acid And Its Derivatives | −0.86014 |
| Com_155_Neg | Indoleacetaldehyde | Organoheterocyclic Compounds | −0.82646 |
| Com_2_Neg | Indolelactate | Indole And Its Derivatives | −0.78699 |
| Com_410_Pos | 5-Hydroxytryptophol | Indole And Its Derivatives | −0.76389 |
| Com_539_Pos | Testosterone | Hormones | −0.76328 |
| Com_275_Neg | Alpha-Ketoglutaric Acid | Tca Cycle | −0.69826 |
| Com_62_Neg | Acetoacetate | Organic Acid And Its Derivatives | −0.62387 |
| Com_129_Neg | Alpha-Benzylsuccinic Acid | Organic Acid And Its Derivatives | −0.60988 |
| Com_195_Neg | Thymidine 5′-Diphosphate | Nucleotide And Its Derivates | −0.60937 |
3.3. Identification of testosterone in adult males
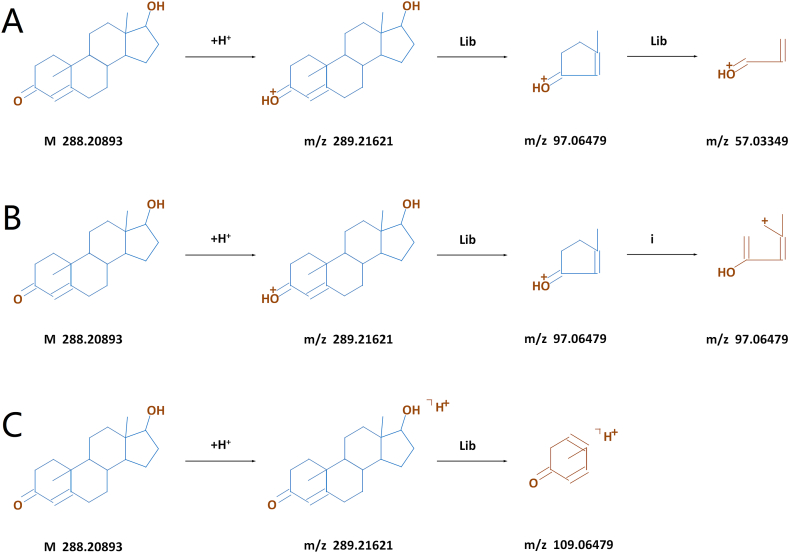
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) revealed the cleavage of testosterone molecules and the mass-to-charge ratio (m/z) of the testosterone ions. The testosterone molecules were first positively charged to produce a parent ion (m/z = 289.21), and then fractured to generate several fragment ions (m/z = 97.06, 57.03, and 109.06) (Fig. 3A–C).
Fig. 3.
Structures of testosterone molecules and their cleavage into fragment ions revealed by LC-MS/MS. A, Charge of testosterone and its cleavage at m/z 57.03. B, Charge of testosterone and its cleavage at m/z 97.04. C, Charge of testosterone and its cleavage at m/z 109.06.
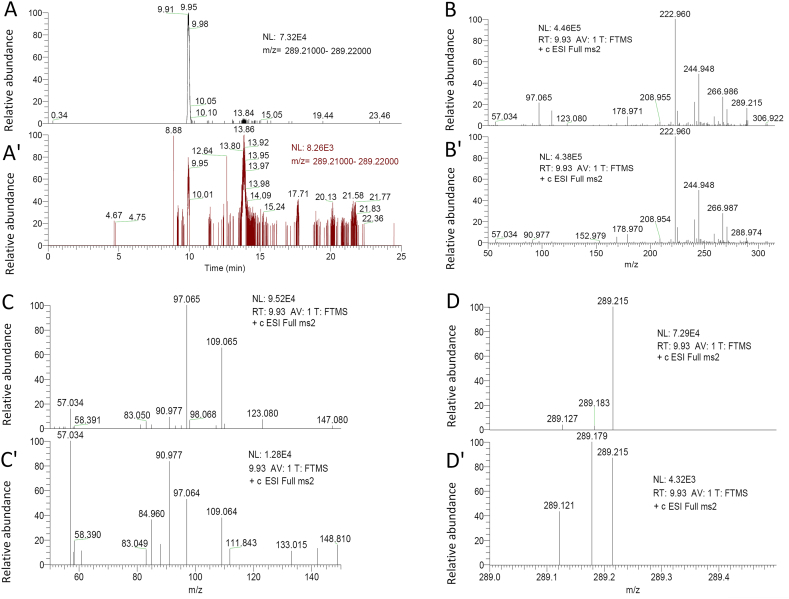
Extracted ion chromatography (EIC) of the testosterone standard showed a testosterone retention time of 9.95 min (Fig. 4A). The EIC of the testosterone sample from 5-day-old males showed multiple chromatographic peaks due to the presence of impurities in the sample, including an obvious peak at 9.95 min (Fig. 4A’). The testosterone fragment ion at m/z 57.034 was detected in testosterone standard and sample (Fig. 4B and B′). Furthermore, both the testosterone standard and sample in 5-day-old males contained ions at m/z 57.03, 97.06, 109.06 (Fig. 4C and C′), and 289.21 (Fig. 4D and D’). These results suggest that testosterone is present in 5-day-old C. pallens.
Fig. 4.
LC-MS/MS chromatograms of testosterone standard (5 ppm) and samples from 5-day-old C. pallens males. A, Extracted ion chromatogram (EIC) of testosterone standard. A′, EIC of testosterone sample. B, Secondary ion mass spectrum of testosterone standard. B′, Secondary ion mass spectrum of testosterone sample. In B and B′, the testosterone fragment ion at m/z 57.034 is shown. C, Secondary ion mass spectrum obtained from monitoring the testosterone standard transition for m/z 50–150. C′, Secondary ion mass spectrum obtained from monitoring the testosterone sample transition for m/z 50–150. In C and C′, three testosterone fragment ions were detected at m/z 57.03, 97.06 and 109.06. D, Secondary ion mass spectrum obtained from monitoring the testosterone standard transition for m/z 289.0–289.5. D′, Secondary ion mass spectrum obtained from monitoring the testosterone sample transition for m/z 289.0–289.5. In D and D′, the positively charged parent ion of testosterone is shown (m/z = 289.21).
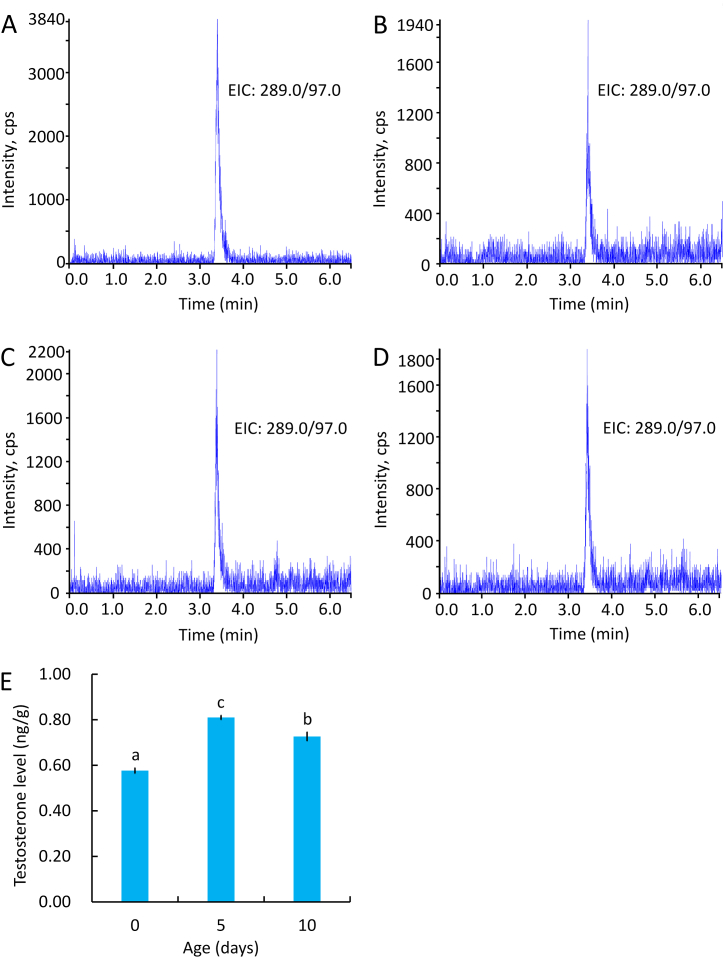
C. pallens males at different developmental stages synthesized testosterone (Fig. 5A–D). The testosterone level on days 0, 5, and 10 post emergence were 0.58 ± 0.01, 0.81 ± 0.01 and 0.73 ± 0.02 ng/g) (Fig. 5E) (F = 65.45, P < 0.01).
Fig. 5.
Quantification of testosterone in C. pallens males using UPLC-MS. A, EIC of testosterone (0.8 ng/ml) for calibration. B, EIC of testosterone in 0-day-old males. C, EIC of testosterone in 5-day-old males. D, EIC of testosterone in 10-day-old males. E, Testosterone level in C. pallens males (one-way ANOVA, n = 3).
3.4. Restoration of fecundity and longevity by testosterone
Topical application of testosterone to dsVg-treated males restored their longevity. The male longevity in the dsgfp-acetone-treated group was significantly longer than that in dsVg-acetone-treated group, but not significantly longer than that in dsVg-testosterone-treated group (Fig. 6A) (F = 2.27, P = 0.11). The use of testosterone in dsVg-treated males also rescued post-mating ovarian development (Fig. 6B) and significantly increased the primary follicle size (Fig. 6C) (F = 19.13, P < 0.01). Reagent, age, and their interaction had significant effects on the daily fecundity of females mated with dsVg-treated males (reagent: df = 2, P < 0.01; age: df = 5, P < 0.01; age × reagent: df = 10, P = 0.01) (Fig. 6D). Relative expression of Vg in females mated with dsVg-treated males was partially recovered by the use of testosterone in males (F = 5.28, P = 0.03) (Fig. 6E).
Fig. 6.
Restore of male longevity and female reproduction by testosterone. Female Reproduction is partially restored by topical testosterone application in males. Male lifespan (A) (one-way ANOVA, n = 29–32), female ovarian development (B), primary follicle size (C) (one-way ANOVA, n = 9), total fecundity (D) (Generalized Linear Model, n = 29–32) and Vg expression on day 10 post emergence (E) (one-way ANOVA, n = 4) post mating were recovered substantially. Scale bar = 1 mm. A, acetone; T, testosterone. Bars (mean ± SE) with different letters represent significant differences.
4. Discussion
In recent years, LC-MS/MS has been widely used for testosterone measurement. Despite its high specificity, mass spectrometry has drawbacks in terms of testosterone measurements. For example, because other endogenous compounds are similar in structure and molecular mass, testosterone must be chromatographically separated for accurate quantification. LC-MS/MS analysis of C. pallens testosterone was performed following the sensitive method previously described [39]. This method was established to detect total testosterone levels in all patients. The accuracy was verified using the American National Institute of Standards and Technology Standard Reference Material 971. In the present study, vertebrate-type testosterone in C. pallens males was identified using quasi-targeted metabolomic analysis. Furthermore, LC-MS/MS revealed that both the testosterone standard and sample in C. pallens males contained ions at m/z 57.03, 97.06, 109.06 (Fig. 4C–F), and 289.21 (Fig. 4G–H), demonstrating that vertebrate-type testosterone was present in C. pallens males. Now, the origin of vertebrate-type steroids in insect tissues still remains concerns. One hypothesis is that they are simply derived from dietary source of the insects [9]. This hypothesis is less likely to hold in the present study because the males treated with dsVg and dsgfp were fed the same diet in the assay, and the vertebrate-type testosterone level in C. pallens males should be independent of dietary source.
The average concentration of total testosterone for all human age groups is 15.36 ± 4.86 nmol/L [40]. That is approximately 4.43 ± 1.40 ng/g. However, the abdominal vertebrate-type testosterone of 5-day-old C. pallens males was 0.81 ng/g, a concentration much lower than that in humans. Taken together, these results suggest that C. pallens can synthesize the vertebrate androgen testosterone, in addition to common hormones such as JH and 20E. Since the first isolation and synthesis of the testosterone molecule in 1935, vertebrate testosterone has been studied for nearly a century [41]. In the past decades, the endocrine system in insects is found much more intricate than previously deemed and sharing many common characteristics with that in vertebrates. The classical sex steroid hormones of higher vertebrates, including progesterone, testosterone, estradiol, has been identified in insect tissues [4,5,42,43]. So far, biosynthesis of vertebrate-type steroids from cholesterol has been documented in two insects, the water beetle Acilius sulcatus (Coleoptera: Dytiscidae) and the tobacco hornworm Manduea sexta (Lepidoptera: Sphingidae). The vertebrate-type steroids in the prothoracic glands of A. sulcatus are linked to defense against vertebrate predators [44,45]. The tobacco hornworm M. sexta has no distinct defensive glands. However, high amounts of a vertebrate-type steroid conjugate, i.e. 20β-diol glucoside, were isolated from ovaries and eggs. To date, nothing is known about a physiological function of the C21 steroid conjugate in ovaries and eggs of this species [46]. In the present study, the abundance of C. pallens vertebrate-type testosterone in males is closely associated with longevity of males and fecundity of post-mating females, suggesting its crucial roles in regulation of insect life-span and reproduction.
Women around the world live longer than men. One possible mechanism leading to sex differences in longevity in humans is that testosterone has a depressing effect on the immune system [40]. However, in the present study, treatment with testosterone significantly extended the life expectancy of Vg-deficient males, demonstrating that the C. pallens testosterone and its counterparts in humans have opposite effects on life expectancy. Topical treatment with testosterone positively affected C. pallens male life span and female reproduction. Although these two events occurred in different sexes, their trends followed the same direction. In most animals, longevity is achieved at the expense of fertility, but social insects, such as the ant Cardiocondyla obscurio (Hymenoptera: Formicidae) and queen honeybee Apis mellifera (Hymenoptera: Apidae), do not have this tradeoff [47,48]. Therefore, life expectancy is positively correlated with the reproductive performance of social insects. This phenomenon was also observed in C. pallens.
Insect Vg is generally known as a female-specific protein fundamental to female reproduction. Males of a small number of species, such as honeybees, have Vg, and its function is associated with social organization [49]. Here, knockdown of C. pallens male Vg significantly shortened the male lifespan and impaired female reproduction, suggesting novel roles for insect Vg. In queen bees, Vg acts as an antioxidant that extends longevity [50]. We conclude that the C. pallens male Vg regulates lifespan via a similar mechanism.
Metabolome profiles indicated that in addition to testosterone, dl-threitol, phenylpyruvic acid, indoleacetaldehyde, indolelactate, and 5-hydroxytryptophol were also downregulated by treatment with dsVg (Table 1). To date, the threitol biosynthetic pathway has not been fully characterized in most organisms. In the Alaskan beetle Upis ceramboides (Coleoptera: Tenebrionidae), threitol accumulation is associated with cold tolerance [51]. In the present study, dl-threitol may be linked to tolerance of adverse conditions in C. pallens males. Phenylpyruvic acid is an intermediate or catabolic by-product of phenylalanine metabolism [52]. In malarial mosquitoes, phenylalanine metabolism regulates reproduction and immunity. Silencing of phenylalanine hydroxylase (PAH), which is involved in the conversion of phenylalanine to tyrosine, significantly reduces the number of eggs, retards vitellogenesis, and impairs melanization of the chorion [53]. However, the role of the phenylalanine metabolism in male insects remains unclear. Here, we demonstrated that it is also associated with vitellogenesis and immunity in C. pallens males.
KEGG analysis revealed that tryptophan metabolism was enriched in metabolites that differentially accumulated between dsgfp-treated and dsVg-treated males (Fig. 2B). Indoleacetaldehyde and indolelactate are distributed in this pathway. In the human body, tryptophan and its metabolites affect immune reactions, possess antioxidant properties, and act as anabolic signals [54]. In insects, tryptophan is the initial precursor for the formation of ommochromes, which regulate the color of insect wings and eyes [55,56]. In the present study, it was difficult to explain the downregulation of tryptophan metabolism after disruption of male Vg. We speculate that C. pallens male Vg is responsible for maintaining stable tryptophan metabolism that may be essential for immunity and spermatogenesis.
Briefly, among the metabolites differentially accumulated between in dsVg-treated- and dsgfp-treated males, dl-threitol, phenylpyruvic acid, indoleacetaldehyde, indolelactate, and testosterone have been implicated in insect or vertebrate adversity tolerance and immunity. Their downregulation leads to a significantly shorter lifespan in Vg-deficient males.
Data availability statement
No data was used for the research described in the article.
Limitations of the study
A major limitation of our study is that we did not rule out the possibility that the vertebrate-type testosterone in male C. pallens may be derived from the dietary source. In this study, the testosterone level is about 41.1 % lower in Vg-deficient males than in dsgfp treated males. This possibility is less likely to hold because the Vg-deficient males and dsgfp treated males were fed the same diet, pea aphids. However, to completely rule out the possibility, the presence of vertebrate-type testosterone in pea aphids should be analyzed using LC-MS/MS. We can be sure that male C. pallens synthesize the vertebrate-type testosterone if the testosterone is not detected in pea aphids.
CRediT authorship contribution statement
Xiaoping Liu: Methodology, Investigation, Formal analysis. Xingkai Guo: Methodology, Investigation. Tingting Zhang: Validation, Supervision, Formal analysis. Jiaqi Duan: Methodology, Investigation. Lisheng Zhang: Supervision, Project administration, Conceptualization. Mengqing Wang: Visualization, Supervision. Yuyan Li: Validation, Supervision. Zhongjian Shen: Visualization, Supervision. Jianjun Mao: Writing – review & editing, Writing – original draft, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by the National Key R&D Program of China (2021YFD1000500 and 2021YFD1400803), Projects of Guizhou Tobacco Corporation (201936, 201937, 201941), and Major Projects of China National Tobacco Corporation (110202001032 ([LS-01]).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32478.
Appendix A. Supplementary data
The following are the supplementary data to this article:
figs1.
References
- 1.Happ G.M. Maturation of the male reproductive system and its endocrine regulation. Annu. Rev. Entomol. 1992;37:303–320. doi: 10.1146/annurev.en.37.010192.001511. [DOI] [PubMed] [Google Scholar]
- 2.Gade G., Hoffmann K.H., Spring J.H. Hormonal regulation in insects: facts, gaps, and future directions. Physiol. Rev. 1997;77:963–1032. doi: 10.1152/physrev.1997.77.4.963. [DOI] [PubMed] [Google Scholar]
- 3.Gillott C. Male accessory gland secretions: modulators of female reproductive physiology and behavior. Annu. Rev. Entomol. 2003;48:163–184. doi: 10.1146/annurev.ento.48.091801.112657. [DOI] [PubMed] [Google Scholar]
- 4.Novak E.J.S., Lambert J.G.D. Pregnenolone, testosterone and estradiol in the migratory locust Locusta migratoria: a gas chromatographical-mass spectrometrical study. Gen. Comp. Endocrinol. 1989;76:73–82. doi: 10.1016/0016-6480(89)90034-8. [DOI] [PubMed] [Google Scholar]
- 5.Bradbrook D.A., Clement C.Y., Cook B., Dinan L. The occurrence of vertebrate-type steroids in insects and a comparison with ecdysteroid levels. Comp. Biochem. Physiol. 1990;95:365–374. [Google Scholar]
- 6.De Clerck D., Diederik H., De Loof A. Identification by capillary gas chromatography-mass spectrometry of eleven non-ecdysteroid steroids in the haemolymph of larvae of Sarcophaga bullata. Insect Biochem. 1984;14:199–208. [Google Scholar]
- 7.De Clerck D., Diederik H., Paesen G., De Loof A. Identification and quantification of C21 and C19 steroids in the haemolymph of Leptinotarsa decemlineata, a phytophagous insect. Insect Biochem. 1988;18:93–99. [Google Scholar]
- 8.Brueggemeier R.W., Yocum G.D., Denlinger D.L. In: Endocrinological Frontiers in Physiological Insect Ecology. Sehnal F., Zabza A., Denlinger D.L., editors. Wroclaw Technical University Press; 1989. Estranes, androstanes and pregnanes in insects and other invertebrates; pp. 885–898. [Google Scholar]
- 9.Swevers L., Lambert J.G.D., De Loof A. Synthesis and metabolism of vertebrate-type steroids by tissues of insects: a critical evaluation. Experientia. 1991;47:687–698. doi: 10.1007/BF01958817. [DOI] [PubMed] [Google Scholar]
- 10.Zirkin B.R., Papadopoulos V. Leydig cells: formation, function, and regulation. Biol. Reprod. 2018;99:101–111. doi: 10.1093/biolre/ioy059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang P.Z., Tsai-Morris C.H., Dufau M.L. Regulation of 3beta-hydroxysteroid dehydrogenase in gonadotropin-induced steroidogenic desensitization of Leydig cells. Endocrinology. 1998;139:4496–4505. doi: 10.1210/endo.139.11.6316. [DOI] [PubMed] [Google Scholar]
- 12.Gilep A.A., Sushko T.A., Usanov S.A. At the crossroads of steroid hormone biosynthesis: the role, substrate specificity and evolutionary development of CYP17. Biochim. Biophys. Acta. 2011;1814:200–209. doi: 10.1016/j.bbapap.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Ge R.S., Hardy M.P. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat Leydig cells. Endocrinology. 1998;139:3787–3795. doi: 10.1210/endo.139.9.6183. [DOI] [PubMed] [Google Scholar]
- 14.De Loof A., Broeck J.V., Swevers L., Schoofs L. In: Molecular Insect Science. Hagedorn H.H., Hildebrand J.G., Kidwell M.G., Law J.H., editors. Springer; 1990. Vertebrate-type neuropeptides and steroids in Locusta migratoria: identification and metabolism; pp. 173–179. [Google Scholar]
- 15.De Clerck D., Eechaute W., Leusen I., De Loof A. Study of the metabolism of steroids in larvae of the fleshfly Sarcophaga bullata. Comp. Biochem. Physiol. 1987;87B:821–826. doi: 10.1016/0305-0491(87)90395-6. [DOI] [PubMed] [Google Scholar]
- 16.Swevers L. Catholic University of Leuven; Belgium: 1990. Search for the Origin and Possible Functions of Vertebrate-type Steroids in a Few Insect Species. Ph.D. Thesis. [Google Scholar]
- 17.Raikhel A. In: Biology of Disease Vectors. Marquardt W., editor. Elsevier; 2005. Vitellogenesis of disease vectors, from physiology to genes; pp. 329–346. [Google Scholar]
- 18.Swevers L., Raikhel A., Sappington T., Shirk P., Iatrou K. In: Comprehensive Insect Physiology, Biochemistry, Pharmacology and Molecular Biology. Gilbert L., Gill S., Iatrou K., editors. Elsevier; 2005. Vitellogenesis and post-vitellogenic maturation of the insect ovarian follicle; pp. 87–155. [Google Scholar]
- 19.Engelmann F. In: Endocrinology of Insects, Liss. Downer R., Laufer H., editors. 1983. Vitellogenesis controlled by juvenile hormone; pp. 259–270. [Google Scholar]
- 20.Hagedorn H. In: Comprehensive Insect Physiology, Biochemistry and Pharmacology. Kerkut G., Gilbert L., editors. Pergamon; 1985. The role of ecdysteroids in reproduction; pp. 205–226. [Google Scholar]
- 21.Hagedorn H. In: Biology of Disease Vectors. Marquardt W., editor. Elsevier; 2005. Mosquito endocrinology; pp. 317–327. [Google Scholar]
- 22.Zhang T., Liu X., Zhang L., Wang M., Li Y., Mao J. Four insulin-like peptides orchestrate reproductive signaling of the green lacewing, Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae) Ann. Entomol. Soc. Am. 2022;115:352–359. [Google Scholar]
- 23.Parthasarathy R., Sheng Z., Sun Z., Palli S.R. Ecdysteroid regulation of ovarian growth and oocyte maturation in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2010;40:429–439. doi: 10.1016/j.ibmb.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gujar H., Palli S.R. Juvenile hormone regulation of female reproduction in the common bed bug, Cimex lectularius. Sci. Rep. 2016;6:1–10. doi: 10.1038/srep35546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S., Saha T.T., Zou Z., Raikhel A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018;63:489–511. doi: 10.1146/annurev-ento-020117-043258. [DOI] [PubMed] [Google Scholar]
- 26.Wu Z., Yang L., He Q., Zhou S. Regulatory mechanisms of vitellogenesis in insects. Front. Cell Dev. Biol. 2021;8:1863. doi: 10.3389/fcell.2020.593613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tufail M., Takeda M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008;54:1447–1458. doi: 10.1016/j.jinsphys.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Feng Y., Liu X., Zhang T., Chen H., Wang M., Liu C., Li Y., Zhang L., Mao J. Identification and expression characteristics of vitellogenin gene in Chrysopa pallens males. Chin. J. Biol. Control. 2021;37:77–85. [Google Scholar]
- 29.Tauber M.J., Tauber C.A., Daane K.M., Hagen K.S. Commercialization of predators: recent lessons from green lacewings (Neuroptera: Chrysopidae: Chrysoperla) Am. Entomol. 2000;46:26–38. [Google Scholar]
- 30.Han B., Zhang T., Feng Y., Liu X., Zhang L., Chen H., Zeng F., Wang M., Liu C., Li Y., Cui J., Li Z., Mao J. Two insulin receptors coordinate oogenesis and oviposition via two pathways in the green lacewing, Chrysopa pallens. J. Insect Physiol. 2020;123 doi: 10.1016/j.jinsphys.2020.104049. [DOI] [PubMed] [Google Scholar]
- 31.Montezano D.G., Specht A., Sosa-Gomez D.R., Roque-Specht V.F., Sousa-Silva J.C., Paula-Moraes S.V., Peterson J.A., Hunt T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018;26:286–300. [Google Scholar]
- 32.Sarkar S.C., Wang E., Zhang Z., Wu S., Lei Z. Laboratory and glasshouse evaluation of the green lacewing, Chrysopa pallens (Neuroptera: Chrysopidae) against the western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae) Appl. Entomol. Zool. 2019;54:115–121. [Google Scholar]
- 33.Cao W., Zang T., Yang H., Xu Z., Gu J., Chen H. Evaluation of predatory function of Chrysopa pallens to larvae of fall armyworm Spodoptera frugiperda. J. Plant Protect. 2020;47:839–844. [Google Scholar]
- 34.Wang J., Li S., Yang J., Guo M., Dai H., Ramirez-Romero R., Jin Z., Wang S. The fitness of mass rearing food on the establishment of Chrysopa pallens in a banker plant system under fluctuating temperature conditions. Insects. 2021;12:1014. doi: 10.3390/insects12111014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Husain M., Rasool K.G., Tufail M., Aldawood A.S. Molecular characterization, expression pattern and RNAi-mediated silencing of vitellogenin receptor gene in almond moth, Cadra cautella. Insect Mol. Biol. 2020;29:417–430. doi: 10.1111/imb.12646. [DOI] [PubMed] [Google Scholar]
- 36.Rasool K.G., Mehmood K., Tufail M., Husain M., Alwaneen W.S., Aldawood A.S. Silencing of vitellogenin gene contributes to the promise of controlling red palm weevil, Rhynchophorus ferrugineus (Olivier) Sci. Rep. 2021;11 doi: 10.1038/s41598-021-01159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak K.J., Schmittgen S.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Sun G., Xue J., Li L., Li X., Cui Y., Qiao B., Wei D., Li H. Quantitative determination of human serum testosterone via isotope dilution ultra-performance liquid chromatography tandem mass spectrometry. Mol. Med. Rep. 2020;22:1576–1582. doi: 10.3892/mmr.2020.11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.French D. Development and validation of a serum total testosterone liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay calibrated to NIST SRM 971. Clin. Chim. Acta. 2013;415:109–117. doi: 10.1016/j.cca.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Kulikov A.V., Arkhipova L.V. Testosterone and life span or why women live longer than men: a hypothesis. Moscow Univ. Biol. Sci. Bull. 2021;76:137–141. [Google Scholar]
- 41.Nieschlag E., Nieschlag S. Endocrine history: the history of discovery, synthesis and development of testosterone for clinical use. Eur. J. Endocrinol. 2019;180:R201–R212. doi: 10.1530/EJE-19-0071. [DOI] [PubMed] [Google Scholar]
- 42.De Loof A., De Clerck D. In: Advances in Invertebrate Reproduction. Porchet M., Andrirs J.C., Dhainaut A., editors. Elsevier Science Publishers; 1986. Vertebrate-type steroids in arthropods: identification, concentrations and possible functions; pp. 117–123. [Google Scholar]
- 43.Denlinger D.L., Brueggemeier R.W., Mechoulam R., Katlic N., Yocum L.B., Yocum G.D. In: Molecular Entomology, Liss. Law J.H., Alan R., editors. 1987. Estrogens and androgens in insects; pp. 189–199. [Google Scholar]
- 44.Schildknecht H. The defensive chemistry of land and water beetles. Angew. Chem. 1970;9:1–9. [Google Scholar]
- 45.Chapman J.C., Lockley W.J.S., Rees H.H., Goodwin T.W. Stereochemistry of olefinic bond formation in defensive, steroids of Acilius sulcatus (Dytiscidae) Eur. J. Biochem. 1977;81:293–298. doi: 10.1111/j.1432-1033.1977.tb11951.x. [DOI] [PubMed] [Google Scholar]
- 46.Thompson M.J., Svoboda J.A., Lushby W.R., Rees H.H., Oliver J.E., Weirich G.E., Wilzer K.R. Biosynthesis of a C21 steroid conjugate in an insect. The conversion of 14C-cholesterol to 5-14C-pregnen-3β, 20β-diol glucoside in the tobacco hornworm, Manduca sexta. J. Biol. Chem. 1985;260:15410–15412. [PubMed] [Google Scholar]
- 47.Corona M., Velarde R.A., Remolina S., Moran-Lauter A., Wang Y., Hughes K.A., Robinson G.E. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc. Natl. Acad. Sci. U.S.A. 2007;104:7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinze J., Giehr J. The plasticity of lifespan in social insects. Phil. Trans. R. Soc. B. 2021;376 doi: 10.1098/rstb.2019.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson C.M., Ihle K.E., Fondrk M.K., Page R.E., Amdam G.V. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007;5:673–677. doi: 10.1371/journal.pbio.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park H.G., Lee K.S., Kim B.Y., Yoon H.J., Choi Y.S., Lee K.Y., Wan H., Li J., Jin B.R. Honeybee (Apis cerana) vitellogenin acts as an antimicrobial and antioxidant agent in the body and venom. Dev. Comp. Immunol. 2018;85:51–60. doi: 10.1016/j.dci.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Walters K.R., Jr., Pan Q., Serianni A.S., Duman J.G. Cryoprotectant biosynthesis and the selective accumulation of threitol in the freeze-tolerant Alaskan beetle, Upis ceramboides. J. Biol. Chem. 2009;284:16822–16831. doi: 10.1074/jbc.M109.013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smit J.A. Specific activity of phenylalanine deaminase in extracts of the Proteus-Providence group. Nature. 1966;211:1003. doi: 10.1038/2111003a0. [DOI] [PubMed] [Google Scholar]
- 53.Fuchs S., Behrends V., Bundy J.G., Crisanti A., Nolan T. Phenylalanine metabolism regulates reproduction and parasite melanization in the malaria mosquito. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanova M., Kohout P. Tryptophan: a unique role in the critically ill. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222111714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koch P.B. Precursors of pattern specific ommatin in red wing scales of the polyphenic butterfly Araschnia levana L.: haemolymph tryptophan and 3-hydroxykynurenine, Insect. Biol. 1991;21:785–794. [Google Scholar]
- 56.Koch P.B. Production of [14C]-labeled 3-hydroxy-L-kynurenine in a butterfly, Heliconius charitonia L. Heliconidae), and precursor studies in butterfly wing ommatins. Pigm. Cell Res. 1993;6:85–90. doi: 10.1111/j.1600-0749.1993.tb00586.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.