Abstract
Exposure to inorganic arsenic (As) is recognized as a risk factor for non-melanoma skin cancer (NMSC). We followed up with 7000 adults for 6 years who were exposed to As. During follow-up, 2.2% of the males and 1.3% of the females developed basal cell carcinoma (BCC), while 0.4% of the male and 0.2% of the female participants developed squamous cell carcinoma (SCC). Using a panel of more than 400 cancer-related genes, we detected somatic mutations (SMs) in the first 32 NMSC samples (BCC = 26 and SCC = 6) by comparing paired (tissue–blood) samples from the same individual and then comparing them to the SM in healthy skin tissue from 16 participants. We identified (a) a list of NMSC-associated SMs, (b) SMs present in both NMSC and healthy skin, and (c) SMs found only in healthy skin. We also demonstrate that the presence of non-synonymous SMs in the top mutated genes (like PTCH1, NOTCH1, SYNE1, PKHD1 in BCC and TP53 in SCC) significantly affects the magnitude of differential expressions of major genes and gene pathways (basal cell carcinoma pathways, NOTCH signaling, IL-17 signaling, p53 signaling, Wnt signaling pathway). These findings may help select groups of patients for targeted therapy, like hedgehog signaling inhibitors, IL17 inhibitors, etc., in the future.
Keywords: somatic mutation, basal cell carcinoma, squamous cell carcinoma, arsenic exposure, hedgehog signaling, notch signaling, iL-17 signaling, gene–environment interaction
1. Introduction
The human epidermis is composed of keratinocytes, which exhibit cuboidal (basaloid) cytology at the lowest (basal) layer and squamous cytology at the suprabasal layers. It also contains Merkel cells and pigment-producing melanocytes. Tumors originating from keratinocytes or Merkel cells are grouped into non-melanoma skin cancer (NMSC). NMSC includes basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). Bowen disease (BD) is a clinical term for squamous cell carcinoma in situ. In 2019, there were 4.0 million cases of BCC and 2.4 million cases of SCC worldwide [1]. In 2012, in the United States (US), 56,987 patients were identified with BCC (39,035 incident and 17,952 prevalent) [2]. NMSC is the most prevalent malignancy in the US, exceeding all other cancers combined with an estimated 2 million new diagnoses each year [3,4]. Both BCC and SCC have low mortality but can have a high recurrence rate and can cause significant disfiguration, particularly in the head and neck regions where they commonly occur [4,5]. BCC of skin is the most common type and may account for about 90% of all skin cancers [6,7,8].
The incidence of BCC shows a strong inverse correlation with geographic latitude combined with the pigment status of its inhabitants [9]. The highest rates are seen in Australia, where over one in two inhabitants will be diagnosed with BCC by the time they reach 70 years of age [10]. The incidence rates in Asia and South America are ten- to hundred-fold lower [11,12,13]. Patients with a BCC have a seventeen-fold increased risk of subsequent BCC compared with the general population, as well as a three-fold increased risk of subsequent SCC and a two-fold increased risk of melanoma [14,15]. The mortality of BCC is extremely low. However, the healthcare cost for NMSC is quite high. A US Medicare expenditure study showed that NMSC was the fifth most costly cancer between 1992 and 1995 [16]. A report estimated the average annual cost of treating NMSC in the US to be USD 4.8 billion from 2007 to 2011, a substantial increase compared with the 2002 to 2006 estimate of USD 2.7 billion [17].
The risk factors for both BCC and SCC include ultraviolet radiation (UVR) and chronic immunosuppression [18,19,20]. UVR is the major known environmental risk factor. Thus, the prevention strategy is photoprotection, which can be both topical and systemic [21]. The ability to repair UV-induced DNA damage reduces with age. Increasing age and the male sex (at older age) are well-known factors for an increased risk of BCC. Molecular biomarkers of NMSC including genomics, transcriptomics, proteomics, and metabolomics have been recently reviewed extensively [22,23]. Arsenic (As) is a known carcinogen that appears in groundwater and is associated with skin cancer [24]. Chronic exposure to As may induce BCC [25,26,27,28]. One study suggested that miR-155-5p regulates the NF-AT1-mediated immunological dysfunction that is involved in the pathogenesis and carcinogenesis of As [29]. Some studies showed that in the presence of As exposure, decreased telomere length predisposes individuals to an increased risk of BCC [30]. As generates reactive oxygen species that cause oxidative stress, leading to DNA damage. Concurrently, As inhibits DNA repair, modifies the epigenetic regulation of gene expression, and targets protein function due to its ability to replace zinc in select proteins [31]. In a recent study, we have shown that high As exposure was associated with impaired DNA replication pathways, cellular response to different DNA damage repair mechanisms, and immune response [32].
In Bangladesh, between 2000 and 2002, 11,746 participants (5042 men and 6704 women) were recruited for the Health Effects of Arsenic Longitudinal Study (HEALS) and were exposed to As through the consumption of As-enriched groundwater. The study found 714 confirmed cases of premalignant skin lesions [33].
UVR-induced cancers, such as BCC and melanoma, exhibit the highest prevalence of somatic mutations. The majority show UVR signatures. Two tumor suppressor genes are important in sporadic BCC: patched 1 (PTCH1) and tumor protein 53 (TP53). Loss of heterozygosity in chromosome 9q22 is the most frequently encountered cytogenic change in BCC. The inactivation of PTCH1 and the up-regulation of hedgehog (Hh) signaling are most likely pivotal events in the pathogenesis [34]. Studies have shown that a large percentage (~85%) of BCC may harbor somatic mutations in Hh pathway genes—PTCH1 (73%), SMO (20%), and SUFU (8%) [3,35]. Mutations in TP53 are also found in >60% of cases [3,35,36]. In BCC, mutations are also reported in other known cancer genes, including NRAS, HRAS, KRAS, PIK3CA, RAC1, FBXW7, RB1, CDKN2A, NOTCH1, NOTCH2, CASP8, and ARID1A [3,36]. Other significantly mutated genes include PTN14, MYCN, RPL22, and PPIAL4G [3]. There is evidence that Wnt pathways play a role in the pathogenesis of NMSCs through the activation of inflammatory processes and the stimulation of cancer cell proliferation and invasion [37]. A recent study highlighted the role of CYFIP2, HOXB5, PTPN3, MARCKSL1, PTCH1, and CDC2 in BCC [38].
Our group conducted a double-blind, placebo-controlled study, the Bangladesh Vitamin E and Selenium Trial (BEST), to evaluate the effect of vitamin E and selenium supplementation in the prevention of NMSC in a population exposed to As who had a clinical manifestation of As toxicity in the form of As-related non-malignant skin lesion [39]. Seven thousand subjects were followed up for 6 years for the development of NMSC. Of them, 1.7% developed BCC (males = 2.2%, females = 1.3%) [32]. Our previous study showed interactions of As exposure and gene expression profiling in BCC in the study group who were exposed to inorganic As through drinking As-contaminated well water [32].
The mutational profile in SCC tissue may be slightly different [40,41,42,43]. In a study on high-risk head and neck SCC patients, TP53 was mutated in 100% of cases; APC, ATM, ERBB4, GNAQ, KIT, RB1, and ALB1 were altered in 60% of cases; and FGF2 mutation was seen in 40% of cases [43]. Interestingly, druggable targets may be found in 60–80% of the cases [40,43].
Gene expression studies [34,44,45,46,47] (mostly with small sample sizes) in NMSC have suggested differential expressions of Wnt and Hh pathway genes [34], TGF-beta signaling, PPAR-Υ signaling pathway genes [48], and MAPK pathways [47] for classical BCC. SCC-like BCC may show differential expressions of immune-response genes and oxidative stress-related genes [34]. There are few transcriptome-wide gene expression studies in SCC [45,49,50,51]. Compared to normal skin, SCC and actinic keratosis show differential expression of a number of genes, including RAB31, MAP4K4, IL-1RN, NMI, and IL4R; however, there was no difference between SCC and actinic keratosis [45].
Studies on NMSC have been mainly performed in Caucasian populations. To our knowledge, no study addresses the molecular profiling of NMSC in a “non-Caucasian population” exposed to As. Moreover, no study in NMSC has yet addressed the fact that sunlight exposure is associated with a large number of somatic mutations in different genes in non-lesional, apparently healthy skin. Therefore, identifying a somatic mutation in NMSC samples does not necessarily establish the association between that mutation and the NMSC pathogenesis. In this study, we have looked for (a) somatic mutations in NMSC tissue by scanning more than 400 cancer-related genes to identify NMSC-associated somatic mutations in a Bangladeshi population exposed to As through drinking As-contaminated water and (b) examined if such mutation(s) were associated with differential expression of gene(s) or pathway(s).
2. Materials and Methods
2.1. Study Population
For this study, we selected the first 32 subjects from the BEST study developing histopathologically confirmed NMSC and had the tumor tissue properly preserved in RNA later, an RNA stabilizing buffer (ThermoFisher Scientific, Waltham, MA, USA). The BEST study included 7000 men and women (m = 2840, f = 4160) who were known to be exposed to As through consuming well water containing As [32,39]. This study included all subjects with clinically visible non-malignant skin lesions (melanosis, leukomelanosis, or keratosis)—a known manifestation of As toxicity. We also collected “non-lesional” or apparently healthy skin tissue surrounding the margin of the arsenical keratosis lesion from 16 independent patients and preserved it in the same way. Throughout the manuscript, we have used the term “healthy skin tissue” for these non-lesional skin tissues. Patient characteristics are shown in Supplementary Table S1. All these patients were followed up every 2 years for a total of 6 years to check for the development of NMSC. The urinary As creatinine ratio (UACR) was also recorded at baseline and follow-up visits. A skin biopsy was performed on patients who had reasonable clinical suspicion of BD or NMSC, including SCC and BCC. All these patients consented to a biopsy. During this follow-up period, 14.7% of the males and 7.5% of the females (p < 0.0001, chi-square test) had a skin biopsy performed. Histopathological examination was performed by two pathologists independently. For the pathological diagnosis, a structured reporting form was used (see Supplementary Form S1). A skin biopsy was performed on a total of 727 participants (m = 417, f = 310). Among them, 37.7% of the biopsies showed BD, 2.9% had invasive SCC, 16.1% showed BCC, and the rest (43.3%) showed arsenical keratosis or other skin conditions [32]. Thus, among the As-exposed study population, 2.2% of the male and 1.3% of the female participants developed BCC, while 0.4% of the male and 0.2% of the female participants developed SCC over the six-year follow-up.
2.2. Arsenic Exposure Measurement
We measured UACR at baseline and 2-year, 4-year, and 6-year follow-up as a measure of As exposure. The urinary total As concentration was measured by inductively coupled plasma mass spectrometry [52]. Urinary creatinine was measured by a colorimetric method based on the Jaffe reaction described by Heinegard and Tiderstrom [53]. The urinary As was measured from a spot urine collection. To take into account the hydration status, we used the UACR as a measure of As exposure. The log2-transformed UACR showed a strong correlation to the log2-transformed well water as a concentration (r = 0.66) [54].
2.3. Nucleic Acid Extraction
DNA was extracted from these RNA later preserved tissues using a Quick-DNA/RNA Microprep Plus kit (Zymo Research, Irvine, CA, USA) following the manufacturer’s protocol. After taking the samples out of the RNA later, the tissue was washed with a 1xPBS buffer and then submerged into the DNA/RNA shield before simultaneous DNA and RNA extraction. RNA and DNA quantification and the 260/280 ratio were checked by NanoDrop 1000.
2.4. Somatic Mutation Assay
For the detection of somatic mutation, we used AmpliSeq for the Illumina comprehensive Cancer Panel Guide (Illumina Inc., San Diego, CA, USA). The total gene list is presented in Supplementary Table S2. There were 4 plates with 4 different sets of primer pools. We made 4 identical plates of DNA with 10 ng input DNA each for 1 pool plate. DNA target regions were amplified in all 4 plates, and then amplified DNA was pooled together in corresponding wells on one plate. In the next step, primer dimer or unused amplicon were digested. After that, i7 and i5 adapters were ligated to the amplicon ends. These products were cleaned up by magnetic beads and then amplified for the 2nd time. The library was then cleaned up to have the final library for sequencing. After pooling, the final library was measured with a fluorometer. Library size and quantity were also measured by a fragment analyzer. Sequencing was performed on the Illumina HiSeq platform (San Diego, CA, USA).
2.5. Gene Expression Assay
For RNA sequencing on the Illumina platform, we used Lexogen’s QuantSeq 3′ mRNA–Seq kit (Vienna, Austria) for library preparation as described previously [32]. The final library was measured by a fluorometer, and after pooling, qPCR was performed to quantify the input library for sequencing on the Illumina HiSeq platform (San Diego, CA, USA).
This study was approved by the Institutional Review Board of The University of Chicago Medicine protocol code IRB19-0724 and was approved on 24 September 2019.
2.6. Statistical Method
Mutation detection: The FASTQ Illumina sequencing data were initially processed by CLC genomics Workbench23 (https://digitalinsights.qiagen.com/ (accessed on 26 April 2023)). After adapter sequencing trimming, default parameters were used for QC. The minimum length was kept at 40. A Targeted Amplicon Sequencing (TAS) module for paired samples was used where we used the tissue sample and the corresponding blood DNA sample as a pair. In this module, initially, the reads were mapped to homo sapiens sequence hg19, and the variants were detected using structural variant caller v1.2 (Biomedical Genomics Analysis 23.1). Variants found in normal samples (in our case—the blood) were removed from the variants detected in the tissue sample. The in-built workflow removed the germline variants found in the public database (db SNP, 1000 genomes project, dbSNPs common, and hapmap) that were found in the mapped reads. Also, variants outside the target region were removed as they are likely to be false positives due to non-specific mapping of sequencing reads. The parameters for the low-frequency variant detection were set at a minimum coverage of 10, a minimum count of 2, and a minimum frequency of 2%. Next, the remaining variants (the “somatic variants”) were annotated with gene names, amino acid changes, conservation scores, and information from ClinVar (variants with clinically relevant association). We used a variant calling quality score of Q60 as the cut-off for the list of somatic mutations.
Transcriptome data were processed using Partek Flow (version 10.0) (https://www.partek.com/partek-flow/, accessed on 11 November 2022). A STAR aligner was used for alignment, and the final gene count data were expressed as the count per million reads (CPM) and were log2 transformed for the ANOVA using Partek Genomics Suite (version 7.0) (https://www.partek.com/partek-genomics-suite/, accessed on 22 April 2024). For statistical analyses, IBM SPSS Statistics version 29 was used. We also used Partek Genomis Suit for ANOVA and Gene set ANOVA as described in a previous paper [32]. In the GO enrichment analysis, we tested if the differentially expressed genes (as per the set criteria) fell into a Gene Ontology category more often than expected by chance. We used a chi-square test for comparison. The negative log of the p-value for this test was used as the enrichment score. In addition to GO enrichment analysis, we also examined the differential expression of “gene sets” using the Gene Set Enrichment Analysis (GSEA) [55]. Given an a priori-defined set of genes “S” (sharing the same GO category or the KEGG pathway), the goal of GSEA was to determine whether the members of “S” were randomly distributed throughout the ranked list or primarily found at the top or bottom. For further statistical comparison of the magnitudes of the differential expression of the “Gene set” in the absence or presence of a factor (mutation), we used “Gene set ANOVA”, which offers an introduction to the interaction terms in the model. Gene set ANOVA is a mixed model ANOVA to test the expression of a set of genes (sharing the same category or functional group) instead of an individual gene in different groups. The analysis is performed at the gene level, but the result is expressed at the level of the Gene set category by averaging the member genes’ results. The equation for the model is as follows:
| Model: Y = μ + T + G + TxG + TxMut + ε |
where Y represents the expression status of a Gene set category, μ is the common effect or average expression of the Gene set category, T is the tissue-to-tissue (tumor/normal) effect, G is the gene-to-gene effect, TxG is the differential pattern of gene expression in different tissue types, TxMut is the interaction term, and ε represents the random error.
3. Results
The diagnoses of BCC (n = 26) and SCC (n = 6) were confirmed by skin biopsy. There was consensus between two pathologists for all 32 cases.
3.1. Somatic Mutation
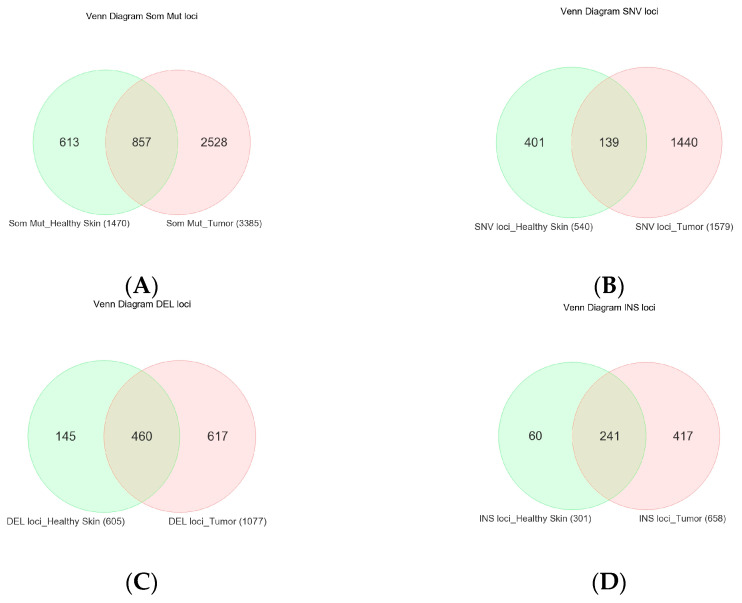
Considering the fact that even healthy-looking skin tissue is also exposed to sunlight and may develop UVR-induced somatic mutations, for each tissue sample, we compared the tissue DNA with the corresponding whole blood DNA (a proxy for germline) from the same patient for the detection of a somatic mutation. In 32 tumor tissue samples, we found a total of 6829 somatic mutations (in 3385 unique genomic loci, see Figure 1A). In 16 healthy skin tissues, we found a total of 2530 somatic mutations in 1470 unique genomic loci (see Figure 1A). Some of the variant metrics are shown in Table 1.
Figure 1.
Venn diagram showing the overlap of unique somatic mutation loci among NMSC tissue (in pink) and normal skin tissue (in light green). All types of mutations are shown in the upper left (A), SNVs are shown in the upper right (B), deletions (DELs) are shown in the lower left (C), and insertions (INSs) are shown in the lower right panel (D).
Table 1.
Some of the variant metrics by somatic variant type and tissue type.
| Somatic Variant Type | Variant Metrics | Median (BCC) | Median (SCC) | Median (Healthy Skin) |
|---|---|---|---|---|
| All Somatic Variants | Count | 10.00 | 44.00 | 15.00 |
| Coverage at the variant loci | 169.00 | 772.50 | 245.00 | |
| Frequency of the variant | 4.74 | 6.15 | 4.71 | |
| Q-score of the variant allele | 37.00 | 36.72 | 36.96 | |
| QUAL of the variant call | 200.00 | 200.00 | 200.00 | |
| SNVs | Count | 14.00 | 357.50 | 22.00 |
| Coverage at the variant loci | 114.00 | 974.50 | 154.00 | |
| Frequency of the variant | 8.33 | 43.89 | 17.78 | |
| Q-score of the variant allele | 37.00 | 36.86 | 37.00 | |
| QUAL of the variant call | 200.00 | 200.00 | 200.00 | |
| Insertion | Count | 9.00 | 37.50 | 15.00 |
| Coverage at the variant loci | 201.00 | 795.50 | 317.00 | |
| Frequency of the variant | 4.12 | 4.84 | 4.63 | |
| Q-score of the variant allele | 37.00 | 36.75 | 36.93 | |
| QUAL of the variant call | 200.00 | 200.00 | 200.00 | |
| Deletion | Count | 8.00 | 26.00 | 13.00 |
| Coverage at the variant loci | 189.00 | 616.00 | 297.00 | |
| Frequency of the variant | 4.17 | 4.07 | 3.91 | |
| Q-score of the variant allele | 37.00 | 36.32 | 36.69 | |
| QUAL of the variant call | 200.00 | 200.00 | 200.00 |
The median number of somatic mutations per BCC sample was 148; for SCC, it was 180.5 per sample; and for healthy skin tissue, it was 140 per sample (p = 0.73, Kruskal–Wallis test). Considering the target sequence region of 1.7 Mb, the calculated median tumor mutation burden (TMB) was 87 mutations/Mb for BCC tissue, 106 mutations/Mb for SCC tissue, and 82 mutations/Mb for healthy skin tissue (p = 0.58, Kruskal–Wallis test). When we compared the TMB in BCC, SCC, and healthy skin tissue by sex, the difference was not statistically different, although the TMB appeared to be higher in females.
We generated a list of somatic mutations in NMSC cases (BCC and SCC) and a list of somatic mutations in healthy skin tissue from an independent set of participants. Then, by comparing the somatic mutations in tumor tissue and healthy skin tissue, we looked for (a) NMSC-associated somatic mutations, (b) somatic mutations potentially associated with NMSC, which are found in tumor tissue as well as in healthy skin tissue, and (c) somatic mutations present only in healthy skin tissue.
The overlap of the total unique somatic mutation loci between tumor tissue and healthy skin tissue and the mutations stratified by type (SNV, Del, and INS) are shown in Figure 1A. Among the SNVs, irrespective of BCC, SCC, or healthy skin tissue, the most common type of substitution was C > T (median 15.7% of substitutions/sample, 95% CI 1.6–37.8%) and G > A (median 15.8% of substitutions/sample, 95% CI 0–42.7%) without statistical difference between the tissue types (Supplementary Figure S1). This high prevalence of C > T + G > A substitution is consistent with the mutational signature for NMSC usually related to sunlight exposure.
3.2. NMSC-Associated Somatic SNVs
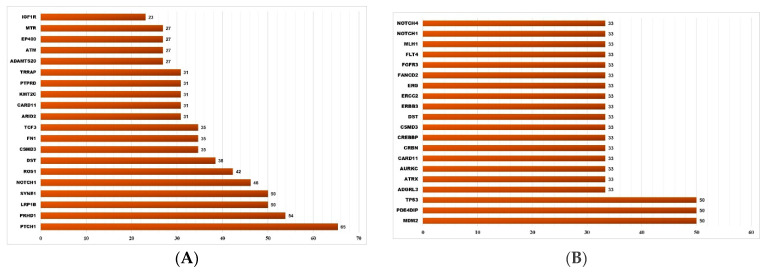
There were a total of 1611 somatic SNVs (representing 1440 unique SNV loci in 361 genes) detected only in NMSC samples (total n = 32, of which BCC = 26, SCC = 6) and not in healthy skin tissue (Figure 1B). All of these were in the gene coding regions; 321 were found in the ClinVar database, 628 were also found in TCGA skin cancer samples and reported in the COSMIC database, and 604 were “non-synonymous” SNVs. Among these 1611 NMSC-associated somatic SNVs, 1344 SNVs (covering 1222 loci in 344 genes) were found in BCC and the other 267 SNVs (covering 261 loci in 153 genes) were found in SCC. Some 43 unique loci were common in BCC and SCC but not in normal skin tissue. The list of the top 20 genes harboring these BCC-associated and SCC-associated somatic mutations are shown in Figure 2A and Figure 2B, respectively.
Figure 2.
The top 20 genes that had BCC-associated somatic mutations (shown on the left panel (A)) and SCC-associated somatic mutations (shown on the right panel (B)). The x-axis shows the percentage of samples harboring NMSC-associated somatic mutations in a given gene. The y-axis shows the gene name.
3.3. Somatic Mutation SNVs Common in NMSC and Healthy Skin Tissue
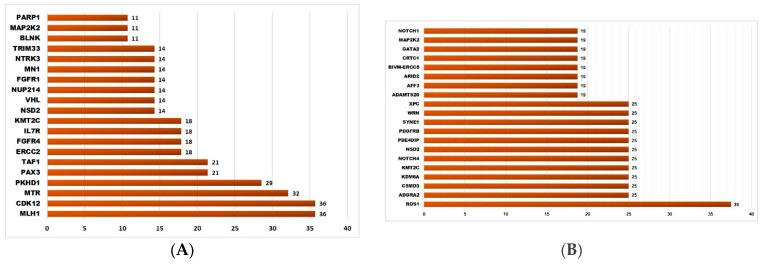
We detected 277 somatic SNVs (representing 139 unique SNV loci in 95 different genes) found in both NMSC and healthy skin (Figure 1B). A total of 96 of them were reported in ClinVar; 66 were also found in TCGA skin cancer samples and reported in the COSMIC database; and 34 were “non-synonymous” SNVs. The list of the top twenty genes harboring these somatic mutations potentially associated with NMSC is shown in Figure 3A.
Figure 3.
The top 20 genes that had somatic mutations in NMSC and healthy skin (shown on left panel (A)) and the top 20 genes that had somatic mutations only in healthy skin tissue (shown on the right panel (B)). The x-axis shows the percentage of samples harboring somatic mutations in a given gene. The y-axis shows the gene name.
3.4. Somatic Mutation SNVs Detected Only in Healthy Skin Tissue
We detected 426 somatic SNVs (representing 401 unique SNV loci in 192 different genes), which were found only in healthy skin and not in any NMSC tissue (see Figure 1B). A total of 87 of them were reported in ClinVar; 146 were also found in TCGA skin cancer samples and reported in the COSMIC database; and 83 were “non-synonymous” SNVs. The list of the top twenty genes harboring these somatic mutations potentially associated with NMSC is shown in Figure 3B.
3.5. Association of Somatic Mutation and Differential Gene Expression in NMSC
In the next step, we asked if the absence or presence of somatic mutation(s) in the tissue showed a difference in differential gene expression patterns in tumor tissue compared to healthy skin tissue. Considering the fact that non-synonymous SNVs (causing amino acid change) may have functional effects, we restricted the analysis to NMSC-associated non-synonymous SNVs only. So, a tumor tissue was only considered a mutant for PTCH1 (for example) if that sample harbored at least one of the non-synonymous SNVs in PTCH1 but not if it harbored only some other SNVs in the PTCH1 gene.
3.5.1. Gene Level Analysis
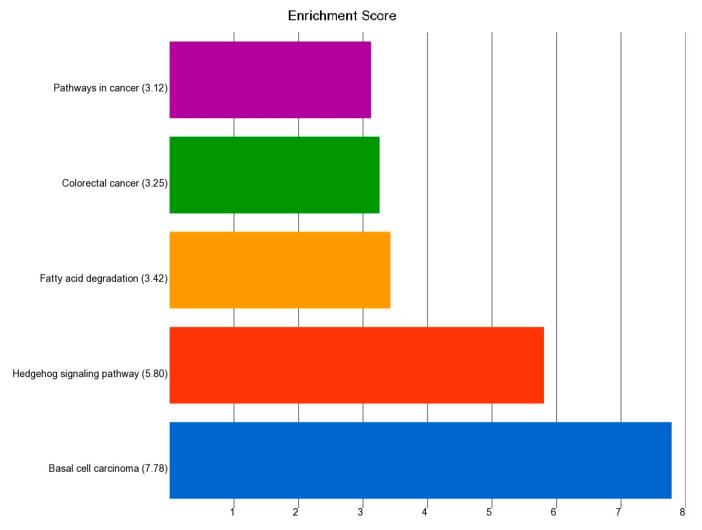
A comparison of gene expression data between BCC (n = 26) and healthy skin tissue (n = 16) showed that 118 genes were differentially expressed at least by a fold change (FC) of 3 and an FDR level of ≤0.05 (see Supplementary Table S3). Gene Ontology (GO) or enrichment analysis of this gene list is shown in Figure 4. The list was enriched in genes involved in “Basal cell carcinoma”, “Hedgehog signaling pathway”, and “pathways in cancer”. It may be mentioned that GSEA analysis (see Supplementary Table S4) also confirmed the enrichment of these pathway genes.
Figure 4.
GO enrichment analysis of the top 118 differentially expressed genes (FC ≥ 3 and FDR ≤ 0.05) in BCC tissue compared to healthy skin tissue. The x-axis represents the enrichment score and the y-axis is the group of genes.
Next, in the ANOVA model(s), we entered an interaction term “tissue (0 = healthy, 1 = BCC) x nonsynonymous mutation in PTCH1 (0 = no mutation, 1 = mutation)” to find out the genes that had a different magnitude of differential expression in the BCC tissue in the absence or presence of the mutation. The differential expression of these same 118 differentially expressed genes in the absence (n = 14) and the presence of the non-synonymous somatic mutation (n = 12) in the PTCH1 gene compared to the same normal skin (n = 16) are presented in Table 2 along with the interaction p-values. In the combined analysis, the PTCH1 gene was overexpressed in BCC tissue by FC = 4 (95% CI 2.2–7.2) compared to healthy skin tissue (see Supplementary Table S3); but, in the absence of any non-synonymous somatic mutation in PTCH1 in BCC tissue (n = 14), the FC was 2.8 (95% CI 1.5–5.3), and in presence of a non-synonymous somatic mutation in PTCH1 in the BCC tissue (n = 12), the FC was 6 (95% CI 3.1–11.8) (interaction p = 0.03, see Table 2). The result showed that the magnitude of differential expression for 40 out of these 118 genes was statistically different if the tumor had the non-synonymous mutation in PTCH1 (see the interaction p column in Table 2). In fact, the effect of this somatic mutation in PTCH1 was more pronounced for other genes.
Table 2.
The top 118 differentially expressed genes in BCC tissue compared to healthy skin tissue by at least an FC of 3 at an FDR level of 0.05. The comparison of FCs (95% CI) in BCC tissue without a PTCH1 somatic mutation and BCC tissue with a PTCH1 mutation status are shown. The genes are arranged in the same order as in Supplementary Table S3, where the genes are arranged in ascending order of their p-value for the combined analysis. The significant interactions are shown in red.
| BCC without PTCH1 Mutation | BCC with PTCH1 Mutation | ||||
|---|---|---|---|---|---|
| Gene Symbol | Fold Change | (95% CI) | Fold Change | (95% CI) | Interaction |
| p | |||||
| CHGA | 37.3 | (8.04–172.69) | 296.6 | (59.86–1469.36) | 0.0150 |
| MPPED1 | 35.6 | (8.13–156.0) | 51.3 | (10.99–239.68) | 0.6443 |
| CHCHD7 | 3.0 | (1.73–5.17) | 7.4 | (4.18–13.12) | 0.0035 |
| LGR5 | 20.5 | (4.81–87.16) | 84.4 | (18.63–382.35) | 0.0734 |
| ABI3BP | 6.3 | (2.39–16.80) | 27.3 | (9.88–75.45) | 0.0075 |
| IRS4 | 16.4 | (3.67–73.60) | 108.6 | (22.71–518.88) | 0.0229 |
| LEF1 | 9.6 | (3.02–30.61) | 20.4 | (6.1–68.31) | 0.2286 |
| RP11-368P15.3 | 15.4 | (3.83–62.07) | 36.6 | (8.55–156.32) | 0.2506 |
| DUSP10 | 12.0 | (3.05–47.20) | 41.5 | (9.96–173.2) | 0.0960 |
| PALM | 11.1 | (2.83–43.27) | 48.6 | (11.71–201.7) | 0.0480 |
| DIO2 | 3.1 | (1.57–6.05) | 8.4 | (4.14–16.91) | 0.0083 |
| VCAN | 5.4 | (1.94–15.17) | 26.2 | (8.95–76.53) | 0.0064 |
| RGCC | 25.0 | (5.30–117.63) | 30.5 | (6.05–153.7) | 0.8094 |
| LRRCC1 | 2.4 | (1.40–4.01) | 5.1 | (2.95–8.84) | 0.0090 |
| SOX4 | 2.4 | (1.39–3.97) | 5.0 | (2.88–8.58) | 0.0105 |
| SETBP1 | 2.0 | (1.26–3.05) | 4.9 | (3.1–7.82) | 0.0003 |
| DCP1B | 17.0 | (3.81–75.70) | 35.8 | (7.54–170.36) | 0.3530 |
| RP11-157G21.2 | 12.8 | (3.57–45.78) | 16.4 | (4.34–62.12) | 0.7144 |
| NNMT | 15.5 | (3.28–73.10) | 53.8 | (10.66–271.7) | 0.1390 |
| TGS1 | 2.8 | (1.61–4.86) | 3.6 | (2.04–6.44) | 0.3826 |
| SPON2 | 4.1 | (1.60–10.47) | 23.4 | (8.79–62.22) | 0.0012 |
| PROCR | 11.1 | (2.71–45.07) | 41.0 | (9.45–177.31) | 0.0875 |
| LPL | 12.4 | (2.97–51.52) | 35.6 | (8.04–157.49) | 0.1714 |
| BNC2 | 2.5 | (1.34–4.77) | 9.5 | (4.9–18.47) | 0.0003 |
| SYNJ1 | 7.1 | (2.21–22.57) | 21.2 | (6.3–71.02) | 0.0833 |
| TM4SF1 | 5.0 | (1.82–13.9) | 18.7 | (6.49–54.02) | 0.0196 |
| PTCH1 | 2.8 | (1.47–5.33) | 6.0 | (3.07–11.77) | 0.0311 |
| MMP11 | 11.4 | (2.45–53.18) | 77.3 | (15.54–384.35) | 0.0245 |
| MEX3A | 3.7 | (1.48–9.08) | 17.6 | (6.86–45.33) | 0.0023 |
| RP11-433O3.1 | 16.0 | (3.03–84.15) | 60.4 | (10.68–341.94) | 0.1400 |
| TUBA1A | 3.2 | (1.53–6.52) | 6.6 | (3.11–14.06) | 0.0622 |
| NXN | 2.8 | (1.51–5.11) | 4.3 | (2.29–8.13) | 0.1822 |
| TMCO3 | 10.3 | (2.53–42.01) | 30.9 | (7.14–133.59) | 0.1497 |
| PYCR2 | 14.3 | (2.87–71.24) | 51.0 | (9.55–272.2) | 0.1444 |
| ALCAM | 2.7 | (1.42–5.22) | 5.6 | (2.85–11.02) | 0.0433 |
| RP11-74M13.4 | −13.6 | (−56.71–3.24) | −20.3 | (-90.17–4.55) | 0.6003 |
| MARCKSL1 | 9.8 | (2.15–44.09) | 59.8 | (12.38–288.3) | 0.0294 |
| SCAMP5 | 8.2 | (2.07–32.72) | 38.7 | (9.18–163.16) | 0.0412 |
| VASH2 | 8.2 | (2.06–32.65) | 38.9 | (9.21–164.60) | 0.0403 |
| HDGFRP3 | 9.6 | (2.24–41.29) | 40.4 | (8.83–184.54) | 0.0715 |
| KRT17 | 4.7 | (1.74–12.72) | 12.2 | (4.31–34.25) | 0.0802 |
| DCLRE1A | 9.4 | (2.30–38.67) | 31.3 | (7.19–136.32) | 0.1176 |
| ATP5F1 | 4.7 | (1.81–12.24) | 9.7 | (3.56–26.24) | 0.1641 |
| RP11-366L20.2 | 11.2 | (2.50–50.45) | 36.1 | (7.53–172.85) | 0.1519 |
| BASP1 | 2.1 | (1.20–3.61) | 5.8 | (3.24–10.23) | 0.0012 |
| DSC2 | 3.2 | (1.4–7.29) | 10.7 | (4.5–25.24) | 0.0091 |
| IGF2BP2 | 2.2 | (1.21–3.94) | 6.3 | (3.39–11.55) | 0.0017 |
| FBN3 | 8.1 | (1.67–39.62) | 151.1 | (28.98–788.07) | 0.0013 |
| CSPG4 | 7.1 | (1.83–27.53) | 39.1 | (9.51–161.04) | 0.0228 |
| CCDC152 | 5.2 | (1.77–15.01) | 13.8 | (4.54–42.13) | 0.0902 |
| IGF2BP1 | −12.0 | (−48.57–2.94) | −17.7 | (−76.48–4.1) | 0.6008 |
| SOBP | 6.5 | (1.99–21.18) | 16.0 | (4.67–54.9) | 0.1587 |
| TMEM176B | 9.5 | (2.11–42.44) | 45.4 | (9.48–217.22) | 0.0565 |
| RP11-301G23.1 | 8.8 | (1.97–39.52) | 52.7 | (11.03–251.33) | 0.0307 |
| ACAA2 | 11.5 | (2.30–57.16) | 53.7 | (10.05–287.19) | 0.0781 |
| SHOX2 | 4.7 | (1.53–14.21) | 22.7 | (7.12–72.64) | 0.0108 |
| COA7 | 21.1 | (5.65–78.70) | 7.1 | (1.79–27.97) | 0.1268 |
| RUNX1T1 | 3.6 | (1.43–8.85) | 12.4 | (4.78–32.02) | 0.0139 |
| ITFG3 | 3.2 | (1.65–6.35) | 3.9 | (1.94–7.9) | 0.6011 |
| GRM5 | −9.3 | (−34.5–2.49) | −16.0 | (−62.86–4.05) | 0.4416 |
| PLAG1 | 9.6 | (2.26–41.08) | 29.8 | (6.57–135.5) | 0.1504 |
| SKIV2L | 12.8 | (3.14–52.23) | 15.2 | (3.5–65.88) | 0.8202 |
| HMGN1P38 | 5.5 | (2.1–14.27) | 6.5 | (2.41–17.76) | 0.7300 |
| CTB-167B5.2 | 9.9 | (2.25–43.22) | 31.5 | (6.75–147.23) | 0.1471 |
| VPS37D | 6.1 | (1.71–21.50) | 27.5 | (7.35–103.18) | 0.0302 |
| OSCP1 | 13.7 | (2.88–64.61) | 25.9 | (5.12–131.33) | 0.4421 |
| C1QTNF1 | 19.1 | (3.73–97.99) | 22.7 | (4.13–124.97) | 0.8438 |
| ACADL | −29.6 | (−148.16–5.92) | −13.3 | (−71.41–2.48) | 0.3556 |
| GJB6 | 5.5 | (1.93–15.78) | 9.7 | (3.24–29.02) | 0.3184 |
| PTCH2 | 9.5 | (1.8–49.61) | 101.3 | (17.97–570.53) | 0.0104 |
| RAB28 | 12.7 | (2.75–58.71) | 24.4 | (4.94–120.48) | 0.4273 |
| CASC14 | 12.1 | (2.4–60.91) | 39.6 | (7.33–213.81) | 0.1754 |
| LTBP1 | 2.9 | (1.25–6.59) | 12.1 | (5.09–28.84) | 0.0023 |
| ALYREF | 13.3 | (3.0–58.87) | 18.0 | (3.81–85.04) | 0.7035 |
| LITAF | 4.9 | (1.91–12.46) | 6.6 | (2.46–17.44) | 0.5564 |
| TMEM104 | 6.2 | (1.54–25.25) | 50.2 | (11.68–215.66) | 0.0078 |
| NUDCD1 | 12.4 | (2.95–52.09) | 15.2 | (3.39–67.72) | 0.7944 |
| SLCO2A1 | 13.7 | (2.77–67.39) | 26.5 | (5.02–140.11) | 0.4391 |
| SH3BP4 | 5.3 | (1.92–14.42) | 7.8 | (2.71–22.29) | 0.4691 |
| RP4-765C7.2 | 12.0 | (2.29–63.0) | 43.8 | (7.77–246.52) | 0.1502 |
| RP11-5N11.5 | 5.2 | (1.29–20.68) | 87.7 | (20.63–372.88) | 0.0004 |
| FMO2 | 6.4 | (1.95–20.71) | 12.3 | (3.59–42.36) | 0.2968 |
| AC005013.1 | 5.8 | (1.82–18.61) | 12.8 | (3.79–42.87) | 0.2120 |
| AC187652.1 | 11.9 | (2.99–47.46) | 11.8 | (2.78–49.71) | 0.9851 |
| GALNT15 | 9.6 | (2.41–38.38) | 15.6 | (3.67–65.88) | 0.5174 |
| EDN1 | 5.1 | (1.77–14.59) | 9.5 | (3.16–28.55) | 0.2714 |
| CREB5 | 2.3 | (1.13–4.65) | 8.7 | (4.15–18.04) | 0.0010 |
| RP11-73E6.2 | −8.1 | (−28.01–2.33) | −10.7 | (−39.29–2.93) | 0.6692 |
| TBX1 | 6.3 | (1.53–25.63) | 39.1 | (9.01–169.72) | 0.0190 |
| RAC3 | 13.1 | (3.15–54.56) | 11.9 | (2.69–52.64) | 0.8978 |
| RP3-512B11.3 | 9.3 | (2.06–41.58) | 27.4 | (5.71–131.35) | 0.1821 |
| C1QA | 11.8 | (2.11–66.46) | 52.4 | (8.66–316.86) | 0.1128 |
| COA3 | 13.0 | (2.8–60.32) | 18.3 | (3.69–90.76) | 0.6775 |
| RP11-384C21.9 | −7.9 | (−31.63–1.98) | −20.2 | (−85.61–4.76) | 0.2112 |
| TGFB2 | 8.3 | (2.22–31.06) | 13.6 | (3.42–53.58) | 0.4900 |
| STMN1 | 2.6 | (1.19–5.42) | 8.0 | (3.61–17.48) | 0.0072 |
| GLI2 | 7.5 | (1.86–30.38) | 22.6 | (5.26–96.75) | 0.1466 |
| UBAC2 | 7.2 | (2.09–24.74) | 11.3 | (3.11–40.92) | 0.4971 |
| SOX18 | 10.9 | (1.99–59.42) | 48.9 | (8.33–286.95) | 0.1039 |
| IFT88 | 5.4 | (1.86–15.56) | 8.0 | (2.65–24.26) | 0.4840 |
| RP11-496I9.1 | 11.6 | (2.19–60.82) | 35.5 | (6.27–201.05) | 0.2107 |
| RN7SL776P | −13.7 | (−57.39–3.25) | −10.8 | (−48.48–2.42) | 0.7635 |
| FSCN1 | 7.0 | (1.96–25.13) | 13.4 | (3.542–50.60) | 0.3471 |
| SLC7A2 | 3.8 | (1.37–10.49) | 12.3 | (4.24–35.44) | 0.0361 |
| RBP1 | 10.9 | (2.52–46.79) | 15.4 | (3.36–70.83) | 0.6533 |
| TMEM217 | −10.5 | (−45.1–2.45) | −15.7 | (−71.82–3.44) | 0.6061 |
| LRIG3 | 4.7 | (1.6–13.51) | 10.1 | (3.31–30.56) | 0.1816 |
| PELI2 | 4.1 | (1.44–11.52) | 11.5 | (3.89–34.03) | 0.0676 |
| TRIL | 8.3 | (1.98–34.59) | 20.2 | (4.55–89.76) | 0.2476 |
| ARPC1B | 3.8 | (1.52–9.69) | 7.3 | (2.78–19.23) | 0.1974 |
| CAV1 | 2.2 | (1.13–4.06) | 5.7 | (2.94–11.13) | 0.0062 |
| GPX8 | 7.5 | (1.92–29.08) | 17.1 | (4.15–70.41) | 0.2594 |
| INPP4A | 5.2 | (1.75–15.31) | 9.0 | (2.92–28.01) | 0.3391 |
| ADAM23 | 8.3 | (1.84–36.98) | 27.6 | (5.79–131.96) | 0.1376 |
| PNMA1 | 9.1 | (1.93–43.07) | 29.3 | (5.8–148.32) | 0.1646 |
| MGAT5 | 3.7 | (1.50–8.99) | 6.6 | (2.6–16.79) | 0.2251 |
| IRX1 | 5.4 | (1.22–23.96) | 74.3 | (15.73–351.2) | 0.0020 |
| UST | 8.4 | (1.92–36.55) | 22.6 | (4.86–104.94) | 0.2130 |
Similarly, we asked whether the absence or presence of somatic mutations in NOTCH1 (Supplementary Table S5), SYNE1 (Supplementary Table S6), PKHD1 (Supplementary Table S7), and EP400 (Supplementary Table S8) in BCC tissue was associated with a difference in magnitude of the differential expression of genes. The result shows that the non-synonymous somatic mutations of each of these genes have a significant association with functional effects in terms of differential gene expression. In the next step, we wanted to see the effect on the gene pathway level.
3.5.2. Pathway Level Analysis
In this step, we examined if a set of genes (e.g., in the KEGG pathway) was differentially expressed in NMSC tissue compared to normal skin tissue and if the magnitude of differential expression in NMSC compared to normal was significantly different in the absence or presence of non-synonymous somatic mutations in tumor tissue. First, we looked at the mutation of the PTCH1 gene. Table 3 shows the BCC-associated non-synonymous somatic mutations in the PTCH1 gene found only in tumor tissue but not in healthy skin tissue.
Table 3.
BCC-associated non-synonymous somatic mutations in the PTCH1 gene. The coding region changes and the amino acid changes are reported in multiple sources. An “*” in the amino acid change column indicates a translation termination codon.
| Coordinate | Reference Allele | Variant Allele | Exact Match | Coding Region Change | Amino Acid Change |
|---|---|---|---|---|---|
| chr9:98211572 | T | A | clinvar_hg19 | NM_000264.3:c.3583A>T; NM_001083602.1:c.3385A>T; NM_001083603.1:c.3580A>T; NM_001083604.1:c.3130A>T; NM_001083605.1:c.3130A>T; NM_001083606.1:c.3130A>T; NM_001083607.1:c.3130A>T | NP_000255.2:p.Thr1195Ser; NP_001077071.1:p.Thr1129Ser; NP_001077072.1:p.Thr1194Ser; NP_001077073.1:p.Thr1044Ser; NP_001077074.1:p.Thr1044Ser; NP_001077075.1:p.Thr1044Ser; NP_001077076.1:p.Thr1044Ser |
| chr9:98239132 | G | T | clinvar_hg19 | NM_000264.3:c.1511C>A; NM_001083602.1:c.1313C>A; NM_001083603.1:c.1508C>A; NM_001083604.1:c.1058C>A; NM_001083605.1:c.1058C>A; NM_001083606.1:c.1058C>A; NM_001083607.1:c.1058C>A | NP_000255.2:p.Pro504Gln; NP_001077071.1:p.Pro438Gln; NP_001077072.1:p.Pro503Gln; NP_001077073.1:p.Pro353Gln; NP_001077074.1:p.Pro353Gln; NP_001077075.1:p.Pro353Gln; NP_001077076.1:p.Pro353Gln |
| chr9:98242779 | C | A | NM_000264.3:c.838G>T; NM_001083602.1:c.640G>T; NM_001083603.1:c.835G>T; NM_001083604.1:c.385G>T; NM_001083605.1:c.385G>T; NM_001083606.1:c.385G>T; NM_001083607.1:c.385G>T | NP_000255.2:p.Glu280*; NP_001077071.1:p.Glu214*; NP_001077072.1:p.Glu279*; NP_001077073.1:p.Glu129*; NP_001077074.1:p.Glu129*; NP_001077075.1:p.Glu129*; NP_001077076.1:p.Glu129* | |
| chr9:98242797 | G | A | NM_000264.3:c.820C>T; NM_001083602.1:c.622C>T; NM_001083603.1:c.817C>T; NM_001083604.1:c.367C>T; NM_001083605.1:c.367C>T; NM_001083606.1:c.367C>T; NM_001083607.1:c.367C>T | NP_000255.2:p.Gln274*; NP_001077071.1:p.Gln208*; NP_001077072.1:p.Gln273*; NP_001077073.1:p.Gln123*; NP_001077074.1:p.Gln123*; NP_001077075.1:p.Gln123*; NP_001077076.1:p.Gln123* | |
| chr9:98248001 | G | A | NM_000264.3:c.550C>T; NM_001083602.1:c.352C>T; NM_001083603.1:c.547C>T; NM_001083604.1:c.97C>T; NM_001083605.1:c.97C>T; NM_001083606.1:c.97C>T; NM_001083607.1:c.97C>T | NP_000255.2:p.Gln184*; NP_001077071.1:p.Gln118*; NP_001077072.1:p.Gln183*; NP_001077073.1:p.Gln33*; NP_001077074.1:p.Gln33*; NP_001077075.1:p.Gln33*; NP_001077076.1:p.Gln33* | |
| chr9:98244299 | A | T | NM_000264.3:c.678T>A; NM_001083602.1:c.480T>A; NM_001083603.1:c.675T>A; NM_001083604.1:c.225T>A; NM_001083605.1:c.225T>A; NM_001083606.1:c.225T>A; NM_001083607.1:c.225T>A | NP_000255.2:p.Cys226*; NP_001077071.1:p.Cys160*; NP_001077072.1:p.Cys225*; NP_001077073.1:p.Cys75*; NP_001077074.1:p.Cys75*; NP_001077075.1:p.Cys75*; NP_001077076.1:p.Cys75* | |
| chr9:98268818 | T | A | NM_000264.3:c.265A>T; NM_001083602.1:c.67A>T; NM_001083603.1:c.262A>T; NM_001083604.1:c.-189A>T; NM_001083605.1:c.-189A>T; NM_001083606.1:c.-189A>T; NM_001083607.1:c.-189A>T | NP_000255.2:p.Lys89*; NP_001077071.1:p.Lys23*; NP_001077072.1:p.Lys88* | |
| chr9:98241336 | C | T | clinvar_hg19 | NM_000264.3:c.1161G>A; NM_001083602.1:c.963G>A; NM_001083603.1:c.1158G>A; NM_001083604.1:c.708G>A; NM_001083605.1:c.708G>A; NM_001083606.1:c.708G>A; NM_001083607.1:c.708G>A | NP_000255.2:p.Trp387*; NP_001077071.1:p.Trp321*; NP_001077072.1:p.Trp386*; NP_001077073.1:p.Trp236*; NP_001077074.1:p.Trp236*; NP_001077075.1:p.Trp236*; NP_001077076.1:p.Trp236* |
| chr9:98268719 | C | A | NM_000264.3:c.364G>T; NM_001083602.1:c.166G>T; NM_001083603.1:c.361G>T; NM_001083604.1:c.-90G>T; NM_001083605.1:c.-90G>T; NM_001083606.1:c.-90G>T; NM_001083607.1:c.-90G>T | NP_000255.2:p.Glu122*; NP_001077071.1:p.Glu56*; NP_001077072.1:p.Glu121* | |
| chr9:98215785 | C | A | NM_000264.3:c.3424G>T; NM_001083602.1:c.3226G>T; NM_001083603.1:c.3421G>T; NM_001083604.1:c.2971G>T; NM_001083605.1:c.2971G>T; NM_001083606.1:c.2971G>T; NM_001083607.1:c.2971G>T | NP_000255.2:p.Gly1142*; NP_001077071.1:p.Gly1076*; NP_001077072.1:p.Gly1141*; NP_001077073.1:p.Gly991*; NP_001077074.1:p.Gly991*; NP_001077075.1:p.Gly991*; NP_001077076.1:p.Gly991* | |
| chr9:98218658 | C | T | NM_000264.3:c.3206G>A; NM_001083602.1:c.3008G>A; NM_001083603.1:c.3203G>A; NM_001083604.1:c.2753G>A; NM_001083605.1:c.2753G>A; NM_001083606.1:c.2753G>A; NM_001083607.1:c.2753G>A | NP_000255.2:p.Gly1069Asp; NP_001077071.1:p.Gly1003Asp; NP_001077072.1:p.Gly1068Asp; NP_001077073.1:p.Gly918Asp; NP_001077074.1:p.Gly918Asp; NP_001077075.1:p.Gly918Asp; NP_001077076.1:p.Gly918Asp | |
| chr9:98239830 | T | A | NM_000264.3:c.1502A>T; NM_001083602.1:c.1304A>T; NM_001083603.1:c.1499A>T; NM_001083604.1:c.1049A>T; NM_001083605.1:c.1049A>T; NM_001083606.1:c.1049A>T; NM_001083607.1:c.1049A>T | NP_000255.2:p.Gln501Leu; NP_001077071.1:p.Gln435Leu; NP_001077072.1:p.Gln500Leu; NP_001077073.1:p.Gln350Leu; NP_001077074.1:p.Gln350Leu; NP_001077075.1:p.Gln350Leu; NP_001077076.1:p.Gln350Leu |
In a previous study, using the gene expression data from the same 26 BCC samples, we have shown that the top differential pathways in BCC include the “hedgehog signaling pathway” and the “basal cell carcinoma pathway”, and there was an interaction with the degree of As exposure in this population [32].
Association of the Somatic ns Mutation in the PTCH1 Gene and Dysregulated Pathways in BCC
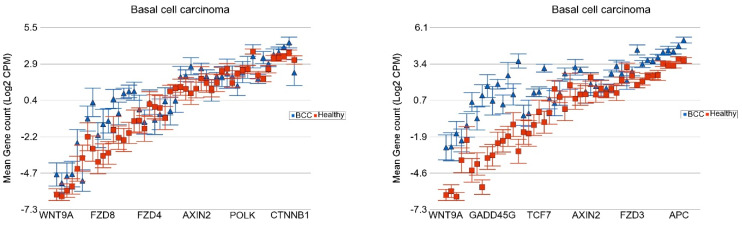
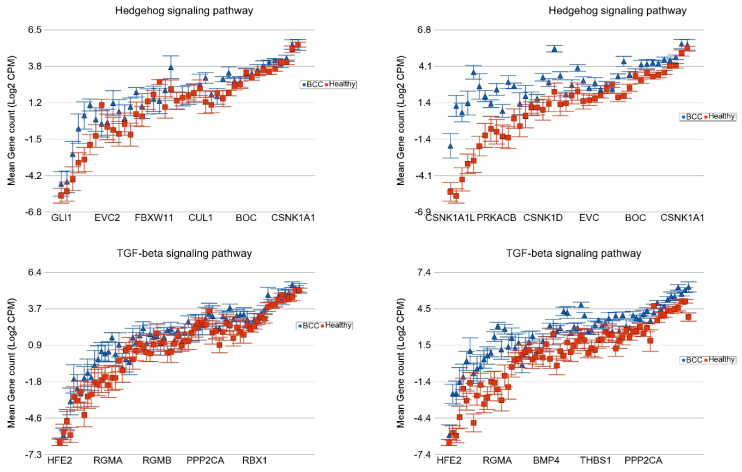
The detailed results from Gene set ANOVA for all the KEGG pathways are presented in Supplementary Table S9. Compared to healthy skin tissue, in the BCC samples without the PTCH1 non-synonymous somatic mutation (n = 14), the genes in the “Basal cell carcinoma pathway” were overexpressed by FC 1.62 (95% CI 1.34–1.96), whereas in BCC samples with the PTCH1 non-synonymous somatic mutation (n = 12), the same pathway genes were overexpressed by FC 3.95 (95% CI 3.24–4.82). This shows a significant association (interaction p = 2.48 × 10−17) between PTCH1 mutation status and the overexpression of the “basal cell carcinoma pathway”. Among the other major pathways that are markedly overexpressed in the presence of the PTCH1 mutation include the “hedgehog signaling pathway” and the “TGF-beta signaling pathway” (see Figure 5).
Figure 5.
Differential expression of gene pathways in BCC (in blue) compared to healthy skin tissue (in red) by PTCH1 mutational status. BCC tissues with no non-synonymous somatic mutations in PTCH1 are shown on the left panel and those with mutations are shown on the right panel. Genes are arranged on the x-axis by expression level, and the log2-transformed gene count per million (CPM) is shown on the y-axis. Gene symbols for all the genes could not be shown on the x-axis.
We also conducted the rank-based analysis—GSEA for patients without the PTCH1 ns mutation (Supplementary Table S10) and for patients with the PTCH1 ns mutation (Supplementary Table S11). It was interesting to note that in the GSEA analysis, too, many of the pathways found in the above-mentioned Gene set ANOVA were also seen to be more significantly enriched in the presence of the PTCH1 mutation.
Association of the Somatic ns Mutation in the NOTCH1 Gene and Dysregulated Pathways in BCC
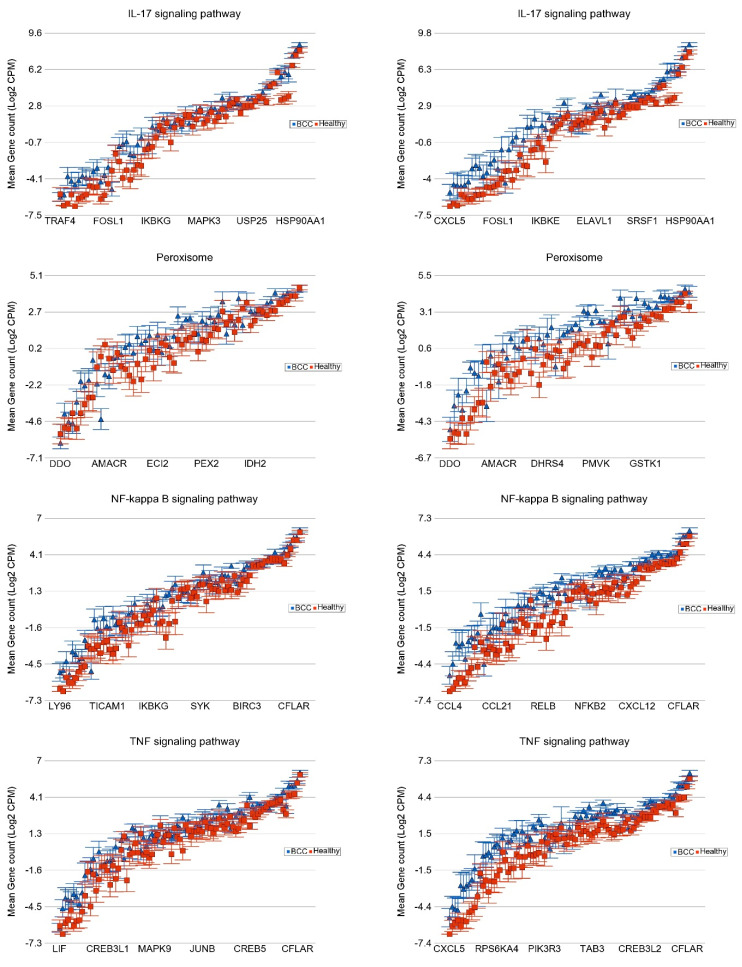
In the same way, we looked at the NOTCH1 mutation status (nineteen BCC without and seven BCC with the NOTCH1 mutation) and compared it to the same healthy skin tissue (n = 16). The major pathways that were more markedly overexpressed in the presence of NOTCH1 mutation include the “IL-17 signaling pathway”, “peroxisome related genes”, and “NF-Kappa beta signaling pathway”, and the “TGF beta signaling pathway” (see Figure 6).
Figure 6.
Differential expression of gene pathways in BCC (in blue) compared to healthy skin tissue (in red) by NOTCH1 mutational status. BCC tissues with no non-synonymous somatic mutations in NOTCH1 are shown on the left panel and those with mutations are shown on the right panel. Genes are arranged on the x-axis by expression level, and the log2-transformed gene count per million (CPM) is shown on the y-axis. Gene symbols for all the genes could not be shown on the x-axis.
Association of the Somatic ns Mutation in SYNE1 and PKHD1 Genes and Dysregulated Pathways in BCC
For BCC, we also looked for associations with other frequently mutated genes, such as SYNE1 (see Figure 7) and PKHD1 mutations (Supplementary Table S12).
Figure 7.
Differential expression of gene pathways in BCC (in blue) compared to healthy skin tissue (in red) by SYNE1 mutational status. BCC tissues with no non-synonymous somatic mutations in SYNE1 are shown on the left panel and those with mutations are shown on the right panel. Genes are arranged on the x-axis by expression level, and the log2-transformed gene count per million (CPM) is shown on the y-axis. Gene symbols for all the genes could not be shown on the x-axis.
Association of the Somatic ns Mutation in the TP53 Gene and Dysregulated Pathways in SCC
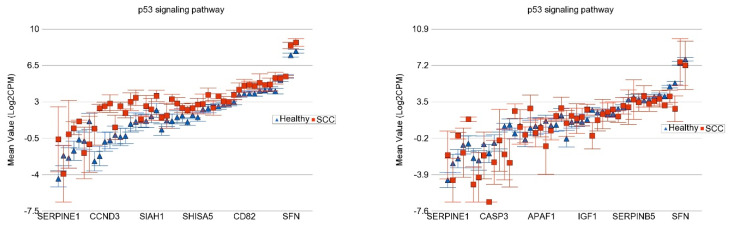
The detailed analysis for all the KEGG pathways is presented in Supplementary Table S13. Compared to healthy skin tissue, in the SCC samples without the TP53 ns somatic mutation (n = 3), the genes in the “p53 signaling pathway” were significantly overexpressed by FC 2.21 (95% CI 1.64–2.98), whereas in SCC samples with the TP53 ns somatic mutation (n = 3), the same pathway genes were somewhat under-expressed by FC −1.32 (95% CI −1.78 to 1.01, see Figure 8). This shows a significant association (interaction p = 5.53 × 10−8) between TP53 mutation status and the “p53 signaling pathway”, where the TP53 mutation is associated with impaired tumor suppression activity.
Figure 8.
Differential expression of gene pathways in SCC (in red) compared to healthy skin tissue (in blue) by TP53 mutational status. SCC tissues with no non-synonymous somatic mutations in TP53 are shown on the left panel and those with mutations are shown on the right panel. Genes are arranged on the x-axis by expression level, and the log2-transformed gene count per million (CPM) is shown on the y-axis. Gene symbols for all the genes could not be shown on the x-axis.
3.6. Gene–Environmental Interaction: Interaction of the Somatic Mutation and Degree of As Exposure on Gene Expression Pathways
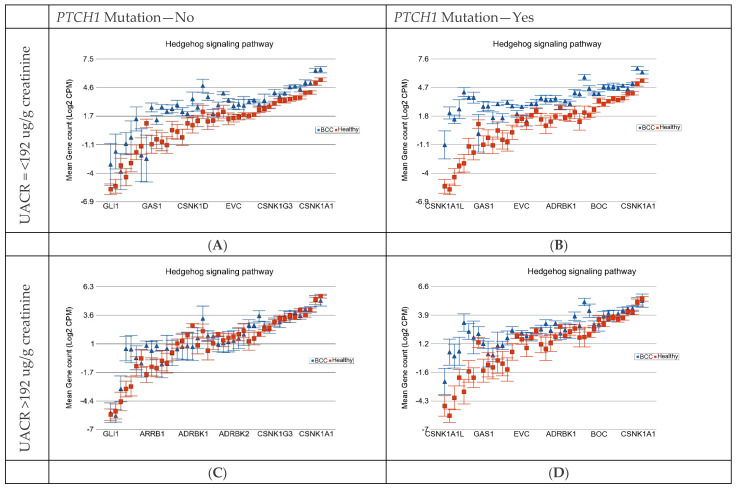
All our participants were exposed to As, so to explore the effect of As, we used the UACR at baseline below 192 µg/g creatinine and above 192 µg/g creatinine for comparison. Using the somatic mutation status (no vs. yes) and the baseline UACR level (≤192 µg/g creatinine or low vs. >192 µg/g creatinine or high), we divided the BCC patients into four categories (see Table 4) and compared the expression of the different gene pathways of each group of tissues to the same 16 healthy skin tissues. The overall FC (95% CI) of the pathways in each group are presented in Table 4. The result shows that the presence of the PTCH1 somatic mutation increases the magnitude of the differential expression of genes in the “basal cell carcinoma pathway” and “hedgehog pathway” in both low and high As exposure groups. It also shows that high As exposure decreases the magnitude of the differential expression in the absence or presence of the PTCH1 somatic mutation. The differences in the magnitudes of differential expressions were statistically significant, indicated by the interaction p-value.
Table 4.
Effect of the PTCH1 somatic mutation and As exposure on the differential expression of gene pathways in BCC. Result from Gene set ANOVA analysis showing the FC (95% CI) of different pathways in BCC samples compared to healthy skin tissue. Patients were divided by PTCH1 somatic mutation status (no vs. yes) and level of As exposure—baseline UACR (low: ≤192 µg/g creatinine vs. high: >192 µg/g creatinine).
| Basal Cell Carcinoma | Hedgehog Signaling Pathway | Antigen Processing and Presentation | ||||||
|---|---|---|---|---|---|---|---|---|
| UACR | PTCH1 Mut −ve | PTCH1 Mut +ve | PTCH1 Mut −ve | PTCH1 Mut +ve | PTCH1 Mut −ve | PTCH1 Mut +ve | ||
| ≤192 µg/g | FC | 4.05 | 6.92 | 2.93 | 5.58 | 4.77 | 6.25 | |
| 95% CI | (3.03–5.4) | (5.30–9.04) | (2.23–3.85) | (4.34–7.17) | (3.72–6.09) | (4.98–7.83) | ||
| ≥192 µg/g | FC | 1.33 | 2.98 | 1.37 | 3.01 | 1.08 | 2.05 | |
| 95% CI | (1.08–1.65) | (2.35–3.7) | (1.12–1.67) | (2.40–3.76) | (−1.098–1.30) | (1.67–2.50) | ||
| Interaction p | 1.68 × 10−31 | 3.17 × 10−24 | 2.75 × 10−52 | |||||
Figure 9 shows the gene expression profiles of the genes in the hedgehog pathway and how their differential expressions are affected by PTCH1 mutation status and the As-exposure level.
Figure 9.
Differential gene expression of hedgehog signaling pathway genes in BCC tissue (in blue) compared to healthy skin tissue (in red). BCC tissues with no somatic mutations in PTCH1 and low As exposure are shown on the left upper plot (A). BCC tissues with somatic mutations in PTCH1 and low As exposure are shown on the right upper plot (B). BCC tissues with no somatic mutations in PTCH1 and high As exposure are shown on the left lower plot (C). BCC tissues with somatic mutations in PTCH1 and high As exposure are shown on the right lower plot (D). Genes are arranged on the x-axis by expression level, and the log2-transformed gene count per million (CPM) is shown on the y-axis. Gene symbols for all the genes could not be shown on the x-axis.
In the same way, we also checked the interaction of the NOTCH1 somatic mutation and As exposure. Table 5 shows how the NOTCH1 somatic mutation and As exposure status influence the immune response pathways like the “IL-17 signaling pathway”, “Antigen processing and presentation”, and the “p53 signaling pathway”. These results also show a similar trend that the somatic mutation increases the differential expression and high As exposure decreases the magnitude of the differential expression of these pathways.
Table 5.
Effect of NOTCH1 somatic mutations and As-exposure on the differential expression of gene pathways in BCC. Result from Gene set ANOVA analysis showing the FC (95% CI) of different pathways in BCC samples compared to healthy skin tissue. Patients were divided by NOTCH1 somatic mutation status (no vs. yes) and level of As exposure—baseline UACR (low: ≤192 µg/g creatinine vs. high: >192 µg/g creatinine).
| IL17 Signaling Pathway | Antigen Processing and Presentation | p53 Signaling Pathway | ||||||
|---|---|---|---|---|---|---|---|---|
| UACR | NOTCH1 Mut −ve | NOTCH1 Mut +ve | NOTCH1 Mut −ve | NOTCH1 Mut +ve | NOTCH1 Mut −ve | NOTCH1 Mut +ve | ||
| ≤192 µg/g | FC | 5.13 | 6.45 | 5.44 | 6.09 | 4.96 | 5.77 | |
| 95% CI | (4.18–6.31) | (4.93–8.43) | (4.39–6.71) | (4.62–8.02) | (4.04–6.08) | (4.42–7.53) | ||
| ≥192 µg/g | FC | 1.12 | 2.29 | 1.17 | 2.58 | 1.26 | 2.66 | |
| 95% CI | (−1.04–1.32) | (1.80–2.91) | (−1.01–1.38) | (2.01–3.30) | (1.07–1.48) | (2.09–3.37) | ||
| Interaction p | 6.27 × 10−60 | 2.45 × 10−54 | 2.49 × 10−48 | |||||
4. Discussion
While UVR exposure and skin sensitivity are known risk factors for NMSC, especially among Caucasians, As exposure through contaminated drinking water may be a major risk factor in other populations. To our knowledge, our current study presents the most comprehensive molecular profiling (more than 400 cancer-related genes) for NMSC in a non-Caucasian population exposed to As. We are unaware of any previous study on NMSC that has considered the fact that apparently healthy, non-lesional human skin exposed to sunlight actually harbors somatic mutations. Our study addressed this fact and identified NMSC-associated somatic mutations that are not found in healthy skin tissue. We acknowledge the weakness of the small sample size and the fact that it would have been ideal if we could sequence normal tumor pairs for all the patients.
A variant seen in a given tissue that is not seen in germline DNA (blood may be used as a proxy) is considered a “somatic mutation”. Because of exposure to UV rays from sunlight, even healthy skin tissue may show a multitude of such somatic mutations resembling a UVR signature. Therefore, unlike many somatic mutations seen in other internal organ cancers, the detection of a somatic mutation in NMSC tissue does not necessarily mean that the detected mutation is a “cancer-associated somatic mutation”. Our study confirms this fact, and by excluding those somatic mutations in healthy skin, we could identify NMSC-associated somatic mutations. Looking at the top 20 genes showing somatic mutations in our study in an As-exposed population from Southeast Asia, we could see that many of the genes are also seen to be mutated in NMSC patients from Caucasian populations worldwide. We did not have patients who were not exposed to As and cannot comment on the cause of mutation or NMSC pathogenesis. Unfortunately, we also did not have any tissue left for measuring As content in the tumor tissue, which could have shed some light if As exposure was associated with NMSC pathogenesis.
Unlike some other cancers, like colorectal or thyroid cancer, where a single-point mutation (like KRAS rs#112445441 or BRAF V600E) is found in a large proportion of samples, in NMSC, there is no single mutation that is seen in a large number of samples. Rather, sequencing of large genomic regions is needed to detect somatic mutations in a given gene (e.g., PTCH1 or NOTCH1) because the mutations are at different locations in different samples. But, it is interesting to note that, regardless of the difference in position and amino acid change (e.g., c.3583 A>T causing Thr1129Ser change in one sample and c.1313C>A causing Pro504Gln change in another sample), when the samples were grouped together based on BCC-associated PTCH1 negative or positive non-synonymous somatic mutations, we see a marked difference of the differential expression of many relevant gene pathways. This allows us to utilize these genomic markers for the individualization of targeted therapy if and when they are needed. For example, hedgehog signaling pathway inhibitor small molecules (vismodegib, sonidegib) may be most effective in BCC patients with PTCH1 mutations who are not exposed to high As, whereas the same therapy may show the least or no response in BCC patients without PTCH1 somatic mutations exposed to high As (see Table 4 and Figure 8). Currently, both vismodegib and sonidegib are only approved for metastatic or locally advanced BCC [56,57]. But, PTCH1 mutation status may be used for selecting patients for individualized targeted therapy. In the same line, our data suggest a molecular basis for the potential use of IL-17 inhibitors in BCC patients with low As exposure with NOTCH1 somatic mutations (see Table 5). Transcriptomic data were not strongly suggestive of great potential for immune checkpoint inhibitors in these BCC patients; however, they suggested a lower chance of platinum drug resistance in BCC patients with high UACR compared to high platinum drug resistance potential in patients with lower UACR [32].
In a study utilizing ultra-deep sequencing of 74 cancer genes from skin biopsies of normal skin across 234 biopsies of sun-exposed eyelid epidermis from four individuals, Martincorena et al. looked for somatic mutations [58]. The burden of somatic mutations averaged two to six mutations per megabase per cell, similar to that seen in many cancers, and exhibited characteristic signatures of UVR exposure. There was a predominance of C>T mutations and high rates of CC>TT dinucleotide substitutions. NOTCH1 was the most frequently mutated gene, and 20% of normal skin cells carried a driver mutation in NOTCH1 [58]. In SCC of the skin and other organs, both copies of NOTCH1 are frequently inactivated, typically through point mutation combined with copy number alteration. Other frequently-mutated genes include RBM10, FGFR3, CDKN2A, and NOTCH2 [58].
We found few studies where investigators used fresh-frozen BCC tissue to look at the somatic mutations. In one study, fresh-frozen BCC tumor tissues were obtained from 191 patients, and corresponding normal-appearing skin was available from 115 patients [59]. PCR and Sanger sequencing were performed, and they detected 137 PTCH1 mutations in 105 tumors with some loss of heterozygosity. For TP53, 31% of BCC carried mutations, mostly of the missense type. TERT and DPH3 promoter mutations were present in 113 and 73 cases, respectively. Gene expression analysis found statistically significant higher TERT mRNA levels in BCC tumors with TERT promoter mutations compared to the tumors without mutations (p < 0.001) [59].
In another study, whole genome exome sequencing was performed on a total of 27 pairs of tissue (tumor and normal adjacent healthy skin) [60]. They identified 84,571 cancer sample-specific somatic mutations, of which 42,380 (50.1%) were located in protein-coding regions, and the remaining 42,191 (49.9%) were located in non-coding regions. They showed the relation between the different pathways and mutations, like hedgehog pathways (PTCH1, GL12, SMO), MYCN regulation genes (MYCN, MTOR, DYRk3, AMBRA1), filaggrin genes (FLG, FLG2), and NOTCH genes (NOTCH1, NOTCH2, NOTCH3). They also detected mutations in the non-coding region of BAD, DHODH, SPHK2, CHCHD2 (also known as MNRR1), and RPS27. Promoter mutations of TERT and DPH3 were also detected. Mutations were also found in TP53, PTPRD, LATS1, and ARIDIA [60]. They found mutations in TNFAIP2, which encodes a multifunctional protein playing a role in angiogenesis, inflammation, cell migration and invasion, cytoskeleton remodeling, and cell membrane protrusion formation. In the coding region, they also detected somatic mutations in EZH2 and KNSTRN [60]. Whole-exome sequencing of secondary tumors arising from nevus sebaceous revealed additional genomic alterations in addition to RAS mutations [61].
In SCC, one study found highly mutated PDE4DIP, SYNE1, and NOTCH1 genes [62]. They analyzed somatic mutations in SCC in smokers and non-smokers. Mutations of the ATM, RNF213, DST, RET, CYP2C19, PKHD1, PTPRD, SETD2, ATR, CDKN2A, TP53, KAT6B, FGFR3, NOTCH2, and NOTCH4 genes, which were common to SCC, were foundin their study [62].
The relation of PTCH1 mutations and mRNA expression was studied in twenty cases of nevoid basal cell carcinoma (Gorlin) syndrome, an autosomal dominant disorder, using cancer tissue, surrounding healthy tissue, blood DNA, and skin tissue from four healthy people. They detected twelve genomic and five somatic mutations of the PTCH1 gene. Quantitative PCR was used to determine the mRNA expression levels of PTCH1, SMO, GLI3, and CCND1 genes in relation to the PTCH1 mutation. The mRNA expression was highest in BCC tissue, followed by surrounding healthy tissue and the skin tissue of healthy people [63]. They also showed the effect of PTCH1 mutations on gene expression. In surrounding healthy tissue with PTCH1 mutations, the mRNA expression was lower for PHCH1 and GLI3 genes. On the other hand, they found higher SMO and CCND1 mRNA expressions in the same group. BCC tumors with germline and somatic mutations of PTCH1 expression levels of PTCH1, SMO, and GLI3 were higher compared to those with germline mutations only, but CCND1 levels were lower in that group [63].
The list of somatic mutations detected in BCC and SCC depends on the number of target genes sequenced in a particular study, the use of whole exome sequencing or whole genome sequencing, and the strictness of criteria for the detection of a somatic mutation. However, some of the most frequently mutated genes are common among the published studies. In that respect, our current study confirms the findings of many of the past studies and also detects some new mutations. We report the NMSC-associated somatic mutations after excluding the somatic mutations seen in the healthy skin tissue, and this study was performed in a Bangladeshi population exposed to As. We acknowledge the fact that we did not perform ultra-deep sequencing to capture rare variants, so we might have missed very rare variants (below 2% frequency). On the other hand, the somatic mutations detected in our study had reasonably high frequency, giving us confidence that the reported mutations are real. Importantly, the associations of the non-synonymous mutations within the frequently mutated genes with the differential expression of genes and major gene pathways further underscore the importance of the findings.
Surgical excision of the NMSC is the first line of management for most of the cases. However, for some recurrent or locally advanced or metastatic cases, targeted therapy may be considered. Keeping that in mind, we analyzed the molecular genomic data in a manner that helps understand the pathogenesis and the utilization of the mutation data for the potential selection of patients for some targeted therapies in the future. We acknowledge that the small sample size did not allow us to test such associations for low-frequency mutations and gene pathways. In the future, we plan to carry out a larger study utilizing the already available biological samples and the clinical follow-up data from the parent BEST study.
5. Conclusions
We present the result of somatic mutation detection in NMSC tissue and paired blood samples from participants exposed to As through drinking As-contaminated water and compare them with somatic mutations in healthy skin to identify NMSC-associated mutations. We also show the effect of these mutations on different genes and pathways that may be helpful for targeted therapy in the future.
Acknowledgments
We acknowledge the support and help from all the patients included in this study. We thank the University of Chicago Research, Bangladesh (URB) staff for handling and shipping of all the study material to the University of Chicago molecular genomics laboratory.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells13121056/s1. Supplementary Form S1: Skin Biopsy Pathology Report; Supplementary Figure S1: Substitution changes by category; Supplementary Table S1: Patient Characteristics; Supplementary Table S2: Total gene list of the targeted NGS panel; Supplementary Table S3: ANOVA_BCC vs Healthy FC3FDR0.05; Supplementary Table S4: GSEA combined p0.05_by NES; Supplementary Table S5: Gene interaction with NOTCH1; Supplementary Table S6: Gene interaction with SYNE1; Supplementary Table S7: Gene interaction with PKHD1; Supplementary Table S8: Gene interaction with EP400; Supplementary Table S9: PTCH1 mutation and dysregulated KEGG pathways in BCC; Supplementary Table S10: GSEA PTCH1wild_p0.05_by NES; Supplementary Table S11: GSEA PTCH1mutant_p0.05_by NES; Supplementary Table S12: PKHD1 mutation and dysregulated KEGG pathways in BCC; Supplementary Table S13: TP53 mutation and dysregulated KEGG pathways in SCC.
Author Contributions
Conceptualization, F.J., M.A., H.A. and M.G.K.; methodology, F.J. and C.R.S.; formal analysis, M.G.K.; investigation, F.J., T.I., M.R.-Z., M.S. and C.R.S.; resources, H.A.; data curation, M.R.-Z., T.I. and M.S.; writing—original draft preparation, F.J., Y.K. and M.G.K.; writing—review and editing, M.A., C.R.S. and H.A.; supervision, H.A., M.G.K. and M.A.; funding acquisition, H.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of The University of Chicago Medicine (protocol code IRB19-0724, approved 24 September 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All the supporting data are presented in the tables presented in the main manuscript and Supplementary Materials.
Conflicts of Interest
Author Muhammad Rakibuz-Zaman was employed by the company Pulse Infoframe. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This study was partially supported by NIH funds P30ES027792 and R01CA107431.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhang W., Zeng W., Jiang A., He Z., Shen X., Dong X., Feng J., Lu H. Global, regional and national incidence, mortality and disability-adjusted life-years of skin cancers and trend analysis from 1990 to 2019: An analysis of the Global Burden of Disease Study 2019. Cancer Med. 2021;10:4905–4922. doi: 10.1002/cam4.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg G., Karagiannis T., Palmer J.B., Lotya J., O’Neill C., Kisa R., Herrera V., Siegel D.M. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: A retrospective cohort study. J. Am. Acad. Dermatol. 2016;75:957–966.e2. doi: 10.1016/j.jaad.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Bonilla X., Parmentier L., King B., Bezrukov F., Kaya G., Zoete V., Seplyarskiy V.B., Sharpe H.J., McKee T., Letourneau A., et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat. Genet. 2016;48:398–406. doi: 10.1038/ng.3525. [DOI] [PubMed] [Google Scholar]
- 4.Kim R.H., Armstrong A.W. Nonmelanoma skin cancer. Dermatol. Clin. 2012;30:125–139. doi: 10.1016/j.det.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Madan V., Lear J.T., Szeimies R.M. Non-melanoma skin cancer. Lancet. 2010;375:673–685. doi: 10.1016/s0140-6736(09)61196-x. [DOI] [PubMed] [Google Scholar]
- 6.Fahradyan A., Howell A.C., Wolfswinkel E.M., Tsuha M., Sheth P., Wong A.K. Updates on the Management of Non-Melanoma Skin Cancer (NMSC) Healthcare. 2017;5:82. doi: 10.3390/healthcare5040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paolino G., Donati M., Didona D., Mercuri S.R., Cantisani C. Histology of Non-Melanoma Skin Cancers: An Update. Biomedicines. 2017;5:71. doi: 10.3390/biomedicines5040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones O.T., Ranmuthu C.K.I., Hall P.N., Funston G., Walter F.M. Recognising Skin Cancer in Primary Care. Adv. Ther. 2020;37:603–616. doi: 10.1007/s12325-019-01130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verkouteren J.A.C., Ramdas K.H.R., Wakkee M., Nijsten T. Epidemiology of basal cell carcinoma: Scholarly review. Br. J. Dermatol. 2017;177:359–372. doi: 10.1111/bjd.15321. [DOI] [PubMed] [Google Scholar]
- 10.Staples M.P., Elwood M., Burton R.C., Williams J.L., Marks R., Giles G.G. Non-melanoma skin cancer in Australia: The 2002 national survey and trends since 1985. Med. J. Aust. 2006;184:6–10. doi: 10.5694/j.1326-5377.2006.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 11.Abarca J.F., Casiccia C.C. Skin cancer and ultraviolet-B radiation under the Antarctic ozone hole: Southern Chile, 1987–2000. Photodermatol. Photoimmunol. Photomed. 2002;18:294–302. doi: 10.1034/j.1600-0781.2002.02782.x. [DOI] [PubMed] [Google Scholar]
- 12.Rawashdeh M.A., Matalka I. Basal cell carcinoma of the maxillofacial region: Site distribution and incidence rates in Arab/Jordanians, 1991 to 2000. J. Oral Maxillofac. Surg. 2004;62:145–149. doi: 10.1016/j.joms.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 13.King C., Fowler J.C., Abnizova I., Sood R.K., Hall M.W.J., Szeverényi I., Tham M., Huang J., Young S.M., Hall B.A., et al. Somatic mutations in facial skin from countries of contrasting skin cancer risk. Nat. Genet. 2023;55:1440–1447. doi: 10.1038/s41588-023-01468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flohil S.C., van der Leest R.J., Arends L.R., de Vries E., Nijsten T. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma: A systematic review and meta-analysis. Eur. J. Cancer. 2013;49:2365–2375. doi: 10.1016/j.ejca.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Van der Leest R.J., Flohil S.C., Arends L.R., de Vries E., Nijsten T. Risk of subsequent cutaneous malignancy in patients with prior melanoma: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2015;29:1053–1062. doi: 10.1111/jdv.12887. [DOI] [PubMed] [Google Scholar]
- 16.Housman T.S., Feldman S.R., Williford P.M., Fleischer A.B., Jr., Goldman N.D., Acostamadiedo J.M., Chen G.J. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J. Am. Acad. Dermatol. 2003;48:425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 17.Guy G.P., Jr., Machlin S.R., Ekwueme D.U., Yabroff K.R. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am. J. Prev. Med. 2015;48:183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai V., Cranwell W., Sinclair R. Epidemiology of skin cancer in the mature patient. Clin. Dermatol. 2018;36:167–176. doi: 10.1016/j.clindermatol.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Savoia P., Veronese F., Camillo L., Tarantino V., Cremona O., Zavattaro E. Multiple Basal Cell Carcinomas in Immunocompetent Patients. Cancers. 2022;14:3211. doi: 10.3390/cancers14133211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zambrano-Román M., Padilla-Gutiérrez J.R., Valle Y., Muñoz-Valle J.F., Valdés-Alvarado E. Non-Melanoma Skin Cancer: A Genetic Update and Future Perspectives. Cancers. 2022;14:2371. doi: 10.3390/cancers14102371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyeraci M., Papanikolau E.S., Grimaldi M., Ricci F., Pallotta S., Monetta R., Minafò Y.A., Di Lella G., Galdo G., Abeni D., et al. Systemic Photoprotection in Melanoma and Non-Melanoma Skin Cancer. Biomolecules. 2023;13:1067. doi: 10.3390/biom13071067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolouzakis T.K., Falzone L., Lasithiotakis K., Krüger-Krasagakis S., Kalogeraki A., Sifaki M., Spandidos D.A., Chrysos E., Tsatsakis A., Tsiaoussis J. Current and Future Trends in Molecular Biomarkers for Diagnostic, Prognostic, and Predictive Purposes in Non-Melanoma Skin Cancer. J. Clin. Med. 2020;9:2868. doi: 10.3390/jcm9092868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azimi A., Fernandez-Peñas P. Molecular Classifiers in Skin Cancers: Challenges and Promises. Cancers. 2023;15:4463. doi: 10.3390/cancers15184463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naujokas M.F., Anderson B., Ahsan H., Aposhian H.V., Graziano J.H., Thompson C., Suk W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013;121:295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo H.R., Yu H.S., Hu H., Monson R.R. Arsenic in drinking water and skin cancers: Cell-type specificity (Taiwan, ROC) Cancer Causes Control. 2001;12:909–916. doi: 10.1023/a:1013712203455. [DOI] [PubMed] [Google Scholar]
- 26.Leonardi G., Vahter M., Clemens F., Goessler W., Gurzau E., Hemminki K., Hough R., Koppova K., Kumar R., Rudnai P., et al. Inorganic arsenic and basal cell carcinoma in areas of Hungary, Romania, and Slovakia: A case-control study. Environ. Health Perspect. 2012;120:721–726. doi: 10.1289/ehp.1103534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maloney M.E. Arsenic in Dermatology. Dermatol. Surg. 1996;22:301–304. doi: 10.1111/j.1524-4725.1996.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 28.Choudhury M.I.M., Shabnam N., Ahsan T., Ahsan S.M.A., Kabir M.S., Khan R.M., Miah M.A., Uddin M.K., Liton M.A.R. Cutaneous Malignancy due to Arsenicosis in Bangladesh: 12-Year Study in Tertiary Level Hospital. BioMed Res. Int. 2018;2018:4678362. doi: 10.1155/2018/4678362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng Q., Zhang A. Assessing potential mechanisms of arsenic-induced skin lesions and cancers: Human and in vitro evidence. Environ. Pollut. 2020;260:113919. doi: 10.1016/j.envpol.2020.113919. [DOI] [PubMed] [Google Scholar]
- 30.Srinivas N., Rachakonda S., Hielscher T., Calderazzo S., Rudnai P., Gurzau E., Koppova K., Fletcher T., Kumar R. Telomere length, arsenic exposure and risk of basal cell carcinoma of skin. Carcinogenesis. 2019;40:715–723. doi: 10.1093/carcin/bgz059. [DOI] [PubMed] [Google Scholar]
- 31.Speer R.M., Zhou X., Volk L.B., Liu K.J., Hudson L.G. Arsenic and cancer: Evidence and mechanisms. Adv. Pharmacol. 2023;96:151–202. doi: 10.1016/bs.apha.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kibriya M.G., Jasmine F., Munoz A., Islam T., Ahmed A., Tong L., Rakibuz-Zaman M., Shahriar M., Kamal M., Shea C.R., et al. Interaction of Arsenic Exposure and Transcriptomic Profile in Basal Cell Carcinoma. Cancers. 2022;14:5598. doi: 10.3390/cancers14225598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahsan H., Chen Y., Parvez F., Zablotska L., Argos M., Hussain I., Momotaj H., Levy D., Cheng Z., Slavkovich V., et al. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: Baseline results from the Health Effects of Arsenic Longitudinal Study. Am. J. Epidemiol. 2006;163:1138–1148. doi: 10.1093/aje/kwj154. [DOI] [PubMed] [Google Scholar]
- 34.Jee B.A., Lim H., Kwon S.M., Jo Y., Park M.C., Lee I.J., Woo H.G. Molecular classification of basal cell carcinoma of skin by gene expression profiling. Mol. Carcinog. 2015;54:1605–1612. doi: 10.1002/mc.22233. [DOI] [PubMed] [Google Scholar]
- 35.Jayaraman S.S., Rayhan D.J., Hazany S., Kolodney M.S. Mutational landscape of basal cell carcinomas by whole-exome sequencing. J. Investig. Dermatol. 2014;134:213–220. doi: 10.1038/jid.2013.276. [DOI] [PubMed] [Google Scholar]
- 36.Pellegrini C., Maturo M.G., Di Nardo L., Ciciarelli V., Gutierrez Garcia-Rodrigo C., Fargnoli M.C. Understanding the Molecular Genetics of Basal Cell Carcinoma. Int. J. Mol. Sci. 2017;18:2485. doi: 10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Bartolomeo L., Vaccaro F., Irrera N., Borgia F., Li Pomi F., Squadrito F., Vaccaro M. Wnt Signaling Pathways: From Inflammation to Non-Melanoma Skin Cancers. Int. J. Mol. Sci. 2023;24:1575. doi: 10.3390/ijms24021575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y., Liu H., Bian Q. Identification of Potential Biomarkers Associated with Basal Cell Carcinoma. BioMed Res. Int. 2020;2020:2073690. doi: 10.1155/2020/2073690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Argos M., Rahman M., Parvez F., Dignam J., Islam T., Quasem I., K. Hore S., T. Haider A., Hossain Z., I. Patwary T., et al. Baseline comorbidities in a skin cancer prevention trial in Bangladesh. Eur. J. Clin. Investig. 2013;43:579–588. doi: 10.1111/eci.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Rohil R.N., Tarasen A.J., Carlson J.A., Wang K., Johnson A., Yelensky R., Lipson D., Elvin J.A., Vergilio J., Ali S.M., et al. Evaluation of 122 advanced-stage cutaneous squamous cell carcinomas by comprehensive genomic profiling opens the door for new routes to targeted therapies. Cancer. 2016;122:249–257. doi: 10.1002/cncr.29738. [DOI] [PubMed] [Google Scholar]
- 41.Li Y.Y., Hanna G.J., Laga A.C., Haddad R.I., Lorch J.H., Hammerman P.S. Genomic analysis of metastatic cutaneous squamous cell carcinoma. Clin. Cancer Res. 2015;21:1447–1456. doi: 10.1158/1078-0432.Ccr-14-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.South A.P., Purdie K.J., Watt S.A., Haldenby S., den Breems N., Dimon M., Arron S.T., Kluk M.J., Aster J.C., McHugh A., et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J. Investig. Dermatol. 2014;134:2630–2638. doi: 10.1038/jid.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zilberg C., Lee M.W., Yu B., Ashford B., Kraitsek S., Ranson M., Shannon K., Cowley M., Iyer N.G., E Palme C., et al. Analysis of clinically relevant somatic mutations in high-risk head and neck cutaneous squamous cell carcinoma. Mod. Pathol. 2018;31:275–287. doi: 10.1038/modpathol.2017.128. [DOI] [PubMed] [Google Scholar]
- 44.Asplund A., Gry Bjorklund M., Sundquist C., Stromberg S., Edlund K., Östman A., Nilsson P., Pontén F., Lundeberg J. Expression profiling of microdissected cell populations selected from basal cells in normal epidermis and basal cell carcinoma. Br. J. Dermatol. 2008;158:527–538. doi: 10.1111/j.1365-2133.2007.08418.x. [DOI] [PubMed] [Google Scholar]
- 45.Nindl I., Dang C., Forschner T., Kuban R.J., Meyer T., Sterry W., Stockfleth E. Identification of differentially expressed genes in cutaneous squamous cell carcinoma by microarray expression profiling. Mol. Cancer. 2006;5:30. doi: 10.1186/1476-4598-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Driscoll L., McMorrow J., Doolan P., McKiernan E., Mehta J.P., Ryan E., Gammell P., Joyce H., O’Donovan N., Walsh N., et al. Investigation of the molecular profile of basal cell carcinoma using whole genome microarrays. Mol. Cancer. 2006;5:74. doi: 10.1186/1476-4598-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu M., Zloty D., Cowan B., Shapiro J., Haegert A., Bell R.H., Warshawski L., Carr N., McElwee K.J. Superficial, nodular, and morpheiform basal-cell carcinomas exhibit distinct gene expression profiles. J. Investig. Dermatol. 2008;128:1797–1805. doi: 10.1038/sj.jid.5701243. [DOI] [PubMed] [Google Scholar]
- 48.Heller E.R., Gor A., Wang D., Hu Q., Lucchese A., Kanduc D., Katdare M., Liu S., Sinha A. Molecular signatures of basal cell carcinoma susceptibility and pathogenesis: A genomic approach. Int. J. Oncol. 2013;42:583–596. doi: 10.3892/ijo.2012.1725. [DOI] [PubMed] [Google Scholar]
- 49.Dazard J.E., Gal H., Amariglio N., Rechavi G., Domany E., Givol D. Genome-wide comparison of human keratinocyte and squamous cell carcinoma responses to UVB irradiation: Implications for skin and epithelial cancer. Oncogene. 2003;22:2993–3006. doi: 10.1038/sj.onc.1206537. [DOI] [PubMed] [Google Scholar]
- 50.Dooley T.P., Reddy S.P., Wilborn T.W., Davis R.L. Biomarkers of human cutaneous squamous cell carcinoma from tissues and cell lines identified by DNA microarrays and qRT-PCR. Biochem. Biophys. Res. Commun. 2003;306:1026–1036. doi: 10.1016/s0006-291x(03)01099-4. [DOI] [PubMed] [Google Scholar]
- 51.Serewko M.M., Popa C., Dahler A.L., Smith L., Strutton G.M., Coman W., Dicker A.J., A Saunders N. Alterations in gene expression and activity during squamous cell carcinoma development. Cancer Res. 2002;62:3759–3765. [PubMed] [Google Scholar]
- 52.Nixon D.E., Mussmann G.V., Eckdahl S.J., Moyer T.P. Total arsenic in urine: Palladium-persulfate vs nickel as a matrix modifier for graphite furnace atomic absorption spectrophotometry. Clin. Chem. 1991;37:1575–1579. doi: 10.1093/clinchem/37.9.1575. [DOI] [PubMed] [Google Scholar]
- 53.Heinegård D., Tiderström G. Determination of serum creatinine by a direct colorimetric method. Clin. Chim. Acta. 1973;43:305–310. doi: 10.1016/0009-8981(73)90466-x. [DOI] [PubMed] [Google Scholar]
- 54.Kibriya M.G., Jasmine F., Parvez F., Argos M., Roy S., Paul-Brutus R., Islam T., Ahmed A., Rakibuz-Zaman M., Shinkle J., et al. Association between genome-wide copy number variation and arsenic-induced skin lesions: A prospective study. Environ. Health. 2017;16:75. doi: 10.1186/s12940-017-0283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bisceglia M., Panniello G., Galliani C.A., Centola M., D’Errico M.M., Minenna E., Tucci F.A., Ben-Dor D.J. Metastatic Basal Cell Carcinoma of the Skin: A Comprehensive Literature Review, Including Advances in Molecular Therapeutics. Adv. Anat. Pathol. 2020;27:331–353. doi: 10.1097/pap.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 57.Gambini D., Passoni E., Nazzaro G., Beltramini G., Tomasello G., Ghidini M., Kuhn E., Garrone O. Basal Cell Carcinoma and Hedgehog Pathway Inhibitors: Focus on Immune Response. Front. Med. 2022;9:893063. doi: 10.3389/fmed.2022.893063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martincorena I., Jones P.H., Campbell P.J. Constrained positive selection on cancer mutations in normal skin. Proc. Natl. Acad. Sci. USA. 2016;113:E1128–E1129. doi: 10.1073/pnas.1600910113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maturo M.G., Rachakonda S., Heidenreich B., Pellegrini C., Srinivas N., Requena C., Serra-Guillen C., Llombart B., Sanmartin O., Guillen C., et al. Coding and noncoding somatic mutations in candidate genes in basal cell carcinoma. Sci. Rep. 2020;10:8005. doi: 10.1038/s41598-020-65057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nawrocka P.M., Galka-Marciniak P., Urbanek-Trzeciak M.O., M-Thirusenthilarasan I., Szostak N., Philips A., Susok L., Sand M., Kozlowski P. Profile of Basal Cell Carcinoma Mutations and Copy Number Alterations-Focus on Gene-Associated Noncoding Variants. Front. Oncol. 2021;11:752579. doi: 10.3389/fonc.2021.752579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim Y.S., Park G.S., Chung Y.J., Lee J.H. Whole-exome sequencing of secondary tumors arising from nevus sebaceous revealed additional genomic alterations besides RAS mutations. J. Dermatol. 2023;50:1072–1075. doi: 10.1111/1346-8138.16784. [DOI] [PubMed] [Google Scholar]
- 62.Rawal R.M., Joshi M.N., Bhargava P., Shaikh I., Pandit A.S., Patel R.P., Patel S., Kothari K., Shah M., Saxena A., et al. Tobacco habituated and non-habituated subjects exhibit different mutational spectrums in head and neck squamous cell carcinoma. 3 Biotech. 2015;5:685–696. doi: 10.1007/s13205-014-0267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez M.F., Romano M.V., Martinez A.P., González A., Muchnik C., Stengel F.M., Mazzuoccolo L.D., Azurmendi P.J. Nevoid Basal Cell Carcinoma Syndrome: PTCH1 Mutation Profile and Expression of Genes Involved in the Hedgehog Pathway in Argentinian Patients. Cells. 2019;8:144. doi: 10.3390/cells8020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the supporting data are presented in the tables presented in the main manuscript and Supplementary Materials.