Abstract
We report that lysosomotropic agents and cysteine protease inhibitors inhibited protease-resistant prion protein accumulation in scrapie-infected neuroblastoma cells. The inhibition occurred without either apparent effects on normal prion protein biosynthesis or turnover or direct interactions with prion protein molecules. The findings introduce two new classes of inhibitors of the formation of protease-resistant prion protein.
The transmissible spongiform encephalopathies (TSEs), or prion diseases, constitute a group of related neurodegenerative disorders characterized by the accumulation in the central nervous system of an abnormal protease-resistant prion protein (PrP-res), which is made posttranslationally from its normal endogenous protease-sensitive isoform, PrP-sen, by an apparent conformational alteration rather than a modification of the covalent structure (for review, see reference 5). The accumulation of PrP-res is a central event in TSE pathogenesis, because it is correlated with infectivity and neurodegeneration (4, 19).
Recent outbreaks in younger people of new variant Creutzfeldt-Jakob disease (31) and of iatrogenic Creutzfeldt-Jakob disease by cadaveric dura grafting (1) have urged that therapies be developed for TSE diseases. One possible strategy for TSE therapy is to inhibit PrP-res formation in the infected host. Polyanions like sulfated glycans and Congo red inhibit PrP-res formation and scrapie agent replication in scrapie-infected neuroblastoma (ScNB) cells (6, 7, 9). Tetrapyrrole compounds have been recently identified as potent inhibitors of PrP-res formation in ScNB cells and in a cell-free system (11). Such polyanions and other classes of potential drugs, such as the polyene antibiotics and anthracycline, are also protective against scrapie in rodents when administered near the time of infection. However, these compounds have no therapeutic benefit if administered after the infection has been established (14–16, 18, 20, 29).
We have attempted to find a new class of inhibitors of PrP-res accumulation, not only for TSE therapy, but also for elucidating the mechanism of PrP-res accumulation. In this article, we report that lysosomotropic agents and cysteine protease inhibitors inhibit PrP-res accumulation in ScNB cells and, therefore, are new classes of potential anti-TSE drugs.
The compounds used in the study were obtained from Sigma, Aldrich, or Peptide Institute, Inc. (Osaka, Japan), and were used as received. The ScNB cultures were grown in minimal essential medium supplemented with 10% fetal bovine serum as described previously (24). Lysosomotropic agents and cysteine protease inhibitors, shown in Table 1, were added at various concentrations to the medium of cells seeded at 5% confluent density, and the cultures were allowed to grow to confluence for 4 days. The cells were then harvested and analyzed for PrP-res content by immunoblotting as described previously (9), except that an enhanced chemifluorescence reagent (JBL Scientific, Inc.) and a Storm PhosphorImager instrument (Molecular Dynamics) were used for visualizing and quantifying the PrP-res signals on the blots. Both the concentration of a compound giving 50% inhibition of PrP-res accumulation relative to the control (IC50) and the maximal concentration of a compound that does not affect the rate of cell growth to confluence were estimated from three independent experiments.
TABLE 1.
Inhibition of PrP-res accumulation in ScNB cells by lysosomotropic agents and cysteine protease inhibitors
| Compound | IC50 (μM)a | MC (μM)a |
|---|---|---|
| Lysosomotropic agents | ||
| Quinacrine | 0.4 ± 0.02 | 2.0 ± 0.25 |
| Tilorone | 1.3 ± 0.21 | 9.2 ± 1.44 |
| Chloroquine | 2.3 ± 0.53 | 4.2 ± 1.44 |
| Suramine | 12.3 ± 2.30 | >50b |
| Cysteine protease inhibitors | ||
| E-64d | 0.5 ± 0.11 | >100b |
| E-64 | 2.0 ± 0.30 | >625b |
| Leupeptin | 25.2 ± 4.12 | >125b |
Mean ± standard deviation.
MC, maximal tested concentration at which the cell growth to confluence is still tolerant.
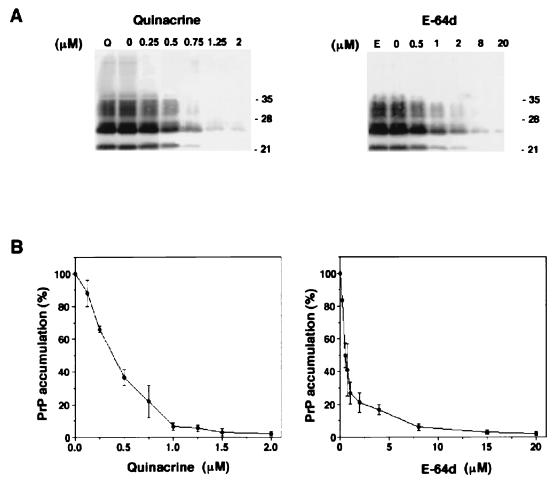
Among the compounds tested here, quinacrine and E-64d had better IC50s of 0.4 and 0.5 μM, respectively. E-64d did not show toxicity to cell growth at concentrations up to 100 μM, although quinacrine inhibited cell growth at more than 2.0 μM (Table 1 and Fig. 1).
FIG. 1.
Immunoblots of inhibition of PrP-res accumulation in ScNB cultures grown in quinacrine or E-64d (A) and normalized percent PrP-res accumulation versus concentration of quinacrine or E-64d (B). (A) ScNB cells were grown to confluence in the presence of the designated concentrations of quinacrine or E-64d. PrP-res was isolated from the cells and analyzed by immunoblotting as described in the text. For control experiments to examine the interference of the compounds with the detection of PrP-res, ScNB cell lysates were treated with 50 μM quinacrine (lane Q) or E-64d (lane E) before PK treatment and extraction for the detection of PrP-res by immunoblotting. The positions of molecular mass markers are designated in kilodaltons on the right. (B) PrP-res band intensities of the blots were quantified with a Storm PhosphorImager instrument. Normalization is relative to 100% for control experiments that contained only vehicle, and each data point represents the mean ± standard deviation of data from three independent experiments.
To control for the possibility that these effects were a result of artifactual interference with the detection of PrP-res, quinacrine or E-64d was added at 50 μM (about 100-fold higher than the IC50) to cell lysates before the addition of proteinase K (PK) and further processing for the detection of PrP-res. No effect on the PrP-res immunoblot band intensity was observed in comparison with that of untreated control cell lysates (Fig. 1A, lanes Q and E), indicating that neither quinacrine nor E-64d interfered with the PrP-res detection.
Because phospholipase-sensitive, cell surface PrP-sen is the precursor of PrP-res (3, 8), it was possible that the inhibition of PrP-res accumulation by these compounds could be due indirectly to an effect on PrP-sen metabolism. To investigate the specificity of the inhibition of PrP-res accumulation, we tested quinacrine and E-64d for effects on the metabolic labeling of cellular proteins and on the biosynthesis and turnover of PrP-sen. The [35S]methionine labeling of the cells and the immunoprecipitation of total 35S-PrP-sen were performed as described previously (9). For the cell surface PrP-sen detection, PrP-sen was immunoprecipitated from media treated with phosphatidylinositol-specific phospholipase C (PIPLC) to release pulse-35S-labeled PrP-sen from the cell surface as described previously (9). Quinacrine and E-64d were maintained at 1.5 and 15 μM, respectively, in the labeling media of all but the control cells.
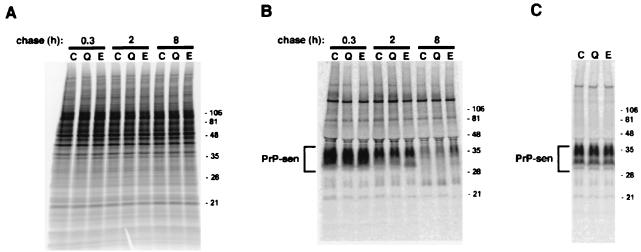
As shown in Fig. 2, little difference either in the overall profile of 35S-labeled proteins in the cells or in the whole or cell surface 35S-PrP-sen band intensities was observed. Thus, the inhibition of PrP-res accumulation by these compounds was not a result of effects on the protein biosynthesis in general or on the metabolic labeling and turnover of PrP-sen in particular.
FIG. 2.
Lack of effect of quinacrine or E-64d on the metabolic labeling of total proteins (A), total PrP-sen (B), and PIPLC-sensitive, cell surface PrP-sen (C). (A) Control flasks of ScNB cells (lanes C) were pulse-labeled as described previously (9) and then incubated in chase medium for the indicated chase time. The total lysate proteins were methanol precipitated from the detergent lysates of the cells and were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The quinacrine-treated flasks (lanes Q) and E-64d-treated flasks (lanes E) were treated identically, except that the preincubation, pulse, and chase media contained 1.5 μM quinacrine and 15 μM E-64d, respectively. Equal flask equivalents were loaded onto all lanes in each panel. The positions of molecular mass markers are designated in kilodaltons on the right. (B) PrP-sen was isolated from the total lysate proteins by immunoprecipitation as described previously (9) and was analyzed by SDS-PAGE. (C) PrP-sen was immunoprecipitated from the medium treated with PIPLC as described previously (9). Quinacrine or E-64d was included in all media, starting with the preincubation, except in the case of the control cells.
Previously reported PrP-res inhibitors, such as sulfated polyanions, Congo red, and tetrapyrrole compounds, appear to inhibit PrP-res formation by direct interactions with PrP molecules (6, 7, 9, 11). To study whether the lysosomotropic agents and cysteine protease inhibitors inhibit PrP-res formation in a similar manner, the effects of quinacrine or E-64d on PrP-res formation were examined in a highly specific, cell-free conversion reaction. PrP-res isolated from scrapie-infected hamster brain tissue was used to induce the conversion of immunoprecipitated hamster 35S-PrP-sen to 35S-PrP-res. Conversions in the absence or the presence of guanidine hydrochloride (GdnHCl) were performed as described previously (11).
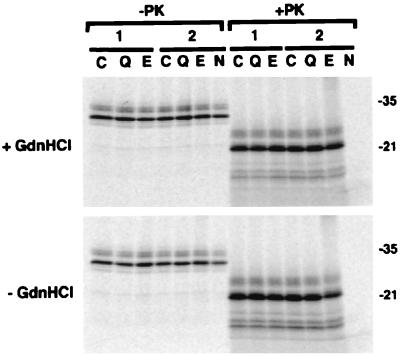
Quinacrine or E-64d was added at 2.5 mM (about 5,000-fold higher than the IC50 in ScNB cells) to the conversion reaction mixture containing 100 ng of PrP-res and 20 kcpm (about a 3.3-ng equivalent) of 35S-labeled PrP-sen in a total volume of 20 μl. No effect on the PrP-res formation in conversion reactions either with or without GdnHCl was observed in comparison with that in control conversion reactions (Fig. 3), indicating that neither quinacrine nor E-64d inhibited the PrP-res formation directly.
FIG. 3.
Lack of effect of quinacrine or E-64d on PrP-res formation in the cell-free conversion reactions under GdnHCl-containing (+GdnHCl) or GdnHCl-free (−GdnHCl) conditions. 35S-PrP-sen was incubated with unlabeled PrP-res in the absence (lanes C) or the presence of 2.5 mM quinacrine (lanes Q) or E-64d (lanes E) as described previously (11). One-tenth of the reaction mixture was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis without PK digestion (−PK), and the remainder was digested with PK (+PK). The designations 1 and 2 correspond to duplicate experiments. Controls containing no unlabeled PrP-res in the reaction mixtures are shown in lanes N. The positions of molecular mass markers are designated in kilodaltons on the right.
The findings in this article suggest that the lysosomotropic agents and cysteine protease inhibitors prevent PrP-res formation indirectly or reduce the PrP-res metabolic half-life, rather than either affecting normal PrP metabolism or blocking direct interactions of PrP molecules. Studies with ScNB cells have shown that the conversion from PrP-sen to PrP-res occurs at the plasma membrane or along an endocytic pathway to the lysosomes (8, 10) and that PrP-res appears to accumulate in the secondary lysosomes (23). Sulfated glycosaminoglycans (GAGs) especially heparan sulfate GAG, may play an essential role in the PrP-res formation or stabilization (9). Considering these findings, the following possible mechanisms of PrP-res inhibition by the lysosomotropic agents and cysteine protease inhibitors can be envisioned.
One possible mechanism is competitive inhibition of GAG-PrP interaction by the formation of insoluble GAG or of GAG-drug complexes. Most of the compounds tested here are known to cause lysosomal storage of insoluble sulfated GAG or GAG-drug complexes (13, 17, 22, 32). Another possible mechanism is unfolding of PrP-res by accumulated chaperone-like factor or factors, whose degradation in the endosome or lysosome is blocked by the compounds. An example of such a phenomenon would be the observation that yeast Sup35 is converted into the yeast prion-like factor [psi+] with the help of chaperone hsp104, but [psi+] is refolded into normal Sup35 and its amount is reduced by overexpressed hsp104 (12).
Another possible mechanism is destabilization of PrP-res conformation by an alteration of lysosomal pH. Some of the lysosomotropic agents are known to cause an increase in lysosomal pH (26). PrP molecules can be converted into a beta-sheet-rich form reminiscent of PrP-res by mild acidification (28, 33), indicating that acidic pH conditions may be important for PrP-res formation. However, a previous study (10) found that another lysosomotropic amine, NH4Cl, partially blocked the N-terminal truncation of PrP-res, but did not inhibit its formation under conditions similar to those used in the present study. This observation suggests either that elevation of lysosomal pH alone is not sufficient for inhibition of PrP-res formation or that quinacrine, tilorone, chloroquine, and suramine have more profound effects on lysosomal pH than NH4Cl.
Drug pharmacokinetics, side effects, toxicity, and delivery to the target organ are important therapeutic parameters. Some of the compounds tested herein are used in clinical medicine or are known to have medical applications. E-64d or E-64 derivatives were used and showed definite improvement in experimental animals with dystrophic myopathy or glomerulonephritis, in which increased activities of cysteine proteases are involved in pathogenesis (2, 21, 27, 30). These compounds were also used in humans in therapeutic trials for treatment of progressive muscular dystrophy, although clinical application of the compounds failed to prove the effects on the patients (25). An ability to penetrate the blood-brain barrier is expected to be helpful, because the central nervous system is a target organ where most of the PrP-res accumulates and TSE pathogenesis occurs. However, data on the penetration of the blood-brain barrier by these compounds are limited.
In conclusion, we have demonstrated that lysosomotropic agents and cysteine protease inhibitors block PrP-res accumulation in ScNB cells. The most potent of these compounds were quinacrine and E-64d. The inhibition was not caused by interference with the biosynthesis or turnover of PrP-sen and was not a result of direct interactions with PrP molecules.
Acknowledgments
We thank Hidemi Sasaki, Gregory Raymond, and Lynne Raymond for technical assistance.
This work was supported by a grant from the Japan Intractable Diseases Research Foundation (K.D.) and a grant from the Ministry of Health and Welfare, Japan (K.D.).
REFERENCES
- 1.Anonymous. Creutzfeldt-Jakob disease associated with cadaveric dura mater grafts—Japan, January 1979–May 1996. Morbid Mortal Weekly Rep. 1997;46:1066–1069. [PubMed] [Google Scholar]
- 2.Baricos W H, O'Connor S E, Cortez S L, Wu L T, Shah S V. The cysteine proteinase inhibitor, E-64, reduces proteinuria in an experimental model of glomerulonephritis. Biochem Biophys Res Commun. 1988;155:1318–1323. doi: 10.1016/s0006-291x(88)81285-3. [DOI] [PubMed] [Google Scholar]
- 3.Borchelt D R, Scott M, Taraboulos A, Stahl N, Prusiner S B. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990;110:743–752. doi: 10.1083/jcb.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruce M E, McBride P A, Farquhar C F. Precise targeting of the pathology of the sialoglycoprotein, PrP, and vacuolar degeneration in mouse scrapie. Neurosci Lett. 1989;102:1–6. doi: 10.1016/0304-3940(89)90298-x. [DOI] [PubMed] [Google Scholar]
- 5.Caughey B, Chesebro B. Prion protein and the transmissible spongiform encephalopathies. Trends Cell Biol. 1997;7:56–62. doi: 10.1016/S0962-8924(96)10054-4. [DOI] [PubMed] [Google Scholar]
- 6.Caughey B, Ernst D, Race R E. Congo red inhibition of scrapie agent replication. J Virol. 1993;67:6270–6272. doi: 10.1128/jvi.67.10.6270-6272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caughey B, Race R E. Potent inhibition of scrapie-associated PrP accumulation by congo red. J Neurochem. 1992;59:768–771. doi: 10.1111/j.1471-4159.1992.tb09437.x. [DOI] [PubMed] [Google Scholar]
- 8.Caughey B, Raymond G J. The scrapie-associated form of PrP is made from a cell surface precursor that is both protease- and phospholipase-sensitive. J Biol Chem. 1991;266:18217–18223. [PubMed] [Google Scholar]
- 9.Caughey B, Raymond G J. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J Virol. 1993;67:643–650. doi: 10.1128/jvi.67.2.643-650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caughey B, Raymond G J, Ernst D, Race R E. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J Virol. 1991;65:6597–6603. doi: 10.1128/jvi.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caughey W S, Raymond L D, Horiuchi M, Caughey B. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc Natl Acad Sci USA. 1998;95:12117–12122. doi: 10.1073/pnas.95.21.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chernoff Y O, Lindquist S L, Ono B, Inge V S, Liebman S W. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+] Science. 1995;268:880–884. doi: 10.1126/science.7754373. [DOI] [PubMed] [Google Scholar]
- 13.Christensen B, Lullmann R R. On the alcianophilia of the drug suramin used as a tool for inducing experimental mucopolysaccharidosis. Histochemistry. 1988;89:365–367. doi: 10.1007/BF00500638. [DOI] [PubMed] [Google Scholar]
- 14.Demaimay R, Adjou K T, Beringue V, Demart S, Lasmézas C I, Deslys J-P, Seman M, Dormont D. Late treatment with polyene antibiotics can prolong the survival time of scrapie-infected animals. J Virol. 1997;71:9685–9689. doi: 10.1128/jvi.71.12.9685-9689.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehlers B, Diringer H. Dextran sulphate 500 delays and prevents mouse scrapie by impairment of agent replication in spleen. J Gen Virol. 1984;65:1325–1330. doi: 10.1099/0022-1317-65-8-1325. [DOI] [PubMed] [Google Scholar]
- 16.Farquhar C F, Dickinson A G. Prolongation of scrapie incubation period by an injection of dextran sulphate 500 within the month before or after infection. J Gen Virol. 1986;67:463–473. doi: 10.1099/0022-1317-67-3-463. [DOI] [PubMed] [Google Scholar]
- 17.Hein L, Lullmann R R. Mucopolysaccharidosis and lipidosis in rats treated with tilorone analogues. Toxicology. 1989;58:145–154. doi: 10.1016/0300-483x(89)90004-8. [DOI] [PubMed] [Google Scholar]
- 18.Ingrosso L, Ladogana A, Pocchiari M. Congo red prolongs the incubation period in scrapie-infected hamsters. J Virol. 1995;69:506–508. doi: 10.1128/jvi.69.1.506-508.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jendroska K, Heinzel F P, Torchia M, Stowring L, Kretzschmar H A, Kon A, Stern A, Prusiner S B, DeArmond S J. Proteinase-resistant prion protein accumulation in Syrian hamster brain correlates with regional pathology and scrapie infectivity. Neurology. 1991;41:1482–1490. doi: 10.1212/wnl.41.9.1482. [DOI] [PubMed] [Google Scholar]
- 20.Kimberlin R H, Walker C A. Suppression of scrapie infection in mice by heteropolyanion 23, dextran sulfate, and some other polyanions. Antimicrob Agents Chemother. 1986;30:409–413. doi: 10.1128/aac.30.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Komatsu K, Inazuki K, Hosoya J, Satoh S. Beneficial effect of new thiol protease inhibitors, epoxide derivatives, on dystrophic mice. Exp Neurol. 1986;91:23–29. doi: 10.1016/0014-4886(86)90022-1. [DOI] [PubMed] [Google Scholar]
- 22.Lullmann-Rauch R, Pods R, von Witzendorff B. The antimalarials quinacrine and chloroquine induce weak lysosomal storage of sulphated glycosaminoglycans in cell culture and in vivo. Toxicology. 1996;110:27–37. doi: 10.1016/0300-483x(96)03319-7. [DOI] [PubMed] [Google Scholar]
- 23.McKinley M P, Taraboulos A, Kenaga L, Serban D, Stieber A, DeArmond S J, Prusiner S B, Gonatas N. Ultrastructural localization of scrapie prion proteins in cytoplasmic vesicles of infected cultured cells. Lab Investig. 1991;65:622–630. [PubMed] [Google Scholar]
- 24.Race R E, Caughey B, Graham K, Ernst D, Chesebro B. Analyses of frequency of infection, specific infectivity, and prion protein biosynthesis in scrapie-infected neuroblastoma cell clones. J Virol. 1988;62:2845–2849. doi: 10.1128/jvi.62.8.2845-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satoyoshi E. Therapeutic trials on progressive muscular dystrophy. Intern Med. 1992;31:841–846. doi: 10.2169/internalmedicine.31.841. [DOI] [PubMed] [Google Scholar]
- 26.Schneider P, Korolenko T A, Busch U. A review of drug-induced lysosomal disorders of the liver in man and laboratory animals. Microsc Res Tech. 1997;36:253–275. doi: 10.1002/(SICI)1097-0029(19970215)36:4<253::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Sugita H, Higuchi I, Sano M, Ishiura S. Trial of a cysteine proteinase inhibitor, EST, in experimental chloroquine myopathy in rats. Muscle Nerve. 1987;10:516–523. doi: 10.1002/mus.880100605. [DOI] [PubMed] [Google Scholar]
- 28.Swietnicki W, Petersen R, Gambetti P, Surewicz W K. pH-dependent stability and conformation of the recombinant human prion protein PrP(90-231) J Biol Chem. 1997;272:27517–27520. doi: 10.1074/jbc.272.44.27517. [DOI] [PubMed] [Google Scholar]
- 29.Tagliavini F, McArthur R A, Canciani B, Giaccone G, Porro M, Bugiani M, Lievens P M, Bugiani O, Peri E, Dall'Ara P, Rocchi M, Poli G, Forloni G, Bandiera T, Varasi M, Suarato A, Cassutti P, Cervini M A, Lansen J, Salmona M, Post C. Effectiveness of anthracycline against experimental prion disease in Syrian hamsters. Science. 1997;276:1119–1122. doi: 10.1126/science.276.5315.1119. [DOI] [PubMed] [Google Scholar]
- 30.Tamai M, Omura S, Kimura M, Hanada K, Sugita H. Prolongation of life span of dystrophic hamster by cysteine proteinase inhibitor, loxistation (EST) J Pharmacobio-Dyn. 1987;10:678–681. doi: 10.1248/bpb1978.10.678. [DOI] [PubMed] [Google Scholar]
- 31.Will R G, Ironside J W, Zeidler M, Cousens S N, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith P G. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. doi: 10.1016/s0140-6736(96)91412-9. [DOI] [PubMed] [Google Scholar]
- 32.Yanagishita M. Inhibition of intracellular degradation of proteoglycans by leupeptin in rat ovarian granulosa cells. J Biol Chem. 1985;260:11075–11082. [PubMed] [Google Scholar]
- 33.Zhang H, Stockel J, Mehlhorn I, Groth D, Baldwin M A, Prusiner S B, James T L, Cohen F E. Physical studies of conformational plasticity in a recombinant prion protein. Biochemistry. 1997;36:3543–3553. doi: 10.1021/bi961965r. [DOI] [PubMed] [Google Scholar]