Abstract
In many herpesviruses, genome segments flanked by inverted repeats invert during DNA replication. It is not known whether this inversion is a consequence of an inherently recombinagenic replicative mechanism common to all herpesviruses or whether the replication enzymes of viruses with invertible segments have specifically evolved additional enzymatic activities to drive inversion. By artificially inserting a fusion of terminal sequences into the genome of a virus which normally lacks invertible elements (murine cytomegalovirus), we created a genome composed of long and short segments flanked by 1,359- and 543-bp inverted repeats. Analysis of genomic DNA from this virus revealed that inversion of both segments generates equimolar amounts of four isomers during the viral propagation necessary to produce DNA for analysis from a single viral particle. We conclude that a herpesvirus which naturally lacks invertible elements is able to support efficient segment inversion. Thus, the potential to invert is probably inherent in the replication machinery of all herpesviruses, irrespective of genome structure, and therefore genomes with invertible elements could have evolved simply by acquisition of inverted repeats and without concomitant evolution of enzymatic activities to mediate inversion. Furthermore, the recombinagenicity of herpesvirus DNA replication must have some importance independent of genome segment inversion.
The genomes of herpesviruses vary significantly with respect to the presence and arrangement of inverted and directly repeated sequences (48). The most complex viral genomes, those of herpes simplex virus type 1 (HSV-1), HSV-2, and human cytomegalovirus (CMV), contain two sets of large inverted repeats that flank segments of unique sequences (48). Frequent inversion of the segments bordered by these repeats results in equimolar amounts of four genomic isomers (12, 20, 24, 51, 52, 66). Viruses such as varicella-zoster virus, pseudorabies virus, and equine herpesvirus 1 have slightly less complex genomes that contain single sets of inverted repeats bordering unique segments, and inversion of these segments results in equimolar amounts of two genomic isomers (3, 8, 9, 17, 21, 48, 59, 65).
Inversion is not essential for herpesvirus replication. Many herpesviruses do not contain inverted repeats or invertible elements (48), and in HSV-1, mutants unable to invert due to deletion of the internal repeats are fully replication competent in vitro (23, 41, 42). Inversion does, however, require that the substrate DNA undergo replication; more specifically, the replication must be by a herpesvirus-directed mechanism (14, 37, 49, 62, 63). This requirement does not appear to stem from a need for local initiation of DNA synthesis, since deletion of the two origins that lie within HSV-1 inverted repeats does not alter their inversion efficiency (54). The observations that inverted segments can be detected within the replicative concatemers of several herpesviruses (1, 2, 30, 31, 50, 53, 54, 68), at the earliest time points when HSV-1 concatemeric DNA can be detected (68), and within concatemers formed by HSV-1 mutants unable to cleave and package DNA (26, 29, 67) indicate that inversion occurs within viral concatemeric DNA during or shortly after concatemer formation and does not involve unit genomes or the cleavage and packaging process. That inversion could occur by recombination between different concatemers is suggested by the observation that concatemers generated from different HSV-1 replication templates frequently recombine (54).
Inversion requires homologous inverted repeats flanking the invertible segments (56, 57), and hence the mechanism is believed to involve homologous recombination; however, the importance of specific cis elements within the repeated sequences has not been clearly established. A variety of sequences, both viral and nonviral, can mediate inversion or recombination in the context of herpesvirus DNA replication (6, 7, 10, 11, 13–15, 37, 39, 43, 44, 49, 55–57, 60, 62, 63). The fact that some sequences mediate inversion with reduced efficiency (25, 28, 55) and some not at all (18, 19, 27, 36, 43) indicates that although no specific sequences are essential, cis sequences within the repeats can have some influence. For example, the HSV-1 terminal repeat, or a sequence, can support inversion despite having a size considerably below the minimum 700 bp needed for inversion mediated by certain nonviral sequences (7, 55, 60, 62). Quantitative studies have further shown that the a sequence can mediate both inversion and recombination with significantly greater efficiency than can nonviral sequences having a similar size and G+C content (5, 13–15, 36, 37, 49, 62). These findings are consistent with the proposal that the a sequence may function as the target for a virus-encoded sequence-specific recombinase (7, 36–39). However, because the a sequence also serves as the site for DNA cleavage during genome packaging, its recombinagenic properties might also stem from the recombination-enhancing effects of free DNA ends (49, 60). Whatever the cause of its recombinagenisity, the contribution of the a sequence to HSV-1 inversion in its natural context appears to be minimal, since deletion of the a sequences from the large HSV-1 inverted repeats results in a virus that still inverts with normal efficiency (29). It therefore appears that the importance of cis sequences may be manifested only when the repeats are very small, with large repeats having little or no sequence dependence. This argues against a need for an a sequence-specific recombinase but leaves open the possibility that some herpesviruses encode a non-sequence-specific recombinase to mediate segment inversion.
The observation that inversion occurs when DNA replication is driven solely by the seven minimal HSV-1 DNA replication genes in the absence of other viral gene products indicates that if a virally encoded recombinase activity is needed for inversion, it must be present within these seven HSV-1 replication proteins (62). We reasoned that if such an activity evolved strictly to support inversion, it is unlikely to exist in viruses that lack invertible elements. Alternatively, if inversion is simply a consequence of the recombinagenic nature of herpesvirus DNA replication, then all herpesviruses, irrespective of their genome structures, should be able to support inversion when presented with inverted repeats. To date, studies of inversion have utilized viruses that naturally contain invertible elements, and therefore the putative recombinase activity would be predicted to be present. To determine if a virus with a simple genome structure has the ability to mediate segment inversion, we introduced both direct and inverted repeats into the murine CMV (MCMV) genome, which normally lacks invertible segments (16, 35), and analyzed the resulting viruses for genome segment inversion.
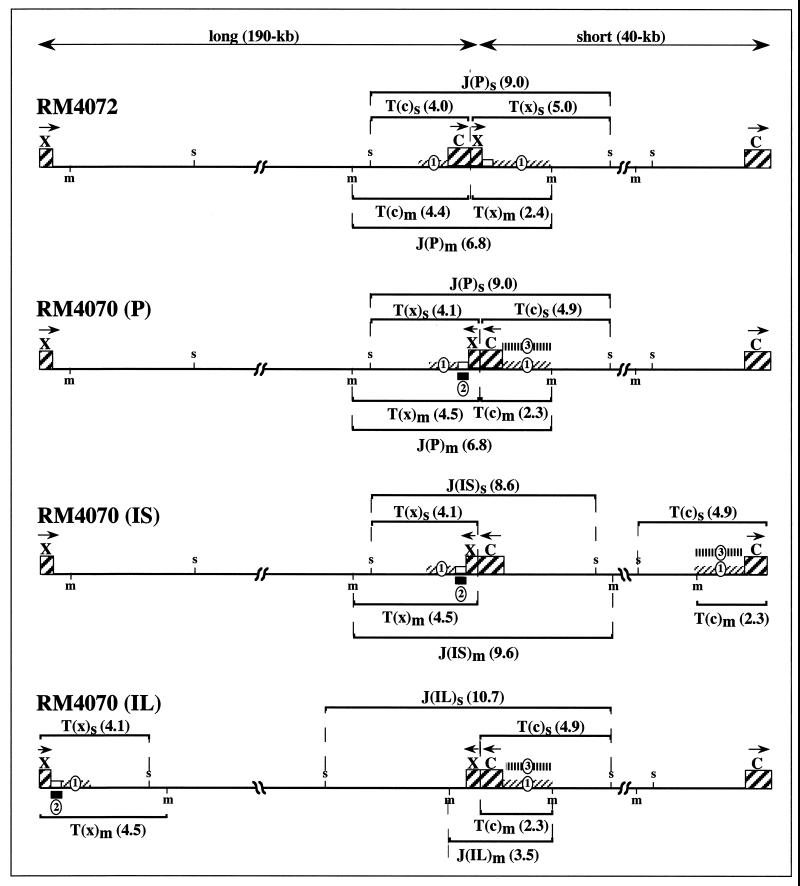
Two recombinant MCMVs were constructed that contain internal duplications of terminal sequences engineered in opposite orientations at a location about 40 kb from the right end of the genome (Fig. 1). In both viruses, 543 bp from the X terminus (nucleotides 1 to 543) was fused with 1,359 bp from the C terminus (nucleotides 228920 to 230278) to create a 1,902-bp fragment that was inserted adjacent to an expression cassette for Escherichia coli xanthine-guanine phosphoribosyltransferase (gpt) (used for recombinant virus selection) at nucleotide position 187889 (nucleotide positions are as designated by the published MCMV genomic sequence [46]). Virus RM4072 was constructed by homologous recombination with plasmid pON4072 and selection for gpt expression as previously described (33, 61). Virus RM4070 was constructed from plasmid pON4070 in the same way. Plasmids pON4070 and pON4072 were derived from the same blunt-end ligation, as previously described (33), and represent insertion of the gpt/ectopic site-containing fragment into MCMV sequences in opposite orientations. Thus, in virus RM4070, the inserted sequences form 1,359- and 543-bp internal inverted repeats of the terminal sequences, whereas in virus RM4072, the same sequences form internal direct repeats (Fig. 1). The insertions had no apparent effect on virus viability, since both viruses routinely attained titers in excess of 107 PFU/ml, which is comparable to titers attained by the parental virus.
FIG. 1.
Structures of recombinant viruses. The genome of virus RM4072 and the prototype (P), inverted-long (IL), and inverted-short (IS) isomers of RM4070 are shown. Hatched boxes represent C- and X-terminal sequences inserted within the genome to generate internal repeats, and double-ended arrows at the top delineate long and short segments created by the repeats. The relative orientations of the repeats are indicated by arrows over the C and X boxes. Sequences contained in hybridization probes are indicated by diagonally hatched bars (probe 1), solid black bars (probe 2), or vertically hatched bars (probe 3). Ectopic terminal (T) and junction (J) MluI (m) and SacI (s) fragments are indicated, with their predicted sizes (in kilobases) shown in parentheses.
In both viruses, the internal repeats, being fusions of the termini, act as functional “ectopic” cleavage sites (33, 34). During cleavage and packaging of viral concatemers, a head-full restriction leads to formation of equimolar amounts of two genome isomers. Prototype (P) isomers result from cleavage at natural cleavage sites, producing natural termini and leaving ectopic cleavage sites uncleaved, whereas cleavage at ectopic cleavage sites produces ectopic termini and leaves junctions of natural termini uncleaved, generating inverted-long/inverted-short (ISIL) isomers that contain an apparent inversion of both the long and short segments relative to the P arrangement (33, 34). Thus, both viruses were predicted to contain three restriction fragments at the ectopic site: terminal fragments T(x) and T(c) and a junction fragment, J(P), representing their fusion within the prototype (P) genome arrangement (Fig. 1). If segment inversion occurs by homologous recombination between the inverted repeats of the RM4070 genome, two additional junction fragments, J(IL) and J(IS), were predicted to arise from genome isomers containing only inverted-long (IL) or inverted-short (IS) segments, as shown in Fig. 1.
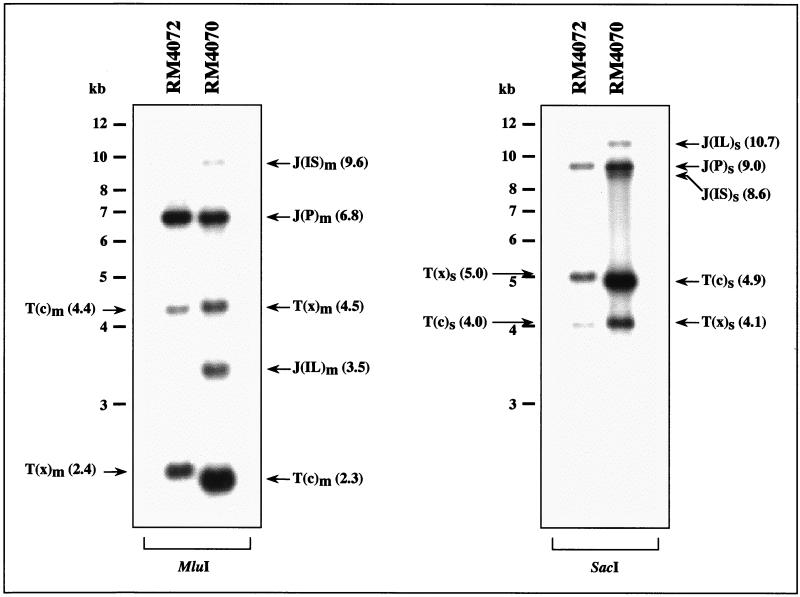
RM4070 and RM4072 were used to infect murine NIH 3T3 cells (ATCC CRL1658) at a multiplicity of infection of 3 as previously described (58). Virion DNAs from both viruses were prepared from extracellular culture supernatants as previously described (32) and digested with MluI or SacI. The resulting DNA fragments were separated using a 0.6% agarose gel, transferred to a Nytran nylon membrane (Schleicher & Schuell), and hybridized with 32P-labeled DNA probes as previously described (31). Probe 1 consisted of a 1.3-kb BamHI-MluI fragment that was gel purified from plasmid pON432 (58) and contained sequences on each side of the inserted sequences (Fig. 1). When hybridized with probe 1, RM4070 and RM4072 DNA contained the predicted T(x) and T(c) terminal fragments as well as the J(P) junction fragments from the prototype orientation (Fig. 2). RM4070 DNA also contained MluI and SacI fragments that were absent from RM4072 DNA and had molecular weights predicted for the ectopic junction fragments J(IS) and J(IL) resulting from IS and IL genome structures, respectively (Fig. 2). Their presence suggests that both the long and short arms of RM4070 undergo inversion.
FIG. 2.
DNA blot analysis of recombinant viruses. Virion DNAs were digested with MluI or SacI, separated by agarose gel electrophoresis, transferred to a nylon membrane, and hybridized with probe 1 (Fig. 1). Terminal (T) and junction (J) MluI (m) and SacI (s) fragments are indicated, with their predicted sizes (in kilobases) shown in parentheses.
Although the stocks used in this experiment were derived after several rounds of limiting-dilution cloning (33), the formal possibility remained that the fragments observed represented a mixture of different stable viral genomes. To confirm that the different genome isomers can arise rapidly from a single progenitor virus, serial dilutions of the RM4070 stock were used to infect 96-well plate cultures of NIH 3T3 cells. On one plate, viral replication was evidenced by cytopathic effect in 4 out of 96 wells, indicating that each well had a high probability of containing a virus population derived from a single virus. The viruses from two of these wells were amplified by cell culture to generate sufficient virion DNA for DNA blot hybridization as described above. Both DNAs exhibited MluI and SacI patterns identical to those shown for RM4070 in Fig. 2 (not shown), indicating that single RM4070 particles, presumably containing only one of the four genome isomers, were able to rapidly give rise to a population of viruses containing all four genome isomers.
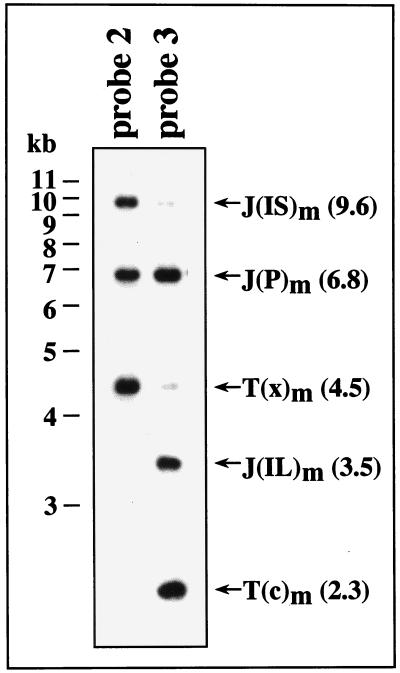
The efficiency of inversion is difficult to surmise from the results shown in Fig. 2, since J(P), J(IL), and J(IS) contain different sequences with homology to probe 1 and the efficiency of hybridization to each fragment differs. To confirm the identities of these fragments and to better assess the efficiency of inversion using probes that hybridize to sequences that are identical within the fragments from the different isomers, membranes containing agarose-separated MluI fragments of RM4070 virion DNA were sequentially hybridized with probes 2 and 3. Probe 2 consisted of gpt sequences cloned in plasmid pON1101 (61) and specific for the T(x) side of the cleavage site (Fig. 1), whereas probe 3 consisted of plasmid pMA34, containing a HindIII-XhoI fragment of MCMV sequences subcloned from pON432 (33), specific for the T(c) side of the cleavage site (Fig. 1). As predicted, the T(x), J(P), and J(IS) fragments hybridized to probe 2 but the T(c) and J(IL) fragments did not (Fig. 3). Conversely, T(c), J(P), and J(IL) fragments hybridized to probe 3 but T(x) and J(IS) did not (Fig. 3). Analogous results were observed when the DNAs were digested with SacI instead of MluI (data not shown). These observations indicate that the identities of these fragments have been correctly assigned. Moreover, the nearly equivalent signals from hybridization of probe 2 to J(P) and J(IS) and of probe 3 to J(P) and J(IL) (Fig. 3) indicate that the P, IL, and IS isomers are present in nearly equimolar amounts. Junctions from the fourth isomer, ISIL, in which both the long and short segments are inverted, cannot be detected by these probes but have been detected in equimolar amounts using a terminal sequence probe (not shown).
FIG. 3.
DNA blot analysis of junction fragments within RM4070. Virion DNAs were digested with MluI, separated by agarose gel electrophoresis, transferred to a nylon membrane, and hybridized with probe 2 (Fig. 1). After autoradiography, probe 2 was removed by methods previously described (31) and the membrane was rehybridized with probe 3 (Fig. 1). Terminal (T) and junction (J) fragments are indicated, with their predicted sizes (in kilobases) shown in parentheses.
These results demonstrate that a herpesvirus that normally lacks invertible segments can be converted to a complex genome structure having two invertible elements simply by introduction of inverted repeats. However, because the inserted sequences also contain a cleavage site, two of the four genome isomers (i.e., P and ISIL) probably arise not by recombination but, rather, by differential use of overlapping cleavage frames (22). Even though cleavage does not appear to contribute to inversion of the HSV-1 genome (26, 29, 67), cleavage could become significant when small or inefficiently recombinagenic sequences comprise the repeats. This is exemplified by a PrV mutant in which translocation of terminal sequences created 1,400-bp inverted repeats and resulted in the apparent inversion of the unique segment bordered by these repeats (25). The translocation, however, also introduced an internal, alternative cleavage site similar to those of the two MCMV mutants described here. A 68-bp deletion both inactivated cleavage at this site and eliminated inversion but did not significantly reduce the size of the inverted repeats, strongly suggesting that cleavage rather than recombination was responsible for the apparent inversion of the segment bordered by the repeats (25). In our studies, the contribution of cleavage at the ectopic site could at most account for the P and ISIL isomers; the IS or IL isomers almost certainly result from inversion via homologous recombination between one or both pairs of inverted repeats. The presence of all four isomers in equimolar amounts indicates that these comparatively small inverted repeats (543 and 1,359 kbp) are able to support efficient inversion, at least to an extent sufficient to generate equimolar amounts of the isomers during the viral propagation necessary to produce DNA for analysis from a single viral particle.
The observation that MCMV, a virus that lacks invertible elements, has the protein machinery necessary to mediate efficient segment inversion suggests that this property is probably common to all herpesviruses. It is therefore unlikely that the replication mechanisms or enzymatic machinery of viruses such as HSV-1 have evolved unique activities that permit genome segment inversion. This further implies that the recombinagenisity of herpesvirus DNA replication did not evolve strictly to support inversion but, rather, must be either an essential component of the herpesvirus DNA replication process or a de facto consequence of that process. One role that has been postulated for recombination is the priming of new replication forks by strand invasion (4, 64), as occurs during bacteriophage T4 concatemer synthesis (40). However, because recombination between direct repeats generally results in deletion of circular molecules, another attractive hypothesis is that recombination serves to amplify templates for DNA synthesis by deleting unit genomic or multimeric circles from concatemers, which, irrespective of genome structure, consist of directly repeated genomes.
With regard to the evolution of herpesvirus genomes, our data suggest that viruses with one set of inverted repeats may have obtained their present genome structures in a single step by acquiring internal inverted duplications of sequences from one terminus; viruses with two sets of inverted repeats could then have evolved through a second duplication of the other terminus. Once acquired, no enzymatic modifications to the viral DNA replication machinery would have been necessary to begin inversion of the segments bordered by the repeats. In PrV, acquisition of an additional invertible segment (and an internal cleavage site) provides a growth advantage in chicken cells and in chickens and a disadvantage in rabbit cells and in mice, apparently due to alternations in concatemer structure and genome cleavage and packaging (45, 47); however, the presence of invertible segments confers no apparent growth disadvantage or advantage to HSV-1 (23, 42) or to MCMV (this study) in vitro. Why these complex genome structures have been retained and what advantages they confer in vivo are as yet largely unknown.
Acknowledgments
We thank Edward Mocarski for kindly providing plasmids pON1101 and pON432 and for his helpful comments during the preparation of the manuscript.
D.R. was a field experience mentorship student from the Governor's School for Government and International Studies, Richmond, Va. This work was supported by Public Health Service grant R21 AI43527-01 and in part by the A. D. Williams Fund of the Medical College of Virginia, Virginia Commonwealth University (both to M.A.M.).
REFERENCES
- 1.Bataille D, Epstein A. Herpes simplex virus replicative concatemers contain L components in inverted orientation. Virology. 1994;203:384–388. doi: 10.1006/viro.1994.1498. [DOI] [PubMed] [Google Scholar]
- 2.Bataille D, Epstein A L. Equimolar generation of the four possible arrangements of adjacent L components in herpes simplex virus type 1 replicative intermediates. J Virol. 1997;71:7736–7743. doi: 10.1128/jvi.71.10.7736-7743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Porat T. Replication of herpesvirus DNA. Curr Top Microbiol Immunol. 1981;91:81–107. doi: 10.1007/978-3-642-68058-8_4. [DOI] [PubMed] [Google Scholar]
- 4.Boehmer P E, Lehman I R. Herpes simplex virus DNA replication. Annu Rev Biochem. 1997;66:347–384. doi: 10.1146/annurev.biochem.66.1.347. [DOI] [PubMed] [Google Scholar]
- 5.Bruckner R C, Dutch R E, Zemelman B V, Mocarski E S, Lehman I R. Recombination in vitro between herpes simplex virus type 1 a sequences. Proc Natl Acad Sci USA. 1992;89:10950–10954. doi: 10.1073/pnas.89.22.10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chou J, Roizman B. Characterization of DNA sequence-common and sequence-specific proteins binding to cis-acting sites for cleavage of the terminal a sequence of the herpes simplex virus 1 genome. J Virol. 1989;63:1059–1068. doi: 10.1128/jvi.63.3.1059-1068.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou J, Roizman B. Isomerization of herpes simplex virus 1 genome: identification of the cis-acting and recombination sites within the domain of the a sequence. Cell. 1985;41:803–811. doi: 10.1016/s0092-8674(85)80061-1. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury S I, Buhk H J, Ludwig H, Hammerschmidt W. Genomic termini of equine herpesvirus 1. J Virol. 1990;64:873–880. doi: 10.1128/jvi.64.2.873-880.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 10.Davison A J, Wilkie N M. Inversion of the two segments of the herpes simplex virus genome in intertypic recombinants. J Gen Virol. 1983;64:1–18. doi: 10.1099/0022-1317-64-1-1. [DOI] [PubMed] [Google Scholar]
- 11.Davison A J, Wilkie N M. Nucleotide sequences of the joint between the L and S segments of herpes simplex virus types 1 and 2. J Gen Virol. 1981;55:315–331. doi: 10.1099/0022-1317-55-2-315. [DOI] [PubMed] [Google Scholar]
- 12.Delius H, Clements J B. A partial denaturation map of herpes simplex virus type 1 DNA: evidence for inversions of the unique DNA regions. J Gen Virol. 1976;33:125–133. doi: 10.1099/0022-1317-33-1-125. [DOI] [PubMed] [Google Scholar]
- 13.Dutch R E, Bianchi V, Lehman I R. Herpes simplex virus type 1 DNA replication is specifically required for high-frequency homologous recombination between repeated sequences. J Virol. 1995;69:3084–3089. doi: 10.1128/jvi.69.5.3084-3089.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutch R E, Bruckner R C, Mocarski E S, Lehman I R. Herpes simplex virus type 1 recombination: role of DNA replication and viral a sequences. J Virol. 1992;66:277–285. doi: 10.1128/jvi.66.1.277-285.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutch R E, Zemelman B V, Lehman I R. Herpes simplex virus type 1 recombination: the Uc-DR1 region is required for high-level a-sequence-mediated recombination. J Virol. 1994;68:3733–3741. doi: 10.1128/jvi.68.6.3733-3741.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebeling A, Keil G M, Knust E, Koszinowski U H. Molecular cloning and physical mapping of murine cytomegalovirus DNA. J Virol. 1983;47:421–433. doi: 10.1128/jvi.47.3.421-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ecker J R, Hyman R W. Varicella zoster virus DNA exists as two isomers. Proc Natl Acad Sci USA. 1982;79:156–160. doi: 10.1073/pnas.79.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibson M G, Spear P G. Insertion mutants of herpes simplex virus have a duplication of the glycoprotein D gene and express two different forms of glycoprotein D. J Virol. 1983;48:396–404. doi: 10.1128/jvi.48.2.396-404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harland J, Brown S M. A HSV-1 variant (1720) generates four equimolar isomers despite a 9200-bp deletion from TRL and sequences between 9200 np and 97,000 np in inverted orientation being covalently bound to sequences 94,000–126,372 np. Virus Genes. 1992;6:291–299. doi: 10.1007/BF01702567. [DOI] [PubMed] [Google Scholar]
- 20.Hayward G S, Jacob R J, Wadsworth S C, Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci USA. 1975;72:4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry B E, Robinson R A, Dauenhauer S A, Atherton S S, Hayward G S, O'Callaghan D J. Structure of the genome of equine herpesvirus type 1. Virology. 1981;115:97–114. doi: 10.1016/0042-6822(81)90092-1. [DOI] [PubMed] [Google Scholar]
- 22.Jacob R J, Morse L S, Roizman B. Anatomy of herpes simplex virus DNA. XII. Accumulation of head-to-tail concatemers in nuclei of infected cells and their role in the generation of the four isomeric arrangements of viral DNA. J Virol. 1979;29:448–457. doi: 10.1128/jvi.29.2.448-457.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins F J, Roizman B. Herpes simplex virus 1 recombinants with noninverting genomes frozen in different isomeric arrangements are capable of independent replication. J Virol. 1986;59:494–499. doi: 10.1128/jvi.59.2.494-499.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kilpatrick B A, Huang E S. Human cytomegalovirus genome: partial denaturation map and organization of genome sequences. J Virol. 1977;24:261–276. doi: 10.1128/jvi.24.1.261-276.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kupershmidt S, Rall G F, Lu Z Q, Ben-Porat T. Cleavage of concatemeric DNA at the internal junction of “translocation” mutants of pseudorabies virus and inversion of their L component appear to be linked. Virology. 1992;187:223–232. doi: 10.1016/0042-6822(92)90310-l. [DOI] [PubMed] [Google Scholar]
- 26.Lamberti C, Weller S K. The herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology. 1996;226:403–407. doi: 10.1006/viro.1996.0668. [DOI] [PubMed] [Google Scholar]
- 27.Lee G T, Pogue-Geile K L, Pereira L, Spear P G. Expression of herpes simplex virus glycoprotein C from a DNA fragment inserted into the thymidine kinase gene of this virus. Proc Natl Acad Sci USA. 1982;79:6612–6616. doi: 10.1073/pnas.79.21.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longnecker R, Roizman B. Generation of an inverting herpes simplex virus 1 mutant lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and the alpha 47 gene. J Virol. 1986;58:583–591. doi: 10.1128/jvi.58.2.583-591.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin D W, Weber P C. The a sequence is dispensable for isomerization of the herpes simplex virus type 1 genome. J Virol. 1996;70:8801–8812. doi: 10.1128/jvi.70.12.8801-8812.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez R, Sarisky R T, Weber P C, Weller S K. Herpes simplex virus type 1 alkaline nuclease is required for efficient processing of viral DNA replication intermediates. J Virol. 1996;70:2075–2085. doi: 10.1128/jvi.70.4.2075-2085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McVoy M A, Adler S P. Human cytomegalovirus DNA replicates after early circularization by concatemer formation, and inversion occurs within the concatemer. J Virol. 1994;68:1040–1051. doi: 10.1128/jvi.68.2.1040-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McVoy M A, Nixon D E, Adler S P. Circularization and cleavage of guinea pig cytomegalovirus genomes. J Virol. 1997;71:4209–4217. doi: 10.1128/jvi.71.6.4209-4217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McVoy M A, Nixon D E, Adler S P, Mocarski E S. Sequences within the herpesvirus-conserved pac1 and pac2 motifs are required for cleavage and packaging of the murine cytomegalovirus genome. J Virol. 1998;72:48–56. doi: 10.1128/jvi.72.1.48-56.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McVoy M A, Nixon D E, Jur J K, Adler S P. The ends on herpesvirus DNA replicative concatemers contain pac2 cis cleavage/packaging elements and their formation is controlled by terminal cis sequences. J Virol. 2000;74:1587–1592. doi: 10.1128/jvi.74.3.1587-1592.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mercer J A, Marks J R, Spector D H. Molecular cloning and restriction endonuclease mapping of the murine cytomegalovirus genome (Smith strain) Virology. 1983;129:94–106. doi: 10.1016/0042-6822(83)90398-7. [DOI] [PubMed] [Google Scholar]
- 36.Mocarski E S, Post L E, Roizman B. Molecular engineering of the herpes simplex virus genome: insertion of a second L-S junction into the genome causes additional genome inversions. Cell. 1980;22:243–255. doi: 10.1016/0092-8674(80)90172-5. [DOI] [PubMed] [Google Scholar]
- 37.Mocarski E S, Roizman B. Herpesvirus-dependent amplification and inversion of cell-associated viral thymidine kinase gene flanked by viral a sequences and linked to an origin of viral DNA replication. Proc Natl Acad Sci USA. 1982;79:5626–5630. doi: 10.1073/pnas.79.18.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mocarski E S, Roizman B. Site-specific inversion sequence of the herpes simplex virus genome: domain and structural features. Proc Natl Acad Sci USA. 1981;78:7047–7051. doi: 10.1073/pnas.78.11.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mocarski E S, Roizman B. Structure and role of the herpes simplex virus DNA termini in inversion, circularization and generation of virion DNA. Cell. 1982;31:89–97. doi: 10.1016/0092-8674(82)90408-1. [DOI] [PubMed] [Google Scholar]
- 40.Mosig G. Recombination and recombination-dependent DNA replication in bacteriophage T4. Annu Rev Genet. 1998;32:379–413. doi: 10.1146/annurev.genet.32.1.379. [DOI] [PubMed] [Google Scholar]
- 41.Poffenberger K L, Roizman B. A noninverting genome of a viable herpes simplex virus 1: presence of head-to-tail linkages in packaged genomes and requirements for circularization after infection. J Virol. 1985;53:587–595. doi: 10.1128/jvi.53.2.587-595.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poffenberger K L, Tabares E, Roizman B. Characterization of a viable, noninverting herpes simplex virus 1 genome derived by insertion and deletion of sequences at the junction of components L and S. Proc Natl Acad Sci USA. 1983;80:2690–2694. doi: 10.1073/pnas.80.9.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pogue-Geile K L, Lee G T, Spear P G. Novel rearrangements of herpes simplex virus DNA sequences resulting from duplication of a sequence within the unique region of the L component. J Virol. 1985;53:456–461. doi: 10.1128/jvi.53.2.456-461.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pogue-Geile K L, Spear P G. Enhanced rate of conversion or recombination of markers within a region of unique sequence in the herpes simplex virus genome. J Virol. 1986;58:704–708. doi: 10.1128/jvi.58.2.704-708.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rall G F, Lu Z Q, Sugg N, Veach R A, Ben-Porat T. Acquisition of an additional internal cleavage site differentially affects the ability of pseudorabies virus to multiply in different host cells. J Virol. 1991;65:6604–6611. doi: 10.1128/jvi.65.12.6604-6611.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rawlinson W D, Farrell H E, Barrell B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reilly L M, Rall G, Lomniczi B, Mettenleiter T C, Kuperschmidt S, Ben-Porat T. The ability of pseudorabies virus to grow in different hosts is affected by the duplication and translocation of sequences from the left end of the genome to the UL-US junction. J Virol. 1991;65:5839–5847. doi: 10.1128/jvi.65.11.5839-5847.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roizman B. The herpesviridae, a brief introduction. In: Roizman B, Whitley R J, Lopez C, editors. The human herpesviruses. New York, N.Y: Raven Press; 1993. pp. 1–9. [Google Scholar]
- 49.Sarisky R T, Weber P C. Requirement for double-strand breaks but not for specific DNA sequences in herpes simplex virus type 1 genome isomerization events. J Virol. 1994;68:34–47. doi: 10.1128/jvi.68.1.34-47.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Severini A, Morgan A R, Tovell D R, Tyrrell D L. Study of the structure of replicative intermediates of HSV-1 DNA by pulsed-field gel electrophoresis. Virology. 1994;200:428–435. doi: 10.1006/viro.1994.1206. [DOI] [PubMed] [Google Scholar]
- 51.Sheldrick P, Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harbor Symp Quant Biol. 1975;39:667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- 52.Skare J, Summers W C. Structure and function of herpesvirus genomes. II. EcoRI, SbaI, and HindIII endonuclease cleavage sites on herpes simplex virus. Virology. 1977;76:581–595. doi: 10.1016/0042-6822(77)90240-9. [DOI] [PubMed] [Google Scholar]
- 53.Slobedman B, Simmons A. Concatemeric intermediates of equine herpesvirus type 1 DNA replication contain frequent inversions of adjacent long segments of the viral genome. Virology. 1997;229:415–420. doi: 10.1006/viro.1997.8447. [DOI] [PubMed] [Google Scholar]
- 54.Slobedman B, Zhang X, Simmons A. Herpes simplex virus genome isomerization: origins of adjacent long segments in concatemeric viral DNA. J Virol. 1999;73:810–813. doi: 10.1128/jvi.73.1.810-813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smiley J R, Duncan J, Howes M. Sequence requirements for DNA rearrangements induced by the terminal repeat of herpes simplex virus type 1 KOS DNA. J Virol. 1990;64:5036–5050. doi: 10.1128/jvi.64.10.5036-5050.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smiley J R, Fong B S, Leung W C. Construction of a double-jointed herpes simplex viral DNA molecule: inverted repeats are required for segment inversion, and direct repeats promote deletions. Virology. 1981;113:345–362. doi: 10.1016/0042-6822(81)90161-6. [DOI] [PubMed] [Google Scholar]
- 57.Smiley J R, Lavery C, Howes M. The herpes simplex virus type 1 (HSV-1) a sequence serves as a cleavage/packaging signal but does not drive recombinational genome isomerization when it is inserted into the HSV-2 genome. J Virol. 1992;66:7505–7510. doi: 10.1128/jvi.66.12.7505-7510.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoddart C A, Cardin R D, Boname J M, Manning W C, Abenes G B, Mocarski E S. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol. 1994;68:6243–6253. doi: 10.1128/jvi.68.10.6243-6253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Straus S E, Aulakh H S, Ruyechan W T, Hay J, Casey T A, Vande Woude G F, Owens J, Smith H A. Structure of varicella-zoster virus DNA. J Virol. 1981;40:516–525. doi: 10.1128/jvi.40.2.516-525.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varmuza S L, Smiley J R. Signals for site-specific cleavage of HSV DNA: maturation involves two separate cleavage events at sites distal to the recognition sequences. Cell. 1985;41:793–802. doi: 10.1016/s0092-8674(85)80060-x. [DOI] [PubMed] [Google Scholar]
- 61.Vieira J, Farrell H E, Rawlinson W D, Mocarski E S. Genes in the HindIII J fragment of the murine cytomegalovirus genome are dispensable for growth in cultured cells: insertion mutagenesis with a lacZ/gpt cassette. J Virol. 1994;68:4837–4846. doi: 10.1128/jvi.68.8.4837-4846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber P C, Challberg M D, Nelson N J, Levine M, Glorioso J C. Inversion events in the HSV-1 genome are directly mediated by the viral DNA replication machinery and lack sequence specificity. Cell. 1988;54:369–381. doi: 10.1016/0092-8674(88)90200-0. [DOI] [PubMed] [Google Scholar]
- 63.Weber P C, Levine M, Glorioso J C. Recombinogenic properties of herpes simplex virus type 1 DNA sequences resident in simian virus 40 minichromosomes. J Virol. 1990;64:300–306. doi: 10.1128/jvi.64.1.300-306.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weller S K. Herpes simplex virus DNA replication and genome maturation. In: Cooper B M, Temin R G, Sugden B, editors. The DNA provirus: Howard Temin's scientific legacy. Washington D.C.: American Society for Microbiology; 1995. pp. 189–213. [Google Scholar]
- 65.Whalley J M, Robertson G R, Davison A J. Analysis of the genome of equine herpesvirus type 1: arrangement of cleavage sites for restriction endonucleases EcoRI, BglII and BamHI. J Gen Virol. 1981;57:307–323. doi: 10.1099/0022-1317-57-2-307. [DOI] [PubMed] [Google Scholar]
- 66.Wilkie N M. Physical maps for herpes simplex virus type 1 DNA for restriction endonucleases HindIII, HpaI, and XbaI. J Virol. 1976;20:222–233. doi: 10.1128/jvi.20.1.222-233.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu D, Sheaffer A K, Tenney D J, Weller S K. Characterization of ICP6::lacZ insertion mutants of the UL15 gene of herpes simplex virus type 1 reveals the translation of two proteins. J Virol. 1997;71:2656–2665. doi: 10.1128/jvi.71.4.2656-2665.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X, Efstathiou S, Simmons A. Identification of novel herpes simplex virus replicative intermediates by field inversion gel electrophoresis: implications for viral DNA amplification strategies. Virology. 1994;202:530–539. doi: 10.1006/viro.1994.1375. [DOI] [PubMed] [Google Scholar]