Abstract
Background
Comirnaty, Pfizer-BioNTech's polyethylene-glycol (PEG)-containing Covid-19 vaccine, can cause hypersensitivity reactions (HSRs), or rarely, life-threatening anaphylaxis in a small fraction of immunized people. A causal role of anti-PEG antibodies (Abs) has been proposed, but causality has not yet proven in an animal model. The aim of this study was to provide such evidence using pigs immunized against PEG, which displayed very high levels of anti-PEG antibodies (Abs). We also aimed to find evidence for a role of complement activation and thromboxane A2 release in blood to explore the mechanism of anaphylaxis.
Methods
Pigs (n = 6) were immunized with 0.1 mg/kg PEGylated liposome (Doxebo) i.v., and the rise of anti-PEG IgG and IgM were measured in serial blood samples with ELISA. After ∼2–3 weeks the animals were injected i.v. with 1/3 human dose of the PEGylated mRNA vaccine, Comirnaty, and the hemodynamic (PAP, SAP) cardiopulmonary (HR, EtCO2,), hematological (WBC, granulocyte, lymphocyte and platelet counts) parameters and blood immune mediators (anti-PEG IgM and IgG antibodies, thromboxane B2, C3a) were measured as endpoints of HSRs (anaphylaxis).
Results
The level of anti-PEG IgM and IgG rose 5–10-thousand-fold in all of 6 pigs immunized with Doxebo by day 6, after which time all animals developed anaphylactic shock to i.v. injection of 1/3 human dose of Comirnaty. The reaction, starting within 1 min involved maximal pulmonary hypertension and decreased systemic pulse pressure amplitude, tachycardia, granulo- and thrombocytopenia, and skin reactions (flushing or rash). These physiological changes or their absence were paralleled by C3a and TXB2 rises in blood.
Conclusions
Consistent with previous studies, these data show a causal role of anti-PEG Abs in the anaphylaxis to Comirnaty, which involves complement activation, and, hence, it represents C activation-related pseudo-anaphylaxis. The setup provides the first large-animal model for mRNA-vaccine-induced anaphylaxis in humans.
Keywords: Covid-19, SARS-CoV-2, mRNA, Anaphylaxis, Neutralizing antibodies, Spike protein, Hypersensitivity reactions, ELISA, Anti-PEG IgG, IgM
Introduction
The reported incidence rate of anaphylaxis to the mRNA-lipid nanoparticle (mRNA-LNP)-based Covid-19 vaccines, Comirnaty and Spikevax, ranges in different studies between 3–234 cases per million vaccinees, which rates are considered as rare adverse events [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17].
Regarding the mechanism of the phenomenon, confirmed PEG allergy does occur [18], but there is consensus in the literature that the overwhelming majority of these reactions are not classical type-1 allergies but represent IgE-independent “pseudoallergies” [9], [10], [16], [19]. These allergy-like reactions arise without prior sensitization, by way of direct and/or indirect stimulation of mast cells and also, macrophages, platelets, granulocytes, which are typically not involved in Type-1 allergy [20], [21], [22], [23]. These allergy-mediating immune cells can be triggered by direct binding of reactive nanoparticles (NPs), but also via anaphylatoxin (C3a, C5a) binding to their specific surface receptors. Because anaphylatoxins are byproducts of complement (C) activation, these reactions were dubbed as C activation-related pseudoallergy (CARPA) [24], wherein C activation may be sole cause or a co-trigger [25].
In support of the involvement of CARPA in Comirnaty-induced HSRs, it was pointed out that at least three components delivered by SARS-CoV-2 mRNA vaccines (PEGylated LNP carrier, polyanionic nucleic acid and the ionizable lipid) can activate the C system [26], [27], and in fact, Comirnaty turned out to be a strong activator of porcine C [28]. Accordingly, i.v. administration of the vaccine in pigs was shown to mimic the typical hemodynamic, hematological and blood thromboxane B2 changes in CARPA caused by C-activating liposomes [28]. One of the mechanism by which PEGylated NPs activate C is the binding of anti-PEG Abs to NP surface PEG, which has been shown to cause damage in the NPs [29], [30]. The possible causal role of anti-PEG Abs in mRNA-LNP vaccine-induced HSRs/anaphylaxis was raised in numerous studies [1], [8], [15], [16], [18], [27], [31], but conclusive experimental or clinical evidence has not been presented to date. Consistent with a role of anti-PEG Abs in mRNA-LNP-induced HSRs and anaphylaxis, we found significant correlation between the blood levels of anti-PEG Abs and rise of HSR/anaphylaxis in recipients of Comirnaty and Spikevax [32], providing indirect proof of a causal role of anti-PEG Abs in Comirnaty-induced HSRs. The goal of the present study was to obtain direct evidence for causality, using an anti-PEG hyperimmune pig model [33], which showed the acceleration of HSR to anaphylaxis to PEGylated liposomal doxorubicin (Doxil) if the blood level of anti-PEG IgM had been increased by prior vaccination with drug-free Doxil (Doxebo) [33]. It should be emphasized that we administered the vaccine i.v., although the Comirnaty vaccine is administered intramuscularly (i.m.) in humans. As addressed in the Discussion in detail, a small fraction of the vaccine enters into the bloodstream within minutes even after i.m. administration, whose potential biological effects are reproduced by i.v. injection of a part of the full vaccine dose, as applied in this study.
METHODS
Materials
Comirnaty was from Pfizer/BioNtech, the vaccine used for human vaccinations against SARS-Cov-2 infections. The preparation is characterized in detail in the prescription information and other public information on the vaccine.
The porcine C3a kit was obtained from TECOMedical AG, Sissach, Switzerland (Cat No: TE1078). Zymosan, Dulbecco’s phosphate-buffered saline (PBS) without Ca2+/Mg2+ and bovine calf serum, and biotin-labeled goat polyclonal anti-porcine IgM were from Sigma Chemical Co. (St. Louis, MO, USA).
Preparation of Doxebo
The preparation and characteristics of Doxebo were described earlier [33]. In brief, the freeze-dried lipid components of Doxil were hydrated in 10 mL sterile pyrogen-free normal saline by vortexing for 2-3 min at 70°C to form multilamellar vesicles (MLVs). The MLVs were downsized through 0.4 and 0.1 μm polycarbonate filters in two steps, 10 times each, using a 10 mL extruder barrel from Northern Lipids (Vancouver, British Columbia, Canada) at 62 °C. Liposomes were suspended in 0.15 M NaCl/10 mM histidine buffer (pH 6.5). The size distribution (Z-average): 81.17 nm and phospholipid concentration (12.6 mg/mL) were determined as described earlier [6].
Animals
Mixed-breed Yorkshire/Hungarian White Landrace pigs of both sexes (2-3 months old, 20-28 kg) were obtained from the Animal Breeding, Nutrition and Meat Science Research Institute, Hungarian University of Agriculture and Life Sciences (Herceghalom, Hungary).
Treatment protocol
As outlined in Fig. 1, baseline (“pre-immune”) blood samples were taken from 6 pigs followed by immunization by way of infusion of 0.1 mg PL/kg Doxebo via the ear vein (suspended in 20 mL of saline) at a speed of 1 mL/min. The animals were then placed back into their cages until the 2nd blood sampling 9–10 days later, to screen for anti-PEG Ab induction. From 3 days later, within a period of 13 days, the animals showing seroconversion (all 6) were subjected to the “CARPA induction” protocol. In short, the animals were sedated with Ketamin/Xilazine, and then anesthetized with isoflurane (2-3 % in O2). This was followed by intubation with endotracheal tubes to maintain free airways and to enable controlled ventilation if spontaneous breathing stopped during the experiment. After iodine (10 %, povidone) disinfection of the skin, the pigs were subjected to surgery to insert various catheters into their circulation, namely: (a) a Swan-Ganz catheter (Arrow AI-07124 single-lumen balloon wedge pressure catheter 5 Fr., 110 cm, Arrow International Inc, Reading, PA, USA), into the pulmonary artery via the right external jugular vein (in order to measure the pulmonary arterial pressure (PAP); (b) the left femoral artery to record the systemic arterial pressure (SAP); (c) the left external jugular vein for saline and drug infusion; (d) into the left femoral vein for blood sampling; and (e) the right common carotid artery for arterial blood gas analysis. After 15–30 min adaptation the animals were treated by 5 consecutive i.v. injections into the pulmonary artery of (1) 5 mL PBS (to provide baseline for the hemodynamic changes), (2) bolus injection of 1/3 human dose of Comirnaty (to trigger the immune reaction), (3, 4) 2 repeats of the same dose of Comirnaty (to establish tachyphylaxis, i.e., self-induced tolerance), and finally (5) with 0.1 mg/kg zymosan, as positive control. All injections were administered under 30 s. Among the injections 15–60 min breaks were taken to allow the hemodynamic parameters return to baseline. The latter, as well as the ECG data, were recorded by the physiological monitoring systems of Pulsion Medical Systems SE (Munich, Germany) and Powerlab (ADInstruments, Bella Vista, Australia). The arterial blood gas analysis was executed with a Roche COBAS B221 benchtop analyzer (Roche Diagnostics, Rotkreuz ZG, Switzerland). End-tidal pCO2, O2 saturation, ventilation rate and body temperature were also continuously measured. At the end of the experiments the animals were sacrificed with pentobarbital (120 mg/kg iv.) and concentrated potassium chloride.
Fig. 1.
Timeline of the experimental protocol testing the physiological effects of i.v. Comirnaty in anti-PEG hyperimmune pigs.
Blood cell assays
For the blood cells assays 10 mL venous blood samples were drawn from the pigs at different times into EDTA containing vacuum blood collection tubes (K3EDTA Vacuette, Greiner 367 Bio-One Hungary, Mosonmagyaróvár, Hungary) and aliquoted to 0.5 mL Eppendorf tubes. The white blood cell (WBC), granulocyte (GR) and lymphocyte (LY), platelet (PLT) and red blood cell (RBC) counts and hemoglobin (Hgb) concentration were determined using an ABACUS Junior Vet hematology analyzer (Diatron, Budapest, Hungary).
ELISA of Anti-PEG antibodies
For the analysis of anti-PEG Abs, blood was taken from the ear vein before pretreatment and then at different times specified in the Results. Anticoagulation was done with K3-EDTA tubes. Polysorp (Nunc) plates were coated with 1.25 μg/well DSPE-PEG2000 in 100 μL of bicarbonate buffer (4.46 μM) (pH ∼9.0) overnight at 4 °C, followed by blocking of the wells with 150 μL of PBS/0.05 % Tween-20 + 2 % bovine serum albumin (BSA) at 37 °C for 1.5 h. Before and after blocking, wells were washed two and three times with 300 μL of wash buffer containing PBS/0.05 % Tween-20 for 1 min, respectively. The EDTA-anti-coagulated plasma samples were diluted by PBS/0.05 % Tween-20 + 1 % BSA in the 10-19500-fold range and incubated in the wells for 1.5 h at 37 °C, with slow shaking. Wells were washed five times with 300 μL of wash buffer for 1 min. After staining with 100 μL of HRP-conjugated anti-porcine IgM (2000 × dilution, Sigma) or IgG (800 × dilution, Sigma) for 1 h, wells were washed again five times with wash buffer as mentioned. The antibodies were stained by incubation with 100 μL of substrate solution (Neogen) containing 3,3′,5,5′-tetramethylbenzidine (TMB) and hydrogen peroxide for 15 min in dark. The reaction was stopped with 50 μL of 2 N H2SO4, and A450 was read with a Fluostar Omega 96-well plate reader (BMG Labtech, Ortenberg, Germany). The titer unit was defined as the dilution at which the blank-corrected OD was 0.1 [5].
ELISA of blood levels of TXB2 and C3a
For measuring thromboxane B2 (TXB2), a stable metabolite of thromboxane A2 (TXA2), 4 μg indomethacin (diluted in 2 ul of 96 % ethanol) was mixed to 2 mL of EDTA-anticoagulated blood to prevent TXA2 release from WBC before centrifugation at 2000g, for 4 min at 4 °C. The plasma samples were aliquoted, frozen, and stored at -70°C until the TXB2 assay was performed using a kit from Cayman Chemicals (Ann Arbor, MI, USA) and a FLUOstar Omega microplate reader (BMG 379 Labtech).
To measure porcine C3a in EDTA-anticoagulated blood samples, we used a porcine specific C3a ELISA obtained from TECOMedical AG, Sissach, Switzerland (Cat No: TE1078).
Statistical methods
Values at all time points were compared to their baseline (-01 min), and the significance of differences was determined by nonparametric Paired Samples Wilcoxon test. Depletion of immunoglobulins was tested with Trend analysis. Reactions to repeated injections of 1/3 human vaccine dose (HVD) of Comirnaty were compared with Friedman-test, followed by Wilcoxon post-hoc test. Correlation among parameters was examined with Spearman’s method. Trend analysis was carried out in Graphpad Prism, while further statistical analysis was performed in R. A P-value of less than 0.05 was considered statistically significant.
Ethics
The investigation conformed to the EU Directive 2010/63/EU and the Guide for the Care and Use of Laboratory Animals used by the US National Institutes of Health (NIH Publication No.85– 23, revised 1996). The experiments were approved by the Ethical Committee of Hungary for Animal Experimentation (permission number: PE/EA/843-7/2020.
Results
Raising of blood anti-PEG Ab levels by immunization with Doxebo
Fig. 2A and C shows the absolute levels of anti-PEG IgM and IgG on days 0 (Pre-Immune) and 9–10 (Screen) on a logarithmic scale. Immunization was successful in all animals, although with some individual variation. Fig. 2B and D displays IgM and IgG levels on the day of the experiment 12–23 days after immunization with Doxebo preceeding the first repeat (r0), second (r1) and third repeat (r2) injections of 1/3 HVD of Comirnaty.
Fig. 2.
Panels A and C shows the absolute levels of anti-PEG IgM and IgG on a logarithmic scale just before (PreImmun) and 9–10 days after (Screen) the immunization with Doxebo. Panels B and D show anti-PEG IgM and IgG levels on the day of the experiment preceeding the first (r0), second (r1) and third (r2) injection of 1/3 HVD of CMT.
These data demonstrate that the immunization was effective in each animal, implying that the Comirnaty challenge on the 12–23 days postvaccination interval was performed in anti-PEG Ab hyperimmune animals. Furthermore, our analysis has identified a significant downward trend in case of both antibodies, suggesting progressive depletion caused by the reactions to repeated (r0, r1, r2) injections.
Induction of anaphylaxis by Comirnaty in anti-PEG hyperimmune pigs: Characteristics of the reaction
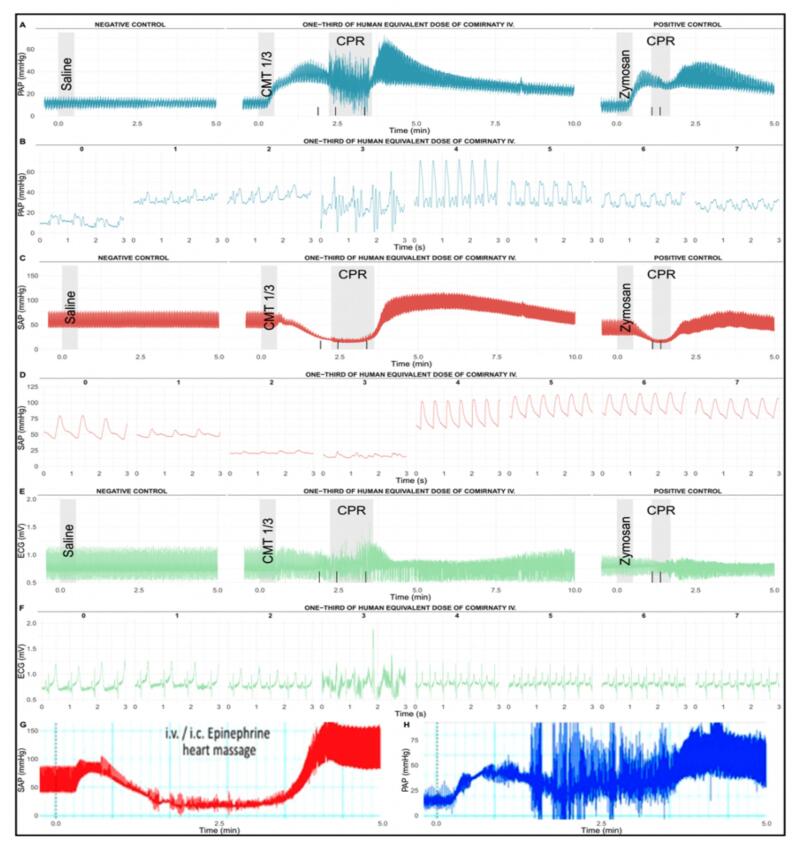
Fig. 3 shows that 1/3 human vaccine dose (HVD) of Comirnaty caused robust hemodynamic changes leading to shock within 1–2 min after i.v. injection. The reaction involved maximal rise of PAP within 1 min after the vaccine's injection, with initially unchanged pulmonary arterial pulse pressure amplitude (pPAP) (A-B), which was paralleled by an abrupt decline in systemic arterial pulse pressure amplitude (pSAP) shortly followed by falling SAP (C-D). The 3-second snapshots from the third minute after the injection highlight the massive signal distortions of PAP, SAP and ECG wave morphology due to cardiopulmonary resuscitation (CPR) involving chest compressions and noradrenaline administration followed by tachycardia and rebound systemic hypertension (B, D, F).
Fig. 3.
Typical real-time hemodynamic and ECG tracings in 1 of 6 pigs injected i.v. with 1/3 HVD (containing 0.01 mg mRNA and 0,26 mg lipid) of Comirnaty 16 days after immunization with 0.1 mg/kg Doxebo, as described in the Methods. Saline (PBS) bolus to establish the baseline; vertical gray-shaded boxes (CPR) correspond to the period of cardiopulmonary resuscitation (CPR), involving injections of noradrenaline in 1:100 dilution at times shown with vertica black lines on the X axis. CMT 1/3 i.v. bolus injection means the injection of 1/3 human dose of Comirnaty; CPR, cardiopulmonary resuscitation; Zymosan, bolus injection of 0.1 mg/kg zymosan. To illustrate the identity of hemodynamic changes caused by Comirnaty and Doxebo in immunized pigs with high anti-PEG IgM in their blood, panel G and H show the SAP and PAP responses of an anti-PEG hyperimmune pig to Doxebo, data reproduded from Ref. [33].
The CPR salvaged the animal, which could be later injected two more times (2 repeat vaccine injections, see below), and finally with 0.1 mg/kg zymosan. The latter caused similar, although less intense hemodynamic changes than that caused by the vaccine at 0.01 mg/kg, implying >10-times greater vascular reactogenicity than that of zymosan, one of the most reactive innate immue stimulators. Fig. 3G-H reproduces the SAP and PAP changes caused by Doxebo in anti-PEG hyperimmune pigs [5], highlighting the practical identity of vaccine- and liposome-induced reactions that was considered as pseudo-anaphylaxis [5].
Fig. 4 summarizes the hemodynamic alterations in 6 pigs after the first injection of 1/3 HVD of Comirnaty. Boxplots of mean pressure and pulse pressure derived from PAP and SAP (A-D), as well as HR, R wave amplitude and ST height derived from the ECG signal (E-G) are shown. The highly reproducible, statistically significant rises of mean PAP and HR, as well as the declines of mean SAP, R amplitude and ST heights, are typical symptoms of CARPA in this model [24], [34], [35], [36], [37], [38]. Panel H highlights the pathologic proximity of mean PAP and SAP 1.5 min into the reaction, i.e., near identity of blood pressures in the pulmonary and systemic circulation. Furthermore, the marked shrinkage of pulse amplitude of SAP seems to occur immediately, detected as early as 0.5 min after injection of Comirnaty, while a significant drop in mean SAP was only detected at 1.5 min. On the contrary, mean PAP rose without delay upon injection of 1/3 HVD of Comirnaty, while the pulse amplitude of PAP was unaffected by the ongoing reaction, surging only after successful CPR. The sluggishness of decline in mean SAP may postpone the detection of a severe reaction, leaving a smaller margin of error for the timely decision of initiation of CPR. Since in a clinical setting, measurement of PAP is problematic, monitoring of early changes in the pulse amplitude of SAP may serve as a better early warning signal.
Fig. 4.
Boxplots of hemodynamic and ECG changes in 6 Doxebo-immunized, anti-PEG hyperimmune pigs injected i.v. with 1/3 HVD of Comirnaty 12–23 days after treatment with 0.1 mg/kg Doxebo, as described in the Methods. mPAP, mSAP, pPAP, pSAP denote mean and pulse pressure of PAP and SAP. The opposing arrows on panel H highlight the near equivalence of PAP and SAP 1.5 min after the injection. All other abbreviations and labels are the same as in Fig. 3. Values at all time points were compared to their baseline (−1 min), and the significance of differences was determined by nonparametric Paired Samples Wilcoxon test (*p < 0.05).
Fig. 5A-D and F-I show the respiratory and hematologic endpoints, among which the significant drops of etCO2 (A), platelet (E) and WBC (F) counts are also typical symptoms of CARPA [24], [34], [35], [36], [37], [38], while the lack of changes in oxygen saturation (B), RBC count (C), hemoglobin (D) and relative abundance of granulocytes (G) or lymphocytes (H) are not known to be CARPA-dependent variables on the time scale of minutes. The ventilation with 2–3 % isoflurane in O2 further stabilized the O2 saturation.
Fig. 5.
Summarized respiratory (A,B) and hematologic (C-I) changes in 6 Doxebo-immunized, anti-PEG hyperimmune pigs injected i.v. with 1/3 HVD of Comirnaty. All other details are explained in the Methods and other figure legends. Values at all time points were compared to their baseline (-1 min), and the significance of differences was determined by nonparametric Paired Samples Wilcoxon test (*p < 0.05).
In addition to the above hyperimmune animals we injected a control, “naive” pig with 5X human dose of Comirnaty. This Doxebo-non-pretreated animal showed negligible physiological changes (Supplementary SFig.S1).
Taken together, these data provide strong evidence that i.v. injection of 1/3 HVD of Comirnaty can cause typical CARPA symptoms in anti-PEG hyperimmune pigs.
Comirnaty-induced hemodynamic changes are partly tachyphylactic in anti-PEG hyperimmune pigs
Fig. 6 shows the effects of 2nd and 3rd repeat injections of 1/3HVD of Comirnaty after the 1st injection, all expressed as % of baseline. The repeated injections caused significant decrease of PAP and SAP responses whose first signs were diminished increase of mean PAP and reduced decrease of the pulse amplitude of SAP. Thus, tachyphylaxis was initially only partial, and became full only after the second repeat injection.
Fig. 6.
Boxplots showing the gradual decrease of cardiopulmonary response to 1/3 HVD of Comirnaty in 6 anti-PEG hyperimmune pigs. Key: r (repeat) 0, r1 and r2 represents the 1st, 2nd and 3rd injection of the vaccine. Values normalized to baseline preceding each injection (−1) are displayed. Reactions to repeated injections of 1/3 HVD of Comirnaty were compared with Friedman-test, followed by Wilcoxon post-hoc test. *, significantly (p < 0.05) ameliorated r1 or r2 response due to partial tachyphylaxis compared to r0. Coloring of the * corresponds to the repeat reaction (r1 / r2) with the same color value.
Comirnaty-induced changes in plasma TXB2 and C3a
Fig. 7 shows the time course of changes of plasma TXB2 and C3a after Comirnaty administration in anti-PEG hyperimmune pigs, both inflammatory mediators rising and declining on the same time course of minutes, in close parallelism with the hemodynamic changes. Levels of pSAP and mPAP, the two most sensitive parameters of the CARPA reaction evoked by the injection of 1/3 HVD of Comirnaty showed significant correlation with C3a and TXB2 as well (Fig. 7).
Fig. 7.
Boxplots of the time course of C3a (A) and TXB2 (B) rise following first i.v. injection of 1/3 HVD of Comirnaty in anti-PEG hyperimmune pigs. TXB2 data is shown on logarithmic scales. Injection resulted in significant and maintained elevation of C3a and TXB2 levels. Values at all time points were compared to their baseline (−1 min), and the significance of differences was determined by nonparametric Paired Samples Wilcoxon test (*p < 0.05). Spearman correlation of mean pulmonary arterial pressure (mPAP) and systemic arterial pulse pressure (pSAP) with C3a and TXB2 levels. TXB2 data is shown on logarithmic scales.
Discussion
Clinical relevance
Beyond the clear merits of COVID-19 vaccinations in reducing the morbidity and mortality of SARS-CoV-2 infections, the record number of vaccinations worldwide brought along a scientific benefit, namely, new insights into the mechanism of the occasional anaphylactic reactions to the vaccine. The increased risk for such reactions to Comirnaty was recognized soon after the introduction of the vaccine in December 2019 [31], leading to the exclusion of people with allergy to a vaccine component, or because of genetic proneness for anaphylaxis. Yet, anaphylactic reactions to Comirnaty have continued to occur; in fact, they have been listed on top in the manufacturer's adverse effect list [39].
Regarding the prevalence of anaphylaxis, the worst outcome of HSRs that entails death or disability in 1.7 % of reactors [40], the ∼ 1.8 billion mRNA-LNP injections given worldwide in 3 years places even the lowest estimate of the sheer number of anaphylaxis cases in the multiple thousand range and Calculating with the median of estimated anaphylaxis rate (123 cases/million) [32], yields 223,200 anaphylaxis with ∼3,800 death or disability worldwide putting vaccine-induced anaphylaxis into the first pandemic of an the category of life-threatening rare (orphan) diseases. To prevent its occurrence in the future, when new vaccines arise, the phenomenon needs to be better understood and more efficiently prevented.
The anti-PEG hyperimmune pig CARPA model
Since its first description in 1999 [24], pigs have been used to study liposome- and other NP-induced HSRs [28], [33], [34], [35], [41], [42], [43], [44], [45]. Although criticized for overt sensitivity, this feature is uniquely beneficial when rare diseases need to be studied [38], such as vaccine-induced anaphylaxis. Among the pig studies in the near past, two led to the present investigation. In the first, the mechanism of PEGylated liposome (Doxil)-induced HSRs was studied, and we immunized pigs with PEGylated liposomes (Doxebo) to induce the rise of anti-PEG Abs in blood [33]. This treatment led to several thousand-fold rise of blood anti-PEG IgM level in 6–7 days, at which time both Doxil and Doxebo caused life-threatening anaphylactic shock in all animals within minutes after i.v. administration. The second study [28] explored the pigs' response to i.v. administered Comirnaty and found that i.v. administration of 5X human dose of Comirnaty caused typical CARPA symptoms in 8 of 14 animals, with 1 anaphylaxis. However, these were naive animals regarding anti-PEG immunity, and the blood levels of anti-PEG Abs were low and highly variable, which we could not correlate with the reactions. Fusing the two protocols and studying the reactogenicity of Comirnaty in anti-PEG-hyperimmune versus naive animals was expected to provide direct evidence for a causal role of anti-PEG Abs in anaphylaxis. As the data showed, it is in fact what we observed.
Features of reactogenicity of Comirnaty in anti-PEG hyperimmune pigs
Our previous study injecting 5X HVD of Comirnaty in 14 naive pigs led to one anaphylaxis. In sharp contrast, in the present study using anti-PEG hyperimmune pigs, a 15-fold lower dose led to anaphylaxis in 6 of 6 pigs. This difference, taken together with the lack of reaction in the non-immunized control animal against 5X HVD Comirnaty in the present study, provides strong direct evidence that anti-PEG Abs play a causal role in vaccine-induced anaphylaxis. There is, however, a major difference in the dose-dependence of Doxebo and Comirnaty-induced anaphylaxis in immunized pigs. Notably, the PEG lipids in Doxil/Doxebo and Comirnaty are 2 K-PEG-1,2-distearoylphosphatidylethanolamine (DSPE) and 2 K-PEG-N,N-ditetradecylacetamide (ALC-0159), respectively, and the amount of 2 K-PEG lipid administered to pigs with Doxil/Doxebo and Comirnaty were 25.0 and 0.68 g/kg, respectively, implying that the amount of 2 K-PEGylated lipid in Comirnaty was ∼37-fold less than that in equi-reactive Doxil/Doxebo. Strengthening the claim that Comirnaty is a relatively strong activators of the innate immune system, the above comparison of equi-anaphylactogenic amounts of PEG on Comirnaty and Doxebo is consistent with the increased weight-normalized vasoreactivity of Comirnaty relative to zymosan in pigs (see above) and increased concentration-normalized C activation by Comirnaty compared to Doxil/Doxebo in pig [28] and human [46] serum.
Mechanism proposed
As mentioned, mRNA vaccine-induced HSRs represent in most cases pseudoallergy [9], [10], [15], [16], [19], [27], which can proceed with or without the involvement of C activation. The observations on significant C activation by Comirnaty in pig serum in vitro [28] and in the present study in vivo, the latter proceeding in close parallelism with the development of anaphylaxis, provide direct evidence for the involvement of C activation in these reactions. The steps involved in CARPA that lead to vasoreactivity and ultimately to anaphylaxis were outlined in many previous studies [24], [34], [35], [36], [37], [38], and is illustrated for Comirnaty-induced reactions in the scheme in Fig. 8.
Fig. 8.
Schematic illustration of the possible mechanisms of HSRs/anaphylaxis by mRNA-LNP COVID-19 vaccines,.adapted from [33]. After i.v. injection, the PEGylated vaccine NPs (solid black spheres with a crown) bind anti-PEG IgG and Ig Abs, which leads to C activation via the classical pathway. In addition, the vaccine NPs alo activate the alternative pathway. The liberated cleavage products (C1q, C3a, C5a, C3b, C3d, C5b-9) stimulate a variety of allergy-mediating innate immune cells (AMICs, e.g., mast cells, PIM cells in pig lung, macrophages, basophils, granulocytes, platelets) via different receptors (C1qR, C3aR, C5aR, CR1 (CD35), CR2 (CD21), CR3 (CD11b/CD18, C5b-9R), illustrated with different colors and discussed in more detail in Ref. [33]. These signaling pathways cause CARPA, but AMICs could also be activated without the involvement of C, among others, via the FcgR (FcγRIIB (CD32)/FcγR (CD351) binding the Fcγ of anti-PEG Abs bound to Comirnaty NPs (also illustrated in the figure). Also, PEGylated NPs can bind to pattern recognition receptors (PRRs), e.g., Toll-like receptor 2 and/or 6 and/or other PRR, as a consequence of mimicking pathogen-associated molecular patterns (PAMPs) (not shown in Fig. 8). The specified vasoactive secretory products released by AMICs explain the symptoms of HSR/anaphylaxis [33].
It should be noted regarding the mechanism of HSRs that in murine models, C activation is not necessarily involved in the hemodynamic changes. C-independent pseudoallergy (CIPA) was described for Abelcet and AmBisome-induced hypotension in NMRI mice [47], thus, a contribution of this mechanism to the human HSR to mRNA vaccines cannot be excluded, and it is likely that in most HSRs CARPA and CIPA are simultaneously contributing to the reactions (double hit hypothesis) [48], [49].
Human relevance of findings
The pig model applied in this study deviates from the human vaccination practice in that we administered the vaccine i.v., while people are vaccinated i.m., via the deltoid muscle. The models’ human relevance is supported by the fact that in experimental animals a varying fraction of the vaccine injected i.m. can get into the blood on a time scale of minutes. Evidence for this claim includes a 2015 study by Pardi et al. [50] showing that 24 min after deep muscle injection of a luciferase-mRNA carrying LNP in mice, the majority of luciferase was expressed in the liver. It was also observed in this experiment that superficial muscle injection entailed less protein translation in the liver, suggesting that the injection site and depth are critical variables in vaccine intravasation [50]. Another study attesting to rapid entry of LNPs into blood after i.m. administration [51] utilized a tritiated lipid marker to establish the biodistribution of Comirnaty-equivalent luciferase-mRNA-LNP in rats, showed 2.8 % of radioactivity in the plasma 15 min after the injection of LNPs, reaching peak between 1–4 h post-dose and distribution of LNPs mainly into the liver, adrenal glands, spleen and ovaries over 48 h [51]. Further animal and human data on rapid biodistribution of RNA vaccine NPs are reviewed by Pateev et al. [52]. Regarding the correspondence of reaction-triggering vaccine amounts in humans and pigs, it should be reminded that allergic reactions are complex self-amplified cascadic processes, the reactions depend on individually variable sensitivity, and are not necessarily dose-limited. Thus, interspecies comparison of dose–effect relationships is difficult, if possible, at all. In our case, the hyperimmune pig is a functional model for the anaphylactic reactions of anti-PEG Ab “supercarrier”[33] people who have extremely high anti-PEG Ab levels in their blood, up to ∼ 3 % of the normal population [33]. In these, mostly also atopic people, miniscule amounts of PEG can trigger anaphylactic shock, and not only vaccine-associated PEG, but many other PEGylated drugs. In this sense, Comirnaty in the present study represents a functional model for many other PEGylated proteins and nanomedicines.
Yet another unique benefit of the model is that the hemodynamic and cardiopulmonary changes mimic those human circulatory abnormalities (mainly cardiopulmonary distress, acute myocardial infarction), that make cardiac anaphylaxis life-threatening. Furthermore, the reactogenic effect of high anti-PEG Ab levels in pigs may provide a model for the severe allergy in humans, which increases the risk of anaphylaxis to PEGylated vaccines [13]. For all these reasons we propose that the anti-PEG hyperimmune porcine CARPA model has high human relevance, enabling basic studies aimed to understand PEGylated vaccine-induced allergic reactions and develop methods for their prevention. In fact, to our best knowledge, the technique, recently updated with regulatory-compatible, standardizable specifications [53], represents the first large animal model for drug-induced severe HSRs and anaphylaxis that may better enable solving this problem than the current mouse and murine models.
Funding
The financial support by the European Union Horizon 2020 projects 825828 (Expert) and 952520 (Biosafety) are acknowledged. This project was supported by a grant from the National Research, Development, and Innovation Office (NKFIH) of Hungary (2020-1.1.6-JÖVŐ-2021-00013). TKP2021-EGA-23 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme. JS thanks the logistic support by the Applied Materials and Nanotechnology, Center of Excellence, Miskolc University, Miskolc, Hungary. The project was supported by the ÚNKP-23-3-II-SE-13 New National Excellence Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund to BAB.
CRediT authorship contribution statement
Bálint András Barta: Methodology, Software, Data curation, Validation, Formal analysis, Investigation, Visualization, Writing - original draft. Tamás Radovits: Conceptualization, Methodology, Validation, Funding acquisition, Investigation, Project administration, Supervision, Resources, Writing - original draft. Attila Balázs Dobos: Project administration, Resources, Methodology, Validation, Investigation, Writing - review & editing. Gergely Tibor Kozma: Conceptualization, Investigation, Data curation, Supervision, Writing – review & editing. Tamás Mészáros: Conceptualization, Investigation, Validation, Supervision, Data curation, Writing – review & editing. Petra Berényi: Formal analysis, Investigation, Data curation, Writing – review & editing. Réka Facskó: Formal analysis, Investigation, Data curation, Writing – review & editing. Tamas Fulop: Conceptualization, Resources, Writing – review & editing. Béla Merkely: Conceptualization, Funding acquisition, Resources, Supervision, Data curation, Writing – review & editing. János Szebeni: Conceptualization, Funding acquisition, Resources, Methodology, Formal analysis, Data curation, Project administration, Supervision, Writing - original draft, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors affiliated with SeroScience LLC are involved in the company’s contract research service activity providing studies that were applied here.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2024.100497.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Garvey L.H., Nasser S. Anaphylaxis to the first COVID-19 vaccine: is polyethylene glycol (PEG) the culprit? Br J Anesthesia. 2020 doi: 10.1016/j.bja.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banerji A., Wickner P.G., Saff R., Stone C.A., Jr., Robinson L.B., Long A.A., et al. mRNA Vaccines to Prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2020 doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren C.M., Snow T.T., Lee A.S., Shah M.M., Heider A., Blomkalns A., et al. Assessment of allergic and Anaphylactic Reactions to mRNA COVID-19 vaccines with confirmatory testing in a US regional health system. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.25524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine - United States, December 21, 2020-January 10, 2021. Am J Transplant. 2021;21:1326–1331. doi: 10.1111/ajt.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine - United States, December 14–23, 2020. Am J Transplant. 2021;21:1332–1337. doi: 10.1111/ajt.16516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Team CC-R, Food and Drug A. Allergic reactions including anaphylaxis after receipt of the first dose of moderna COVID-19 Vaccine - United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125-129. [DOI] [PMC free article] [PubMed]
- 7.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA. 2021;325:780–781. doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kounis N.G., Koniari I., de Gregorio C., Velissaris D., Petalas K., Brinia A., et al. Allergic reactions to current available COVID-19 vaccinations: Pathophysiology, causality, and therapeutic considerations. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9030221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim X.R., Leung B.P., Ng C.Y.L., Tan J.W.L., Chan G.Y.L., Loh C.M., et al. Pseudo-anaphylactic reactions to Pfizer BNT162b2 vaccine: report of 3 cases of anaphylaxis post Pfizer BNT162b2 vaccination. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krantz M.S., Bruusgaard-Mouritsen M.A., Koo G., Phillips E.J., Stone C.A., Jr., Garvey L.H. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: Don't give up on the second dose! Allergy. 2021;76:2916–2920. doi: 10.1111/all.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blumenthal K.G., Robinson L.B., Camargo C.A., Jr., Shenoy E.S., Banerji A., Landman A.B., et al. Acute allergic reactions to mRNA COVID-19 vaccines. JAMA. 2021;325:1562–1565. doi: 10.1001/jama.2021.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nittner-Marszalska M., Rosiek-Biegus M., Kopec A., Pawlowicz R., Kosinska M., Lata A., et al. Pfizer-BioNTech COVID-19 vaccine tolerance in allergic versus non-allergic individuals. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9060553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shavit R., Maoz-Segal R., Iancovici-Kidon M., Offengenden I., Haj Yahia S., Machnes Maayan D., et al. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gringeri M., Mosini G., Battini V., Cammarata G., Guarnieri G., Carnovale C., et al. Preliminary evidence on the safety profile of BNT162b2 (Comirnaty): new insights from data analysis in EudraVigilance and adverse reaction reports from an Italian health facility. Hum Vaccin Immunother. 2021;17:2969–2971. doi: 10.1080/21645515.2021.1917236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Risma KA, Edwards KM, Hummell DS, Little FF, Norton AE, Stallings A, et al.. Potential mechanisms of anaphylaxis to COVID-19 mRNA vaccines. J Allergy Clin Immunol. 2021;147:2075-2082 e2. [DOI] [PMC free article] [PubMed]
- 16.Luxi N., Giovanazzi A., Arcolaci A., Bonadonna P., Crivellaro M.A., Cutroneo P.M., et al. Allergic reactions to COVID-19 vaccines: risk factors, frequency, mechanisms and management. BioDrugs. 2022;36:443–458. doi: 10.1007/s40259-022-00536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anis E., Preis S.A., Cedar N., Tal Y., Hershkowitz I., Hershko A.Y. Reporting of allergic reactions during Pfizer-BioNTech BNTT162B2 vaccination in Israel. J Allergy Clin Immunol Pract. 2022;10:2969–2976. doi: 10.1016/j.jaip.2022.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McSweeney M.D., Mohan M., Commins S.P., Lai S.K. Anaphylaxis to Pfizer/BioNTech mRNA COVID-19 vaccine in a patient with clinically confirmed PEG allergy. Front Allergy. 2021;2 doi: 10.3389/falgy.2021.715844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfson AR, Robinson LB, Li L, McMahon AE, Cogan AS, Fu X, et al. First-Dose mRNA COVID-19 Vaccine Allergic Reactions: Limited Role for Excipient Skin Testing. J Allergy Clin Immunol Pract. 2021;9:3308-3320 e3. [DOI] [PMC free article] [PubMed]
- 20.Warren S.L. A new look at type I immediate hypersensitivity immune reactions. Ann Allergy. 1976;36:337–341. [PubMed] [Google Scholar]
- 21.Smith T.F. Allergy and pseudoallergy: an overview of basic mechanisms. J Prim Care. 1987;14:421–434. [PubMed] [Google Scholar]
- 22.Zuberbier T. Pseudoallergy or nonallergic hypersensitivity. J Allergy. 1999;54:397–398. doi: 10.1034/j.1398-9995.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 23.Patterson A, Kendall P, Monroy J, C. P, Ren Z, Maya Jerath M. Patients with pseudoallergic reactions following COVID-19 vaccination are able to tolerate subsequent dosing. JACI. 2022;149:DOI: 10.1016/j.jaci.2021.12.57.
- 24.Szebeni J., Fontana J.L., Wassef N.M., Mongan P.D., Morse D.S., Dobbins D.E., et al. Hemodynamic changes induced by liposomes and liposome-encapsulated hemoglobin in pigs: a model for pseudoallergic cardiopulmonary reactions to liposomes. Role of complement and inhibition by soluble CR1 and anti-C5a antibody. Circulation. 1999;99:2302–2309. doi: 10.1161/01.cir.99.17.2302. [DOI] [PubMed] [Google Scholar]
- 25.Szebeni J. Complement activation-related pseudoallergy: a stress reaction in blood triggered by nanomedicines and biologicals. Mol Immunol. 2014;61:163–173. doi: 10.1016/j.molimm.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 26.Yu J., Yuan X., Chen H., Chaturvedi S., Braunstein E.M., Brodsky R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;2020(18):2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szebeni J., Storm G., Ljubimova J.Y., Castells M., Phillips E.J., Turjeman K., et al. Applying lessons learned from nanomedicines to understand rare hypersensitivity reactions to mRNA-based SARS-CoV-2 vaccines. Nat Nanotechnol. 2022;17:337–346. doi: 10.1038/s41565-022-01071-x. [DOI] [PubMed] [Google Scholar]
- 28.Dezsi L, Meszaros T, Kozma G, M HV, Olah CZ, Szabo M, et al.. A naturally hypersensitive porcine model may help understand the mechanism of COVID-19 mRNA vaccine-induced rare (pseudo) allergic reactions: complement activation as a possible contributing factor. Geroscience. 2022;44:597-618. [DOI] [PMC free article] [PubMed]
- 29.Chen E, Chen BM, Su YC, Chang YC, Cheng TL, Barenholz Y, et al. Premature drug release from polyethylene glycol (PEG)-coated liposomal doxorubicin via formation of the membrane attack complex. ACS Nano. 2020. 10.1021/acsnano.9b07218. [DOI] [PubMed]
- 30.Estape Senti M., de Jongh C.A., Dijkxhoorn K., Verhoef J.J.F., Szebeni J., Storm G., et al. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J Control Release. 2022;341:475–486. doi: 10.1016/j.jconrel.2021.11.042. [DOI] [PubMed] [Google Scholar]
- 31.Vrieze Jd. Suspicions grow that nanoparticles in Pfizer’s COVID-19 vaccine trigger rare allergic reactions. Science. 2020. https://www.sciencemag.org/news/2020/12/suspicions-grow-nanoparticles-pfizer-s-covid-19-vaccine-trigger-rare-allergic-reactions.
- 32.Kozma G.T., Mészáros T., Berényi P., Facskó R., Patkó Z., Oláh C., et al. Role of anti-polyethylene glycol (PEG) antibodies in the allergic reactions to PEG-containing Covid-19 vaccines: Evidence of immunogenicity of PEG. Vaccine. 2023;41:4561–4570. doi: 10.1016/j.vaccine.2023.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozma G.T., Meszaros T., Vashegyi I., Fulop T., Orfi E., Dezsi L., et al. Pseudo-anaphylaxis to polyethylene Glycol (PEG)-coated liposomes: roles of anti-PEG IgM and Complement activation in a porcine model of human infusion reactions. ACS Nano. 2019;13:9315–9324. doi: 10.1021/acsnano.9b03942. [DOI] [PubMed] [Google Scholar]
- 34.Szebeni J., Bedocs P., Rozsnyay Z., Weiszhar Z., Urbanics R., Rosivall L., et al. Liposome-induced complement activation and related cardiopulmonary distress in pigs: factors promoting reactogenicity of Doxil and AmBisome. Nanomedicine. 2012;8:176–184. doi: 10.1016/j.nano.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Szebeni J., Bedocs P., Urbanics R., Bunger R., Rosivall L., Toth M., et al. Prevention of infusion reactions to PEGylated liposomal doxorubicin via tachyphylaxis induction by placebo vesicles: a porcine model. J Control Release. 2012;160:382–387. doi: 10.1016/j.jconrel.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 36.Urbanics R., Szebeni J. Lessons learned from the porcine CARPA model: constant and variable responses to different nanomedicines and administration protocols. Eur J Nanomedicine. 2015;7:219–231. [Google Scholar]
- 37.Szebeni J., Bedőcs P., Dézsi L., Urbanics R. A porcine model of complement activation-related pseudoallergy to nano-pharmaceuticals: Pros and cons of translation to a preclinical safety test. Prec Nanomed. 2018;1:63–73. [Google Scholar]
- 38.Szebeni J., Bawa R. Human clinical relevance of the porcine model of pseudoallergic infusion reactions. Biomedicines. 2020;8(4) doi: 10.3390/biomedicines8040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfizer Pfizer. Responds to Research Claims 2023; Jan; 27: 2023. https://wwwpfizercom/news/announcements/pfizer-responds-research-claims.
- 40.Klosko RC, Lynch SE, Cabral DL, Nagaraju K, Johnston YA, Steinberg JD et al. Death and disability reported with cases of vaccine anaphylaxis stratified by administration setting: an analysis of the vaccine adverse event reporting system from 2017 to 2022. Vaccines (Basel). 2023;11. [DOI] [PMC free article] [PubMed]
- 41.Bodo M., Szebeni J., Baranyi L., Savay S., Pearce F.J., Alving C.R., et al. Cerebrovascular involvement in liposome-induced cardiopulmonary distress in pigs. J Liposome Res. 2005;15:3–14. doi: 10.1081/lpr-64523. [DOI] [PubMed] [Google Scholar]
- 42.Szebeni J., Baranyi L., Savay S., Bodo M., Milosevits J., Alving C.R., et al. Complement activation-related cardiac anaphylaxis in pigs: role of C5a anaphylatoxin and adenosine in liposome-induced abnormalities in ECG and heart function. Am J Physiol Heart Circ Physiol. 2006;290:H1050–H1058. doi: 10.1152/ajpheart.00622.2005. [DOI] [PubMed] [Google Scholar]
- 43.Jackman J.A., Meszaros T., Fulop T., Urbanics R., Szebeni J., Cho N.J. Comparison of complement activation-related pseudoallergy in miniature and domestic pigs: foundation of a validatable immune toxicity model. Nanomedicine. 2016;12:933–943. doi: 10.1016/j.nano.2015.12.377. [DOI] [PubMed] [Google Scholar]
- 44.Meszaros T., Kozma G.T., Shimizu T., Miyahara K., Turjeman K., Ishida T., et al. Involvement of complement activation in the pulmonary vasoactivity of polystyrene nanoparticles in pigs: unique surface properties underlying alternative pathway activation and instant opsonization. Int J Nanomedicine. 2018;13:6345–6357. doi: 10.2147/IJN.S161369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fulop T., Kozma G.T., Vashegyi I., Meszaros T., Rosivall L., Urbanics R., et al. Liposome-induced hypersensitivity reactions: Risk reduction by design of safe infusion protocols in pigs. J Control Release. 2019;309:333–338. doi: 10.1016/j.jconrel.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Bakos T., Mészáros T., Kozma G.T., Berényi P., Facskó R., Farkas H., et al. mRNA-LNP COVID-19 vaccine lipids induce complement activation and production of proinflammatory cytokines: Mechanisms, effects of complement inhibitors, and relevance to adverse reactions. Int J Mol Sci. 2024;25(7):3595. doi: 10.3390/ijms25073595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orfi E., Meszaros T., Hennies M., Fulop T., Dezsi L., Nardocci A., et al. Acute physiological changes caused by complement activators and amphotericin B-containing liposomes in mice. Int J Nanomedicine. 2019;14:1563–1573. doi: 10.2147/IJN.S187139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szebeni J., Bedocs P., Csukas D., Rosivall L., Bunger R., Urbanics R. A porcine model of complement-mediated infusion reactions to drug carrier nanosystems and other medicines. Adv Drug Deliv Rev. 2012;64:1706–1716. doi: 10.1016/j.addr.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Szebeni J. Mechanism of nanoparticle-induced hypersensitivity in pigs: complement or not complement? Drug Discov Today. 2018;23:487-492.49. [DOI] [PubMed]
- 50.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K., et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfizer Australia Pty Ltd. Nonclinical Evaluation Report: BNT162b2 [mRNA] COVID-19 vaccine (COMIRNATYTM). https://www.tga.gov.au/sites/default/files/foi-2389-06.pdf 2021.
- 52.Pateev I., Seregina K., Ivanov R., Reshetnikov V. Biodistribution of RNA vaccines and of their products: evidence from human and animal studies. Biomedicines. 2023;12(1) doi: 10.3390/biomedicines12010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szebeni, J. Evaluation of the Acute Anaphylactoid Reactogenicity of Nanoparticle-Containing Medicines and Vaccines Using the Porcine CARPA Model. In Characterization of Nanoparticles Intended for Drug Delivery, ttps://doi.org/10.1007/978-1-0716-3786-9_23,, Jeffrey D. Clogston et al. (eds.) Ed.; Methods in Molecular Biology, 2024. Ch 23, pp. 229-245. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.